SUMMARY
Immune thrombocytopenia (ITP) is an autoimmune disorder. Besides platelet count, immature platelet fraction (IPF) can be used as a tool to predict megakaryocytic activity in ITP patients. The aim of the study was to evaluate the utility of extended platelet indices in ITP diagnosis and their association with disease persistence and severity. This case-control study (1:1), conducted from January 2015 to December 2017, included 111 ITP patients and 111 healthy controls. ITP patients were grouped as newly diagnosed ITP, persistent ITP, chronic ITP, and refractory ITP patients. Peripheral blood was collected and complete blood profile parameters were recorded using Sysmex XN 1000. Significant (p≤0.05) difference between the groups of ITP patients and healthy control subjects was determined by Fisher exact test, while Pearson correlation was used to evaluate platelet count correlation with IPF using SPSS ver. 23. Low hemoglobin and platelet counts with high total leukocyte count and IPF were detected in ITP patients as compared to healthy subjects (p≤0.001). Among all groups of ITP patients, very low platelet count (6.9±6.02.x109/L) with highest mean IPF (27.1±19.2%) was observed in newly diagnosed ITP group. Other platelet parameters including mean platelet volume (MPV), plateletcrit, platelet large cell ratio (P-LCR) and platelet distribution width values were also altered in patient groups. Pearson correlation revealed negative relationship between platelet count and IPF in all patient groups. With the advent of new, sophisticated hematologic analyzers, the IPF and other platelet parameters provide simple, reliable and easier tools for predicting platelet disorders such as ITP, and to some extent the disease severity. Besides IPF, the MPV and P-LCR seemed to predict disease severity, treatment responsiveness, and duration of the disease to some extent.
Key words: Immune thrombocytopenia purpura, Chronic immune thrombocytopenia, Mean platelet volume, Immature platelet fraction
Introduction
Platelets are non-nucleated, membrane bound, disk-like structures. They activate coagulation factors by activating their membrane phospholipids in blood clotting due to impaired blood vessel flow (2). Thrombocytopenia or decreased platelet count (i.e. <1,50000/mm3) is a common symptom associated with different autoimmune diseases or microbial infections. Thrombocytopenia can be divided into mild (<100000/mm3), moderate (20000-50000/mm3) and severe (<20000/mm3). It usually occurs due to increased destruction of platelets in diseases such as disseminated intravascular coagulation, thrombotic thrombocytopenic purpura and immune thrombocytopenia (ITP), or decreased/reduced platelet production related with other bone marrow diseases (3).
Immune thrombocytopenia is an immune disorder in which bleeding generally correlates with the severity of thrombocytopenia (3, 4). To date, the exact etiology of ITP is unknown. It has been proposed that various factors including excessive platelet destruction due platelet autoantibody production, T-cell mediated or oxidative stress dependent platelet destruction, and cessation of megakaryopoiesis cause ITP. Bleeding is the commonest clinical manifestation that occurs with or without bruises and epistaxis (1, 5).
With technology advancement, the new generations of automated hematologic analyzers have incorporated new parameters in the complete blood count (CBC) test including extended platelet indices such as platelet count (PLT), plateletcrit (PCT), platelet distribution width (PDW), mean platelet volume (MPV), immature platelet fraction (IPF), and platelet large cell ratio (P-LCR). The IPF represents a population of newly formed platelets or reticulated platelets (RP) with a high concentration of residual RNA due to excessive peripheral platelet destruction (6). The RP were previously enumerated by flow cytometry, which was time consuming (7, 8). Although an increased IPF may be an early tool in the differential diagnosis between hypo- and hyperproliferative thrombocytopenia including ITP, there are no supporting reports on the utility of IPF in predicting the severity and chronicity of ITP (9). At present, differentiation of hypoproliferative and hyperdestructive thrombocytopenia can be made by a useful yet simple indicator of the platelet size. Collective interpretation of PLT, PDW, MPV, PCT, P-LCR and IPF by automated cell counters such as XN-1000 can be used as a convenient approach in differentiating thrombocytopenia due to ITP and its severity. These are simple, quick, cost-effective, noninvasive, easy to perform and reliable tools. The average size of platelets is MPV. Generally, an increased MPV, i.e. >13 fl, occurs in platelet hyper-destruction, while MPV <8 fl is indicative of platelet hypo-production. The best cut-off value for MPV in ITP is generally >9.7 fl. MPV along with morphological examination can be used in differential diagnosis of ITP (10). PCT is a measure of total platelet mass; its low values between 0.2% and 0.36% indicate quantitative abnormalities of platelets in ITP and other thrombocytopenias. The release of larger, younger, active platelets in response to excessive platelet destruction in ITP may be the cause of variability in platelet size as expressed by higher PDW (11). P-LCR and MPV are directly associated with PDW. In the past, an increase in P-LCR was observed in destructive thrombocytopenias compared to hypoproliferative thrombocytopenia. The previously reported diagnostic accuracy, sensitivity and specificity for most of these parameters (MPV, IPF) confirmed their possible utility in discriminating and evaluating the severity of ITP (7, 11-13). The aim of this case-control study was to further elucidate diagnostic value of IPF and other platelet indices in the groups of ITP patients and to compare them with those recorded in healthy control subjects.
Subjects and Methods
This study was conducted at the National Institute of Blood Diseases and Bone Marrow Transplantation (NIBD), Karachi, Pakistan, a tertiary care hospital with specialty in diagnosing, treating and managing hematologic disorders in Pakistan.
Study participants
Participation in the study was voluntary and informed consent was required after the institutional Research Ethics Committee (NIBD-REC) approval of the study. This case-control study was carried out from January 2015 to December 2017, including 111 ITP patients (61 women and 50 men) as a test group and 111 healthy participants of either gender as a control group. Based on clinical and laboratory investigations, ITP patients were divided into four groups, as follows: newly diagnosed ITP (ND-ITP), diagnosed within the past three months; persistent ITP (P-ITP), diagnosed within 3-12 months; chronic ITP (C-ITP), persisting for a longer time, usually more than 12 months; and refractory ITP (R-ITP), having treatment failure after splenectomy or have relapsed thereafter, or exhibiting severe ITP, clinically relevant bleeding, or have a risk of bleeding according to the International Working Group (IWG) guidelines (1). Moreover, control group subjects had no systemic diseases. A unique number was given to each patient and healthy subjects. Medical records were reviewed and data collected including demographic (age, gender), laboratory (CBC), and outcome measurements (bleeding type, sites, etc.).
Laboratory analysis
Blood samples drawn in K2EDTA anticoagulant tube were used to analyze CBC on a Sysmex XN-1000 (Sysmex Corporation, Kobe, Japan) with extended CBC parameters including PLT, PDW, PCT, MPV, P-LCR and IPF. All samples were analyzed soon after collection from study participants. Peripheral smear was also examined to rule out pseudo-thrombocytopenia.
Statistical analysis
Mean, standard deviation, median and interquartile range (IQR) were used to describe the parameters. Kruskal-Wallis test with Dunn’s test was used to evaluate significant difference (p≤0.05) between ITP patients and healthy control group, and Spearman’s correlation test was performed to evaluate PLT correlation with IPF using SPSS ver. 23.
Results
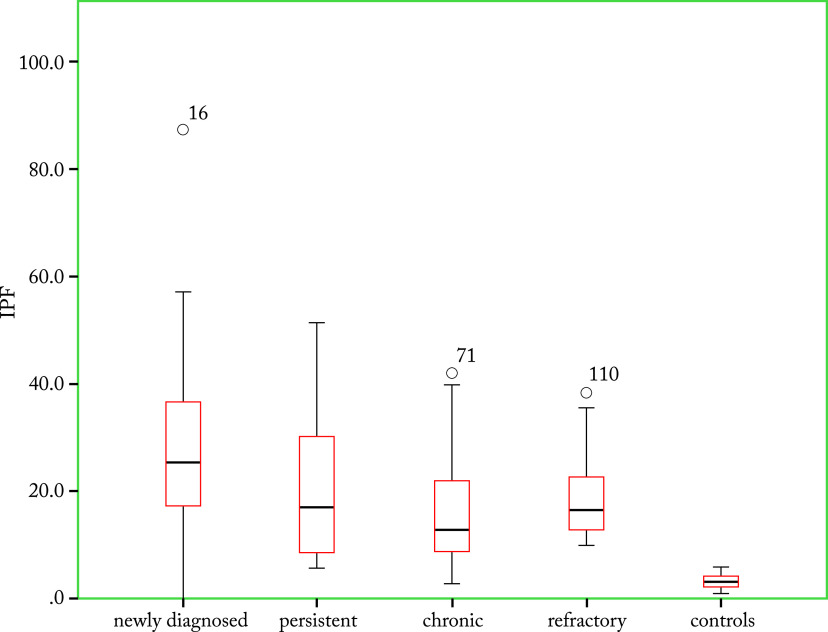
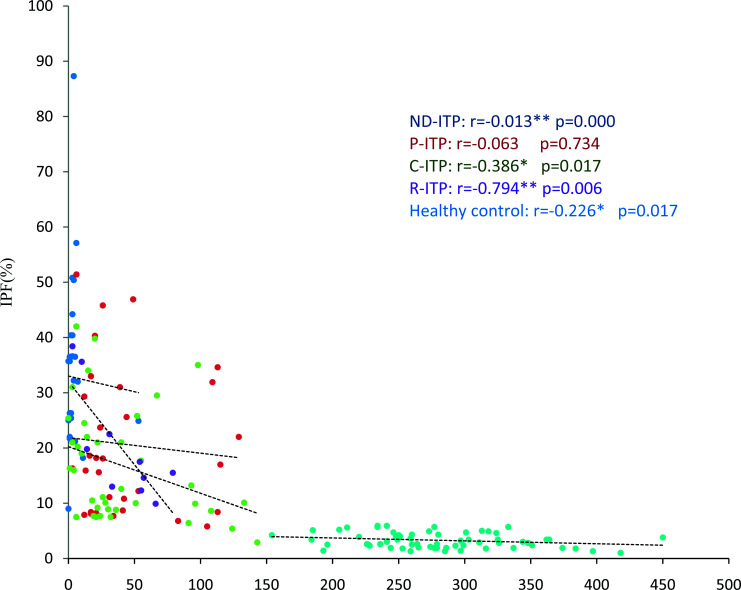
The study included 111 ITP patients and 111 healthy control subjects with the same gender distribution. Median age of the ITP patients and healthy control group was 23±17.1 and 29±8.5 years, respectively. Out of 111 patients, there were 70 (76.2%) females and 41 (36.9%) males. There were 38 (34%) patients with chronic ITP, 32 (29%) with newly diagnosed ITP, 31 (28%) with persistent ITP, and 10 (9%) with refractory ITP. Mean hemoglobin, TLC and PLT were evaluated and significant difference was observed (Table 1). Mean hemoglobin, total leukocyte count (TLC) and PLT were recorded and significant difference was observed in all ITP groups compared with healthy controls (Table 1). A significantly higher IPF was observed in ITP patients (p≤0.001) as compared with healthy controls, suggesting increased reticulated platelets (Table 2). The IPF was highest (27.1±19.2%) in ND-ITP patients. Decreased PLT and increased IPF indicated increased peripheral destruction in ITP. An inverse correlation of PLT and IPF was observed in all study groups with r values of -0.013 (p=0.000), -0.063 (p=0.734), -0.386 (p=0.017), -0.794 (p=0.006) and -0.226 (p=0.017) in ND-ITP, P-ITP, C-ITP, R-ITP and healthy control subjects, respectively (Fig. 1). Other platelet parameters such as MPV, PCT, P-LCR and PDW also showed significant differences between the ITP patients and control group (Table 2). Interestingly, due to the very low PLT in ND-ITP patients, the MPV, PCT, P-LCR and PDW were not recorded by the Sysmex XN-1000 (Sysmex Corporation, Kobe, Japan). The MPV and P-LCR were lowest (11.35 fl; IQR 1.7) in P-ITP patients and highest in R-ITP patients (Table 2). A slightly different trend was observed for PDW and PCT. The ranges for these two parameters were highest in the C-ITP subgroup followed by R-ITP subgroup (Table 2). The MPV and P-LCR were relatively higher in the R-ITP group, reflecting the possible complexity of this ITP subgroup (Figure 2).
Table 1. Hematologic findings in ITP patients and healthy controls.
| Parameter | Newly diagnosed ITP Mean±SD |
Persistent ITP Mean±SD |
Chronic ITP Mean±SD |
Refractory ITP Mean±SD |
Healthy controls Mean±SD |
p-value |
|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 10.4±2.3 | 11.8±2.10 | 11.4±2.5 | 11.2±2.6 | 14.4±1.14 | 1 ≤0.001 |
| 2 ≤0.001 |
||||||
| 3 ≤0.001 | ||||||
| 4 ≤0.001 | ||||||
| TLC (x109/L) | 11.3±5.7 | 12.07±6.2 | 10.8±6.13 | 10.4±7.25 | 7.49±1.65 | 1 ≤0.001 |
| 2 ≤0.001 |
||||||
| 3 ≤0.001 | ||||||
| 4 ≤0.001 | ||||||
| Platelet count (x109/L) | 6.9±6.02 | 44.3±38.2 | 41.3±40.4 | 40.2±25.7 | 277±58.7 | 1 ≤0.001 |
| 2 ≤0.001 |
||||||
| 3 ≤0.001 | ||||||
| 4 ≤0.001 | ||||||
| Reticulocytes (%) | 2.4±1.8 | 1.35±0.81 | 1.55±1.64 | 1.74±1.54 | 1.13±0.40 | 1 ≤0.001 |
| 2 ≤0.05 |
||||||
| 3 ≤0.05 | ||||||
| 4 ≤0.05 | ||||||
| Neutrophils (x109/L) | 6.7±4.39 | 7.9±6.6 | 7.4±5.98 | 7.36±6.34 | 4.19±1.07 | 1 ≤0.05 |
| 2 ≤0.05 |
||||||
| 3 ≤0.05 | ||||||
| 4 ≤0.05 | ||||||
| Lymphocytes (x109/L) | 3.45±2.64 | 53±12.2 | 2.55±1.66 | 2.29±1.17 | 2.93±4.11 | 1 ≥0.05 |
| 2 ≤0.05 |
||||||
| 3 ≥0.05 | ||||||
| 4 ≥0.05 |
ITP = immune thrombocytopenia; TLC = total leukocyte count; SD = standard deviation; Fisher exact test was used to determine significant difference between the groups; 1 = comparison between newly diagnosed ITP and healthy controls; 2 = comparison between persistent ITP and healthy controls; 3 = comparison between chronic ITP and healthy controls; 4 = comparison between refractory ITP and healthy controls
Table 2. Variability in extended platelet parameters in ITP patients and healthy controls.
| Parameter | Newly diagnosed ITP Median (IQR) |
Persistent ITP Median (IQR) | Chronic ITP Median (IQR) |
Refractory ITP Median (IQR) |
Healthy controls Median (IQR) | p-value |
|---|---|---|---|---|---|---|
| PDW (fl) | 0 (0) | 14.75 (2.6) Range: 8.1-18.2 |
13.35 (2.8) Range: 10.3-22 |
15.2 (7.8) Range: 9.1-21.3 |
11.7 (1.8) Range: 9.1-14 |
1 ≤0.05 |
| 2 ≤0.05 |
||||||
| 3 ≤0.05 | ||||||
| 4 ≤0.05 | ||||||
| MPV (fl) | 0 (0) | 11.35 (1.7) Range: 8-12.8 |
11.4 (1.7) Range: 9-14.2 |
12.4 (1.77) Range: 10.7-13.6 |
10.2 (0.95) Range: 8.8-11.1 |
1 ≤0.05 |
| 2 ≤0.05 |
||||||
| 3 ≤0.05 | ||||||
| 4 ≤0.05 | ||||||
| P-LCR (%) | 0 (0) | 39.2 (14.4) Range: 11.8-46.7 |
35.9 (16.2) Range: 21.8-52.9 |
43.65 (18.9) Range: 30.2-56.3 |
26.2 (6.65) Range: 14.5-43.2 |
1 ≤0.05 |
| 2 ≤0.05 |
||||||
| 3 ≤0.05 | ||||||
| 4 ≤0.05 | ||||||
| PCT (%) | 0 (0) | 0.025 (0.12) Range: 0.01-0.58 |
0.15 (0.26) Range: 0.03-0.5 |
0.045 (0.02) Range: 0.04-0.08 |
0.27 (0.07) Range: 0.26-0.37 |
1 ≤0.05 |
| 2 ≤0.05 |
||||||
| 3 ≤0.05 | ||||||
| 4 ≤0.05 | ||||||
| IPF (%) | 25.4 (19.8) Range: 0-87.3 |
17 (22.6) Range: 5.8-51.4 |
12.9 (13.9) Range: 2.9-42 |
16.5 (13) Range: 9.9-38.4 |
3.1 (1.9) Range: 1-5.9 |
1 ≤0.05 |
| 2 ≤0.05 |
||||||
| 3 ≤0.05 | ||||||
| 4 ≤0.05 |
ITP = immune thrombocytopenia; IQR = interquartile range; PDW = platelet distribution width; MPV = mean platelet volume; P-LCR = platelet large cell ratio; PCT = plateletcrit; IPF = immature platelet fraction; 1 = comparison between newly diagnosed ITP and healthy controls; 2 = comparison between persistent ITP and healthy controls; 3 = comparison between chronic ITP and healthy controls; 4 = comparison between refractory ITP and healthy controls
Fig. 1.
Comparison of immature platelet fraction between immune thrombocytopenia (ITP) subgroups and control group – it was significantly higher in ITP patients as compared to healthy controls.
Fig. 2.
Correlation of platelet count and immature platelet fraction (IPF): newly diagnosed immune thrombocytopenia (ND-ITP) subgroup; persistent ITP (P-ITP) subgroup; chronic ITP (C-ITP) subgroup; refractory ITP (R-ITP) subgroup; and healthy control group.
Discussion
Platelets are an important component of blood and difference in their absolute count, size, ratio, maturity, etc. may depict thrombocytopenia, which is a significant marker in malignancies, cardiovascular events, prothrombotic and proinflammatory diseases including autoimmune diseases such as systemic lupus erythematosus and ITP (10, 13, 14). Bone marrow collection just for examination of platelet morphology is an invasive procedure. Nowadays, it has been replaced greatly by extended platelet indices as offered by modern hematologic analyzers. Many retrospective studies confirming the utility of platelet indices such as IPF, MPV and P-LCR as biomarkers for differential diagnosis of ITP and a range of hematologic disorders have been published to date (15-17). The analyzer based variability in the cut-off values of these indices for differential diagnosis of diseases of complex pathophysiology with platelet hypoproduction or hyperdestruction has been a matter of concern (18-20). None of the studies to date has evaluated the possible clinical utility of IPF, MPV, PDW and P-LCR within the subtypes of ITP as per 2008 IWG classification (1). Only a few studies included a healthy control group with a substantial comparable number of patients with ITP and hypoproductive thrombocytopenia (19-21). In this view, a 1:1 case-control comparison between ITP patients (n=111) and healthy controls (n=111) was performed. Variability of these indices among the ITP subtypes was also recorded. It was interesting to observe that the device was unable to calculate most platelet indices except for PLT and IPF in ND-ITP patients (Table 2). Such limitation of automated analyzers has also been reported by Noris et al. In that study, the researchers observed that Sysmex XE-2100 (Sysmex Corporation, Kobe, Japan), an impedentiometric counter, failed to analyze MPV in 10 patients due to a very large platelet size (91.5% specificity) (12).
It has been accepted that higher platelet RNA content detected as increased IPF% by automated analyzers directly correlates with megakaryocytic activity specially observed in the conditions of thrombocytopenia (18, 22). IPF as a relative difference in immature platelets in ITP subtypes was investigated in this study to assess the possible association with the disease severity and duration. In this study, significantly higher values of IPF were observed in different ITP groups compared to healthy controls (Table 2). PLT and IPF% showed a significant inverse correlation in patient groups, i.e. the lower the PLT, the higher was the IPF%; similarly, lower IPF% with high PLT was observed in control group (Fig. 1). The values were highest in the ND-ITP subgroup (mean 27.1%) with PLT range of 0-55x0 (9)/L. In contrast, higher IPF values were documented by Adly et al. in C-ITP in pediatric patients (19). Previously, a cut-off value of 9.4% for IPF was proposed as a diagnostic modality for differential diagnosis of ITP (median 7.7%) compared to hypoproductive thrombocytopenia and gender-matched healthy subjects as control. Comparatively lower IPF% in C-ITP among the ITP subtypes may be due to treatment induced platelet recovery since most of our C-ITP patients were in remission (n=16; 14.4% of patients). Abe et al. also found normal absolute IPF in ITP patients in complete remission (23). Thus, platelet recovery may also be predicted by improved IPF values. Furthermore, in another study, higher IPF% has been reported as a favorable predictive marker for earlier good response to treatment in ITP subtypes leading to complete recovery but unlike the present study, case-control comparison was not performed (24).
The MPV was reported for the first time in 1983 as an important diagnostic criterion for thrombocytopenia and is now considered as an important differential parameter for the diagnosis of ITP (25). In ITP, increased platelet production by bone marrow compensates for the excessive peripheral platelet destruction leading to increased circulation of large-size younger platelets, resulting in an overall increased MPV (16, 20). It even has applicability in determination of the risk of ITP relapse, as reported from China where in a group of 233 de novo ITP patients, MPV values ≤21 fl were found to be an independent marker of the increased risk of relapse after 6 months (26).
Furthermore, in an Ethiopian study, the authors declared various platelet indices recorded with Sysmex XT-2000 (a five-part differential analyzer, Sysmex Corporation, Kobe, Japan) as a powerful tool in differentiating thrombocytopenia of hypoproductive and hyperdestructive origin (21). The mean MPV of 12.4±3.6 fl in ITP patients showed a sensitivity of 82%, which is in line with our finding of the mean cumulative MPV of 12.5±1.47 fl in the ITP group compared to controls (10.1±0.61 fl). The highest MPV was observed in the R-ITP subgroup, inferring abnormal platelet production and increased peripheral destruction in this subgroup with prolonged disease.
Both MPV and PDW are important for understanding the physiological role of platelets in ITP. Most recently, in 2019, Lee et al. hypothesized that peripheral platelet hyperdestruction in ITP may be due to hyperactivation of platelets irrespective of their age and size (20). The compensatory response of bone marrow to excessive platelet destruction may cause circulation of both mature and immature platelets, increasing the PDW in ITP patients compared to controls. Similarly, a higher range of PDW was observed in ITP groups compared to controls, with the highest range of 10.3-22 (IQR 2.2) in the C-ITP group (Table 2).
The P-LCR as a relative measure of platelet size is directly correlated with MPV and PDW. The utility of P-LCR in ITP diagnosis has been under debate for decades. In 2005, Kaito et al. studied comparative reliability of MPV, PDW and P-LCR in the diagnosis of ITP and concluded that P-LCR was a far better differential parameter than MPV and PDW (16). This claim was later opposed by Ntaios et al. when determining diagnostic accuracy of different P-LCR cut-off values (15). The Youden index obtained for P-LCR in that study was 60.8% compared to 100% for MPV and PDW. Significantly higher P-LCR was observed in ITP compared to healthy controls in the present study. The R-ITP group expressed highest P-LCR (range: 30.2-56.3), which may be linked to more impaired platelet function in this group of ITP patients.
Although slightly lower PCT values were observed in ITP groups in comparison to control group, the PCT or relative platelet mass seemed to be the least useful discriminatory index compared to MPV, PDW and P-LCR, which is in accordance with previous studies (19). On the contrary, Tang et al. considered PCT (74.8% sensitivity) along with MPV and PDW as a reliable tool in the differential diagnosis of ITP compared to myelodysplasia (27). Diagnostic accuracy of any of the studied platelet indices was not determined as evaluated by Aponte-Barrios et al. for clinical application of these parameters in pediatric ITP group (18).
In conclusion, based on the variability in IPF, MPV, PDW, P-LCR and PCT, the ITP may be diagnosed easily. The higher ranges of PDW and PCR were observed in the C-ITP subgroup, while MPV and P-LCR were highest in the R-ITP group, which may reflect the severity, complexity and duration of disease in these groups. A limitation of the present study could be the fact that the impact of age as an etiologic factor was not studied separately in detail with respect to increased indices in pediatric and adult groups. A comparative study between these two age groups within ITP subtypes with a larger sample size is suggested to evaluate the possible impact of age dependent immune dysfunction as a potential cause of disease severity and chronicity. Future investigations on other hematologic analyzers along with a larger number of ND-ITP patients are required to confirm whether MPV and other indices could be used as an important tool in predicting ITP in ND-ITP patients since these parameters were not expressed by XN-1000 in the present study.
References
- 1.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009. March 12;113(11):2386–93. 10.1182/blood-2008-07-162503 [DOI] [PubMed] [Google Scholar]
- 2.Di Paola JA, Buchanan GR. Immune thrombocytopenic purpura. Pediatr Clin North Am. 2002. October;49(5):911–28. 10.1016/S0031-3955(02)00027-5 [DOI] [PubMed] [Google Scholar]
- 3.Sekhon SS, Roy V. Thrombocytopenia in adults: a practical approach to evaluation and management. South Med J. 2006 May;99(5):491-8; quiz 9-500, 33. DOI: 10.1097/01.smj.0000209275.75045.d4 10.1097/01.smj.0000209275.75045.d4 [DOI] [PubMed]
- 4.Kayal L, Jayachandran S, Singh K. Idiopathic thrombocytopenic purpura. Contemp Clin Dent. 2014. July;5(3):410–4. 10.4103/0976-237X.137976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011. April 21;117(16):4190–207. org/ 10.1182/blood-2010-08-302984 [DOI] [PubMed] [Google Scholar]
- 6.Buttarello M, Plebani M. Automated blood cell counts: state of the art. Am J Clin Pathol. 2008. July;130(1):104–16. 10.1309/EK3C7CTDKNVPXVTN [DOI] [PubMed] [Google Scholar]
- 7.Naz A, Mukry SN, Shaikh MR, Bukhari AR, Shamsi TS. Importance of immature platelet fraction as predictor of immune thrombocytopenic purpura. Pak J Med Sci. 2016. May-June;32(3):575–9. 10.12669/pjms.323.9456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rinder HM, Munz UJ, Ault KA, Bonan JL, Smith BR. Reticulated platelets in the evaluation of thrombopoietic disorders. Arch Pathol Lab Med. 1993. June;117(6):606–10. [PubMed] [Google Scholar]
- 9.Hoffmann JJ. Reticulated platelets: analytical aspects and clinical utility. Clin Chem Lab Med. 2014. August;52(8):1107–17. 10.1515/cclm-2014-0165 [DOI] [PubMed] [Google Scholar]
- 10.Sit M, Aktas G, Ozer B, Kocak MZ, Erkus E, Erkol H, et al. Mean platelet volume: an overlooked herald of malignant thyroid nodules. Acta Clin Croat. 2019. September;58(3):417–20. 10.20471/acc.2019.58.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, Koutroubakis IE, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001. March;96(3):776–81. 10.1111/j.1572-0241.2001.03621.x [DOI] [PubMed] [Google Scholar]
- 12.Noris P, Klersy C, Gresele P, Giona F, Giordano P, Minuz P, et al. Platelet size for distinguishing between inherited thrombocytopenias and immune thrombocytopenia: a multicentric, real life study. Br J Haematol. 2013. July;162(1):112–9. 10.1111/bjh.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowles KM, Cooke LJ, Richards EM, Baglin TP. Platelet size has diagnostic predictive value in patients with thrombocytopenia. Clin Lab Haematol. 2005. December;27(6):370–3. 10.1111/j.1365-2257.2005.00726.x [DOI] [PubMed] [Google Scholar]
- 14.Korniluk A, Koper-Lenkiewicz OM, Kaminska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074. DOI.org/10.1155/2019/9213074 10.1155/2019/9213074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ntaios G, Papadopoulos A, Chatzinikolaou A, Saouli Z, Karalazou P, Kaiafa G, et al. Increased values of mean platelet volume and platelet size deviation width may provide a safe positive diagnosis of idiopathic thrombocytopenic purpura. Acta Haematol. 2008;119(3):173–7. 10.1159/000135658 [DOI] [PubMed] [Google Scholar]
- 16.Kaito K, Otsubo H, Usui N, Yoshida M, Tanno J, Kurihara E, et al. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol. 2005. March;128(5):698–702. org/ 10.1111/j.1365-2141.2004.05357.x [DOI] [PubMed] [Google Scholar]
- 17.Barsam SJ, Psaila B, Forestier M, Page LK, Sloane PA, Geyer JT, et al. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. 2011. May 26;117(21):5723–32. org/ 10.1182/blood-2010-11-321398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aponte-Barrios NHL-BA, Sarmiento-Urbina IC, Uribe-Botero GI. Evaluation of the diagnostic performance of platelet-derived indices for the differential diagnosis of thrombocytopenia in pediatrics. Rev Fac Med (Caracas). 2014;62(4):547–52. org/ 10.15446/revfacmed.v62n4.43754 [DOI] [Google Scholar]
- 19.Adly AA, Ragab IA, Ismail EA, Farahat MM. Evaluation of the immature platelet fraction in the diagnosis and prognosis of childhood immune thrombocytopenia. Platelets. 2015;26(7):645–50. DOI.org/10.3109/09537104.2014.969220 10.3109/09537104.2014.969220 [DOI] [PubMed] [Google Scholar]
- 20.Lee E, Jeon K, Lee J, Lee JS, Kim HS, Kang HJ, et al. Mean platelet volume, platelet distribution width, and platelet count, in connection with immune thrombocytopenic purpura and essential thrombocytopenia. Lab Med. 2019;50(3):279–85. 10.1093/labmed/lmy082 [DOI] [PubMed] [Google Scholar]
- 21.Negash M, Tsegaye A. G/Medhin A. Diagnostic predictive value of platelet indices for discriminating hypo productive versus immune thrombocytopenia purpura in patients attending a tertiary care teaching hospital in Addis Ababa, Ethiopia. BMC Hematol. 2016;16:18. DOI.org/10.1186/s12878-016-0057-5 10.1186/s12878-016-0057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YG, Lee JH, Kim DS, Lee HS, Choi SI. Clinical usefulness of the simple technique to diagnose thrombocytopenia using immature platelet fraction. Korean J Lab Med. 2007. February;27(1):1–6. DOI.org/10.3343/kjlm.2007.27.1.1 [DOI] [PubMed] [Google Scholar]
- 23.Abe Y, Wada H, Tomatsu H, Sakaguchi A, Nishioka J, Yabu Y, et al. A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF). Thromb Res. 2006;118(4):463–9. 10.1016/j.thromres.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 24.Ghemlas IA, Klaassen RJ, Barrowman N, Leung EW. Utility of the immature platelet fraction in predicting recovery with or without treatment in pediatric immune thrombocytopenia. Blood. 2013;122(21):3546. 10.1182/blood.V122.21.3546.3546 [DOI] [Google Scholar]
- 25.Norrasethada L, Khumpoo W, Rattarittamrong E, Rattanathammethee T, Chai-Adisaksopha C, Tantiworawit A. The use of mean platelet volume for distinguishing the causes of thrombocytopenia in adult patients. Hematol Rep. 2019 Feb 19;11(1):7732. DOI.org/10. 4081/hr.2019.7732 [DOI] [PMC free article] [PubMed]
- 26.Chen C, Song J, Wang Q, Wang L-H, Guo P-X. Mean platelet volume at baseline and immune thrombocytopenia relapse in Chinese newly-diagnosed patients: a retrospective cohort study. Hematology. 2018 2018/10/21;23(9):646-52. DOI: 10.1080/10245332.2018.1461317 10.1080/10245332.2018.1461317 [DOI] [PubMed]
- 27.Tang YT, He P, Li YZ, Chen HZ, Chang XL, Xie QD, et al. Diagnostic value of platelet indices and bone marrow megakaryocytic parameters in immune thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2017. January;28(1):83–90. 10.1097/MBC.0000000000000612 [DOI] [PubMed] [Google Scholar]