Abstract
Objective
We investigated the safety of a novel interlobar fissure division technique using the da Vinci vessel sealing system in robot-assisted pulmonary lobectomy.
Methods
The medical records of patients who underwent robotic pulmonary lobectomy with node dissection for primary lung cancer between 2018 and 2020 were reviewed. The inclusion criteria were fulfilled by 111 patients, whose perioperative factors and postoperative results were compared with those previously reported. Furthermore, the new robotic lung interlobar division technique using the da Vinci vessel sealing system without a robotic stapler was evaluated in patients with low-grade incomplete fissure. We considered the Craig and Walker classification of lung fissures grades 1 and 2 as a good adaptation for the vessel sealing system interlobar fissure division.
Results
The vessel sealing system group had shorter mean operative and console times (P = .03 and P = .01, respectively) and lesser median intraoperative blood loss (20 mL vs 50 mL; P = .01). The vessel sealing system group had lower surgical complication rates (2.2% vs 20.0%; P = .01). The incidence of persistent postoperative air leak was lower (0% vs 10.0%; P = .06), and fewer robotic stapler cartridges were used during surgery (3.4 vs 5.6; P < .001) in the vessel sealing system group than in the stapler group.
Conclusions
We report the safety of using the da Vinci vessel sealing system as an alternative to the use of robotic staples for interlobar fissure division in robot-assisted pulmonary lobectomy. This technique seems easy and feasible though limited to the low-grade incomplete fissure.
Key Words: RATS, lung cancer, lung resection, fused fissure, fissure division
Abbreviations and Acronyms: CT, computed tomography; LN, lymph node; RATS, robot-assisted thoracic surgery; VATS, video-assisted thoracic surgery; VSS, vessel sealing systems
Robotic lung interlobar division using the da Vinci vessel-sealing system without staplers.
Central Message.
The new robotic lung interlobar division using the da Vinci vessel-sealing system without a stapler seems feasible in limited patients with low-grade incomplete fissure.
Perspective.
Safe fissure division is crucial in surgical treatment of lung cancer. To the best of our knowledge, this is the first report to apply the VSS fissure division in RATS lobectomy. We observed a shorter operative time in the VSS group, with a decrease in complications and a tendency for reduced air leak. It should be noted that this study was limited to patients with low-grade incomplete fissure.
See Commentary on page 217.
The major advantages of robot-assisted thoracic surgery (RATS) are the excellent field of view enabled by high-precision 3D images and the maneuverability provided by robotic arms that allow precise movement even in a narrow surgical field. In video-assisted thoracic surgery (VATS), the assistant operates the camera, but it might be challenging to achieve the operative field desired by the operator. Therefore, RATS might contribute to surgical safety. RATS is still a relatively new technique, and there are only few high-quality large-scale randomized trials that have tested RATS. Although retrospective studies that compared RATS and VATS are increasing, the results are conflicting, and many reports include a small number of cases.1, 2, 3 With the rapid increase in the number of robot-assisted thoracic surgeries, new and useful devices such as da Vinci staplers and vessel sealing systems (VSS) have been developed. However, the surgical techniques using these devices need more exploration. In RATS, the console surgeon can perform the procedure with excellent maneuverability provided by the robot arms. However, it is difficult for the assistant to perform procedures blocked by the robotic arms, thereby lacking the control of the video camera even when compared with VATS. To address this shortcoming, we performed solo surgery by the operator (RATS solo lobectomy), which has lesser dependence on assistants in the surgical field.
In lobectomy, the interlobar fissure is usually divided with mechanical staplers or monopolar electrocautery, whereas few reports have described an interlobar fissure division technique with VSS. Many studies indicate that RATS is more expensive than VATS or thoracotomy, and reducing the number of mechanical staplers used is an easy way to reduce the cost of RATS lobectomy. To reduce the assistant's stapler use, we used the da Vinci VSS for fissure division in selected patients with low-grade incomplete fissure.
We hypothesized that the use of da Vinci VSS for fissure division in selected patients would be safe and would not increase the air leakage. Therefore, in this study we evaluated the perioperative outcomes of our novel method of RATS lobectomy for lung cancer using the da Vinci VSS.
Methods
Patients and Study Design
Our institutional review board approved the study protocol and waived the need for written informed consent (number 322-265, approval date: February 4, 2021). A retrospective analysis was conducted on 111 patients between April 2018 and June 2021. All patients underwent pulmonary lobectomy with node dissection for non–small cell lung cancer. Patients who underwent sublobar resection, including segmentectomy, were excluded from the study. Medical records of the patients were reviewed and the following characteristics were recorded: age, sex, body height, body mass index, tumor location, extent of node dissection, smoking status, interlobar fissure completeness, number of stapler cartridges, p stage, intraoperative blood loss, operative time, chest tube drainage duration, length of postoperative stay, and morbidity for the entire study period. The fissures were prospectively graded by the surgeon at the time of surgery. In our surgical database, we record the information about the lobe fissure completeness with Craig and Walker classification. We have used these data in this study.
To further elucidate the effectiveness of interlobar fissure division with VSS in RATS lobectomy, we divided the 76 patients into 2 groups after excluding the patients who had thick incomplete fissure with Craig and Walker classification grades of 3, 4, and 1 who did not need interlobar division (Table 1).4 After ports placements, the completeness of a fissure was evaluated. They were grouped into 4 stages according to Craig and Walker classification: grade 1, complete fissure with entirely separate lobes; grade 2, complete visceral cleft but parenchymal fusion at the base of the fissure; grade 3, visceral cleft evident on part of the fissure; and grade 4, complete fusion of the lobes with no evident fissure line. The 2 groups were as follows: the VSS (n = 46) and stapler (n = 30) groups. We then compared patient characteristics and outcomes.
Table 1.
Demographic characteristics of patients with Craig and Walker grade 2 fissure (N = 76)
| VSS (n = 46) | Staple (n = 30) | P value | |
|---|---|---|---|
| Age, y | 70 (66-74) | 70 (61-74) | .510 |
| Sex, male/female | 22/24 | 18/12 | .352 |
| Height, cm | 161 (155-167) | 164 (159-168) | .164 |
| BMI | 23.2 (21.2-25.5) | 23.3 (20.7-25.5) | .392 |
| Smoking status | .806 | ||
| Ever | 32 | 20 | |
| Never | 14 | 10 | |
| VC, % | 111 (105-121) | 114 (104-124) | .820 |
| FEV1, % | 74.7 (71.3-80.7) | 74.0 (68.7-78.1) | .411 |
| COPD | 7 (15.2) | 9 (30.0) | .154 |
| ILD | 4 (8.7) | 3 (10.0) | .447 |
| Tumor location | .161 | ||
| RUL | 13 | 15 | |
| RML | 3 | 3 | |
| RLL | 15 | 3 | |
| LUL | 10 | 6 | |
| LLL | 5 | 3 |
Data are presented as mean (interquartile range), n (%), or n, except where otherwise noted. VSS, Vessel-sealing system; BMI, body mass index; VC, vital capacity; FEV1, forced expiratory volume in 1 second; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
The indications for RATS lobectomy were clinical T1 to T3, N0 to N1, and M0. All patients underwent preoperative assessments comprised of chest computed tomography (CT) imaging, standard hematology and blood chemistry, cardiological examination, pulmonary function test, brain magnetic resonance imaging or CT, and bronchoscopy. Positron emission tomography (PET)/CT was performed in almost all patients, and patients who did not undergo positron emission tomography/CT and abdominal ultrasonography underwent bone scanning. We evaluated the perioperative outcomes of our novel method to divide the interlobar fissure using the da Vinci VSS in RATS lobectomy.
Surgical Procedure
At our institution, RATS is usually performed via a single utility incision (30-40 mm) at the level of the fourth intercostal space and 4 ports. Surgery was performed with patients under general anesthesia with selective lung ventilation, and lobectomy was performed basically using 4-arm robotic techniques as described by Cerfolio.5 The assistant surgeon was positioned on the ventral side of the patient. The pulmonary veins, larger pulmonary arteries, and bronchus were divided by the robotic stapler by the operator in all cases. In initial cases, we used the handheld staplers to divide the bronchus, which was performed by the assistant. Using the vessel sealer, the small pulmonary artery branch and lymph node (LN) dissection areas were sealed in all cases. Our VATS technique is similar to an open resection (through the fissure first, and dissecting along the pulmonary artery). After ports placements, the surgeon prospectively graded the fissures. In limited patients who had almost complete fissure, the interlobar fissure was divided by the vessel sealer (Intuitive Surgical). In our methods, the interlobar division line is placed on the resected lobe at 1 to 2 mm away from the actual interlobar line (Figure 1, Video 1). The method cannot be used when no interlobar line is observed, and the tumor is located near the interlobar fissure. Even in such cases, we used the VSS for the remainder of the surgery. Thick incomplete fissures were divided by the robotic staplers or a combination of staplers and the da Vinci VSS as deemed appropriate (these patients constituted the stapler group). Considering safety, it was necessary to change to stapler when sealing was incomplete, and there was a concern about air leakage when using VSS. We emphasize that the decision regarding the use of VSS versus stapling was at the discretion of the console surgeon. Bertolaccini and colleagues6 reported that fissure division with LigaSure (Medtronic) was safely performed in VATS lobectomy with no cases of mortality or major complications in 50 consecutive patients. Therefore, we used the LigaSure for fissure division in VATS lobectomy.6,7 Therefore, we decided to apply this technique to RATS lobectomy. We considered the Craig and Walker classification of lung fissures grades 1 and 2 as a good adaptation for the VSS interlobar fissure division. We had no previous experience of surgery with the da Vinci S; therefore, this study was limited to surgery with the da Vinci Xi. Mediastinal node dissection was performed at a lobar-specific station. Upper and lower mediastinal LN dissection were performed in cases N1 node metastasis was confirmed by frozen section diagnosis. At the end of the surgery, air leakage was checked by submerging the resected lung in sterile warm saline, followed by inflation with a pressure of 20 cm H2O. Air leak was controlled with suturing, soft coagulation, or fibrin glue reinforcement.8 Postoperative analgesia was administered using an epidural catheter.
Figure 1.
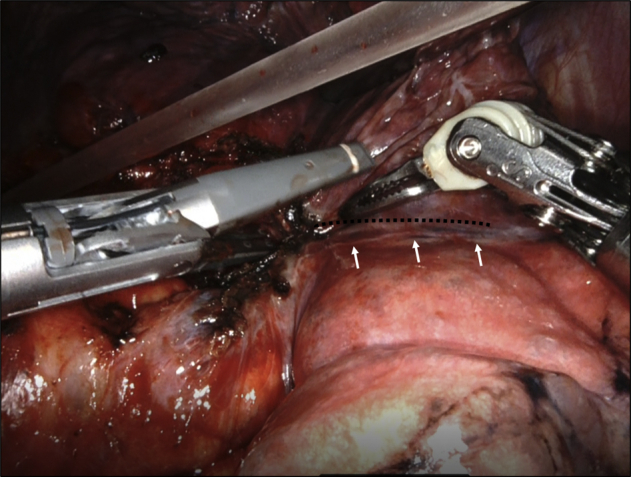
Lung interlobar fissure division using the vessel sealer in a Craig and Walker classification grade 2 case during right upper lobectomy. Black dotted line indicates the division line. White arrows indicate the actual interlobar fissure.
Perioperative Management and Definition of Postoperative Complications
Aspiration pressure of 5 cm H2O was applied on the chest tube. The chest tube was removed when no air leak was observed, and nonchylous and nonbloody discharge was <2 cc/kg in 8 hours or 6 cc/kg per day. Postoperative persistent air leak was defined as a continuous air leak for more than 5 days. Complications were defined as grade III or higher according to the Clavien–Dindo classification.9 Complications were included for analysis if the complications occurred within 30 days from the date of surgery or during the same admission, whichever was longer. Complications were further grouped as major or minor complications.
Cost Evaluation
Actual costs for disposables, operating room use, and daily hospital costs were obtained by the hospital cost center (Table 2). Data on disposables use in the operating room were retrospectively collected. Cost of buying/renting nondisposable tools (videoscope, monitors, surgical instruments, etc) was not included considering these are equipment usually available in all the thoracic surgeries. The total cost and medical material cost of patients were calculated and assessed. The difference between VSS group (n = 46) and the stapler group (n = 30) was evaluated.
Table 2.
Cost-related outcomes of the patients with fused fissure (N = 76)
| VSS (n = 46) | Staple (n = 30) | P value | |
|---|---|---|---|
| Postoperative stay, d | 9 (7-10) | 10 (7-11) | .321 |
| Mean number of stapler cartridges used ± SD | 3.4 ± 1.0 | 5.6 ± 1.5 | <.001 |
| Mean number of stapler cartridges used for interlobar division ± SD | 0 | 2.0 ± 1.3 | <.001 |
| Cost | |||
| Material cost | 4241 (3965-4505) | 4867 (4665-5242) | <.001 |
| Total cost | 16,275 (15,604-16,885) | 16,955 (16,668-17,524) | <.01 |
Data are presented as mean (interquartile range) except where otherwise noted. All of the direct medical costs related to the procedure were calculated in yen and converted to US dollars considering 1 dollar = 110 yen. VSS, Vessel-sealing system; SD, standard deviation.
Statistical Analysis
The descriptive statistics are summarized as median or mean for continuous variables, and as frequency and percentage for categorical variables. All statistical analyses were performed using SPSS for Windows (version 22.0; SPSS, Inc). Mann–Whitney U test, χ2 test, or Fisher exact test were used for analysis.
Results
Characteristics of the Patients With Fused Fissure
To further elucidate the effectiveness of interlobar fissure division with VSS in RATS lobectomy, we divided 76 patients into 2 groups after excluding 7 patients with a thick incomplete fissure in Craig and Walker classification grades 3 and 4. The 2 groups were the VSS group (n = 46) and the stapler group (n = 30). We also excluded another 28 patients with grade 1 classification who did not need fissure division. The average pulmonary function (percent volume capacity) was similar to that of the usual cases. Chronic obstructive pulmonary disease and interstitial lung disease were present in 15.2% and 8.7% of the VSS group, and 30.0% and 10.0% of the stapler group, respectively. Age, sex, body mass index, smoking status, forced expiratory volume in 1 second, and primary lobe were well balanced between the groups (Table 1).
Surgery-Related Outcomes of Patients With Fused Fissure
Table 3 shows a summary of the surgery-related outcomes of patients with fused fissures. The mean operative time and console time were shorter in the VSS group (200 minutes vs 225 minutes [P = .030] and 132 minutes vs 158 minutes [P = .012], respectively). The median intraoperative blood loss was reduced in the VSS group (20 mL vs 50 mL; P = .011). The surgical complication rates were also reduced in the VSS group (2.2% vs 20.0%; P = .013). Other surgery-related outcomes were as follows: mean chest tube duration, 1.4 days versus 1.8 days (P = .076); incidence of persistent air leak, 0% versus 10.0% (P = .058); intervention for air leak control, 2.2% versus 6.7% (P = .558) in the VSS and stapler groups, respectively.
Table 3.
Surgery-related outcomes of the patients with fused fissure (N = 76)
| VSS (n = 46) | Staple (n = 30) | P value | |
|---|---|---|---|
| Operation time, min | 200 (174-227) | 225 (196-256) | .030 |
| Console time, min | 132 (112-150) | 158 (136-168) | .012 |
| Bleeding amount, mL | 20 (5-35) | 50 (6-65) | .011 |
| Mean duration of chest tube ± SD | 1.4 ± 0.8 | 1.8 ± 1.2 | .076 |
| Postoperative complication | 1 (2.2) | 6 (20.0) | .013 |
| Major | |||
| Cerebral infarction | 1 | ||
| Arrhythmia | 1 | ||
| Minor | |||
| Pneumonia | 1 | ||
| Pneumothorax | 1 | ||
| Air leak | 1 | 3 | |
| Persistent postoperative air leak | 0 (0) | 3 (10.0) | .058 |
| Intervention for air leak | 1 (2.2) | 2 (6.7) | .558 |
Data are presented as mean (interquartile range), n (%), or n, except where otherwise noted. VSS, Vessel-sealing system; SD, standard deviation.
Cost-Related Outcomes of Patients With Fused Fissure
Table 2 shows a summary of the cost-related outcomes of patients with fused fissures. The median postoperative stay were not different between 2 groups, 9 days versus 10 days (P = .321). The total number of robotic stapler cartridges used during surgery were reduced in the VSS group, 3.4 versus 5.6 (P < .001). Regarding the medical cost, material cost, and total cost were reduced in the VSS group ($4241 vs $4867 [P < .001] and $16,275 vs $16,955 [P < .01], respectively).
Discussion
Thoracoscopic lung resection for lung cancer has shown improved perioperative outcomes similar to those of open thoracotomy.10,11 The major advantages of RATS are the unobstructed field of view provided by high-precision 3D images and the excellent operability provided by the robot arms, which enable precise movement in a narrow surgical field.8 These features of RATS allow thorough LN dissection, which is technically demanding, relatively easy. However, limited and conflicting perioperative outcomes have been reported for different cohorts.2,3
We compared our surgical results with those of previous studies on robotic lobectomy for lung cancer.12, 13, 14, 15, 16 We had no conversion to thoracotomy in this study (previous studies: 7.5%-15.8%; this study: 0%). This might have been affected because this study consists of our initial experience with robotic surgery. Our results also showed the lowest morbidity among these studies (previous: 10.5%-28.3%; this study: 9.9%). We confirmed that robotic lobectomy for lung cancer could be performed safely. Although the duration of hospital stay might appear to be long for lung resection, it is shorter than 15.5 days, which was reported by the 2020 Japanese Hospital Intelligence Agency database.17 In Japan, the National Health Insurance covers almost 90% of the cost of a patient's hospital stay for lung cancer resection. The duration of hospitalization gradually decreased according to the national medical cost reduction policy.
While using the handheld stapler in robotic lobectomy, the assistant experienced considerable difficulty when the operative field was blocked by the robotic arms. These difficult and time-consuming procedures can be eliminated with RATS solo lobectomy. Considering the da Vinci camera, it is not completely flexible compared with the VATS scope, which could be inserted at every port or via minithoracotomy and it can obtain a field of view with no blind spots without any restrictions.
Furthermore, tunneling and stapler insertion can be technically demanding in some cases according to the length of the stapler. Therefore, if the VSS division does not increase complications or prolong air leaks, it should be preferred for interlobar fissure division in robotic lobectomy.
Sakuragi and colleagues18 used another vessel-sealing technology (BiClamp bipolar coagulation forceps; Erbe Elektromedizin GmbH) for dividing the lung parenchyma in 60 patients with fused fissures during VATS lobectomy and compared them with stapler-divided cases. In that study, the rates of prolonged air leak and pneumonia were similar different between both groups (6.9% and 3.4% in the staple group vs 10.6% and 9.1% in the BiClamp group). They divided the interlobar fissure straight, and the division line might include the part of the lobe that should be preserved. In our novel method, using VSS, the interlobar division line is placed on the resected lobe at 1 to 2 mm away from the actual interlobar line. This strategy might have reduced the complications and the tendency for reduced air leak in the VSS fissure division.
We have also reported that VSS for lung cancer resection could decrease chest tube duration, blood loss, and incidence of chylothorax in VATS lobectomy.19 To the best of our knowledge, this is the first report to apply the VSS fissure division in RATS lobectomy. In this study, we observed a shorter operative time in the VSS group, with a decrease in complications and a tendency for reduced air leak. Moreover, the number of stapler cartridges used was reduced by approximately 2 cartridges in the VSS group. Both of these could contribute to reducing the cost of robot-assisted surgery. We performed simple cost analysis and the material cost was lower by approximately $626 for the VSS group, probably because of the lesser number of stapler cartridges used (5.6 vs 3.4); it should be noted that stapler cartridges are expensive in Japan (approximately $250). The total cost for the VSS group was lower by approximately $680.
This study has some limitations. First, the results were on the basis of retrospective data obtained by reviewing medical records; prospective randomized studies are required to further validate application of robotic pulmonary resections. Second, we divided the almost complete fissure using VSS and chose the stapler for thick incomplete fissure. However, the discretion of the surgeon regarding the use of VSS or stapler could be detrimental for each procedure. Therefore, the inherent selection bias cannot be eliminated. Third, the robotic data in our series included the initial experience, which could skew the outcome. Prospective randomized studies at experienced centers are required to further validate the application of RATS lobectomy.
Conclusions
We report herein the safety of the use of da Vinci VSS as an alternative to robotic staples for interlobar fissure division in RATS pulmonary lobectomy in selected patients. This interlobar fissure division technique seems easy and feasible, although it is limited to the patients with low-grade incomplete fissure. We also confirmed that robotic lobectomy for lung cancer could be performed safely and effectively.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Lung interlobar fissure division using the vessel sealer in Craig and Walker classification grade 2 case during left lower lobectomy. The interlobar division line is placed on the resected lobe at 1-2 mm away from the actual interlobar line. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00125-0/fulltext.
References
- 1.Farivar A.S., Cerfolio R.J., Vallières E., Knight A.W., Bryant A., Lingala V., et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10–15. doi: 10.1097/IMI.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 2.Louie B.E., Farivar A.S., Aye R.W., Vallières E. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg. 2012;93:1598–1604. doi: 10.1016/j.athoracsur.2012.01.067. discussion: 1604-5. [DOI] [PubMed] [Google Scholar]
- 3.Jang H.J., Lee H.S., Park S.Y., Zo J.I. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single institution case series matching study. Innovations (Phila) 2011;6:305–310. doi: 10.1097/IMI.0b013e3182378b4c. [DOI] [PubMed] [Google Scholar]
- 4.Walker W.S., Craig S.R. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb. 1997;42:233–234. [PubMed] [Google Scholar]
- 5.Cerfolio R.J. Total port approach for robotic lobectomy. Thorac Surg Clin. 2014;24:151–156. doi: 10.1016/j.thorsurg.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Bertolaccini L., Viti A., Cavallo A., Terzi A. Results of Li-Tho trial: a prospective randomized study on the effectiveness of LigaSure® in lung resections. Eur J Cardiothorac Surg. 2014;45:693–698. doi: 10.1093/ejcts/ezt445. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe A., Miyajima M., Kawaharada N., Higami T. Two separate thoroscopic segmentectomies with vessel sealing system. Eur J Cardiothorac Surg. 2012;41:e62–e64. doi: 10.1093/ejcts/ezr332. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y., Saito A., Sakuma Y., Tada M., Maki R., Takahashi M., et al. Treatment of air leakage using the VIO soft coagulation system: a mouse pulmonary air leak model. Surg Today. 2021;51:1521–1529. doi: 10.1007/s00595-021-02251-3. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceppa D.P., Kosinski A.S., Berry M.F., Tong B.C., Harpole D.H., Mitchell J.D., et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg. 2012;256:487–493. doi: 10.1097/SLA.0b013e318265819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith C.B., Kale M., Mhango G., Neugut A.I., Hershman D.L., Mandeli J.P., et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol. 2014;9:383–389. doi: 10.1097/JTO.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 12.Park B.J., Flores R.M., Rusch V.W. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg. 2006;131:54–59. doi: 10.1016/j.jtcvs.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Cerfolio R.J., Bryant A.S., Skylizard L., Minnich D.J. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg. 2011;142:740–746. doi: 10.1016/j.jtcvs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi G., Galetta D., Maisonneuve P., Melfi F., Schmid R.A., Borri A., et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg. 2010;140:19–25. doi: 10.1016/j.jtcvs.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Giulianotti P.C., Buchs N.C., Caravaglios G., Bianco F.M. Robot-assisted lung resection: outcomes and technical details. Interact Cardiovasc Thorac Surg. 2010;11:388–392. doi: 10.1510/icvts.2010.239541. [DOI] [PubMed] [Google Scholar]
- 16.Nelson D.B., Mehran R.J., Mitchell K.G., Rajaram R., Correa A.M., Bassett R.L., Jr., et al. Robotic-assisted lobectomy for non-small cell lung cancer: a comprehensive institutional experience. Ann Thorac Surg. 2019;108:370–376. doi: 10.1016/j.athoracsur.2019.03.051. [DOI] [PubMed] [Google Scholar]
- 17.Hospital intelligence agency Diagnosis procedure combination statistics. https://hospia.jp/dpc
- 18.Sakuragi T., Takeda Y., Teishikata T., Sakoda K., Morita S. Is bipolar thermofusion an acceptable option for unseparated interlobar fissure division in pulmonary lobectomy? Interact Cardiovasc Thorac Surg. 2013;17:26–31. doi: 10.1093/icvts/ivt113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyajima M., Maki R., Tada M., Tsuruta K., Takahashi Y., Arai W., et al. Vessel sealing system for video-assisted lung resection for cancer reduces chylothorax and bleeding. J Thorac Dis. 2021;13:3458–3466. doi: 10.21037/jtd-21-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lung interlobar fissure division using the vessel sealer in Craig and Walker classification grade 2 case during left lower lobectomy. The interlobar division line is placed on the resected lobe at 1-2 mm away from the actual interlobar line. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00125-0/fulltext.


