Abstract
People recovered from COVID-19 may still present complications including respiratory and neurological sequelae. In other viral infections, cognitive impairment occurs due to brain damage or dysfunction caused by vascular lesions and inflammatory processes. Persistent cognitive impairment compromises daily activities and psychosocial adaptation. Some level of neurological and psychiatric consequences were expected and described in severe cases of COVID-19. However, it is debatable whether neuropsychiatric complications are related to COVID-19 or to unfoldings from a severe infection. Nevertheless, the majority of cases recorded worldwide were mild to moderate self-limited illness in non-hospitalized people. Thus, it is important to understand what are the implications of mild COVID-19, which is the largest and understudied pool of COVID-19 cases. We aimed to investigate adults at least four months after recovering from mild COVID-19, which were assessed by neuropsychological, ocular and neurological tests, immune markers assay, and by structural MRI and 18FDG-PET neuroimaging to shed light on putative brain changes and clinical correlations. In approximately one-quarter of mild-COVID-19 individuals, we detected a specific visuoconstructive deficit, which was associated with changes in molecular and structural brain imaging, and correlated with upregulation of peripheral immune markers. Our findings provide evidence of neuroinflammatory burden causing cognitive deficit, in an already large and growing fraction of the world population. While living with a multitude of mild COVID-19 cases, action is required for a more comprehensive assessment and follow-up of the cognitive impairment, allowing to better understand symptom persistence and the necessity of rehabilitation of the affected individuals.
Subject terms: Diagnostic markers, Neuroscience
Introduction
The majority of worldwide cases of SARS-CoV-2 infection were mild to moderate, self-limited illness in non-hospitalized people. In the beginning of the pandemic, the WHO–China Joint Mission on COVID-19 reported 80% of the 55 924 patients with laboratory-confirmed COVID-19 in China to Feb 20 (2020), had mild-to-moderate disease, while 13.8% developed severe disease and 6.1% evolved to critical stage requiring intensive care [1]. As of February 10th, 2022, there has been estimated more than 402 million confirmed cases of COVID-19 reported to WHO. It is expected that more than 320 million (80%) had mild to moderate COVID-19. Now, more than 24 months after the start of the events that overturned the health systems across the world, new worries are emerging.
Vaccine distribution worldwide is heterogeneous, so the emergence of new variants has been the new status quo. These new variants were more transmissible, leading to a sharp increase in cases in shorter periods and infecting a larger number of people with less severe presentations [2]. However, mild COVID-19 has been much less studied than the moderate and severe forms.
An increasing concern is the long-lasting presentation of COVID-19 which has been identified in about 5% of COVID-19 infected individuals a month after infection, and in up to 2% after four months [3]. Symptoms include fatigue, headache, cognitive compromise, dyspnea, and anosmia [3]. The association with higher number of symptoms [4] and the slow decrease of people having lasting symptoms [5] suggests that the pathophysiological drivers underlying the presence of symptoms might be transient, such as inflammatory response. However, recent data shows that mild COVID-19 is related to important long-lasting symptoms, including neurological and psychiatric manifestations, and persistent sequelae, in about 30% of those patients, with increasing prevalence in aged individuals [6]. In England, a modeling of health economic impact of the long-COVID symptoms estimated the government willingness-to-pay cost could reach more than 32 billion pounds to avoid the potential 557,764 QALY’s loss in population [7].
Undoubtedly, beyond the COVID-19 pandemic obvious consequences, it also carries a significant psychological stressor with a tremendous impact on individuals and society. Death and insecurity about the future are powerful psychological stressors and social isolation results in loss of educational activities, structured work, and mental health problems [8]. Historical records suggest that previous influenza pandemics of the XVIII and XIX centuries were marked by increased incidence of neuropsychiatric syndromes, such as insomnia, anxiety, depression, mania, psychosis, suicide, delirium [9, 10], and neuromuscular or demyelinating processes. These usually appear during the acute viral phase or at subsequent periods after infection, in recovered patients. As an example, lethargic encephalitis had a surge in cases following the Spanish flu of 1918 [11]. In the XXI century, there were reports of neuropsychiatric sequelae, such as narcolepsy, convulsions, encephalitis, encephalopathy, Guillain-Barre syndrome (GBS) and other neuromuscular and demyelinating processes, associated with SARS-CoV-1, H1N1 and MERS-CoV, virus from the same genus of the actual SARS-CoV-2 [12–14]. Thus, there were plenty of reasons to assume long-lasting neuropsychiatric symptoms associated with COVID-19.
Magnetic resonance imaging data in patients with severe or mild forms of COVID-19 demonstrated brain lesions [15, 16]. A multimodal study including neuropsychological evaluation, MRI and PET-CT imaging in 29 hospitalized patients observed frontoparietal damage with a distinctive pattern of lesions from sepsis, without attentional and processing speed worsening and with persistent changes in language and visual testes up to a month of discharge [17]. Individuals who recovered from suspected or confirmed COVID-19 had a worse performance on cognitive tests in multiple domains when matched with non-COVID-19 subjects, showing evident deficits even amongst those without severe disease [18]. In a preliminary study, we demonstrated important deficits in the visuospatial processing in around 25% of mild (not requiring hospitalization) COVID-19 patients [19]. Here, we report results of the baseline of a prospective observational cohort study of individuals with mild COVID-19 cases. They were investigated using neuropsychological tests, PET-CT and MRI neuroimaging, and immune markers analysis aiming to shed light on the mechanisms of long standing symptoms and related findings.
Methods
Research design and procedures
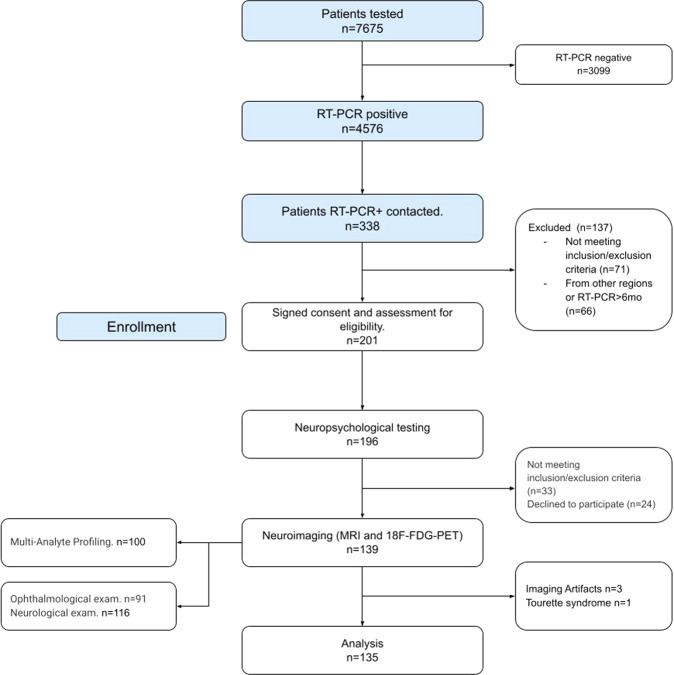
Initially, we assessed clinical status, mental health and history of neurological symptoms with online questionnaires and scales, using the REDCap platform. All included individuals had positive RT-PCR for SARS-CoV-2 and mild COVID-19 presentation. After answering the questions, participants were assigned to subsequent procedures in two different visits. On the first one, neuropsychological assessment, neurological examination and brain structural magnetic resonance imaging (MRI) were performed. On the second visit, blood was collected and 18-FDG-PET brain imaging was performed. Overall research design is shown in Fig. 1.
Fig. 1. Research design and subsamples for each research procedure.
Neuropsychological tests were available for 191 participants (one patient was unable to perform the tests due to anxiety symptoms). Neuroimaging data was available for 166 participants––excluding five as previously mentioned––other 26 images were excluded due to technical problems during data acquisition (6 MRI datasets and 20 FDG-PET datasets), which led to a final subsample of neuroimage data of 135 participants. Lastly, immunological data was acquired for 100 participants which had both neuropsychological and neuroimaging data.
Participants
The study was approved by the IRB of Universidade Federal de Minas Gerais (UFMG) (CAAE3768820.1.0000.5149). Written informed consent was provided by all participants. COVID-19 disease severity 1 and 2 according to the WHO clinical ordinal scale was referred to as mild COVID-19 [20]. Initial data collection was conducted using the Research Electronic Data Capture Platform (REDCap) and followed data protection regulation [21]. Volunteers were recruited through the NUPAD of Faculdade de Medicina-UFMG (FM-UFMG). We contacted a total of 338 patients with RT-PCR confirmed diagnosis of COVID-19. The average time between RT-PCR confirmation and inclusion was 4.35 (±2.45) months. Recruiting and sample details are described in the Supplementary Methods and Supplementary Tables 1 and 2.
Psychiatric assessment
Two questions at the REDcap online form were the initial screening used as exclusion criteria: “Have you ever been diagnosed with a mental disorder” and if the answer is positive, “Does this disorder persist to this day?”. Even if the participant did not report previous or current mental disorder we adopted two other screening measures to document possible psychiatric symptoms. The DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure-Adult [22] and Self Reporting Questionnaire - SRQ-20 [23].
Cognitive assessment
We adopted standardized psychometric measures previously validated for the Brazilian population. To assess the subjective perception of cognitive change (worsening) we adopted the AD8 scale [24]. Although usually adopted in cases of Alzheimer’s disease, we adopted the test for self-report and its original instructions, asking the participant to report cognitive changes after the recovery of COVID-19.
Following the more commonly adopted guidelines regarding cognitive impairment we adopted the −1.5 standard-deviation below demographically adjusted normative values as indicative criteria of deficits. The cutoff score 1 (“normal”)/2 (“impairment”) was adopted for the classification of our participants. The summed score was also used. This is usually the recommended threshold for minor neurocognitive disorders (APA, 2013) or mild cognitive impairment [25]. In our neuropsychological assessment protocol we classified the observed impairment regarding main cognitive functions assessed by each test: language (verbal fluency), visuoconstructive (Rey-Osterrieth Complex Figure Test (ROCF) Copy) [26], memory (Logical Memory and ROCF recall) [26, 27], attention (Trail Making Test) [28], executive functions (Verbal Fluency Switching and Five Point Test) [29, 30] and working memory (Digit Span) [31].
Neurologic and ophthalmological exams
Neurological evaluation was performed by two neurologists (STC and HO), encompassing mental status, cranial nerves, motor and sensory function, tendon reflexes, coordination, gait and stance. A routine ophthalmological examination was performed by an ophthalmologist (LCM) to rule out possible ocular conditions that could interfere with the assessments: refraction, eye alignment and motility, pupil, visual confrontation field, examination of the external eye, attachments, previous and posterior segments.
Neuroimaging
Magnetic resonance imaging (MRI)
Brain imaging acquisitions were performed for every patient on a 3.0 T MRI system (Skyra; Siemens, Erlangen, Germany) with a 20-channel receive head coil. The protocol included isotropic three-dimensional (3D) T1, T2 and T2-FLAIR sequences, performed at Hermes Pardini Institute. T1-weighted spin-echo sequence, isotropic 3D T2-WI turbo spin-echo (SPACE), isotropic 3D fluid-attenuated inversion recovery (FLAIR), diffusion-weighted MRI (DW-MRI), and susceptibility-WI imaging (SWI). Gadolinium-contrast was not administered.
PET/CT
Resting-state 18F-FDG PET/CT brain images were acquired in a GE D690 (GE Healthcare, Milwaukee, WI, USA) PET/CT scanner. Blood glucose level was checked and only patients with <140 mg/dl were injected with 0.09 mCi/kg of 18F-FDG. After 50 min in a quiet and dark room with minimum stimuli, PET brain images were acquired for 10 min, and reconstructed using the OSEM (Ordered Subsets Expectation Maximization) algorithm. Attenuation correction was performed using the CT image.
LUMINEX immunoassay
Peripheral blood samples were obtained in EDTA vacuum tubes. Plasma samples were prepared by centrifugation of venous blood (3000 g for 15 min at 4 °C), divided in aliquots and stored at −80 °C until analysis. Biomarkers including chemokines, inflammatory cytokines, regulatory cytokines and growth factors were analyzed at Instituto René Rachou–Fiocruz using the MultiPlex kit 45-Plex Human ProcartaPlex™ (Thermo Fisher Scientific. USA. EPXR450-12171-901) according to the manufacturer’s instructions (the full list of markers can be found in the Supplementary Material. The biomarker concentrations were determined according to standard curves using a 5-parameter logistic fit and the results were expressed as pg/mL.
Statistical analyses
A detailed description of the statistical analysis is provided in Supplementary Information.
Results
Sociodemographic data and report of COVID-19 symptoms
Our sample (n = 192) was predominantly female (n = 71%), relatively young (on average 38.17 ± 9.82 years), highly educated (66% with a college degree or post graduation) and average socioeconomic status according to Brazilian standards (62%). Regarding the COVID-19 infection 6% were referred to hospitalization during the acute phase of the disease. The most reported symptoms were headache (77%), myalgia (68%) and anosmia (64%).
Mental health, ophthalmologic and neurological symptoms
About 8% of our sample has a history of mental disorders (n = 15), mostly depression and anxiety disorders. According to the SRQ-20 screening 91 participants (48%) showed non-psychotic psychiatric symptoms. Similar results were seen in the self-reported DSM-5 screening where a relatively similar number of participants showed signs of depression (49%), anxiety (53%), anger (47%) and sleep disorders (50%). Other symptoms are shown in Supplementary Table 3. Although these values are considerably high they do not refer to mental disorders per se, but a higher number of symptoms when compared to the general population. Isolated and nonspecific neurological findings were encountered, such as optokinetic nystagmus, absence of ankle reflexes, indifferent plantar responses, decreased pinprick sensitivity on distal extremity of toes, global tendon hyperreflexia, unsustained ankle clonus, postural tremor and intention tremor.
Regarding cognitive changes, 51% of our sample reported subjective daily problems with thinking/or memory, 39% problems with judgment and 34% in remembering appointments.
Neuropsychological assessment results
The frequency of cognitive impairment is shown on Table 1. We did not find significant differences in most of the neuropsychological tests, with frequencies of impairment around 8% (the expected for the criterion −1.5 standard-deviations below normative data). However, we found an atypically high rate of impairment in the copy ROCFT (26%). Matched controls showed about 6% of impairment in the same task.
Table 1.
Neuropsychological impairment in COVID-19 patients (n = 191).
| Test | Impairmenta | Patientsb | Controlsb | t-testc | ||
|---|---|---|---|---|---|---|
| n | % | M(SD) | M(SD) | p | d | |
| Verbal Fluency (animals) | 16 | 8% | 19.35 (5.59) | 19.76 (4.79) | 0.699 | −0.08 |
| Verbal Fluency (fruits) | 5 | 3% | 17.00 (4.14) | 16.24 (3.88) | 0.354 | 0.19 |
| Switching fluency (pairs) | 5 | 3% | 17.00 (4.14) | 8.84 (1.68) | 0.820 | −0.05 |
| ROCFT - Copy | 48 | 24% | 34.14 (2.95) | 29.22 (4.41) | 0.001 | 1.31 |
| ROCFT - Immediate Recalld | 9 | 5% | 20.61 (6.19) | 17.22 (5.42) | 0.003 | 0.58 |
| ROCFT - Delayed Recalld | 14 | 7% | 21.04 (5.98) | 16.41 (5.28) | 0.001 | 0.82 |
| Logical Memory - Immediate Recall | 17 | 9% | 10.69 (3.55) | 11.76 (2.26) | 0.184 | −0.27 |
| Logical Memory - Delayed Recall | 10 | 5% | 9.82 (3.92) | 10.27 (4.10) | 0.581 | −0.11 |
| Digit Span Forward | 0 | 0% | 51.16 (22.88) | 50.22 (26.42) | 0.851 | 0.04 |
| Digit Span Backward | 0 | 0% | 24.82 (16.45) | 28.51 (15.09) | 0.250 | −0.23 |
| Five Point Test (unique) | 0 | 0% | 29.08 (11.6) | 27.45 (12.47) | 0.505 | −0.23 |
| Trail Making Test A | 21 | 11% | 37.45 (14.08) | 39.41 (14.46) | 0.498 | −0.23 |
| Trail Making Test B | 19 | 10% | 96.37 (54.78) | 85.67 (43.38) | 0.263 | −0.23 |
M mean, SD standard-deviation, ROCFT Rey-Osterrieth Complex Figure Test.
aCompared to Brazilian normative data.
bMatched by age, education and sex (n = 49 for each group).
cIndependent samples t-tests and effect size calculated by the Cohens’s d equation.
dThis differences were not significant after controlling for the copy impairment in an analysis of covariance (ANCOVA) model.
When compared to the matched control sample we observed significant differences in this test. The copy trial was the most affected (t = 6.40, p < 0.001, d = 1.31), while the immediate (t = 2.88, p = 0.003, d = 0.58) and delayed recalls (t = 4.06, p = 0.001, d = 0.82) showed less prominent differences. No other statistically significant difference was observed between patients and matched controls (p values ranged from 0.184 to 0.870). We compared patients and controls in both memory trials using analysis of covariance (ANCOVA) controlling for the copy score, to investigate if the memory difficulties in the task were secondary to the visuospatial impairment. The analysis showed no differences in immediate (F = 0.474, p = 0.506) or delayed recall (F = 0.219, p = 0.650) after controlling for the copy score, which suggests a more specific impairment in the visuoconstructional processes of COVID-19 patients. Some examples of abnormal ROCFT copies are shown in Supplementary Fig. 1.
In order to test if the cognitive deficits were restricted to the visuoconstructional processes or explained by symptoms of mental disorders or sociodemographic factors, we computed spearman rank-order correlations between neuropsychological tests Z-scores between these factors (Supplementary Fig. 2). Most correlations were not statistically significant but we found a weak positive association of socioeconomic status and test performance (rho = 0.290, p < 0.01). However, this coefficient was relatively similar in most neuropsychological tests, and did not seem a factor specifically related to the visuoconstructional measure. Even when patients and matched-controls were compared covariariating socioeconomic status the difference remained significant (p < 0.001).
The neuropsychological profile of the COVID-19 showed a specific pattern of impairment in visuoconstructional processes, measured by the ROCF, in about 26% of the patients. To further investigate this deficit and its neurobiological correlates we stratified our sample in patients with and without impairment.
Luminex multiplex assay findings
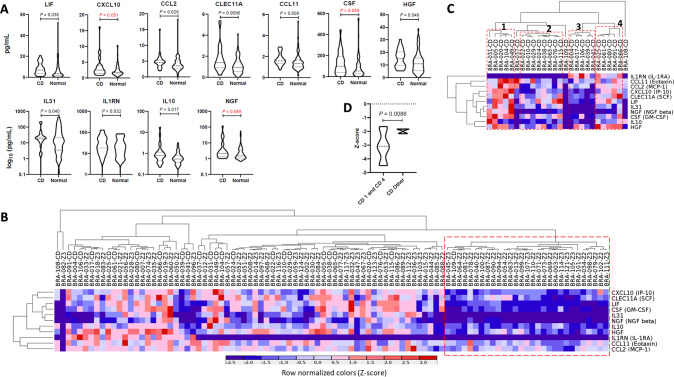
Eleven biomarkers, namely LIF Interleukin 6 Family Cytokine (LIF), C-X-C motif chemokine ligand 10 (CXCL10), chemokine (C-C-motif) ligand 2 (CCL2), C-type lectin domain containing 11 A (CLEC11A), C-C motif chemokine ligand 11 (CCL11), granulocyte-macrophage colony-stimulating factor (CSF), hepatocyte growth factor (HGF), interleukin 31 (IL31), interleukin 1 receptor antagonist (IL1RN), interleukin 10 (IL10) and nerve growth factor (NGF) were upregulated in the plasma of individuals with COVID-19 that showed visuoconstructional impairment in the copy of the ROCF test when compared to patients without this deficit. Figure 2A shows the multiplex results.
Fig. 2. Plasma Biomarkers and Hierarchical clustering of individuals with visuoconstructive deficit or normal outcome after mild COVID-19 and the differentially expressed plasma biomarkers.
A Differentially expressed plasma biomarkers associated with visuoconstructive deficit after mild COVID-19. Dashed lines = medians; dotted lines = quartiles. CD = visuoconstructive deficit. N for CD = 26; N for normal = 74. B Hierarchical clustering of individuals with visuoconstructive deficit or normal outcome after mild COVID-19 and the differentially expressed plasma biomarkers. C Hierarchical clustering of individuals with visuoconstructive deficit after mild COVID-19 and their upregulated plasma biomarkers. D Comparison of Z-scores at the Rey–Osterrieth Complex Figure (ROCFT) test compared with Kolmogorov-Smirnov statistical test (interquartile).
Individuals were grouped based on the expression levels of 11 plasma biomarkers upregulated in those with visuoconstructive deficit when compared with those without deficit. In the hierarchical clustering, individuals were segregated in two main groups, one with low frequency of visuoconstructive deficit and relatively low levels of the 11 plasma biomarkers, the other with high incidence of deficit and higher levels of these proteins. This result suggests that lower expression of the 11 biomarkers is associated with protection against cognitive impairment (Fig. 2B). Among the individuals with visuoconstructive deficit, at least four clusters were found (Fig. 2C), indicating that the highly expressed 11 plasma biomarkers associated with cognitive impairment can be present at distinct combinatorial patterns. Individuals with visuoconstructive deficit from clusters 1 and 4 performed poorly at the ROCF test (Fig. 2D).
Significant correlation was observed between the lowest values of the ROCF test and the highest plasma levels of SCF (c = 0.39, p = 0.048), CSF (c = 0.46, p = 0.020), HGF (c = 0.40, p = 0.041) and IL1RA (c = 0.59, p = 0.001), which was not observed in the group of individuals without visuoconstructive deficit (p ≥ 0,30 for all). The ROCF test values did not correlate with the age and the gender of the subjects as well as with the levels of the biomarkers (p ≥ 0,31 for all), but CCL11 increased plasma levels correlated with increasing age (c = 0.41, r = 0.001).
Neuroimaging findings
We observed no structural changes in the MRI (no signs of thromboembolism, atrophy, acute encephalitis or leptomeningeal enhancement) in any of the 135 patients. The VBM-based analysis of GM images showed no voxel clusters of significant positive or negative correlations with scores on the ROCF test, at the threshold of p < 0.0005 and 10 contiguous voxels.
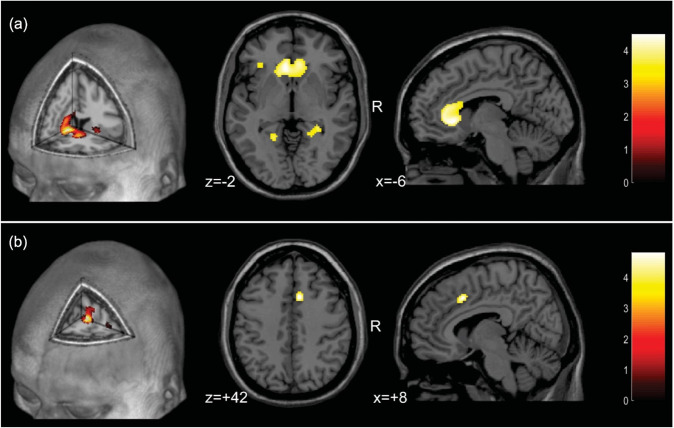
The analysis of WM images also showed no voxel clusters of significant positive correlation with scores on the ROCF test. Conversely, there was a large number of voxels (n = 1,848) in which there were significant negative correlations between regional volumes and Z-scores on the ROCF test, indicating a widespread pattern of inverse relationship between test performance and WM volumes (see Fig. 3). These voxels were aggregated in nine clusters (Table 2), the largest of which (n = 1426 voxels) encompassing the subgenual portion of the corpus callosum and the cingulum on both hemispheres (Fig. 3). The additional clusters involved WM portions of the inferior frontal gyrus and the fronto-occipital fasciculus bilaterally, as well as the right fusiform gyrus and the bilateral lingual gyri (Table 2).
Fig. 3. Brain correlates of visuoconstructional performance in mild COVID-19 patients.
a Findings of negative correlation between performance on the Rey-Osterrieth Complex Figure (ROCF) test and white matter volume (filtered at the Z > 3.29 threshold). The foci show the peak of the greatest significance within the cluster (highlighted in yellow), located in the left and right genu of the corpus callosum, extending to the cingulum bundle; (b) Findings of negative correlation between performance on the ROCF test and glucose metabolism (filtered at the Z > 3.29 threshold). The foci show the peak of the greatest significance within the cluster (highlighted in yellow), located in the right dorsal anterior cingulate gyrus. The colored bar represents the T value. Foci of significance were overlaid on axial brain slices spatially normalized into an approximation to the Talairach and Tournoux stereotactic atlas (Talairach and Tornoux, 1988). Abbreviations: R right. Statistical details are provided in Table 2.
Table 2.
Significant correlations between performance on the Rey–Osterrieth Complex Figure Test and neuroimaging measurements of gray and white matter volumes (MRI) and glucose metabolism (FDG-PET).
| Brain regiona | Direction of significant correlation | Cluster sizeb | Coordinatesc | Peak Z-scored | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Gray matter volume | ||||||
| No significant correlations. | – | – | – | – | – | – |
| White matter volume | ||||||
| Left and right genu of the corpus callosum, extending to the cingulum bundle | Negative | 1426 | −6 | 26 | −2 | 4.32 |
| Right fusiform gyrus | Negative | 93 | 32 | −22 | −26 | 4.01 |
| Right lingual gyrus | Negative | 127 | 30 | −44 | −8 | 3.84 |
| Right inferior frontal gyrus | Negative | 98 | 40 | 6 | 16 | 3.76 |
| Left lingual gyrus | Negative | 41 | −18 | −52 | 2 | 3.73 |
| Left inferior frontal gyrus | Negative | 20 | −34 | 30 | −2 | 3.48 |
| Left inferior fronto-occipital fasciculus | Negative | 15 | −24 | 4 | −8 | 3.47 |
| Left inferior fronto-occipital fasciculus | Negative | 15 | −32 | −10 | −8 | 3.44 |
| Right inferior fronto-occipital fasciculus | Negative | 13 | 32 | −10 | −8 | 3.43 |
| Glucose metabolism (FDG-PET) | ||||||
| Left inferior temporal gyrus | Positive | 34 | −56 | −46 | −22 | 3.96 |
| Left inferior occipital gyrus (superior portion) | Positive | 53 | −48 | −68 | −16 | 3.92 |
| Right dorsal anterior cingulate gyrus | Negative | 69 | 8 | 16 | 42 | 4.61 |
| Right Rolandic operculum and opercular part of the inferior frontal gyrus | Negative | 57 | 52 | 8 | 12 | 4.48 |
| Right inferior occipital gyrus | Negative | 62 | 38 | −74 | −6 | 4.15 |
| Left calcarine and lingual gyri | Negative | 66 | −14 | −92 | −8 | 3.88 |
| Left superior frontal gyrus | Negative | 50 | −16 | 60 | −8 | 3.75 |
| Left inferior occipital gyrus (inferior portion) | Negative | 19 | −30 | −82 | −8 | 3.46 |
| Right medial frontal and orbital frontal gyri | Negative | 20 | 18 | 54 | −4 | 3.43 |
aFor the analysis of white matter volumes, the brain regions where voxel clusters were located were identified according to the MRI Atlas of Human White Matter (Oishi et al., 2010). For the analysis of glucose metabolism, brain regions were identified according to the Automatic Anatomical Labeling Toolbox for SPM12 (Rolls et al., 2015).
bNumber of contiguous voxels in each cluster that surpassed the initial cutoff of p <= 0.0005.
cMNI coordinates of the voxel of maximal statistical significance within each cluster.
dZ-score for the voxel of maximal statistical significance in each region.
FDG-PET images indicated nine clusters of voxels displaying significant correlations with Z-scores on the ROCF test (see Fig. 3 and Table 2). Two of those were clusters of significant positive correlation, involving the left inferior temporal gyrus and the left inferior occipital gyrus (Table 2). The six clusters of significant negative correlation encompassed frontal (right dorsal anterior cingulate, Rolandic operculum and ventrolateral frontal cortices, and left superior lateral frontal cortex) and occipital regions (bilateral inferior occipital cortex, and left calcarine/lingual gyri (Table 2).
Discussion
Cognitive deficits following COVID-19 infection have been documented across studies of patients with different ages, disease severity and recovery time [32]. A smaller number of studies analyzed more specific cognitive functions, such as episodic memory, executive functions, verbal fluency [17] and sustained attention [33]. On the other hand, Mattioli and colleagues [34] reported no significant differences between patients and controls in neuropsychological tests, four months after the infection. However, there are inconsistencies regarding affected cognitive functions and severity of deficits [35]. We observed significant cognitive impairment only in the ROCF, a drawing task test used to assess visuospatial abilities, executive functions and memory. The deficits observed in the ROCF could not be explained by socio-demographic factors, ophthalmologic deficits or psychiatric symptoms, suggesting cognitive deficit secondary to SARS-CoV-2 infection. Other factors which may influence performance, such as motor coordination, spatial neglect, visual attention, semantic knowledge, intelligence and executive functions were not likely to explain the observed difficulties, since we did not find any significant differences in other non-verbal (Trail Making Test and Five Points Test) and verbal tests (verbal fluency, digit span) also related to these processes.
Visuoconstructive deficits are usually defined as an atypical difficulty in using visual and spatial information to guide complex behaviors like drawing, assembling objects or organizing multiple pieces of a more sophisticated stimuli. In drawing a complex figure, as in the ROCFT, the patient must organize visual and spatial information in a planned manner to execute the drawing per se, a processes that demand several more specific cognitive abilities related to perceiving, processing, storing and recalling visuospatial information, both regarding shape and position, as well the planning and execution of the drawing per se.
These processes involve multiple brain regions, including the occipito-parietal regions, the dorsal and ventral streams and connections with the cingulate, medial temporal and frontal cortices, integrating the perception and interpretation of the visual information with memory and executive systems [36, 37]. Drawing tasks have been getting attention lately for their sensitivity to study visuospatial deficits, which were shown to be early biomarkers of neurodegenerative disorders, such as Alzheimer’s and Parkison’s disease [38–40].
The COVID-19 individuals investigated herein were often unable to produce a proper copy of Rey’s figure, and had difficulties in memory. Immediate and delayed recall seems to be secondary to the copy impairment. The lack of ability to assemble or organize parts into a whole object or figure is considered constructional apraxia, a neuropsychological syndrome which results in inability to accurately reproduce two-dimensional or three-dimensional visual models [41]. Constructional apraxia might follow acquired brain lesions or aging-related neurodegenerative diseases which affect the parietal or frontal lobes, but it is very uncommon in younger patients as the ones in our study, with a mean age of 38 years. Constructional apraxia is a sign of a divergence between the intended action and the actual performance, which may be seen in tests of free drawing or standardized tests of copy, including the ROCF. The performance on visuoconstruction and memory tests, such as the ROCF, are associated with different aspects of everyday life, including the capacity to learn, problem-solving skills, and activities of daily living [42]. A more comprehensive assessment and follow-up of the visuoconstructive impairment should allow better understanding of symptom persistence and the need of rehabilitation.
HCoVs have molecular structure and mode of replication similar to neuro-invasive animal coronaviruses [43], which can reach the CNS and induce different types of neuropathology. MHV is the best known coronavirus involved in short- and long-term neurological disorders [44]. It is plausible to consider their involvement in neuropsychiatric symptoms and possible post-viral sequelae. The angiotensin-converting enzyme 2 (ACE2 or Ace2) has been identified as a primary entry receptor for SARS-CoV-2, indicating that SARS-CoV-2 may be able to infect the brain and result in CNS symptoms in patients with COVID-19 [45].
In other viral infections, such as HTLV-1, a correlation between the proviral load in peripheral blood mononuclear cells was observed with brain white matter lesions, and deficits in tasks requiring integrity of subcortical activation, including constructive praxis [46]. Persistent neuroinflammation was considered a possible explanation for white matter lesion and cognitive impairment in HTLV-1-infected patients [46]. The disruption of cytokines and chemokines signaling in the CNS can contribute to the dysfunctional host-viral immune function and pathogenesis that occurs in inflammatory diseases such as HTLV-1 infection [47]. In infections with influenza A virus (IAV), neuropsychiatric complications were reported after infection with either neurotropic or non-neurotropic variants [48], suggesting that viral infections can provoke neuroinflammation via peripherally-produced cytokines.
Cytokines produced by the peripheral innate immune system can trigger a secondary neuroinflammatory response in the CNS, depicted by activation of microglia and production of proinflammatory cytokines (IL-1 beta, IL-6, and TNF-alpha) [49–51]. IL-6 and IL-1 beta are typical features from the innate immune response. IL-6 acts as a major proinflammatory mediator for the induction of the acute phase response and IL-1 beta was identified as a severity marker of the COVID-19 progress. We did not find significant differences between participants with/without visuoconstructional impairment, regarding these markers. However, after recovery from the acute phase high levels of IL-6 or IL-1 beta are not expected, especially in individuals with mild COVID-19 at post-acute phase (at least after 4 months) such as in our sample.
Neuroinflammation was shown to severely affect cognition and behavior in animal models [48, 52, 53]. It is mediated by the increase of cytokines and chemokines, reactive oxygen species (ROS) and second lipid messengers produced by astrocytes and microglia, endothelial and peripheral immune cells [54]. Chronic neuroinflammation implies persistent activation of microglia and other immune cells in the CNS with potential damage [55, 56], such as neuropathological changes and psychiatric complications, such as depression and cognitive deficits [53, 57, 58].
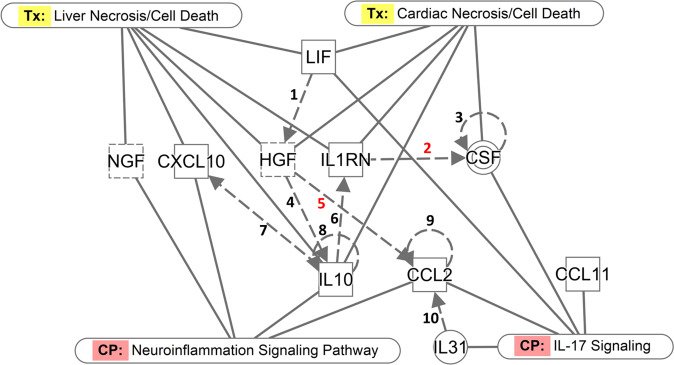
Among the 11 upregulated plasma biomarkers in individuals with visuoconstructional deficit, 10 composed a functional interaction network where they up- or downregulate each other (Fig. 4). These interactions are represented by the broken edges of the network connecting the nodes, which, in turn, represent the protein biomarkers. Four biomarkers are components of the canonical “Neuroinflammation Signaling Pathway” and four of the “IL-17 Signaling pathway”. Furthermore, six of the 10 biomarkers in the network are related to hepatic necrosis and five to cardiac necrosis. The fact that 11 plasma biomarkers were associated with visuoconstructive deficit and 10 of them composed a functional network lend considerable support to the relevance of the immunological markers, which may act either in the protection or increasing risk to cognitive impairment. The association was not only by binary comparison, but also by comparing their levels and presence of visuoconstructive deficit.
Fig. 4. Network of functional interactions between the differentially expressed plasma biomarkers (plain squares for cytokines or chemokines; dashed squares for growth factors, double circle for complex/group), canonical pathways (CP) and clinical pathology endpoints (Tx).
(1) LIF protein increases expression of HGF mRNA [91]; (2) IL1RN protein decreases production of CSF protein [92]; (3) CSF protein increases its own dimerization [93]; (4) HGF increases secretion of IL10 protein [94]; (5) HGF protein decreases expression of CCL2 mRNA [95]; (6) IL10 protein increases expression of IL1RN mRNA [96]; (7) IL10 protein increases release of CXCL10 protein [97]; (8) IL10 protein increases expression of human IL10 mRNA [98]; (9) CCL2 mRNA is increased by CCL2 protein [99]; (10) IL31 protein increases expression of CCL2 mRNA [100, 101]).
Several upregulated biomarkers, identified in the present study, act on the central nervous system promoting protection or damage to the brain. HGF is a neuronal growth and survival factor capable of preventing neuronal death through its pro-angiogenic, anti-inflammatory and immunoregulatory activities and stimulating neuroregeneration by acting on neural stem cells and also promoting synaptogenesis [59]. Also part of the comprehensive multiplex assay, another relevant biomarker found in the present study was NGF, which has anti-inflammatory and homeostatic properties in the central nervous system [60]. However, NGF must be seen as a pleiotropic cytokine in contrast to other specialized cytokines, whose overall effects will be the result of signaling in the different systems expressing NGF receptors, in the CNS [60] or in the periphery, such as the respiratory system [61].
Other identified biomarkers act by promoting neuroinflammation. LIF is able to cross the blood-brain barrier and promote the induction of other pro-inflammatory cytokines in the central nervous system [62]. CXCL10 acts as an mediator for the activation and influx of leukocytes, such as T cells and others, in various inflammatory diseases of the central nervous system [63]. CSF is essential for the pathogenesis of experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis, mediated by encephalitogenic T helper cells that produce IL-17 (Th17 cells) [64]. Cytokine C-C motif chemokine 11 (CCL11, also known as eotaxin-1) can limit neurogenesis and contribute to cognitive impairment [65]. Elevated CCL11 levels were also found in the plasma of long-COVID patients with cognitive deficits compared to those without cognitive symptoms [66]. Notably, in accordance with the study of Villeda et al. (2011), an age-related increased CCL11 in the plasma of the patients with visuoconstructive deficit was observed [65]. With the exception of HGF and IL1RN, all other markers identified in this study are components of the canonical pathways of IL-17 or neuroinflammation signaling (CCL2 participates in both). In addition to the well-established association between neuroinflammation and cognitive impairment, the role of Th17 cells in brain diseases associated with this condition, such as multiple sclerosis, cerebral ischemia and Alzheimer’s disease, is also noteworthy. Th17 cells infiltrate the central nervous system where they induce direct brain cell damage or indirect effects mediated by disruption of the blood-brain barrier and neurovascular dysfunction [67]. In microglial cells, CCL2 decreases the activation of the protein GAD (aspartate 1-decarboxylase) [68]. GAD, in turn, decreases the density of GABAergic neurons [68] and increased GABAergic function in the prefrontal cortex impairs working memory [69]. Versace et al. [70] reported that severe COVID-19 survivors had reduced GABAergic inhibition in the primary motor cortex associated with fatigue and dysexecutive syndrome [70].
CSF assessment might be helpful to identify signs of neuroinfection, neuronal injury and degeneration. However due to the mild nature of COVID-19 symptoms of our participants, we did not include lumbar puncture to collect CSF at the time of IRB approval.
With the exception of CCL2, CCL11 and IL31, the other found biomarkers are also upregulated in liver and heart injury, playing a protective and regenerative role. For example, IL1RN regulates cardiac remodeling by promoting the survival of cardiomyocytes in ischemic regions [71] and is also hepatoprotective [72]. We cannot rule out that individuals with visuoconstructive impairment post mild COVID-19 could also have some level of cardiac [73, 74] and/or hepatic damage [75]. COVID-19 has shown to be a multifactorial disease, which we are just starting to scratch the surface of. As no standard solution is yet available to solve this problem, new anti-inflammatory and immunomodulatory strategies will be necessary.
A recent study showed that mice mildly-infected with SARS-CoV-2 lost approximately one third of mature oligodendrocytes of the WM in the cingulum and corpus callosum, which was still present at 7-weeks post-infection [66]. WM-selective microglial reactivity was shown to inhibit neurogenesis, dysregulation of the oligodendrocytes and loss of myelin [76] in both mice and humans following SARS-CoV-2 infection [66]. Our main MRI finding was an inverse relationship between ROCF test copy performance and WM volumes encompassing the subgenual portion of the corpus callosum and the cingulum on both hemispheres. The crossing over characteristics and the corpus callosum findings might have an important role in understanding the correlation with constructive apraxia [77]. Visuoconstructive deficit is a common finding in callosal ischaemic lesions [78] and agenesis [79]. Although spatial abilities are expected to involve most prominently the right brain hemisphere, some tasks elicit bilateral brain activity [37]. Constructive apraxia has been associated with left- and right-hemisphere lesions, both in posterior and anterior regions, and their integration by white matter tracts. Involvement of the right superior parietal lobe, angular gyrus, middle occipital gyrus have been previously associated with poorer performance in the ROCF copy task in pathological conditions, including vascular lesions [80], epilepsy [81], and traumatic brain lesions [82]. However, we found additional clusters involving WM portions of the inferior frontal gyrus and the fronto-occipital fasciculus bilaterally, as well as the right fusiform gyrus and the bilateral lingual gyri (Table 2).
Although increased volume seems paradoxical, since the correlation between brain volume and cognitive functioning is usually positive [83], there is evidence that COVID-19 patients might show an increased brain volume when compared to matched controls [84]. As reported by Lu and colleagues [85], recovered patients might show increased cerebral volume across different brain regions, including olfactory cortex, hippocampus, insula, left Rolandic operculum, left Heschl’s gyrus and right cingulate gyrus. In diseases known to affect the white matter, such as multiple sclerosis, there are reports of transient increases in brain volume during periods of neuroinflammation relapse [86]. On the other hand, in a large imaging study, comparing brain scans from individuals before and after SARS-CoV-2 infection, it was found greater reduction in GM thickness in the orbitofrontal cortex and parahippocampal gyrus and greater reduction in global brain size [16]. As we lacked pre-COVID-19 imaging and all our participants had confirmed SARS-CoV-2 infection, we were not able to detect these reductions in GM or global brain size. Instead, we were able to identify changes correlated with a selective visuoconstructional impairment revealed by the ROCF test.
Brain metabolic changes have been documented across multiple studies of COVID-19 patients, although with inconsistent results [17, 87]. Patterns of hypo- or hypermetabolism across different cortical and subcortical symptoms are related to neuropsychiatric symptoms of the disease, including anosmia [88], fatigue [89] and cognitive impairment [17]. Concerning resting brain glucose metabolism measured with 18FDG-PET and copy-ROCF performance, we found the most significant cluster to have a negative correlation, located in the right dorsal anterior cingulate gyrus, but we also found clusters of significant positive correlation, involving the left inferior temporal gyrus and the left inferior occipital gyrus. Hosp and colleagues analyzed the pattern o covariance of PET-FDG brain images between COVID-19 patients and controls and reported several regions of cortical hypometabolism in the frontal and parietal lobes, as well as the caudate nuclei, and hypermetabolism in the white matter, cerebellum, brainstem and the mesial temporal lobe, and this pattern was predictive of cognitive deficits [17].
There was not much attention directed to the large population affected by mild COVID-19, since they apparently had recovered well. More than two years into the pandemic, it is still underinvestigated. It was reported that home-isolated young adults (16–30 years old), with mild COVID-19, had persistent symptoms at 6 months, including fatigue, impaired concentration and memory problems [90]. As we observed roughly 25% of our mild COVID-19 patients presenting visuoconstructive impairment, we can expect millions of people worldwide potentially suffering from this kind of deficit. It is imperative to approach populational samples to better understand the extension, causes and persistence of the dysfunction. Why is that so worrying? Constructive apraxia might stay undiagnosed without specific visuospatial testing, which does not mean it has no functional implications in daily life. Visuospatial ability is key to several daily living activities, such as driving, planning, drawing, to locate oneself in a place, and several occupations rely on good visuospatial perception, such as artists, surgeons, designers, pilots, among others. The functional adaptability must be evaluated to plan rehabilitation strategies. Investigation of neuropsychiatric impacts and the pathophysiological drivers underlying the risk factors associated with COVID-19 are important in surveillance and in the development of evidence-based therapeutic strategies.
Factors affecting enrollment into our prospective cohort study would not be expected to introduce selection bias, however, as our findings occurred when establishing the baseline, we must consider the potential to have a selection bias, causing an overestimate, since included individuals were invited for a neuropsychiatric study. Nevertheless, our findings provide evidence of putative neuroinflammatory burden, in an already large and growing fraction of the world population with mild COVID-19, putatively afflicted by long COVID, which requires urgent confirmation, comprehension, and planning for mitigating actions.
Supplementary information
Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (processo 88881.504749/2020-01. 9951-Programa Estratégico Emergencial de Prevenção e Combate a Surtos. Endemias. Epidemias e Pandemias Edital de Seleção Emergencial I -Prevenção e Combate a Surtos. Endemias. Epidemias e Pandemias). Brazil; DMM, MARS, LADM, DMMQ, GB and WM are research fellows of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Brazil, FAPEMIG (APQ-00735-19), INOVA Fiocruz. The authors thank CDTN (agreement number 23072.047899/2018-13) for providing 18F-FDG doses; and Hospital Risoleta Tolentino Neves and Hospital Odilon Behrens.
Author contributions
Study conception and/or design: JJP, DMM and MAR-S. Clinical assessment: JJP, RERPP, HSDT, STC, LCM. Data acquisition: JJP, NGSS, DVR, HMMV, NOC, JBS, MBS, DBO, CM, JNJ, LCS. Data analysis: JJP, RERPP, NGSS, FLSD, RSC, DSC, PRD, DMO, LCS, DMMQ, WMJr, GB, DMM, MAR-S. Manuscript writing and/or revision: JJP, RSC, LADM, CM, DMMQ, GB, DMM, MAR-S.
Data availability
To protect the data privacy of the study participants, the dataset cannot be made publicly available. Specific data needed for reproducing results is available from the corresponding author upon reasonable request.
Code availability
Code needed for reproducing results is available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01632-5.
References
- 1.Gomes C Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). Brazilian Journal of Implantology and Health Sciences [Internet]. 2020 [cited 2021 Dec 14];2. Available from: https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19).
- 2.Elliott P, Bodinier B, Eales O, Wang H, Haw D, Elliott J, et al. Rapid increase in Omicron infections in England during December 2021: REACT-1 study. Science. 2022;375:1406–11. doi: 10.1126/science.abn8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–31. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4:e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin CJ, McDonald S, Luteijn M, Robertson J, Letton W. A model framework for projecting the prevalence and impact of Long-COVID in the UK. medRxiv. 2021;16:e0260843. doi: 10.1371/journal.pone.0260843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho PMdeM, de Medeiros Carvalho PM, Moreira MM, de Oliveira MNA, Landim JMM, Neto MLR. The psychiatric impact of the novel coronavirus outbreak. Psychiatry Res. 2020;286:112902. doi: 10.1016/j.psychres.2020.112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menninger KA. Influenza and schizophrenia. Am J Psychiatry. 1926;82:469–529. doi: 10.1176/ajp.82.4.469. [DOI] [PubMed] [Google Scholar]
- 10.Honigsbaum M. ‘An inexpressible dread’: psychoses of influenza at fin-de-siècle. Lancet. 2013;381:988–9. doi: 10.1016/S0140-6736(13)60701-1. [DOI] [PubMed] [Google Scholar]
- 11.Economo CV, V. Economo C. Bemerkungen zur Frage der infektiösen nicht eitrigen Enzephalitiden. Dtsch Z Für Nervenheilkd. 1932;124:84–7. doi: 10.1007/BF01652904. [DOI] [Google Scholar]
- 12.Kim KH, Tandi TE, Choi JW, Moon JM, Kim MS. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: epidemiology, characteristics and public health implications. J Hospital Infect. 2017;95:207–13. doi: 10.1016/j.jhin.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjunatha N, Kumar C, Math S. Coronavirus disease 2019 pandemic: Time to optimize the potential of telepsychiatric aftercare clinic to ensure the continuity of care. Indian J Psychiatry. 2020;62:320. doi: 10.4103/psychiatry.IndianJPsychiatry_236_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai L-K, Hsieh S-T, Chao C-C, Chen Y-C, Lin Y-H, Chang S-C, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61:1669–73. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 15.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–83. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosp JA, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144:1263–76. doi: 10.1093/brain/awab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampshire A, Trender W, Chamberlain SR, Jolly A, Grant JE, Patrick F, et al. Cognitive deficits in people who have recovered from COVID-19 relative to controls: An N = 84,285 online study. 2021;39:101044. 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed]
- 19.de Paula JJ, Paiva RERP, Costa D de S, Souza e Silva NG, Rosa DV, Januário JN, et al. Visuospatial processing impairment following mild COVID-19. medRxiv. 2021 doi: 10.1101/2021.02.18.21251442. [DOI] [Google Scholar]
- 20.Son K-B, Lee T-J, Hwang S-S. Disease severity classification and COVID-19 outcomes, Republic of Korea. Bull World Health Organ. 2021;99:62–6. doi: 10.2471/BLT.20.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brazilian General Data Protection Law (LGPD, English translation). https://iapp.org/resources/article/brazilian-data-protection-law-lgpd-english-translation/. Accessed 11 Jan 2022.
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. 2013.
- 23.de Jesus Mari J, Williams P. A validity study of a psychiatric screening questionnaire (SRQ-20) in primary care in the city of Sao Paulo. Br J Psychiatry. 1986;148:23–6. doi: 10.1192/bjp.148.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–64. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–55. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin M-S, Park S-Y, Park S-R, Seol S-H, Kwon JS. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc. 2006;1:892–9. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- 27.Nitrini R. Immediate recall of short stories depends on educational level. Dement Neuropsychol. 2008;2:310–4. doi: 10.1590/S1980-57642009DN20400014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1:2277–81. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 29.de Paula JJ, Paiva GCdeC, Costa DdeS. Use of a modified version of the switching verbal fluency test for the assessment of cognitive flexibility. Dement Neuropsychol. 2015;9:258–64. doi: 10.1590/1980-57642015dn93000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strauss E, Strauss of PE, Neuropsychologist and Adjunct Assistant Professor Departments of Paediatrics and Clinical Neurosciences Elisabeth M S Sherman, Sherman EMS, Spreen O, Both Professors of Psychology Otfried Spreen. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; 2006.
- 31.Kessels RPC, van den Berg E, Ruis C, Brands AMA. The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment. 2008;15:426–34. doi: 10.1177/1073191108315611. [DOI] [PubMed] [Google Scholar]
- 32.Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. PLoS One. 2021;16:e0246590. doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattioli F, Stampatori C, Righetti F, Sala E, Tomasi C, De Palma G. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J Neurol. 2021;268:4422–8. doi: 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderlind WM, Rabinovitz BB, Miao IY, Oberlin LE, Bueno-Castellano C, Fridman C, et al. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Curr Opin Psychiatry. 2021;34:420–33. doi: 10.1097/YCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semnic MD, Semnic R, Nikolasevic Z, Bugarski-Ignjatovic VV, Vujanic-Stankov TZ, Kostic S, et al. Performance on the Rey-Osterrieth complex figure test and the correlation with the magnetic resonance imaging brain lesion volume in multi-infract versus small vessel disease dementia. Vojnosanit Pregl. 2021;78:40–6. doi: 10.2298/VSP191220039S. [DOI] [Google Scholar]
- 37.Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12:217–30. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandal PK, Joshi J, Saharan S. Visuospatial perception: an emerging biomarker for Alzheimer’s disease. J Alzheimers Dis. 2012;31:S117–35. doi: 10.3233/JAD-2012-120901. [DOI] [PubMed] [Google Scholar]
- 39.Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Prim. 2021;7:47. doi: 10.1038/s41572-021-00280-3. [DOI] [PubMed] [Google Scholar]
- 40.Bai S, Liu W, Guan Y. The visuospatial and sensorimotor functions of posterior parietal cortex in drawing tasks: a review. Front Aging Neurosci. 2021;13:717002. doi: 10.3389/fnagi.2021.717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gainotti G, Trojano L. Constructional apraxia. Handb Clin Neurol. 2018;151:331–48. doi: 10.1016/B978-0-444-63622-5.00016-4. [DOI] [PubMed] [Google Scholar]
- 42.Davies SR, Field ARJ, Andersen T, Pestell C. The ecological validity of the Rey–Osterrieth Complex Figure: Predicting everyday problems in children with neuropsychological disorders. J Clin Exp Neuropsychol. 2011;33:820–31. doi: 10.1080/13803395.2011.574608. [DOI] [PubMed] [Google Scholar]
- 43.Brian DA, Baric RS. Coronavirus genome structure and replication. Curr Top Microbiol Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowley TJ, Weiss SR. Murine coronavirus neuropathogenesis: determinants of virulence. J Neurovirol. 2010;16:427–34. doi: 10.1007/BF03210848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen R, Wang K, Yu J, Howard D, French L, Chen Z, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 2020;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalil RS, Vasconcellos I, Rosadas C, Cony A, Lima DP, Gonçalves CCA, et al. Association between high proviral load, cognitive impairment, and white matter brain lesions in HTLV-1-infected individuals. J Neurovirol. 2021;27:810–9. doi: 10.1007/s13365-021-00944-6. [DOI] [PubMed] [Google Scholar]
- 47.Lepoutre V, Jain P, Quann K, Wigdahl B, Khan ZK. Role of resident CNS cell populations in HTLV-1-associated neuroinflammatory disease. Front Biosci. 2009;14:1152–68. doi: 10.2741/3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosseini S, Wilk E, Michaelsen-Preusse K, Gerhauser I, Baumgärtner W, Geffers R, et al. Long-term neuroinflammation induced by influenza a virus infection and the impact on hippocampal neuron morphology and function. J Neurosci. 2018;38:3060–80. doi: 10.1523/JNEUROSCI.1740-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson CA, McColl A, Cavanagh J, Graham GJ. Peripheral inflammation is associated with remote global gene expression changes in the brain. J Neuroinflammation. 2014;11:73. doi: 10.1186/1742-2094-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenza-Ferrer H, Magno LAV, Romano-Silva MA, da Silva JF, Gomez MV. Phα1β spider toxin reverses glial structural plasticity upon peripheral inflammation. Front Cell Neurosci. 2019;13:306. doi: 10.3389/fncel.2019.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riester K, Brawek B, Savitska D, Fröhlich N, Zirdum E, Mojtahedi N, et al. In vivo characterization of functional states of cortical microglia during peripheral inflammation. Brain Behav Immun. 2020;87:243–55. doi: 10.1016/j.bbi.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han KM, Ham BJ. How inflammation affects the brain in depression: a review of functional and structural MRI studies. J Clin Neurol. 2021;17:503–15. doi: 10.3988/jcn.2021.17.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139:136–53. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartlage-Rübsamen M, Waniek A, Meißner J, Morawski M, Schilling S, Jäger C, et al. Isoglutaminyl cyclase contributes to CCL2-driven neuroinflammation in Alzheimer’s disease. Acta Neuropathologica. 2015;129:565–83. doi: 10.1007/s00401-015-1395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–16. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desole C, Gallo S, Vitacolonna A, Montarolo F, Bertolotto A, Vivien D, et al. HGF and MET: from brain development to neurological disorders. Front Cell Dev Biol. 2021;9:683609. doi: 10.3389/fcell.2021.683609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villoslada P, Genain CP. Role of nerve growth factor and other trophic factors in brain inflammation. Prog Brain Res. 2004;146:403–14. doi: 10.1016/S0079-6123(03)46025-1. [DOI] [PubMed] [Google Scholar]
- 61.Liu P, Li S, Tang L. Nerve growth factor: a potential therapeutic target for lung diseases. Int J Mol Sci. 2021;22:9112. doi: 10.3390/ijms22179112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan W, Yu C, Hsuchou H, Zhang Y, Kastin AJ. Neuroinflammation facilitates LIF entry into brain: role of TNF. Am J Physiol Cell Physiol. 2008;294:C1436–42.. doi: 10.1152/ajpcell.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vazirinejad R, Ahmadi Z, Kazemi Arababadi M, Hassanshahi G, Kennedy D. The biological functions, structure and sources of CXCL10 and its outstanding part in the pathophysiology of multiple sclerosis. Neuroimmunomodulation. 2014;21:322–30. doi: 10.1159/000357780. [DOI] [PubMed] [Google Scholar]
- 64.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–7. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 65.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–4. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee M-H, Wood J, et al. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. bioRxiv. 2022:2022.01.07.475453.
- 67.Cipollini V, Anrather J, Orzi F, Iadecola C. Th17 and cognitive impairment: possible mechanisms of action. Front Neuroanat. 2019;13:95. doi: 10.3389/fnana.2019.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crowley T, Cryan JF, Downer EJ, O’Leary OF. Inhibiting neuroinflammation: the role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav Immun. 2016;54:260–77. doi: 10.1016/j.bbi.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Bañuelos C, Sofia Beas B, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, et al. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci. 2014;34:3457–66. doi: 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Versace V, Sebastianelli L, Ferrazzoli D, Romanello R, Ortelli P, Saltuari L, et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin Neurophysiol. 2021;132:1138–43. doi: 10.1016/j.clinph.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vecile E, Dobrina A, Salloum FN, Van Tassell BW, Falcione A, Gustini E, et al. Intracellular function of interleukin-1 receptor antagonist in ischemic cardiomyocytes. PLoS One. 2013;8:e53265. doi: 10.1371/journal.pone.0053265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lodder J, Denaës T, Chobert M-N, Wan J, El-Benna J, Pawlotsky J-M, et al. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11:1280–92. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–73. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azevedo RB, Botelho BG, Hollanda JVG, de, Ferreira LVL, Junqueira de Andrade LZ, Oei SSML, et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35:4–11. doi: 10.1038/s41371-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amin M. COVID-19 and the liver: overview. Eur J Gastroenterol Hepatol. 2021;33:309–11. doi: 10.1097/MEG.0000000000001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibson EM, Monje M. Microglia in cancer therapy-related cognitive impairment. Trends Neurosci. 2021;44:441–51. doi: 10.1016/j.tins.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seydell-Greenwald A, Ferrara K, Chambers CE, Newport EL, Landau B. Bilateral parietal activations for complex visual-spatial functions: Evidence from a visual-spatial construction task. Neuropsychologia. 2017;106:194–206. doi: 10.1016/j.neuropsychologia.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giroud M, Dumas R. Clinical and topographical range of callosal infarction: a clinical and radiological correlation study. J Neurol Neurosurg Psychiatry. 1995;59:238–42. doi: 10.1136/jnnp.59.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Antonio F, Pagani G, Familiari A, Khalil A, Sagies T-L, Malinger G, et al. Outcomes associated with isolated agenesis of the corpus callosum: a meta-analysis. Pediatrics. 2016;138:e20160445. doi: 10.1542/peds.2016-0445. [DOI] [PubMed] [Google Scholar]
- 80.Biesbroek JM, van Zandvoort MJE, Kuijf HJ, Weaver NA, Kappelle LJ, Vos PC, et al. The anatomy of visuospatial construction revealed by lesion-symptom mapping. Neuropsychologia. 2014;62:68–76. doi: 10.1016/j.neuropsychologia.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 81.Salanova V. Parietal lobe epilepsy. J Clin Neurophysiol. 2012;29:392–6. doi: 10.1097/WNP.0b013e31826c9ebc. [DOI] [PubMed] [Google Scholar]
- 82.Ashton VL, Donders J, Hoffman NM. Rey complex figure test performance after traumatic brain injury. J Clin Exp Neuropsychol. 2005;27:55–64. doi: 10.1080/138033990513636. [DOI] [PubMed] [Google Scholar]
- 83.Oschwald J, Guye S, Liem F, Rast P, Willis S, Röcke C, et al. Brain structure and cognitive ability in healthy aging: a review on longitudinal correlated change. Rev Neurosci. 2019;31:1–57. doi: 10.1515/revneuro-2018-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Najt P, Richards HL, Fortune DG. Brain imaging in patients with COVID-19: a systematic review. Brain Behav Immun Health. 2021;16:100290. doi: 10.1016/j.bbih.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu Y, Li X, Geng D, Mei N, Wu P-Y, Huang C-C, et al. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheriyan J, Kim S, Wolansky LJ, Cook SD, Cadavid D. Impact of inflammation on brain volume in multiple sclerosis. Arch Neurol. 2012;69:82–8. doi: 10.1001/archneurol.2011.674. [DOI] [PubMed] [Google Scholar]
- 87.Kiatkittikul P, Promteangtrong C, Kunawudhi A, Siripongsatian D, Siripongboonsitti T, Ruckpanich P, et al. Abnormality Pattern of F-18 FDG PET Whole Body with Functional MRI Brain in Post-Acute COVID-19. Nucl Med Mol Imaging. 2022;56:1–13. doi: 10.1007/s13139-021-00730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morbelli S, Chiola S, Donegani MI, Arnaldi D, Pardini M, Mancini R, et al. Metabolic correlates of olfactory dysfunction in COVID-19 and Parkinson’s disease (PD) do not overlap. Eur J Nucl Med Mol Imaging. 2022;49:1939–50. doi: 10.1007/s00259-021-05666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sollini M, Gelardi F, Biroli M, Chiti A. Patients’ findings after COVID-19 infection and vaccinations: what to expect from [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2022;49:791–5. doi: 10.1007/s00259-021-05652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blomberg B, Mohn KG-I, Brokstad KA, Zhou F, Linchausen DW, Hansen B-A, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–13. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomida M, Saito T. The human hepatocyte growth factor (HGF) gene is transcriptionally activated by leukemia inhibitory factor through the Stat binding element. Oncogene. 2004;23:679–86. doi: 10.1038/sj.onc.1207190. [DOI] [PubMed] [Google Scholar]
- 92.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. doi: 10.1182/blood.V87.6.2095.bloodjournal8762095. [DOI] [PubMed] [Google Scholar]
- 93.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/S0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 94.Singhal E, Kumar P, Sen P. A novel role for Bruton’s tyrosine kinase in hepatocyte growth factor-mediated immunoregulation of dendritic cells. J Biol Chem. 2011;286:32054–63. doi: 10.1074/jbc.M111.271247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gong R, Rifai A, Tolbert EM, Biswas P, Centracchio JN, Dworkin LD. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J Am Soc Nephrol. 2004;15:2868–81. doi: 10.1097/01.ASN.0000141962.44300.3A. [DOI] [PubMed] [Google Scholar]
- 96.Tamassia N, Castellucci M, Rossato M, Gasperini S, Bosisio D, Giacomelli M, et al. Uncovering an IL-10-dependent NF-kappaB recruitment to the IL-1ra promoter that is impaired in STAT3 functionally defective patients. FASEB J. 2010;24:1365–75. doi: 10.1096/fj.09-145573. [DOI] [PubMed] [Google Scholar]
- 97.Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol. 2000;165:2783–9. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 98.Staples KJ, Smallie T, Williams LM, Foey A, Burke B, Foxwell BMJ, et al. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol. 2007;178:4779–85. doi: 10.4049/jimmunol.178.8.4779. [DOI] [PubMed] [Google Scholar]
- 99.Monzon ME, Forteza RM, Casalino-Matsuda SM. MCP-1/CCR2B-dependent loop upregulates MUC5AC and MUC5B in human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2011;300:L204–15.. doi: 10.1152/ajplung.00292.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dai X, Okazaki H, Hanakawa Y, Murakami M, Tohyama M, Shirakata Y, et al. Eccrine sweat contains IL-1α, IL-1β and IL-31 and activates epidermal keratinocytes as a danger signal. PLoS One. 2013;8:e67666. doi: 10.1371/journal.pone.0067666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ip WK, Wong CK, Li MLY, Li PW, Cheung PFY, Lam CWK. Interleukin-31 induces cytokine and chemokine production from human bronchial epithelial cells through activation of mitogen-activated protein kinase signalling pathways: implications for the allergic response. Immunology. 2007;122:532–41. doi: 10.1111/j.1365-2567.2007.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To protect the data privacy of the study participants, the dataset cannot be made publicly available. Specific data needed for reproducing results is available from the corresponding author upon reasonable request.
Code needed for reproducing results is available from the corresponding author upon reasonable request.