Abstract
Objective:
This study aimed to assess the efficacy and safety of various neoadjuvant regimens for patients diagnosed with early-stage or locally advanced triple-negative breast cancer (TNBC).
Methods:
Medline, EMBASE, Cochrane Library, and Web of Science were searched in May 2020 to identify randomized controlled trials (RCTs). Bayesian network meta-analysis (NMA) was performed (Registration: PROSPERO CRD42020223012).
Results:
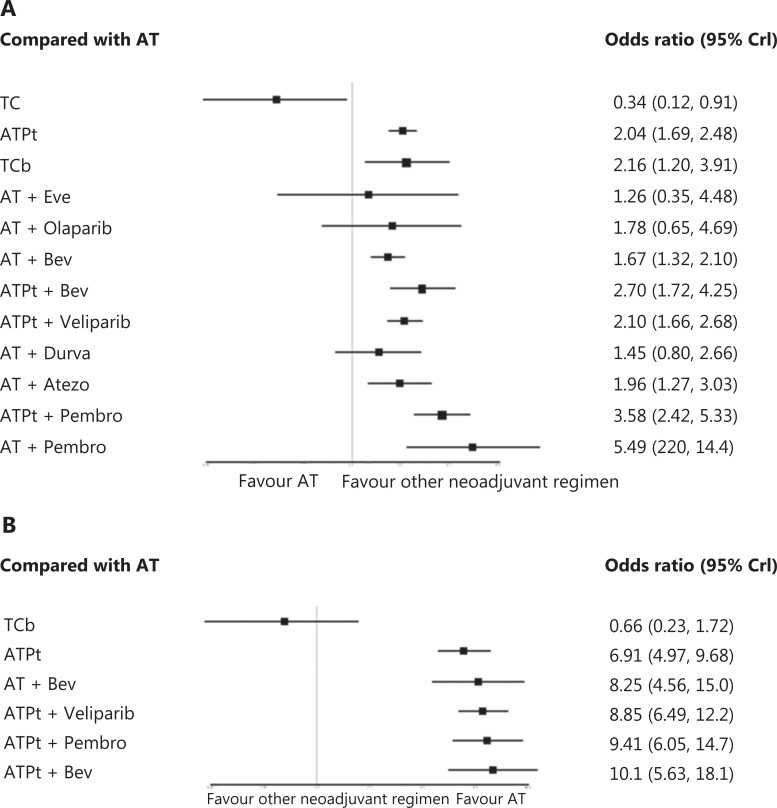
A total of 35 RCTs involving 8,424 participants were reviewed, of which 22 RCTs with 5,203 patients were included in this NMA focusing on pathologic complete response (pCR). An anthracycline-taxane-based (AT) regimen combined with a platinum (ATPt) [odds ratio (OR) = 2.04, 95% credible interval (CrI): 1.69, 2.48] regimen, and a docetaxel regimen combined with a carboplatin (TCb; OR = 2.16, 95% CrI: 1.20, 3.91) regimen improved pCR beyond that with AT only. AT and ATPt combined with targeted therapy [including bevacizumab (Bev), veliparib, atezolizumab, or pembrolizumab] also improved pCR. Five RCTs included in this NMA reported serious adverse events (SAEs) or grade ≥ 3 AEs. TCb was associated with fewer grade ≥ 3 AEs than was AT (OR = 0.66, 95% CrI: 0.23, 1.72) alone. In contrast, ATPt, AT + Bev, ATPt + Bev, ATPt + veliparib, and ATPt + pembrolizumab were associated with more SAEs than was AT alone.
Conclusions:
In patients with TNBC, platinum-based neoadjuvant regimens ATPt and TCb increase pCR beyond that with AT alone, but TCb appears to be better tolerated than either AT or ATPt. Platinum-based regimens combined with targeted therapies (Bev, PARPi, and PD-1/PD-L1 inhibitor) also improve the pCR rate beyond that with AT alone, but this benefit is accompanied by greater toxicity.
Keywords: Breast cancer, triple negative, neoadjuvant, network meta-analysis
Introduction
Triple-negative breast cancer (TNBC) is defined by cancer cells that lack estrogen receptors, progesterone receptors, and human epidermal growth factor receptor type 2 (HER2) expression. TNBC is a genetically heterogeneous, aggressive molecular subgroup of breast cancer (BC) that accounts for approximately 15%–20% of all BCs and often occurs in younger women1. Although many studies have been conducted on TNBC, its prognosis remains poor in the long term. Approximately 25%–30% of patients with early-stage TNBC are estimated to develop distant metastases within 3–5 years after diagnosis2. Although adjuvant therapy remains commonly used, neoadjuvant chemotherapy is now recognized as the standard of care for patients with TNBC3,4.
Neoadjuvant therapy, consisting of systemic therapy before surgical tumor removal, can downstage tumors, thus allowing for breast-conserving surgery and offering a valuable opportunity to monitor individual tumor responses1,5,6. Pathologic complete response (pCR) is used to interpret prognostic information, predict overall outcomes, and guide adjuvant therapy selection and decision-making2,7. Minckwitz et al.8 have reported a pooled analysis exploring the association between pCR and long-term clinical benefits in TNBC. The results indicated that patients who achieve a pCR have significantly better event-free survival and overall survival outcomes than those who do not; however, a similar difference was not observed in hormone receptor (HP)-positive patients. Achieving a pCR is thus highly prognostic in TNBC, because such patients have better survival in the long term. Although the Create-X9 study has recently shown that adding the adjuvant capecitabine after standard neoadjuvant chemotherapy prolongs overall survival in patients with TNBC with residual invasive disease on pathological testing, gaps exist in the medical knowledge regarding how best to increase the pCR rate for TNBC. Therefore, more individualized therapy strategies are needed for patients without pCR.
Several studies combining standard neoadjuvant regimens with platinum or targeted agents, such as bevacizumab (Bev), PARP inhibitors (PARPi), and PD-1/PD-L1 inhibitors, have been shown to improve pCR rates in TNBC3,4,10.
Most drugs used in neoadjuvant regimens can cause serious adverse effects (AEs) that may lead to poorer prognosis or death. Several studies have reported that participants withdrew or discontinued treatment because of severe toxicity11. Common AEs of neoadjuvant regimens include thrombocytopenia, neutropenia, anemia, myelogenous leukemia, alopecia, stomatitis, anorexia, pyrexia, conjunctivitis, cardiac disorder, and pigmentation12.
Because of the heterogeneity of TNBC and the variety of neoadjuvant regimens, finding the optimal neoadjuvant regimen to improve long-term outcomes in patients with early-stage TNBC remains a challenge in clinical practice. A previous meta-analysis13 has shown that a platinum-based regimen may be an option in the neoadjuvant setting; however, the regimen providing the best benefit/risk ratio when combined with targeted agents such as Bev, PARPi, and PD-1/PD-L1 inhibitors remains unknown. The toxicity of neoadjuvant regimens may be a barrier for clinicians, who might prefer to select better tolerated agents and dosages for patients with TNBC. To help clinicians choose appropriate treatments for patients with TNBC, we conducted a network meta-analysis (NMA) to assess the efficacy and safety of various neoadjuvant regimens for patients diagnosed with early-stage or locally advanced TNBC.
Materials and methods
This study was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42020223012)14. The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-NMA checklist15.
Search strategy and selection criteria
Medline, EMBASE, Cochrane Library, and Web of Science were searched from inception to September 2020, without limitations on the date/time, language, or document type. The reference lists of the included studies were examined to identify any additional relevant published or unpublished material not retrieved by the electronic search. Search strategies for all databases are described in detail in Online Appendix 1.
Randomized controlled trials (RCTs) fulfilling the following criteria were included: 1) patients with early or locally advanced TNBC (clinical stage of I–III or M0); 2) any neoadjuvant regimen (concurrent or sequential chemotherapy) including a single drug or a combination of any of the following drugs: paclitaxel, docetaxel, platinum/cisplatin/carboplatin/oxaliplatin, albumin paclitaxel, capecitabine/gemcitabine/5-fluorouracil, doxorubicin/epirubicin, cyclophosphamide, pembrolizumab/nivolumab/atezolizumab, veliparib/olaparib, or everolimus; and 3) any outcomes of interest, namely pCR (ypT0/is ypN0 or ypT0 ypN0), serious AEs (SAEs), or grade ≥ 3 AEs.
Patients in studies with subgroup analysis of TNBC were included only if they were stratified according to receptor status when randomized. Randomized controlled trials (RCTs) published only as abstracts without full articles or detailed reports were excluded from the analysis. Studies in a language other than English were excluded.
Screening, data extraction, and assessment of risk of bias
Four reviewers were divided into 2 groups to independently screen the articles (JL and LC; FQ and YZ), perform data extraction (JL and LC; FQ and YZ), and assess the risk of bias (JL and LC; WT and FQ). Disagreements were resolved by discussion, with assistance from a third party (ZW or ZS) if necessary. The 7 domains of the Cochrane Risk of Bias tool16 were evaluated, comprising sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. More details have been presented in our protocol14.
Statistical analysis
The primary objective was to compare pCR among all included RCT regimens in the network. The second objective was to compare aggregated AEs (defined as total SAEs or grade ≥3 AEs, owing to different AE reports in the RCTs) among all included RCT regimens in the network. A fixed-effect NMA within a Bayesian framework was performed in R 3.6.2 software (gemtc package)17. The pooled estimation and the probability of a given drug being optimal were obtained according to the Markov chain Monte Carlo method. The model convergence was assessed with trace plots and Brooks-Gelman-Rubin plots18. The results of dichotomous outcomes are reported as odds ratios (ORs) and credible intervals (CrIs). The ranking probabilities for all neoadjuvant regimens were estimated and are reported as the area under the cumulative ranking curve (SUCRA). Evidence inconsistency and clinical similarities in patient characteristics and settings across trials were carefully assessed before analysis. Network geometry was performed in STATA 16.0 software.
Results
Results of the search
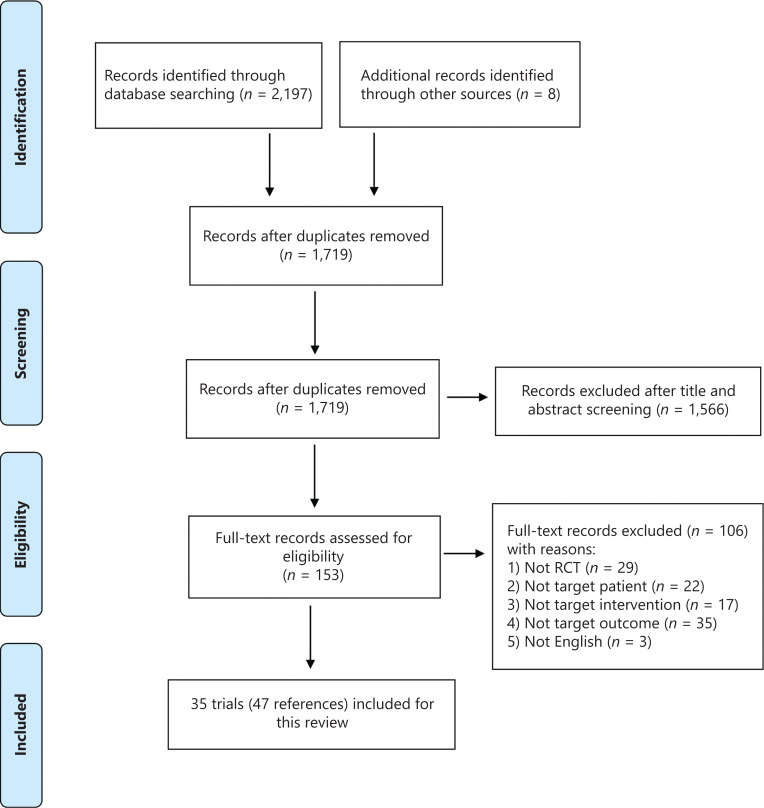
A total of 2,205 articles were identified [2,197 articles identified through an electronic database search in August 2020, and 8 articles identified from abstracts and posters for the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) annual meetings, and the San Antonio Breast Cancer Symposium (SABCS)]. After removal of duplicates, 1,719 articles were identified for screening. An additional 1,566 articles were excluded after inspection of the titles and abstracts. The remaining 153 articles were read in full, and 106 articles were subsequently excluded for various reasons (further details in Figure 1). Thirty-five RCTs (with 47 references) were eligible according to the inclusion criteria; of these, 22 RCTs (with 29 references) were included in the NMA.
Figure 1.
PRISMA flow diagram.
Characteristics of the included studies
A total of 35 RCTs (published from 2012 to 2020) involving 8,424 participants met the inclusion criteria for this review. A total of 28 RCTs (80%) were multicenter trials. Participants were recruited from South America (Brazil, Columbia), Mexico, the United States, Canada, Australia, Europe (including Belgium, Czechia, France, Germany, Hungary, Ireland, Italy, the Netherlands, Poland, Portugal, Russia, Spain, Sweden, Turkey, and the United Kingdom), and Asia (including China, India, Israel, Japan, Korea, and Singapore). The average age of the included participants was approximately 50 years. Table 1 (Online Appendix 4) and Online Appendix 2 (Supplementary Tables S1 and S2) provide more details on the study and population characteristics.
Table 1.
Characteristics of the included RCTs
| Study ID | Country | Center | Sample size at randomization | Clinical stage | Mean age (years) | BRCA (BRCA-1 or BRCA-2) mutation | Direct comparisons | Outcomes reported | Data extracted from subgroup analysis of RCT | Data in network meta-analysis (NMA) | Comparisons in NMA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alba 2012 | Spain | Multi | 94 | Non-metastatic (non-specific) | 47 (median) | NR | EC-T vs. EC-TCb | pCR; AE (grade 3–4) | No | Yes | AT vs. ATPt |
| Ando 2014 | Japan | Multi | 75 | II–IIIA | NR | NR | PCb-FEC vs. P-FEC | pCR | Yes | Yes | AT vs. ATPt |
| Bear 2012 | USA (Puerto Rico), Canada, India | Multi | 490 | T1c-T3; N0-N2a; M0 | NR | NR | T/TX/TG-AC + Bev vs. T/TX/TG-AC | pCR | Yes | No | NA |
| Chen 2016 | China | Multi | 102 | IIB or III | NR | NR | TC vs. TAC | pCR | Yes | Yes | AT vs. TC |
| Earl 2015 | UK | Multi | 248 | Early stage (non-specific) | NR | NR | T-FEC vs. T-FEC + Bev | pCR | Yes | Yes | AT vs. AT + Bev |
| Fasching 2019 | Germany | Multi | 77 | Early stage (non-specific) | NR | NR | P-EC + Ola vs. PCb-EC | pCR | Yes | Yes | AT + Ola vs. ATPt |
| Gerber 2013 | Germany | Multi | 678 | Untreated cT1c-T4d | 48 | 18.3%BRCA1 mutation 15.1%BRCA2 mutation 3.2% | EC-T + Bev vs. EC-T | pCR | Yes | Yes | AT vs. AT + Bev |
| Geyer 2017 | USA, Australia, Belgium, Canada, Czechia, France, Germany, Hungary, Italy, Korea, Netherlands, Russia, Spain, China (Taiwan), UK | Multi | 634 | Early stage (non-specific) | 50 (median) | NR | PCb-AC + Veli vs. PCb-AC vs. P-AC | pCR; AE (grade 3–4) | No | Yes | AT vs. ATPt vs. ATPt + Veli |
| Gianni 2018 | Australia, Germany, Italy, Russia, Singapore, Spain | Multi | 219 | T2N01; T3N0; T3N1; T4 any N; any T N2-3 | NR | NR | P-AC/EC/FEC vs. nabP-AC/EC/FEC | pCR | Yes | No | NA |
| Gigolaeva 2019 | Russia | NR | 192 | IIB–IIIA | 47 (median) | BRCA1 mutation 12.0% | AC-P vs. AC-q3w PCb/EriCb | pCR | No | Yes | AT vs. ATPt |
| Gluz 2018 | Germany | Multi | 336 | I–IV (IV-1.4%) | 50 | NR | q3w nabPG vs. q3w nabPCb | pCR; SAE | No | No | NA |
| Gonzalez-Angulo 2014 | Germany | Single | 62 | IIA–IIIC | 48 | NR | P-FEC vs. P-FEC + Eve | pCR | No | Yes | AT vs. AT + Eve |
| Harbeck 2020 | USA, Australia, Belgium, Brazil, Canada, Germany, Italy, Japan, Korea, Poland, Spain, China (Taiwan), UK | Multi | 333 | II–III | NR | NR | nabP-AC + Atezo vs. nabP-AC | pCR; AE (grade 3–5) | No | Yes | AT vs. AT + Atezo |
| Ishikawa 2016 | Japan | Single | 66 | I–IIIC | 53 | NR | TC vs. FEC-T | pCR | Yes | Yes | AT vs. TC |
| Jovanovic 2017 | USA | Multi | 145 | II or III | 52 | 4.0% | PCis + Eve vs. PCis | pCR; AE (grade 3–5) | No | No | NA |
| Kummel 2017 | Germany | Multi | 131 | cT2-T3 | NR | NR | Caba vs. P | pCR | Yes | No | NA |
| Llombart-Cussac 2015 | France, Germany, Spain | Multi | 141 | II–IIIA | NR | NR | P vs. P + weekly Ini vs. P + q2w Ini | pCR; treatment-related AE (grade 3–4) | No | No | NA |
| Loi 2019† | UK | Multi | 60 | Early stage (non-specific) | 48.5 (median) | NR | nabP-AC + Pembro vs. nabPCb-AC + Pembro vs. PCb-AC + Pembro | pCR; SAE | No | No | NA |
| Loibl 2018 | USA, Australia, Belgium, Canada, Czechia, France, Germany, Hungary, Italy, Korea, Netherlands, Russian, Spain, China (Taiwan), UK | Multi | 634 | II–III | 50 | Deleterious mutation 14.7% | PCb-AC + Veli vs. PCb-AC vs. P-AC | pCR; AE (grade 3–4) | No | Yes | AT vs. ATPt vs. ATPt + Veli |
| Loibl 2019 | Germany | Multi | 174 | Early stage (non-specific) | 49.5 | NR | nabP-AC + Durva vs. nabP-AC | pCR; SAE | No | Yes | AT vs. AT + Durva |
| Martinez 2015 | Mexico | NR | 61 | Locally advanced (non-specific) | 47 (median) | NR | P-FAC vs. PA + Cis | pCR | No | Yes | AT vs. ATPt |
| Mayer 2019 | USA | NR | 140 | I–III | NR | NR | Cis vs. P | pCR | No | No | NA |
| Nahleh 2016 | USA (Puerto Rico), India | Multi | 67 | IIB–IIIC | NR | NR | nabP-AC + Bev vs. AC-nabP | pCR | Yes | Yes | AT vs. AT + Bev |
| Nanda 2020 | USA | Multi | 88 | II–III | NR | NR | P-AC vs. P-AC + Pembro | pCR | Yes | Yes | AT vs. AT + Pembro |
| Rugo 2016 | USA | Multi | 60 | II–III | NR | NR | P-AC vs. PCb-AC + Veli | pCR | Yes | Yes | AT vs. ATPt + Veli |
| Schmid 2020 | USA, Australia, Brazil, Canada, Columbia, France, Germany, Ireland, Israel, Italy, Japan, Korea, Poland, Portugal, Russia, Singapore, Spain, Sweden, China (Taiwan), Turkey, UK | Multi | 1174 | II–III | NR | NR | PCb-AC/EC + Pembro vs. PCb-AC/EC | pCR; AE (grade ≥ 3) | No | Yes | ATPt vs. ATPt + Pembro |
| Schneeweiss 2019 | Germany | Multi | 403 | Early stage (non-specific) | NR | NR | AC-q2wP vs. PA + Cb | pCR | Yes | Yes | AT vs. ATPt |
| Sharma 2019 | USA | Multi | 100 | I–III | 52 (median) | 17.0% | PCb-AC vs. TCb | pCR; AE (grade 3–4) | No | Yes | ATPt vs. TCb |
| Sikov 2015 | USA | Multi | 454 | II–III | NR | NR | P-AC vs. P-AC + Bev vs. PCb-AC vs. PCb-AC + Bev | pCR; SAE | No | Yes | AT vs. ATPt vs. AT + Bev vs. ATPt + Bev |
| Tung 2020 | USA | Multi | 83 | I–III | NR | NR | Cis vs. AC | pCR | Yes | No | NA |
| Untch 2016 | Germany | Multi | 276 | Early stage (non-specific) | NR | NR | nabP-EC vs. P-EC | pCR | Yes | No | NA |
| Von Minckwitz 2014 | Germany | Multi | 315 | II–III | NR | 15.9% | PACb + Bev vs. PA + Bev | pCR | No | No | NA |
| Wu 2018 | China | Single | 128 | I–III | 47 (median) | NR | ET vs. ET + Loba | pCR | No | No | NA |
| Zhang 2016 | China | Single | 91 | II–III | 47 (median) | NR | q3w PCb vs. q3w PE | pCR | No | No | NA |
| Zhang 2020 | USA | Multi | 93 | Early stage (non-specific) | 49 (median) | Deleterious mutation 12.2% | TCb vs. EC-T | pCR | No | Yes | AT vs. TCb |
A, doxorubicin; SAE, serious adverse event; Atezo, atezolizumab; Bev, bevacizumab; BRCA mutation, mutations in 2 genes producing a hereditary breast-ovarian cancer syndrome; BRCA1, the first of these genes to be discovered; BRCA2, the second of these genes to be discovered; C, cyclophosphamide; Caba, cabazitaxel; Cb, carboplatin; Cis, cisplatin; Durva, durvalumab; E, epirubicin; Eve, everolimus; F, 5-fluorouracil; G, gemcitabine; Ini, iniparib; Loba, lobaplatin; nabP, albumin paclitaxel (weekly cycle if not specifically noted); NR, not reported; Ola, olaparib; P (weekly cycle if not specifically noted); Pt, platinum; pCR, pathologic complete response; Pembro, pembrolizumab; q2/3w: every 2/3 weeks; T, docetaxel; X, capecitabine. According to previous reports, guidelines, and clinical practice, a reasonable combination was made to maximize the inclusion of RCTs in NMA, which included the following: doxorubicin and epirubicin regarded as equal, cisplatin and carboplatin regarded as equal, TAC and AC-T regarded as equal, different sequential sequences regarded as equal (such as AC-P equal to P-AC, etc.). Citations for included RCTs are presented in Online Appendix 4. We excluded studies with interventions in only one study from this network meta-analysis (NMA). †Loi 2019 is a phase Ib study with 6 treatment arms exploring doses for chemotherapy combined with pembrolizumab, whose objective was not the primary focus in this NMA; each arm enrolled only 10 participants. We excluded this study from the outcome description and primary NMA analysis. In network meta-analysis, regimens including FECT, P-FAC, ACT, AC-nabP, and ACP were merged as anthracycline-taxane based (AT) regimens, and regimens including EC-TCb, PA + Cis/Cb, PCb-FEC, and PCb-AC were merged as anthracycline-taxane based + platinum (ATPt) regimens. (Sensitivity analyses were also performed on the basis of detailed regimens; details in Online Appendix 7: Supplementary Figures S7–S11 and Supplementary Tables S17–S22 and Online Appendix 8: Supplementary Figures S12–S15 and Supplementary Tables S23–S28).
All included RCTs reported pCR outcomes (neoadjuvant regimens in 22 RCTs were connected for NMA); 11 RCTs reported SAEs or grade ≥ 3 AEs (neoadjuvant regimens in 5 RCTs were connected for NMA). Data were extracted from subgroup analyses for TNBC in 15 RCTs. More details are presented in Online Appendix 2 (Supplementary Tables S3 and S4). A detailed risk of bias assessment is reported in Online Appendix 3 (Supplementary Figure S1).
Effects of interventions (pCR)
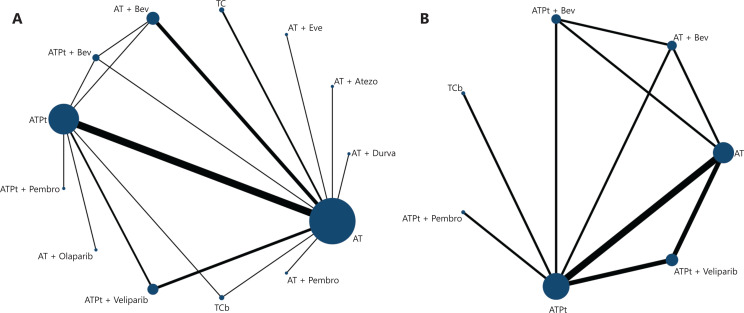
A total of 22 RCTs and 5,203 patients were included in the NMA, and a network plot is shown in Figure 2A (more details in Online Appendix 5: Supplementary Tables S5–8). An improved pCR was detected for the taxane-platinum-anthracycline (ATPt; OR = 2.04, 95% CrI: 1.69, 2.48) and docetaxel-carboplatin (TCb) (OR = 2.16, 95% CrI: 1.20, 3.91) chemotherapy regimens compared with the anthracycline-taxane-based (AT) regimen. The addition of Bev also improved pCR outcomes in patients receiving AT + Bev (OR = 1.67, 95% CrI: 1.32, 2.10) and the ATPt + Bev (OR = 2.70, 95% CrI: 1.72, 4.25). Combination with PARP inhibitors improved pCRs only for the ATPt + veliparib (OR = 2.10, 95% CrI: 1.66, 2.68) regimens. Adding PD-1/PD-L1 inhibitors improved pCRs in the AT + atezolizumab (OR = 1.96, 95% CrI: 1.27, 3.03), the AT + pembrolizumab (OR = 5.49, 95% CrI: 2.20, 14.4), and the ATPt + pembrolizumab (OR = 3.58, 95% CrI: 2.42, 5.33) regimens. (Figure 3A; Online Appendix 5: Supplementary Table S9).
Figure 2.
Network structure (A, pCR; B, aggregated AEs). Notes: Direct comparisons are represented by the black lines connecting the neoadjuvant therapy regimens. Line width is proportional to the number of trials including every pair of neoadjuvant regimens, whereas circle size is proportional to the total number of trials for each neoadjuvant regimen in the network. A, doxorubicin; Atezo, atezolizumab; Bev, bevacizumab; C, cyclophosphamide; Cb, carboplatin; Durva, durvalumab; Pt, platinum; pCR, pathologic complete response; Pembro, pembrolizumab; T, Taxane; In network meta-analysis, regimens including FEC-T, P-FAC, ACT, AC-nabP, and ACP (E, epirubicin; F, 5-fluorouracil; nabP, albumin paclitaxel) were merged as anthracycline-taxane based (AT) regimens, and regimens including EC-TCb, PA + Cis/Cb, PCb-FEC, and PCb-AC (Cis, cisplatin) were merged as anthracycline-taxane based + platinum (ATPt) regimens.
Figure 3.
Network meta-analysis (A, pCR; B, aggregated AEs). A. NMA results for all regimens compared with the AT regimen. B. NMA results for all regimens compared with the AT regimen. Notes: A, doxorubicin; Atezo, atezolizumab; Bev, bevacizumab; C, cyclophosphamide; Cb, carboplatin; Durva, durvalumab; Pt, platinum; pCR, pathologic complete response; Pembro, pembrolizumab; T, Taxane. In network meta-analysis, regimens including FECT, P-FAC, ACT, AC-nabP, and ACP (E, epirubicin; F, 5-fluorouracil; nabP, albumin paclitaxel) were merged as anthracycline-taxane based (AT) regimens, and regimens including EC-TCb, PA + Cis/Cb, PCb-FEC, and PCb-AC (Cis, cisplatin) were merged as anthracycline-taxane based + platinum (ATPt) regimens.
Safety
The incidence of aggregated AEs reported in RCTs is summarized in Table 2. A total of 5 RCTs with 2,965 patients were connected in the NMA, and a network plot is shown in Figure 2B (more details are shown in Online Appendix 6: Supplementary Tables S11–14). The incidence of aggregated AEs was lower with TCb than with AT (OR = 0.66, 95% CrI: 0.23, 1.72), but the difference was not statistically significant. In contrast, a significantly higher incidence of aggregated AE was observed with ATPt (OR = 6.91, 95% CrI: 4.97, 9.68), AT + Bev (OR = 8.25, 95% CrI: 4.56, 15.0), ATPt + Bev (OR = 10.1, 95% CrI: 5.63, 18.1), ATPt + veliparib (OR = 8.85, 95% CrI: 6.49, 12.2), and ATPt + pembrolizumab (OR = 9.41, 95% CrI: 6.05, 14.7) than with AT (Figure 3B; Online Appendix 6: Supplementary Table S15).
Table 2.
Incidence of aggregated AEs
| Study ID | Neoadjuvant regimen | Neoadjuvant regimen in network meta-analysis | No. of participants with aggregated AEs | Sample size | Incidence |
|---|---|---|---|---|---|
| Alba 2012 | EC-T | NA | 25 | 46 | 54.35% |
| Alba 2012 | EC-TCb | NA | 26 | 47 | 55.32% |
| Geyer 2017 | PCb-AC + Veli | ATPt + Veli | 272 | 316 | 86.08% |
| Geyer 2017 | PCb-AC | ATPt | 136 | 160 | 85.00% |
| Geyer 2017 | P-AC | AT | 71 | 158 | 44.94% |
| Gluz 2018 | q3w nabPG | NA | 31 | 178 | 17.42% |
| Gluz 2018 | q3w nabPCb | NA | 16 | 146 | 10.96% |
| Harbeck 2020 | nabP-AC + Atezo | NA | 103 | 165 | 62.42% |
| Harbeck 2020 | nabP-AC | NA | 101 | 168 | 60.12% |
| Jovanovic 2017 | PCis + Eve | NA | 22 | 96 | 22.92% |
| Jovanovic 2017 | PCis | NA | 6 | 49 | 12.24% |
| Llombart-Cussac 2015 | P | NA | 5 | 46 | 10.87% |
| Llombart-Cussac 2015 | P + weekly Ini | NA | 5 | 46 | 22.34% |
| Llombart-Cussac 2015 | P + q2w Ini | NA | 16 | 48 | 33.33% |
| Loibl 2018 | PCb-AC + Veli | ATPt + Veli | 222 | 313 | 70.93% |
| Loibl 2018 | PCb-AC | ATPt | 108 | 158 | 68.35% |
| Loibl 2018 | P-AC | AT | 23 | 157 | 14.65% |
| Loibl 2019 | nabP-AC + Durva | NA | 30 | 92 | 32.61% |
| Loibl 2019 | nabP-AC | NA | 29 | 82 | 35.37% |
| Schmid 2020 | PCb-AC/EC + Pembro | ATPt + Pembro | 633 | 781 | 81.05% |
| Schmid 2020 | PCb-AC/EC | ATPt | 295 | 389 | 75.84% |
| Sharma 2019 | PCb-AC | ATPt | 35 | 48 | 72.92% |
| Sharma 2019 | TCb | TCb | 11 | 52 | 21.15% |
| Sikov 2015 | P-AC | AT | 15 | 107 | 14.02% |
| Sikov 2015 | P-AC + Bev | AT + Bev | 39 | 105 | 37.14% |
| Sikov 2015 | PCb-AC | ATPt | 29 | 111 | 26.13% |
| Sikov 2015 | PCb-AC + Bev | ATPt + Bev | 46 | 110 | 41.82% |
A, doxorubicin; Atezo, atezolizumab; Bev, bevacizumab; C, cyclophosphamide; Cb, carboplatin; Cis, cisplatin; Durva, durvalumab; E, epirubicin; Eve, everolimus; G, gemcitabine; Ini, iniparib; nabP, albumin paclitaxel (weekly cycle if not specifically noted); No.: number; P, paclitaxel (weekly cycle if not specifically noted); Pt, platinum; Pembro, pembrolizumab; q2/3w: every 2/3 weeks; T, docetaxel; Veli, veliparib. In network meta-analysis, regimens including FECT, P-FAC, ACT, AC-nabP, and ACP were merged as anthracycline-taxane based (AT) regimens, and regimens including EC-TCb, PA + Cis/Cb, PCb-FEC, and PCb-AC were merged as anthracycline-taxane based + platinum (ATPt) regimens.
The trace plot and density plot showed a good degree of convergence (Online Appendix 5: Supplementary Figure S4 and Online Appendix 6: Supplementary Figure S6). Except for interventions in which the loop could not be constructed, we observed no significant inconsistencies between the direct and indirect results [inconsistency test results in Online Appendix 5 (Supplementary Figure S3)]. Online Appendix 5 and Online Appendix 6 show the mean SUCRA values for providing the hierarchy ranking of the different neoadjuvant regimens in terms of pCR (Supplementary Table S10 and Supplementary Figure S2) and aggregated AEs (Supplementary Table S16 and Supplementary Figure S5). The ranking might be highly biased, and interpretation should be made with caution. Funnel plots were not constructed because the number of included studies in one comparison was less than 10.
Discussion
TNBC presents a more proliferative pattern with a poorer prognosis than that of the HR-positive pattern, and the biological characteristics of TNBC remain unclear. Some studies have examined the biological characteristics of TNBC and their links to different treatment responses19. However, to date, chemotherapy-based treatment remains the first choice to decrease the risk of relapse, and insufficient evidence is available to recommend the routine addition of target drugs, such as immune checkpoint inhibitors, to neoadjuvant chemotherapy in patients with early-stage TNBC6.
Currently, neoadjuvant therapy is a standard treatment strategy that can decrease the relapse rate and prolong survival20. According to breast cancer guidelines, all adjuvant treatment regimens may be used20. To date, many RCTs evaluating neoadjuvant therapy in TNBC have been reported, and no evidence has indicated that any one regimen is superior to others.
As a surrogate for long-term survival21, pCR has been used as the primary endpoint in many neoadjuvant clinical trials. This NMA is the first to compare the efficacy and safety of neoadjuvant RCTs combining chemotherapy with VEGF inhibitors, PARP inhibitors, immunotherapy, and other drugs. In this NMA, most of the included studies used the pCR definition of ypT0/is ypN0, which is the most commonly used definition according to the Miller and Payne criteria. Several studies using ypT0 ypN0 were included; this limitation was a result of changes in the pCR definition over the years. In several articles reviewed herein22–27, to include as many studies in the NMA as possible, we considered 2 pCR definitions to be coincident, according to clinical practice. This NMA provides several findings of interest for physicians, because it compares neoadjuvant regimens that could not have been compared through conventional meta-analyses, owing to a lack of head-to-head evidence. Before analysis, clinical heterogeneity was fully discussed for the various regimens, and sensitivity analysis was conducted to assess the consistency of the conclusions (Online Appendix 7: Supplementary Figures S7–11 and Supplementary Tables S17–22 and Online Appendix 8: Supplementary Figures S12–15 and Supplementary Tables S23–28). The network inconsistency was also low in this analysis. In addition, we performed a comprehensive search with no limitations on language, date, document type, or publication status to identify all relevant published or unpublished RCTs. Four reviewers divided into 2 groups performed the screening, data extraction, and assessment independently to minimize possible bias in the review process.
NCCN guidelines20 recommend AC (where A indicates doxorubicin, and C indicates cyclophosphamide) followed by biweekly or weekly paclitaxel as the preferred regimens for HER2-negative breast cancer. AC-T (where T indicates docetaxel) (q3w) or TAC are both commonly used regimens for neoadjuvant/adjuvant therapy in clinical practice. The combination of carboplatin with paclitaxel/docetaxel can be used in patients with TNBC in preoperative settings but is not routinely recommended for most patients.
In this NMA, we found that adding platinum to an AT-based regimen resulted in a significantly greater pCR than observed with the AT regimen alone. Removing anthracycline from the taxane-platinum regimen showed a pCR benefit comparable to that of ATPt, but with a relatively better safety profile, possibly because it combines only 2 chemotherapeutic agents. We additionally conducted an analysis without combining similar regimens (Supplementary Figure S8). An improvement effect of pCR was detected for chemotherapy regimens including AC-nabP (where nabP indicates albumin paclitaxel; OR = 1.82, 95% CrI: 1.27, 2.65), TCb (OR = 2.38, 95% CrI: 1.03, 5.46), and PCb-AC (OR = 2.60, 95% CrI: 2.02, 3.36), as compared with AC-P (where P indicates paclitaxel) (Supplementary Figure S8A). Including a platinum agent in TNBC neoadjuvant therapy appears to be important to improve pCR benefits, and TCb appears to be effective but better tolerated than an ATPt regimen.
The VEGF inhibitor Bev combined with chemotherapy has demonstrated an improvement over chemotherapy alone, with respect to patient outcomes in several cancers, such as NSCLC28 and colorectal cancer29. In breast cancer, NCCN guidelines recommend Bev in combination with chemotherapy for only selected patients with recurrent or stage IV disease20. In this NMA, we report an improvement in pCR when Bev is added to chemotherapy in the neoadjuvant setting; adding platinum to AT plus Bev appears to be associated with even higher pCR rates, but this benefit is accompanied by higher toxicity (Online Appendix 5: Supplementary Table S9).
This NMA also suggests that the use of PD-1/PD-L1 inhibitors (including atezolizumab, pembrolizumab, and veliparib) combined with various chemotherapy regimens, compared with AT alone, significantly improves pCR in patients with TNBC. However, no clear difference was identified between AT plus durvalumab and AT alone. In addition, none of the regimens including a PD-1/PD-L1 inhibitor showed superiority to TCb (Online Appendix 5: Supplementary Table S9). Head-to-head trials are needed to confirm these data. In all reported studies, the chemotherapy regimens combined with PD-1/PD-L1 inhibitors were paclitaxel- or nab-paclitaxel-based dose dense regimens, but this combination was associated with a high incidence of aggregated AEs (Table 2)25,30,31. Additional clinical trials are thus needed to define the optimal chemotherapy regimen to be combined with a PD-1/PD-L1 inhibitor. The superiority of TCb vs. AT supports future clinical trials combining TCb with immunotherapy. The results of the NeoPACT (NCT03639948) study, an ongoing phase II single-arm clinical trial combining TCb with pembrolizumab in neoadjuvant treatment of TNBC, are awaited. Immunotherapy may also result in different responses according to the PD-L1 expression level, and patients with PD-L1-positive expression have been found to have higher pCR rates25,31,32. Therefore, additional research is needed to define which patients would benefit most from immunotherapy.
This NMA has some limitations. First, the methods (particularly random process and allocation, and the blinding of outcome assessment) were not always adequately reported in the included studies; thus, the risk was unclear for several domains of bias risk. Second, owing to a lack of head-to-head evidence and insufficient data in the included studies, we were unable to explore the comparative effects in some subgroups, such as BRCA mutation, dosage, and treatment duration. Third, owing to limited reports on survival data, long-term survival outcomes should be further assessed.
Conclusions
In conclusion, the key messages of this NMA are as follows. First, adding platinum to TNBC neoadjuvant therapy (ATPt and TCb) significantly increases pCR beyond that with AT alone. TCb and ATPt show comparable pCR rates, but TCb is better tolerated than ATPt. Second, adding Bev, veliparib, and PD-1/PD-L1 inhibitors to AT and ATPt improves pCR rates. We observed no significant differences between regimens, including PD-1/PD-L1, but ATPt plus PD-1/L1 inhibitor led to relatively higher rates of aggregated AEs. The increased efficacy of regimens should be balanced with patients’ quality of life.
Supporting Information
Acknowledgements
We thank all parties who provided help with this article and who are not mentioned above.
Grant support
This work was supported by funding from Sanofi. The funders had no involvement in any parts of this study. All authors confirm the independence of the researchers from funding sources.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Junjie Li, Li Chen, Wei Tan, Fang Qi, Yang Zhang, Zhonghua Wang, Zhimin Shao
Collected the data: Junjie Li, Li Chen, Yang Zhang, Zhonghua Wang
Contributed data or analysis tools: Junjie Li, Li Chen, Wei Tan, Fang Qi
Performed the analysis: Junjie Li, Li Chen, Wei Tan, Fang Qi
Wrote the paper: Junjie Li, Li Chen, Wei Tan, Fang Qi, Zhimin Shao
References
- 1.Tan AR. Triple-negative breast cancer: a clinician’s guide. Cham: Springer International Publishing AG; 2018. [Google Scholar]
- 2.Chaudhary LN. Early stage triple negative breast cancer: management and future directions. Semin Oncol. 2020;47:201–8. doi: 10.1053/j.seminoncol.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li ZH, Qiu YR, Lu WQ, Jiang Y, Wang J. Immunotherapeutic interventions of triple negative breast cancer. J Transl Med. 2018;16:147. doi: 10.1186/s12967-018-1514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 5.van la Parra RFD, Tadros AB, Checka CM, Rauch GM, Lucci A, Jr, Smith BD, et al. Baseline factors predicting a response to neoadjuvant chemotherapy with implications for non-surgical management of triple-negative breast cancer. Br J Surg. 2018;105:535–43. doi: 10.1002/bjs.10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. :JCO.20.03399. doi: 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabbs DJa. Breast pathology. 2nd ed. Philadelphia, Pennsylvania: Elsevier; 2017. [Google Scholar]
- 8.Cortazar PC, Zhang LJ, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 9.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Yost SE, Yuan Y. Neoadjuvant treatment for triple negative breast cancer: recent progresses and challenges. Cancers. 2020;12:1404. doi: 10.3390/cancers12061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caramelo O, Silva C, Caramelo F, Frutuoso C, Almeida-Santos T. The effect of neoadjuvant platinum-based chemotherapy in BRCA mutated triple negative breast cancers-systematic review and meta-analysis. Hered Cancer Clin Pract. 2019;17:11. doi: 10.1186/s13053-019-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wecker Le, Taylor DAe, Theobald RJ., Jr . Brody’s human pharmacology: mechanism-based therapeutics. 6th ed. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 13.Poggio F, Bruzzone M, Ceppi M, Pondé NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497–508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 14.Li JJC, Chen L, Wang ZH, Shao ZM. Different neoadjuvant treatment regimens of early triple negative breast cancer: a systematic review and network meta-analysis. Available from Netlibrary: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020223012. [Google Scholar]
- 15.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011] London: The Cochrane Collaboration; 2011. [Google Scholar]
- 17.Coreteam R. R: A Language and Environment for Statistical Computing. Available from Netlibrary: https://www.R-project.org/ [Google Scholar]
- 18.Gelman AR, Donald B. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7:457–72. [Google Scholar]
- 19.Jiang YZ, Ma D, Suo C, Shi JX, Xue MZ, Hu X, et al. Genomic and transcriptomic landscape of triple-negative breast cancers: subtypes and treatment strategies. Cancer Cell. 2019;35:428–40.e5. doi: 10.1016/j.ccell.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 20.NCCN. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (Version 1.2021) Available from Netlibrary: https://www.genomeweb.com/sites/default/files/nccn_bc_clinical_practice_guidelines.pdf. [Google Scholar]
- 21.Huang M, O’Shaughnessy J, Zhao J, Haiderali A, Fasching PA, Cortes J, et al. Evaluation of pathologic complete response as a surrogate for long-term survival outcomes in triple-negative breast cancer. J Natl Compr Canc Netw. 2020;18:1096–104. doi: 10.6004/jnccn.2020.7550. [DOI] [PubMed] [Google Scholar]
- 22.von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–56. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 23.Untch M, Jackisch C, Schneeweiss A, Loibl S, Salat C, Denkert C, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17:345–56. doi: 10.1016/S1470-2045(15)00542-2. [DOI] [PubMed] [Google Scholar]
- 24.Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, et al. Comparison of neoadjuvant nab-paclitaxel + carboplatin vs nab-paclitaxel + gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. J Natl Cancer Inst. 2018;110:628–37. doi: 10.1093/jnci/djx258. [DOI] [PubMed] [Google Scholar]
- 25.Schmid P, Cortes J, Pusztai L, Mcarthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–21. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 26.Fasching PA, Link T, Hauke J, Seither F, Jackisch C, Klare P, et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study) Ann Oncol. 2021;32:49–57. doi: 10.1016/j.annonc.2020.10.471. [DOI] [PubMed] [Google Scholar]
- 27.Loi S, Schmid P, Cortes J, Muñoz-Couselo E, Kim SB, Sohn J, et al. Abstract P3-10-09: Relationship between tumor infiltrating lymphocytes (TILs) and response to pembrolizumab (Pembro)+chemotherapy (Chemo) as neoadjuvant treatment (NAT) for triple-negative breast cancer (TNBC): phase Ib KEYNOTE-173 trial. Cancer Res. 2019;79:3–10. [Google Scholar]
- 28.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 29.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 30.Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30:1279–88. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 31.Harbeck N, Zhang H, Barrios CH, Saji S, Mittendorf E. LBA11 - IMpassion031: results from a phase III study of neoadjuvant (neoadj) atezolizumab + chemotherapy in early triple-negative breast cancer (TNBC) Ann Oncol. 2020;31:S1142–215. [Google Scholar]
- 32.Bianchini G, Huang C, Egle D, Bermejo B, Zamagni C, Thill M, et al. Tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Presented at: 2020 ESMO Virtual Congress; September 19-21; LBA13; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.