Abstract
Objective
Despite the recent increase in the use of minimally invasive approaches to mitral valve surgery in patients with a prior sternotomy, the outcomes of the robotic approach to mitral valve surgery in this patient population have not been examined.
Methods
We retrospectively reviewed 342 consecutive patients who underwent mitral valve surgery after a prior sternotomy between 2013 and 2020, in which the robotic approach was used in 21 patients (6.1%). We reviewed the clinical details of these 21 patients.
Results
The median age was 71 years [interquartile range 64.00, 74.00 years], and mean Society of Thoracic Surgeons Predicted Risk of Mortality was 4.2% ± 3.8%. The indication for mitral valve surgery was degenerative mitral valve disease in 33.3% (7/21), functional disease in 28.6% (6/21), mixed disease in 4.8% (1/21), rheumatic disease in 9.5% (2/21), and failed repair for degenerative disease in 23.8% (5/21). No cases required conversion from robotic assistance to alternative approaches, there were no intraoperative deaths, and intraoperative transesophageal echocardiogram confirmed complete elimination of mitral regurgitation in 90.5% (19/21) of cases. Thirty-day mortality was 0.0% (0/21), and 1-year mortality was 4.8% (1/21). There were no strokes or wound infections at 30 days, and 14.3% (3/21) of patients received intraoperative blood product transfusions.
Conclusions
The results of this retrospective review suggest that the robotic approach to mitral valve surgery in patients with a prior sternotomy is safe in experienced hands. Although some centers have considered prior sternotomy a relative contraindication to robotic mitral valve surgery, this approach is feasible and can be considered an option for experienced surgeons.
Key Words: robotic mitral valve surgery, prior sternotomy, redo sternotomy, mitral valve, reoperation
Abbreviations and Acronyms: CABG, coronary artery bypass graft; LOS, length of stay; MR, mitral regurgitation
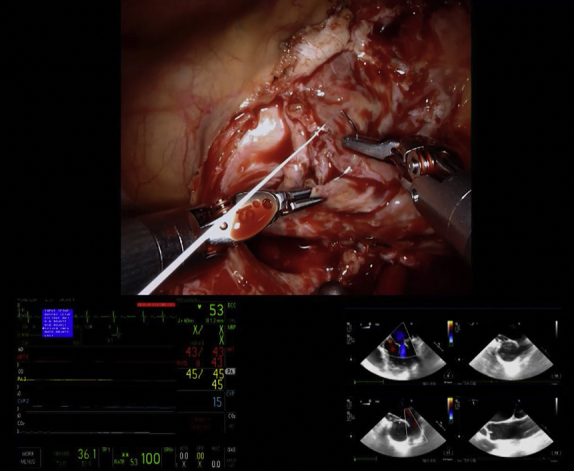
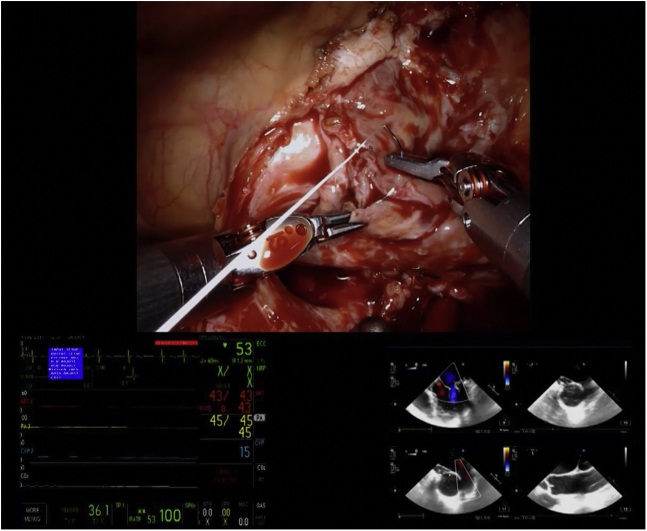
Mitral valve repair with corresponding echocardiographic imaging.
Central Message.
Although some centers have considered prior sternotomy a relative contraindication to robotic mitral valve surgery, this approach is feasible and can be considered an option for experienced surgeons.
Perspective.
The findings of this study indicate that robotic approach to mitral valve surgery in patients who have had a prior sternotomy is safe and feasible. In experienced hands, this approach can be considered as a treatment option.
See Commentary on page 52.
Reoperation occurs in 10% of patients undergoing cardiac surgeries and is associated with an increased risk of perioperative morbidity and mortality relative to the index procedure.1,2 Reoperations for cardiac surgery have traditionally been performed via a median sternotomy. However, this approach is invasive and associated with increased blood loss and length of stay (LOS) relative to minimally invasive approaches.1 These risks, along with increased experience with minimally invasive strategies, have led to more frequent use of the port-access approach for mitral valve surgery in the setting of prior sternotomy.3, 4, 5, 6, 7 At some centers, there has also been a growing experience with robotic-assisted minimally invasive approaches in patients undergoing mitral valve surgery.8, 9, 10, 11 However, prior sternotomy has been considered a relative contraindication to robotic mitral valve surgery for reasons related to adhesions and lack of experience with robotic techniques. Despite these concerns, the robotic technique has been applied to reoperative mitral valve surgery in a small number of cases, but the safety of this approach has not been closely examined.12, 13, 14 As our center has gained more experience with robotic mitral valve surgery, we have selected this strategy for a number of patients with a prior sternotomy. Thus, we reviewed our series of patients with prior sternotomy who underwent robotic mitral valve surgery to evaluate the safety and feasibility of this approach.
Methods
Patient Selection
A retrospective review of consecutive patients with a history of prior sternotomy undergoing robotic-assisted mitral valve surgery performed by the senior author (R.L.S.) at Baylor Scott & White The Heart Hospital Plano from 2013 to 2020 was performed. Patient demographics, baseline characteristics, and in-hospital outcomes were defined and coded according to the Society of Thoracic Surgeons Adult Cardiac Surgery Database, versions 2.6-2.8. This study was approved by the Baylor Scott & White Research Institute institutional review board (#014-209, approved July 27, 2021). The requirement for informed consent was waived due to the retrospective nature of the study.
Study End Points
The outcomes of interest were success of robotic mitral valve surgery without open conversion, planned mitral valve repair without conversion to replacement, and reduction of mitral regurgitation on echocardiographic imaging. Further outcomes of interest included intraoperative blood use, postoperative intensive care unit LOS, and total hospital LOS. We also evaluated postoperative complications including new-onset atrial fibrillation, neurologic events, renal failure, and reoperation for bleeding or valve dysfunction. Finally, we ascertained short- and long-term vital status via systematic chart review and patient phone calls according to a previously validated protocol.15
Statistical Analysis
Categorical variables are presented as proportions and continuous variables as mean ± standard deviation or median [interquartile range] after evaluating whether the data were normally distributed. Survival time with censoring status, as well as 30-day and 1-year mortality, were calculated based on the date of surgery and date of last seen or called.
Results
A total of 342 patients underwent mitral valve surgery after a prior sternotomy, 120 (35.1%) with a median sternotomy and 222 (64.9%) with a minimally invasive approach. Of these 222 patients, robotic assistance was used in 21 (9.5%). The median age was 71 [64.0, 74.0] years, and 66.7% (14/21) of patients were male (Table 1). Mean Society of Thoracic Surgeons Predicted Risk of Mortality was 4.2% ± 3.8%. Prior sternotomy was performed for isolated coronary artery bypass graft (CABG) in 42.9% (9/21), isolated mitral valve repair in 28.6% (6/21), concomitant aortic valve replacement and CABG in 19.0% (4/21), and for atrial septal defect closures in 9.5% (2/21). The median time between the prior sternotomy and the robotic mitral valve surgery was 136,16 years. Preoperative echocardiography showed severe mitral regurgitation (MR) in 90.5% (19/21) of patients, moderate-to-severe MR in 4.8% (1/21), and moderate MR in 4.8% (1/21). In addition, 95.2% (20/21) of patients had no mitral stenosis, and 4.8% (1/21) had moderate mitral stenosis.
Table 1.
Baseline characteristics
| Variables | All patients (n = 21) |
|---|---|
| Age | 71 [64.0, 74.0] |
| Male | 14 (66.7) |
| Peripheral arterial disease | 3 (14.3) |
| Cerebrovascular accident | 1 (4.8) |
| CVD transient ischemic attack | 1 (4.8) |
| Hypertension | 18 (85.7) |
| Diabetes | 2 (9.5) |
| Dialysis-dependence | 0 (0.0) |
| Endocarditis | 0 (0.0) |
| Atrial fibrillation | 16 (76.2) |
| Coronary artery disease | 12 (57.1) |
| LVEF, % | 37.95 ± 19.78 |
| STS score, % | 4.2 ± 3.8 |
| Previous myocardial infarction | 7 (33.3) |
| Previous sternotomy procedure | |
| Isolated CABG | 9 (42.9) |
| Isolated mitral valve repair | 6 (28.6) |
| SAVR + CABG | 4 (19.0) |
| Atrial septal defect closure | 2 (9.5) |
| Mitral regurgitation grade | |
| Moderate | 1 (4.8) |
| Moderate-to-severe | 1 (4.8) |
| Severe | 19 (90.4) |
| Mitral stenosis grade | |
| None | 20 (95.2) |
| Moderate | 1 (4.8) |
Values are median [IQR], mean ± SD, or absolute count (%), as appropriate. CVD, Cerebrovascular disease; LVEF, left ventricular ejection fraction; STS, Society of Thoracic Surgeons; CABG, coronary artery bypass graft; SAVR, surgical aortic valve replacement; IQR, interquartile range; SD, standard deviation.
In the 15 patients without prior mitral valve repair, the indication for surgery was degenerative mitral valve disease in 46.7% (7/15), functional disease in 40.0% (6/15), mixed disease in 6.7% (1/15), and rheumatic disease in 6.7% (1/15) (Table 2). In the 6 patients who had previously undergone mitral valve repair, the indication for surgery was failed repair for degenerative disease in 83.3% (5/6) and rheumatic mitral valve insufficiency in 16.7% (1/6). No patients had endocarditis.
Table 2.
Operative data
| Variable | All patients (n = 21) |
|---|---|
| Etiology | |
| Degenerative | 7 (33.3) |
| Failed prior MV procedure | 5 (23.8) |
| Functional | 6 (28.6) |
| Rheumatic | 2 (9.5) |
| Mixed | 1 (4.8) |
| MV procedure type | |
| Repair | 17 (81.0) |
| Replacement | 4 (19.0) |
| Cannulation method | |
| Axillary artery | 2 (9.5) |
| Femoral artery | 19 (90.5) |
| Concomitant procedure | |
| Tricuspid valve surgery | 8 (38.1) |
| Cryoablation | 3 (14.3) |
| Aortic occlusion | 16 (76.2) |
| Aortic occlusion method | |
| Balloon | 16 (100.0) |
| Cold fibrillation | 5 (23.8) |
| CPB time, min | 196 ± 40 |
| Aortic occlusion time, min | 108 ± 33 |
Values are median [IQR], mean ± SD, or absolute count (%), as appropriate. MV, Mitral valve; CPB, cardiopulmonary bypass; IQR, interquartile range; SD, standard deviation.
Bypass was established peripherally through a femoral artery in 90.5% of patients (19/21) and through an axillary artery in 9.5% (2/21). Endoaortic balloon occlusion with both antegrade and retrograde del Nido cardioplegia was used in 76.2% (16/21) of patients, and cold fibrillatory arrest was used in 23.8% (5/21). Indications for cold fibrillatory arrest were porcelain aorta in 20.0% (1/5) and concerns about achieving adequate delivery of cardioplegia due to prior CABG in 80.0% (4/5). Concomitantly, 14.3% (3/21) of patients underwent cryoablation to treat long-standing persistent or paroxysmal atrial fibrillation, and 38.1% (8/21) underwent tricuspid valve repair. Mean cardiopulmonary bypass time was 196 ± 40 minutes in all patients, and aortic occlusion time was 108 ± 33 minutes in patients not undergoing cold fibrillatory arrest.
A mitral valve replacement was planned preoperatively in 3 patients: 2 had MR, 1 due to rheumatic disease and the other due to a failed repair of a congenital anterior leaflet cleft complicated by significant fibrosis, and 1 had mitral stenosis and regurgitation due to rheumatic disease. Of the remaining patients, all of whom were planned for mitral valve repair, successful repair was performed in 94.4% (17/18) (Figure 1; Video 1). One patient underwent a conversion to mitral valve replacement following inadequate reduction in MR secondary to leaflet calcification limiting the mobility of the anterior and commissural leaflets after attempted repair. No cases required conversion from robotic assistance to alternative approaches, and there were no intraoperative deaths. Intraoperative transesophageal echocardiogram confirmed complete elimination of MR in 90.5% (19/21) of cases (Table 3). The remaining 2 patients’ intraoperative transesophageal echocardiogram showed trace/trivial MR. Before discharge, repeat echocardiography was performed, showing 57.1% (12/21) with no MR, 28.6% (6/21) with trace/trivial MR, and 14.3% (3/21) with mild MR. No patients had postoperative mitral stenosis.
Figure 1.
Mitral valve repair with corresponding echocardiographic imaging.
Table 3.
Postoperative echocardiographic data
| Variable | Postoperative (n = 21) | Discharge (n = 21) |
|---|---|---|
| Mitral regurgitation grade | ||
| None | 19 (90.5) | 12 (57.1) |
| Trace/trivial | 2 (9.5) | 6 (28.6) |
| Mild | 0 (0.0) | 3 (14.3) |
Values are absolute count (%), as appropriate.
Thirty-day mortality was 0.0% (0/21), and 1-year mortality was 4.8% (1/21) (Table 4). There were no strokes or wound infections at 30 days, and 14.3% (3/21) received intraoperative blood product transfusions. Postoperatively, 14.3% (3/21) of patients developed atrial fibrillation, 4.8% (1/21) required permanent pacemaker implantation, and 4.8% (1/21) developed acute kidney injury that did not require dialysis. One patient was readmitted with acute heart failure symptoms and was found to have a dehisced mitral valve ring. This patient initially underwent a mitral valve repair for functional mitral insufficiency and a rigid ring was placed. The patient was taken emergently for a port access mitral valve replacement 15 days postoperatively and was discharged home in stable condition. Discharge to home was achieved in 85.7% (18/21), and 14.3% (3/21) were discharged to in-patient rehabilitation. Median LOS was 6 [5, 7] days. The median follow-up time was 3 [2, 4] years.
Table 4.
Clinical outcomes
| Variables | All patients (n = 21) |
|---|---|
| Survival | |
| In-hospital mortality | 0 (0.0) |
| 30-d mortality | 0 (0.0) |
| 1-y mortality | 1 (4.8) |
| 30-d morbidity | |
| Stroke | 0 (0.0) |
| New-onset atrial fibrillation | 3 (14.3) |
| New permanent pacemaker | 1 (4.8) |
| Renal failure | 1 (4.8) |
| Other outcomes | |
| Duration of ICU stay, h | 56.16 [42.1, 100] |
| Postoperative vent time, h | 6.3 [4.0, 13.3] |
| Postoperative LOS, d | 6 [5, 7] |
| Discharge location | |
| Home | 18 (85.7) |
| Rehab | 3 (14.3) |
Values are median [IQR], mean ± SD, or absolute count (%), as appropriate. ICU, Intensive care unit; LOS, length of stay; IQR, interquartile range; SD, standard deviation.
Discussion
In this report, we retrospectively analyzed 21 patients with a prior sternotomy who underwent robotic mitral valve surgery. Our primary findings were the following: (1) conversion to a median sternotomy or port-access approach was unnecessary, (2) mitral valve repair was successful in 94.4% of cases in which repair was intended preoperatively, and (3) intraoperative blood use was minimal. Furthermore, the need for reoperation was infrequent, occurring in one patient.
The correction of mitral valve regurgitation can be achieved with 1 of 4 main approaches: standard median sternotomy, nonrobotic right thoracotomy, robotically assisted right thoracotomy, and transcatheter intervention (MitraClip). The MitraClip repair is typically reserved for high-risk and inoperable patients, as the repair may not be as durable over time. Relative to the minimally invasive surgical approaches, a notable advantage of mitral valve surgery via a standard median sternotomy is increased visualization, enhancing the ability to perform concomitant procedures and control myocardial protection. However, minimally invasive approaches, when compared with sternotomy, are associated with less blood loss, shorter LOS, and decreased time of recovery. Within minimally invasive mitral valve surgery, the robotic approach may offer further advantages over the port access approach, including improved visualization. Disadvantages of the robotic approach, however, include a potentially steep technical learning curve, the risk of emergent conversion, increased cost, and limited ability to perform concomitant procedures. Overall, there is no difference in the type of procedures that can be performed when comparing the robotic approach with the port-access approach.
Given the increased invasiveness of sternotomy, the minimally invasive right thoracotomy approach to mitral valve surgery in patients with a prior sternotomy has been increasingly reported.1,3,5, 6, 7 In the reoperative setting specifically, sternotomy also poses a risk of injury to mediastinal structures, particularly those which may be in close opposition to the chest wall including the aorta, right ventricle, and bypass grafts.1, 2, 3,16, 17, 18, 19 However, while a small number of cases have been described,7,12, 13, 14 the safety and efficacy of robotic assisted mitral valve surgery in the setting of prior sternotomy have not been closely examined.
We have not considered prior sternotomy an absolute contraindication to a robotic approach in our patients, including patients with prior right-sided grafts, if they are otherwise appropriate candidates for this technique. In such cases, intra-thoracic adhesions in the presence of a right chest thoracostomy are taken down with electrocautery and sharp dissection via both the robotic and port access approaches. Furthermore, in patients who have previously undergone revascularization, computed tomography angiography of the chest, abdomen, and pelvis are performed to plan peripheral cannulation strategy, and cold fibrillatory arrest is used in patients with fragile coronary anatomy. When mitral valve repair is planned preoperatively, the senior author prefers a robotic approach over port access or sternotomy for patients undergoing mitral valve surgery, whether or not the patient has a history of prior sternotomy. However, if a mitral valve replacement is planned preoperatively, we commonly default to a port-access approach without robotic assistance, as the incision needed to accommodate a space-occupying prosthesis minimizes the cosmetic advantages of the robotic approach. In the 3 cases in this series in which a mitral valve replacement was planned preoperatively, the patients specifically requested use of the robot.
Our data suggest that the robotic approach is safe and feasible when performed by an experienced robotic mitral valve surgical team. There was nearly uniform technical success given the reduction of MR, and a high rate of mitral valve repair was achieved. Perioperative morbidity and intraoperative blood use were low, and the majority of patients were able to be discharged home following surgery. Because our experience with this approach is still limited, our primary focus was on safety, leading to longer LOS. Surgeons developing a robotic mitral valve surgery program may seek more straightforward cases to build their early experience. However, as their experience grows, prior sternotomy may not represent a strong contraindication to a robotic approach in an otherwise appropriate candidate.
Our study has a number of limitations to be acknowledged. It is a retrospective study derived from a single surgeon's experience with a limited sample size. Thus, our results may not be generalizable to other settings, particularly to centers and surgeons without significant experience in robotic mitral valve surgery. In addition, when using robotic surgery, cost is an important consideration that was not evaluated in this study. While the data support the safety and feasibility of this approach with an experienced team, no conclusions can be drawn regarding the relative superiority or inferiority of the robotic approach as compared to other approaches in patients with prior sternotomy.
Conclusions
This retrospective review of 21 consecutive patients with prior sternotomy undergoing robotic mitral valve surgery suggests that this approach is safe in experienced hands. Technical success and mitral valve repair rates were excellent, and postoperative outcomes including 30-day and 1-year mortality were acceptable despite a high-risk patient profile. Although prior sternotomy has been considered a contraindication to robotic surgery at some centers, this approach is feasible and can be considered an option for experienced robotic surgical teams.
Conflict of Interest Statement
R.L.S. reports honoraria from Edwards Lifesciences, Abbott, and CryoLife; institutional grants from Abbott; is a member of the advisory board of Edwards Lifesciences; and national trial leadership, including principal investigator of the CLASP IID, SITRAL, and ENRAPT-US trials. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
The efforts of T.G.M., A.T.L., J.J.S., E.S., and M.H. were supported by a philanthropic grant from Satish and Yasmin Gupta.
T. G. Meidan and A. T. Lanfear contributed equally to this article.
Contributor Information
Talia G. Meidan, Email: Talia.meidan@bswhealth.org.
the Redo Robotic Mitral Valve Surgery Collaborative:
Timothy J. George, Kelley A. Hutcheson, Emily Shih, Allison I. Aldrich, Lena Hasson, and Sri Charitha Koneru
Supplementary Data
Dr Robert L. Smith using a robotic approach to perform a redo mitral valve repair with excision of a dehisced prior annuloplasty ring. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00104-3/fulltext.
References
- 1.Mehaffey H.J., Hawkins R.B., Schubert S., Fonner C., Yarboro L.T., Quader M., et al. Contemporary outcomes in reoperative mitral valve surgery. Heart. 2018;104:652–656. doi: 10.1136/heartjnl-2017-312047. [DOI] [PubMed] [Google Scholar]
- 2.Seeburger J., Borger M.A., Falk V., Passage J., Walther T., Doll N., et al. Minimally invasive mitral valve surgery after previous sternotomy: experience in 181 patients. Ann Thorac Surg. 2009;87:709–714. doi: 10.1016/j.athoracsur.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Kilic A., Acker M.A., Gleason T.G., Sultan I., Vemulapalli S., Thibault D., et al. Clinical outcomes of mitral valve reoperations in the United States: an analysis of the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2019;107:754–759. doi: 10.1016/j.athoracsur.2018.08.083. [DOI] [PubMed] [Google Scholar]
- 4.Botta L., Cannata A., Bruschi G., Fratto P., Taglieri C., Russo C.F., et al. Minimally invasive approach for redo mitral valve surgery. J Thorac Dis. 2013;5(suppl 6):S686–S693. doi: 10.3978/j.issn.2072-1439.2013.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Losenno K.L., Jones P.M., Valdis M., Fox S.A., Kiaii B., Chu M.W. Higher-risk mitral valve operations after previous sternotomy: endoscopic, minimally invasive approach improves patient outcomes. Can J Surg. 2016;59:399–406. doi: 10.1503/cjs.004516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q., Xue X., Yang J., Yang Q., Wang P., Wang L., et al. Right mini-thoracotomy approach reduces hospital stay and transfusion of mitral or tricuspid valve reoperation with non-inferior efficacy: evidence from propensity-matched study. J Thorac Dis. 2018;10:4789–4800. doi: 10.21037/jtd.2018.07.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy D.A., Moss E., Miller J., Halkos M.E. Repeat robotic endoscopic mitral valve operation: a safe and effective strategy. Ann Thorac Surg. 2018;105:1704–1709. doi: 10.1016/j.athoracsur.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Chemtob R.A., Wierup P., Mick S.L., Javorski M.J., Burns D.J.P., Blackstone E.H., et al. A conservative screening algorithm to determine candidacy for robotic mitral valve surgery. J Thorac Cardiovasc Surg. December 17, 2020 doi: 10.1016/j.jtcvs.2020.12.036. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Ramzy D., Trento A., Cheng W., De Robertis M.A., Mirocha J., Ruzza A., et al. Three hundred robotic-assisted mitral valve repairs: the Cedars-Sinai experience. J Thorac Cardiovasc Surg. 2014;147:228–235. doi: 10.1016/j.jtcvs.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 10.Murphy D.A., Moss E., Binongo J., Miller J.S., Macheers S.K., Sarin E.L., et al. The expanding role of endoscopic robotics in mitral valve surgery: 1,257 consecutive procedures. Ann Thorac Surg. 2015;100:1675–1682. doi: 10.1016/j.athoracsur.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 11.Hamandi M., Hafan L., Squiers J.J., Lanfear A.T., DiMaio J.M., Smith R.L. A review of robotic mitral valve surgery. Structural Heart. 2020;5:151–157. [Google Scholar]
- 12.Patel H., Lewis C.T.P., Stephens R.L., Angelillo M., Sibley D.H. Minimally invasive redo mitral valve replacement using a robotic-assisted approach. Innovations (Phila) 2017;12:375–377. doi: 10.1097/IMI.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 13.Spiller R., Symalla T., McCrorey M., Balkhy H.H. Robotic-assisted third-time redo mitral valve replacement. Ann Thorac Surg. 2019;108:e245–e247. doi: 10.1016/j.athoracsur.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Arcidi J.M., Jr., Rodriguez E., Elbeery J.R., Nifong L.W., Efird J.T., Chitwood W.R., Jr. Fifteen-year experience with minimally invasive approach for reoperations involving the mitral valve. J Thorac Cardiovasc Surg. 2012;143:1062–1068. doi: 10.1016/j.jtcvs.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Wooley J., Neatherlin H., Mahoney C., Squiers J.J., Tabachnick D., Suresh M., et al. Description of a method to obtain complete one-year follow up in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Am J Cardiol. 2018;121:758–761. doi: 10.1016/j.amjcard.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Meyer S.R., Szeto W.Y., Augoustides J.G., Morris R.J., Vernick W.J., Paschal D., et al. Reoperative mitral valve surgery by the port access minithoracotomy approach is safe and effective. Ann Thorac Surg. 2009;87:1426–1430. doi: 10.1016/j.athoracsur.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 17.Modi P., Hassan A., Chitwood W.R., Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2008;34:943–952. doi: 10.1016/j.ejcts.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 18.Casselman F.P., La Meir M., Jeanmart H., Mazzarro E., Coddens J., Van Praet F., et al. Endoscopic mitral and tricuspid valve surgery after previous cardiac surgery. Circulation. 2007;116:I270–I275. doi: 10.1161/CIRCULATIONAHA.106.680314. [DOI] [PubMed] [Google Scholar]
- 19.Murzi M., Miceli A., Di Stefano G., Cerillo A.G., Farneti P., Solinas M., et al. Minimally invasive right thoracotomy approach for mitral valve surgery in patients with previous sternotomy: a single institution experience with 173 patients. J Thorac Cardiovasc Surg. 2014;148:2763–2768. doi: 10.1016/j.jtcvs.2014.07.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dr Robert L. Smith using a robotic approach to perform a redo mitral valve repair with excision of a dehisced prior annuloplasty ring. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00104-3/fulltext.