Abstract
Dementia is a devastating disease associated with aging. Alzheimer’s disease is the most common form of dementia, followed by vascular dementia. In addition to clinically diagnosed dementia, cognitive dysfunction has been reported in diabetic patients. Recent studies are now beginning to recognize type 2 diabetes mellitus, characterized by chronic hyperglycemia and insulin resistance, as a risk factor for Alzheimer’s disease and other cognitive disorders. While studies on insulin action have remained traditionally in the domain of peripheral tissues, the detrimental effects of insulin resistance in the central nervous system on cognitive dysfunction are increasingly being reported by recent clinical and preclinical studies. The findings from these studies suggest that antidiabetic drugs have the potential to be used to treat dementia. In this review, we discuss the physiological functions of insulin in the brain, studies on the evaluation of cognitive function under conditions of insulin resistance, and reports on the beneficial actions of antidiabetic drugs in the brain. This review covers clinical studies as well as investigations in animal models and will further highlight the emerging link between insulin resistance and neurodegenerative disorders.
Keywords: Insulin resistance, Metabolic syndrome, Obesity, Diabetes, Dementia, Alzheimer’s disease
Introduction
Chronic hyperglycemia, measured as glycosylated hemoglobin A1c (HbA1c), directly correlates with diabetic complications including peripheral neuropathy, retinopathy, nephropathy, and cardiovascular diseases. However, how diabetes affects brain function has not been a subject of serious investigation for a long time. With the increasing awareness and the availability of a vast array of antidiabetic medications, diabetic patients are living longer and in the process are at greater risk for aging-associated diseases, including dementia. This is evident from the increasing number of recent studies on cognitive function in diabetic patients. Studies on insulin action have primarily remained in the domain of peripheral diseases. Although insulin was known to regulate feeding behavior, it was generally considered that the brain is not a major target of insulin action. However, studies are beginning to recognize that insulin plays a role in cognition and neuroprotection. Brain function can be also affected indirectly by insulin action on peripheral tissues. For example, the circulating factors in obesity and diabetes can pass through blood brain barrier (BBB) and cause dysfunction of several brain cell types, including neurons, astrocytes and microglia. Insufficient metabolites may be also generated in the periphery for utilization in the brain in conditions of insulin resistance.
Insulin Signaling in the Brain
Insulin Transport across BBB
Insulin, produced and released by the pancreatic β cells, is known to act in the brain in addition to peripheral tissues. Insulin is readily transported across the BBB via a receptormediated process. However, it has been suggested that this transport pathway can be saturable given that the cerebrospinal fluid (CSF) insulin levels are non-linearly correlated with plasma insulin levels [1]. Levels of insulin in the brain vary dramatically, with hypothalamus and olfactory bulb having insulin concentrations higher than plasma insulin levels [2]. Ependymal cells and brain endothelial cells (BECs) that make up the BBB and blood-CSF barrier possess insulin-binding sites, allowing insulin to be transported across the BBB [3]. Insulin receptors (IR) on endothelial cells are likely to be essential for the transport of circulating insulin to its target tissues. Interestingly, using the same animal model in which the IR gene is deleted in endothelial cells, two studies came up with different findings on insulin transport across BBB. Konishi et al. (2017) reported that in response to systemic insulin stimulation, delayed insulin signaling was observed in skeletal muscle, hypothalamus, hippocampus and prefrontal cortex but not in liver or olfactory bulb [4]. The delay in insulin signaling in the hypothalamus resulted in increased food intake and obesity. However, employing kinetic methods, another study demonstrated that the endothelial IR gene is not required for insulin transport across the BBB [5]. Although the findings of these studies appear to contradict, one study [5] measured the transport of insulin directly whereas the other study [4] examined insulin action in the target tissue. In vitro transport models using purified BECs in monoculture or BECs co-cultured with astrocytes, found that the IR is responsible for physically transporting insulin across the BBB [6]. Decreased transport of insulin into brain has been observed in obese animals. The transport rate of insulin across the BBB is about half that in obese mice compared to lean mice [7]. Unlike obesity, diabetes mellitus was shown to increase BBB insulin transport and endothelial insulin binding [8]. Streptozotocin-induced diabetic mice were observed to have enhanced transport of insulin across the BBB and this was not attributed to altered levels of serum factors such as glucose or insulin. Thus, the transport of insulin across BBB is likely to involve multiple mechanisms.
Insulin Production in the Brain
The brain is also a source of insulin in addition to peripheral insulin getting transported across the BBB. Insulin was shown to be widely distributed in the human cadaveric brain by radioimmunoassay [9]. Birch et al. (1984) later identified two forms of immunoreactive insulin in cultures of fetal mouse brain [10]. The major component resembled proinsulin which was converted by trypsin to the minor form, similar to pancreatic insulin. Deltour et al. (1993) examined the expression of proinsulin genes and detected proinsulin II mRNA in the mouse brain [11]. The authors suggested that insulin may act as a growth factor in the developing mouse brain. Similarly, another study examined the expression of Insulin I and II mRNA in multiple rat tissues and reported the presence of insulin II mRNA but not insulin I mRNA in the rat brain [12]. The same group later confirmed the presence of insulin mRNA in CA1 and CA3 regions of the hippocampus and in granule cell layer of the olfactory bulbs of the neonatal rabbit brain by in situ hybridization [13]. Another study identified the presence of insulin mRNA in both mouse and human brain samples [14]. In the post-mortem brain cortex of elderly and AD subjects, c-peptide and insulin concentrations were found to be directly correlated and decrease with aging [15]. These findings led the authors to conclude that, at least in part, cerebral insulin was a product of the brain itself. Neuronal cells derived from the hippocampus and olfactory bulb have been shown to produce insulin [16]. An interesting study demonstrated the presence of insulin mRNA in the GABAergic neurogliaform cells of rat brain, using single-cell quantitative RT-PCR [17]. Importantly, they also observed that the quantity of insulin II mRNA per cell was greater when the concentration of extracellular glucose was increased. The authors also demonstrated that the effects of locally generated insulin can be blocked with insulin receptor antagonist S961. Thus, there are sufficient experimental evidences to suggest that insulin is produced by the brain.
Insulin Receptor Expression in the Brain
Havrankova et al. (1978) were the first to report the presence of IR in the rat brain, based on insulin binding studies [2]. A later study reported the autoradiographic localization of IR in rat brain by 125I-labeled insulin binding experiments [18]. The insulin binding was shown to be prominent in the neocortex, accessory motor areas of the basal ganglia and the cerebellum. Further studies showed that IRs are abundantly distributed throughout the brain, especially in regions that regulate appetite (arcuate nucleus of the hypothalamus), cognitive function, autonomic activity, and olfaction (olfactory bulb) [19, 20]. They are also selectively distributed in the cerebral cortex and cerebellum. The presence of IR/A and IR/B, and the isoforms of IR have been demonstrated by in situ RT-PCR/FISH assay in neurons of adult human brain [21]. Expression of IR/IGF-IR hybrid receptor in neurons, astrocytes and microglia has been reported in mouse brain [22]. A study with fluorescently labeled insulin has shown that intranasally administered insulin reaches CNS targets and activates IR [23]. Thus, previous studies present sufficient experimental evidences to suggest that IR is expressed in the brain.
Insulin and Glucose Uptake in the Brain
It has been well established that insulin stimulates glucose uptake in peripheral tissues (Fig. 1), such as skeletal muscle and adipose tissue, through GLUT4, a glucose transporter [24]. However, studies suggest that insulin may not be involved in the cellular glucose uptake in the brain [25]. The majority of glucose uptake by the central nervous system (CNS) occurs via GLUT3 in neurons, GLUT1 in astrocytes, and GLUT5 in microglia [26]. Insulin-insensitive GLUT2 is found in some cells in the hypothalamus. GLUT4 is largely limited to a few regions of the brain, including the olfactory bulb, hippocampus, hypothalamus, and other regions associated with motor movement in the cerebellum [27-29]. Deletion of GLUT4 from the CNS in mice (BG4KO) resulted in impaired glucose sensing and reduced glucose uptake in the brain [30]. In rats, GLUT4 is co-expressed with GLUT3 in the hippocampus, amygdala, basal forebrain, and, to lesser degrees, in the cerebellum and cerebral cortex [31]. All these regions are related to cognitive behaviors. GLUT4 has been suggested to play key role in hippocampal memory formation [32]. However, Talbot et al. (2012) reported that insulin had no effect on GLUT4 translocation to the membrane and was unable to induce cellular glucose uptake in neuronal cells [33]. Insulin and IGF-1 were found to synergistically stimulate the translocation of GLUT1 to the plasma membrane, promoting astrocytic glucose uptake [34]. Similarly, insulin may facilitate neuronal glucose uptake by translocation of GLUT3 to the cell membrane [35]. However, Gould et al. (1992) reported that GLUT3 mediated glucose uptake in the neurons of mice occurs independent of insulin action [36]. Therefore, previous studies on the regulation of glucose transport by insulin have reported mixed findings. It appears that insulin may not play a significant role in the uptake of glucose in the brain.
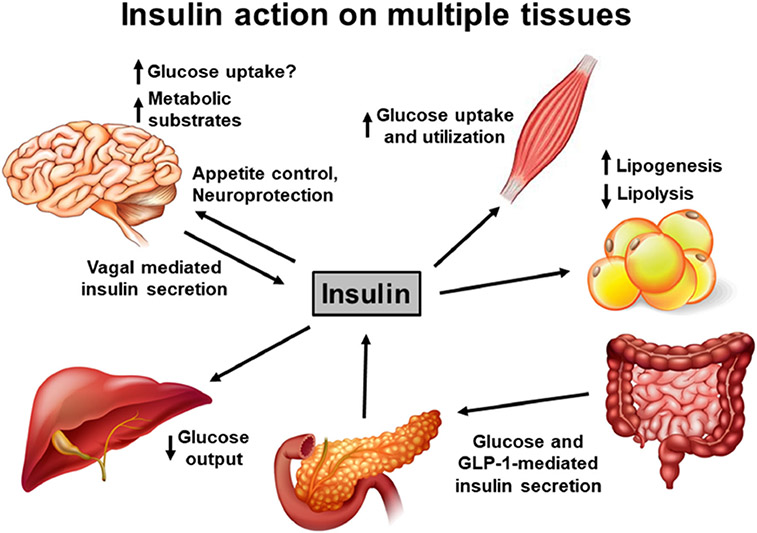
Fig. 1.
Insulin action on multiple tissues. Insulin is primarily produced by the β cells of the pancreatic islets and secreted in response to glucose. In addition, food consumption promotes intestinal secretion of GLP-1, which acts on the β cells to enhance insulin release. Insulin release may also be modulated by the vagus nerve. Insulin acts on multiple tissues to regulate glucose metabolism. It stimulates glucose uptake and utilization in the muscle, while suppressing hepatic glucose release. Insulin promotes lipogenesis and suppresses lipolysis in the adipose tissue. While the effect of insulin action in the brain on appetite control is known, it may not play a significant role in glucose uptake. Insulin action on neuroprotection and cognitive function are being recognized recently
Insulin Action on Eating Behavior, Learning, and Memory
Hypothalamic insulin action is known to regulate eating behavior. Dodd et al. (2019) examined the effects of enhancing hypothalamic insulin sensitivity on feeding behavior in a mouse model [37]. Deletion of tyrosine phosphatases that downregulate insulin signaling resulted in restrained eating. Intranasal administration of inhibitors of tyrosine phosphatases also produced similar effects. In a 9-year follow-up study [38], the authors demonstrated that improving insulin sensitivity before lifestyle intervention results in enhanced reduction in visceral fat. It has been shown that either peripheral or intracerebroventricular administration of insulin leads to positive effects on learning and memory [39]. These improvements in cognition via insulin administration have also been shown to activate IR and its downstream signaling cascade [40]. A double-blind study investigated the effects of intranasal insulin administration for 8 weeks on memory, attention and mood in healthy subjects [41]. Improvement in delayed recall of words and signs of enhanced mood were observed in the absence of systemic side effects. The same group later tested the effects of a rapid acting insulin analog and reported better memory improvement in healthy subjects [42]. Insulin can also act as a neuroprotective agent by preventing neuronal apoptosis and by inhibiting of Aβ fibril formation [43]. A single dose of intranasal insulin was shown increase brain glucose metabolism as measured by FDG-PET and activated Akt in wild type mice [44]. Thus, previous studies have suggested a physiological role of insulin in the normal brain function.
Insulin Signaling and Alzheimer’s Disease
Insulin signaling and AD lesions have been shown to have a two-way relationship. While brain insulin resistance plays a role in Alzheimer’s pathogenesis, AD lesions also have deleterious effects on peripheral insulin signaling and glucose homeostasis, leading to a vicious cycle. Alzheimer’s patients are reported to have higher levels of insulin in the blood, a marker for peripheral insulin resistance, and decreased insulin in the cerebrospinal fluid, compared to healthy controls [45]. Lower cerebral insulin levels and central insulin resistance are in part due to decreased insulin transport across the BBB and excessive amyloid accumulation [8]. In addition, the brains of AD patients have decreased levels of both insulin and insulin-like growth factors (IGF)-I and -II and reduced expression of IR mRNA, IRS-associated PI3K, and activated Akt [46]. The environment in the brain during neurodegenerative changes is not conducive to insulin signaling. For example, neuroinflammation is a key component of Alzheimer’s pathogenesis [47, 48] and cytokines are known to interfere with insulin action [49]. Another causative factor in the neurodegenerative brain is oxidative stress which can also interfere with insulin action [50].
Furthermore, Aβ generated in the Alzheimer’s brain is known to downregulate insulin signaling. For example, it has been demonstrated that neuronal IR signal transduction is sensitive to disruption by soluble Aβ oligomers [51]. A study examining IR binding, showed that both Aβ40 and Aβ42 peptides compete with insulin for IR binding, leading to reduced IR autophosphorylation [52]. Studies with cultured porcine brain capillary endothelial cells and 3XTg-AD mice show that Aβ causes lysosomal degradation of both IR and lipoprotein receptor-related protein-1, a target of insulin signaling at the BBB [53]. Neuronal response to insulin, as measured by evoked IR tyrosine autophosphorylation, was found to be greatly inhibited by soluble Aβ oligomers [51]. Insulin resistance, as shown by decreased Akt phosphorylation, was observed in neuroblastoma 2a cell line overexpressing human APP as a result of increased Aβ production [54].
Aβ has been shown, by in vitro and in vivo studies, to induce hepatic insulin resistance via activation of JAK2/STAT3/SOCS-1 pathway [55, 56]. In a recent study, we have demonstrated that the brain insulin resistance observed in metabolic syndrome is further exacerbated by amyloid pathology in the brain of APP/PS1/Sirt3−/− mice, a comorbid Alzheimer’s mouse model, generated in our lab [57]. In this study, we also observed peripheral insulin resistance as shown by hyperinsulinemia and glucose intolerance in APP/PS1 mice with amyloid pathology. Similarly, tau pathology can also impair insulin signaling. Hull et al. (2020) reported hyperglycemia in mice expressing human tau mutant (PLB2Tau), suggesting a progressive diabetic phenotype [58]. Tau pathology has also been suggested to be a trigger for insulin resistance in the brain as well as peripheral tissues [59]. Thus, the two-way communications between AD lesions and insulin signaling can lead to a loop resulting in exacerbated cognitive dysfunction.
Apolipoprotein E4 and Insulin Signaling
The link between diabetes and amyloid pathology in AD has been shown to be stronger among carriers of the Apolipoprotein E4 (ApoE4) allele. ApoE4 was shown to interfere with insulin signaling in vivo in human ApoE4-targeted replacement mice [60] and this effect was further exacerbated following HFD feeding. In addition, the authors demonstrated, in cultured neurons, that ApoE4 interferes with IR trafficking by trapping it in the endosomes. Another study demonstrated that mice expressing human ApoE4 and human mutant APP are characterized by acceleration of cognitive deficits and impaired insulin signaling [61]. Conversely, insulin signaling was not affected in ApoE3/APP mice. The authors also demonstrated that insulin signaling is impaired in isolated hippocampal neurons from ApoE4 mice, whereas neurons from ApoE3 mice show normal insulin response. The expressions of insulin network polypeptides were shown to be significantly less in the post-mortem samples of AD patients with the ApoE4 phenotype [62].
Insulin Resistance and Cognitive Dysfunction-Clinical Studies
Although limited information is available on the physiological actions of insulin in cognition, studies under conditions of insulin resistance strongly suggest a role for insulin in cognitive function. In this section, we have reviewed studies examining cognitive function under conditions of metabolic syndrome (MetS), type 2 diabetes, and obesity. All these conditions are characterized by insulin resistance. We have also included interactions of these conditions with AD.
Metabolic Syndrome (MetS)
MetS is the precondition for obesity, diabetes and cardiovascular disease. It is characterized by hypertension, insulin resistance, hypertriglyceridemia, low HDL-cholesterol and central obesity. MetS is highly prevalent in developed countries with one in three adults having at least three of these risk factors. A 15-year long-term study that examined the relationship between metabolic syndrome and cognitive decline in 1759 elderly women observed that patients with all five components of MetS were three to four times more likely to develop cognitive dysfunction than those with no risk factors [63]. Employing the category fluency test (CFT) to determine cognitive ability, this study found that higher fasting plasma glucose levels are associated with poorer CFT scores. Insulin resistance, as determined by the Homeostatic Model Assessment for insulin Resistance (HOMA-IR) index, was also associated with increased cognitive dysfunction. A clinical study aimed at understanding the impact of variation in MetS status and risk of dementia found that individuals whose metabolic condition worsened over the 5-year study had a significantly greater chance of developing dementia [64]. Another clinical study with over four million participants investigated the relationship between changes in MetS status over a 2-year period and occurrence of dementia [65]. Specifically, this study observed that hyperglycemia and hypertension were impactful in raising the risk of dementia. Another study examined the associations between individual components of MetS and the risk for dementia and AD [66]. The authors found a significant linear relationship between decrease in cognitive scores with increased number of MetS risk factors. Among the components of MetS, hyperglycemia showed maximum effects on cognitive decline, followed by hypertension.
MetS has also been shown to be a risk factor for AD. For example, a clinical study observed significantly more cognitive decline among AD patients with MetS [67]. Vascular endothelial dysfunction and insulin resistance were characteristic features of AD patients with coexisting MetS in this study. A Finnish population-based study reported that midlife insulin resistance is an independent risk factor for brain amyloid accumulation 15 years later [68]. This association was observed in carriers as well as noncarriers of the ApoE4 allele. However, a recent meta-analysis of nine longitudinal studies reported that there was no significant association between MetS and the occurrence of AD, although MetS increased the likelihood of developing VaD and the risk of mild cognitive impairment (MCI) progressing to dementia [69]. Thus, the association between MetS and AD may be contentious.
Type 2 Diabetes
Several clinical studies have reported that cognitive performance is affected by type 2 diabetes. A study involving nearly seven thousand older male and females reported that the presence of type 2 diabetes is associated with poor performance in cognitive tests [70]. Diabetic patients with good glycemic control, as shown by HbA1c levels under 53 mmol/mol, did significantly better in cognitive performance than those with higher HbA1c levels. This study also found that both increased body mass index (BMI) and duration of diabetes were directly linked with worse cognitive performance. Similarly, a meta-analysis of twelve studies in sixty-eight hundred patients examined the impact of diabetes in the progression of MCI to dementia [71]. The authors observed that having diabetes for five or more years had a 40–60% increased risk for dementia than those recently diagnosed. Diabetic patients, taking statins and oral hypoglycemic agents, were at reduced risk of developing dementia. A recent meta-analysis of twenty-four studies revealed that the presence of diabetes, obesity, hypertension, hypercholesterolemia in mid-life were associated with development of dementia in late life [72]. Conversely, lifestyle changes including exercise and consumption of a healthy diet in mid-life were associated with lowered risk of dementia. Pekkala et al. (2020) examined the association between markers of type 2 diabetes with amyloid accumulation in the brain using PiB PET scans [73]. They observed a correlation of amyloid status with insulin resistance index (HOMA-IR), but not with HbA1c levels. A recent study has reported that brain insulin resistance in Down syndrome (DS) patients can trigger early onset AD [74]. In this study, the authors examined proteins belonging to the insulin signaling pathway in post-mortem brain samples of DS patients before and after the development of AD. They observed markers of dysregulated insulin signaling, especially before the onset of AD pathology, suggesting that brain insulin resistance in DS may contribute to cognitive impairment.
Obesity
Obesity has been shown to cause cognitive dysfunction independent of type 2 diabetes. For example, Whitmer et al. (2008) reported that abdominal obesity alone has a direct correlation with the risk of developing dementia [75]. Several studies have suggested that obesity in midlife causes cognitive decline later in life [76-78]. An Australian study projected that by decreasing mid-life obesity by 20%, dementia in old age could be lowered by 10% [79]. However, late-age obesity may be protective against AD. For example, a study utilizing over twelve million patient data from a Korean health database found that obese subjects who were aged sixty or more had a lower risk of developing dementia and AD than non-obese subjects [80]. Higher incidence of dementia was observed among elderly underweight patients when compared to normal weight and obese patients, although this study did observe other components of MetS to be associated with higher dementia risk, especially vascular dementia. Overall, previous studies have suggested that subjects who developed mid-life obesity are significantly more likely to develop cognitive dysfunction than those who developed late-life obesity. While overt hyperglycemia is not commonly seen in obese individuals, compared to diabetic patients, hypertriglyceridemia is a common feature of both conditions. A study with radiolabeled triglycerides has shown that they can cross the BBB and play a role in brain leptin and insulin resistance [81]. A review characterizing the role of non-esterified fatty acids in developing dementia describe how they are able to the cross the BBB, leading to neuronal dysfunction and toxic effects in different brain cell types [82].
Cognitive Dysfunction in Genetic Animal Models of Insulin Resistance
Zucker Diabetic Fatty Rat
The CNS effect of insulin resistance is also suggested by studies in several genetic models of obesity and diabetes. Zucker diabetic fatty (ZDF) rats are a model for type 2 diabetes. These rats are characterized by obesity, hyperinsulinemia, and hypertriglyceridemia. Obese Zucker rats were found to show impaired performance in a hippocampal-dependent learning and memory test [83]. There was also reduced association of insulin sensitive GLUT4 in the plasma membrane of the hippocampus, although there was no change in the total GLUT4 and insulin receptor expression. Another study reported reduction in the number of BBB insulin receptors in these rats [84]. Because these receptors are involved in the transport of insulin across BBB, this may account for a decrease in CSF insulin levels previously observed in these rats. Impairments in the cerebral cholinergic system were observed in ZDF rats, as shown by increased choline acetyltransferase and decreased acetylcholinesterase activities [85]. These rats were also found to have impaired mitochondrial function, increased ROS production and redox homeostasis complications, and reduced ATP content in the brain [86]. Figlewicz et al. (1985) reported a decrease in insulin binding in the olfactory bulb in the obese rats when compared to the lean controls [87]. However, insulin binding in cerebral cortex and hippocampus were comparable in both groups. A PET-[18F]FDG study reported the lack of acute metabolic flexibility in brain glucose disposal of Zucker diabetic rats as a result of chronic overexposure to glucose [88]. Another study observed astrogliosis in the cortex and hippocampal regions of obese Zucker rats [89]. Increased production of ROS and nitric oxide (NO), along with reduced ATP synthesis, were observed in the brain of Zucker diabetic rats [86]. We have reported significant increases in the expression of genes related to the pathways of oxidative stress and inflammation, as well as defective CREB phosphorylation, resulting in reduced levels of CREB target proteins including Bcl-2, BDNF, and BIRC3 in the brain of ZDF rats [90].
db/db Mouse
Another model for type 2 diabetes is the leptin receptor-deficient db/db mouse. Impaired spatial memory, as measured by the Morris water maze test (MWMT), was observed in db/db mice [91]. A study using NMR-based metabolomic approach reported that cognitive decline in db/db mice correlated with region-specific changes in brain metabolism [92]. Specifically, the authors observed an increase in anaerobic glycolysis and a decrease in tricarboxylic acid (TCA) pathway. Another study examined the domains of executive function in db/db mice, and showed impaired cognitive flexibility in the Morris water maze reversal phase [93]. Studies have also shown that db/db mice are more susceptible to injury in stroke models. For example, distal middle cerebral occlusion (dMCAO)-induced ischemic stroke caused massive BBB leakage in db/db mice, compared to lean controls [94]. When rFGF21 was administered 6 h after stroke, all abnormal changes were significantly reversed, including PPAR DNA-binding activity and mRNA levels of tight junction proteins. Another study observed increased mortality in db/db mice, compared to lean controls, after hypoxic/ischemic (H/I) insult [95]. This study attributed increased infiltration of neutrophils into the brain as the probable cause of increased mortality because depletion of circulating neutrophils resulted in smaller infarct size.
ob/ob Mouse
Leptin plays a critical role in energy homeostasis. Mice deficient in leptin (ob/ob) develop obesity and insulin resistance without overt fasting hyperglycemia. Studies have examined changes in the CNS of this model. A study reported tau hyperphosphorylation at multiple epitopes in the brain of ob/ob mice [96]. The authors suggest impaired thermoregulation due to hypothermia as a potential cause for the findings because normothermia restored the tau phosphorylation to control levels. Long-term potentiation (LTP) in the lateral nucleus of the amygdala was increased in the ob/ob mouse brain, suggesting that emotional state may be affected by diabetes [97]. Spatial memory impairment was observed in ob/ob mice compared to lean controls following chronic unpredictable mild stress [98]. This observation was accompanied by higher levels of proinflammatory cytokines and low levels of antiinflammatory cytokines. Takeda et al. (2010) generated a novel comorbid mouse model by crossing ob/ob mice with Alzheimer’s transgenic mice (APP23) [99]. Diabetes exacerbated cognitive dysfunction in this model without an increase in amyloid deposition in the brain. There was also increased cerebrovascular inflammation and amyloid angiopathy. Interestingly, these mice showed accelerated diabetic phenotype, compared to ob/ob mice, suggesting that amyloid pathology can aggravate diabetes as well. Aβ1-42 was shown to decrease the lifespan of ob/ob mice, following the cross of ob/ob mice with AppNL-F/wt knock-in mice [100].
Goto-Kakizaki Rat
The Goto-Kakizaki (GK) rat is a non-obese and genetic type 2 diabetic model generated by repeated selective breeding of glucose intolerant Wistar rats. Brain energy metabolism was investigated by 13C magnetic resonance spectroscopy in GK rats following 13C glucose administration [101]. GK rats displayed lower rates of brain glutamate synthesis and glutamate-glutamine cycle and TCA cycle rates in neurons. However, the TCA cycle rate was higher in astrocytes, suggesting impairment of glutamate-glutamine cycle between neurons and astrocytes. Higher susceptibility to lipid peroxidation, as assessed by formation of malondialdehyde (MDA) and TBARS, was observed in the brain as compared to liver of GK rats [102]. The mitochondria isolated from the brain of these diabetic rats were also more susceptible to oxidative damage in vitro. Impaired glycogen synthesis in the hippocampus and hypothalamus of GK rats was demonstrated by magnetic resonance spectroscopy [103]. Diabetes also caused a significant reduction in the glucose transport rate as well as the cerebral metabolic rate of glucose. Another study reported that brain mitochondrial dysfunction observed in the GK rats is further exacerbated by aging [104]. In addition, isolated brain mitochondria, were more prone to injury following exposure to Aβ in vitro. Lasting cognitive impairment was observed in GK rats following the induction of ischemia [105]. Markers of neurodegeneration were also observed in these diabetic rats. Similarly, another study reported cognitive dysfunction in GK rats along with decreased activation of CREB [106]. Increased p-tau and Aβ levels were observed in the brain of aged GK rats along with decreased synaptic protein levels [107].
Effects of Antidiabetic Drugs on Cognitive Function
Diabetic patients are living longer because of the availability of a vast array of antidiabetic medications. Studies with antidiabetic drugs have generally focused on diabetic complications including neuropathy, nephropathy, retinopathy and cardiovascular diseases. Recent studies are beginning to examine the cognitive dysfunction as well. As chronic hyperglycemia in diabetes plays a key role in causing cognitive dysfunction, treatment with antidiabetic drugs that improve glycemic control is expected to improve cognitive function (Fig. 2). It is also possible that some of these drugs could have independent beneficial effects in the brain and slow down cognitive decline. In this section, we review the findings from previous reports on the assessment of cognitive function with various antidiabetic treatments. We have excluded sulfonylureas because they may produce hypoglycemic episodes [108] which can affect cognitive function.
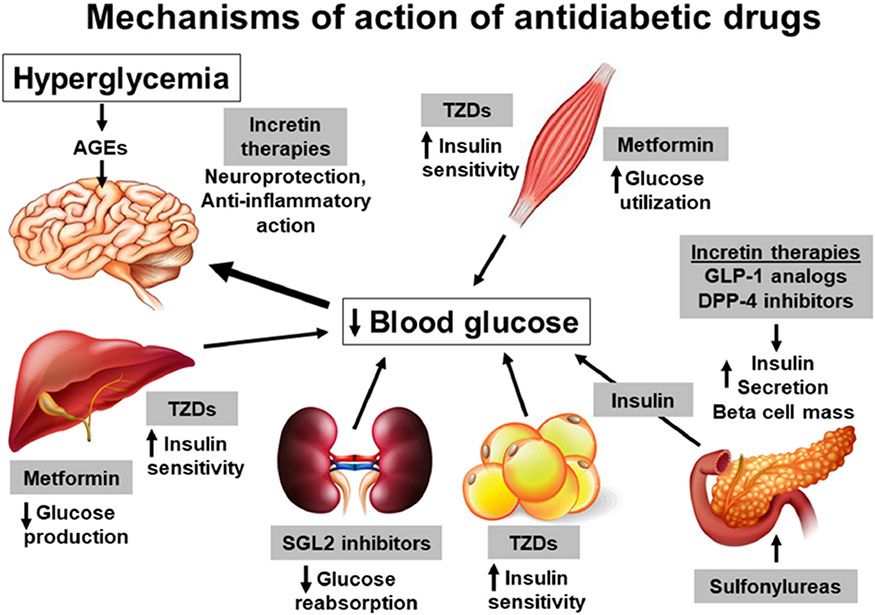
Fig. 2.
Mechanisms of action of antidiabetic drugs. The lifespan of type 2 diabetic patients is increasing steadily because of the availability of a wide range of antidiabetic drugs. These drugs decrease blood glucose levels by multiple mechanisms. Metformin, a widely used hypoglycemic drug, acts by increasing glucose utilization in the peripheral tissues and by reducing hepatic glucose output. Sulfonylureas stimulate insulin secretion from pancreatic β cells. The use of this class of drug is associated with hypoglycemic episodes. Thiazolidinediones (TZDs) improve the sensitivity of adipose tissue, skeletal muscles, and the liver towards insulin. Incretin therapies, such as DPP-4-resistant GLP-1 analogs and DPP-4 inhibitors that enhance endogenous GLP-1 levels, promote pancreatic release of insulin. In addition, they are reported to have insulin-independent actions in other tissues, including the brain. Recently introduced SGLT2 inhibitors increase glucose excretion in the urine by preventing glucose reabsorption in the renal tubules. Overall, these antidiabetic drugs have been shown to have beneficial actions in the brain either directly or indirectly through glycemic control
Intranasal Insulin
Although insulin action in the brain can be improved by peripheral administration of insulin, this approach is limited by its hypoglycemic effects. Diabetic patients receiving insulin peripherally may experience hypoglycemic episodes along with increased risk of dementia [109]. Intranasal (IN) insulin administration on the other hand has shown promise as an effective treatment option to facilitate insulin action in the brain. IN insulin is non-invasive and delivers insulin to the brain parenchyma quickly and effectively. Cerebral levels of insulin are significantly higher following IN administration than after intra venous administration. Several studies have taken this approach and reported significant improvement in cognitive function. For example, Avgerinos et al. (2018) analyzed seven studies with 293 patients that tested the effectiveness of IN insulin in improving cognitive function and observed positive effects in patients with dementia or MCI [110]. These effects appear to be limited to patients with the ApoE4 allele. This study also describes how ApoE4 (−) patients had improved results based on the authors’ primary memory composite score, while ApoE4 (+) patients had worse outcomes. A randomized controlled trial of over one hundred MCI and AD patients found amelioration of delayed memory following IN treatment [111]. However, another study reported significant improvements in verbal working memory and visuospatial working memory following IN insulin administration, regardless of ApoE4 allele status [112]. A study in patients with MCI reported that low dose IN insulin improved cognitive function which had a positive correlation with insulin signaling mediators in plasma neuronal-enriched extracellular vesicles [113]. There are also negative reports on the lack of insulin action in cognition. A recent phase 2/3 multisite randomized clinical trial with 289 participants found no cognitive or functional benefits associated with IN insulin treatment over a 12-month period [114]. Participants in this trial included adults aged 55 to 85 years with a diagnosis of amnestic MCI or AD, a score of 20 or higher on the Mini-Mental State Examination (MMSE) and a clinical dementia rating of 0.5 or 1.0.
The effectiveness of IN insulin is further supported by studies in preclinical models. The effects of IN insulin was examined by a study in 9-month-old 3xTg-AD mice, a mouse model with both amyloid and tau pathologies [115]. The authors observed restoration of brain insulin signaling, increased synaptic proteins, reduced Aβ levels, and inhibition of microglia activation in mice, leading to reduced inflammation. Ruegsegger et al. (2019) demonstrated how insulin reduction in the brain contributes to cognitive impairment, upregulation of proteins involved in Tau phosphorylation and neurodegeneration in streptozotocin-induced diabetic mice [116]. IN insulin administration in these mice resulted in enhanced mitochondrial ATP production and cognitive function. A study in wild type mice showed that peripheral administration of insulin modulates the trafficking of Aβ across BBB [117]. The authors also demonstrated in an in vitro model of the BBB that insulin is able to regulate the luminal uptake of Aβ. Traumatic brain injury (TBI) being a risk factor for AD, Franklin et al. (2019) examined insulin responsiveness in isolated hippocampal synaptosomes after TBI injury in a rat model [118]. They observed that insulin is able to block LTP inhibition by Aβ and tau oligomers and that its effect is lost in TBI rats.
Metformin
Metformin, a widely used oral hypoglycemic drug for the treatment of diabetes, acts by increasing glucose utilization in the peripheral tissues and by reducing hepatic glucose output. Several clinical studies have assessed the impact of this drug on cognitive dysfunction. Hsu et al. (2011) observed that metformin treatment significantly reduced the risk of dementia in type 2 diabetic patients aged fifty and older, after controlling for cerebrovascular disease [119]. In addition, when metformin was given in combination with sulfonylureas, the risk of dementia was reduced by 35% over an 8-year period [119]. The Sydney Memory and Aging Study has recently reported decreased incidence of dementia in older diabetic patients treated with metformin [120]. In this prospective observational study, community-dwelling 70–90-year-old participants without dementia were followed for 6 years. Among them, diabetic patients receiving metformin had slower cognitive decline compared to diabetic patients without metformin treatment. However, a U.K. clinical study found long-term chronic use of metformin associated with an increased risk of developing AD, while long-term use of other drugs including sulfonylureas, thiazolidinediones, or insulin were found to have no effect on the risk of dementia [121]. Thus, clinical studies have reported mixed findings on the effectiveness of this drug in improving cognitive function.
SGLT2 Inhibitors
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a new class of antidiabetic drugs. They act by increasing glucose excretion in the urine by inhibition of the reabsorption of glucose in renal tubules. Empagliflozin and dapagliflozin are such drugs, currently being used in the treatment of diabetic patients. The effect of empagliflozin administration has been tested in APP/PS1/db/db mice, a model with AD and diabetes [122]. The authors observed a decrease of neuronal loss and senile plaque burden in the brain of treated mice, along with amelioration of cognitive deficits. Glycemic control with empagliflozin was shown to prevent cognitive impairment and amelioration of cardiovascular injury in db/db mice following 10 weeks of treatment [123]. Another study reported improved brain mitochondrial function and prevention of cognitive decline in high-fat diet (HFD)-fed rats after treatment with dapagliflozin [124]. This SGLT2 inhibitor ameliorated mitochondrial dysfunction, peripheral insulin resistance, inflammation, and neuronal apoptosis in treated rats. The effects of SGLT2 inhibitions on the CNS are likely to be secondary to glucose lowering effects.
Thiazolidinediones
Thiazolidinediones, another class of oral hypoglycemic drugs, act as agonists of PPAR-ɣ and promote insulin responsiveness in type 2 diabetic patients. They improve the sensitivity of adipose tissue and skeletal muscles towards insulin and inhibit hepatic gluconeogenesis [125]. Both clinical and preclinical animal model studies suggest beneficial effects of these drugs in improving cognition. Rosiglitazone and pioglitazone belong to the thiazolidinedione class of drugs.
Rosiglitazone
Rosiglitazone has been shown to improve cognitive function by several clinical studies. For example, a randomized double-blind trial of rosiglitazone demonstrated cognitive function improvement in early-stage AD patients who did not carry the ApoE4 allele [126]. Similarly, a six-month randomized controlled trial in MCI and AD patients observed that rosiglitazone-treated subjects maintained memory function and demonstrated better delayed recall and selective attention, while placebo-assigned subjects showed anticipated memory decline [127]. A study in older diabetic patients with MCI tested the effects of diabetes drugs over a 36-week period [128]. Several neuropsychological tests, including MMSE, Rey Verbal Auditory learning and Trail Making Tests were performed. Patients receiving both metformin and rosiglitazone showed stable results with all the tests, while groups on diet control and on metformin alone showed declining performance in one of the tests, suggesting that rosiglitazone may have positive effects on cognitive function. However, a few other studies found no significant improvement in cognitive function, following rosiglitazone treatment in patients with mild to moderate AD [129, 121].
An interesting study examined the effects of rosiglitazone treatment for 1 month in different age groups of Tg2576 mice, a transgenic Alzheimer’s mouse model [130]. Both peripheral gluco-regulatory status and cognitive function were assessed. Based on the results, the authors concluded that rosiglitazone-mediated reversal of associative learning and memory deficits is independent of its glucose lowering effects. Rosiglitazone was found to reduce Aβ burden in the brain by improving the phagocytic activity of microglia in an Alzheimer’s transgenic mouse model expressing human mutant APP [131]. Impaired recognition and spatial memory were also rescued in the treated mice. Neuronal insulin resistance, induced by a three-month HFD, in rats was reversed by rosiglitazone [132]. Insulin-induced long-term depression was significantly improved, and increased brain mitochondrial ROS production was attenuated in this study. Pathan et al. (2008) reported rosiglitazone’s ability to attenuate cognitive dysfunction and metabolic dysregulation induced by 5 weeks of HFD consumption in rats [133]. Rosiglitazone administration also stimulated mitochondrial biogenesis in the mouse brain by inducing both mitochondrial DNA and estrogen-stimulated related receptor alpha mRNA, a key regulator of this process [134].
Pioglitazone
The effects of pioglitazone on cognitive function have been reported by studies in animal models. Pioglitazone improved learning on the active avoidance task and decreased β-amyloid deposition and tau pathology in 10-month-old triple transgenic mice (3xTg-AD) [135]. Gene microarray analysis of hippocampal tissue revealed that this TZD drug facilitated estrogenic processes. In a study on aged mice expressing human mutant APP (APPSwe,Ind), pioglitazone was administered for 6–8 weeks [136]. While pioglitazone did not improve spatial memory or reduce Aβ levels significantly, it attenuated astroglial activation, reduced oxidative stress, and completely normalized cerebral blood flow. Proteomic analysis of surgically isolated cerebral arteries from 3-month-old APP transgenic mice revealed that nearly 200 cerebrovascular proteins were abnormally expressed [137]. Pioglitazone administration rescued a third of these proteins and they were mostly associated with oxidative stress, promotion of cerebrovascular vasocontractile tone, and vascular compliance. Papadopoulos et al. (2013) tested the effects of pioglitazone in bitransgenic A/T mice that overexpress a human APP mutant (APPSwe,Ind) and constitutively active TGF-β1 [138]. Pioglitazone treatment normalized metabolic and vascular functions and reduced hippocampal microglial activation but did not rescue spatial learning and memory deficits in the MWMT. Another study showed that the sera from AD patients induced apoptosis of human umbilical vein endothelial cells which was prevented by preincubation with pioglitazone [139]. Poor BBB permeability of pioglitazone has been suggested to be an obstrucle for its therapeutic potential in AD. However, Chang et al. (2015) have demonstrated that the levels of pioglitazone in brain tissue can be significantly increased by inhibition of P-glycoprotein (P-gp) [140]. Pioglitazone was also investigated for its stereoselectivity and the concentration of (+) pioglitazone was 46.6% higher than (−) pioglitazone in brain tissue and 67.7% lower than (−) pioglitazone in plasma. Mice that were given pure (+) pioglitazone had 76% higher brain exposure levels than mice given an equivalent dose of racemic pioglitazone. Therefore, P-gp may act as a stereoselective barrier for pioglitazone brain penetration and pure (+)-pioglitazone may result in higher levels of brain exposure to pioglitazone. Pioglitazone has been tested in other models of cognitive dysfunction. For example, pioglitazone rescued impaired cognitive function, as observed by the MWMT in a rat controlled cortical impact model of TBI [141]. In addition, pioglitazone ameliorated several neuropathological aspects including activation of microglia. Long-term fructose-drinking in rats caused insulin resistance, oxidative stress, and cognitive deficit, and pioglitazone treatment significantly reduced insulin resistance and the levels of ROS in the hippocampus and cerebral cortex [142]. Pioglitazone also improved learning and memory functions partially, as observed by the MWMT.
GLP-1 Based Therapies
Glucagon-like peptide-1 (GLP-1), secreted by the intestinal L-cells in response to food, acts on pancreatic β cells to stimulate insulin. GLP-1 action is transient because it is rapidly degraded by dipeptidyl peptidase-4 (DPP-4) [143]. To further enhance GLP-1 action, two types of drugs are being used currently in the treatment of diabetes. DPP-4 inhibitors can be given orally to sustain endogenous GLP-1 levels, whereas DPP-4-resistant GLP-1 analogs need to be injected. The GLP-1 receptor has been shown to be expressed in tissues other than pancreatic β cells including the brain [144]. We have reported the expression of GLP-1 receptors in differentiated human neuroprogenitor cells [145]. Abbas et al. (2009) examined the cognitive function in GLP-1 receptor knockout mice using several behavioral tests [146]. Glp1r−/− mice were found to have impaired cognitive function, based on water maze and novel object recognition tasks, compared to wild type mice. LTP in CA1 area of the hippocampus was also severely impaired in Glp-1−/− mice. Findings of this study and previous reports suggest a physiological role for GLP-1 in neuronal function and some forms of memory formation. Because GLP-1 can pass through BBB, diabetic patients treated with GLP-1-based drugs are likely to have beneficial actions in the CNS in addition to glycemic control.
GLP-1 Analogs
Exenatide/Exendin-4
Li et al. (2010) examined the effects of exendin-4 treatment in 3xTgAD mice with streptozotocin (STZ)-induced diabetes [147]. Exendin-4 decreased HbA1c levels and brain Aβ levels, suggesting its potential therapeutic value in patients with AD and diabetes. Exendin-4 also mitigated the toxicity of Aβ and oxidative challenge in mouse primary neuronal cultures and in SH-SY5Y cells in a concentration-dependent manner. Administration of exenatide for 16 weeks in 5XFAD mice resulted in normalization of mitochondrial dynamics and prevention of cognitive decline [148]. In addition, the drug alleviated Aβ1–42 deposition and synapse damage in the hippocampus of this mouse model of AD. Li et al. (2016) examined the neuroprotective effects of GLP-1 receptor agonists, exendin-4 and liraglutide in db/db mice following middle cerebral artery occlusion (MCAO) injury [149]. The MCAO injury decreased cerebral microcirculation, but this was reversed by the administration of exendin-4 or liraglutide. The mechanism involved was suggested to be through elevation of endothelial NOS levels. Another study reported that blast TBI-induced neurodegeneration and memory deficits were ameliorated by subcutaneous administration of exendin-4 [150]. Similarly, exendin-4 also improved cognitive function, as assessed by the MWMT in a fluid percussion injury rat model [151]. Exendin-4 has been shown to exert neuroprotective effects when administered to HFD-induced insulin-resistant mice [152]. These treated mice showed improved hippocampal synaptic plasticity and cognitive function. A potential mechanism of direct action of exenatide in the brain was suggested by Bomba et al. (2018), who examined its effects in wild type mice [153]. Following a two-month treatment, exenatide promoted the activation of the BDNF-TrkB neurotrophic axis and inhibited apoptosis by decreasing p75NTR-mediated signaling. We have reported the neuroprotective effects of exendin-4 in cultured human neural stem cell-derived neurons [145]. Exendin-4 decreased Aβ-induced neuronal apoptosis through a mechanism involving the activation of the neuroprotective transcription factor CREB and induction of BDNF promoter activity in a CREB-dependent manner. Furthermore, withdrawal of neurotrophins in the neuronal culture led to the loss of neuronal phenotype of differentiated neuroprogenitor cells but this was prevented by exposure to exendin-4.
Liraglutide
A pilot study found that T2DM patients who were administered liraglutide performed better in short term memory and memory composition scores than patients who had controlled blood glucose levels by diet and exercise, suggesting that liraglutide action on cognitive function is not secondary to glycemic control [154]. A recent study found that obese individuals with T2DM given liraglutide or exenatide for 3 months had significantly improved Montreal Cognitive Assessment (MoCA) scores, olfactory test total scores, and olfactory brain activation [155]. A double-blinded study used fMRI brain scans to demonstrate reduced brain activity and connectivity between brain regions in placebo-assigned middleaged subjects over the course of 1 year, whereas this negative association was not present in those given liraglutide [156]. A 26-week randomized, placebo-controlled, double-blind study with AD patients concluded that liraglutide treatment was able to prevent the decline of cerebral glucose metabolism. The authors, however, were not able to find significant differences in Aβ deposition or cognitive measures between liraglutide-treated and placebo groups [157]. Another study analyzed the benefits of 28-day liraglutide treatment in 10-month-old 3xTg-AD female mice [158]. Liraglutide reduced cortical Aβ1−42 levels and partially attenuated brain oxidative/nitrosative stress. Liraglutide was shown to prevent memory impairment and synapse loss in the hippocampi of 7-month-old APP/PS1 mice following 8 weeks of treatment [159]. In addition, there were significant reductions in β-amyloid plaque deposition and activated microglia numbers in the brain of these mice. In a later study, the same group tested the effects of liraglutide treatment for 2 months in aged APP/PS1 mice [160]. The GLP-1 analog improved spatial memory and reduced amyloid plaque load. In addition, long-term potentiation was significantly enhanced, along with increased synapse numbers in the hippocampus and cortex. Another study examined the effects of liraglutide in a non-human primate model of AD, following infusion of Aβ oligomers into the lateral cerebral ventricle [161]. Loss of synapses and memory impairment by this infusion were partially reversed by liraglutide administration. However, long-term liraglutide administration was found to have no significant impact on β-Amyloid plaque load, as assessed in two AD mouse models expressing “London” (hAPPLon/PS1A246E) and “Swedish” (hAPPSwe/PS1ΔE9) mutants of APP along with co-expression of distinct PS1 variants [162]. Administration of liraglutide to HFD-fed mice [163] or ob/ob mice [164] resulted in significantly improved cognitive function and hippocampal synaptic plasticity, as a result of the preservation of neurogenesis and neuronal survival.
Dulaglutide and Lixisenatide
The effect of dulaglutide on cognitive function was investigated by the Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial [165]. Patients’ cognitive function was assessed at baseline and during follow-up using the MoCA and Digit Symbol Substitution Test (DSST) in this randomized, double-blind placebo-controlled trial at 371 sites in 24 countries. This trial reported that treatment with dulaglutide reduces cognitive impairment in people with type 2 diabetes by 14%. Lixisenatide, another GLP-1 analog, was shown to reduce amyloid plaque deposition, neurofibrillary tangle formation and neuroinflammation in the brain of aged APP/PS1/tau mice, following 2 months of treatment [166]. The authors suggested the activation of PKA-CREB signaling as a possible mechanism. Similarly, this GLP-1 analog reduced amyloid plaque load, prevented the loss of synapses, and improved performance in novel object recognition tasks in APP/PS1 mice after 10 weeks of treatment [167]. Overall, Lixisenatide was equally effective at a lower dose compared to liraglutide in some of the parameters measured. Lixisenatide was shown to reduce peripheral insulin resistance, improve recognition memory, and boost the number of hippocampal dentate gyrus neurons in HFD-fed mice [168].
New GLP-1 Analogs
Some studies have suggested that dual GLP-1/gastric inhibitory peptide (GIP) receptor agonists may be better options for the treatment of AD [169]. A recent study found that DA5-CH, a novel dual GLP-1/GIP receptor agonist, reverses working memory and spatial memory impairments in an intracerebroventricular STZ-induced insulin desensitization AD rat model [170]. The authors observed that DA5-CH can cross the BBB at a rate comparable to other GLP-1 receptor agonists and DA5-CH treatment reduces tauS396 phosphorylation in the hippocampus significantly. The drug also alleviated hippocampal mitochondrial stress and increased the expression of synapse-related proteins in the hippocampal region, further highlighting the neuroprotective effects of DA5-CH. Cao et al. (2018) tested the neuroprotective effects of DA5-CH in the APP/PS1 transgenic mouse model [171]. DA5-CH reduced hippocampal amyloid senile plaques and phosphorylated tau protein levels and activated the PI3K/Akt signaling pathway. The treatment also resulted in improved working memory and long-term spatial memory. A novel triagonist of GLP-1, GIP and glucagon was tested in the 3xTg mouse model of AD [172]. Following a 30-day treatment in 7-month-old AD mice, the authors observed improved long-term spatial memory in the MWMT and improved working memory in a Y-maze task. The triagonist also alleviated the suppression of LTP in the CA1 region of the hippocampus, upregulated the hippocampal levels of S133 p-CREB, T286 p-CAMKII, and S9 p-GSK3β in 3xTg-AD mice and significantly reduced hippocampal pathological damages, including Aβ and phosphorylated tau aggregates. Similarly, a study reported the neuroprotective effects of another triple receptor agonist in the APP/PS1 transgenic mouse model [173]. Decreases were observed in β-amyloid deposition and markers of neuroinflammation and oxidative stress in the brain of the treated AD mice, leading to reversal of memory deficits. The agonist also decreased the levels of the mitochondrial pro-apoptotic BAX, enhanced the anti-apoptotic Bcl-2 and increased the levels of BDNF, resulting in increased levels of synaptophysin.
DPP-4 Inhibitors
DPP-4 inhibitors are another class of drugs under incretin therapies. These drugs act by sustaining the levels of endogenous GLP-1 through inhibition of DPP-4. A large number of DPP-4 inhibitors, including sitagliptin, vildagliptin, alogliptin and linagliptin are currently being used in the treatment of diabetes. A recent network meta-analysis and meta-analysis with over 1.2 million patients analyzed seven antidiabetic agents across twenty-two studies and their impact on the risk of dementia in T2DM patients [174]. The analysis included several drugs including metformin, sulfonylureas, thiazolidinediones, DPP-4 inhibitors, glucosidase inhibitors, benzoic acid derivatives, and insulin, but did not include GLP-1 analogs or SGLT-2 inhibitors due to lack of relevant data. Results of the study indicated that DPP-4 inhibitors and metformin provide a protective effect against the risk of developing dementia, while those treated with insulin had the highest risk for dementia. Overall, DPP-4 inhibitors provided the most significant protective effect among all the antidiabetic agents and were the most effective drug to prevent dementia, followed by metformin and thiazolidinedione.
Sitagliptin
A recent study with nearly fifteen thousand T2DM patients in Taiwan found that patients given DPP-4 inhibitors, such as sitagliptin, are less likely to develop dementia, especially vascular dementia, compared to the placebo group [175]. However, this trend was notably absent for the risk of developing AD. Another study reported that 6-month sitagliptin therapy improves cognitive function, based on MMSE scores, in older diabetic patients with and without AD [176]. The effect of sitagliptin on cognitive function has been tested in animal models. A recent study reported improved performance in the MWMT by APP/PS1 mice after oral administration of sitagliptin for 8 weeks [177]. In addition, treated APP/PS1 mice had significantly reduced amyloid plaque deposition, elevated spine density, and increased protein levels of BDNF in the brain. Sitagliptin was shown to improve insulin sensitivity, reduce inflammation and reverse memory impairment in high-fat-fed mice [178]. Similarly, another study found that, in HFD-induced insulin-resistant rats, DPP-4 inhibitors, vildagliptin or sitagliptin administered individually, decreased oxidative stress levels and improved brain mitochondrial dysfunction in the brain [179]. Tsai et al. (2015) tested CNS effects of sitagliptin in a mouse model of chronic cerebral hypoperfusion, induced by bilateral carotid artery stenosis [180]. Sitagliptin suppressed white matter lesions, microglial activation, and astrocytosis. Furthermore, there was protection against cognitive impairment and a decrease of inflammatory markers.
Vildagliptin
A retrospective longitudinal clinical trial found that vildagliptin administration for 6 months led to better performance in the copying subdomain of the MMSE in patients with T2DM [181]. Another retrospective longitudinal study found that older patients with both T2DM and MCI had reduced glucose level fluctuations and improved cognitive functioning within 2 years of vildagliptin therapy [182]. Six-month administration of vildagliptin with metformin showed protective effects, with better HbA1c control and prevented MMSE score reduction in diabetic elderly patients compared to metformin-only treated patients [183]. Treatments with both vildagliptin and pioglitazone were shown to restore dendritic spines in CA1 hippocampus that were reduced by HFD in rats [184]. Vildagliptin has been also shown to restore neuronal IR phosphorylation, IRS-1 phosphorylation, and Akt phosphorylation, all indicators of insulin signaling, to normal levels in HFD-fed rats [185]. STZ-induced diabetic mice treated with vildagliptin for 8 weeks showed reversal of diabetes-induced memory and learning impairment [186]. In addition, vildagliptin significantly increased the expression of BDNF and SOD and decreased MDA levels in the brain. Similarly, another study observed improvement in memory in STZ-induced diabetic rats, following vildagliptin treatment [187]. There was also decreased apoptosis of hippocampal neurons in the treated rats. The authors suggested the activation of Akt as a potential mechanism of the neuroprotective action of the drug.
Alogliptin
We have reported the dual beneficial actions of alogliptin in pancreatic β cells and in the brain by a common CREB-dependent mechanism in two different studies [90, 188]. The plasma levels of GLP-1 were elevated in alogliptin-treated Zucker diabetic rats, which resulted in improved survival of β cells [188]. Alogliptin activated CREB in insulin positive β cells of islets and increased the levels of CREB-regulated proteins including anti-apoptotic bcl-2, the caspase inhibitor, BIRC3, and IRS-2, an adapter protein required for insulin signaling. We also reported that alogliptin administration in Zucker diabetic rats for 10 weeks led to increases in the levels of CREB-dependent neuroprotective proteins in the brain [90]. Elevated levels of neuroinflammatory markers in the diabetic brain were also decreased, suggesting the potential therapeutic benefits of DPP-4 inhibitors in treating AD.
Linagliptin
A cardiovascular outcome trial with linagliptin did not find any change in cognitive decline [189]. Kosaraju et al. (2017) reported significant beneficial effects of linagliptin in 3xTg-AD mouse model [190]. In this study, 9-month-old 3xTg-AD mice were administered linagliptin orally for 8 weeks. The treated mice showed significant improvement in the Morris Water Maze and Y-maze. Furthermore, linagliptin attenuated β-amyloid accumulation, tau phosphorylation and neuroinflammation in the AD mice. Another study observed improvement in cerebrovascular dysfunction in GK rats, independent of glycemic control [191]. Linagliptin was also shown to ameliorate HFD-induced cognitive decline and increase cerebral blood flow in a tauopathy mouse model [192]. In this study, linagliptin reversed the cognitive impairment and brain atrophy induced by bilateral common carotid artery occlusion-mediated cerebral ischemia in db/db mice [193]. This DPP-4 inhibitor also increased the levels of tight junction protein claudin-5 and prevented neuronal loss in the hippocampus and cortex of these mice.
Conclusions
Recent studies have recognized that the CNS is negatively affected under conditions of insulin resistance. Clinical and preclinical studies have reported cognitive dysfunction during conditions of insulin resistance, including metabolic syndrome, obesity and diabetes. Hyperglycemia in diabetes can generate advanced glycation end products which can pass through the BBB and cause neuronal dysfunction. In addition, there are circulating factors including cytokines and fatty acids, generated by the peripheral tissues, in the diabetic state that can have indirect effects on the CNS. These observations raise an important question on whether treating cognitive dysfunction needs to be directed specifically to the brain cell types or can be accomplished at the systemic level. Recent studies are suggesting that insulin resistance can be targeted therapeutically to prevent cognitive decline. Antidiabetic drugs have been shown to improve cognitive functions by clinical and preclinical studies. Their actions may be directly on the CNS or indirectly through glycemic control. However, there are also reports showing negative effects on cognition with these drugs. Even among those studies, some beneficial actions at the molecular level in the brain are being reported. It is possible that timing of the intervention with these drugs was not early enough to reverse cognitive decline or the effects were not sufficient enough to tilt the scale. Among the antidiabetic drugs, GLP-1-based drugs have demonstrated consistent beneficial actions in the brain. They have the potential to delay cognitive decline in conditions of insulin resistance. Furthermore, these drugs, well tested for use in diabetic patients, can be also repurposed for the treatment of patients with dementia including Alzheimer’s disease.
Acknowledgments
This work was carried out with the use of resources and facilities at the Rocky Mountain Regional Veterans Administration Medical Center. The academic support provided to AT by Lawrence J. Berliner is greatly appreciated. We thank Alpna Tyagi for critically reading the manuscript and for providing feedback.
Funding
This study was supported by a Merit Review grant NEUD-004-07F from the Veterans Administration (to S.P.).
Abbreviations
- 3xTg
triple transgenic
- AD
Alzheimer’s disease
- Ang
Angiotensin
- ApoE4
apolipoprotein E4
- APP
β-amyloid precursor protein
- ARB
Ang II type 1 receptor blocker
- BBB
blood brain barrier
- BDNF
brain-derived neurotrophic factor
- BECs
brain endothelial cells
- CBF
cerebral blood flow
- CFT
category fluency test
- CNS
central nervous system
- CSF
cerebrospinal fluid
- dMCAO
distal middle cerebral occlusion
- DPP-4
dipeptidyl peptidase-4
- DS
Down syndrome
- GIP
gastric inhibitory polypeptide
- GK
Goto-Kakizaki
- GLP-1
glucagon-like peptide-1
- HbA1c
hemoglobin A1c
- H/I
hypoxic/ischemic
- HFD
high-fat diet
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- IGF
insulin-like growth factor
- IN
intranasal
- IR
insulin receptor
- IRS-1
insulin receptor substrate-1
- LTP
long-term potentiation
- MCAO
middle cerebral artery occlusion
- MCI
mild cognitive impairment
- MDA
malondialdehyde
- MetS
metabolic syndrome
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- MWMT
Morris water maze test
- P-gp
P-glycoprotein
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- SAMP8
senescence-accelerated mouse prone 8
- SGLT2
sodium-glucose co-transporter 2
- STZ
streptozotocin
- T2DM
type 2 diabetes mellitus
- TBI
traumatic brain injury
- TCA
tricarboxylic acid
- VaD
vascular dementia
- ZDF
Zucker diabetic fatty
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Consent for Publication The authors give consent for publication.
References
- 1.Hersom M, Helms HC, Schmalz C, Pedersen TA, Buckley ST, Brodin B (2018) The insulin receptor is expressed and functional in cultured blood-brain barrier endothelial cells but does not mediate insulin entry from blood to brain. Am J Physiol Endocrinol Metab 315(4):E531–E542. 10.1152/ajpendo.00350.2016 [DOI] [PubMed] [Google Scholar]
- 2.Havrankova J, Roth J, Brownstein M (1978) Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272(5656):827–829. 10.1038/272827a0 [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Owen JB, Erickson MA (2012) Insulin in the brain: there and back again. Pharmacol Ther 136(1):82–93. 10.1016/j.pharmthera.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konishi M, Sakaguchi M, Lockhart SM, Cai W, Li ME, Homan EP, Rask-Madsen C, Kahn CR (2017) Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proc Natl Acad Sci U S A 114(40): E8478–E8487. 10.1073/pnas.1710625114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhea EM, Rask-Madsen C, Banks WA (2018) Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J Physiol 596(19):4753–4765. 10.1113/JP276149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray SM, Aylor KW, Barrett EJ (2017) Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia 60(8):1512–1521. 10.1007/s00125-017-4285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urayama A, Banks WA (2008) Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology 149(7):3592–3597. 10.1210/en.2008-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks WA, Jaspan JB, Kastin AJ (1997) Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides 18(10):1577–1584. 10.1016/s0196-9781(97)00238-6 [DOI] [PubMed] [Google Scholar]
- 9.Dorn A, Bernstein HG, Rinne A, Ziegler M, Hahn HJ, Ansorge S (1983) Insulin- and glucagonlike peptides in the brain. Anat Rec 207(1):69–77. 10.1002/ar.1092070108 [DOI] [PubMed] [Google Scholar]
- 10.Birch NP, Christie DL, Renwick AG (1984) Proinsulin-like material in mouse foetal brain cell cultures. FEBS Lett 168(2):299–302. 10.1016/0014-5793(84)80266-5 [DOI] [PubMed] [Google Scholar]
- 11.Deltour L, Leduque P, Blume N, Madsen O, Dubois P, Jami J, Bucchini D (1993) Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc Natl Acad Sci U S A 90(2):527–531. 10.1073/pnas.90.2.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaskar SU, Singh BS, Carnaghi LR, Rajakumar PA, Giddings SJ (1993) Insulin II gene expression in rat central nervous system. Regul Pept 48(1–2):55–63. 10.1016/0167-0115(93)90335-6 [DOI] [PubMed] [Google Scholar]
- 13.Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS (1994) Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem 269(11):8445–8454 [PubMed] [Google Scholar]
- 14.Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A et al. (2012) Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab 16(6):723–737. 10.1016/j.cmet.2012.10.019 [DOI] [PubMed] [Google Scholar]
- 15.Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A et al. (1998) Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna) 105(4–5):423–438. 10.1007/s007020050068 [DOI] [PubMed] [Google Scholar]
- 16.Kuwabara T, Kagalwala MN, Onuma Y, Ito Y, Warashina M, Terashima K, Sanosaka T, Nakashima K et al. (2011) Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. EMBO Mol Med 3(12):742–754. 10.1002/emmm.201100177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnar G, Farago N, Kocsis AK, Rozsa M, Lovas S, Boldog E, Baldi R, Csajbok E et al. (2014) GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci 34(4): 1133–1137. 10.1523/JNEUROSCI.4082-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill JM, Lesniak MA, Pert CB, Roth J (1986) Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience 17(4):1127–1138. 10.1016/0306-4522(86)90082-5 [DOI] [PubMed] [Google Scholar]
- 19.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA (1987) Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121(4):1562–1570. 10.1210/endo-121-4-1562 [DOI] [PubMed] [Google Scholar]
- 20.Marks JL, Porte D Jr, Stahl WL, Baskin DG (1990) Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127(6):3234–3236. 10.1210/endo-127-6-3234 [DOI] [PubMed] [Google Scholar]
- 21.Spencer B, Rank L, Metcalf J, Desplats P (2018) Identification of insulin receptor splice variant B in neurons by in situ detection in human brain samples. Sci Rep 8(1):4070. 10.1038/s41598-018-22434-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Rachadell L, Aguilera A, Perez-Domper P, Pignatelli J, Fernandez AM, Torres-Aleman I (2019) Cell-specific expression of insulin/insulin-like growth factor-I receptor hybrids in the mouse brain. Growth Hormon IGF Res 45:25–30. 10.1016/j.ghir.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 23.Lochhead JJ, Kellohen KL, Ronaldson PT, Davis TP (2019) Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci Rep 9(1):2621. 10.1038/s41598-019-39191-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leto D, Saltiel AR (2012) Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol 13(6): 383–396. 10.1038/nrm3351 [DOI] [PubMed] [Google Scholar]
- 25.Blazquez E, Velazquez E, Hurtado-Carneiro V, Ruiz-Albusac JM (2014) Insulin in the brain: its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol (Lausanne) 5:161. 10.3389/fendo.2014.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEwen BS, Reagan LP (2004) Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol 490(1–3):13–24. 10.1016/j.ejphar.2004.02.041 [DOI] [PubMed] [Google Scholar]
- 27.El Messari S, Leloup C, Quignon M, Brisorgueil MJ, Penicaud L, Arluison M (1998) Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J Comp Neurol 399(4):492–512 [DOI] [PubMed] [Google Scholar]
- 28.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA (1998) GLUT4 glucose transporter expression in rodent brain: Effect of diabetes. Brain Res 797(1): 1–11. 10.1016/s0006-8993(98)00103-6 [DOI] [PubMed] [Google Scholar]
- 29.Grillo CA, Piroli GG, Hendry RM, Reagan LP (2009) Insulinstimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res 1296:35–45. 10.1016/j.brainres.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reno CM, Puente EC, Sheng Z, Daphna-Iken D, Bree AJ, Routh VH, Kahn BB, Fisher SJ (2017) Brain GLUT4 knockout mice have impaired glucose tolerance, decreased insulin sensitivity, and impaired hypoglycemic counterregulation. Diabetes 66(3): 587–597. 10.2337/db16-0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apelt J, Mehlhorn G, Schliebs R (1999) Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res 57(5):693–705 [PubMed] [Google Scholar]
- 32.McNay EC, Pearson-Leary J (2020) GluT4: A central player in hippocampal memory and brain insulin resistance. Exp Neurol 323:113076. 10.1016/j.expneurol.2019.113076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR et al. (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122(4):1316–1338. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez AM, Hernandez-Garzon E, Perez-Domper P, Perez-Alvarez A, Mederos S, Matsui T, Santi A, Trueba-Saiz A et al. (2017) Insulin regulates astrocytic glucose handling through cooperation with IGF-I. Diabetes 66(1):64–74. 10.2337/db16-0861 [DOI] [PubMed] [Google Scholar]
- 35.Uemura E, Greenlee HW (2006) Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Exp Neurol 198(1):48–53. 10.1016/j.expneurol.2005.10.035 [DOI] [PubMed] [Google Scholar]
- 36.Gould GW, Brant AM, Kahn BB, Shepherd PR, McCoid SC, Gibbs EM (1992) Expression of the brain-type glucose transporter is restricted to brain and neuronal cells in mice. Diabetologia 35(4):304–309. 10.1007/BF00401196 [DOI] [PubMed] [Google Scholar]
- 37.Dodd GT, Xirouchaki CE, Eramo M, Mitchell CA, Andrews ZB, Henry BA, Cowley MA, Tiganis T (2019) Intranasal targeting of hypothalamic PTP1B and TCPTP reinstates leptin and insulin sensitivity and promotes weight loss in obesity. Cell Rep 28(11): 2905–2922 e2905. 10.1016/j.celrep.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 38.Kullmann S, Valenta V, Wagner R, Tschritter O, Machann J, Haring HU, Preissl H, Fritsche A et al. (2020) Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat Commun 11(1):1841. 10.1038/s41467-020-15686-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park CR, Seeley RJ, Craft S, Woods SC (2000) Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav 68(4):509–514. 10.1016/s0031-9384(99)00220-6 [DOI] [PubMed] [Google Scholar]
- 40.Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL (1999) Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem 274(49):34893–34902. 10.1074/jbc.274.49.34893 [DOI] [PubMed] [Google Scholar]
- 41.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W (2004) Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29(10):1326–1334. 10.1016/j.psyneuen.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 42.Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W (2007) Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 32(1):239–243. 10.1038/sj.npp.1301193 [DOI] [PubMed] [Google Scholar]
- 43.Rensink AA, Otte-Holler I, de Boer R, Bosch RR, ten Donkelaar HJ, de Waal RM, Verbeek MM, Kremer B (2004) Insulin inhibits amyloid beta-induced cell death in cultured human brain pericytes. Neurobiol Aging 25(1):93–103. 10.1016/s0197-4580(03)00039-3 [DOI] [PubMed] [Google Scholar]
- 44.Gabbouj S, Natunen T, Koivisto H, Jokivarsi K, Takalo M, Marttinen M, Wittrahm R, Kemppainen S et al. (2019) Intranasal insulin activates Akt2 signaling pathway in the hippocampus of wild-type but not in APP/PS1 Alzheimer model mice. Neurobiol Aging 75:98–108. 10.1016/j.neurobiolaging.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 45.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D Jr (1998) Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 50(1):164–168. 10.1212/wnl.50.1.164 [DOI] [PubMed] [Google Scholar]
- 46.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR et al. (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis 7(1):63–80. 10.3233/jad-2005-7107 [DOI] [PubMed] [Google Scholar]
- 47.Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16(6):358–372. 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- 48.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T et al. (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116(7):1793–1801. 10.1172/JCI29069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L et al. (2009) Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119(3):573–581. 10.1172/JCI37048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL (2008) Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 22(1): 246–260. 10.1096/fj.06-7703com [DOI] [PubMed] [Google Scholar]
- 52.Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R (2002) Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci 22 (10):RC221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gali CC, Fanaee-Danesh E, Zandl-Lang M, Albrecher NM, Tam-Amersdorfer C, Stracke A, Sachdev V, Reichmann F et al. (2019) Amyloid-beta impairs insulin signaling by accelerating autophagy-lysosomal degradation of LRP-1 and IR-beta in blood-brain barrier endothelial cells in vitro and in 3XTg-AD mice. Mol Cell Neurosci 99:103390. 10.1016/j.mcn.2019.103390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Najem D, Bamji-Mirza M, Yang Z, Zhang W (2016) Abeta-induced insulin resistance and the effects of insulin on the cholesterol synthesis pathway and Abeta secretion in neural cells. Neurosci Bull 32(3):227–238. 10.1007/s12264-016-0034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Zhou B, Zhang F, Wu J, Hu Y, Liu Y, Zhai Q (2012) Amyloid-beta induces hepatic insulin resistance by activating JAK2/STAT3/SOCS-1 signaling pathway. Diabetes 61(6):1434–1443. 10.2337/db11-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Zhou B, Deng B, Zhang F, Wu J, Wang Y, Le Y, Zhai Q (2013) Amyloid-beta induces hepatic insulin resistance in vivo via JAK2. Diabetes 62(4):1159–1166. 10.2337/db12-0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyagi A, Mirita C, Taher N, Shah I, Moeller E, Tyagi A, Chong T, Pugazhenthi S (2020) Metabolic syndrome exacerbates amyloid pathology in a comorbid Alzheimer’s mouse model. Biochim Biophys Acta Mol basis Dis 1866(10):165849. 10.1016/j.bbadis.2020.165849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hull C, Dekeryte R, Koss DJ, Crouch B, Buchanan H, Delibegovic M, Platt B (2020) Knock-in of mutated hTAU causes insulin resistance, inflammation and proteostasis disturbance in a mouse model of frontotemporal dementia. Mol Neurobiol 57(1): 539–550. 10.1007/s12035-019-01722-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goncalves RA, Wijesekara N, Fraser PE, De Felice FG (2019) The link between tau and insulin signaling: implications for Alzheimer’s disease and other tauopathies. Front Cell Neurosci 13:17. 10.3389/fncel.2019.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao N, Liu CC, Van Ingelgom AJ, Martens YA, Linares C, Knight JA, Painter MM, Sullivan PM et al. (2017) Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron 96(1):115–129 e115. 10.1016/j.neuron.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan ES, Shetty MS, Sajikumar S, Chen C, Soong TW, Wong BS (2016) ApoE4 expression accelerates hippocampus-dependent cognitive deficits by enhancing Abeta impairment of insulin signaling in an Alzheimer’s disease mouse model. Sci Rep 6:26119. 10.1038/srep26119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robbins J, Busquets O, Tong M, de la Monte SM (2020) Dysregulation of insulin-linked metabolic pathways in Alzheimer’s disease: co-factor role of apolipoprotein E varepsilon4. J Alzheimers Dis Rep 4(1):479–493. 10.3233/ADR-200238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neergaard JS, Dragsbaek K, Christiansen C, Nielsen HB, Brix S, Karsdal MA, Henriksen K (2017) Metabolic syndrome, insulin resistance, and cognitive dysfunction: does your metabolic profile affect your brain? Diabetes 66(7): 1957–1963. 10.2337/db16-1444 [DOI] [PubMed] [Google Scholar]
- 64.Fan YC, Chou CC, You SL, Sun CA, Chen CJ, Bai CH (2017) Impact of worsened metabolic syndrome on the risk of dementia: a nationwide cohort study. J Am Heart Assoc 6(9). 10.1161/JAHA.116.004749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JE, Shin DW, Han K, Kim D, Yoo JE, Lee J, Kim S, Son KY et al. (2020) Changes in metabolic syndrome status and risk of dementia. J Clin Med 9(1). 10.3390/jcm9010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsai CK, Kao TW, Lee JT, Wu CJ, Hueng DY, Liang CS, Wang GC, Yang FC et al. (2016) Increased risk of cognitive impairment in patients with components of metabolic syndrome. Medicine (Baltimore) 95(36):e4791. 10.1097/MD.0000000000004791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hishikawa N, Fukui Y, Sato K, Kono S, Yamashita T, Ohta Y, Deguchi K, Abe K (2016) Cognitive and affective functions in Alzheimer’s disease patients with metabolic syndrome. Eur J Neurol 23(2):339–345. 10.1111/ene.12845 [DOI] [PubMed] [Google Scholar]
- 68.Ekblad LL, Johansson J, Helin S, Viitanen M, Laine H, Puukka P, Jula A, Rinne JO (2018) Midlife insulin resistance, APOE genotype, and late-life brain amyloid accumulation. Neurology 90(13): e1150–e1157. 10.1212/WNL.0000000000005214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atti AR, Valente S, Iodice A, Caramella I, Ferrari B, Albert U, Mandelli L, De Ronchi D (2019) Metabolic syndrome, mild cognitive impairment, and dementia: a meta-analysis of longitudinal studies. Am J Geriatr Psychiatry 27(6):625–637. 10.1016/j.jagp.2019.01.214 [DOI] [PubMed] [Google Scholar]
- 70.Mallorqui-Bague N, Lozano-Madrid M, Toledo E, Corella D, Salas-Salvado J, Cuenca-Royo A, Vioque J, Romaguera D et al. (2018) Type 2 diabetes and cognitive impairment in an older population with overweight or obesity and metabolic syndrome: baseline cross-sectional analysis of the PREDIMED-plus study. Sci Rep 8(1): 16128. 10.1038/s41598-018-33843-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pal K, Mukadam N, Petersen I, Cooper C (2018) Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 53(11):1149–1160. 10.1007/s00127-018-1581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li XY, Zhang M, Xu W, Li JQ, Cao XP, Yu JT, Tan L (2019) Midlife modifiable risk factors for dementia: a systematic review and meta-analysis of 34 prospective cohort studies. Curr Alzheimer Res 16(14):1254–1268. 10.2174/1567205017666200103111253 [DOI] [PubMed] [Google Scholar]
- 73.Pekkala T, Hall A, Mangialasche F, Kemppainen N, Mecocci P, Ngandu T, Rinne JO, Soininen H et al. (2020) Association of peripheral insulin resistance and other markers of type 2 diabetes mellitus with brain amyloid deposition in healthy individuals at risk of dementia. J Alzheimers Dis 76(4):1243–1248. 10.3233/JAD-200145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tramutola A, Lanzillotta C, Di Domenico F, Head E, Butterfield DA, Perluigi M, Barone E (2020) Brain insulin resistance triggers early onset Alzheimer disease in Down syndrome. Neurobiol Dis 137:104772. 10.1016/j.nbd.2020.104772 [DOI] [PubMed] [Google Scholar]
- 75.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K (2008) Central obesity and increased risk of dementia more than three decades later. Neurology 71(14): 1057–1064. 10.1212/01.wnl.0000306313.89165.ef [DOI] [PubMed] [Google Scholar]
- 76.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr, Yaffe K (2005) Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 330(7504):1360. 10.1136/bmj.38446.466238.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hassing LB, Dahl AK, Thorvaldsson V, Berg S, Gatz M, Pedersen NL, Johansson B (2009) Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes 33(8):893–898. 10.1038/ijo.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anstey KJ, Cherbuin N, Budge M, Young J (2011) Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev 12(5):e426–e437. 10.1111/j.1467-789X.2010.00825.x [DOI] [PubMed] [Google Scholar]
- 79.Nepal B, Brown LJ, Anstey KJ (2014) Rising midlife obesity will worsen future prevalence of dementia. PLoS One 9(9):e99305. 10.1371/journal.pone.0099305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JY, Han K, Han E, Kim G, Cho H, Kim KJ, Lee BW, Kang ES et al. (2019) Risk of incident dementia according to metabolic health and obesity status in late life: a population-based cohort study. J Clin Endocrinol Metab 104(7):2942–2952. 10.1210/jc.2018-01491 [DOI] [PubMed] [Google Scholar]
- 81.Banks WA, Farr SA, Salameh TS, Niehoff ML, Rhea EM, Morley JE, Hanson AJ, Hansen KM et al. (2018) Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int J Obes 42(3):391–397. 10.1038/ijo.2017.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mukamal KJ (2020) Nonesterified fatty acids, cognitive decline, and dementia. Curr Opin Lipidol 31(1):1–7. 10.1097/MOL.0000000000000656 [DOI] [PubMed] [Google Scholar]
- 83.Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS (2005) Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci 119(5): 1389–1395. 10.1037/0735-7044.119.5.1389 [DOI] [PubMed] [Google Scholar]
- 84.Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D Jr (1990) Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides 11(3):467–472. 10.1016/0196-9781(90)90044-6 [DOI] [PubMed] [Google Scholar]
- 85.Ohkuma S, Ma FH, Kishi M, Kuriyama K (1990) Alteration of acetylcholine metabolism in the brain of Zucker fatty rat. Neurochem Int 16(1):99–103. 10.1016/0197-0186(90)90129-h [DOI] [PubMed] [Google Scholar]
- 86.Raza H, John A, Howarth FC (2015) Increased oxidative stress and mitochondrial dysfunction in Zucker diabetic rat liver and brain. Cell Physiol Biochem 35(3):1241–1251. 10.1159/000373947 [DOI] [PubMed] [Google Scholar]
- 87.Figlewicz DP, Dorsa DM, Stein LJ, Baskin DG, Paquette T, Greenwood MR, Woods SC, Porte D Jr (1985) Brain and liver insulin binding is decreased in Zucker rats carrying the ‘fa’ gene. Endocrinology 117(4):1537–1543. 10.1210/endo-117-4-1537 [DOI] [PubMed] [Google Scholar]
- 88.Liistro T, Guiducci L, Burchielli S, Panetta D, Belcari N, Pardini S, Del Guerra A, Salvadori PA et al. (2010) Brain glucose overexposure and lack of acute metabolic flexibility in obesity and type 2 diabetes: a PET-[18F]FDG study in Zucker and ZDF rats. J Cereb Blood Flow Metab 30(5):895–899. 10.1038/jcbfm.2010.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomassoni D, Nwankwo IE, Gabrielli MG, Bhatt S, Muhammad AB, Lokhandwala MF, Tayebati SK, Amenta F (2013) Astrogliosis in the brain of obese Zucker rat: a model of metabolic syndrome. Neurosci Lett 543:136–141. 10.1016/j.neulet.2013.03.025 [DOI] [PubMed] [Google Scholar]
- 90.Qin L, Chong T, Rodriguez R, Pugazhenthi S (2016) Glucagonlike peptide-1-mediated modulation of inflammatory pathways in the diabetic brain: relevance to Alzheimer’s disease. Curr Alzheimer Res 13(12):1346–1355. 10.2174/1567205013666160401114751 [DOI] [PubMed] [Google Scholar]
- 91.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T (2002) Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 113(3):607–615. 10.1016/s0306-4522(02)00162-8 [DOI] [PubMed] [Google Scholar]
- 92.Zheng H, Zheng Y, Zhao L, Chen M, Bai G, Hu Y, Hu W, Yan Z et al. (2017) Cognitive decline in type 2 diabetic db/db mice may be associated with brain region-specific metabolic disorders. Biochim Biophys Acta Mol basis Dis 1863(1):266–273. 10.1016/j.bbadis.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 93.Yermakov LM, Griggs RB, Drouet DE, Sugimoto C, Williams MT, Vorhees CV, Susuki K (2019) Impairment of cognitive flexibility in type 2 diabetic db/db mice. Behav Brain Res 371: 111978. 10.1016/j.bbr.2019.111978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang Y, Lin L, Liu N, Wang Q, Yuan J, Li Y, Chung KK, Guo S et al. (2020) FGF21 protects against aggravated blood-brain barrier disruption after ischemic focal stroke in diabetic db/db male mice via cerebrovascular PPARgamma activation. Int J Mol Sci 21(3). 10.3390/ijms21030824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumari R, Bettermann K, Willing L, Sinha K, Simpson IA (2020) The role of neutrophils in mediating stroke injury in the diabetic db/db mouse brain following hypoxia-ischemia. Neurochem Int 139:104790. 10.1016/j.neuint.2020.104790 [DOI] [PubMed] [Google Scholar]
- 96.Gratuze M, El Khoury NB, Turgeon A, Julien C, Marcouiller F, Morin F, Whittington RA, Marette A et al. (2017) Tau hyperphosphorylation in the brain of ob/ob mice is due to hypothermia: Importance of thermoregulation in linking diabetes and Alzheimer’s disease. Neurobiol Dis 98:1–8. 10.1016/j.nbd.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 97.Schepers J, Gebhardt C, Bracke A, Eiffler I, von Bohlen Und Halbach O (2020) Structural and functional consequences in the amygdala of leptin-deficient mice. Cell Tissue Res. 10.1007/s00441-020-03266-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qin X, Wang W, Wu H, Liu D, Wang R, Xu J, Jiang H, Pan F (2020) PPARgamma-mediated microglial activation phenotype is involved in depressive-like behaviors and neuroinflammation in stressed C57BL/6J and ob/ob mice. Psychoneuroendocrinology 117:104674. 10.1016/j.psyneuen.2020.104674 [DOI] [PubMed] [Google Scholar]
- 99.Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M et al. (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A 107(15):7036–7041. 10.1073/pnas.1000645107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shinohara M, Tashiro Y, Shinohara M, Hirokawa J, Suzuki K, Onishi-Takeya M, Mukouzono M, Takeda S et al. (2020) Increased levels of Abeta42 decrease the lifespan of ob/ob mice with dysregulation of microglia and astrocytes. FASEB J 34(2): 2425–2435. 10.1096/fj.201901028RR [DOI] [PubMed] [Google Scholar]
- 101.Girault FM, Sonnay S, Gruetter R, Duarte JMN (2019) Alterations of brain energy metabolism in type 2 diabetic Goto-Kakizaki rats measured in vivo by (13)C magnetic resonance spectroscopy. Neurotox Res 36(2):268–278. 10.1007/s12640-017-9821-y [DOI] [PubMed] [Google Scholar]
- 102.Santos MS, Santos DL, Palmeira CM, Seica R, Moreno AJ, Oliveira CR (2001) Brain and liver mitochondria isolated from diabetic Goto-Kakizaki rats show different susceptibility to induced oxidative stress. Diabetes Metab Res Rev 17(3):223–230. 10.1002/dmrr.200 [DOI] [PubMed] [Google Scholar]
- 103.Soares AF, Nissen JD, Garcia-Serrano AM, Nussbaum SS, Waagepetersen HS, Duarte JMN (2019) Glycogen metabolism is impaired in the brain of male type 2 diabetic Goto-Kakizaki rats. J Neurosci Res 97(8):1004–1017. 10.1002/jnr.24437 [DOI] [PubMed] [Google Scholar]
- 104.Moreira PI, Santos MS, Moreno AM, Seica R, Oliveira CR (2003) Increased vulnerability of brain mitochondria in diabetic (Goto-Kakizaki) rats with aging and amyloid-beta exposure. Diabetes 52(6):1449–1456. 10.2337/diabetes.52.6.1449 [DOI] [PubMed] [Google Scholar]
- 105.Moreira T, Cebers G, Pickering C, Ostenson CG, Efendic S, Liljequist S (2007) Diabetic Goto-Kakizaki rats display pronounced hyperglycemia and longer-lasting cognitive impairments following ischemia induced by cortical compression. Neuroscience 144(4):1169–1185. 10.1016/j.neuroscience.2006.10.054 [DOI] [PubMed] [Google Scholar]
- 106.Li XH, Xin X, Wang Y, Wu JZ, Jin ZD, Ma LN, Nie CJ, Xiao X et al. (2013) Pentamethylquercetin protects against diabetes-related cognitive deficits in diabetic Goto-Kakizaki rats. J Alzheimers Dis 34(3):755–767. 10.3233/JAD-122017 [DOI] [PubMed] [Google Scholar]
- 107.Pintana H, Apaijai N, Kerdphoo S, Pratchayasakul W, Sripetchwandee J, Suntornsaratoon P, Charoenphandhu N, Chattipakorn N et al. (2017) Hyperglycemia induced the Alzheimer’s proteins and promoted loss of synaptic proteins in advanced-age female Goto-Kakizaki (GK) rats. Neurosci Lett 655:41–45. 10.1016/j.neulet.2017.06.041 [DOI] [PubMed] [Google Scholar]
- 108.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A et al. (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35(6):1364–1379. 10.2337/dc12-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weinstein G, Davis-Plourde KL, Conner S, Himali JJ, Beiser AS, Lee A, Rawlings AM, Sedaghat S et al. (2019) Association of metformin, sulfonylurea and insulin use with brain structure and function and risk of dementia and Alzheimer’s disease: Pooled analysis from 5 cohorts. PLoS One 14(2):e0212293. 10.1371/journal.pone.0212293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Avgerinos KI, Kalaitzidis G, Malli A, Kalaitzoglou D, Myserlis PG, Lioutas VA (2018) Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment: a systematic review. J Neurol 265(7):1497–1510. 10.1007/s00415-018-8768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M et al. (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: apilot clinical trial. Arch Neurol 69(1):29–38. 10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M et al. (2015) Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 44(3):897–906. 10.3233/JAD-141791 [DOI] [PubMed] [Google Scholar]
- 113.Mustapic M, Tran J, Craft S, Kapogiannis D (2019) Extracellular vesicle biomarkers track cognitive changes following intranasal insulin in Alzheimer’s disease. J Alzheimers Dis 69(2):489–498. 10.3233/JAD-180578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Craft S, Raman R, Chow TW, Rafii MS, Sun CK, Rissman RA, Donohue MC, Brewer JB et al. (2020) Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 10.1001/jamaneurol.2020.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y, Zhao Y, Dai CL, Liang Z, Run X, Iqbal K, Liu F, Gong CX (2014) Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Abeta level and microglia activation in the brains of 3xTg-AD mice. Exp Neurol 261:610–619. 10.1016/j.expneurol.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 116.Ruegsegger GN, Manjunatha S, Summer P, Gopala S, Zabeilski P, Dasari S, Vanderboom PM, Lanza IR et al. (2019)Insulin deficiency and intranasal insulin alter brain mitochondrial function: a potential factor for dementia in diabetes. FASEB J 33(3):4458–4472. 10.1096/fj.201802043R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Swaminathan SK, Ahlschwede KM, Sarma V, Curran GL, Omtri RS, Decklever T, Lowe VJ, Poduslo JF et al. (2018) Insulin differentially affects the distribution kinetics of amyloid beta 40 and 42 in plasma and brain. J Cereb Blood Flow Metab 38(5):904–918. 10.1177/0271678X17709709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Franklin W, Krishnan B, Taglialatela G (2019) Chronic synaptic insulin resistance after traumatic brain injury abolishes insulin protection from amyloid beta and tau oligomer-induced synaptic dysfunction. Sci Rep 9(1):8228. 10.1038/s41598-019-44635-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hsu CC, Wahlqvist ML, Lee MS, Tsai HN (2011) Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 24(3):485–493. 10.3233/JAD-2011-101524 [DOI] [PubMed] [Google Scholar]
- 120.Samaras K, Makkar S, Crawford JD, Kochan NA, Wen W, Draper B, Trollor JN, Brodaty H et al. (2020) Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: The Sydney Memory and Ageing Study. Diabetes Care. 10.2337/dc20-0892 [DOI] [PubMed] [Google Scholar]
- 121.Imfeld P, Bodmer M, Jick SS, Meier CR (2012) Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc 60(5):916–921. 10.1111/j.1532-5415.2012.03916.x [DOI] [PubMed] [Google Scholar]
- 122.Hierro-Bujalance C, Infante-Garcia C, Del Marco A, Herrera M, Carranza-Naval MJ, Suarez J, Alves-Martinez P, Lubian-Lopez S et al. (2020) Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimers Res Ther 12(1):40. 10.1186/s13195-020-00607-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T et al. (2014) Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol 13:148. 10.1186/s12933-014-0148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sa-Nguanmoo P, Tanajak P, Kerdphoo S, Jaiwongkam T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2017) SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol 333:43–50. 10.1016/j.taap.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 125.Nanjan MJ, Mohammed M, Prashantha Kumar BR, Chandrasekar MJN (2018) Thiazolidinediones as antidiabetic agents: a critical review. Bioorg Chem 77:548–567. 10.1016/j.bioorg.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 126.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA et al. (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J 6(4):246–254. 10.1038/sj.tpj.6500369 [DOI] [PubMed] [Google Scholar]
- 127.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ et al. (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 13(11):950–958. 10.1176/appi.ajgp.13.11.950 [DOI] [PubMed] [Google Scholar]
- 128.Abbatecola AM, Lattanzio F, Molinari AM, Cioffi M, Mansi L, Rambaldi P, DiCioccio L, Cacciapuoti F et al. (2010) Rosiglitazone and cognitive stability in older individuals with type 2 diabetes and mild cognitive impairment. Diabetes Care 33(8): 1706–1711. 10.2337/dc09-2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G et al. (2010) Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord 30(2):131–146. 10.1159/000318845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodriguez-Rivera J, Denner L, Dineley KT (2011) Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav Brain Res 216(1):255–261. 10.1016/j.bbr.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez de Maturana R, Garcia-Osta A, Ricobaraza A, Perez-Mediavilla A et al. (2010) Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology 35(7): 1593–1604. 10.1038/npp.2010.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 153(1):329–338. 10.1210/en.2011-1502 [DOI] [PubMed] [Google Scholar]
- 133.Pathan AR, Gaikwad AB, Viswanad B, Ramarao P (2008) Rosiglitazone attenuates the cognitive deficits induced by high fat diet feeding in rats. Eur J Pharmacol 589(1–3): 176–179. 10.1016/j.ejphar.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 134.Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, Ghosh S, Nock C et al. (2007) Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis 11(1):45–51. 10.3233/jad-2007-11108 [DOI] [PubMed] [Google Scholar]
- 135.Searcy JL, Phelps JT, Pancani T, Kadish I, Popovic J, Anderson KL, Beckett TL, Murphy MP et al. (2012) Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J Alzheimers Dis 30(4): 943–961. 10.3233/JAD-2012-111661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E (2008) Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci 28(37):9287–9296. 10.1523/JNEUROSCI.3348-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Badhwar A, Brown R, Stanimirovic DB, Haqqani AS, Hamel E (2017) Proteomic differences in brain vessels of Alzheimer’s disease mice: normalization by PPARgamma agonist pioglitazone. J Cereb Blood Flow Metab 37(3):1120–1136. 10.1177/0271678X16655172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Papadopoulos P, Rosa-Neto P, Rochford J, Hamel E (2013) Pioglitazone improves reversal learning and exerts mixed cerebrovascular effects in a mouse model of Alzheimer’s disease with combined amyloid-beta and cerebrovascular pathology. PLoS One 8(7):e68612. 10.1371/journal.pone.0068612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dehghani L, Meamar R, Askari G, Khorvash F, Shaygannejad V, Pour AF, Javanmard SH (2013) The effect of pioglitazone on the Alzheimer’s disease-induced apoptosis in human umbilical vein endothelial cells. Int J Prev Med 4(Suppl 2):S205–S210 [PMC free article] [PubMed] [Google Scholar]
- 140.Chang KL, Pee HN, Yang S, Ho PC (2015) Influence of drug transporters and stereoselectivity on the brain penetration of pioglitazone as a potential medicine against Alzheimer’s disease. Sci Rep 5:9000. 10.1038/srep09000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG (2011) Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol 227(1):128–135. 10.1016/j.expneurol.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yin QQ, Pei JJ, Xu S, Luo DZ, Dong SQ, Sun MH, You L, Sun ZJ et al. (2013) Pioglitazone improves cognitive function via increasing insulin sensitivity and strengthening antioxidant defense system in fructose-drinking insulin resistance rats. PLoS One 8(3): e59313. 10.1371/journal.pone.0059313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Drncker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368(9548):1696–1705. 10.1016/S0140-6736(06)69705-5 [DOI] [PubMed] [Google Scholar]
- 144.Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S (2015) Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab 4(10): 718–731. 10.1016/j.molmet.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Velmurugan K, Bouchard R, Mahaffey G, Pugazhenthi S (2012) Neuroprotective actions of glucagon-like peptide-1 in differentiated human neuroprogenitor cells. J Neurochem 123(6):919–931. 10.1111/jnc.12036 [DOI] [PubMed] [Google Scholar]
- 146.Abbas T, Faivre E, Holscher C (2009) Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res 205(1):265–271. 10.1016/j.bbr.2009.06.035 [DOI] [PubMed] [Google Scholar]
- 147.Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T et al. (2010) GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis 19(4):1205–1219. 10.3233/JAD-2010-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.An J, Zhou Y, Zhang M, Xie Y, Ke S, Liu L, Pan X, Chen Z (2019) Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5xFAD mouse model of Alzheimer’s disease. Behav Brain Res 370:111932. 10.1016/j.bbr.2019.111932 [DOI] [PubMed] [Google Scholar]
- 149.Li PC, Liu LF, Jou MJ, Wang HK (2016) The GLP-1 receptor agonists exendin-4 and liraglutide alleviate oxidative stress and cognitive and micturition deficits induced by middle cerebral artery occlusion in diabetic mice. BMC Neurosci 17(1):37. 10.1186/s12868-016-0272-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rachmany L, Tweedie D, Rubovitch V, Li Y, Holloway HW, Kim DS, Ratliff WA, Saykally JN et al. (2017) Exendin-4 attenuates blast traumatic brain injury induced cognitive impairments, losses of synaptophysin and in vitro TBI-induced hippocampal cellular degeneration. Sci Rep 7(1):3735. 10.1038/s41598-017-03792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eakin K, Li Y, Chiang YH, Hoffer BJ, Rosenheim H, Greig NH, Miller JP (2013) Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats. PLoS One 8(12):e82016. 10.1371/journal.pone.0082016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gault VA, Porter WD, Flatt PR, Holscher C (2010) Actions of exendin-4 therapy on cognitive function and hippocampal synaptic plasticity in mice fed a high-fat diet. Int J Obes 34(8): 1341–1344. 10.1038/ijo.2010.59 [DOI] [PubMed] [Google Scholar]
- 153.Bomba M, Granzotto A, Castelli V, Massetti N, Silvestri E, Canzoniero LMT, Cimini A, Sensi SL (2018) Exenatide exerts cognitive effects by modulating the BDNF-TrkB neurotrophic axis in adult mice. Neurobiol Aging 64:33–43. 10.1016/j.neurobiolaging.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 154.Vadini F, Simeone PG, Boccatonda A, Guagnano MT, Liani R, Tripaldi R, Di Castelnuovo A, Cipollone F et al. (2020) Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes 44(6): 1254–1263. 10.1038/s41366-020-0535-5 [DOI] [PubMed] [Google Scholar]
- 155.Zhang Z, Zhang B, Wang X, Zhang X, Yang QX, Qing Z, Zhang W, Zhu D et al. (2019) Olfactory dysfunction mediates adiposity in cognitive impairment of type 2 diabetes: insights from clinical and functional neuroimaging studies. Diabetes Care 42(7):1274–1283. 10.2337/dc18-2584 [DOI] [PubMed] [Google Scholar]
- 156.Watson KT, Wroolie TE, Tong G, Foland-Ross LC, Frangou S, Singh M, McIntyre RS, Roat-Shumway S et al. (2019) Neural correlates of liraglutide effects in persons at risk for Alzheimer’s disease. Behav Brain Res 356:271–278. 10.1016/j.bbr.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 157.Gejl M, Gjedde A, Egefjord L, Moller A, Hansen SB, Vang K, Rodell A, Braendgaard H et al. (2016) In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci 8:108. 10.3389/fnagi.2016.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Duarte AI, Candeias E, Alves IN, Mena D, Silva DF, Machado NJ, Campos EJ, Santos MS et al. (2020) Liraglutide protects against brain amyloid-beta1-42 accumulation in female mice with early Alzheimer’s disease-like pathology by partially rescuing oxidative/nitrosative stress and inflammation. Int J Mol Sci 21 (5). 10.3390/ijms21051746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McClean PL, Parthsarathy V, Faivre E, Holscher C (2011) The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci 31(17):6587–6594. 10.1523/JNEUROSCI.0529-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McClean PL, Holscher C (2014) Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 76 Pt A:57–67. 10.1016/j.neuropharm.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 161.Batista AF, Forny-Germano L, Clarke JR, Lyra ESNM, Brito-Moreira J, Boehnke SE, Winterborn A, Coe BC et al. (2018) The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol 245(1):85–100. 10.1002/path.5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hansen HH, Fabricius K, Barkholt P, Kongsbak-Wismann P, Schlumberger C, Jelsing J, Terwel D, Termont A et al. (2016) Long-term treatment with liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, has no effect on beta-amyloid plaque load in two transgenic APP/PS1 mouse models of Alzheimer’s disease. PLoS One 11(7):e0158205. 10.1371/journal.pone.0158205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Porter DW, Kerr BD, Flatt PR, Holscher C, Gault VA (2010) Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes Metab 12(10):891–899. 10.1111/j.1463-1326.2010.01259.x [DOI] [PubMed] [Google Scholar]
- 164.Porter WD, Flatt PR, Holscher C, Gault VA (2013) Liraglutide improves hippocampal synaptic plasticity associated with increased expression of Mash1 in ob/ob mice. Int J Obes 37(5): 678–684. 10.1038/ijo.2012.91 [DOI] [PubMed] [Google Scholar]
- 165.Cukierman-Yaffe T, Gerstein HC, Colhoun HM, Diaz R, Garcia-Perez LE, Lakshmanan M, Bethel A, Xavier D et al. (2020) Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol 19(7): 582–590. 10.1016/S1474-4422(20)30173-3 [DOI] [PubMed] [Google Scholar]
- 166.Cai HY, Yang JT, Wang ZJ, Zhang J, Yang W, Wu MN, Qi JS (2018) Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochem Biophys Res Commun 495(1): 1034–1040. 10.1016/j.bbrc.2017.11.114 [DOI] [PubMed] [Google Scholar]
- 167.McClean PL, Holscher C (2014) Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology 86:241–258. 10.1016/j.neuropharm.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 168.Lennox R, Flatt PR, Gault VA (2014) Lixisenatide improves recognition memory and exerts neuroprotective actions in high-fat fed mice. Peptides 61:38–47. 10.1016/j.peptides.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 169.Holscher C (2020) Brain insulin resistance: role in neurodegenerative disease and potential for targeting. Expert Opin Investig Drugs 29(4):333–348. 10.1080/13543784.2020.1738383 [DOI] [PubMed] [Google Scholar]
- 170.Li C, Liu W, Li X, Zhang Z, Qi H, Liu S, Yan N, Xing Y et al. (2020) The novel GLP-1/GIP analogue DA5-CH reduces tau phosphorylation and normalizes theta rhythm in the icv. STZ rat model of AD. Brain Behav 10(3):e01505. 10.1002/brb3.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cao Y, Holscher C, Hu MM, Wang T, Zhao F, Bai Y, Zhang J, Wu MN et al. (2018) DA5-CH, a novel GLP-1/GIP dual agonist, effectively ameliorates the cognitive impairments and pathology in the APP/PS1 mouse model of Alzheimer’s disease. Eur J Pharmacol 827:215–226. 10.1016/j.ejphar.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 172.Li T, Jiao JJ, Holscher C, Wu MN, Zhang J, Tong JQ, Dong XF, Qu XS et al. (2018) A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer’s disease. Hippocampus 28(5):358–372. 10.1002/hipo.22837 [DOI] [PubMed] [Google Scholar]
- 173.Tai J, Liu W, Li Y, Li L, Holscher C (2018) Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Brain Res 1678:64–74. 10.1016/j.brainres.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 174.Zhou JB, Tang X, Han M, Yang J, Simo R (2020) Impact of antidiabetic agents on dementia risk: a Bayesian network meta-analysis. Metabolism 109:154265. 10.1016/j.metabol.2020.154265 [DOI] [PubMed] [Google Scholar]
- 175.Chen KC, Chung CH, Lu CH, Tzeng NS, Lee CH, Su SC, Kuo FC, Liu JS et al. (2020) Association between the use of dipeptidyl peptidase 4 inhibitors and the risk of dementia among patients with type 2 diabetes in Taiwan. J Clin Med 9(3). 10.3390/jcm9030660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Isik AT, Soysal P, Yay A, Usarel C (2017) The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes Res Clin Pract 123:192–198. 10.1016/j.diabres.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 177.Dong Q, Teng SW, Wang Y, Qin F, Li Y, Ai LL, Yu H (2019) Sitagliptin protects the cognition function of the Alzheimer’s disease mice through activating glucagon-like peptide-1 and BDNF-TrkB signalings. Neurosci Lett 696:184–190. 10.1016/j.neulet.2018.12.041 [DOI] [PubMed] [Google Scholar]
- 178.Gault VA, Lennox R, Flatt PR (2015) Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab 17(4):403–413. 10.1111/dom.12432 [DOI] [PubMed] [Google Scholar]
- 179.Pintana H, Apaijai N, Chattipakorn N, Chattipakorn SC (2013) DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J Endocrinol 218(1): 1–11. 10.1530/JOE-12-0521 [DOI] [PubMed] [Google Scholar]
- 180.Tsai TH, Sun CK, Su CH, Sung PH, Chua S, Zhen YY, Leu S, Chang HW et al. (2015) Sitagliptin attenuated brain damage and cognitive impairment in mice with chronic cerebral hypoperfusion through suppressing oxidative stress and inflammatory reaction. J Hypertens 33(5):1001–1013. 10.1097/HJH.0000000000000529 [DOI] [PubMed] [Google Scholar]
- 181.Ates Bulut E, Sahin Alak ZY, Dokuzlar O, Kocyigit SE, Soysal P, Smith L, Isik AT (2020) Cognitive and metabolic outcomes of vildagliptin addition to the therapy in patients with type 2 diabetes mellitus: 26 week follow-up study. Arch Gerontol Geriatr 88: 104013. 10.1016/j.archger.2020.104013 [DOI] [PubMed] [Google Scholar]
- 182.Rizzo MR, Barbieri M, Boccardi V, Angellotti E, Marfella R, Paolisso G (2014) Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. J Gerontol A Biol Sci Med Sci 69(9): 1122–1131. 10.1093/gerona/glu032 [DOI] [PubMed] [Google Scholar]
- 183.Borzi AM, Condorelli G, Biondi A, Basile F, Vicari ESD, Buscemi C, Luca S, Vacante M (2019) Effects of vildagliptin, a DPP-4 inhibitor, in elderly diabetic patients with mild cognitive impairment. Arch Gerontol Geriatr 84:103896. 10.1016/j.archger.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 184.Sripetchwandee J, Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2014) DPP-4 inhibitor and PPARgamma agonist restore the loss of CA1 dendritic spines in obese insulin-resistant rats. Arch Med Res 45(7):547–552. 10.1016/j.arcmed.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 185.Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2013) DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci 37(5):839–849. 10.1111/ejn.12088 [DOI] [PubMed] [Google Scholar]
- 186.El Batsh MM, El Batch MM, Shafik NM, Younos IH (2015) Favorable effects of vildagliptin on metabolic and cognitive dysfunctions in streptozotocin-induced diabetic rats. Eur J Pharmacol 769:297–305. 10.1016/j.ejphar.2015.11.033 [DOI] [PubMed] [Google Scholar]
- 187.Zhang DD, Shi N, Fang H, Ma L, Wu WP, Zhang YZ, Tian JL, Tian LB et al. (2018) Vildagliptin, a DPP4 inhibitor, alleviates diabetes-associated cognitive deficits by decreasing the levels of apoptosis-related proteins in the rat hippocampus. Exp Ther Med 15(6):5100–5106. 10.3892/etm.2018.6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pugazhenthi S, Qin L, Bouchard R (2015) Dipeptidyl peptidase-4 inhibition in diabetic rats leads to activation of the transcription factor CREB in beta-cells. Eur J Pharmacol 755:42–49. 10.1016/j.ejphar.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 189.Biessels GJ, Verhagen C, Janssen J, van den Berg E, Zinman B, Rosenstock J, George JT, Passera A et al. (2019) Effect of linagliptin on cognitive performance in patients with type 2 diabetes and cardiorenal comorbidities: the CARMELINA randomized trial. Diabetes Care 42(10) 1930–1938. 10.2337/dc19-0783 [DOI] [PubMed] [Google Scholar]
- 190.Kosaraju J, Holsinger RMD, Guo L, Tam KY (2017) Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer’s disease. Mol Neurobiol 54(8):6074–6084. 10.1007/s12035-016-0125-7 [DOI] [PubMed] [Google Scholar]
- 191.Hardigan T, Yasir A, Abdelsaid M, Coucha M, El-Shaffey S, Li W, Johnson MH, Ergul A (2016) Linagliptin treatment improves cerebrovascular function and remodeling and restores reduced cerebral perfusion in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 311(3):R466–R477. 10.1152/ajpregu.00057.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nakaoku Y, Saito S, Yamamoto Y, Maki T, Takahashi R, Ihara M (2019) The dipeptidyl Peptidase-4 inhibitor linagliptin ameliorates high-fat induced cognitive decline in Tauopathy model mice. Int J Mol Sci 20(10). 10.3390/ijms20102539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ma M, Hasegawa Y, Koibuchi N, Toyama K, Uekawa K, Nakagawa T, Lin B, Kim-Mitsuyama S (2015) DPP-4 inhibition with linagliptin ameliorates cognitive impairment and brain atrophy induced by transient cerebral ischemia in type 2 diabetic mice. Cardiovasc Diabetol 14:54. 10.1186/s12933-015-0218-z [DOI] [PMC free article] [PubMed] [Google Scholar]