Abstract
Behavioral components of chromosome 22q11.2 deletion syndrome (22q), caused by the most common human microdeletion, include cognitive and adaptive functioning impairments, heightened anxiety, and an elevated risk of schizophrenia. We investigated how interactions between executive function and the largely overlooked factor of emotion regulation might relate to the incidence of symptoms of psychotic thinking in youth with 22q. We measured neural activity with event-related potentials (ERPs) in variants of an inhibitory function (Go/No-Go) experimental paradigm that presented affective or non-affective stimuli. The study replicated inhibition impairments in the 22q group that were amplified in the presence of stimuli with negative, more than positive affective salience. Importantly, the anterior N2 conflict monitoring ERP significantly increased when youth with 22q viewed angry and happy facial expressions, unlike the typically developing participants. This suggests that youth with 22q may require greater conflict monitoring resources when controlling their behavior in response to highly salient social signals. This evidence of both behavioral and neurophysiological differences in affectively influenced inhibitory function suggests that frequently anxious youth with 22q may struggle more with cognitive control in emotionally charged social settings, which could influence their risk of developing symptoms of psychosis.
Keywords: 22q11.2 deletion, emotion regulation, event-related potentials (ERP), executive function, psychosis
1 |. INTRODUCTION
Chromosome 22q11.2 deletion syndrome (22q) is the most common human chromosomal microdeletion, affecting as many as 1 in every 2,000–3,000 live births (Grati et al., 2015; Kobrynski & Sullivan, 2007; Shprintzen, 2008). Much work has been dedicated to understanding the cognitive profile of 22q and has highlighted evidence of intellectual impairments and difficulties with attentional and spatiotemporal processing abilities in individuals with 22q compared to typically developing (TD) individuals (De Smedt et al., 2007; Simon, 2008; Simon et al., 2005). A comprehensive understanding of these difficulties is particularly important for the 22q population, since studies have indicated that these individuals face an increased risk of developing both positive and negative psychosis symptoms (Baker & Skuse, 2005; Vorstman et al., 2006). Prevalence estimates have suggested that approximately 25–30% of those with 22q will develop schizophrenia by adulthood (Bassett & Chow, 2008; Green et al., 2009). Recent work has suggested that these rates have been overestimated because some negative psychosis symptoms, including decreased ideational richness and reduced occupational functioning, also reflect characteristics which are already part of the 22q phenotype (Schneider et al., 2018). However, individuals with 22q manifest more negative psychosis symptoms than individuals with genetic disorders such as Williams syndrome or those with idiopathic developmental disability (Mekori-Domachevsky et al., 2017). This suggests that intellectual or developmental disabilities alone cannot explain the higher levels of negative symptoms in those with 22q. So, since no clear explanation exists for the increased incidence of both positive and negative psychosis symptoms in youth or adults with 22q, the current study investigated how neurocognitive and affective processing mechanisms and their interactions may differ in those with 22q compared to TD individuals, and how these differences may impact psychosis-proneness.
Cognitive control impairments have been reported in young people with 22q and in patients with schizophrenia, and thus reduced executive function abilities are a potential risk factor for the development of psychosis (Gottesman & Gould, 2003; Lencz et al., 2006; Thoma, Wiebel, & Daum, 2007; Vorstman et al., 2015). Cognitive control refers to the ability to actively maintain representations of goals and use them to modulate ongoing cognitive processing (Miller, 2000). In TD individuals, cognitive control capabilities improve linearly with age (Shapiro, Tassone, Choudhary, & Simon, 2014; Somerville, Hare, & Casey, 2011). For example, work with 7- to 14-year-old children demonstrated better response inhibition in older TD children relative to younger TD children. However, this relationship was not found in children with 22q (Shapiro et al., 2014). Instead, children with 22q exhibited impairments across tasks measuring response inhibition, cognitive flexibility, and working memory relative to TD children (Bish, Ferrante, McDonald-McGinn, Zackai, & Simon, 2005; Campbell et al., 2010; Lewandowski, Shashi, Berry, & Kwapil, 2007; Shapiro et al., 2014; Shapiro, Wong, & Simon, 2013; Sobin, Kiley-Brabeck, & Karayiorgou, 2005). Similar cognitive control impairments were observed in individuals with schizophrenia, which suggests they may be associated with the development of psychosis (Gottesman & Gould, 2003; Lencz et al., 2006; Thoma et al., 2007). Despite this, in people who experience non-syndromic schizophrenia, those cognitive control impairments are actually losses from previous typical levels of functioning. By contrast, those with 22q do not, in general, appear to lose cognitive control abilities with age but instead develop them at a slower rate than their TD peers. However, these individuals have shown evidence of significant reductions in full-scale IQ with age (particularly verbal IQ) relative to individuals with 22q who did not develop psychotic thinking (Vorstman et al., 2015). Therefore, we argue that cognitive control impairments are part of a complex risk profile for the development of prodromal psychosis symptoms and, in a smaller percentage of the 22q population, conversion to full-blown schizophrenia.
Importantly, a comprehensive understanding of cognitive control processes should also consider the impact of emotion regulation, which has yet to be studied in detail among youth with 22q. Emotion regulation is a core feature of cognitive control (Hare & Casey, 2005; Somerville & Casey, 2010; Tottenham, Hare, & Casey, 2011) that refers to the dynamic way in which emotions influence other psychological and physiological processes. Optimal emotion regulation requires individuals to identify the emotional significance of a stimulus, while also regulating their behavior in the context of emotional information. However, the ability to maintain cognitive control can be influenced by emotionally charged situations. For example, in an emotional version of the Go/No-Go (GNG) task (Tottenham et al., 2011), TD children (5–12 years), adolescents (13–18 years), and adults (19–28 years) responded to pictures of facial expressions (happy, fearful, sad, angry, and neutral). Results demonstrated that all age groups were more likely to falsely respond to a No-Go trial when an emotional rather than neutral expression was presented. However, this impairment decreased with increasing age, suggesting that emotion regulation follows a protracted developmental trajectory relative to typical cognitive control. As a result, young people may be less able to regulate their emotional responses in situations which also require significant cognitive control. This period of reduced ability may be extended in those with developmental delay, as is the case for the majority of youth with 22q.
Despite it being a core feature of cognitive control, emotion regulation is rarely studied within the 22q phenotype. This is an unfortunate omission, given past research showing that individuals with 22q experience elevated levels of impairing anxiety throughout their lives (Angkustsiri et al., 2012; Fabbro, Rizzi, Schneider, Debbane, & Eliez, 2012; Jolin, Weller, & Weller, 2012; Schneider et al., 2014; Stephenson, Beaton, Weems, Angkustsiri, & Simon, 2015). We propose that this elevated anxiety and stress interacts with cognitive and adaptive impairments in 22q to increase allostatic load, which may trigger the onset of psychosis in those most severely affected (Beaton & Simon, 2011). This idea is supported by research demonstrating that elevated anxiety is associated with poorer adaptive function and may exacerbate working memory impairments in those with 22q (Angkustsiri et al., 2012; Fabbro et al., 2012; Sanders, Hobbs, Stephenson, Laird, & Beaton, 2017; Stephenson et al., 2015). It is also supported by studies showing that elevated anxiety may increase their risk for developing psychosis (Gothelf et al., 2007, 2013; Tang et al., 2017). Our thesis is consistent with the Triple Network Model (Menon, 2011; Menon & Uddin, 2010), which suggests that psychopathologies such as psychosis emerge via an interaction between autonomic and cognitive-affective processes. This interaction is driven by a salience network which detects, integrates, and filters relevant affective information, via the anterior insula and the anterior cingulate cortex (ACC). Therefore, the elevated anxiety observed among individuals with 22q may lead them into a phase of “pathological salience” and induced stress, in which they begin to misinterpret mundane events as having high salience (Menon & Uddin, 2010). By this notion, cognitive control impairments may be enhanced in situations containing an emotional component, especially with negative valence, in a population of individuals who already experience elevated anxiety.
We can examine the interaction between cognitive control and emotion regulation, and its relationship to psychosis, by combining traditional behavioral measures with event-related potentials (ERPs) in an emotional and nonemotional version of the GNG task. In this task, participants press a button in response to a frequently presented category of visual stimuli (Go trials) and withhold their button presses to an infrequently presented category of visual stimuli (No-Go trials). Our previous research asked 7- to 14-year-old children to take part in a GNG task using child-friendly stimuli (Casey et al., 1997). Relative to TD children, children with 22q exhibited typical prospective cognitive control, in which they sufficiently monitored the task and prepared for an upcoming inhibitory response, but exhibited poorer inhibitory cognitive control, in which they were less able to physically inhibit their motor response during No-Go trials. Our results also suggested that while this response inhibition improved with age in the TD children, this was not the case for children with 22q. Instead, a subgroup of older children with 22q performed like their younger TD peers rather than their same-age TD peers (Shapiro et al., 2013, 2014). Consequently, poorer GNG performance among a subgroup of individuals with 22q may be associated with the development of psychotic thinking, which could help us to subtype individuals with 22q for risk or protection against psychosis. Moreover, given the poor emotion regulation observed in the 22q population, adolescents with 22q may show an even greater impairment in cognitive control with emotional stimuli, and that failures in cognitive control over emotion may better predict psychosis proneness. We therefore implemented an emotional and nonemotional version of the GNG task in a sample of adolescents with and without 22q.
While research has been conducted to understand these cognitive processes at the behavioral level (Shapiro et al., 2013, 2014), we know little about the precise underlying brain function of such processes within the 22q population. In TD individuals the underlying brain function associated with cognitive and affective processes is commonly examined using task-based functional MRI. However, such functional magnetic resonance imaging (fMRI) studies can be uncomfortable, tiring, and anxiety-inducing—making them particularly demanding for individuals with 22q. Because of these practical difficulties, researchers can only gather data from those with the greater tolerance and attentional vigilance required to complete an fMRI experiment successfully. Therefore, of the few studies which have been successfully conducted with participants with 22q, many are underpowered by small sample sizes and likely report on unrepresentative subsamples of the population. In addition, fMRI BOLD signal represents an indirect measure of neural activity as it measures the level of oxygenated blood to specific brain regions during a task. This may be a problematic signal when studying individuals with 22q, given the high incidence of heart defects observed in this population (Poirsier et al., 2016). To our knowledge, just one previous study successfully used fMRI to examine the brain function of individuals with 22q during a GNG task (Gothelf et al., 2007). Their results suggested that participants with 22q were compensating for executive dysfunction by over-recruiting the left parietal regions relative to TD individuals. This highlights a significant gap in the literature, as the practical difficulties associated with fMRI have contributed to a lack of understanding regarding cognitive and affective processing in the 22q population beyond what can be observed from participants’ behavior.
In contrast, ERPs are relatively simple to acquire, cause minimal discomfort, and directly measure the extracellular potentials generated during neurotransmission, which presents an opportunity to examine the precise neural processes that underlie behavior. ERPs are particularly useful for examining the neural responses associated with correct No-Go trials, as these trials do not include a behavioral response. Decades of previous ERP research provides evidence for the usefulness of multiple possible ERP components that could index underlying neural processing in the GNG task. For example, the anterior N2 is a measure of inhibitory processing (conflict or novelty detection), possibly arising from the ACC (Bekker, Kenemans, & Verbaten, 2005; Folstein & Van Petten, 2008). Previous work has shown N2 amplitude enhancement to be associated with greater trait and state anxiety in response to No-Go trials (Righi, Mecacci, & Viggiano, 2009; Sehlmeyer et al., 2010). We suggest the N2 may be amplified when more anxious individuals respond to negative emotional stimuli, due to the increased salience of the negative stimuli. In addition, the late positive potential (LPP) indexes ongoing attention toward emotional stimuli (Hajcak & Dennis, 2009; Hajcak, Dunning, & Foti, 2009), which may be modulated during emotional Go trials. To better understand the potential interaction between cognitive and affective processing and the increased risk of psychosis in those with 22q, we can examine associations between anterior N2/LPP components and measures of anxiety, adaptive functioning, and psychosis-proneness.
Therefore, the current study seeks to examine inhibitory control using both emotional and nonemotional versions of the GNG task in adolescents with 22q and their TD peers, indexing neurocognitive and affective processing with behavioral and ERP measures. First, we predicted that adolescents with 22q would show poorer cognitive control on the GNG task as measured by their accuracy when inhibiting their responses on No-Go trials (Shapiro et al., 2013, 2014), as well as smaller N2 amplitudes (reflecting reduced inhibitory processing) relative to TD adolescents. Second, we predicted that smaller N2 amplitudes would be associated with a greater degree of psychosis symptoms. Third, we predicted that LPP amplitudes would be larger in adolescents with 22q and larger in response to angry versus happy blocks, as participants elevated anxiety may lead to heightened attention to negatively valenced stimuli. We also explored the relationship between our ERP measures and measures of adaptive function (Adaptive Behavior Assessment System, Second Edition—ABAS-2; Harrison & Oakland, 2003), anxiety (Spence Children’s Anxiety Survey—SCAS; Spence, 1998), and psychosis-proneness (Structured Interview for Prodromal Syndromes—SIPS; McGlashan et al., 2001) to better understand the interaction between cognitive-affective processes and psychosis.
2 |. METHODS
2.1 |. Participants
Fifty-seven adolescents with 22q and 50 TD adolescents (aged 12–18 years) participated in this study (Table 1). Participants were recruited using flyers posted at the UC Davis Medical Center and Sacramento public library, the MIND Institute Subject Tracking System, and through social media posts. Previous participants in studies from our laboratory were also invited to take part. Inclusion criteria for all participants included no history of head trauma and no prior history of antipsychotic medication. In addition, TD participants were only included if they had no known Axis 1 disorders. All participants needed a verbal IQ of 70 or above, to ensure that they could understand the questions being asked during their psychological interviews. IQ was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI-II; Wechsler, 2011). Because the verbal comprehension index screened participants for a verbal IQ of 70 or above, this part of the WASI was completed via video call before their visit to the MIND Institute. To avoid practice effects, participants did not complete the WASI-II in this study if they had completed this assessment within the past 2 years. As a result, a small portion of participants with 22q (n = 9) had an IQ score measured using the Wechsler Intelligence Scale for Children-fifth edition (WISC-V; Wechsler, 2014), as this was the most recent IQ assessment available for them. T-tests showed that WASI-II and WISC-V scores did not significantly differ for verbal IQ, t(12.06) = 0.28, 95% confidence interval [CI]: 7.31, 9.48, p = .783, or full-scale IQ, t(11.05) = 0.55, 95% CI: 7.06, 11.79, p = .592. As a result, WASI-II and WISC-V scores were subsequently aggregated in Table 1. Participants with 22q had significantly lower verbal IQ t(83.34) = 10.94, 95% CI: 22.45, 32.42, p < .001, and full scale IQ t(95.87) = 14.56, 95% CI: 30.98, 40.77, p < .001 relative to TD participants. This study formed part of a 2-day testing battery, which was approved by the University of California, Davis, Institutional Review Board and conformed to institutional and federal guidelines for protection of human participants (IRB Protocol no. 721614). Written informed consent from the parents and assent from the participants were obtained before testing.
TABLE 1.
Participants characteristics
| 22q, mean (SD) | TD, mean (SD) | |
|---|---|---|
| n | 57 | 50 |
| Age (y) | 14.87 (2.17) | 14.80 (1.99) |
| Female % | 45.61 | 56.00 |
| IQ (full scale)** | 80.13 (11.42) | 116 (13.68) |
| IQ (verbal)** | 87.81 (10.00) | 115.24 (15.06) |
Note: Age (years), biological sex (percent female), and IQ (WASI/WISC-V Full Scale, Verbal) for participants with 22q and those who were TD.
p < .001.
Abbreviations: SD, standard deviation; TD, typically developing; WASI, Wechsler Abbreviated Scale of Intelligence; WISC-V, Wechsler Intelligence scale for children fifth edition.
2.2 |. Stimuli and task procedure
Stimuli were presented through E-Prime version 2.0.10.353 on a 27-in. (1920 × 1080) monitor running Windows 7. Participants were seated approximately 57 cm from the monitor. Behavioral responses were recorded using a Logitech Precision gamepad, and EEG data were recorded using a Brain Products actiCHamp system. The EEG was filtered online with a cascaded integrator-comb filter to prevent aliasing (half-power cutoff of 260 Hz) and digitized at 1000 Hz using the PyCorder software (version 1.0.9). Electrodes were mounted in an electrode cap (EasyCap2-C). The data were recorded reference-free and were referenced to the average of the left and right mastoid electrodes once offline. Vertical and horizontal eye movements were monitored using additional facial electrodes placed above and below the right eye and adjacent to the left and right lateral canthi.
2.2.1 |. Nonemotional GNG
To examine response inhibition, an index of cognitive control, we used a nonemotional variant of the GNG task, designed as a whack-a-mole game. In this task, participants were presented with a series of moles (ranging 1–5 in a row) and were occasionally presented with a picture of a vegetable (e.g., cabbage, radish). Participants were required to press a button in response to the moles but withhold their button press in response to vegetables. On 83.33% of trials 1, 3, or 5 moles were presented (27.78% each) before a vegetable was presented. However, the task was made less predictable by also including eight trials where 2 moles were presented in a row, and 6 trials where 4 moles were presented in a row. The visual stimuli were presented in the center of the computer monitor and subtended 12 × 11 degrees of visual angle.
During each trial, each mole or vegetable was presented for 500 ms and was followed by a jittered interstimulus interval of 600–1100 ms (continuous rectangular distribution). Participants completed three practice trials to ensure they understood the task, before completing 306 stimulus events (230 Go, 76 No-Go). To reduce fatigue and maintain participants’ concentration, trials were presented in five pseudorandomized blocks, taking approximately 3 min each to complete. This task was previously used to examine response inhibition in younger children with 22q (Shapiro et al., 2013, age range = 7- to 14-year-olds, 2014).
2.2.2 |. Emotional GNG
To examine the impact of emotionally valenced stimuli on response inhibition, we used an emotional variant of the GNG task (eGNG), designed with the participant acting as a photographer taking pictures of people with emotional (happy or angry) but not calm facial expressions. In each task block, participants were presented with a series of happy or angry faces (1–5 times in a row) and were occasionally presented with a calm face. On 83.33% of Trials 1, 3, or 5, emotional faces were presented (27.78% each) before a calm face was presented. To make the pattern less predictable, we included 16 trials where two emotional faces were presented in a row and 12 trials where four emotional faces were presented in a row. The emotional facial expressions of 36 actors (18 male, 18 female) were taken from the NimStim battery (Tottenham et al., 2009; Tottenham, Borscheid, Ellertsen, Marcus, & Nelson, 2002). Each actor displayed one happy, one angry, and one calm facial expression. The faces of multiple different actors were used on each trial. All images were adjusted to be black-and-white, matched for size and luminance (18 cd/m2) and were presented in the center of the screen, subtending 14 × 17 degrees of visual angle.
For each trial (Figure 1), face stimuli were presented for 500 ms, followed by a jittered ISI of 600–1,100 ms. Participants completed three practice trials for angry faces and three practice trials for happy faces before completing 612 stimulus events (230 Go-happy, 230 Go-angry, 76 No-Go calm on happy, 76 No-Go calm on angry). Trials were divided into 10 blocks (5 happy, 5 angry), taking approximately 3 min each to complete.
FIGURE 1.
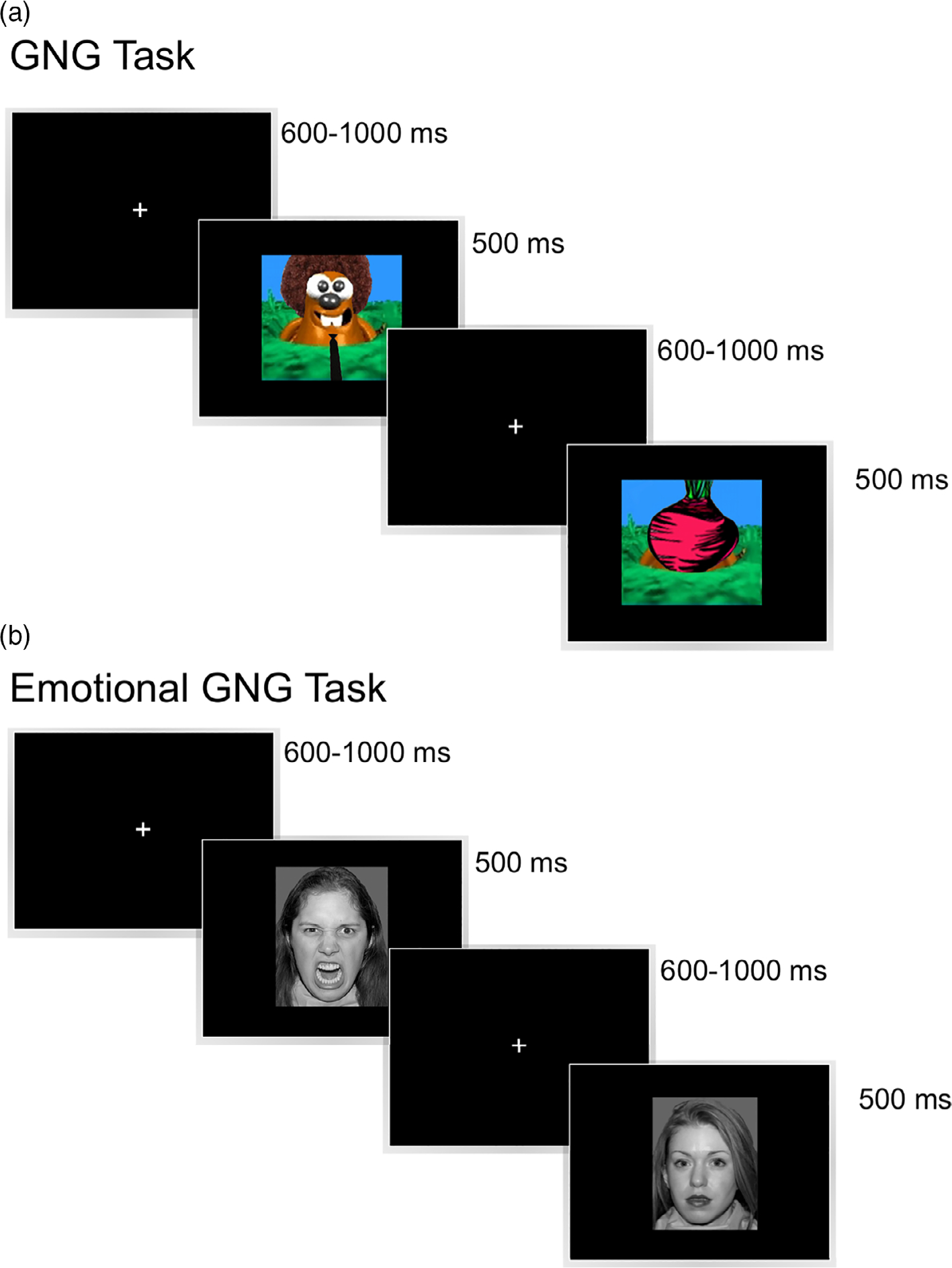
Experimental design of the nonemotional Go/No-Go (GNG) task. (a) Participants were presented with a series of moles and were occasionally presented with a picture of a vegetable. Participants were required to press a button in response to the moles, but withhold their button press in response to vegetables. (b) Experimental design of the emotional GNG task. Participants were presented with a series of emotional facial expressions (happy or angry) and were occasionally presented with a calm face. Participants were required to press a button in response to emotional faces, but withhold their button press in response to calm faces [Color figure can be viewed at wileyonlinelibrary.com]
2.3 |. Outcome measures
2.3.1 |. Psychological assessments
To examine potential relationships between neurocognitive/affective processes and psychosis-proneness, participants with 22q and their parents completed the SIPS with a clinician or staff member trained by one of our team (T.A.N or K.B), and all participants completed the Prodromal Questionnaire—Brief Version PQ-B (Loewy, Bearden, Johnson, Raine, & Cannon, 2005; Loewy, Pearson, Vinogradov, Bearden, & Cannon, 2011). The daily function of all participants was also assessed using the ABAS-2 which was completed by their parents, and anxiety was measured using the SCAS (child and parent forms).
2.3.2 |. Accuracy and response time
To examine response inhibition, we examined participants’ accuracy during No-Go trials (i.e., successfully withholding a prepotent response), and to examine prospective inhibition, we examined response time (RT) during Go trials. We removed trials which had an RT of <200 ms (reflecting anticipatory responses), and trials where the RT was over 2.5 standard deviations from the participant’s mean RT (reflecting disengagement from the task). Participants with less than 50% accuracy on over 50% of conditions were excluded. There were no significant differences between the exclusion rates for each group for the GNG (eight with 22q and four TD), but for the eGNG, significantly more participants with 22q were removed (Fisher’s exact test p = .034; 17 with 22q and 6 TD).
2.3.3 |. EEG data processing and ERP analysis
Once collected, the continuous EEG data were processed using ERPLAB (version 7.0.0, Lopez-Calderon & Luck, 2014) and EEGLAB (version 14.1.0b; Delorme & Makeig, 2004). The data were first downsampled to 500 Hz and were bandpass-filtered using a second-order Butterworth filter with half-amplitude bandpass of 0.1–30 Hz. Electrical line noise was removed using a 60 Hz notch filter, and the resulting EEG signal was referenced to the average of the two mastoid electrodes. Data were visually inspected and major artifacts (e.g., yawning, head movements) were removed. The data were then subjected to an independent component analysis, and components which were characteristic of eyeblinks or eye movements were removed. To ensure we only kept trials where participants could see the stimulus, we also removed trials containing blinks or eye movements within 200 ms of the stimulus presentation. These trials were detected using ERPLAB’s blink rejection tool on the vertical eye channel (cross covariance threshold = 0.7), and using ERPLAB’s steplike artifact detection tool on the horizontal eye channel (threshold = 100 μV).
From the processed EEG data, we extracted stimulus-locked epochs for the Go and No-Go conditions (−200 to 800 ms). Epochs were baseline corrected according to the average activity in the 200 ms time window prior to stimulus onset, and then averaged ERPs were computed. To measure the anterior N2, we averaged across anterior and parietal electrodes (Fz, F3, F4, C3, Cz, C4, Pz, P3, P4). To measure the LPP, we measured the activity from electrode Pz. Participants were excluded at this stage if more than 50% of their trials were contaminated with artifacts, which led to three with 22q and zero TD participants being removed from the GNG and four with 22q and zero TD participants being removed from the eGNG. There was no significant difference between the number of participants excluded at this stage (all Fisher’s Exact test ps > .05). For the GNG, we excluded significantly more artifactual ERP trials among individuals with 22q (mean = 17.58%, SD = 9.60%) compared to TD individuals (mean = 13.19%, SD = 6.51%), t(93.70) = 2.75, p = .007, d = 0.54. Similarly, for the eGNG, we excluded significantly more artifactual ERP trials among individuals with 22q (angry blocks mean = 19.65%, SD = 10.66%; happy blocks mean = 19.37%, SD = 10.09%) compared to TD individuals (angry blocks mean = 14.22%, SD = 7.47%; happy blocks mean = 14.58%, SD = 7.04%), t(93.48) = 3.00, p = .003, d = 0.59, with no significant difference between emotional conditions (ps > .05).
We selected ERP time windows for analysis by first generating a grand-averaged ERP waveform of all participants and conditions (i.e., a collapsed localizer, see Luck & Gaspelin, 2017). Inspection of this grand-averaged ERP revealed an anterior N2 between 280 and 380 ms in the GNG and between 290 and 380 ms in the eGNG. In addition, however, because the No-Go trials were rare in this study, an oddball P3 component was generated by the No-Go stimuli. The P3 component is believed to index fundamental attention and memory processes as participants make comparisons between frequent (Go) trials and rare (No-Go) trials (Polich, 2012). We therefore also examined the P3 component by comparing the mean amplitude at electrode Pz for 300–600 ms on the Go and No-Go trials during the GNG. The LPP was measured for the eGNG between 300 and 600 ms, based on the previous literature (Hajcak et al., 2009; Hajcak & Dennis, 2009; Kappenman, MacNamara, & Proudfit, 2014).
2.3.4 |. Correlations with other measures
To examine potential interactions between cognitive and affective processing and psychosis-proneness, ERP measures were correlated with SCAS, ABAS, and SIPS scores. All correlational analyses were bootstrapped using bias-corrected and accelerated 95% CIs based on 1,000 samples.
3 |. RESULTS
3.1 |. Psychological assessments
Participants with 22q had significantly poorer adaptive functioning skills relative to TD participants across all ABAS subscales (Table S1). In addition, participants with 22q reported significantly higher anxiety on child SCAS measures of OCD, panic agoraphobia, fears of physical injury, separation anxiety, and total anxiety symptoms (Table S2). On the parent SCAS participants with 22q had significantly higher anxiety on all measures compared to TD participants (Table S3).
3.2 |. Accuracy and RT
3.2.1 |. Go/No-Go
Mean No-Go accuracy scores and Go RTs for participants with 22q and their TD peers during the GNG are displayed in Table 2. To examine whether participants with 22q differ in their inhibitory control processes relative to TD participants during the GNG, we computed two linear mixed effects models. The first model examined accuracy during No-Go trials, with diagnosis (22q and TD) and the number of preceding Go trials and their interaction as fixed effects, and with participant as a random effect (285 observations on 95 individuals). The second model examined RT on Go trials, with diagnosis, Go trial number, and their interaction as fixed effects, and with participant as a random effect (474 observations on 95 individuals).
TABLE 2.
Mean No-Go accuracy scores and Go RTs for both groups during the GNG
| 22Q, mean (SD) | TD, mean (SD) | |
|---|---|---|
| No-Go accuracy (Go trial #) | ||
| 1 | 0.74 (0.18) | 0.82 (0.18) |
| 3 | 0.73 (0.14) | 0.80 (0.19) |
| 5 | 0.71 (0.14) | 0.81 (0.20) |
| Go RT (trial #) | ||
| 1 | 335.36 (52.67) | 357.80 (70.60) |
| 2 | 347.61 (45.70) | 369.02 (59.95) |
| 3 | 351.56 (46.48) | 364.63 (52.81) |
| 4 | 358.53 (40.63) | 375.45 (51.69) |
| 5 | 323.52 (47.03) | 343.32 (88.86) |
Abbreviations: GNG, Go/No-Go; RT, response time; SD, standard deviation; TD, typically developing.
For No-Go accuracy, we observed a significant main effect of diagnosis (coefficient = 0.08, t[93] = 3.00, p = .003) with participants with 22q exhibiting lower accuracy relative to TD participants (Figure 2a). For RT on Go trials, we also observed a significant main effect of diagnosis (coefficient = 22.20, t[93] = 2.40, p = .019) with participants with 22q responding faster on Go trials relative to TD participants (Figure 2a). All other main effects and interactions for these models were nonsignificant (all ps > .05). In addition, we examined whether No-Go accuracy improves with age and found that while accuracy was significantly positively correlated with age in the TD participants following one Go trial (r[44] = 0.40, p = .006), three Go trials (r[44] = 0.34, p = .02), and five Go trials (r[44] = 0.44, p = .002), all correlations in the 22q group were nonsignificant (all ps > .05, Figure S1).
FIGURE 2.

Go/No-Go (GNG) behavioral results. (a) Participants with 22q exhibited significantly lower accuracy in response to No-Go trials and faster response times (RTs) in response to Go trials, relative to typically developing (TD) participants. (b) Emotional GNG (eGNG) behavioral results. Participants with 22q exhibited significantly lower accuracy in response to No-Go trials and faster RTs in response to Go trials when compared with TD participants and exhibited significantly lower accuracy during angry versus happy face blocks. Both groups had significantly slower RTs in response to angry versus happy face blocks. Error bars represent SEM [Color figure can be viewed at wileyonlinelibrary.com]
To better understand why participants with 22q exhibited faster overall RTs during Go trials relative to TD participants (Figure 2a), we plotted overall No-Go accuracy as a function of RT and examined associations between them. These analyses demonstrated a significant positive association between accuracy and RT in participants with 22q (r[47] = .30, p = .037) such that more accurate participants had slower RTs. However, this association was not significant in the TD group (r[44] = .17, p = .270), which suggests faster overall RTs in participants with 22q may have resulted from a speed-accuracy trade-off.
3.2.2 |. Emotional GNG
Mean No-Go accuracy scores and Go RTs for participants with 22q and their TD peers during the eGNG are displayed in Table 3. To examine whether highly salient emotional facial expressions would impact inhibitory processing during the GNG in participants with 22q relative to TD participants, we computed two linear mixed effects models. The first model examined accuracy during No-Go trials, with diagnosis (22q and TD), the emotion of the Go trials during a given trial block (angry vs happy), the number of preceding Go trials, and their interaction as fixed effects, and with participant as a random effect (504 observations on 84 individuals). The second model examined RT on Go trials, with diagnosis, the emotion of the Go trials, Go trial number, and their interaction as fixed effects, and with participant as a random effect (837 observations on 84 individuals).
TABLE 3.
Mean No-Go accuracy scores and Go RTs for both groups during the eGNG (happy and angry blocks)
| Happy |
Angry |
|||
|---|---|---|---|---|
| 22Q, mean (SD) | TD, mean (SD) | 22Q, mean (SD) | TD, mean (SD) | |
| No-Go accuracy (trial #) | ||||
| 1 | 0.71 (0.14) | 0.75 (0.15) | 0.65 (0.16) | 0.75 (0.15) |
| 3 | 0.69 (0.10) | 0.79 (0.15) | 0.67 (0.11) | 0.79 (0.14) |
| 5 | 0.69 (0.14) | 0.77 (0.17) | 0.64 (0.15) | 0.78 (0.14) |
| Go RT (trial #) | ||||
| 1 | 367.31 (67.87) | 430.22 (74.36) | 377.66 (78.87) | 437.59 (77.44) |
| 2 | 377.93 (48.47) | 432.11 (52.97) | 390.81 (60.73) | 434.93 (53.62) |
| 3 | 375.78 (47.97) | 430.68 (55.54) | 389.00 (52.55) | 434.69 (53.39) |
| 4 | 379.06 (45.54) | 424.92 (53.62) | 399.28 (65.62) | 441.17 (63.21) |
| 5 | 366.69 (48.08) | 425.33 (80.92) | 367.81 (51.93) | 416.18 (74.98) |
Abbreviations: eGNG, emotional Go/No-Go; RT, response time; SD, standard deviation; TD, typically developing.
For No-Go accuracy, we observed a significant main effect of diagnosis (coefficient = 0.09, t[82] = 3.02, p = .003), with participants with 22q exhibiting lower accuracy relative to TD participants. The key finding was a significant interaction between diagnosis and emotion, with participants with 22q showing a larger difference between happy and angry faces compared to TD participants (TD×Happy coefficient = −0.04, t[415] = −2.51, p = .013, Figure 2b). More specifically, while TD participants exhibited virtually identical accuracy for happy and angry faces, accuracy dropped for angry faces relative to happy faces in participants with 22q. All other main effects and interactions were nonsignificant (all ps > .05).
For Go RT, we observed a significant main effect of diagnosis (coefficient = 54.15, t[82] = 4.26, p < .001) with participants with 22q responding faster compared to TD participants. We also observed a significant main effect of emotion (coefficient = 16.15, t[748] = 2.61, p = .009), with both groups responding faster during happy compared to angry blocks. There was a numerical trend for the slowing of angry faces relative to happy faces to be greater in participants with 22q than in TD participants, mirroring the accuracy data, but this effect was not statistically significant (coefficient = 7.25, t[748] 1.50, p = .134).
3.3 |. Event-related potentials
3.3.1 |. Go/No-Go
The mean N2 area amplitudes and P3 mean amplitudes observed for both groups during the GNG are displayed in Table 4, and the grand average ERP waveforms are shown in Figure 3a. These anterior ERP waveforms begin with an N1 wave, followed by a P2 wave, an N2 wave, and finally a P3 wave. In TD participants the N2 No-Go minus Go difference wave appears larger and the P3 difference wave appears smaller in area relative to participants with 22q.
TABLE 4.
Mean N2 area amplitudes and P3 mean amplitudes or both groups during the GNG
| 22Q, mean (SD) | TD, mean (SD) | |
|---|---|---|
| Anterior N2 area amplitude (No-Go minus Go difference) | 0.19 (0.22) | 0.22 (0.23) |
| P3 mean amplitude (Go trials) | 7.81 (7.13) | 6.78 (4.91) |
Abbreviations: GNG, Go/No-Go; SD, standard deviation; TD, typically developing.
FIGURE 3.

Go/No-Go (GNG) event-related potential (ERP) results. (a) All participants exhibited similar Anterior N2 area amplitudes in response to Go vs No-Go trials (p = .509), and (b) exhibited greater late positive potential (LPP) mean amplitudes in response to Go vs No-Go trials. (c) Grand average ERP waveforms to show the anterior N2 component. *p < .001. Error bars represent SEM [Color figure can be viewed at wileyonlinelibrary.com]
To examine the potential differences in a neural measure of inhibitory processing in the GNG task, we measured group differences in the size of the anterior N2, as measured using negative area under the curve, for the No-Go minus Go waveforms. Although the anterior N2 was numerically reduced in participants with 22q relative to TD participants during the GNG task, the difference between the groups was small relative to the within-group variability, and the difference between the groups did not approach significance, t(101.9) = 0.66, 95% CI [−0.12, 0.06], p = .509 (Figure 3b).
To examine the P3 wave, we computed a model with fixed main effects of diagnosis (22q versus TD) and trial type (Go vs No-Go), their interaction, and a random effect of participant (208 observations on 104 individuals). This P3 wave was significantly larger on No-Go trials than on Go trials in this window (coefficient = 5.80, t[102] = 9.53, p < .001) (Figure 3c). There was no main or interaction effect of this measure with diagnosis.
3.3.2 |. Emotional GNG
The mean N2 area amplitudes and LPP mean amplitudes observed for both groups during the eGNG are displayed in Table 5, and the grand average ERP waveforms for the N2 and LPP are shown in Figure 4a, b. The anterior ERP waveforms begin with an N1 wave, followed by a P2 wave, an N2 wave, and a P3 wave. TD participants exhibited much more prominent N1 and P2 ERPs when compared with participants with 22q. However, participants with 22q are exhibiting a larger average N2 difference wave between No-Go and Go trials for both happy and angry face blocks when compared with TD participants. The posterior ERP waveforms clearly show an LPP extending across several hundred milliseconds for both groups.
TABLE 5.
Mean N2 and LPP amplitudes for both groups during the eGNG (happy and angry blocks)
| Happy |
Angry |
|||
|---|---|---|---|---|
| 22Q, mean (SD) | TD, mean (SD) | 22Q, mean (SD) | TD, mean (SD) | |
| Anterior N2 area amplitude (No-Go minus go difference) | 0.24 (0.35) | 0.11 (0.16) | 0.25 (0.40) | 0.07 (0.14) |
| LPP mean amplitude (Go trials) | 9.60 (7.71) | 8.12 (7.35) | 9.59 (8.33) | 8.68 (7.24) |
Abbreviations: eGNG, emotional Go/No-Go; LPP, late positive potential; SD, standard deviation; TD, typically developing.
FIGURE 4.

Emotional GNG (eGNG) event-related potential (ERP) results. (a) Participants with 22q exhibited significantly larger anterior N2 area amplitudes in response to No-Go trials which were preceded by both angry and happy Go trials relative to typically developing (TD) participants. (b) Both groups of participants exhibited similar late positive potential (LPP) amplitudes in response to angry and happy Go trials. (c) Grand average ERP waveforms to show the anterior N2 component. (d) Grand average ERP waveforms to show the LPP component. *p < .001. Error bars represent SEM [Color figure can be viewed at wileyonlinelibrary.com]
To examine whether differences in the anterior N2 would be observed when participants were presented with emotional facial expressions, we computed a mixed effects linear model for the N2 No-Go minus Go difference waves using fixed main effects for diagnosis (22q vs TD) and emotion (angry vs happy), their interaction, and a random effect for participant (206 observations on 103 individuals). There was a significant main effect for diagnosis, with participants with 22q showing a larger N2 than TD participants (coefficient = 0.17, t[101] = 3.03, p = .003) (Figure 4c) in response to both angry and happy emotional expressions. The main effect of emotion was nonsignificant (coefficient = 0.01, t[101] = 0.23, p = .817) as was the interaction (coefficient = 0.05, t[101] = 0.69, p = .493). Given that TD participants did not show (on average) an N2 (as visible in Figure 4a), our use of the negative area measure may be problematic, as the TD participant data will contain more zeros than the 22q group, resulting in a nonnormal distribution of scores across groups. However, a non-parametric analysis of N2 area amplitude and an analysis of N2 mean amplitudes produced the same result (see Data S1), which strengthens the reliability of our original outcome.
Examination of the waveforms in Figure 4a shows a large P2 for the TD group but not the 22q group during the eGNG. This could partially explain the smaller N2 wave in the TD group. To investigate further, we conducted an additional exploratory analysis of the P2 wave. Reinspection of the collapsed localizer revealed a P2 wave between 180 and 280 ms. We extracted positive signed area amplitudes at this time window for both groups during this task, and used these values to compute a mixed effects linear model for the P2 No-Go minus Go difference waves using fixed main effects for diagnosis (22q vs TD), emotion (angry versus happy), their interaction, and a random effect for participant (206 observations of 103 individuals). This analysis revealed nonsignificant main effects of diagnosis (coefficient = 0.04, t[101] = 0.84, p = .404), emotion (coefficient = 0.87, t[101] = 0.87, p = .389), and a nonsignificant interaction effect (coefficient = 0.01, t[101] = 0.12, p = .904).
Given the significant difference that was observed between the P3 wave between Go and No-Go trials during the nonemotional GNG, we restricted our LPP analyses in the eGNG to the emotional Go trials and did not examine the calm, No-Go trials. Thus, to examine whether the LPP would be modulated differently during the eGNG between participants with 22q and TD participants, a mixed effects linear model was generated for LPP on Go trials, using a main effect of diagnosis (22q vs TD), emotion (Angry vs Happy), their interaction, and a random effect of participant (206 observations and 103 individuals). All main effects and interactions were nonsignificant (all ps > .05, Figure 4d).
3.4 |. Correlations with other measures
3.4.1 |. Go/No-Go
For GNG, among participants with 22q, a greater anterior N2 was associated with significantly lower child-reported fear of physical injury (r[51] = −0.35, p = .011) and was associated with a greater ABAS community use score (r[50] = 0.30, p = .032). Similarly among TD participants, a greater anterior N2 was associated with a greater ABAS community use score (r[44] = .33, p = .023; Table S4 and Figure S2).
3.4.2 |. Emotional GNG
For eGNG angry blocks, among participants with 22q, a greater anterior N2 was associated with a significantly greater ABAS community use score (r[49] = 0.33, p = .017), and a greater LPP in response to angry Go trials was associated with significantly greater scores on the following ABAS scales: community use (r[49] = 0.33, p = .017), functional academics (r[49] = .34, p = .014), home living (r[49] = .30, p = .034), health and safety (r[49] = .41, p = .003), leisure (r[49] = .38, p = .006), self-direction (r[49] = .32, p = .023), and social skills (r[49] = .33, p = .019). In addition, a greater LPP in response to angry Go trials was also associated with significantly greater score on SIPS disorganized communication (r[45] = .30, p = .039), and a lower score on SIPS social anhedonia (r[46] = −.35, p = .017).
For eGNG angry blocks, among TD participants, a greater anterior N2 was associated with a significantly greater ABAS community use (r(44) = .31, p = .035). A greater LPP in response to angry Go trials was associated with significantly lower child-reported OCD (r[48] = −0.29, p = .043) and greater parent-reported panic agoraphobia (r[45] = 0.43, p = .002), fear of physical injury (r[45] = 0.32, p = .028), general anxiety (r[45] = 0.32, p = .030), and overall total anxiety symptoms (r[45] = 0.31, p = .032). Lastly, a greater LPP in response to angry Go trials was associated with significantly greater scores on the following ABAS scales: leisure (r(44) = −.40, p = .006), self-care (r(44) = −.43, p = .003), self-direction (r(44) = −.38, p = .010) (Table S5 and Figure S3).
For eGNG happy blocks, among participants with 22q, a greater anterior N2 was associated with significantly lower parent-reported social phobia (r[49] = −0.29, p = .038) and lower total anxiety symptoms (r[49] = −0.29, p = .041). In addition, a greater anterior N2 was associated with greater ABAS home living (r[49] = .31, p = .025), and greater SIPS trouble with focus and attention (r[45] = .32, p = .027). A greater LPP in response to happy Go trials was associated with significantly greater scores on the following ABAS subscales: community use (r[49] = 0.32, p = .021), functional academics (r[49] = .44, p = .001), home living (r[49] = .38, p = .006), health and safety (r[49] = .39, p = .005), leisure (r[49] = .36, p = .009), self-direction (r[49] = .40, p = .004), and social skills (r[49] = .38, p = .006). In addition, a greater LPP in response to happy Go trials was associated with greater SIPS disorganized communication (r[45] = .33, p = .022), lower SIPS social anhedonia (r[46] = −.30, p = .038), and lower SIPS occupational functioning (r[46] = −.29, p = .05).
For eGNG happy blocks, among TD participants, a greater anterior N2 was associated with greater ABAS leisure score (r(44) = .31, p = .039). A greater LPP in response to happy Go trials was associated with greater parent-reported panic agoraphobia (r[45] = 0.33, p = .025) and fear of physical injury (r[45] = 0.34, p = .021), and was associated with greater ABAS leisure (r(44) = −.39, p = .007), and lower ABAS self-care (r(44) = −.34, p = .019) (Table S6 and Figure S4).
4 |. DISCUSSION
The present study aimed to investigate neurocognitive and affective processing mechanisms in 22q to better understand their elevated risk of psychosis. To achieve this aim, we compared inhibitory control in both emotional and nonemotional contexts in 12- to 18-year-olds with and without 22q using a GNG paradigm. Overall, we observed reduced adaptive function and increased anxiety in participants with 22q relative to TD participants. When examining their behavior during the nonemotional GNG, participants with 22q exhibited impaired response inhibition to No-Go trials, which did not improve with age, relative to TD participants. There was an added impact of emotion during the eGNG, whereby participants with 22q exhibited impaired response inhibition to No-Go trials during angry face blocks vs happy face blocks. When examining participants ERPs during the nonemotional GNG, we observed no significant group differences in the size of the anterior N2 response during No-Go trials. However, during the eGNG, anterior N2 responses were larger in participants with 22q relative to TD participants, with no significant group differences observed in the size of the LPP during this task.
Together, our findings provide evidence of behavioral and neurophysiological differences in cognitive control abilities in those with 22q relative to TD individuals. Our finding of increased anxiety and reduced adaptive functioning in participants with 22q is consistent with previous work which found that greater anxiety, rather than lower IQ, was associated with reduced adaptive functioning in 7- to 14-year-old children with 22q (Angkustsiri et al., 2012). Our behavioral results are consistent with studies showing that children with 22q are impaired across multiple measures of response inhibition, cognitive flexibility, and working memory relative to TD children (Bish et al., 2005; Campbell et al., 2010; Lewandowski et al., 2007; Shapiro et al., 2013, 2014; Sobin et al., 2005). Moreover, our finding that No-Go accuracy improved with age in 12- to 18-year-old TD participants but not in participants with 22q is consistent with our previous work (Shapiro et al., 2013, 2014) that used the same GNG task with 7- to 14-year-old children with 22q. Like the current study, Shapiro et al. observed improved response inhibition in older TD children relative to younger TD children, with no such relationship observed in children with 22q. These results suggest that individuals with 22q continue to exhibit impairments in their cognitive control abilities into late adolescence.
Consistent with impairments in response inhibition in participants with 22q, the anterior N2 was numerically reduced in these participants compared to TD participants. A review of studies which elicited the anterior N2 component suggested this component arises from the ACC and is related to cognitive control (specifically conflict monitoring) during No-Go trials (Folstein & Van Petten, 2008), rather than strictly indexing response inhibition (Donkers & van Boxtel, 2004). Conflict monitoring refers to the way participants monitor for the occurrence of conflicts in information processing and translates the occurrence of conflict into compensatory adjustments in control (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004). However, in this study, there was considerable within-group variability in anterior N2 amplitude, and the difference between groups did not reach significance. Thus, it is not possible at this time to determine whether this neural measure of conflict monitoring is reduced in the 22q population relative to the typical population in the nonemotional GNG task.
However, additional evidence from the eGNG task suggests that both conflict monitoring and response inhibition were impacted by emotionally salient stimuli in participants with 22q relative to TD participants. During the eGNG, the TD participants showed approximately equal accuracy in the context of angry faces compared to the context of happy faces, but participants with 22q showed lower accuracy in the context of angry faces, leading to a significant interaction between diagnosis and emotion. Furthermore, participants with 22q exhibited larger anterior N2 responses than TD participants in the context of both angry and happy faces. Taken together, these results suggest that youth with 22q increase their conflict monitoring when they must distinguish between emotional faces and calm faces. This notion is supported by previous combined EEG-fMRI work which demonstrated that the emotional salience of a stimulus can impact conflict monitoring in the GNG task by recruiting additional resources in the ventral ACC, thereby speeding up the processing of conflict (Kanske & Kotz, 2011).
This idea is consistent with previous work showing that patients diagnosed with an anxiety disorder have larger anterior N2 components relative to nonanxious individuals during a nonemotional GNG task (Sehlmeyer et al., 2010), which suggests that anxiety may interact with conflict monitoring to adversely impact task performance. Furthermore, recent work from our laboratory has suggested that emotional faces impact youth with 22q differently to TD individuals (Popa et al., 2019). In a dot probe threat bias task, 7- to 17-year-old participants were asked to respond to a target appearing at one of two screen locations which could be preceded by emotional (angry or happy) or neutral facial expressions. An examination of gaze patterns between early and late trials demonstrated that TD children gradually shifted their gaze from the center of the screen toward other areas of the faces over time regardless of their emotional valence. In contrast, children with 22q demonstrated no such shifts in attention. This was interpreted as potential evidence that children with 22q are less able to habituate their emotional responses to the face stimuli and so are less able to freely explore their content. This was supported by pupillometry data which showed TD children exhibiting increased pupil contraction to happy faces following repeated exposure to them, which may signal a decrease in arousal/less effortful processing over time. Children with 22q showed no such change, suggesting they experienced a similar degree of arousal/effortful processing throughout the experiment. This work provides a potential explanation for the increased anterior N2 response observed in the eGNG, as participants with 22q may have experienced greater arousal and/or continued to engage in effortful processing of the emotional faces during the task.
According to our original thesis, elevated anxiety and stress may be interacting with cognitive and adaptive functioning impairments in 22q to increase allostatic load (i.e., the detrimental effects of too much stress) which may trigger the onset of psychosis in those most severely affected. The current study demonstrates evidence of significant impairments in a cognitive control process as well as increased anxiety and reduced adaptive functioning among youth with 22q relative to their TD peers. These findings are important because elevated anxiety and cognitive control impairments appear to precede the onset of psychosis in the general population (Gothelf et al., 2007, 2013; Gottesman & Gould, 2003; Lencz et al., 2006; Tang et al., 2017; Thoma et al., 2007). So, if elevated anxiety and cognitive control impairments are prevalent in the 22q population relative to TD individuals (which has been demonstrated in our study), these may be two key drivers of increased psychosis risk in this population. This idea is consistent with the Triple Network Model (Menon & Uddin, 2010), which suggests that an interaction between autonomic and cognitive-affective processes may drive the development of psychopathologies such as psychosis—driven by a salience network which detects, integrates, and filters relevant affective information via the anterior insula and ACC.
In those with 22q, we observed a number of significant relationships between the ERPs and measures of psychosis-proneness using the SIPS. First, a greater anterior N2 in the context of happy faces was associated with greater trouble with focus and attention. Given that the anterior N2 is believed to index conflict monitoring (Folstein & Van Petten, 2008) and that participants with 22q exhibited greater anterior N2 responses following the presentation of emotional faces, greater conflict monitoring may come at a cost of attentional focus in a subset of participants with 22q. Second, a greater LPP to happy and angry faces was associated with greater disorganized communication, which could suggest that greater sustained processing of emotional faces in 22q (as indexed by the LPP) is related to difficulties keeping track of their train of thought or communicating in a coherent manner. Third, a greater LPP to happy and angry faces was associated with lower social anhedonia, which could suggest that participants with higher social anhedonia/withdrawal may find it easier to disengage from the sustained attentional processing of these emotional faces or may be actively avoiding them altogether. Lastly, a greater LPP to happy faces was also associated with fewer issues with occupational functioning, which could mean that the ability to sustain attention to positively valenced faces is related to a better ability to perform well in school/work. These are exploratory analyses so our interpretations are currently highly speculative. However, these relationships will be followed up when participants return for their second visit, which should help us to determine whether these relationships persist with age and whether they worsen over time in a subset of participants with 22q.
Some limitations should be considered when evaluating our results. An important limitation of the study relates to differences in IQ between the two study groups. Participants with 22q had significantly lower Full Scale and Verbal IQ than their TD peers. This was expected, given that IQ scores will be lower in almost all individuals with 22q compared to a TD group (e.g., De Smedt et al., 2007), making it impossible to IQ-match participants across both groups. Although it may seem advisable to attempt to control for differences in IQ by entering it as a covariate in our analyses, there are strong and principled reasons for avoiding this in most neurodevelopmental studies (see Dennis et al., 2009, for full discussion). Specifically, the inclusion of a covariate in a linear model is a statistical method designed to minimize preexisting group differences in situations where group characteristics such as IQ occur by chance. However, lower IQ is an intrinsic characteristic of the 22q cognitive phenotype and is therefore not separable from the condition. Attempting to adjust group differences in neurocognitive function, which is another measure of intellectual ability, by covarying for IQ would reduce the group differences because of the shared variance between the predictor variable (22q vs TD) and the covariate (IQ), thus creating false negatives in the results. Such adjustments would violate the statistical assumption for covariate analysis that states that these variables should be independent of one another and would also go against widely cited arguments from Miller and Chapman (2001) that suggest groups should not differ on the level of the covariate. In line with these arguments, we suggest that IQ does not meet the requirements for a covariate in the analyses of neurocognitive function in youth with 22q presented in this study. Instead, we wish to highlight the complex role of IQ in neurocognitive and adaptive function (Angkustsiri et al., 2012) and ask readers to consider our findings within the context of reduced IQ in the 22q population.
In addition to the limitation concerning group differences in IQ, we did not observe a difference in the size of the LPP component between angry and happy Go trials. However, LPP amplitudes are generally greater for both unpleasant and pleasant images relative to neutral images, which suggests the LPP is typically related to arousal rather than to valence (Hajcak, Weinberg, MacNamara, & Foti, 2012). As such, it seems the lack of emotion effect on the LPP may be typical in this regard. Still, however, future work could examine this further by replicating the current study design and using stimuli from the International Affective Picture System, as these stimuli are more typically used for examining modulation of the LPP (Lang, Bradley, & Cuthbert, 1999, 2008). Finally, although we do report significant correlations between the LPP to angry and happy Go trials and a number of measures of anxiety, adaptive functioning, and psychosis-proneness using the SIPS, the lack of a significant LPP emotion effect suggests these correlations should be interpreted with some caution.
Regarding future work, our study participants are currently undergoing a second assessment 2.5 years after first taking part in this study. Results from their second assessment will be used to examine how their behavioral/ERP responses change and how such change may relate to a worsening of psychosis symptoms in the 22q population. From these data, we aim to identify a set of risk and protective factors for psychosis-proneness among youth with 22q. In addition, future ERP work could further isolate the neural process(es) underlying response inhibition impairments in 22q by examining group differences in a response-related ERP during the GNG task. For example, the error-related negativity component is believed to be sensitive to response conflict and may also arise from the ACC (Yeung, Botvinick, & Cohen, 2004), which may enable a more precise understanding of cognitive control impairments in 22q and the impact of emotionally salient stimuli.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank participants who took part in this study and their families. Thanks also go to Research Assistants Angela Bassal, Veena Do, Courtney Durdle, Danielle Harris, Hannah Morgan, Srey Luch Sam, Anthony Schmiedeler, and Postdoctoral Researcher Danessa Mayo for their involvement in data collection, data processing, and/or clinical interviewing. Funding for the current study was made possible by NIH grant R01-MH107018 PI (T.J.S.) and the NIH-funded MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125).
Funding information
MIND Institute Intellectual and Developmental Disabilities Research Center, Grant/Award Number: U54 HD079125; National Institutes of Health, Grant/Award Number: R01-MH107018
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
Data Availability Statement
The data that support the findings of this study will be openly available in NIMH Data Archive at https://nda.nih.gov, reference number C2303.
REFERENCES
- Angkustsiri K, Leckliter I, Tartaglia N, Beaton EA, Enriquez J, & Simon TJ (2012). An examination of the relationship of anxiety and intelligence to adaptive functioning in children with chromosome 22q11. 2 deletion syndrome. Journal of Developmental and Behavioral Pediatrics, 33(9), 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, & Skuse DH (2005). Adolescents and young adults with 22qll deletion syndrome: Psychopathology in an at-risk group. The British Journal of Psychiatry, 186(2), 115–120. [DOI] [PubMed] [Google Scholar]
- Bassett AS, & Chow EW (2008). Schizophrenia and 22q11. 2 deletion syndrome. Current Psychiatry Reports, 10(2), 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton EA, & Simon TJ (2011). How might stress contribute to increased risk for schizophrenia in children with chromosome 22q11. 2 deletion syndrome? Journal of Neurodevelopmental Disorders, 3(1), 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, & Verbaten MN (2005). Source analysis of the N2 in a cued Go/NoGo task. Brain Research. Cognitive Brain Research, 22(2), 221–231. 10.1016/j.cogbrainres.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Bish JP, Ferrante SM, McDonald-McGinn D, Zackai E, & Simon TJ (2005). Maladaptive conflict monitoring as evidence for executive dysfunction in children with chromosome 22q11. 2 deletion syndrome. Developmental Science, 8(1), 36–43. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. 10.1037/0033-295X.108.3.624 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Campbell L, McCabe K, Leadbeater K, Schall U, Loughland C, & Rich D (2010). Visual scanning of faces in 22q11.2 deletion syndrome: Attention to the mouth or the eyes? Psychiatry Research, 177, 211–215. 10.1016/j.psychres.2009.06.007 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, … Cohen JD (1997). A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience, 9(6), 835–847. [DOI] [PubMed] [Google Scholar]
- De Smedt B, Devriendt K, Fryns JP, Vogels A, Gewillig M, & Swillen A (2007). Intellectual abilities in a large sample of children with Velo-cardio-facial syndrome: An update. Journal of Intellectual Disability Research, 51, 666–670. 10.1111/j.1365-2788.2007.00955.x [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15(3), 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FCL, & van Boxtel GJM (2004). The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition, 56(2), 165–176. 10.1016/j.bandc.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Fabbro A, Rizzi E, Schneider M, Debbane M, & Eliez S (2012). Depression and anxiety disorders in children and adolescents with velo-cardio-facial syndrome (VCFS). European Child & Adolescent Psychiatry, 21(7), 379–85. [DOI] [PubMed] [Google Scholar]
- Folstein JR, & Van Petten C (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology, 45(1), 152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, … Reiss AL (2007). Risk factors for the emergence of psychotic disorders in adolescents with 22q11. 2 deletion syndrome. American Journal of Psychiatry, 164(4), 663–669. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, … Reiss AL (2007). Risk factors for the emergence of psychotic disorders in adolescents with 22q11. 2 deletion syndrome. American Journal of Psychiatry, 164(4), 663–669. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Schneider M, Green T, Debbané M, Frisch A, Glaser B, … Eliez S (2013). Risk factors and the evolution of psychosis in 22q11. 2 deletion syndrome: A longitudinal 2-site study. Journal of the American Academy of Child & Adolescent Psychiatry, 52(11), 1192–1203. e3. [DOI] [PubMed] [Google Scholar]
- Gottesman II, & Gould TD (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry, 160(4), 636–645. [DOI] [PubMed] [Google Scholar]
- Grati FR, Molina Gomes D, Ferreira JCPB, Dupont C, Alesi V, Gouas L, … Vega AG (2015). Prevalence of recurrent pathogenic microdeletions and microduplications in over 9500 pregnancies. Prenatal Diagnosis, 35(8), 801–809. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, … Eliez S (2009). Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11. 2 deletion) syndrome. Journal of the American Academy of Child & Adolescent Psychiatry, 48(11), 1060–1068. [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Dennis TA (2009). Brain potentials during affective picture processing in children. Biological Psychology, 80(3), 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, & Foti D (2009). Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology, 120(3), 505–510. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, & Foti D (2012). ERPs and the study of emotion. In Kappenman ES & Luck SJ (Eds.), The Oxford handbook of event-related potential components (Vol. 441, p. 474). New York, NY: Oxford University Press. [Google Scholar]
- Hare TA, & Casey BJ (2005). The neurobiology and development of cognitive and affective control. Brain, 9(3), 273–286. [Google Scholar]
- Harrison P, & Oakland T (2003). Manual for the adaptive behavior assessment system. San Antonio: The Psychological Corporation. [Google Scholar]
- Jolin EM, Weller RA, & Weller EB (2012). Occurrence of affective disorders compared to other psychiatric disorders in children and adolescents with 22q11. 2 deletion syndrome. Journal of Affective Disorders, 136(3), 222–228. [DOI] [PubMed] [Google Scholar]
- Kanske P, & Kotz SA (2011). Emotion speeds up conflict resolution: A new role for the ventral anterior cingulate cortex? Cerebral Cortex, 21, 911–919. 10.1093/cercor/bhq157 [DOI] [PubMed] [Google Scholar]
- Kappenman ES, MacNamara A, & Proudfit GH (2014). Electrocortical evidence for rapid allocation of attention to threat in the dot-probe task. Social Cognitive and Affective Neuroscience, 10(4), 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrynski LJ, & Sullivan KE (2007). Velocardiofacial syndrome, DiGeorge syndrome: The chromosome 22q11.2 deletion syndromes. The Lancet, 370, 1443–1452 10.1016/S0140-6736(07)61601-8 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1999). International affective picture system (IAPS): Instruction manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida. [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, & Cornblatt BA (2006). Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biological Psychiatry, 59(9), 863–871. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Shashi V, Berry PM, & Kwapil TR (2007). Schizophrenic-like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 144(1), 27–36. [DOI] [PubMed] [Google Scholar]
- Loewy RL, Bearden CE, Johnson JK, Raine A, & Cannon TD (2005). The prodromal questionnaire (PQ): Preliminary validation of a self-report screening measure for prodromal and psychotic syndromes. Schizophrenia Research, 79(1), 117–125. 10.1016/j.schres.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Loewy RL, Pearson R, Vinogradov S, Bearden CE, & Cannon TD (2011). Psychosis risk screening with the prodromal questionnaire—Brief version (PQ-B). Schizophrenia Research, 129(1), 42–46. 10.1016/j.schres.2011.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, & Gaspelin N (2017). How to get statistically significant effects in any ERP experiment (and why you shouldn’t). Psychophysiology, 54(1), 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T, Miller T, Woods S, Rosen J, Hoffman R, & Davidson L (2001). Structured interview for prodromal syndromes. New Haven, CT: PRIME Research Clinic, Yale School of Medicine. [Google Scholar]
- Mekori-Domachevsky E, Guri Y, Yi J, Weisman O, Calkins ME, Tang SX, … Gothelf D (2017). Negative subthreshold psychotic symptoms distinguish 22q11.2 deletion syndrome from other neurodevelopmental disorders: A two-site study. Schizophrenia Research, 188, 42–49. 10.1016/j.schres.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK (2000). The prefontral cortex and cognitive control. Nature Reviews Neuroscience, 1, 59–65. [DOI] [PubMed] [Google Scholar]
- Miller GA, & Chapman JP (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110(1), 40–48. [DOI] [PubMed] [Google Scholar]
- Poirsier C, Besseau-Ayasse J, Schluth-Bolard C, Toutain J, Missirian C, Le Caignec C, … Catty M (2016). A French multicenter study of over 700 patients with 22q11 deletions diagnosed using FISH or aCGH. European Journal of Human Genetics, 24(6), 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J (2012). Neuropsychology of P300. In Kappenman ES & Luck SJ (Eds.), The Oxford handbook of event-related potential components (pp. 159–188). New York, NY: Oxford University Press. [Google Scholar]
- Popa A, Cruz J, Wong LM, Harvey D, Angkustsiri K, Leckliter I, … Simon TJ (2019). Seeing eye to eye with threat: Atypical threat bias in children with 22q11.2 deletion syndrome. American Journal on Intellectual and Developmental Disabilities, 124, 549–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi S, Mecacci L, & Viggiano MP (2009). Anxiety, cognitive self-evaluation and performance: ERP correlates. Journal of Anxiety Disorders, 23, 1132–1138. 10.1016/j.janxdis.2009.07.018 [DOI] [PubMed] [Google Scholar]
- Sanders AF, Hobbs DA, Stephenson DD, Laird RD, & Beaton EA (2017). Working memory impairments in chromosome 22q11. 2 deletion syndrome: The roles of anxiety and stress physiology. Journal of Autism and Developmental Disorders, 47(4), 992–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Armando M, Schultze-Lutter F, Pontillo M, Vicari S, Debbané M, & Eliez S (2018). Prevalence, course and psychosis-predictive value of negative symptoms in 22q11. 2 deletion syndrome. Schizophrenia Research, 206, 386–393. [DOI] [PubMed] [Google Scholar]
- Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, Van Den Bree MBM, … Kates WR (2014). Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: Results from the international consortium on brain and behavior in 22q11.2 deletion syndrome. American Journal of Psychiatry, 171(6), 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Konrad C, Zwitserlood P, Arolt V, Falkenstein M, & Beste C (2010). ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia, 48(9), 2488–2495. [DOI] [PubMed] [Google Scholar]
- Shapiro HM, Tassone F, Choudhary NS, & Simon TJ (2014). The development of cognitive control in children with chromosome 22q11. 2 deletion syndrome. Frontiers in Psychology, 5, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro HM, Wong LM, & Simon TJ (2013). A cross-sectional analysis of the development of response inhibition in children with chromosome 22q11. 2 deletion syndrome. Frontiers in Psychiatry, 4, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ (2008). Velo-cardio-facial syndrome: 30 years of study. Developmental Disabilities Research Reviews, 14, 3–10. 10.1002/ddrr.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ (2008). A new account of the neurocognitive foundations of impairments in space, time, and number processing in children with chromosome 22q11. 2 deletion syndrome. Developmental Disabilities Research Reviews, 14(1), 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bish JP, Bearden CE, Ding L, Ferrante S, Nguyen V, … Emanuel BS (2005). A multilevel analysis of cognitive dysfunction and psychopathology associated with chromosome 22q11.2 deletion syndrome in children. Development and Psychopathology, 17, 753–784. 10.1017/S0954579405050364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, & Karayiorgou M (2005). Lower prepulse inhibition in children with the 22q11 deletion syndrome. American Journal of Psychiatry, 162(6), 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, & Casey BJ (2010). Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology, 20(2), 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, & Casey BJ (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH (1998). A measure of anxiety symptoms among children. Behaviour Research and Therapy, 36(5), 545–566. [DOI] [PubMed] [Google Scholar]
- Stephenson DD, Beaton EA, Weems CF, Angkustsiri K, & Simon TJ (2015). Identifying patterns of anxiety and depression in children with chromosome 22q11. 2 deletion syndrome: Comorbidity predicts behavioral difficulties and impaired functional communications. Behavioural Brain Research, 276, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, Moore TM, Calkins ME, Yi JJ, McDonald-McGinn DM, Zackai EH, … Gur RE (2017). Emergent, remitted and persistent psychosis-spectrum symptoms in 22q11.2 deletion syndrome. Translational Psychiatry, 7(7), e1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Wiebel B, & Daum I (2007). Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophrenia Research, 92(1–3), 168–180. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, & Nelson CA (2002). Categorization of facial expressions in children and adults: Establishing a larger stimulus set. Journal of Cognitive Neuroscience, 14, 74. [Google Scholar]
- Tottenham N, Hare TA, & Casey BJ (2011). Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Frontiers in Psychology, 2, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JA, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, … Hooper SR (2015). Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry, 72(4), 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Beemer FA, Swaab H, … van EngelanD H (2006). The 22q11.2 deletion in children: High rate of autistic disorders and early onset of psychotic symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 45(9), 1104–1113. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp. [Google Scholar]
- Wechsler D (2014). WISC-V: Technical and interpretive manual. Bloomington, MN: Pearson. [Google Scholar]
- Yeung N, Botvinick MM, & Cohen JD (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931–959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study will be openly available in NIMH Data Archive at https://nda.nih.gov, reference number C2303.


