SUMMARY
There are different options for surgical treatment of brain abscess, mainly standard craniotomy and stereotactic aspiration. It has not yet been established which of these options is associated with a more favorable outcome under similar baseline conditions of patients. Demographic characteristics, microbiology, clinical presentation, and treatment outcome were analyzed for surgically treated adult patients with brain abscess over a 14-year period. A propensity score model was applied to account for baseline conditions that may determine the choice of neurosurgical method. The propensity score was included in the prediction of a favorable outcome, defined as a Glasgow Outcome Scale (GOS) score 4 or 5. We analyzed 91 adult surgically treated patients, of which 53 had standard craniotomy and 38 stereotactic aspiration of brain abscess. Focal neurological deficit was the most common symptom present in 60 (65.9%) patients on admission. Sixty-seven (73.6%) patients had GOS 4 or 5, and seven (7.7%) patients died. The choice of surgery did not influence the outcome (OR 1.181, 95% CI 0.349-3.995), neither did the time elapsed from diagnosis to surgery (OR 0.998, 95% CI 0.981-1.015). Propensity towards standard craniotomy procedure did not influence outcome in brain abscess patients (OR 1.181, 95% CI 0.349-3.995). Worse outcome (GOS below 4) was independently associated with Glasgow Coma Score (GCS) on admission (OR 0.787, CI 0.656-0.944). The choice of neurosurgical procedure did not influence the outcome in patients with brain abscess. Patients with brain abscess who had lower GCS on admission also had worse outcome.
Key words: Brain abscess, Craniotomy, Stereotactic aspiration, Glasgow Outcome Scale
Introduction
Brain abscess is an intracerebral infection which starts as a localized area of inflammation and develops into a pus collection surrounded by a capsule (1, 2). During the last thirty years, mortality of brain abscess has fallen from 46% to 10%, with the help of modern neuroradiological imaging, development of neurosurgical techniques and equipment, and with the use of targeted antibiotic regimens (1-3). The outcome in these patients is still influenced by numerous factors, among which are neurosurgical techniques of standard craniotomy and stereotactic aspiration (3). Most studies on this issue are retrospective and observational, and because of non-random treatment assignment, estimates may be biased when comparing outcomes associated with different treatments (2-9). The aim of the study was to define the effect of different surgical methods and patient characteristics on the outcome using the propensity score model.
Patients and Methods
An institutional review board approved a retrospective analysis of data on patients aged ≥18 with the diagnosis of brain abscess, who were admitted to the Dr. Fran Mihaljević University Hospital for Infectious Diseases between July 1, 2000 and July 1, 2014. The study was approved by the Ethics Committees of Dr. Fran Mihaljević University Hospital for Infectious Diseases and Zagreb University Hospital Center. Cases were ascertained through the International Classification of Diseases-10 (ICD-10) discharge codes and medical records were reviewed by paper and electronic data query. Brain abscess was defined as a localized collection of pus surrounded by a well-vascularized capsule which could be seen on a computerized tomography (CT) scan or magnetic resonance imaging (MRI). Inclusion criteria were also evidence of pus in brain tissue material from surgery and appropriate microbiological specimens (cerebrospinal fluid, blood, wound swab). Patients younger than 18 and those with subdural or epidural empyema were excluded from the study by database search engine. We also excluded patients with missing medical records, those treated conservatively (only with antibiotic regimen), and patients with post-neurosurgical brain abscess. Post-neurosurgical brain abscess was defined as a brain abscess which could be seen within one month after the neurosurgical procedure (via CT/MRI scan or clinically presented) (6, 10).
Surgically treated patients were divided into two groups of patients having undergone standard craniotomy and patients having undergone stereotactic aspiration procedure. Standard craniotomy was defined as evacuation of abscess with excision of abscess capsule. Stereotactic aspiration was defined as aspiration of the pus via burr hole or small craniotomy with capsule left in place of brain abscess. Optimal therapy was defined as therapy which led to improvement of the patient clinical status and/or control CT scan finding with significant improvement (no visible abscess). If the patient underwent multiple procedures of stereotactic aspiration combined with subsequent craniotomy, he was included into the craniotomy group.
The outcome of patients was measured with Glasgow Outcome Scale score (GOS 1-5) at discharge (11). According to GOS, patients were divided into two groups of those with favorable outcome (GOS 4 and 5) and unfavorable outcome (GOS 1-3).
Statistical analysis
For continuous variables, median and interquartile values were calculated. Categorical data were shown as frequencies and percentages. The primary outcome was GOS value. Kruskal-Wallis and Mann-Whitney tests were used for quantitative variables, and the χ2-test and Fisher exact test for categorical variables, when appropriate. The study tested how a particular type of surgery influenced outcome in brain abscess patients. For minimizing treatment selection bias (or confounding by indication bias), the propensity score model was used. Stepwise logistic regression analysis derived a model, which was then used to predict propensity score for standard craniotomy for each patient using baseline variables. Baseline variables were defined as variables that were present at the time of inclusion in the study (admission to hospital), so each patient had a propensity score that reflected the probability to undergo standard craniotomy. Summary measures of goodness of fit were evaluated using Hosmer-Lemeshow test. Two-tailed p-values less than 0.05 were considered statistically significant. Data were analyzed using SAS version 9.3 (SAS Institute, Carry, NC, USA).
Results
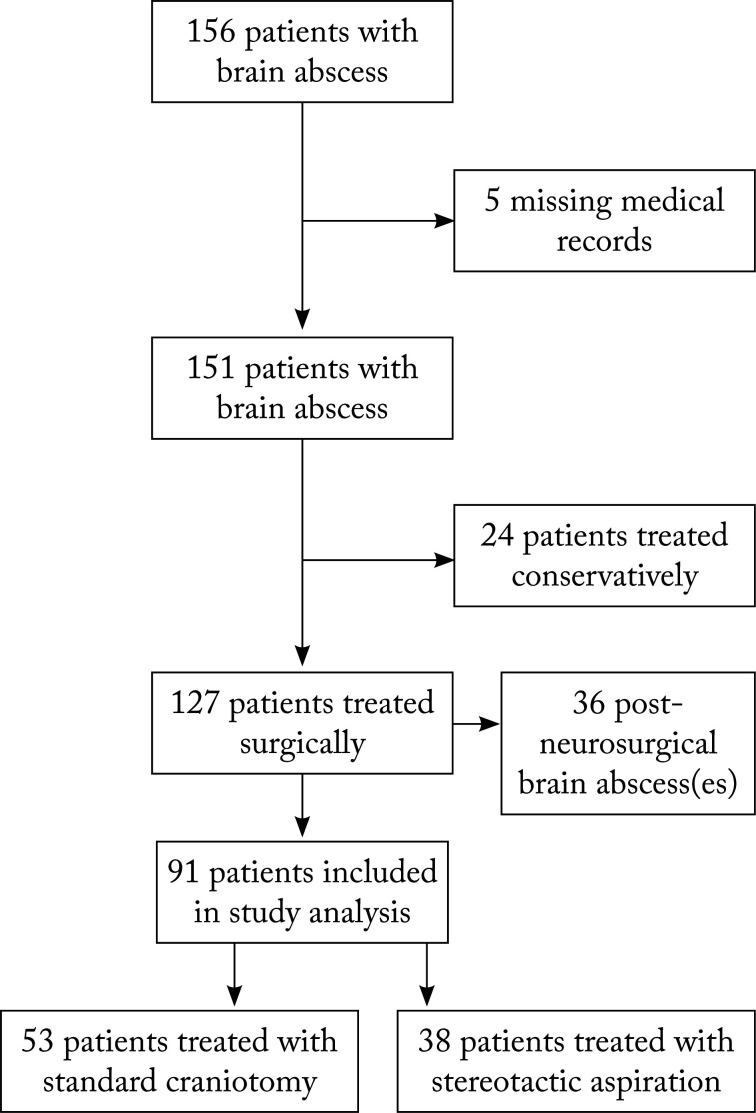
During the 14-year period, there were 156 patients with brain abscess treated at Dr. Fran Mihaljević University Hospital for Infectious Diseases in Zagreb, Croatia. Patients younger than 18 years and those diagnosed with subdural or epidural empyema were excluded from the study at the beginning of the search. Out of 156 patients, five had missing medical records. Out of 151 accessible records, 24 patients were treated only with antibiotics and thus not included in the study. There were 127 surgically treated patients, among which there were 36 post-neurosurgical brain abscesses. We excluded post-neurosurgical population due to the potential bias. Ninety-one patients met the study criteria and had community acquired brain abscess(es). This study flow is shown in Figure 1. Out of 91 surgically treated brain abscess patients, there were 53 patients having undergone standard craniotomy and 38 patients having undergone stereotactic procedure. Three patients having undergone repeated stereotactic aspirations (4 or more) and standard craniotomy as the final procedure were included in the standard craniotomy group.
Fig. 1.
Study flow of adult brain abscess patients during the period from July 1, 2000 to July 1, 2014, treated at Dr. Fran Mihaljević University Hospital for Infectious Diseases, Zagreb, Croatia.
The median age of patients in this study was 47 (interquartile range [IQR] 30-60) years and there were 67 (73.6%) male patients. Eighty-two (90.1%) patients were referred from another hospital. Comorbidities were present in 63 (69.2%) patients, among which cerebral diseases (brain tumors, cerebrovascular diseases, etc.) and chronic pulmonary diseases were most common, being present in 23 (25.3%) and 18 (19.8%) patients, respectively (Table 1).
Table 1. Basic demographic and clinical characteristics of surgically treated patients with brain abscess.
| Demographic and clinical characteristics, N=91 | Neurosurgical approach | p-value | |
|---|---|---|---|
| Standard craniotomy, n=53 | Stereotactic aspiration, n=38 |
||
| Age (yrs), median (IQR)a | 44.0 (29.0-55.0) | 50.5 (32.0-60.0) | 0.273 |
| Sex, male (%)b | 40 (75.5%) | 27 (71.1%) | 0.895 |
| Chronic diseasesb: | |||
| Alcoholism | 6 (11.3%) | 6 (15.8%) | 0.774 |
| Arteriovenous malformations | 3 (5.7%) | 6 (15.8%) | 0.277 |
| Cerebral diseases | 14 (26.4%) | 9 (23.7%) | 0.974 |
| Diabetes mellitus | 2 (3.8%) | 4 (10.5%) | 0.419 |
| Cardiovascular diseases | 8 (15.1%) | 5 (13.2%) | 1.000 |
| Chronic pulmonary diseases | 7 (13.2%) | 11 (28.9%) | 0.189 |
| Hematologic malignancy | 1 (1.9%) | 3 (7.9%) | 0.403 |
| Brain abscess originb: | |||
| Otogenic infection/sinusitis | 20 (37.7%) | 4 (10.5%) | 0.013 |
| Pulmonary infection | 2 (3.8%) | 4 (10.5%) | 0.419 |
| Meningitis | 9 (17.0%) | 5 (13.2%) | 0.928 |
| Head trauma | 2 (3.8%) | 0.0% | 0.668 |
| Odontogenic foci | 2 (3.8%) | 4 (10.5%) | 0.419 |
| Skin and soft tissue infection | 1 (1.9%) | 0.0% | 1.000 |
| Origin unknown | 17 (32.1%) | 21 (55.3%) | 0.090 |
IQR = interquartile range; aKruskal-Wallis test; bFisher exact test.
There were nine (9.9%) patients with arteriovenous (AV) malformation, six of them with Osler-Weber-Rendu syndrome and three with AV malformation in cerebral lobes as the only sites reported. There were no differences between the groups of standard craniotomy and stereotactic aspiration according to age, gender and underlying diseases.
Clinical, radiological and microbiological characteristics of brain abscess
The most common clinical sign of brain abscess was focal neurological deficit (hemiplegia, aphasia, cranial nerve paralysis, etc.), which was present in 60 (65.9%) patients (Table 2).
Table 2. Clinical presentation and brain abscess characteristics of surgically treated patients.
| Neurosurgical approach | p-value | ||
|---|---|---|---|
| Standard craniotomy, n=53 | Stereotactic aspiration, n=38 |
||
| Clinical presentation: | |||
| GCS on admission, median (IQR)a | 15.0 (13.0-15.0) | 15.0 (14.0-15.0) | 0.806 |
| Seizuresb | 17 (32.1%) | 7 (18.4%) | 0.359 |
| Focal neurological signsb | 34 (64.2%) | 26 (68.4%) | 0.916 |
| Feverb | 27 (50.9%) | 24 (63.2%) | 0.535 |
| Headacheb | 36 (67.9%) | 22 (57.9%) | 0.624 |
| Meningismb | 15 (28.3%) | 10 (26.3%) | 1.000 |
| Vomitingb | 9 (17.0%) | 9 (23.7%) | 0.711 |
| Brain abscess characteristics: | |||
| Formed capsule (%)b | 45 (84.9%) | 37 (97.4%) | 0.074 |
| Multiloculated abscess (%)b | 5 (9.4%) | 0 | 0.073 |
| Multiple abscesses (%)b | 14 (26.4%) | 15 (39.5%) | 0.254 |
| Intraventricular rupture on admission (%)b | 1 (1.9%) | 6 (15.8%) | 0.020 |
| Largest diameter of the largest abscess (mm), median (IQR)a, c | 30.0 (14.5-40.0) | 30.0 (20.0-35.0) | 0.851 |
IQR = interquartile range; aMann-Whitney test; bFisher exact test; cabscess diameter was available in 78 (85.7%) study patients, i.e. 44 (75.9%) and 34 (89.5%) patients with surgical excision and aspiration, respectively.
It was followed by headache and fever, present in 58 (63.7%) and 51 (56.0%) patients, respectively. Median Glasgow Coma Scale score (GCS) on admission was 15.0 (IQR 14.0-15.0). There were 76 (83.5%) patients with GCS between 13 and 15, and only 41 (45.1%) patients had GCS less than 15 on admission. The initial GCS did not differ between the groups of standard craniotomy and stereotactic aspiration (p= 0.806).
Neuroradiological investigations altogether revealed multilobularity in five (5/91, 5.5%), multiple abscesses in 29 (31.9%) and intraventricular rupture in seven (7.7%) patients (Table 2). The largest abscess diameter was available in 78 (85.7%) examined patients, and it was 30.0 mm (median, IQR 14.5-40.0). The observed groups differed only by intraventricular rupture of abscess, as seen on CT scan on admission (p=0.020). Most abscesses were of unknown origin (n=38, 41.8%). The most common route of infection was continuous spread of otogenic or sinonasal infection causing brain abscess formation in 24 (26.4%) patients. Other common origins of brain abscess were meningitis in 14 (15.4%) patients, and pulmonary and odontogenic infections present in six (6.6%) patients each. Patients with brain abscess as a complication of otogenic or sinonasal infection were more often treated with standard craniotomy (p=0.013). In 50 (54.9%) patients, the causative microorganism was isolated with most of the specimens taken intraoperatively (positive in 36 patients, 39.6%). There were 55 different isolates, with Streptococcus species (n=22, 40.0%) as the most commonly isolated group (Table 3). Mixed cultures were present in 11 cases.
Table 3. Microbiological characteristics of patients with brain abscess.
| Pathogen species | Number of isolatesa (%) |
|---|---|
| Streptococcus spp. | 22 (40%) |
| S. milleri group | 12 |
| S. viridans | 4 |
| Enterococcus spp. | 3 |
| Other | 3 |
| Staphylococcus spp. | 4 (7.3%) |
| Coagulase-negative staphylococcus | 4 |
| Gram-negative bacteria | 12 (21.8%) |
| P. mirabilis | 5 |
| Pseudomonas spp. | 2 |
| K. pneumoniae | 1 |
| H. influenzae | 3 |
| Other | 1 |
| Anaerobic bacteria | 16 (29.1%) |
| Peptostreptococcus spp. | 7 |
| Bacteroides | 2 |
| Fusobacterium | 2 |
| Propionibacterium | 2 |
| Other | 5 |
| Other pathogens | 4 (8.0%) |
aSome patients had more than one microbiological organism isolated.
Treatment characteristics and outcome analysis
Timing of diagnosis and treatment was calculated for both surgically treated groups. There was significant difference between the groups in days from disease onset to surgery (Mann-Whitney test, p=0.004). In craniotomy group, it was 9.0 days (IQR 5.0-20.0) and in stereotactic group 16.0 days (IQR 8.0-33.0). Other timing intervals between the two surgical groups were similar (Table 4). Median length to optimal therapy was 12.0 days (IQR 7.0-20.0) in craniotomy group and 17.0 days (IQR 8.0-33.0) in stereotactic group. There was no statistically significant difference in total duration of antimicrobial treatment between the operated groups (Mann-Whitney test, p=0.122), with median time 43.0 days (IQR 36.0-57.0) in craniotomy group and 52 days (IQR 42.0-63.0) in aspiration group.
Table 4. Timing and outcome in surgically treated patients with brain abscess.
| Neurosurgical approach | p-value | ||
|---|---|---|---|
| Standard craniotomy, n=53 | Stereotactic aspiration, n=38 |
||
| Timinga: | |||
| Days from disease onset to optimal therapy, median (IQR) | 12.0 (7.0-20.0) | 17.0 (8.0-33.0) | 0.084 |
| Days from disease onset to surgery, median (IQR) | 9.0 (5.0-20.0) | 16.0 (8.0-33.0) | 0.004 |
| Duration of antimicrobial treatment after surgery (days), median (IQR) | 36.0 (30.0-45.0) | 35.0 (28.0-46.0) | 0.942 |
| Total duration of antimicrobial treatment (days), median (IQR) | 43.0 (36.0-57.0) | 52.0 (42.0-63.0) | 0.122 |
| Outcome: | |||
| Favorable outcomeb,c | 41 (77.4%) | 26 (68.4%) | 0.348 |
| Mortality (%)b | 4 (7.5%) | 3 (7.9%) | 1.000 |
IQR = interquartile range; aMann-Whitney test; bFisher exact test; cfavorable outcome was defined as Glasgow Outcome Score (GOS) 4 and 5.
Seven (7.7%) patients died during the treatment of brain abscess, i.e. four (7.5%) patients from the standard craniotomy group and three (7.9%) patients from the stereotactic aspiration group (Table 4).
The study also compared two groups of patients according to the outcome; favorable group included 67 (73.6%) patients and unfavorable group 24 (26.4%) patients (Table 5). Analysis of the outcome revealed that GCS on admission was a factor associated with unfavorable outcome (Mann-Whitney test, p<0.001). Another factor associated with unfavorable outcome was diabetes mellitus (Fisher exact test, p=0.009). There was no statistically significant difference in mortality between the patients treated with standard craniotomy and with stereotactic aspiration (Fisher exact test, p=1.00). Timing between the favorable and unfavorable groups was significantly longer in days from disease onset to optimal therapy (Mann-Whitney test, p=0.008) and to surgery (Mann-Whitney test, p=0.007) (Table 5).
Table 5. Comparison of patients with favorable (GOS 4 and 5) and unfavorable outcome (GOS 1 to 3) by demographic, clinical and timing features.
| Unfavorable outcome (GOS 1-3), n=24 (26.4%) | Favorable outcome (GOS 4 and 5), n=67 (73.6%) | p-value | |
|---|---|---|---|
| Age in years, median (IQR)a | 54.5 (39.5-63.5) | 45.0 (28.0-55.0) | 0.111 |
| Male gender (%)b | 20 (83.3%) | 47 (70.1%) | 0.453 |
| Comorbidityb: | |||
| Diabetes mellitus | 5 (20.8%) | 1 (1.5%) | 0.009 |
| Clinical presentationa: | |||
| GCS at admission, median (IQR) | 13.0 (10.5-14.5) | 15.0 (14.0-15.0) | <0.001 |
| Timinga: | |||
| Days from disease onset to optimal therapy, median (IQR) | 21.5 (11.5-40.5) | 12.0 (6.0-21.0) | 0.008 |
| Days from disease onset to surgery, median (IQR) | 16.0 (11.0-33.0) | 10.0 (5.0-20.0) | 0.007 |
GOS = Glasgow Outcome Score; GCS = Glasgow Coma Score; IQR = interquartile range; aMann-Whitney test; bFisher exact test
Multivariate logistic regression (LR) analysis was used to estimate independent impact of neurosurgical intervention on patient outcome. Propensity score for standard craniotomy was calculated using a non-parsimonious logistic regression model which included age, GCS, gender, diameter of the largest abscess, seizures, presence of capsule, number of abscesses, spread into ventricles, days from the diagnosis to optimal antimicrobial therapy, and days from diagnosis to surgery. The final LR model included GCS, days from diagnosis to surgery, type of surgery (standard craniotomy vs. stereotactic aspiration), and propensity score for standard craniotomy as independent variables. The model fitted well (Hosmer-Lemeshow test, p=0.205) and had a satisfactory explanatory value (c=0.745). Timing, as in days from disease onset to surgery, was not associated with the outcome odds ratio (OR) 0.998, 95% CI (confidence interval) 0.981-1.015, p=0.825) (Table 6).
Table 6. Logistic regression analysis of variables that might influence the outcome: time from diagnosis to surgery (days), GCS score, type of surgery, and propensity score for standard craniotomy.
| Variable | OR | 95% Wald confidence limits |
p-value | |
|---|---|---|---|---|
| Days from disease onset to surgery | 0.998 | 0.981 | 1.015 | 0.825 |
| GCS at admission | 0.787 | 0.656 | 0.944 | 0.010 |
| Propensity score for standard craniotomy | 0.125 | 0.011 | 1.454 | 0.097 |
GOS = Glasgow Outcome Score; GCS = Glasgow Coma Score; OR = odds ratio; favorable outcome was defined as GOS 4 or 5.
The choice of neurosurgical treatment did not influence the outcome OR 1.181, 95% CI 0.349-3.995, p=0.789), nor did the propensity to standard craniotomy procedure (OR 0.125, 95% CI 0.011-1.454, p=0.097). Worse outcome (GOS 1-3) was independently associated with GCS on admission (OR 0.787, 95% CI 0.656-0.944, p=0.010).
Discussion
Treatment of brain abscess is primarily focused on eradication of the causative pathogen and reducing intracranial pressure. The management of brain abscess is influenced by characteristics of brain abscess (size, location, number, stage of formation), patient characteristics (age, underlying disease, neurological status), and surgeon preferences (knowledge, education, availability of medical equipment, etc.). Stereotactic aspiration is used as both therapeutic and diagnostic procedure and is usually performed in patients with brain abscess in eloquent areas, deeply seated brain abscess, or multiple brain abscesses (1, 9, 12-15). It can be done regardless of capsule formation (2, 15). In cases of emergency, decompression of larger abscesses is possible via burr-hole aspiration, combined with sampling for microbiological analysis and possible institution of antimicrobial agent. Aspiration can be repeated several times; in some series, most of the patients needed 2 or 3 subsequent aspirations (1, 15-17). Although the number of repeated aspirations was not counted in the study, there were only three patients who required standard craniotomy procedure due to unsuccessful aspirations. Failure of aspiration could be due to immunodeficiency, lack of catheter drainage, inappropriate antibiotic therapy, or inadequate first (and subsequent) aspiration (15, 18). Standard craniotomy is still the procedure of choice in some patients, i.e. those with large superficial abscesses, those with adhesions to the dura, those that affect large surface of brain, or posterior fossa brain abscess (1, 3, 13-17). It is also performed in patients with multiloculated brain abscesses or when there is high suspicion of specific microorganisms (such as Mycobacterium tuberculosis, fungi, actinomyces or Nocardia species) (1, 12, 15). Nowadays, there is a noticeable growth of immunodeficient population, such as patients with organ transplants, bone marrow transplants, patients treated with immunosuppressants, and human immunodeficiency virus-positive patients, who change the epidemiology and etiology of brain abscess (2, 4, 12). In these patients, we can expect unexpected microorganisms and sometimes prolonged treatment. We had one patient with heart transplant who had had brain abscess caused by Nocardia spp. one year after cardiac surgery. Another patient in the study with nocardial brain abscess was a 64-year-old female with Osler-Weber-Rendu syndrome. A recent work by Mathis et al. shows that patients with hereditary hemorrhagic telangiectasia usually have solitary lobar brain abscess that is caused by anaerobic microorganisms (19). In that study, there were four members of a family with Osler-Weber-Rendu syndrome who had brain abscesses within a period of two years caused by different types of microorganisms. It is interesting that three of these patients had multiple abscesses, which differs from previous findings (19).
We performed detailed analysis of the characteristics and management of brain abscess in relation to the outcome and identified epidemiological and microbiological characteristics in our setting. Favorable outcome (GOS 4 and 5) was measured in 67 (73.6%) patients, and only seven (7.7%) patients died, which is comparable with previous series (4, 7, 14). Demographic data and symptoms of brain abscess present on admission to the hospital did not differ from other studies (4, 7, 9, 20-26). The current study showed male predominance, which is consistent with current literature, since men are more prone to predisposing factors for brain abscess (4, 7, 9, 20-27). However, the study did not find any correlation between gender and outcome. The choice of surgical procedure in the study was influenced by the abscess size and location, predisposed pathogenesis and/or microorganism, patient general condition on admission to the hospital, as well as surgeon preference and knowledge. The study analyzed differences between the two groups of differently treated patients. Standard craniotomy was more often performed in patients who had complication of otogenic and sinonasal infection with intracranial spread, resulting in brain abscess (p=0.013). Although we excluded patients with subdural empyema in frontal lobes as a complication of acute rhinosinusitis, we still had a substantial number of patients with chronic otitis media and temporal lobe involvement. However, the origin of infection in patient brain abscess did not influence the outcome (p=0.945). Intraventricular rupture (IVR) on admission was a more often recognized factor in stereotactic group (p=0.020). It is interesting that we did not find link between IVR and poor outcome (p=0.540), which could be due to the small number of IVR cases in the study.
When comparing outcomes in patients treated with standard craniotomy and stereotactic aspiration, several studies report on better outcome in patients treated with stereotactic aspiration and some report that there are no differences in the outcome regarding surgical strategies (1, 3-5, 7-9, 12, 14-18, 21, 25-27). Important characteristics that might influence the outcome and studies reporting such impact are presented in Table 7.
Table 7. Studies that identify factors which influence outcome in patients with brain abscess.
| Prognostic factor | Age | Gender | GCS | Comorbidities | Type of NRS procedure | Microbiological agents | Time aspects | Sepsis | Other factors |
|---|---|---|---|---|---|---|---|---|---|
| Takeshita, 1998 | - | - | + | - | - | - | - | - | + Deep seated abscesses |
| Lu, 2002 | - | - | - | 0 | - | 0 | - | + | - |
| Kao PT, 2003 | - | - | - | - | 0 | 0 | + | 0 | - |
| Xiao, 2005 | - | - | + | + | + | - | - | - | 0 |
| Hakan, 2006 | - | - | + | - | + | - | - | 0 | + Size of the abscess |
| Tseng, 2006 | - | + | + | - | - | + | - | + | - |
| Tonon, 2006 | - | - | + | 0 | - | - | - | - | 0 |
| Carpenter, 2007 | - | - | - | + | - | - | - | - | + Pathogenesis |
| Ratnaike, 2011 | - | - | - | - | + | - | - | - | - |
| Helweg-Larsen, 2012 | - | - | + | + | - | - | - | - | + IVRA |
| Zhang, 2014 | - | + | - | + | - | 0 | 0 | 0 | + Number of abscesses |
GCS = Glasgow Coma Scale; NRS = neurosurgical procedure; IVRA = intraventricular rupture of abscess; (-) non-relevant; (+) relevant; (0) not included in research
A recent literature review found that aspiration had lower mortality than craniotomy, which makes aspiration the procedure of choice in patients with supratentorial parenchymal brain abscess (3). A drawback of observational studies in patients with brain abscess is their design, thus randomized controlled studies in this field are lacking. To reduce selection bias (which is commonly encountered in cardiac surgery as well), some authors suggest using the propensity score model (28-30). Propensity score used as a method of choice in statistical analysis is the probability of a subject to receive the treatment conditional on the measure of baseline characteristics (29). It gives a probability that the patient will have a certain type of surgery, reducing the treatment bias, which is often present in observational studies (28, 29). Propensity score for standard craniotomy was calculated for each patient using a non-parsimonious logistic regression model which including age, GCS, gender, diameter of the largest abscess, seizures, presence of capsule, number of abscesses, intraventricular rupture of the abscess, days from disease onset to optimal therapy, and days from disease onset to surgical intervention. Afterwards, we applied final LR model including GCS, days from disease onset to surgical intervention, type of surgical procedure, and propensity score for radical surgery as independent variables. In the presented series, propensity towards standard craniotomy did not influence the outcome (OR 0.125, 95% CI 0.011-1.454, p=0.097).
Other factors that might influence the outcome according to recent literature are severely altered mental status on admission, rapidly progressing neurological impairment, and underlying disease (comorbidities) (1, 2, 4, 7, 9, 15, 20-25, 31, 32). In the presented series, patients with more pronounced mental status alteration (lower GCS score) had unfavorable outcome (OR 0.787, 95% CI 0.656-0.944). Several earlier studies confirmed the initial GCS score as an independent factor influencing the outcome of brain abscess (7, 9, 15, 21-25). The current study did not find that underlying diseases were associated with unfavorable outcome, although earlier studies did. We did find that there were more diabetic patients in the group with unfavorable outcome (p=0.009), but there were only six diabetic patients altogether, so we did not include them in the final LR analysis model. Diabetes mellitus and alcoholism rise susceptibility to infection and are commonly present in patients with worse outcome in other studies (4, 6, 8, 20). Although Smith et al. emphasize the importance of timing of surgery in patients with brain abscess, we did not find such a connection (31). There was a statistically significant difference between the groups of standard craniotomy and stereotactic aspiration (p=0.004) in days from disease onset to surgery. This delay in surgery could be explained by brain abscess characteristics, patient general condition, surgeon preferences, and assessment. We did include this finding in our final LR model, which did not find link between timing and poor outcome (OR 0.998, 95% CI 0.981-1.015).
In conclusion, brain abscess is still a life-threatening disease that requires prompt diagnosis and treatment of patients. Unfavorable outcome (GOS below 4) is not influenced by the choice of neurosurgical procedure; however, it is influenced by poor GCS on patient admission.
References
- 1.Tunkel AR. Brain abscess. In: Mendell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases, 7th edn. Philadelphia: Churchill Livingstone; 2010: p. 1265-78. [Google Scholar]
- 2.Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–79. 10.1086/515541 [DOI] [PubMed] [Google Scholar]
- 3.Ratnaike TE, Das S, Gregson BA, Mendelow AD. A review of brain abscess surgical treatment – 78 years: aspiration versus excision. World Neurosurg. 2011;76:431–6. 10.1016/j.wneu.2011.03.048 [DOI] [PubMed] [Google Scholar]
- 4.Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1–11. 10.1007/s10096-006-0236-6 [DOI] [PubMed] [Google Scholar]
- 5.Yang SY. Brain abscess: a review of 400 cases. J Neurosurg. 1981;55:794–9. 10.3171/jns.1981.55.5.0794 [DOI] [PubMed] [Google Scholar]
- 6.Yang KY, Chang WN, Ho JT, Wang HC, Lu CH. Postneurosurgical nosocomial bacterial brain abscess in adults. Infection. 2006;34:247–51. 10.1007/s15010-006-5607-5 [DOI] [PubMed] [Google Scholar]
- 7.Helweg-Larsen J, Astradsson A, Richhall H, Erdal J, Laursen A, Brennum J. Pyogenic brain abscess, a 15 year survey. BMC Infect Dis. 2012;12:332. doi: 10.1186/1471-2334-12-332. doi: 10.1186/1471-2334-12-332. PMID: 23193986; PMCID: PMC3536615. 10.1186/1471-2334-12-332 [DOI] [PMC free article] [PubMed]
- 8.Kao PT, Tseng HK, Liu CP, Su SC, Lee CM. Brain abscess: analysis of 53 cases. J Microbiol Immunol Infect. 2003;36:129–36. [PubMed] [Google Scholar]
- 9.Tseng JH, Tseng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006;65:557–62. 10.1016/j.surneu.2005.09.029 [DOI] [PubMed] [Google Scholar]
- 10.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–40. 10.1016/0196-6553(88)90053-3 [DOI] [PubMed] [Google Scholar]
- 11.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975. March 1;1(7905):480–4. 10.1016/S0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 12.Brouwer MC, Tunkel AR, McKhann GM, van de Beek D. Brain abscess. N Engl J Med. 2014;371:447–56. 10.1056/NEJMra1301635 [DOI] [PubMed] [Google Scholar]
- 13.Dyste GN, Hitchon PW, Menezes AH, VanGilder JC, Greene GM. Stereotaxic surgery in the treatment of multiple brain abscesses. J Neurosurg. 1988;69:188–94. 10.3171/jns.1988.69.2.0188 [DOI] [PubMed] [Google Scholar]
- 14.Cavuşoglu H, Kaya RA, Turkmenoglu ON, Colak I, Aydin Y. Brain abscess: analysis of results in a series of 51 patients with combined surgical and medical approach during 11-year period. Neurosurg Focus. 2008;24:E9. 10.3171/FOC/2008/24/6/E9 [DOI] [PubMed] [Google Scholar]
- 15.Lu CH, Chang WN, Lui CC. Strategies for the management of bacterial brain abscess. J Clin Neurosci. 2006;13:979–85. 10.1016/j.jocn.2006.01.048 [DOI] [PubMed] [Google Scholar]
- 16.Sarmast AH, Showkat HI, Kirmani AR, Bhat AR, Patloo AM, Ahmad SR, et al. Aspiration versus excision: a single center experience of forty-seven patients with brain abscess over 10 years. Neurol Med Chir (Tokyo). 2012;52:724–30. 10.2176/nmc.52.724 [DOI] [PubMed] [Google Scholar]
- 17.Takeshita M, Kagawa M, Izawa M, Takakura K. Current strategies and factors influencing outcome in patients with bacterial brain abscess. Acta Neurochir (Wien). 1998;140:1263–70. 10.1007/s007010050248 [DOI] [PubMed] [Google Scholar]
- 18.Kondziolka D, Duma CM, Lunsford LD. Factors that enhance the likelihood of successful stereotactic treatment of brain abscess. Acta Neurochir (Wien). 1994;127:85–90. 10.1007/BF01808553 [DOI] [PubMed] [Google Scholar]
- 19.Mathis S, Dupuis-Girod S, Plauchu H, Giroud M, Barroso B, Ly KH, et al. Cerebral abscesses in hereditary haemorrhagic telangiectasia: a clinical and microbiological evaluation. Clin Neurol Neurosurg. 2012;114:235–40. 10.1016/j.clineuro.2011.10.036 [DOI] [PubMed] [Google Scholar]
- 20.Lu CH, Chang WN, Lin YC, Tsai NW, Liliang PC, Su TM, et al. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutical outcomes. QJM. 2002;95:501–9. 10.1093/qjmed/95.8.501 [DOI] [PubMed] [Google Scholar]
- 21.Mampalam TJ, Rosenblum ML. Trends in the management of bacterial brain abscesses: a review of 102 cases over 17 years. Neurosurgery. 1988;23:451–8. 10.1227/00006123-198810000-00008 [DOI] [PubMed] [Google Scholar]
- 22.Seydoux C, Francioli P. Bacterial brain abscess: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394–401. 10.1093/clind/15.3.394 [DOI] [PubMed] [Google Scholar]
- 23.Takeshita M, Kawamata T, Izawa M, Hori T. Prodromal signs and clinical factors influencing outcome in patients with intraventricular rupture of purulent brain abscess. Neurosurgery. 2001;48:310–6. [PubMed] [Google Scholar]
- 24.Tonon E, Scotton PG, Galluci M, Vaglia A. Brain abscess: clinical aspects of 100 patients. Int J Infect Dis. 2006;10:103–9. 10.1016/j.ijid.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Xiao F, Tseng MY, Teng LJ, Tseng HM, Tsai JC. Brain abscess: clinical experience and analysis of prognostic factors. Surg Neurol. 2005;63:442–9. 10.1016/j.surneu.2004.08.093 [DOI] [PubMed] [Google Scholar]
- 26.Antić S, Vargek-Solter V, Trkanjec Z, et al. Acute cerebrovascular incident caused by septic emboli: a case report. Acta Clin Croat. 2009;48(3):325–8. [PubMed] [Google Scholar]
- 27.Zadravec D, Gregurić T, Smoljan M, Mustapić M, Miličić G, Jović A, et al. Evaluation of the head multislice computed tomography scan in Emergency Department. Acta Clin Croat. 2017;56(2):284–91. 10.20471/acc.2017.56.02.12 [DOI] [PubMed] [Google Scholar]
- 28.Austin PC, Platt RW. Survivor treatment bias, treatment selection bias, and propensity scores in observational research. J Clin Epidemiol. 2010;63:136–8. 10.1016/j.jclinepi.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 29.Liem YS, Wong JB, Hunink MM, de Charro FT, Winkelmayer WC. Propensity scores in the presence of effect modification: a case study using the comparison of mortality on hemodialysis versus peritoneal dialysis. Emerg Themes Epidemiol. 2010;7:1. 10.1186/1742-7622-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tleyjeh IM, Ghomrawi HK, Steckelberg JM, Hoskin TL, Mirzoyev Z, Anavekar NS, et al. The impact of valve surgery on 6-month mortality in left sided infective endocarditis. Circulation. 2007;115:1721–8. 10.1161/CIRCULATIONAHA.106.658831 [DOI] [PubMed] [Google Scholar]
- 31.Smith SJ, Ughratdar I, MacArthur DC. Never go to sleep on undrained pus: a retrospective review of surgery for intraparenchymal cerebral abscess. Br J Neurosurg. 2009;23:412–7. 10.1080/02688690902887549 [DOI] [PubMed] [Google Scholar]
- 32.Roche M, Humphreys H, Smyth E, Philips J, Cunney R, McNamara E, et al. A twelve- year review of central nervous system bacterial abscesses; presentation and aetiology. Clin Microbiol Infect. 2003;9:803–9. 10.1046/j.1469-0691.2003.00651.x [DOI] [PubMed] [Google Scholar]