Abstract
Benefits of the omega-3 polyunsaturated fatty acids (PUFA) on a number of clinical disorders, including autoimmune diseases, are widely reported in the literature. One major dietary source of PUFA are fish, particularly the small oily fish, like anchovy, sardine, mackerel and others. Unfortunately, fish (particularly the large, top-predator fish like swordfish) are also a source of pollutants, including the heavy metals. One relevant heavy metal is mercury, a known environmental trigger of autoimmunity that is measurable inside the thyroid. There are a number of interactions between the omega-3 PUFA and thyroid hormones, even at the level of the thyroid hormone transport proteins. Concerning the mechanisms behind the protection from/amelioration of autoimmune diseases, including thyroiditis, that are caused by the omega-3 PUFA, one can be the decreased production of chemokines, a decrease that was reported in the literature for other nutraceuticals. Recent studies point also to the involvement of resolvins. The intracellular increase in resolvins is associated with the tissue protection from inflammation that was observed in experimental animals after coadministration of omega-3 PUFA and thyroid hormone. After having presented data on fish consumption at the beginning, we conclude our review by presenting data on the market of the dietary supplements/nutraceuticals. The global omega-3 products market was valued at USD 2.10 billion in 2020, and was projected to go up at a compound annual growth rate of 7.8% from 2020 to 2028. Among supplements, fish oils, which are derived mainly from anchovies, are considered the best and generally safest source of omega-3. Taking into account (i) the anti-autoimmunity and anti-cancer properties of the omega-3 PUFA, (ii) the increasing incidence of both autoimmune thyroiditis and thyroid cancer worldwide, (iii) the predisposing role for thyroid cancer exerted by autoimmune thyroiditis, and (iv) the risk for developing metabolic and cardiovascular disorders conferred by both elevated/trendwise elevated serum TSH levels and thyroid autoimmunity, then there is enough rationale for the omega-3 PUFA as measures to contrast the appearance and/or duration of Hashimoto’s thyroiditis as well as to correct the slightly elevated serum TSH levels of subclinical hypothyroidism.
Keywords: fish, nutraceuticals, omega-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), autoimmune thyroid diseases, endocrine disruptors, mercury
Introduction
After having reminded the benefits of the omega-3 polyunsaturated fatty acids (PUFA) on several autoimmune diseases, we aim to review their benefits in the thyroid setting both at experimental and clinical levels. We start our work by providing a snapshot of fish consumption since fishes represent a major dietary source of omega-3 PUFA. The other side of the coin (or the other face of Janus, upon comparing fish to this god of the Ancient Roman mythology) is that eating fish means also “eating pollutants”. One relevant pollutant is mercury (Hg), a trigger of autoimmunity that is measurable inside the human thyroid. However, in fish the two faces have unequal size. Indeed, in small oily fish, such as anchovy and sardine, the good face represented by the omega-3 PUFA is much larger than the bad face represented by the pollutants. Just the opposite is true for the large, top predator fish, such as swordfish and tuna. Thus, we also provide a review of studies showing thyroid consequences from eating fish. Finally, after having reminded the interactions between the omega-3 PUFA and thyroid hormones at several levels, and having reminded that other natural compounds have shown benefits in the setting of thyroid autoimmunity, we talk about the commercial side of the benefits of all such substances, namely the expanding market of nutraceuticals. Overall, we think that the use of supplements containing omega-3 in the clinical thyroid setting has enough scientific rationale.
Consumption of fish
The European Market Observatory for Fisheries and Aquaculture Products (EUMOFA) considers fish and seafood as “the aggregation of finfish, crustaceans and mollusks and cephalopods”, regardless of being fresh or chilled and frozen (1). Examples of crustaceans are lobsters, shrimps and crabs; examples of mollusks are oysters and clams; examples of cephalopods are octopus, squid, and cuttlefish (1). Household expenditure on fishery and aquaculture products grew by 17% from 2019 to 2020, much greater than the 2.1% inflation of prices for such products (1). This increase was most likely due to the increased at-home consumption due to the closing of the hotels and restaurants because of the COVID-19 pandemic (1). In 2017, the expenditure on fish in the European Union (EU) was equal to euro 54262 million, which was the highest in the world. However, in terms of per capita expenditure, with euro 106 EU ranked 8th after Iceland, Japan, Korea, Norway, Australia, Israel, and Switzerland (1). Excluding out-of-home consumption, household nominal expenditure on fishery and aquaculture products in 2020 (with % variation over 2019 given in parentheses) was led by Spain (euro 13608 million, +39%) and Italy (euro 12277 million, +3%), with Slovenia (euro 90 million, +1%) and Malta (euro 58 million, +4%) at the bottom of the ranking.
Consolidated data for consumption and other items are available up to 2019 (1). Consumption of fishery and aquaculture products in the EU dropped to 12.30 million tonnes of live weight equivalent (LWE) in 2019, continuing a declining trend that started in 2017. Wild products accounted for 9.41 million tonnes LWE (76% of 12.30), and farm products for 2.89 million tonnes LWE (the remaining 24%). Per capita apparent consumption, estimated at 23.97 kg LWE of mostly wild-caught products, was almost stable in 2019 compared with 2018. Portugal remains the major EU consumer, with 59.91 Kg per capita (-2% vs 2018). Portugal is followed by Spain (46.02 Kg, unchanged), Denmark (42.56 Kg, +6%), France (33.26 Kg, -0.5%), Luxembourg (32.84 Kg, -3%), and Italy (31.21 Kg, +1%). At the other extreme, Hungary and the Czech Republic consume 6.28 and 6.0 Kg (+3% and +7% vs 2018, respectively) (1).
Concerning the most consumed fish, 16 products were considered, and they were listed in decreasing order of consumption: tuna, salmon, cod, Alaska pollock, shrimp, mussel, hake, herring, squid, surimi, sardine mackerel, trout, sprat (brisling), saithe (coalfish) and other products. Illustrative per capita consumption in 2019 (with percentage change vs 2018 given in parentheses) were 3.10 Kg (+ 2%) for tuna, 2.11 Kg (-1%) for cod, 1.02 Kg (+ 2%) for hake, 0.98 Kg (-17%) for herring, 0.58 Kg (+1%) for sardine, 0.53 Kg (-12%) for mackerel, and 6.58 Kg (-2%) for other products (1). In 2020, over 80% of the total volume of fresh fishery and aquaculture products consumed by households in 11 EU countries analyzed was accounted for by Spain, Italy, and France. In decreasing order, the top five fresh fish species consumed by households in 2020 were: hake, salmon, sardine, European seabass, and gilthead seabream in Spain; gilthead seabream, mussels, salmon, European seabass, and anchovy in Italy; salmon, cod, saithe (coalfish), trout and gilthead seabream in France. Data for swordfish, a seafood species that will be mentioned subsequently in our review, were not provided.
Based on a document by CBI (Centre for the Promotion of Imports from developing countries) the swordfish fishery is very important for Southern Europe, especially Spain and Italy, the two European countries with the highest consumption of swordfish (2). With 22,676 tonnes in 2017, Spain is the leading European producer of swordfish, followed by Italy and Portugal. The top importers from non-European countries of frozen swordfish are Portugal, Italy and Spain (6,316, 3,762 and 2,130 tonnes, respectively). Italy’s imports of swordfish have increased by 18% since 2014 with most deliveries coming from Spain. Based on a document by Oceana (3), which is the no-profit largest international ocean conservation organization, Greece, Italy, and Spain are the European countries where swordfish is consumed the most, but numbers were not provided. According to a Spanish paper (4), which in turn reports data published in 1995, the consumption of swordfish by Spanish adults averaged 0.35 g/day, with marked differences between communities, ranging from areas of no swordfish consumption to two areas of highest consumption (1.06 g/day and 1.17 g/day). On an annual basis, the said 0.35, 1.06 and 1.17 g/day correspond to 0.13, 0.39 and 0.40 Kg/year. In Italy, swordfish accounts for 4.9% of the national consumption of fresh fish (5), and in the year 2017 it was the fifth species most consumed (5.7%), following European seabass (17%) (6). Thus, of the 29.80 Kg of fish consumed by Italians in 2017, 1.46 Kg were accounted for by swordfish. A recent Italian survey of 560 consumers (7), led to the identification of 24 seafood species that were commonly purchased. The highest preference was for gilthead bream (Sparus auratus), European seabass (Dicentrarchus labrax), swordfish (Xiphias gladius), and European pilchard (Sardina pilchardus), with approximately 13% of consumers who purchased swordfish.
The Good Face of Janus
There is abundant literature, which is accruing over the years, about the benefits of the omega-3 fatty acids as such or omega-3-rich-foods on some disorders (Table 1), including autoimmune diseases (8–18). An example of the clinical studies that show such benefits for illustrative autoimmune diseases is summarized in Table 2 (19–36), with more details provided for type-1 diabetes (T1D) due to the endocrine nature of this disorder. Table 1 also illustrates the magnitude of the literature on other topics of this review, such as fish consumption.
Table 1.
Magnitude of the literature retrievable on PubMed at two indicative time points.
| Entries | Number of articles | |
|---|---|---|
| as of May 21, 2015 | as of Jan 04, 2022 | |
| Omega-3 fatty acids | 20,521 | 32,757 (+ 59.6%) |
| Protective effects of omega-3 fatty acids | 768 | 4,951 (+ 545%) |
| Protective effects of DHA | 297 | 2,347 (+ 690%) |
| Protective effects of fish oil | 809 | 5,527 (+ 583%) |
| Consumption of omega-3 fatty acids | 2,070 | 3,405 (+ 64.5%) |
| Consumption of fish | 10,481 | 17,546 (+ 67.4%) |
| Protective effects of fish consumption | 214 | 1,519 (+ 610%) |
| Omega-3 fatty acids and autoimmunity | 36 | 291 (+ 708%) |
| Omega-3 fatty acids and autoimmune diseases | 433 | 640 (+ 47.8%) |
| Fish consumption and autoimmune diseases | 56 | 105 (+ 87.5%) |
| Fish consumption and autoimmune thyroiditis | 3 | 8 (+ > 167%) |
| Fish consumption and thyroiditis | Zero | 123 (+ > 100%) |
Table 2.
Example literature on the effects of fish/fish oil consumption or supplements containing omega-3 polyunsaturated fatty acids (PUFA) on representative autoimmune diseases. *
| Disease | Effects | Reference |
|---|---|---|
| Rheumatoid arthritis (RA) | Evidence that higher consumption of olive oil, oil-rich fish, fruit, and vegetables protect from the development of RA. | Pattison et al (19) |
| Consuming high amounts of omega-3 fatty acids and fish is one of the modifiable factors that are recommended to prevent the risk for the development of RA. | Koller-Smith et al. (20) | |
| Compared with the lowest category of fish consumption, the highest category was inversely associated with risk of RA (RR: 0.89). Furthermore, a 100 g/day increment in fish intake was associated with a 15% decreased risk of RA. | Asoudeh et al. (21) | |
| An intake of dietary long-chain n-3 PUFAs >0.21 g/day (lowest quintile) was associated with a 35% decreased risk of developing RA (RR= 0.65) compared with a lower intake. Long-term intake consistently above 0.21 g/day was associated with a 52% decreased risk. Consistent long-term consumption of fish ≥1 serving per week compared with<1 serving per week was associated with a 29% decrease in risk (RR 0.71). | Di Giuseppe et al. (22) | |
| RA patients consuming fish ≥2 times/week had a significantly lower disease activity score (DAS) compared with patients who ate fish never or <1 time/month (difference -0.49). For each additional serving of fish per week, DAS was significantly reduced by 0.18. | Tedeschi et al. (23) | |
| RA patients in the fish oil group (10 g/day for 6 months) reported a significantly decreased consumption of non-steroidal anti-inflammatory drugs at 3 and 6 months, and; their status of global arthritic activity improved at 3 months. In contrast, control patients reported an increased global arthritic activity at 6 months. | Sköldstam et al. (24) | |
| Multiple sclerosis (MS) | Consuming fish/seafood at least once a week or at least once a month with regular fish oil use was associated with 44% reduced odds of MS compared with consuming fish/seafood less than once a month and no fish oil supplementation. | Langer-Gould et al. (25) |
| Dietary intake of at least 0.5 servings of fish per week during adolescence and after could reduce the risk of MS. Consumption of fish decreases the risk of MS [OR= 0.77] compared with controls. | Rezaeizadeh et al. (26) | |
| Higher total fish consumption (30 g/day, equivalent to two servings/week) was associated with an 18% reduced risk of central nervous system demyelination (FCD), a precursor to MS (adjusted OR 0.82). Higher tinned fish consumption (30 g/day) was associated with a 41% reduced risk of FCD (adjusted OR 0.59). Tinned fish is predominantly oily. Oily fish is high in vitamin D and long-chain n-3 PUFAs. | Black et al. (27) | |
| Frequent fatty fish intake was associated with decreased occurrence of MS (adjusted OR 0.82). The association between intake of lean fish and MS was not significant. | Bäärnhielm et al. (28) | |
| Type 1 diabetes (T1D) | 6081 Finnish newborn infants with HLA-DQB1-conferred susceptibility to T1D were followed up to 15 years of age. Over the whole period, the risk of advanced islet autoimmunity (IA) in high fish consumers tended to be lower compared to low fish consumers. The overall hazard ratio of advanced IA for high fish consumers, compared to low fish consumers, was 0.68 and of T1D 0.45. The number of children with T1D was 15 (1.6%, n= 941) among the high fish consumers and 180 (3.9%, n= 4604) among the low fish consumers, a significant difference (P < 0.001). The potential benefits of fish consumption could be related to the n-3 fatty acids. | Syrjälä et al. (29) |
| 75 male Albino rats were divided into three groups of 25 each: a negative control group; a group injected with 150-mg/kg body weight of recrystallized alloxan to induce hyperglycemia; a group injected with that dose of alloxan to induce hyperglycemia and treated with insulin injection. Each group was divided into five subgroups. The 1st subgroup was fed on a casein diet, while the 2nd, 3rd, 4th and 5th subgroups were fed a basal diet containing mackerel, sardines, herring, and bolti, respectively. Feeding diabetic rats with the different types of diet (fish diet) resulted in an improvement of the nutritional parameters (serum glucose, triglycerides, cholesterol, LDL-c, HDL-c, VLDL-c, urea nitrogen, uric acid, transaminases) decreased in all treated groups, especially in the rats receiving the mackerel diet and those receiving sardine diet, as compared to the casein diet-fed control rats. Furthermore, diabetic rats that were treated with a low insulin dose and fed on the mackerel diet, showed non-significant differences in any parameter, as compared to non-diabetic rats. | Abdel-Megeid et al. (30) | |
| Review of the molecular mechanisms and signaling pathways induced by ω-3 PUFAs and the beneficial effects of ω-3 PUFAs intake in preventing and treating T1D. Neonates of T1D mothers had lower plasma levels of DHA and other fatty acids compared to neonates of non-diabetic mothers. At least two major mechanisms can explain the benefits of the ω-3 PUFA in T1D: anti-inflammatory and anti-autoimmunity action. One of the several molecular targets for the anti-inflammatory effects of ω-3 PUFA is peroxisome proliferator-activated receptor-gamma (PPARγ). PPARγ activation prevents NF-κB nuclear translocation and reduces inflammatory responses. In the NOD mice (the murine model of T1D), administration of ω-3 PUFA resulted in the modulation of the differentiation of T helper (Th, CD4+) cells and regulatory T cells (Tregs), also alleviating the inflammatory burden by decreasing IFN-γ, IL-6, IL-17, and TNF-α levels. Similar effects were reported on the differentiation of Th cells isolated from human peripheral blood mononuclear cells (PBMC) by reducing the Th1 cells population, balancing the Th1/Th2 ratio and suppressing IL-17A production, and increasing IL-4 and IL-10 secretion. In the NOD mice, Daily intake of EPA and DHA (3.6 g/kg b.w., as fish oil) for 35 weeks reduced the incidence of T1D (33% of the treated mice compared with 80% of the control group). EPA and DHA decreased significantly the incidence of severe insulitis and elevated insulin secretion. In the fat-1 transgenic mouse model, the islets cells contain higher levels of ω-3 PUFA and lower levels of ω-6 PUFA compared to non-transgenic cells. The transgenic islets were resistant to cytokine-induced cell death when exposed to IL-1β, IFN-γ, and TNF-α. |
Purdel C et al. (31) | |
| Vitamin D (1000 IU/day) and ω-3 PUFA (DHA and EPA at 60 mg/kg/day) co-supplementation for 12 months improves T1D by attenuating autoimmunity and counteracting inflammation. The co-supplementation decreased significantly insulin demand, especially as pre-meal boluses, and insulin-dose adjusted HbA1c. | Cadario F et al. (32) | |
| The authors explored the preventative and therapeutic effects of ω-3 PUFA on T1D. Female NOD mice were fed a diet enriched in DHA/EPA for 35 weeks, starting at 5 weeks of age. Further to a control group fed a regular diet, a separate group of animals was fed a diet containing equal levels of arachidonic acid (AA, a ω-6 PUFA). At a preventative level, the islets from the DHA/EPA-fed mice had a significantly reduced incidence of severe insulitis compared with mice maintained on an AA-enriched diet or a regular diet. Furthermore, ω-3 PUFA modulated the differentiation of Th cells and Tregs and decreased the levels of IFN-γ, IL-6, IL-17, and TNF-α. To test the therapeutic effects, the authors delivered, into NOD mice, a lentiviral vector carrying a modified Caenorhabditis elegans cDNA, mfat-1 (mice that are referred as lenti-mfat-1), that encodes an ω-3 fatty acid desaturase. Such lenti-mfat-1 have elevated endogenous levels of ω-3 PUFAs with a concomitant decrease in ω-6 PUFAs. Around 3 to 4 weeks after lenti-mfat-1 treatment, nonfasting blood glucose levels had gradually dropped in most mice. Simultaneously with the normalization of glycemia, serum insulin levels in the lenti-mfat-1–treated group and the DHA/EPA-enriched diet group were completely restored. Lymphocyte infiltration of neopancreatic islets in the lenti-mfat-1–treated or DHA/EPA-enriched dietary group was much lower compared with lenti-control-treated groups. A high proportion of regenerated islets (~40%) had essentially all β cells, with very few α cells. The authors concluded by underscoring the clinical potential of gene therapy or nutritional supplementation of ω-3 PUFAs - in particular DHA and EPA - in preventing and reversing the development of autoimmunity and T1D, and perhaps other autoimmune diseases. |
Bi et al. (33) | |
| This study, termed DAISY (Diabetes Autoimmunity Study in the Young), involved 1770 children at increased risk for TID. The authors investigated a direct association between the ω-3 or ω-6 PUFA intake and the development of islet autoimmunity (IA). They found that the intake of ω-3 PUFA increased the content of ω-3 PUFA in erythrocyte membranes. Furthermore, in children with familial T1D, the long-term dietary intake of ω-3 PUFA starting from 1 year of age was shown to be associated with a reduced risk of IA. | Norris et al. (34) | |
| Male Wistar rats were injected with citrate buffer (control group) or 55 mg/kg streptozotocin (STZ). Control and diabetic groups (STZ) were fed with n-6/n-3 ratio of ≈ 7, STZ + n6 (2.5% sunflower oil) with n-6/n-3 ratio ≈ 60 and STZ + DHA with n-6/n-3 ratio of ≈ 1 containing 19% DHA and 16% EPA. Extensive vacuolization of distal tubular cells (DTCs) was found in T1D, but it was attenuated in the STZ + DHA group, which had the highest renal NF-kB expression. The ectopic lipid accumulation was observed in proximal tubular cells (PTCs) of all diabetic animals, but it became worse in the STZ + n6 group. Thus, the early phase of diabetic nephropathy is characterized by extensive damage and vacuolization of DTCs, which could be attenuated by n-3 PUFA supplementation. | Vitlov Uljević et al. (35) | |
| Participants with previously diagnosed T1D supplemented their diet with a 10 mL dose of seal oil ω-3 PUFAs containing 2,330 mg of essential fatty acids (1,020 mg DHA, 750 mg EPA, 560 mg docosapentaenoic acid [DPA]) for 12 months. Of the 40 participants enrolled, aged 48 ± 14 years, with T1D duration of 27 ± 18 years, 32 completed the full 12-month protocol. Baseline corneal nerve fiber length (CNFL) was 8.3 ± 2.9 mm/mm2 and increased significantly to 10.1 ± 3.7 mm/mm2 after supplementation. Corneal nerve branch density (CNBD) also increased significantly (from 10.6 ± 12.5 to 19.6 ± 19.7 br/mm2). Furthermore, 12 months of seal oil ω-3 PUFA prevented the progression of clinical disease symptoms and prevented declines in small and large sensory fibers and functional measures. | Lewis et al. (36) |
*Keywords of relevance are highlighted by the bold-face print.
As well known, fish (particularly seafood) is a good source of high-quality proteins, vitamins, and minerals, including minerals relevant to thyroid physiology (iodine and selenium). As also well known, fish is a major source of the long-chain omega-3 polyunsaturated fatty acids (abbreviated as either LC n-3 PUFA or omega-3 PUFA), particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Figure 1 illustrates the content of EPA and DHA in representative seafood (17) while Figure 2 illustrates the oily fish (with their scientific, English and Italian names), which will be referred to in some sections of this review. Italian names are reported because in Italy we refer to the oily fish as “pesce azzurro” (“azure fish”) due to the blue-green color of the green in the dorsal and later scales (37). In the absence of a scientific definition, their definition is culinary (37). In contrast, the n-6 (omega-6 PUFA), such as arachidonic acid and linoleic acid, are derived largely from plant sources. Plants (and therefore plant oils and seeds) are also a source of another LC n-3 PUFA: α-linolenic acid (ALA). Tissues can convert ALA into EPA and DHA, but because the corresponding conversions are only 1 to 10% and 0.5-5% in human tissues, this conversion process is inefficient in humans (38). Thus, the main dietary source of EPA and DHA is cold-water oily fish such as mackerel, salmon, herring, sardines, anchovies, etc…. Canned fish contains omega-3 PUFA, but some amounts may be removed during processing (39). A similar decline in content of the omega-3 PUFA occurs with frying (39, 40).
Figure 1.
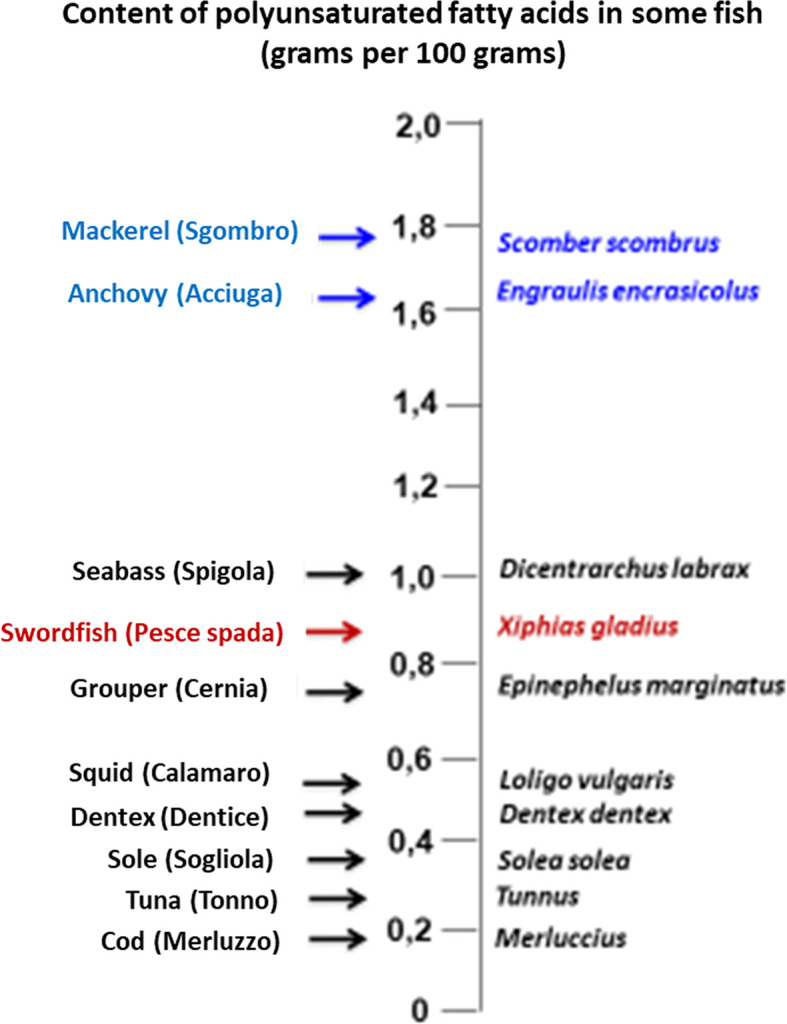
Content of polyunsaturated fatty acids in illustrative species of fish. Oily fish (which are referred to as “pesce azzurro” [“azure fish”] in Italy) are typed in blue, while swordfish (the top predator fish that is mentioned more frequently in our review) is typed in red.
Figure 2.
Illustration of oily fish (“pesce azzurro” [“azure fish”] in Italy), with the English names typed in blue, Italian in black, and scientific/Latin name in italics. Source: https://it.wikipedia.org/wiki/Pesce_azzurro.
The Bad Face of Janus
Fish and seafood contain many contaminants, since anthropic activities (e.g., agriculture, industry, mining) increase their concentration in the aquatic ecosystem (17, 41–43). Clearly, because of biomagnification, pollutants are mostly concentrated in the longer-living and larger predatory fish species such as swordfish, tuna, and shark. Classical fish contaminants are the heavy metals, with Hg being worthy of mention in this review because it acts as an environmental trigger of autoimmunity (17, 44–51), a mechanism that has also been observed for cadmium (Cd) (51–55). In predatory marine fish, approximately 90% of Hg is methylated (methylmercury), while the remainder consists of minimal amounts of inorganic mercury, ethylmercury, and phenylmercury (4).
A recent Italian study on the muscle tissue of 30 Mediterranean swordfish determined that the rank order of toxic metals was Hg, Cd and lead (Pb), while the rank order of essential metals was zinc (Zn), copper (Cu), nickel (Ni) and chrome (Cr) (56). Particularly, Hg, Cd, and Pb levels exceeded the respective critical values 1.02, 0.30 and 0.25 μg/wet weight (that is, the legal safety limits established by the European Community) in eight, three, and two of the swordfish specimens examined, respectively. In a Canadian study, Hg was detected in all samples of swordfish, tuna, marlin, and shark purchased from major supermarket outlets and fish retailers, with swordfish containing the greatest concentration (57). In a Food and Drug Administration document on “Mercury Levels in Commercial Fish and Shellfish”, where 68 types of fish are ranked based on their mean content of Hg, the 68th, 67th and 66th positions are occupied by tilefish (1.123 parts per million [ppm]), swordfish (0.995 ppm) and shark (0.979 ppm]) (58). In contrast, sardine and anchovy occupy the 5th and 8th positions with a mere 0.013 and 0.016 ppm concentration of Hg, respectively (58). Thus, it is no surprise that the blood Hg concentrations of 285 adult seafood consumers in Long Island (NY, USA) were positively associated with weekly tuna steak or sushi intake and monthly or weekly swordfish, shark, or marlin intake (59).
With particular reference to thyroid autoimmunity, associations between total blood Hg and positive thyroid autoantibodies (thyroglobulin autoantibodies [TgAb] and thyroperoxidase autoantibodies [TPOAb]) were evaluated using the National Health and Nutrition Examination Survey (NHANES), 2007-2008 (60). Such associations were searched in 2,047 non-pregnant, non-lactating women. Compared to women with the lowest Hg levels (≤0.40 μg/L), those with Hg levels >1.81 μg/L (upper quintile) had 2.24 greater odds (95% CI=1.22, 4.12) for TgAb positivity. In contrast, no significant association was found for TPOAb positivity (60). One study from the Czech Republic in patients with Hg hypersensitivity (61) concluded that removal of Hg-containing dental amalgam may help to successfully treat autoimmune thyroiditis (AIT). In this study, 27/39 AIT patients had Hg hypersensitivity (with amalgam fillings removed only in 15/27), while 12/39 AIT patients had not (controls). Serum TgAb and TPOAb were measured both at baseline and six months later. Compared to baseline, the 15 patients in whom amalgam fillings were removed had a significant decrease in the serum levels of both TgAb and TPOAb (P=0.0007). In contrast, both TgAb and TPOAb did not change in the other two groups (61). Also, for Cd, the other heavy metal that we mentioned above, a direct relationship with thyroid autoantibodies was reported (53). In Chinese women, natural log-transformed blood levels of Cd correlated directly with natural log-transformed serum levels of TgAb (53).
There is evidence for the Hg presence in the thyroid. In a very recent autopsy-based Australian study (62), the presence of intracellular inorganic Hg was searched in paraffin-embedded thyroid tissue blocks from 115 persons (68 males, 47 females; mean age = 54 years, median age = 47 years, range= 1 to 104) with varied clinicopathological conditions. Using autometallography, Hg was found in the thyrocytes with an age-dependent frequency: 4%, 9%, and 38% of persons in the age band 1-29, 30-59, and 60-104 years, respectively. The frequency of thyroid samples containing Hg was similar in males (18%) and females (21%). Laser ablation-inductively coupled plasma-mass spectrometry not only confirmed the presence of Hg, but also detected other metals in six selected samples: cadmium (n= 6), iron (n= 5), lead (n= 4), nickel (n= 2), and silver (n= 2). The authors concluded that Hg can trigger genotoxicity, autoimmunity, oxidative damage, and be involved in the pathogenesis of AIT, hypothyroidism, and thyroid cancer (TC) (62). A previous Italian study on thyroid tissue samples removed at surgery from 77 euthyroid subjects showed that Hg and Cd are significantly more concentrated in the thyroid than in the adjacent muscle and fat of the same individual (63). Worthy of note, this Italian group (64) compared the urine concentration of several metals in a Sicilian area that features an incidence of thyroid-cancer two-fold greater than a control area. The authors found that the geometric mean value in the first area was at least two-fold higher than that in the second area for eight metals, two of which being Hg and Cd (64).
Having mentioned TC is not improper, given the abundant literature on the important predisposing role for such malignancy (particularly, papillary TC) and its advanced stages exerted not only by Hashimoto’s thyroiditis (HT) but also by serum TSH per se, even by TSH levels that are within the upper values of the reference limits (65–88). In regard to TC, it is also pertinent to remind the involvement of chemokines (89–98) and the peroxisome proliferator-activated receptors (PPARs) in the molecular oncogenesis (95–106), so that both types of molecules may serve as novel targets of TC precision therapy or prevention. Quite interestingly, the beneficial health effects of DHA and EPA on metabolic diseases are thought to arise from their binding to and activation of PPARs (107), as it was shown illustratively for T1D in Table 2.
There is cross-talk between the omega-3 PUFA and the thyroid hormone pathways, as exemplified by the augmented thyroid hormone signaling pathways in the liver, this being one mechanism used by n-3 PUFAs to affect lipid metabolism (108). For instance, specific steps of TH signaling in lipid metabolism that are influenced by n-3 PUFA include higher liver expression of the thyroid hormone nuclear receptor TRβ1 and mitochondrial α-glycerophosphate dehydrogenase (109). Starting from their previous observation showing that plasma free fatty acids concentration in some hypothyroid patients is above the normal range and that this higher concentration is associated with less severe symptoms of hypothyroidism, Makino et al. investigated the effect of highly purified EPA ethyl ester (EPA-E) derived from fish oil on thyroid function in rats with methimazole-induced hypothyroidism (110). They found that oral administration of EPA-E inhibited the reduction of thyroid hormone levels and the change of thyroid follicles in the hypothyroid rats, suggesting that n-3 PUFA may prevent methimazole-induced hypothyroidism. In a Dutch study on 13 patients with hypothyroidism caused by the ablation treatment for well-differentiated TC (111), induction of hypothyroidism decreased PUFA levels in plasma, erythrocytes and polymorphonuclear leukocytes. Another site of interaction between PUFA and thyroid hormones can be the thyroid hormone plasma transport proteins. Several studies have shown that PUFA inhibit thyroid hormone binding to such carrier proteins (112, 113), one practical consequence being increased tissue availability of the biologically active protein-unbound, free thyroid hormone. In addition, studies on the brain of aged rats that were fed fish oil (27% DHA content) for one month showed an approximately 10-fold increase in the expression of transthyretin (114). Transthyretin is the second major thyroid hormone plasma carrier, which is synthesized also in the central nervous system. Since transthyretin also operates as an amyloid-beta protein scavenger, transthyretin overexpression could prevent the formation of amyloid aggregates (114). This study is relevant because decreased PUFA levels, particularly DHA, were detected in elderly subjects and patients with Alzheimer’s disease (AD), and because there is epidemiological evidence for an association between fish consumption and low prevalence of AD (114). Of interest, AD is also characterized by brain inflammation and decreased local concentration of specialized pro-resolving mediators (SPM) (115). SPM are derived from PUFA and are key in the resolution of inflammation. Because of the technical difficulties in investigating the microglia function directly, Wang et al. took advantage of the useful model of peripheral blood mononuclear cells (PBMC) (115). In their randomized, double-blind, and placebo-controlled trial on 204 AD patients, Wang et al. administered a placebo or a supplement of DHA (1.7 g) and EPA (0.6 g) daily for 6 months. At the end of treatment, in those who received the n-3 PUFA, the plasma levels of DHA and EPA levels increased. When the culture medium of PBMC incubated with amyloid-β 1-40 was analyzed, levels of the SPMs lipoxin A 4 and resolvin D1 secreted by PBMCs were decreased in the patients supplemented with placebo, but unchanged in the patients supplemented with n-3 PUFA. Changes in the levels of SPM secreted by PBMC were positively correlated to changes in plasma transthyretin, and to cognitive changes as well (115). In the setting of tissue protection by PUFA, experimental studies in rats by Videla and colleagues showed that the combination of DHA plus thyroid hormone (T3) protects from liver injury through a synergistic action that also involves causing increased intrahepatic levels of resolvins (116–120).
Table 3 summarizes human studies that link thyroid disorders with the consumption of contaminated fish (17, 18, 121–127). Overall, the consequences are impairment of thyroid function, as measured by serum levels of thyroid hormones and/or TSH, and triggering of thyroid autoimmunity, as measured by serum levels of thyroid autoantibodies. Only studies by Benvenga and colleagues addressed Hg contamination (17, 18). The group of pregnant women who consumed swordfish selectively or predominantly among other fish species ingested the greatest amounts of Hg and had the greatest both serum levels and rates of positivity for thyroid autoantibodies throughout pregnancy compared to other groups of women (17, 18). As a result, the swordfish eaters had the greatest rate of postpartum thyroiditis, since positivity for thyroid autoantibodies is a major risk factor for such autoimmune type of thyroiditis that develops within the first 12 months after parturition (17, 18).
Table 3.
Studies on the relationship between consumption of contaminated fish and thyroid disorders in humans. *
| Reference | Methods and subjects studied | Main findings |
|---|---|---|
| Sarkar et al. 2015 (121) | The St. Lawrence River, its estuary, and the Gulf of St. Lawrence are heavily polluted with thyroid disrupting chemicals (TDC) from industries, their effluents, and urbanization in the Great Lakes Watershed and along the river. The west and south coasts are in contact with the Gulf of St. Lawrence (GSL). In studies on blubber samples from harbor porpoises collected in 1989-1991, samples from St. Lawrence were more contaminated by older varieties of persistent organic pollutants (POPs) than samples from the Avalon Peninsula (tip of east and south coasts of Newfoundland). Another study found polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethane, and its metabolites (DDTs) infrequently consumed fish (capelin, halibut, tomcod, smelt, herring, flounder), shellfish (shrimp, crab) and mammals (beluga, seal) caught in the estuary and the GSL. Local marine products are a regular diet of the coastal communities of Newfoundland. [In contrast, Newfoundland depends mainly on food imported from the mainland because it has very few agricultural communities, a shorter growing season, and poor soil]. Data on hospitalizations with hypothyroidism (from 1998 to 2012) was obtained from the provincial hospital abstracts held at the Newfoundland and Labrador Centre for Health Information (NLCHI). |
Mean ± SD hypothyroidism rates of the west [91.8 ± 36.7 persons hospitalized with hypothyroidism diagnosis per 100,000 population per year] and south coasts [96.3 ± 52.0/100,00/year] were significantly higher than in the east coast [51.3 ± 20.2/100,000/year], that is 1.8 and 1.9 times respectively. High levels of TDC were detected in marine animals. Hence, consumption of contaminated seafood might trigger hypothyroidism, the most common cause of which is autoimmune thyroiditis. The authors suspected that marine products caught from the GSL and consumed by communities from the west and south coasts were contaminated with TDCs. Such contamination, in turn, could contribute to the development of hypothyroidism in these areas. |
| Schell et al. (122) | The Akwesasne Mohawk Nation has long-lived, fished, planted, and hunted in the St. Lawrence River valley (both USA side and Canada side). Many industries had developed along the St. Lawrence River and its tributaries. The Mohawk Nation has relied heavily on locally caught fish and game. Some local species of fish, birds, amphibians, and mammals have polychlorinated biphenyls (PCB), p,p ´-DDE, hexachlorobenzene (HCB), and mirex levels that exceed the tolerance limits for human consumption established by the U.S. Food and Drug Administration. 115 youths (range 10–17 years) were sampled for PCB and their congeners, TSH, T4, FT4, T3, FT3, TPOAb. Breast-feeding history was taken into account, with 47 youths having been breastfed. |
18 participants (15.6%) had increased TPOAb levels (23% of females, 9% of males). The rate of TPOAb positivity was similar in the breast-fed group and non-breast-fed group (17.0% vs. 14.7%). Among participants who were breastfed (n=47), those with elevated TPOAb levels had significantly higher levels of all PCB groupings, except levels of non-persistent PCBs which did not differ significantly. Levels of p,p’-DDE were also significantly elevated, while HCB and mirex were similar. Participants who were breastfed had significant, positive relationships between TPOAb levels and all PCB groupings, except groups comprised of non-persistent PCBs, and with p,p’-DDE, HCB, and mirex. No effects were evident among nonbreastfed young adults. |
| Turyk et al. (123) | To assess whether polybrominated diphenyl ethers (PBDE) body burdens are related to thyroid and steroid hormone levels, thyroid antibodies, and thyroid disease is frequent and infrequent adult male sport fish consumers. A cohort of 4,206 frequent and infrequent consumers of Great Lakes fish established during the early 1990s in a previous study, was invited to participate in a follow-up study. 405 adult males were tested for PBDE congeners, polychlorinated biphenyls (PCB) congeners, thyroglobulin antibodies (TgAb), TSH, T3, T4, and T4-binding globulin (TBG). Data were collected on demographics, fish consumption, medical diseases, and medication use. |
Data are reported for the 308 men without exclusion criteria. Thus, excluded were also 21/405 men (5.2%) using thyroid hormones or having thyroid disease. PBDE were positively related to levels of T4 and inversely related to levels of T3 and TSH. PBDE were positively associated with the percentage of T4 bound to albumin, and inversely associated with the percentage of T4 bound to TBG. Participants with PBDE above the 95th percentile were more likely to have TgAb, although high PBDE exposure was not associated with thyroid disease. Indeed, TgAb were detected in 7.8% of the full cohort, but in 31.3% of those whose ∑PBDEs exceeded the 95th percentile [odds ratio (OR) = 6.1]. |
| Bloom et al. (124) | Great Lakes sportfish anglers represent a population with potentially elevated dietary exposure to PBDEs. 36 licensed anglers who participated in the New York State Angler Cohort Study completed questionnaires regarding demographic, clinical, and sportfish consumption information. Archived blood samples were analyzed for T4, FT4, T3, TSH, and nine PBDE congeners. | There was a positive association between ΣPBDEs and FT4, which could have been significant with a sample size approximately 9 times greater. |
| Bloom et al. (125) | Study as above (124), except that the pollutants measured in sera of the 36 licensed anglers were polychlorinated dibenzo-p-dioxins (PCDDs), coplanar biphenyls (PCB), dibenzofurans (PCDFs), and PCB IUPAC #153. | There was a significant inverse linear association between the sum of dioxin-like congener concentrations (∑DIOXs) and FT4. |
| Hagmar et al. (126) | For the population living in the coastal areas around the Baltic Sea, consumption of locally caught fatty fish is the main source of exposure to persistent organohalogens (OHS), which are endocrine disruptors. Persons who consume great amounts of contaminated fatty fish from the Baltic Sea may constitute a risk population. The aim of this study was to assess whether high dietary exposure to OHSs affected hormone levels (among which FT3, T3, FT4, T4, and TSH) in adult men. Participants were 110 men who consumed varying amounts of fish (i.e., 0 to 32 meals per month). |
In regard to thyroid function tests, the only significant association was negative, and consisted of the negative correlation between 2,2’,4,4’-tetrabromodiphenyl ether and TSH. |
| Langer et al. (127) | In an area of the Michalovce district in East Slovakia, heavy industrial pollution by polychlorinated biphenyls (PCBs) developed in 1955-1984 and very high PCB levels persist in the environment. Environmental pollution occurred because of activities of chemical factories manufacturing polychlorinated biphenyls (PCBs) and the entirely unlimited dumping of toxic waste to the nearby Laborec river. The average PCBs level found in the serum of 101 chemical factory employees and persons living nearby reached 7300 ng/g of lipids. In 1998, the average values of organochlorines found in predators (e.g., zander [Stizostedion lucioperca], pike [Esax lucius], sheatfish [Silurus glanis], perch [Perca fluviatilis], and asp [Aspius aspius], from the polluted Sirava lake and Laborec river, were 375430 ng/g lipids for a sum of 15 most abundant PCB congeners (upper range limit was 933 770 ng/g which is nearly 1 mg/g lipids) and 15620 ng/g for 2,20-2-bis(4-chlorobiphenyl)-1,1-dichloroethylene (DDE). In contrast, the same fish species from the neighboring Ondava river and Domasa lake with relatively background pollution had an average value of only 5150 ng/g for a sum of PCBs and 8420 ng/g for DDE, still considerably higher than the values of PCBs (1100 to 4600 ng/g) reported for the Baltic herring. 2045 adults from the said polluted area and the surrounding background pollution area were investigated using questionnaire data, thyroid volume by ultrasound (ThV), urinary iodine, and serum levels of 15 PCB congeners, hexachlorobenzene (HCB), α-, β- γ-hexachlorocyclohexane (HCH), 2,2’-bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT), 2,2’-2-bis(4-chlorobiphenyl)-1,1-dichloroethylene (DDE), and thyroid indices (TSH, FT4, anti-thyroperoxidase antibodies [TPOAb]). Information on the frequency of fish meals and approximate annual consumption of fish from local waters was obtained by questionnaires. Both within the high pollution area and the control background area, participants were divided into five groups based on the amount and frequency of fish consumption. Comparisons were made between different groups in the same area and between the same group in the two different areas. |
In the whole cohort of 2045 participants, those with the highest fish consumption had levels of PCB, DDE, and HCB greater than the corresponding levels of those with no or low fish consumption, regardless of the area. However, the same group had greater levels if it belonged to a highly polluted area. For instance, in the group with the highest fish consumption from the polluted area, PCB levels were 4926 ± 971 ng/g lipid compared to 1063 ± 162 in the corresponding group of the background area. Upon continuing the comparison between these two groups, ThV was almost 2 mm greater (11.62 ± 0.58 vs 10.01 ± 0.05 ml), the rate of TPOAb positivity 3-fold greater (34% vs 10.6%) and the rate of FT4 levels above 20 pmol/L almost 50-fold greater (14.4% vs 0.3%). TSH levels were not presented, while they were presented in 16 marital pairs from the high pollution area [see below]. In contrast, in such 16 marital pairs, FT4 levels were not presented. The authors concluded for an association of contaminated fish consumption with very high blood levels of PCBs, HCB and DDE, increased ThV, increased frequency of positive TPOAb, and high levels of FT4. These relationships were confirmed in 16 marital pairs from the high pollution area with very high PCB levels in both members associated with high fish consumption. Actually, in these 32 persons, ThV was 12.60 ± 1.27 ml, the rate of TPOAb was 43.7%, and the rate of subclinical hypothyroidism was 10%. |
| Benvenga S et al. (17) | The Mediterranean Sea (MS) is a semi-closed basin considered to be the most polluted European sea. Top predators caught in the MS tend to accumulate high amounts of toxic metals and other pollutants. Swordfish caught in the main spawning area (Straits of Messina, southern Italy) were contaminated more than swordfish caught in the Atlantic Ocean (Azores islands). Fish consumption and serum thyroglobulin antibodies (TgAb) and thyroperoxidase antibodies (TPOAb) were measured during gestation (first and second trimester) and postpartum (day 4) in 236 thyroid disease-free, nonsmoker Caucasian women with stable dietary habits and stable residence in the Messina province. Women were divided into four groups (A-D) based on the type of fish consumed: groups A (n=48; selective or predominant swordfish consumption), B (n = 52; selective or predominant oily fish consumption), C (n= 68; swordfish plus other fish, with swordfish consumption accounting for <50% of the total monthly fish consumption; if eaten, oily fish also accounted for <50% of the total monthly fish consumption), D (n = 68; consumption of fish other than swordfish and oily fish). |
Fish consumption was quantitatively similar in all groups, equivalent to 7.0 ± 2.6 through 7.8 ± 2.1 times monthly. The leading seafood in group A (swordfish) and group B (oily fish) were consumed with the same frequency (6.2 ± 2.2 vs. 6.1 ± 2.5 times monthly). Positivity rates and serum levels of the two thyroid Ab were the highest in group A and the lowest in group B. For instance, TPOAb positivity at 1st, 2nd trimester of pregnancy and day 4 postpartum was 25%, 17%, and 12% in group A, but always 0% in group B, with intermediate frequencies in groups C and D. Serum levels of both Ab were also the highest in group A and the lowest in group B. The content of mercury in the fish consumed monthly by the four groups was estimated to be the highest in group A and the lowest in group B (approximately 1000 and 25 μg), with values of approximately 250 and 35 μg in groups C and D, respectively. |
| Benvenga S et al. (18) | Study as in ref. 17, but on a larger cohort of pregnant women (n= 412) and with a longer follow-up (end of the 12th month postpartum) to permit evaluation of the primary outcome: frequency of postpartum thyroiditis (PPT) and its evolution into permanent hypothyroidism (PH). Secondary outcomes were serum levels of thyroid autoantibodies and, not done in the previous study, ultrasonography (US) signs of thyroiditis. | The four fish groups remained comparable in terms of frequency of fish consumption (7.5-8.0 times monthly or twice weekly). Frequencies of positivity of TgAb and TPOAb, and serum concentrations of either thyroid Ab confirmed those of the previous study (17). US signs of thyroiditis during gestation were detected more frequently in group A compared to group B (44.6% and 29.4%), with intermediate values in groups C and D. Overall, the frequency of PPT was 15.3%. However, the greatest frequency was recorded in group A (23.9%) and the lowest in group B (4.7%), with intermediate rates in the other groups. Overall, the frequency of PH was 54%, with no difference between the four fish groups (50 to 56.2%), particularly between fish groups A and B (54.5% and 50%). |
*Keywords of relevance are highlighted by the bold-face print.
Fish and Omega-3 PUFA as Protection From Thyroid Disorders
In the preceding section, we have reminded the role of Hg as an autoimmunity trigger (17, 44–51). However, omega-3 PUFA antagonize this effect of Hg (128, 129). Gill et al. (129) showed that dietary ingestion of n-3 PUFA (fish oil) promotes CD95 signaling by upregulating caspase 8 activation and that DHA counteracts the negative effect of Hg on CD95 signaling in T lymphocytes (128).
The clinical benefit of thyroid autoimmune disorders given by consuming Hg-poor, omega-3-rich oily fish or by taking omega-3-based supplements (17, 18, 130–132) is summarized in Table 4. The group of pregnant women who consumed oily fish, selectively or predominantly among other species of fish, ingested the lowest amount of Hg but the greatest amount of omega- PUFA; this group of women also had the lowest both serum levels and rates of positivity for thyroid autoantibodies throughout pregnancy compared to other groups of women (17, 18). As a result, the oily fish eaters had the lowest rate of postpartum thyroiditis.
Table 4.
Studies/reports on the relationship between consuming Hg-poor, omega-3-rich oily fish or by taking omega-3-based supplements and the clinical benefit towards thyroid autoimmune disorders. * §
| Reference | Methods and subjects studied | Main findings |
|---|---|---|
| Benvenga S et al. (17) | See above, Table 3. |
Fish consumption was quantitatively similar across groups, equivalent to an average of 7 to 8 times a month [see above, Table 3]. Positivity rates and serum levels of both TgAb and TPOAb were the lowest in group B and the highest in group A. For instance, TPOAb positivity at 1st, 2nd trimester of gestation, and day 4 postpartum was always 0% in group B, but 25%, 17%, and 12% in group A, with intermediate frequencies in groups C and D. Serum concentrations of both Ab were also the lowest in group B and the greatest in group B. The estimated content of mercury in the fish consumed monthly by the four groups was the lowest in group B and the highest in group A (approximately 25 and 1000 μg, respectively), with values of approximately 250 and 35 μg in groups C and D, respectively. In contrast, the estimated content of omega-3 fatty acids (EPA plus DHA) in the fish consumed monthly was the greatest in group B and the smallest in group A (13.2 ± 5.4 and 6.3 ± 2.1 g, respectively), with values of 6.0 ± 2.8 and 5.1 ± 3.8 g in groups C and D, respectively. |
| Benvenga S et al. (18) | See above, Table 3. | The four fish groups remained comparable in terms of frequency of fish consumption (7.5–8.0 times/month or twice a week) [see above, Table 3]. Results of frequency of positivity and serum concentration of either thyroid autoantibody (TgAb, TPOAb) confirmed those of the previous study. The lowest and the highest rates of detection of US signs of thyroiditis during pregnancy were detected in groups B and A (29.4 and 44.6%, respectively), with intermediate values in groups C and D. Overall, frequency of postpartum thyroiditis (PPT) was 15.3%. However, the smallest frequency was recorded in group B (4.7%) and the greatest in group B (23.9%), with intermediate rates in the other groups. Overall, the frequency of evolution of PPT into permanent hypothyroidism (PH) was 54%, with no difference between the four fish groups (50 to 56.2%), particularly between groups B and A (50% and 54.5%). |
| Breese McCoy, (130) | The author, an adjunct professor of physiology at Oklahoma State University, reports her self-treatment of postpartum Graves’ disease with flaxseed oil. | “Approximately one year into the propylthiouracil (PTU) treatment, I became aware that omega-3 fatty acids are thought to reduce the inflammation associated with certain autoimmune disorders, such as rheumatoid arthritis. … With this information in mind, I began a regimen of flaxseed oil supplements , 5-1,000 mg tablets twice a day. Flaxseed oil is over 50% omega-3 fatty acids (mainly alpha-linolenic acid), but it also contains about 15% omega-6 fatty acids (mainly linoleic acid). Within approximately eight weeks, TSH levels had normalized for the first time (0.31 µIU/mL, reference range 0.30-5.0). PTU was then discontinued, and flaxseed oil tapered to less than half the original dose, but TSH levels slipped below normal again within six months (0.29 uIU/mL, ref. range 0.35-5.00). Symptoms were mild , and T4 was within normal range (1.0 ng/dL, ref. range 0.7-1.9 ng/dL), so I declined to restart PTU therapy.” … The following year, she was pregnant again. “… by the fourth week postpartum, my TSH was again suppressed (0.174 µIU/mL, ref. range 0.30-5.00), becoming undetectable by four months postpartum. This time, however, plasma T4 remained within norma l range (1.7 ng/dL, ref. range 0.7-1.9 ng/dL). Although the physician advised me to take a low dose of PTU, I was experiencing no noticeable symptoms of hyperthyroidism and declined the prescription due to breastfeeding. As before, I then restarted a flaxseed regimen at about six months postpartum (this time, three tablespoons of whole seed on cereal each morning). My condition began improving , and plasma TSH normalized within six months (0.57 µIU/mL, ref. range 0.35-5.00). Flaxseed was then discontinued, and there has been no recurrence over the following four plus years”. |
| Dolan et al. (131) | Management, with omega 3 and other nutraceuticals, of a 34-yr-old Hashimoto’s thyroiditis woman who had declined thyroid replacement therapy. This patient, who was a part-time worker in a wellness clinic, also had a personal history of seasonal allergies, and a multigenerational history of thyroiditis and autoimmune disorders. In addition to supplementation and dietary changes, she performed two acupuncture treatments. |
Before being managed by the team, the patient self-prescribed a vegan diet and dietary supplements. Such supplements consisted of selenium (100-200 µg/day), iron, vitamin D3, probiotics, N-acetyl-L-cysteine. At the first visit, the patient reported feeling “ravenously hungry” having palpitations, bloating, low libido, low energy, cold hands and feet, and mental sluggishness. The authors switched her to nutritional supplementation of vitamins (B complex, D3), coenzyme Q10, α-lipoic acid, zinc, magnesium, omega-3 oil (DHA/EPA), L-glutamine, quercetin, and probiotics (50 billion live organisms from 14 strains) in conjunction with a customized herbal tincture [milky oat spikelet (A sativa), ashwagandha root (W somnifera), holy basil (O sanctum), damiana (T diffusa), cinnamon bark (Cinnamomum spp)] and a customized tea [chamomile flowering tops (M chamomilla), ginger (Z officinalis) rhizome, and agrimony (A eupatorium) herb]. Noteworthy, the vitamin B complex recommended, and to be taken once daily, contained 40 mg inositol. Furthermore, the patient was advised to avoid sensitive foods (gluten and soy) and increase her intake of berries, omega-3 rich foods (sardines, wild salmon, walnuts, organic flax), quality fats (organic: cold-pressed olive oil, coconut oils, butter or ghee), fermented foods (water, cultured coconut milk, kefir), and filtered water. Interpretation is complicated by the multitude of supplements, and by the lack of reference ranges for serum thyroid function tests (TSH, T4, FT4, T3, FT3) and autoantibodies (TgAb, TPOAb). However, serum TSH declined from the baseline level of 4. 91 µIU/ml to 1.62 at month 4 and 1.66 μIU/mL at month 12 post-treatment. The corresponding levels of FT4 were 1.13, 1.1, and 1.02 ng/dL, and those of FT3 were 3.1, 2.5, and 2.4 [no unit of measure provided, but it should be pg/ml]. Serum TgAb fell from 12.0 to 1.4 and 1.1 IU/mL; TPOAb fell from 258 to 115 and 24 IU/mL. In parallel with these biochemical changes, symptoms improved and had disappeared at the 8-month checkup. |
| Woźniak et al. (132)§ | 232 hypothyroid volunteers (age ≥18 years; median= 27 years; 203 women and 29 men) were asked to provide information on their diagnosis, clinical manifestations of the disease, lifestyles, and use of dietary supplements with effect on their health. Supplements were taken by 197/232, with information taken from websites (74%), physicians (52%), family/friends (46%) and social media (43%). |
Hashimoto’s thyroiditis was diagnosed in 49% of the 232 participants, with 93% taking L-T4. The most popular supplements taken were vitamin D (98%), magnesium (21%), omega-3 acids (15%), selenium (14%), multivitamins (14%), vitamin B (13%), iron (10%), vitamin C (9%) and zinc (8%). Supplements were taken for a median period of 1.5 years. The most common symptoms experienced included dry skin (64%), cold intolerance (58%), constipation (28%), somnolence (26%), fatigue (23%), hair loss (22%), headache (21%) and mood swings (14%). Patients were stratified into 8 categories based on the nutraceutical taken (vitamin D, vitamins B, iron, zinc, multivitamins, selenium, omega-3 acids), with data summarized as % of patients reporting a benefit for 8 items (decline of serum TSH, less fatigue, improved memory and concentration, improved hair and nails condition, improved skin condition, improved general well-being, better quality and longer sleep, alignment of menstrual cycles). Overall, 52% of those who took supplements reported health benefits. In regard to the omega-3 category, reported a benefit was improved hair and nails condition (2%), improved general well-being (8%), improved memory and concentration (13%), and a decline of TSH (2%). By comparison, selenium users reported a benefit for all items (2 to 8%) except the better quality and longer sleep (0%), while vitamin D users, vitamin B users and multivitamins users reported benefits for all 8 items (3 to 35%, 2 to 11%, and 4 to 16%, respectively). A decline of TSH occurred in each category (from 2% in the omega-3 to 7% in the vitamin D), except for iron (0%). |
*Keywords of relevance are highlighted by the bold-face print.
§For internal inconsistencies in the results reported by Woźniak et al, see text.
Concerning Breese McCoy’s study (130), one comment is the limitation given by the fact that only thyroid function tests were monitored, while thyroid autoantibodies were not.
Some comments deserve the questionnaire-based study on 232 hypothyroid patients from Poland (132), a country where 15.8% of women and 2.5% of men suffer from thyroid disease, with an increase of 4.0 percent points in the year 2019 compared to 2014 (132). First, as properly described by the authors, 24% of participants were diagnosed with additional diseases (8% with polycystic ovary syndrome, 4% with depression, and 2% with insulin resistance). Second, there was enormous variability in the 197/232 hypothyroid patients who took supplements. Various was not only the spectrum of supplements taken (Table 4 of their paper) but also the periodicity of taking them (every day, 74%; irregularly, 16%; every other day, 10%). Concerning the main source of information on supplements, patients chose websites (74%), physicians (52%), family and friends (46%), and social media (43%). About two-thirds of participants took supplements according to the leaflet (71%), while one-third according to physicians’ or pharmacists’ guidelines. Results for the 197 participants (85% of 232) who took the supplements are illustrated in Table 1 of their paper (132). In that table, patients were stratified into 8 categories based on the nutraceutical taken (vitamin D, vitamins B, iron, zinc, multivitamins, selenium, omega-3 acids), with data summarized as a percent of patients reporting a benefit for 8 items, the denominator being the said 197 participants. We think that the results of this study (132) should be interpreted cautiously, mainly because it is not possible to distinguish the effect of a given nutraceutical taken alone from that of the same nutraceutical taken in association with others, and because the numbers presented in the said Table 1 are internally inconsistent. For instance, in the initial text of the Results section, the authors state that zinc supplementation was taken by 8% of the participants, therefore by 16 participants (0.08 x 197). However, upon adding the percentages reported in Table 1, the sum is 26, meaning that the number of participants claiming a benefit from zinc supplementation was 51 (0.26 x 197). For the omega-3 users, the sum of percentages is 23, meaning that the number of participants claiming a benefit from such supplementation was 45. Yet, from the initial text in the Results section, omega-3 users had to be 29 or 30 (0.15 x 197 = 29.5). Other obvious limitations of this Polish study (132) are the lack of details on the degree of hypothyroidism (overt, subclinical, either), the magnitude of change in serum levels of TSH, and having neglected to obtain information on changes in serum thyroid autoantibodies.
Concerning the mechanism behind the protection from/amelioration of AIT and the decrease in serum levels of thyroid autoantibodies caused by the omega-3 PUFA, two recent studies point to the involvement of resolvins (133, 134). Resolvins, lipoxins, maresins, and protectins are specialized pro-resolving mediators (SPM), which are downstream derivatives of PUFA (133). Resolvins of the E series (RVE1-RVE3) derive from EPA, while resolvins of the D series (RVD1-RVD6) derive from DHA. Concerning RVE-1 (5S,12R,18R-trihydroxy-eicosapentaenoic acid), which is the resolvin investigated by Song et al. (133), it triggers all aspects of the pro-resolution cascade, from inhibiting lymphocyte aggregation at the inflammation site to efferocytosis (that is, the process by which apoptotic cells are removed by phagocytic cells). The cell receptor for REV-1 is chemokine-like receptor 1 (chemR23 or CMKLR) (135), with the ligand-receptor interaction triggering anti-inflammatory responses that include marked inhibition of proinflammatory cytokines and chemokines (136). In one article, Song et al. (133) measured serum levels of REV-1 and serum thyroid parameters (TSH, T4, FT4, FT3, TPOAb, and TgAb) in 30 untreated HT patients (median and interquartile range [IQR] of TSH= 2.62, 1.95–3.05 µIU/ml) and 27 age- and sex-matched healthy controls (TSH= 2.38, IQR= 1.54–2.94, P= 0.178 vs. patients). Levels of RVE1 in patients were significantly lower compared to controls (median and IQR= 24.09, 15.76–34.38 pg/mL vs 28.51, 20.76–51.23 pg/mL). RVE1 levels correlated inversely with increasing TgAb levels in both the unadjusted model (OR= 0.945, P= 0.002) and adjusted models (OR= 0.938, P= 0.005). RVE1 levels were the lowest (19.21, 13.81–27.34 pg/mL) in the highest quartile group of TgAb levels (TgAb >361 IU/mL), and the highest (37.70, 24.66–99.16 pg/mL) in the lowest quartile group (TgAb <34 IU/mL) (19.21, 13.81–27.34 pg/mL vs 37.70, 24.66–99.16 pg/mL). Concerning TPOAb, the association with serum levels of REV1 was an inverted U-shaped curve with the lowest RVE1 levels (median and IQR= 19.21, 15.11–26.01) in the highest quartile of TPOAb levels (>431 U/mL) and the highest 31.39 (23.79–62.31) in the second quartile of TPOAb levels (13.6–106 U/mL). RVE1 levels correlated negatively also with T3 and FT3 (133). In another article, Song et al. (134) addressed RVD1 (7S, 8R, 17S-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid) by measuring its serum levels in 30 patients with HT and 33 healthy controls. Serum concentrations of RVD1 in patients were significantly lower than in controls (median and IQR= 134.76, 85.35-201.36 pg/mL vs 187.64, 131.01-326.85 pg/mL). There was a significantly negative correlation between the TPOAb levels and the RVD1 levels. The authors also measured serum levels of the inflammatory chemokine IP-10/CXCL-10, and found that this chemokine had a significant negative association with TPOAb in the patients (134). Noteworthy, the administration of DHA (daily doses of 300 mg/kg) combined with T3 (0.05 mg/kg) to rats increased significantly the content of RVD1 and RVD2 in the liver, but left unchanged that of RVE1 and RVE2, indicating a synergistic effect compared to treatment with DHA alone and T3 alone (118). The authors concluded that co-administration of T3 and DHA enhances the capacity of liver for the resolution of inflammation by increasing RVD1 and RVD2 availability, so such co-administration constitutes an important hepatoprotective protocol for clinical purposes (118).
Omega-3 PUFA-Based Nutraceuticals in the Setting of Thyroid Disorders: Experimental Setting
Experimental studies that have evaluated the thyroidal effects of the omega-3 PUFA (137–143) are summarized in Table 5. Omega-3 PUFA were shown to have a neuroprotective (138, 139) and cardioprotective (143) action on unfavorable effects caused by thyroid hormone deficiency. On the other hand, the omega-3 PUFA mitigate, ameliorate certain hyperthyroidism-induced unfavorable effects, such as arrhythmias (141) and hepatic dysfunction (137). Such favorable effects of the omega-3 PUFA on consequences in the peripheral tissues that are caused by opposite states of thyroid dysfunction confer to the omega-3 PUFA a modulatory role that is reminiscent of another dietary nutrient, L-carnitine. Indeed, beneficial effects of L-carnitine were reported both in the hyperthyroidism setting (144, 145) and in the hypothyroidism setting (146, 147), with a modulatory role also demonstrated in the tissue glucocorticoid hormone action (148).
Table 5.
Studies in experimental animals on the effects of the omega-3 polyunsaturated fatty acids (PUFA) in the thyroid setting. *
| Reference | Aims and animals studied | Main findings |
|---|---|---|
| Soukup (137) | To review whether i) administration of n-3 PUFA could improve thyroid hormone (TH)-induced pathophysiological changes such as cardiac tissue remodeling and cell-to-cell communication changes, skeletal muscle protein alterations, alterations in the expression of protein kinases, oxidative stress markers, and cell death, mitochondrial functions, changes in serum lipid levels, in activities of key enzymes of TH metabolism and acetylcholine esterase or membrane anisotropy, as well as in thermal sensitivity and mobile behavior. | Soukup et al. think that there is a rationale for n-3 PUFA administration being capable to ameliorate TH-induced pathophysiological changes in settings such as cardiac disorders. For instance, n-3 PUFA intake significantly reduced cardiovascular risk factors, as they suppressed the incidence of ventricular fibrillation and facilitated sinus rhythm restoration in spontaneously hypertensive rats (SHR) in the early and late stages of hypertension. The antiarrhythmic effects of n-3 PUFA can be attributed to the attenuation of abnormal myocardial gap junction protein connexin 43 (Cx43) distribution, expression, and phosphorylation, as well as to positive modulation of PKCϵ and PKCδ signaling and normalization of myosin heavy chain (MyHC) profiles. Indeed, intercellular Cx43 gap junction channels are involved in the increased susceptibility of the heart to arrhythmias caused by increased TH levels; the expression of PKCϵ, which directly phosphorylates Cx43, is affected. |
| Sinha et al. (138) | A murine model of hypothyroidism-induced neuronal apoptosis was used to investigate the role of omega-3-fatty acids (ω-3 FAs) in regulating neuronal apoptosis during brain development. Pregnant and lactating rats with methimazole-induced primary hypothyroidism were supplemented with a mixture of EPA and DHA. Apoptosis was studied on cerebella from postnatal day 16 (d16) pups. | In the hypothyroid pups, Ω-3 FA-supplementation did not significant. The percentages of DHA and EPA in total cerebellar FAs increased significantly in the ω-3 FA-treated hypothyroid pups compared to euthyroid pups (Ep) and untreated hypothyroid pups (UHp). The cerebellar weight of ω-3 FA-treated hypothyroid pups was similar to that of Ep and greater than that of UHp. In the developing cerebellum of the hypothyroid pups, Ω-3 FA-supplementation decreased significantly DNA fragmentation and caspase-3. The protection given by ω-3 FAs was associated with their ability to prevent increased levels of pro-apoptotic basal cell lymphoma protein-2 (Bcl-2)-associated X protein (Bax) in the cerebellum during hypothyroidism. Ω-3 FAs increased the levels of anti-apoptotic proteins like Bcl-2 and Bcl-extra-large (Bcl-x(L)), which were low in UHp. Ω-3 FAs also restored levels of cerebellar phospho (p)-AKT, phospho-c-Jun N-terminal kinase (p-JNK), and phospho-extracellular regulated kinase (p-ERK), which were low in UHp. In sum, data support the anti-apoptotic role of ω-3 FAs during cerebellar development. |
| Pal et al. (139) | To study the effects of iodine and n-3 fatty acids [FA]), separately and together, in the progeny of an iodine-deficient (ID) pregnant rat model. The supplementation diets were: (i) low-iodine diet (LID), (ii) LID+potassium iodide (KI), (iii) LID+n-3 FA, and (iv) LID+KI+n-3 FA. Morphological and biochemical parameters at the peak of cerebellar histogenesis on postnatal day 16 (P16) and for both neurobehavioural and motor coordination parameters at P40 were studied. |
n-3 FA significantly improved morphological, functional and biochemical indices of the developing cerebellum, despite no improvement in circulating thyroid hormone levels. Co-supplementation with n-3 FA and iodine rescued the loss of neurotrophic support, and salvaged motor coordination, learning and memory. This additive effect resulted in significantly improving neurotrophic support and seemed to be mediated by parallel significant increase in TH receptor (TR)α and normalization of TRβ, p75 neurotrophin receptor and retinoic orphan receptor α, as well as prevention of apoptosis and strengthening of anti-oxidative defense. Thus, n-3 FA may play an important mitigating role in iodine deficiency in enhancing TH nuclear receptor-mediated signaling in the developing cerebellum. |
| Abd Allah et al. (140) | To study the effect of hypothyroidism on memory and spatial learning in adult male rats (n= 30), the underlying mechanisms and the possible therapeutic value of omega-3 supplementation. Rats were divided into three groups; hypothyroid, omega-3 treated and controls. |
Omega-3 supplementation increased serum total antioxidant capacity, decreased the structural changes of the hippocampus (diffuse vacuolar degeneration and distortion of the pyramidal cells), and decreased the expression of Cav1.2 (the voltage-dependent LTCC alpha 1c subunit) protein, and improved memory deficits. Thus, omega-3 could be useful neuroprotective agents against the cognitive impairment caused by hypothyroidism. |
| Gomaa et al. (141) | To investigate, in adult male rats, (i) the hyperthyroidism-induced hepatic dysfunction (ii) whether such dysfunction could be ameliorated by the administration of omega-3 on hyperthyroidism-induced hepatic dysfunction, and (iii) the underlying mechanisms of this ameliorative effect. Rats (n= 24) were divided into three groups; control (which received water for 6 weeks), hyperthyroid (which received L-T4 orally for 6 weeks) and hyperthyroid omega-3 treated (which received L-T4 for 2 weeks and then co-treated with L-T4 and an omega-3 oral mixture of EPA+DHA for 4 weeks). | The hyperthyroid omega-3 treated group had significantly increased final body weight and body weight gain, decreased liver weight to body weight ratio, decreased serum T3 level, increased serum TSH level, decreased serum levels of transaminases, and TNFα, decreased hepatic levels of total peroxide and IL-1β and increased hepatic levels of total antioxidant capacity when compared with the hyperthyroid group. Liver histopathology also confirmed marked improvement of the lesions caused by hyperthyroidism. In brief, omega-3 has encouraging therapeutic effects against hyperthyroidism-induced hepatic dysfunction. These effects can be attributed to multiple mechanisms: anti-inflammatory, antioxidant, and anti-fibrotic effects. |
| Rauchová et al. (142) | To investigate whether a 6-week supplementation with n-3 PUFA (200 mg/kg of body weight/day intragastrically) would affect lipid metabolism in Lewis male rats with altered thyroid status. | Supplementation of n-3 PUFA did not significantly modify plasma lipid levels in any thyroid status. Also, n-3 PUFA did not modify thyroid dysfunction-induced altered plasma glucose levels. |
| Awumey et al. (143) | To evaluate some heart parameters in hypothyroid rats and euthyroid controls that had received diets enriched in either n-6 or n-3 fatty acids (FA). | In hypothyroid animals fed the n-3 diet, maximum tension was 105% greater than resting compared to 399% in controls. Similar responses to noradrenaline and adrenaline were observed, that is, maximum tension was significantly greater in both hypothyroid and euthyroid rats fed the n-3 diet, but the tension was depressed in the hypothyroid rats. Binding of the β-adrenoceptor antagonist [3H]-dihydroalprenolol to ventricular membranes had high affinity and was saturable, regardless of thyroid status and diet. However, binding affinity (Kd) was higher in hypothyroid rats fed the n-6 diet. The inotropic response to forskolin was the same in hypothyroid animals, regardless of diet, but the maximum developed tension was significantly greater in euthyroid rats fed the n-6 compared to the n-3 diet. The dose-response curve for forskolin was shifted to the right in hypothyroid rats fed the n-3 diet, indicating decreased sensitivity. In sum, the depressed contractility of the hypothyroid heart may be attributed in part to an altered lipid environment of the β-adrenoceptor complex. Moreover, n-3 FA supplementation can significantly increase maximum developed tension in the hypothyroid state. |
*Keywords of relevance are highlighted by the bold-face print.
Protection From Thyroid Disorders Given by Nutraceuticals Other Than the Omega-3 PUFA
The very few studies available in the English-language literature on the omega-3 PUFA-based nutraceuticals in the clinical setting of autoimmune thyroid disorders (130, 131) (Table 4) contrast with the large number of studies that have tested seleno-L-methionine alone (149–151) or seleno-L-methionine combined with myo-inositol (151–157), which is a hexahydroxycyclohexane (C6H12O6) and one of the nine stereoisomers of inositol that plays a pivotal role in many metabolic pathways (158). In the studies with seleno-L-methionine combined with myo-inositol, the benefit was generally greater than that given by seleno-L-methionine alone or myo-inositol alone. Such benefit was based on the reduction of TSH, TgAb, TPOAb levels, and, when studied (154), on the reduction of serum chemokine levels (CXCL10/IP-10). Myo-inositol+seleno-L-methionine treatment protected blood mononuclear cells (PBMC) of either HT or healthy patients from H2O2–induced stress. Furthermore, the association of myo-inositol and seleno-L-methionine decreased the expression of the chemokines CXCL10/IP-10, CCL2/MCP-1 and CXCL9 (159). In another study, which evaluated the thyroid toxicity of cadmium and protection from this toxicity by nutraceuticals (160), Cd induced a marked overexpression MCP-1/CCL2 and CXCL10 in the thyroid. Again, the protection given by seleno-L-methionine combined with myo-inositol was significantly greater than that of either nutraceutical alone (160). In vitro experiments on blood cells or other cells have shown that omega-3 PUFA decrease the secretion of CXCL10/IP-10 (161–163), CXCL9 (164) and CCL2/MCP-1 (11, 165–176).
Another nutraceutical, Aloe vera (also known as Aloe barbadensis), decreased serum levels of thyroid antibodies and improved subclinical hypothyroidism (177). It is pertinent to remind Aloe vera because, among the several nutritional substances contained in its leaves, there are selenium and PUFA, including the omega-6 linoleic acid and the omega-3 linolenic acid, the latter being the second most abundant after one saturated fatty acid (palmitic acid) (178). Noteworthy, in the Aloe vera juice used in the study by Metro et al. (177) the fat content is 0.2%, with saturated fatty acids being absent. As written in that paper (177), “one of the authors decided to take Aloe Barbadensis Miller juice (ABMJ), at the dose of 50 ml every morning on an empty stomach, as a skin soother and laxative”. This author had a history of HT-associated subclinical hypothyroidism, which she monitored with periodical checks of serum thyroid function tests and thyroid autoantibodies. “At the biochemical check performed three months after having started taking ABMJ, she was struck by the remarkable improvement of all indices (Table 1). The improvement was even more impressive six months later” (177). Based on this experience, the effects of ABMJ administration were tested, in a 9-month duration trial, on HT women with levothyroxine-untreated subclinical hypothyroidism and high levels of TPOAb (n= 30). Controls were 15 HT women with untreated subclinical hypothyroidism who were matched for age and baseline levels of TPOAb, TSH, FT4 and FT3. In the Aloe-treated group, TSH, FT4 and TPOAb improved significantly already at month 3 and further (−61%, +23% and −56%, respectively) at month 9. At baseline, 100% of women had TSH > 4.0 mU/L and TPOAb > 400 U/ml, but at month 9 rates fell to 0% and 37%, respectively. In contrast, the control group had no significant changes in any index (177).
Of the three aforementioned chemokines (CXCL10/IP-10, CCL2/MCP-1 and CXCL9), only one (MCP-1) was evaluated in terms of response to Aloe vera, and only by two studies (179, 180). In one study (179), a wound dressing containing an Aloe vera extract plus gelatin decreased the production of MCP-1 by 75% in human mesenchymal stem cells treated with TGFβ. In the other study (180) the oral administration of two antidiabetic phytosterols isolated from Aloe vera lophenol (lophenol and cycloartanol) decreased serum and hepatic concentrations of MCP-1 in Zucker diabetic fatty (ZDF) rats. Nevertheless, there is abundant literature on Aloe vera being able to decrease the production of pro-inflammatory cytokines and chemokines (181–188).
Omega-3 PUFA and Oily Fish in the Nutraceutical Market
Based on an article of May 2018 by the main Italian press agency ANSA (189), Italy is the first European country for nutraceutical products based on per capita expenditure, that is, euro 40 compared to euro 28 of the EU. Italians spend more than euro 3.2 billion on dietary/supplements/nutraceutical products. A major driver for this expenditure is wishing to prevent and/or treat metabolic diseases (189) Based on the survey “The Italian food supplement supply chain, 2019-2020” by the FederSalus Research Centre (that is the national agency of the Italian producers and distributors of food supplements), in late 2019, the food supplement market in Italy reached a value of approximately euro 3.6 billion (190), corresponding to 27% of the euro 13.2 billion value of the European market, thus preceding Germany and France (18 and 8%, respectively). In particular, 32 million persons used supplements (65% of the adult Italian population) and 261 million packs (equal to 8 per capita) were sold in 2019 (190). At Italian pharmacies, the main sales channel, supplements are confirmed to be the second category after prescription drugs and give the greatest boost to growth, with 28.6 million medical prescriptions issued for food supplements in 2019 (190). Interestingly, the leading medical category that accounts for most of the 28.6 million medical prescriptions is represented by the general practitioners (21%) (190). Endocrinologists are absent in the first 8 positions, but the document fails to specify which categories are represented in the 9th category (“others”), a category that accounts for 12% of the prescriptions (190). In terms of comparison with the United States, based on data from a decade ago, annual supplement sales were USD 23 billion, with approximately 40,000 supplements products being on the market (191). In 2015, the American market for supplements was valued at USD 37 billion (151), but expected to reach USD 56.7 billion by 2024 (192) or USD 117.92 billion by 2027 (193).
With regard to the omega-3 products, their global market was valued at USD 2.10 billion in 2020 (USD 554.8 million in the US alone) (194), and it is projected to go up at a compound annual growth rate (CAGR) of 7.4% during the from 2020 to 2025 (195) and 7.8% from 2020 to 2028 (194). The main factors responsible for such increased consumption include the rising frequency of cardiovascular diseases (CVDs), changing dietary habits, the rising importance of immunity development post-COVID-19 and an increasing number of omega-3-based pharmaceutical product launches. DHA dominated the market in 2020, but EPA is expected to grow faster because of the increasing demand for immunity-boosting supplements (194).
The marine source segment of omega-3 supplements held the largest revenue share of over 82.8% in 2020. Fish oil (which contains both DHA and EPA) is the major marine source, and it is derived mainly from anchovy fish. Prices of omega-3 PUFA reflect the extraction and processing costs of fish oil, and they change depending on the availability of anchovy fish. Increasing contamination of fish by Hg and other pollutants is expected to negatively affect the prices of fish oil (194). Fish oils are currently considered the best and generally a safe source of omega-3 (196). However, the declining fish population due to overfishing has led to searching for more sustainable sources. Krill oil (which contains both DHA and EPA) and algae oil (which contains only DHA) have gained greater importance in recent years as alternatives, with algae serving as an option for the vegetarian population (197). In the US market, 9% of grocery shoppers buy high-omega-3 food or beverages in a typical shopping trip, with the proportion of adults who take fish oil supplements have increased from 8% in 2006 to 17% in 2011 (198). Omega-3 PUFA are also derived from plant sources including walnuts, pumpkin seeds, soybean oil, flaxseed oil, and canola oil, with plant oils being major sources of alpha-linolenic (ALA).
By comparison, the European market of the omega-3 products during the period (2019–2024) is expected to reach USD 14.61 billion by 2024 growing at a CAGR of 7% (199). The European demand for DHA is forecasted to increase significantly due to the favorable regulations in the European Union, which made DHA a mandatory ingredient in infant formula from 2020. Chia seeds are gaining popularity in Europe because of their nutritional and health properties that derive from their content in omega-3. Germany is the top European importer of chia seeds, preceding the Netherlands, Spain, and the United Kingdom (199).
In 2010, Friend of the Sea (FoS), an international nongovernment organization with the mission of promoting environmental conservation, introduced specific standards for producers of fish oil, fishmeal, fish feed and omega-3 supplements (200). Accredited third-party certification bodies certify that the oil originates from fisheries that are compliant with FoS sustainable fishing requirements, and that a full chain of custody occurs throughout the supply and the production chain (200). As of September 2018, 439 companies (compared to only 76 in 2015) adhere voluntarily to FoS standards for fish oil, fishmeal, fish feed and omega-3 supplement. Certified oils originate mostly from approved Peruvian anchovy fisheries and fleet (Engraulis ringens, 29%), Antarctic krill (Euphausia superba, 22%), European sardine (Sardina pilchardus, 8%), European anchovy (Engraulis encrasicolus, 7%), Chub mackerel (Scomber japonicus, 7%), Atlantic cod (Gadus morhua, 3%) (195). FoS presence in the nutraceutical industry has grown considerably in the United States, where it now accounts for over 50% of total FoS certified supplements (268 companies) (195). The United States are followed by France, Canada, Norway, United Kingdom and Italy, these six countries representing the top 6 countries for FoS labeled supplements.
Some studies are described now to illustrate the bioavalability of different types of omega-3 products. A 4-week randomized, placebo-controlled, double-blinded Icelandic study on 99 adults (of whom 77 completed the study) investigated the bioavailability of LC n-3 PUFAs from microencapsulated powder compared with ready-to-eat meals enriched with liquid fish oil (201). Participants were randomized into three groups 38 received 1.5 g/d EPA and DHA as meals enriched with liquid fish oil; 30 received the same amount of these LC n-3 PUFA as microencapsulated fish oil powder and regular meals; and 31 (controls) received placebo powder and regular meals. The authors found similar bioavailability between ready-to-eat meals enriched with liquid fish oil and LC n-3 PUFAs in encapsulated powder (201).
Starting from the fact that no conclusive information had been published on the relative bioavailability of omega-3 supplements taken as fish oil or krill oil, with few studies suggesting that the phospholipid form (krill) is absorbed better than the fish oil ethyl ester (EE) or triglyceride (TG) forms, Yurko-Mauro et al. (202) compared the oral bioavailability of the same dose of both DHA and EPA in fish oil-EE vs. fish oil-TG vs. krill oil after a four-week supplementation. In this double-blind, randomized, parallel study, 66 healthy adults were supplemented with a 1.3 g/day dose of EPA+DHA (approximately 816 mg/d EPA + 522 mg/d DHA, regardless of formulation) as either fish oil-EE (n=22 participants), fish oil-TG (n=22) or krill oil capsules (n=22). The authors found similar plasma and red blood cell levels of EPA+DHA across fish oil and krill oil products when matched for dose, DHA and EPA concentrations, indicating comparable oral bioavailability irrespective of formulation (197). For instance, mean total plasma DHA+EPA at 672 h were 90.9 ± 41 µg/mL (fish oil-EE), 108 ± 40 µg/mL fish oil-TG (fish oil-TG), and 118.5 ± 48 krill oil (krill oil), with a probability value just borderline significant (P= 0.052). Indeed, upon perusing figures in this paper (202), a decreasing hierarchy is evident for both plasma and red blood cell levels of DHA+EPA across all time points: krill oil > fish oil-TG > fish oil-EE. Furthermore, in a Canadian double-blinded, randomized, placebo-controlled, crossover trial on 24 volunteers, krill oil resulted more effective than fish oil in increasing n-3 PUFA both in plasma (P = 0.0043) and in red blood cells (P = 0.0011) (203). This study consisted of three treatment phases including krill or fish oil, each providing 600 mg of n-3 PUFA or placebo control, corn oil in capsule form (203).
DHA and EPA are added to several commercially available foods, such as infant and pet formulas, and they are also supplemented in animal feed to incorporate them in consumer dairy, meat, and poultry products (204). The main sources of EPA and DHA are fish oils or purified preparations from microalgae (204). In a one-month duration Australian study (205), 16 healthy males were provided with a range of foodstuffs naturally containing LC n-3 PUFA (fresh and canned fish, canola oil, flaxseed meal) and items fortified with fish oil (sausages, milk, margarine spread, luncheon meat, French onion dip); food choices were left to the discretion of each participant. Blood and cell levels of ALA, EPA and DHA increased highly significantly after 4 weeks (P<0.001) (205).
Conclusions
Because of the systemic action of thyroid hormones and their pleiotropic effects, thyroid disrupting chemicals (such as those mentioned in Table 3) represent a public health issue, making the comprehension of the mechanisms through which they interfere on thyroid homeostasis considerably importance.
Considering (i) the increasing incidence of both HT and TC worldwide (206–213); (ii) the aforementioned predisposing role for TC (particularly, papillary TC) and its advanced stages exerted not only by HT but also by even trendwise high serum TSH levels per se (65–88); (iii) the risk for developing metabolic and cardiovascular disorders conferred by both elevated/trendwise elevated serum TSH levels and thyroid autoimmunity (214–230), then it would be beneficial to contrast the appearance and/or duration of HT as well as to correct the slightly elevated serum TSH levels of subclinical hypothyroidism, the leading etiology of which is AIT. Furthermore, HT is frequently associated with other endocrine and nonendocrine autoimmune diseases, on which omega-3 PUFA proved to be beneficial [see above, Introduction]. For instance, 19.5% of 3,069 HT patients had evidence of at least one other autoimmune disease compared to 3.6% of 1,023 patients with multinodular goiter (231). As a corollary, there would be the place for the use of nutraceuticals to prevent/delay/minimize the onset/burden of autoimmune thyroiditis and the magnitude of TSH elevation. Taking into account their aforementioned anti-autoimmunity and anti-cancer (including TC) properties, the omega-3 PUFA would be appropriate nutraceuticals to be used, either alone or combined with other supplements.
Author Contributions
Writing - original draft: SB and FF. Writing - review and editing: SB, FF, LP, AA, GB, FV and MM. Supervision: SB, FV and MM. All authors have read and approved the final manuscript and all materials before submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SF declared a past collaboration with the authors AA, SB to the handling editor.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. EUMOFA . THE EU FISH MARKET 2021 EDITION (2022). Available at: https://www.eumofa.eu/documents/20178/477018/EN_The+EU+fish+market_2021.pdf/27a6d912-a758-6065-c973-c1146ac93d30?t=1636964632989 (Accessed January 19, 2022).
- 2. CBI Ministry of Foreign Affairs . The European Market Potential for Tuna by-Catch Species (2022). Available at: https://www.cbi.eu/market-information/fish-seafood/tuna/market-potential (Accessed January 19, 2022).
- 3. Europe Oceana . ICCAT: SWORDFISH (2022) . Available at: https://europe.oceana.org/en/our-work/swordfish/overview (Accessed January 19, 2022).
- 4. Torres-Escribano S, Vélez D, Montoro R. Mercury and Methylmercury Bioaccessibility in Swordfish. Food Addit Contam Part A Chem Anal Control Expo Risk Assess (2010) 27:327–237. doi: 10.1080/19440040903365272 [DOI] [PubMed] [Google Scholar]
- 5. ASA (Associazione Stampa Agroalimentare Italiana) . PRESS. LA BORSA DELLA SPESA (2022). Available at: https://www.asa-press.com/archivio/r-spesa/borsa2.html (Accessed January 19, 2022).
- 6. ESSERE ANIMALI . SPADARE, ARPIONI, PALANGARI: NON C’è TREGUA PER IL PESCE SPADA . Available at: https://www.essereanimali.org/2020/08/spadare-arpioni-palangari-consumo-pesce-spada/ (Accessed January 19, 2022).
- 7. Giosuè C, Gancitano V, Sprovieri M, Bono G, Vitale S. A Responsible Proposal for Italian Seafood Consumers. Eur J Sustain Dev (2018) 7:523–44. doi: 10.14207/ejsd.2018.v7n3p523 [DOI] [Google Scholar]
- 8. Maulu S, Nawanzi K, Abdel-Tawwab M, Khalil HS. Fish Nutritional Value as an Approach to Children's Nutrition. Front Nutr (2021) 8:780844. doi: 10.3389/fnut.2021.780844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Pora BLR, Dong K, Hasjim J. Health Benefits of Docosahexaenoic Acid and its Bioavailability: A Review. Food Sci Nutr (2021) 9:5229–43. doi: 10.1002/fsn3.2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kapoor B, Kapoor D, Gautam S, Singh R, Bhardwaj S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr Nutr Rep (2021) 10:232–42. doi: 10.1007/s13668-021-00363-3 [DOI] [PubMed] [Google Scholar]
- 11. Pisaniello AD, Psaltis PJ, King PM, Liu G, Gibson RA, Tan JT, et al. Omega-3 Fatty Acids Ameliorate Vascular Inflammation: A Rationale for Their Atheroprotective Effects. Atherosclerosis (2021) 324:27–37. doi: 10.1016/j.atherosclerosis.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 12. Chen C, Huang H, Dai QQ, Ren J, Cai HH, Hu WJ, et al. Fish Consumption, Long-Chain Omega-3 Fatty Acids Intake and Risk of Stroke: An Updated Systematic Review and Meta-Analysis. Asia Pac J Clin Nutr (2021) 30:140–52. doi: 10.6133/apjcn.202103_30(1).0017 [DOI] [PubMed] [Google Scholar]
- 13. Chen J, Jayachandran M, Bai W, Xu B. A Critical Review on the Health Benefits of Fish Consumption and its Bioactive Constituents. Food Chem (2022) 369:130874. doi: 10.1016/j.foodchem.2021.130874 [DOI] [PubMed] [Google Scholar]
- 14. Mendivil CO. Fish Consumption: A Review of Its Effects on Metabolic and Hormonal Health. Nutr Metab Insights (2021) 14:11786388211022378. doi: 10.1177/11786388211022378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu C, Ralston NVC. Seafood and Health: What You Need to Know? Adv Food Nutr Res (2021) 97:275–318. doi: 10.1016/bs.afnr.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 16. Li X, Bi X, Wang S, Zhang Z, Li F, Zhao AZ. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front Immunol (2019) 10:2241. doi: 10.3389/fimmu.2019.02241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benvenga S, Vigo MT, Metro D, Granese R, Vita R, Le Donne M. Type of Fish Consumed and Thyroid Autoimmunity in Pregnancy and Postpartum. Endocrine (2016) 52:120–9. doi: 10.1007/s12020-015-0698-3 [DOI] [PubMed] [Google Scholar]
- 18. Benvenga S, Vita R, Di Bari F, Granese R, Metro D, Le Donne M. Stable Consumption of Swordfish Favors, Whereas Stable Consumption of Oily Fish Protects From, Development of Postpartum Thyroiditis. Endocrine (2019) 65:94–101. doi: 10.1007/s12020-019-01882-4 [DOI] [PubMed] [Google Scholar]
- 19. Pattison DJ, Harrison RA, Symmons DP. The Role of Diet in Susceptibility to Rheumatoid Arthritis: A Systematic Review. J Rheumatol (2004) 31:1310–9. [PubMed] [Google Scholar]
- 20. Koller-Smith L, Mehdi AM, March L, Tooth L, Mishra G, Thomas R. Rheumatoid Arthritis is a Preventable Disease: 11 Ways to Reduce Your Patients' Risk. Intern Med J (2021). doi: 10.1111/imj.15537 [DOI] [PubMed] [Google Scholar]
- 21. Asoudeh F, Jayedi A, Kavian Z, Ebrahimi-Mousavi S, Nielsen SM, Mohammadi H. A Systematic Review and Meta-Analysis of Observational Studies on the Association Between Animal Protein Sources and Risk of Rheumatoid Arthritis. Clin Nutr (2021) 40:4644–52. doi: 10.1016/j.clnu.2021.05.026 [DOI] [PubMed] [Google Scholar]
- 22. Di Giuseppe D, Wallin A, Bottai M, Askling J, Wolk A. Long-Term Intake of Dietary Long-Chain N-3 Polyunsaturated Fatty Acids and Risk of Rheumatoid Arthritis: A Prospective Cohort Study of Women. Ann Rheum Dis (2014) 73:1949–53. doi: 10.1136/annrheumdis-2013-203338 [DOI] [PubMed] [Google Scholar]
- 23. Tedeschi SK, Bathon JM, Giles JT, Lin TC, Yoshida K, Solomon DH. Relationship Between Fish Consumption and Disease Activity in Rheumatoid Arthritis. Arthritis Care Res (Hoboken) (2018) 70:327–32. doi: 10.1002/acr.23295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sköldstam L, Börjesson O, Kjällman A, Seiving B, Akesson B. Effect of Six Months of Fish Oil Supplementation in Stable Rheumatoid Arthritis. A Double-Blind, Controlled Study. Scand J Rheumatol (1992) 21:178–85. doi: 10.3109/03009749209099218 [DOI] [PubMed] [Google Scholar]
- 25. Langer-Gould A, Black LJ, Waubant E, Smith JB, Wu J, Gonzales EG, et al. Seafood, Fatty Acid Biosynthesis Genes, and Multiple Sclerosis Susceptibility. Mult Scler (2020) 26:1476–85. doi: 10.1177/1352458519872652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rezaeizadeh H, Mohammadpour Z, Bitarafan S, Harirchian MH, Ghadimi M, Homayon IA. Dietary Fish Intake and the Risk of Multiple Sclerosis: A Systematic Review and Meta-Analysis of Observational Studies. Nutr Neurosci (2022) 25:681–9. doi: 10.1080/1028415X.2020.1804096 [DOI] [PubMed] [Google Scholar]
- 27. Black LJ, Zhao Y, Peng YC, Sherriff JL, Lucas RM, van der Mei I, et al. Higher Fish Consumption and Lower Risk of Central Nervous System Demyelination. Eur J Clin Nutr (2020) 74:818–24. doi: 10.1038/s41430-019-0476-z [DOI] [PubMed] [Google Scholar]
- 28. Bäärnhielm M, Olsson T, Alfredsson L. Fatty Fish Intake is Associated With Decreased Occurrence of Multiple Sclerosis. Mult Scler (2014) 20:726–32. doi: 10.1177/1352458513509508 [DOI] [PubMed] [Google Scholar]
- 29. Syrjälä E, Nevalainen J, Peltonen J, Takkinen HM, Hakola L, Åkerlund M, et al. A Joint Modeling Approach for Childhood Meat, Fish and Egg Consumption and the Risk of Advanced Islet Autoimmunity. Sci Rep (2019) 9:7760. doi: 10.1038/s41598-019-44196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abdel-Megeid AA, Ael-R A, SS E, Ibrahim AM. Effect of Different Types of Fish on Rats Suffering From Diabetes. Nutr Health (2008) 19:257–71. doi: 10.1177/026010600801900402 [DOI] [PubMed] [Google Scholar]
- 31. Purdel C, Ungurianu A, Margina D. Metabolic and Metabolomic Insights Regarding the Omega-3 PUFAs Intake in Type 1 Diabetes Mellitus. Front Mol Biosci (2021) 8:783065. doi: 10.3389/fmolb.2021.783065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cadario F, Pozzi E, Rizzollo S, Stracuzzi M, Beux S, Giorgis A, et al. Vitamin D and ω-3 Supplementations in Mediterranean Diet During the 1st Year of Overt Type 1 Diabetes: A Cohort Study. Nutrients (2019) 11:2158. doi: 10.3390/nu11092158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bi X, Li F, Liu S, Jin Y, Zhang X, Yang T, et al. ω-3 Polyunsaturated Fatty Acids Ameliorate Type 1 Diabetes and Autoimmunity. J Clin Invest (2017) 127:1757–71. doi: 10.1172/JCI87388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, et al. Omega-3 Polyunsaturated Fatty Acid Intake and Islet Autoimmunity in Children at Increased Risk for Type 1 Diabetes. JAMA (2007) 298:1420–8. doi: 10.1001/jama.298.12.1420 [DOI] [PubMed] [Google Scholar]
- 35. Vitlov Uljević M, Starčević K, Mašek T, Bočina I, Restović I, Kević N, et al. Dietary DHA/EPA Supplementation Ameliorates Diabetic Nephropathy by Protecting From Distal Tubular Cell Damage. Cell Tissue Res (2019) 378:301–17. doi: 10.1007/s00441-019-03058-y [DOI] [PubMed] [Google Scholar]
- 36. Lewis EJH, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of Omega-3 Supplementation on Neuropathy in Type 1 Diabetes: A 12-Month Pilot Trial. Neurology (2017) 88:2294–301. doi: 10.1212/WNL.0000000000004033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. WIKIPEDIA . PESCE AZZURRO (2022). Available at: https://it.wikipedia.org/wiki/Pesce_azzurro (Accessed January 19, 2022).
- 38. Greupner T, Kutzner L, Nolte F, Strangmann A, Kohrs H, Hahn A, et al. Effects of a 12-Week High-α-Linolenic Acid Intervention on EPA and DHA Concentrations in Red Blood Cells and Plasma Oxylipin Pattern in Subjects With a Low EPA and DHA Status. Food Funct (2018) 9:1587–600. doi: 10.1039/c7fo01809f [DOI] [PubMed] [Google Scholar]
- 39. Stephen NM, Jeya Shakila R, Jeyasekaran G, Sukumar D. Effect of Different Types of Heat Processing on Chemical Changes in Tuna. J Food Sci Technol (2010) 47:174–81. doi: 10.1007/s13197-010-0024-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zotos A, Kotaras A, Mikras E. Effect of Baking of Sardine (Sardina Pilchardus) and Frying of Anchovy (Engraulis Encrasicholus) in Olive and Sunflower Oil on Their Quality. Food Sci Technol Int (2013) 19:11–23. doi: 10.1177/1082013212442179 [DOI] [PubMed] [Google Scholar]
- 41. Zuluaga Rodríguez J, Gallego Ríos SE, Ramírez Botero CM. Content of Hg, Cd, Pb and As in Fish Species: A Review. Vitae (2015) 22:148–59. doi: 10.17533/udea.vitae.v22n2a09 [DOI] [Google Scholar]
- 42. Al Taee SK, Al-Mallah K, Ismail HHK. Review On Some Heavy Metals Toxicity On Freshwater Fishes. J Appl Vet Sci (2020) 5:78–86. doi: 10.21608/javs.2020.100157 [DOI] [Google Scholar]
- 43. Yousifi RA, Choudhary MI, Ahmed S, Ahmed Q. Bioaccumulation of Heavy Metals in Fish and Other Aquatic Organisms From Karachi Coast, Pakistan. Nusantara Biosci (2021) 13:73–84. doi: 10.13057/nusbiosci/n130111 [DOI] [Google Scholar]
- 44. Amirhosseini M, Alkaissi H, Hultman PA, Havarinasab S. Autoantibodies in Outbred Swiss Webster Mice Following Exposure to Gold and Mercury. Toxicol Appl Pharmacol (2021) 412:115379. doi: 10.1016/j.taap.2020.115379 [DOI] [PubMed] [Google Scholar]
- 45. Wacewicz-Muczyńska M, Socha K, Soroczyńska J, Niczyporuk M, Borawska MH. Cadmium, Lead and Mercury in the Blood of Psoriatic and Vitiligo Patients and Their Possible Associations With Dietary Habits. Sci Total Environ (2021) 757:143967. doi: 10.1016/j.scitotenv.2020.143967 [DOI] [PubMed] [Google Scholar]
- 46. Kern JK, Geier DA, Mehta JA, Homme KG, Geier MR. Mercury as a Hapten: A Review of the Role of Toxicant-Induced Brain Autoantibodies in Autism and Possible Treatment Considerations. J Trace Elem Med Biol (2020) 62:126504. doi: 10.1016/j.jtemb.2020.126504 [DOI] [PubMed] [Google Scholar]
- 47. Bjørklund G, Peana M, Dadar M, Chirumbolo S, Aaseth J, Martins N. Mercury-Induced Autoimmunity: Drifting From Micro to Macro Concerns on Autoimmune Disorders. Clin Immunol (2020) 213:108352. doi: 10.1016/j.clim.2020.108352 [DOI] [PubMed] [Google Scholar]
- 48. Lu M, Khera S. Autoimmune Manifestations of Acute Mercury Toxicity. Clin Pediatr (Phila) (2020) 59:816–8. doi: 10.1177/0009922820915885 [DOI] [PubMed] [Google Scholar]
- 49. Pollard KM, Cauvi DM, Toomey CB, Hultman P, Kono DH. Mercury-Induced Inflammation and Autoimmunity. Biochim Biophys Acta Gen Subj (2019) 1863:129299. doi: 10.1016/j.bbagen.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Crowe W, Allsopp PJ, Watson GE, Magee PJ, Strain JJ, Armstrong DJ, et al. Mercury as an Environmental Stimulus in the Development of Autoimmunity - A Systematic Review. Autoimmun Rev (2017) 16:72–80. doi: 10.1016/j.autrev.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 51. Kindgren E, Guerrero-Bosagna C, Ludvigsson J. Heavy Metals in Fish and its Association With Autoimmunity in the Development of Juvenile Idiopathic Arthritis: A Prospective Birth Cohort Study. Pediatr Rheumatol Online J (2019) 17:33. doi: 10.1186/s12969-019-0344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Popov Aleksandrov A, Mirkov I, Tucovic D, Kulas J, Zeljkovic M, Popovic D, et al. Immunomodulation by Heavy Metals as a Contributing Factor to Inflammatory Diseases and Autoimmune Reactions: Cadmium as an Example. Immunol Lett (2021) 240:106–22. doi: 10.1016/j.imlet.2021.10.003 [DOI] [PubMed] [Google Scholar]
- 53. Nie X, Chen Y, Chen Y, Chen C, Han B, Li Q, et al. Lead and Cadmium Exposure, Higher Thyroid Antibodies and Thyroid Dysfunction in Chinese Women. Environ Pollut (2017) 230:320–8. doi: 10.1016/j.envpol.2017.06.052 [DOI] [PubMed] [Google Scholar]
- 54. Ohsawa M. Heavy Metal-Induced Immunotoxicity and its Mechanisms. Yakugaku Zasshi (2009) 129:305–19. doi: 10.1248/yakushi.129.305 [DOI] [PubMed] [Google Scholar]
- 55. Leffel EK, Wolf C, Poklis A, White KL, Jr. Drinking Water Exposure to Cadmium, an Environmental Contaminant, Results in the Exacerbation of Autoimmune Disease in the Murine Model. Toxicology (2003) 188:233–50. doi: 10.1016/s0300-483x(03)00092-1 [DOI] [PubMed] [Google Scholar]
- 56. Barone G, Dambrosio A, Storelli A, Garofalo R, Busco VP, Storelli MM. Estimated Dietary Intake of Trace Metals From Swordfish Consumption: A Human Health Problem. Toxics (2018) 6:22. doi: 10.3390/toxics6020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Forsyth DS, Casey V, Dabeka RW, McKenzie A. Methylmercury Levels in Predatory Fish Species Marketed in Canada. Food Addit Contam (2004) 21:849–56. doi: 10.1080/02652030400004259 [DOI] [PubMed] [Google Scholar]
- 58. U.S. Food and Drug Administration . Mercury Levels in Commercial Fish and Shellfish 1990-2012 (2022). Available at: https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012 (Accessed January 19, 2022).
- 59. Karimi R, Silbernagel S, Fisher NS, Meliker JR. Elevated Blood Hg at Recommended Seafood Consumption Rates in Adult Seafood Consumers. Int J Hyg Environ Health (2014) 217:758–864. doi: 10.1016/j.ijheh.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 60. Gallagher CM, Meliker JR. Mercury and Thyroid Autoantibodies in U.S. Women, NHANES 2007-2008. Environ Int (2012) 40:39–43. doi: 10.1016/j.envint.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 61. Sterzl I, Prochazkova J, Hrda P, Matucha P, Bartova J, Stejskal V. Removal of Dental Amalgam Decreases Anti-TPO and Anti-Tg Autoantibodies in Patients With Autoimmune Thyroiditis. Neuro Endocrinol Lett (2006) 27:25–30. [PubMed] [Google Scholar]
- 62. Pamphlett R, Doble PA, Bishop DP. Mercury in the Human Thyroid Gland: Potential Implications for Thyroid Cancer, Autoimmune Thyroiditis, and Hypothyroidism. PloS One (2021) 16:e0246748. doi: 10.1371/journal.pone.0246748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Malandrino P, Russo M, Ronchi A, Moretti F, Gianì F, Vigneri P, et al. Concentration of Metals and Trace Elements in the Normal Human and Rat Thyroid: Comparison With Muscle and Adipose Tissue and Volcanic Versus Control Areas. Thyroid (2020) 30:290–9. doi: 10.1089/thy.2019.0244 [DOI] [PubMed] [Google Scholar]
- 64. Malandrino P, Russo M, Gianì F, Pellegriti G, Vigneri P, Belfiore A, et al. Increased Thyroid Cancer Incidence in Volcanic Areas: A Role of Increased Heavy Metals in the Environment? Int J Mol Sci (2020) 21:3425. doi: 10.3390/ijms21103425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Feldt-Rasmussen U. Hashimoto's Thyroiditis as a Risk Factor for Thyroid Cancer. Curr Opin Endocrinol Diabetes Obes (2020) 27:364–71. doi: 10.1097/MED.0000000000000570 [DOI] [PubMed] [Google Scholar]
- 66. Vita R, Ieni A, Tuccari G, Benvenga S. The Increasing Prevalence of Chronic Lymphocytic Thyroiditis in Papillary Microcarcinoma. Rev Endocr Metab Disord (2018) 19:301–9. doi: 10.1007/s11154-018-9474-z [DOI] [PubMed] [Google Scholar]
- 67. Zirilli G, Salzano G, Corica D, Pajno GB, Mignosa C, Pepe G, et al. Thyrotropin Serum Levels and Coexistence With Hashimoto's Thyroiditis as Predictors of Malignancy in Children With Thyroid Nodules. Ital J Pediatr (2019) 45:96. doi: 10.1186/s13052-019-0693-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Penta L, Cofini M, Lanciotti L, Leonardi A, Principi N, Esposito S. Hashimoto's Disease and Thyroid Cancer in Children: Are They Associated? Front Endocrinol (Lausanne) (2018) 9:565. doi: 10.3389/fendo.2018.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abbasgholizadeh P, Naseri A, Nasiri E, Sadra V. Is Hashimoto Thyroiditis Associated With Increasing Risk of Thyroid Malignancies? A Systematic Review and Meta-Analysis. Thyroid Res (2021) 14:26. doi: 10.1186/s13044-021-00117-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hanege FM, Tuysuz O, Celik S, Sakallıoglu O, Arslan Solmaz O. Hashimoto's Thyroiditis in Papillary Thyroid Carcinoma: A 22-Year Study. Acta Otorhinolaryngol Ital (2021) 41:142–5. doi: 10.14639/0392-100X-N1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Resende de Paiva C, Grønhøj C, Feldt-Rasmussen U, von Buchwald C. Association Between Hashimoto's Thyroiditis and Thyroid Cancer in 64,628 Patients. Front Oncol (2017) 7:53. doi: 10.3389/fonc.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ieni A, Vita R, Magliolo E, Santarpia M, Di Bari F, Benvenga S, et al. One-Third of an Archivial Series of Papillary Thyroid Cancer (Years 2007-2015) Has Coexistent Chronic Lymphocytic Thyroiditis, Which Is Associated With a More Favorable Tumor-Node-Metastasis Staging. Front Endocrinol (Lausanne) (2017) 8:337. doi: 10.3389/fendo.2017.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Uhliarova B, Hajtman A. Hashimoto's Thyroiditis - an Independent Risk Factor for Papillary Carcinoma. Braz J Otorhinolaryngol (2018) 84:729–35. doi: 10.1016/j.bjorl.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Graceffa G, Patrone R, Vieni S, Campanella S, Calamia S, Laise I, et al. Association Between Hashimoto's Thyroiditis and Papillary Thyroid Carcinoma: A Retrospective Analysis of 305 Patients. BMC Endocr Disord (2019) 19:26. doi: 10.1186/s12902-019-0351-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ye ZQ, Gu DN, Hu HY, Zhou YL, Hu XQ, Zhang XH. Hashimoto's Thyroiditis, Microcalcification and Raised Thyrotropin Levels Within Normal Range are Associated With Thyroid Cancer. World J Surg Oncol (2013) 11:56. doi: 10.1186/1477-7819-11-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bircan HY, Koc B, Akarsu C, Demiralay E, Demirag A, Adas M, et al. Is Hashimoto's Thyroiditis a Prognostic Factor for Thyroid Papillary Microcarcinoma? Eur Rev Med Pharmacol Sci (2014) 18:1910–5. [PubMed] [Google Scholar]
- 77. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum Thyrotropin Concentration as a Novel Predictor of Malignancy in Thyroid Nodules Investigated by Fine-Needle Aspiration. J Clin Endocrinol Metab (2006) 91:4295–301. doi: 10.1210/jc.2006-0527 [DOI] [PubMed] [Google Scholar]
- 78. Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, et al. Lower Levels of TSH are Associated With a Lower Risk of Papillary Thyroid Cancer in Patients With Thyroid Nodular Disease: Thyroid Autonomy may Play a Protective Role. Endocr Relat Cancer (2009) 16:1251–60. doi: 10.1677/ERC-09-0036 [DOI] [PubMed] [Google Scholar]
- 79. Huang H, Rusiecki J, Zhao N, Chen Y, Ma S, Yu H, et al. Thyroid-Stimulating Hormone, Thyroid Hormones, and Risk of Papillary Thyroid Cancer: A Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev (2017) 26:1209–18. doi: 10.1158/1055-9965.EPI-16-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hu MJ, Zhang C, Liang L, Wang SY, Zheng XC, Zhang Q, et al. Fasting Serum Glucose, Thyroid-Stimulating Hormone, and Thyroid Hormones and Risk of Papillary Thyroid Cancer: A Case-Control Study. Head Neck (2019) 41:2277–84. doi: 10.1002/hed.25691 [DOI] [PubMed] [Google Scholar]
- 81. Kim HI, Jang HW, Ahn HS, Ahn S, Park SY, Oh YL, et al. High Serum TSH Level Is Associated With Progression of Papillary Thyroid Microcarcinoma During Active Surveillance. J Clin Endocrinol Metab (2018) 103:446–51. doi: 10.1210/jc.2017-01775 [DOI] [PubMed] [Google Scholar]
- 82. Tam AA, Ozdemir D, Aydın C, Bestepe N, Ulusoy S, Sungu N, et al. Association Between Preoperative Thyrotrophin and Clinicopathological and Aggressive Features of Papillary Thyroid Cancer. Endocrine (2018) 59:565–72. doi: 10.1007/s12020-018-1523-6 [DOI] [PubMed] [Google Scholar]
- 83. Golbert L, de Cristo AP, Faccin CS, Farenzena M, Folgierini H, Graudenz MS, et al. Serum TSH Levels as a Predictor of Malignancy in Thyroid Nodules: A Prospective Study. PloS One (2017) 12:e0188123. doi: 10.1371/journal.pone.0188123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Soleimanisardoo L, Rouhani M, Sardoo FS, Gozashti MH. The Effect of Thyroid Stimulating Hormone on Stage of Differentiated Thyroid Carcinoma. Endocrinol Diabetes Metab (2021) 4:e00266. doi: 10.1002/edm2.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mao A, An N, Wang J, Wu Y, Wang T, Wang Z, et al. Association Between Preoperative Serum TSH and Tumor Status in Patients With Papillary Thyroid Microcarcinoma. Endocrine (2021) 73:617–24. doi: 10.1007/s12020-021-02690-5 [DOI] [PubMed] [Google Scholar]
- 86. Zhang X, Zhang X, Chang Z, Wu C, Guo H. Correlation Analyses of Thyroid-Stimulating Hormone and Thyroid Autoantibodies With Differentiated Thyroid Cancer. J BUON (2018) 23:1467–71. [PubMed] [Google Scholar]
- 87. Wu X, Lun Y, Jiang H, Gang Q, Xin S, Duan Z, et al. Coexistence of Thyroglobulin Antibodies and Thyroid Peroxidase Antibodies Correlates With Elevated Thyroid-Stimulating Hormone Level and Advanced Tumor Stage of Papillary Thyroid Cancer. Endocrine (2014) 46:554–60. doi: 10.1007/s12020-013-0121-x [DOI] [PubMed] [Google Scholar]
- 88. Duccini K, de Souza MVL, Delfim R, Aguiar AP, Teixeira P, Vaisman M. High Serum Thyrotropin Concentrations Within the Reference Range: A Predictor of Malignancy in Nodular Thyroid Disease. Med Princ Pract (2018) 27:272–7. doi: 10.1159/000488196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qin XJ, Lin X, Xue G, Fan HL, Wang HY, Wu JF, et al. CXCL10 is a Potential Biomarker and Associated With Immune Infiltration in Human Papillary Thyroid Cancer. Biosci Rep (2021) 41:BSR20203459. doi: 10.1042/BSR20203459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee SA, Choi JH, Cho SJ, Chang JW, Maeng YH. The Clinical Usefulness of Chemokine C-X-C Motif Ligand 12 as a Diagnostic Marker for Papillary Thyroid Carcinoma. Indian J Pathol Microbiol (2020) 63:544–50. doi: 10.4103/IJPM.IJPM_722_19 [DOI] [PubMed] [Google Scholar]
- 91. Wu W, Ren F, Guo M, Yang J, Xiao Y, Liu W. Increased Expression of CX3CL1 and CX3CR1 in Papillary Thyroid Carcinoma. Histol Histopathol (2020) 35:1189–96. doi: 10.14670/HH-18-265 [DOI] [PubMed] [Google Scholar]
- 92. Kim MJ, Sun HJ, Song YS, Yoo SK, Kim YA, Seo JS, et al. CXCL16 Positively Correlated With M2-Macrophage Infiltration, Enhanced Angiogenesis, and Poor Prognosis in Thyroid Cancer. Sci Rep (2019) 9:13288. doi: 10.1038/s41598-019-49613-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang W, Chu HY, Zhong ZM, Qi X, Cheng R, Qin RJ, et al. Platelet-Secreted CCL3 and its Receptor CCR5 Promote Invasive and Migratory Abilities of Anaplastic Thyroid Carcinoma Cells via MMP-1. Cell Signal (2019) 63:109363. doi: 10.1016/j.cellsig.2019.109363 [DOI] [PubMed] [Google Scholar]
- 94. Coperchini F, Croce L, Marinò M, Chiovato L, Rotondi M. Role of Chemokine Receptors in Thyroid Cancer and Immunotherapy. Endocr Relat Cancer (2019) 26:R465–78. doi: 10.1530/ERC-19-0163 [DOI] [PubMed] [Google Scholar]
- 95. Fallahi P, Ferrari SM, Piaggi S, Luconi M, Cantini G, Gelmini S, et al. The Paramount Role of Cytokines and Chemokines in Papillary Thyroid Cancer: A Review and Experimental Results. Immunol Res (2018) 66:710–22. doi: 10.1007/s12026-018-9056-x [DOI] [PubMed] [Google Scholar]
- 96. Ferrari SM, Elia G, Piaggi S, Baldini E, Ulisse S, Miccoli M, et al. CCL2 Is Modulated by Cytokines and PPAR-γ in Anaplastic Thyroid Cancer. Anticancer Agents Med Chem (2018) 18:458–66. doi: 10.2174/1871520617666170719152349 [DOI] [PubMed] [Google Scholar]
- 97. Cui D, Zhao Y, Xu J. Activated CXCL5-CXCR2 Axis Promotes the Migration, Invasion and EMT of Papillary Thyroid Carcinoma Cells via Modulation of β-Catenin Pathway. Biochimie (2018) 148:1–11. doi: 10.1016/j.biochi.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 98. Yapa S, Mulla O, Green V, England J, Greenman J. The Role of Chemokines in Thyroid Carcinoma. Thyroid (2017) 27:1347–59. doi: 10.1089/thy.2016.0660 [DOI] [PubMed] [Google Scholar]
- 99. Ferrari SM, Materazzi G, Baldini E, Ulisse S, Miccoli P, Antonelli A, et al. Antineoplastic Effects of Pparγ Agonists, With a Special Focus on Thyroid Cancer. Curr Med Chem (2016) 23:636–49. doi: 10.2174/0929867323666160203114607 [DOI] [PubMed] [Google Scholar]
- 100. Jin JQ, Han JS, Ha J, Baek HS, Lim DJ. Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing P38 MAPK Signaling Pathway. Endocrinol Metab (Seoul) (2021) 36:1095–110. doi: 10.3803/EnM.2021.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen JY, Wang JJ, Lee HC, Chi CW, Lee CH, Hsu YC. Combination of Peroxisome Proliferator-Activated Receptor Gamma and Retinoid X Receptor Agonists Induces Sodium/Iodide Symporter Expression and Inhibits Cell Growth of Human Thyroid Cancer Cells. J Chin Med Assoc (2020) 83:923–30. doi: 10.1097/JCMA.0000000000000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Copland JA, Marlow LA, Kurakata S, Fujiwara K, Wong AK, Kreinest PA, et al. Novel High-Affinity PPARgamma Agonist Alone and in Combination With Paclitaxel Inhibits Human Anaplastic Thyroid Carcinoma Tumor Growth via P21waf1/CIP1. Oncogene (2006) 25:2304–17. doi: 10.1038/sj.onc.1209267 [DOI] [PubMed] [Google Scholar]
- 103. Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T. Ligands for Peroxisome Proliferator-Activated Receptor Gamma Inhibit Growth and Induce Apoptosis of Human Papillary Thyroid Carcinoma Cells. J Clin Endocrinol Metab (2001) 86:2170–7. doi: 10.1210/jcem.86.5.7493 [DOI] [PubMed] [Google Scholar]
- 104. Hayashi N, Nakamori S, Hiraoka N, Tsujie M, Xundi X, Takano T, et al. Antitumor Effects of Peroxisome Proliferator Activate Receptor Gamma Ligands on Anaplastic Thyroid Carcinoma. Int J Oncol (2004) 24:89–95. doi: 10.3892/ijo.24.1.89 [DOI] [PubMed] [Google Scholar]
- 105. Bonofiglio D, Qi H, Gabriele S, Catalano S, Aquila S, Belmonte M, et al. Peroxisome Proliferator-Activated Receptor Gamma Inhibits Follicular and Anaplastic Thyroid Carcinoma Cells Growth by Upregulating P21cip1/WAF1 Gene in a Sp1-Dependent Manner. Endocr Relat Cancer (2008) 15:545–57. doi: 10.1677/ERC-07-0272 [DOI] [PubMed] [Google Scholar]
- 106. Yousefnia S, Momenzadeh S, Seyed Forootan F, Ghaedi K, Nasr Esfahani MH. The Influence of Peroxisome Proliferator-Activated Receptor γ (Pparγ) Ligands on Cancer Cell Tumorigenicity. Gene (2018) 649:14–22. doi: 10.1016/j.gene.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 107. Gani OA. Are Fish Oil Omega-3 Long-Chain Fatty Acids and Their Derivatives Peroxisome Proliferator-Activated Receptor Agonists? Cardiovasc Diabetol (2008) 7:6. doi: 10.1186/1475-2840-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Souza LL, Nunes MO, Paula GS, Cordeiro A, Penha-Pinto V, Neto JF, et al. Effects of Dietary Fish Oil on Thyroid Hormone Signaling in the Liver. J Nutr Biochem (2010) 21:935–40. doi: 10.1016/j.jnutbio.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 109. Souza LL, Cordeiro A, Oliveira LS, de Paula GS, Faustino LC, Ortiga-Carvalho TM, et al. Thyroid Hormone Contributes to the Hypolipidemic Effect of Polyunsaturated Fatty Acids From Fish Oil: In Vivo Evidence for Cross Talking Mechanisms. J Endocrinol (2011) 211:65–72. doi: 10.1530/JOE-11-0142 [DOI] [PubMed] [Google Scholar]
- 110. Makino M, Oda N, Miura N, Imamura S, Yamamoto K, Kato T, et al. Effect of Eicosapentaenoic Acid Ethyl Ester on Hypothyroid Function. J Endocrinol (2001) 171:259–65. doi: 10.1677/joe.0.1710259 [DOI] [PubMed] [Google Scholar]
- 111. van Doormaal JJ, Muskiet FA, Martini IA, Doorenbos H. Changes in Fatty Acid Profiles of Plasma, Erythrocytes and Polymorphonuclear Leukocytes in Induced Hypothyroidism in Man: Indirect Evidence for Altered Delta 6 Desaturase Activity. Clin Chim Acta (1986) 156:299–313. doi: 10.1016/0009-8981(86)90073-2 [DOI] [PubMed] [Google Scholar]
- 112. Benvenga S, Li Calzi L, Robbins J. Effect of Free Fatty Acids and Nonlipid Inhibitors of Thyroid Hormone Binding in the Immunoradiometric Assay of Thyroxin-Binding Globulin. Clin Chem (1987) 33:1752–5. doi: 10.1093/clinchem/33.10.1752 [DOI] [PubMed] [Google Scholar]
- 113. Tabachnick M, Korcek L. Effect of Long-Chain Fatty Acids on the Binding of Thyroxine and Triiodothyronine to Human Thyroxine-Binding Globulin. Biochim Biophys Acta (1986) 881:292–6. doi: 10.1016/0304-4165(86)90016-4 [DOI] [PubMed] [Google Scholar]
- 114. Puskás LG, Kitajka K, Nyakas C, Barcelo-Coblijn G, Farkas T. Short-Term Administration of Omega 3 Fatty Acids From Fish Oil Results in Increased Transthyretin Transcription in Old Rat Hippocampus. Proc Natl Acad Sci USA (2003) 100:1580–5. doi: 10.1073/pnas.0337683100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang X, Hjorth E, Vedin I, Eriksdotter M, Freund-Levi Y, Wahlund LO, et al. Effects of N-3 FA Supplementation on the Release of Proresolving Lipid Mediators by Blood Mononuclear Cells: The OmegAD Study. J Lipid Res (2015) 56:674–81. doi: 10.1194/jlr.P055418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Videla LA, Fernández V, Vargas R, Cornejo P, Tapia G, Varela N, et al. Upregulation of Rat Liver Pparα-FGF21 Signaling by a Docosahexaenoic Acid and Thyroid Hormone Combined Protocol. Biofactors (2016) 42:638–46. doi: 10.1002/biof.1300 [DOI] [PubMed] [Google Scholar]
- 117. Videla LA. Combined Docosahexaenoic Acid and Thyroid Hormone Supplementation as a Protocol Supporting Energy Supply to Precondition and Afford Protection Against Metabolic Stress Situations. IUBMB Life (2019) 71:1211–20. doi: 10.1002/iub.2067 [DOI] [PubMed] [Google Scholar]
- 118. Videla LA, Vargas R, Valenzuela R, Muñoz P, Corbari A, Hernandez-Rodas MC. Combined Administration of Docosahexaenoic Acid and Thyroid Hormone Synergistically Enhances Rat Liver Levels of Resolvins RvD1 and Rvd2. Prostaglandins Leukot Essent Fatty Acids (2019) 140:42–6. doi: 10.1016/j.plefa.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 119. Mardones M, Valenzuela R, Romanque P, Covarrubias N, Anghileri F, Fernández V, et al. Prevention of Liver Ischemia Reperfusion Injury by a Combined Thyroid Hormone and Fish Oil Protocol. J Nutr Biochem (2012) 23:1113–20. doi: 10.1016/j.jnutbio.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 120. Vargas R, Riquelme B, Fernández J, Álvarez D, Pérez IF, Cornejo P, et al. Docosahexaenoic Acid-Thyroid Hormone Combined Protocol as a Novel Approach to Metabolic Stress Disorders: Relation to Mitochondrial Adaptation via Liver PGC-1α and Sirtuin1 Activation. Biofactors (2019) 45:271–8. doi: 10.1002/biof.1483 [DOI] [PubMed] [Google Scholar]
- 121. Sarkar A, Knight JC, Babichuk NA, Mulay S. Skewed Distribution of Hypothyroidism in the Coastal Communities of Newfoundland, Canada. Environ Int (2015) 83:171–5. doi: 10.1016/j.envint.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 122. Schell LM, Gallo MV, Ravenscroft J, DeCaprio AP. Persistent Organic Pollutants and Anti-Thyroid Peroxidase Levels in Akwesasne Mohawk Young Adults. Environ Res (2009) 109:86–92. doi: 10.1016/j.envres.2008.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. Hormone Disruption by PBDEs in Adult Male Sport Fish Consumers. Environ Health Perspect (2008) 116:1635–41. doi: 10.1289/ehp.11707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bloom M, Spliethoff H, Vena J, Shaver S, Addink R, Eadon G. Environmental Exposure to PBDEs and Thyroid Function Among New York Anglers. Environ Toxicol Pharmacol (2008) 25:386–92. doi: 10.1016/j.etap.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 125. Bloom M, Vena J, Olson J, Moysich K. Chronic Exposure to Dioxin-Like Compounds and Thyroid Function Among New York Anglers. Environ Toxicol Pharmacol (2006) 21:260–7. doi: 10.1016/j.etap.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 126. Hagmar L, Björk J, Sjödin A, Bergman A, Erfurth EM. Plasma Levels of Persistent Organohalogens and Hormone Levels in Adult Male Humans. Arch Environ Health (2001) 56:138–43. doi: 10.1080/00039890109604065 [DOI] [PubMed] [Google Scholar]
- 127. Langer P, Kocan A, Tajtaková M, Petrík J, Chovancová J, Drobná B, et al. Fish From Industrially Polluted Freshwater as the Main Source of Organochlorinated Pollutants and Increased Frequency of Thyroid Disorders and Dysglycemia. Chemosphere (2007) 67:S379–385. doi: 10.1016/j.chemosphere.2006.05.132 [DOI] [PubMed] [Google Scholar]
- 128. Gill R, Lanni L, Jen KL, McCabe MJ, Jr, Rosenspire A. Docosahexaenoic Acid Counteracts Attenuation of CD95-Induced Cell Death by Inorganic Mercury. Toxicol Appl Pharmacol (2015) 282:61–7. doi: 10.1016/j.taap.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gill R, Jen KL, McCabe MJ, Rosenspire A. Dietary N-3 PUFAs Augment Caspase 8 Activation in Staphylococcal Aureus Enterotoxin B Stimulated T-Cells. Toxicol Appl Pharmacol (2016) 309:141–8. doi: 10.1016/j.taap.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Breese McCoy SJ. Coincidence of Remission of Postpartum Graves' Disease and Use of Omega-3 Fatty Acid Supplements. Thyroid Res (2011) 4:16. doi: 10.1186/1756-6614-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dolan K, Finley H, Gasta M, Houseman S. Managing Hashimoto's Thyroiditis Through Personalized Care: A Case Report. Altern Ther Health Med (2018) 24:56–61. [PubMed] [Google Scholar]
- 132. Woźniak D, Drzymała S, Przysławski J, Drzymała-Czyż S. Dietary Supplements in Hypothyroidism. Acta Sci Pol Technol Aliment (2021) 20:375–81. doi: 10.17306/J.AFS.2021.0985 [DOI] [PubMed] [Google Scholar]
- 133. Song J, Sun R, Zhang Y, Ke J, Zhao D. Serum Resolvin E1 Levels and its Relationship With Thyroid Autoimmunity in Hashimoto's Thyroiditis: A Preliminary Study. BMC Endocr Disord (2021) 21:66. doi: 10.1186/s12902-021-00730-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Song J, Sun R, Zhang Y, Fu Y, Zhao D. Role of the Specialized Pro-Resolving Mediator Resolvin D1 in Hashimoto's Thyroiditis. Exp Clin Endocrinol Diabetes (2021) 129:791–7. doi: 10.1055/a-1345-0173 [DOI] [PubMed] [Google Scholar]
- 135. Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical Assignment, Antiinflammatory Properties, and Receptor for the Omega-3 Lipid Mediator Resolvin E1. J Exp Med (2005) 201:713–22. doi: 10.1084/jem.20042031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular Circuits of Resolution: Formation and Actions of Resolvins and Protectins. J Immunol (2005) 174:4345–55. doi: 10.4049/jimmunol.174.7.4345 [DOI] [PubMed] [Google Scholar]
- 137. Soukup T. Effects of Long-Term Thyroid Hormone Level Alterations, N-3 Polyunsaturated Fatty Acid Supplementation and Statin Administration in Rats. Physiol Res (2014) 63:S119–131. doi: 10.33549/physiolres.932623 [DOI] [PubMed] [Google Scholar]
- 138. Sinha RA, Khare P, Rai A, Maurya SK, Pathak A, Mohan V, et al. Anti-Apoptotic Role of Omega-3-Fatty Acids in Developing Brain: Perinatal Hypothyroid Rat Cerebellum as Apoptotic Model. Int J Dev Neurosci (2009) 27:377–83. doi: 10.1016/j.ijdevneu.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 139. Pal A, Mohan V, Modi DR, Sinha RA, Rastogi L, Kumar P, et al. Iodine Plus N-3 Fatty Acid Supplementation Augments Rescue of Postnatal Neuronal Abnormalities in Iodine-Deficient Rat Cerebellum. Br J Nutr (2013) 110:659–70. doi: 10.1017/S0007114512005569 [DOI] [PubMed] [Google Scholar]
- 140. Abd Allah ES, Gomaa AM, Sayed MM. The Effect of Omega-3 on Cognition in Hypothyroid Adult Male Rats. Acta Physiol Hung (2014) 101:362–76. doi: 10.1556/APhysiol.101.2014.3.11 [DOI] [PubMed] [Google Scholar]
- 141. Gomaa AM, Abd El-Aziz EA. Omega-3 Fatty Acids Decreases Oxidative Stress, Tumor Necrosis Factor-Alpha, and Interleukin-1 Beta in Hyperthyroidism-Induced Hepatic Dysfunction Rat Model. Pathophysiology (2016) 23:295–301. doi: 10.1016/j.pathophys.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 142. Rauchová H, Vokurková M, Pavelka S, Behuliak M, Tribulová N, Soukup T. N-3 Polyunsaturated Fatty Acids Supplementation Does Not Affect Changes of Lipid Metabolism Induced in Rats by Altered Thyroid Status. Horm Metab Res (2013) 45:507–12. doi: 10.1055/s-0033-1334944 [DOI] [PubMed] [Google Scholar]
- 143. Awumey EM, Paton DM, Pehowich DJ. Thyroid Status and Dietary Fatty Acids Affect Beta-Adrenoceptor Agonist Stimulation of Tension Development in Rat Myocardium. J Auton Pharmacol (1995) 15:73–84. doi: 10.1111/j.1474-8673.1995.tb00293.x [DOI] [PubMed] [Google Scholar]
- 144. Benvenga S. Effects of L-Carnitine on Thyroid Hormone Metabolism and on Physical Exercise Tolerance. Horm Metab Res (2005) 37:566–71. doi: 10.1055/s-2005-870424 [DOI] [PubMed] [Google Scholar]
- 145. Chee R, Agah R, Vita R, Benvenga S. L-Carnitine Treatment in a Seriously Ill Cancer Patient With Severe Hyperthyroidism. Hormones (Athens) (2014) 13:407–12. doi: 10.14310/horm.2002.149 [DOI] [PubMed] [Google Scholar]
- 146. An JH, Kim YJ, Kim KJ, Kim SH, Kim NH, Kim HY, et al. L-Carnitine Supplementation for the Management of Fatigue in Patients With Hypothyroidism on Levothyroxine Treatment: A Randomized, Double-Blind, Placebo-Controlled Trial. Endocr J (2016) 63:885–95. doi: 10.1507/endocrj.EJ16-0109 [DOI] [PubMed] [Google Scholar]
- 147. Benvenga S, Sindoni A. L-Carnitine Supplementation for the Management of Fatigue in Patients With Hypothyroidism on Levothyroxine Treatment. Endocr J (2016) 63:937–8. doi: 10.1507/endocrj.EJ16-0374 [DOI] [PubMed] [Google Scholar]
- 148. Alesci S, De Martino MU, Mirani M, Benvenga S, Trimarchi F, Kino T, et al. L-Carnitine: A Nutritional Modulator of Glucocorticoid Receptor Functions. FASEB J (2003) 17:1553–5. doi: 10.1096/fj.02-1024fje [DOI] [PubMed] [Google Scholar]
- 149. Duntas LH, Benvenga S. Selenium: An Element for Life. Endocrine (2015) 48:756–75. doi: 10.1007/s12020-014-0477-6 [DOI] [PubMed] [Google Scholar]
- 150. Wichman J, Winther KH, Bonnema SJ, Hegedüs L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients With Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid (2016) 26:1681–92. doi: 10.1089/thy.2016.0256 [DOI] [PubMed] [Google Scholar]
- 151. Benvenga S, Feldt-Rasmussen U, Bonofiglio D, Asamoah E. Nutraceutical Supplements in the Thyroid Setting: Health Benefits Beyond Basic Nutrition. Nutrients (2019) 11:2214. doi: 10.3390/nu11092214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nordio M, Pajalich R. Combined Treatment With Myo-Inositol and Selenium Ensures Euthyroidism in Subclinical Hypothyroidism Patients With Autoimmune Thyroiditis. J Thyroid Res (2013) 2013:424163. doi: 10.1155/2013/424163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Nordio M, Basciani S. Myo-Inositol Plus Selenium Supplementation Restores Euthyroid State in Hashimoto’s Patients With Subclinical Hypothyroidism. Eur Rev Med Pharmacol Sci (2017) 21:51–9. doi: 10.1155/2017/2549491 [DOI] [PubMed] [Google Scholar]
- 154. Ferrari SM, Fallahi P, Di Bari F, Vita R, Benvenga S, Antonelli A. Myo-Inositol and Selenium Reduce the Risk of Developing Overt Hypothyroidism in Patients With Autoimmune Thyroiditis. Eur Rev Med Pharmacol Sci (2017) 21:36–42. [PubMed] [Google Scholar]
- 155. Pace C, Tumino D, Russo M, Le Moli R, Naselli A, Borzì G, et al. Role of Selenium and Myo-Inositol Supplementation on Autoimmune Thyroiditis Progression. Endocr J (2020) 67:1093–8. doi: 10.1507/endocrj.EJ20-0062 [DOI] [PubMed] [Google Scholar]
- 156. Porcaro G, Angelozzi P. Myo-Inositol and Selenium Prevent Subclinical Hypothyroidism During Pregnancy: An Observational Study. IJMDAT (2018) 1:e164. [Google Scholar]
- 157. Benvenga S, Nordio M, Laganà AS, Unfer V. The Role of Inositol in Thyroid Physiology and in Subclinical Hypothyroidism Management. Front Endocrinol (Lausanne) (2021) 12:662582. doi: 10.3389/fendo.2021.662582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dinicola S, Unfer V, Facchinetti F, Soulage CO, Greene ND, Bizzarri M, et al. Inositols: From Established Knowledge to Novel Approaches. Int J Mol Sci (2021) 22:10575. doi: 10.3390/ijms221910575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Benvenga S, Vicchio T, Di Bari F, Vita R, Fallahi P, Ferrari SM, et al. Favorable Effects of Myo-Inositol, Selenomethionine or Their Combination on the Hydrogen Peroxide-Induced Oxidative Stress of Peripheral Mononuclear Cells From Patients With Hashimoto's Thyroiditis: Preliminary In Vitro Studies. Eur Rev Med Pharmacol Sci (2017) 21:89–101. [PubMed] [Google Scholar]
- 160. Benvenga S, Marini HR, Micali A, Freni J, Pallio G, Irrera N, et al. Protective Effects of Myo-Inositol and Selenium on Cadmium-Induced Thyroid Toxicity in Mice. Nutrients (2020) 12:1222. doi: 10.3390/nu12051222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jensen KN, Omarsdottir SY, Reinhardsdottir MS, Hardardottir I, Freysdottir J. Docosahexaenoic Acid Modulates NK Cell Effects on Neutrophils and Their Crosstalk. Front Immunol (2020) 11:570380. doi: 10.3389/fimmu.2020.570380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Li Z, Choi JH, Oh HJ, Park SH, Lee JB, Yoon KC. Effects of Eye Drops Containing a Mixture of Omega-3 Essential Fatty Acids and Hyaluronic Acid on the Ocular Surface in Desiccating Stress-Induced Murine Dry Eye. Curr Eye Res (2014) 39:871–8. doi: 10.3109/02713683.2014.884595 [DOI] [PubMed] [Google Scholar]
- 163. Saedisomeolia A, Wood LG, Garg ML, Gibson PG, Wark PA. Anti-Inflammatory Effects of Long-Chain N-3 PUFA in Rhinovirus-Infected Cultured Airway Epithelial Cells. Br J Nutr (2009) 101:533–40. doi: 10.1017/S0007114508025798 [DOI] [PubMed] [Google Scholar]
- 164. Henao Agudelo JS, Baia LC, Ormanji MS, Santos ARP, Machado JR, Saraiva Câmara NO, et al. Fish Oil Supplementation Reduces Inflammation But Does Not Restore Renal Function and Klotho Expression in an Adenine-Induced CKD Model. Nutrients (2018) 10:1283. doi: 10.3390/nu10091283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Baker EJ, Valenzuela CA, De Souza CO, Yaqoob P, Miles EA, Calder PC. Comparative Anti-Inflammatory Effects of Plant- and Marine-Derived Omega-3 Fatty Acids Explored in an Endothelial Cell Line. Biochim Biophys Acta Mol Cell Biol Lipids (2020) 1865:158662. doi: 10.1016/j.bbalip.2020.158662 [DOI] [PubMed] [Google Scholar]
- 166. Harari A, Leikin Frenkel A, Barshack I, Sagee A, Cohen H, Kamari Y, et al. Addition of Fish Oil to Atherogenic High Fat Diet Inhibited Atherogenesis While Olive Oil did Not, in LDL Receptor KO Mice. Nutr Metab Cardiovasc Dis (2020) 30:709–16. doi: 10.1016/j.numecd.2019 [DOI] [PubMed] [Google Scholar]
- 167. Serini S, Cassano R, Facchinetti E, Amendola G, Trombino S, Calviello G. Anti-Irritant and Anti-Inflammatory Effects of DHA Encapsulated in Resveratrol-Based Solid Lipid Nanoparticles in Human Keratinocytes. Nutrients (2019) 11:1400. doi: 10.3390/nu11061400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ferguson JF, Roberts-Lee K, Borcea C, Smith HM, Midgette Y, Shah R. Omega-3 Polyunsaturated Fatty Acids Attenuate Inflammatory Activation and Alter Differentiation in Human Adipocytes. J Nutr Biochem (2019) 64:45–9. doi: 10.1016/j.jnutbio.2018.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Khadge S, Thiele GM, Sharp JG, McGuire TR, Klassen LW, Black PN, et al. Long-Chain Omega-3 Polyunsaturated Fatty Acids Modulate Mammary Gland Composition and Inflammation. J Mammary Gland Biol Neoplasia (2018) 23:43–58. doi: 10.1007/s10911-018-9391-5 [DOI] [PubMed] [Google Scholar]
- 170. Liang P, Henning SM, Schokrpur S, Wu L, Doan N, Said J, et al. Effect of Dietary Omega-3 Fatty Acids on Tumor-Associated Macrophages and Prostate Cancer Progression. Prostate (2016) 76:1293–302. doi: 10.1002/pros.23218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yoshihara T, Shimada K, Fukao K, Sai E, Sato-Okabayashi Y, Matsumori R, et al. Omega 3 Polyunsaturated Fatty Acids Suppress the Development of Aortic Aneurysms Through the Inhibition of Macrophage-Mediated Inflammation. Circ J (2015) 79:1470–8. doi: 10.1253/circj.CJ-14-0471 [DOI] [PubMed] [Google Scholar]
- 172. Monk JM, Liddle DM, De Boer AA, Brown MJ, Power KA, Ma DW, et al. Fish-Oil-Derived N-3 PUFAs Reduce Inflammatory and Chemotactic Adipokine-Mediated Cross-Talk Between Co-Cultured Murine Splenic CD8+ T Cells and Adipocytes. J Nutr (2015) 145:829–38. doi: 10.3945/jn.114.205443 [DOI] [PubMed] [Google Scholar]
- 173. Baranowski M, Enns J, Blewett H, Yakandawala U, Zahradka P, Taylor CG. Dietary Flaxseed Oil Reduces Adipocyte Size, Adipose Monocyte Chemoattractant Protein-1 Levels and T-Cell Infiltration in Obese, Insulin-Resistant Rats. Cytokine (2012) 59:382–91. doi: 10.1016/j.cyto.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 174. Diaz Encarnacion MM, Warner GM, Cheng J, Gray CE, Nath KA, Grande JP. N-3 Fatty Acids Block TNF-α-Stimulated MCP-1 Expression in Rat Mesangial Cells. Am J Physiol Renal Physiol (2011) 300:F1142–1151. doi: 10.1152/ajprenal.00064.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Meijerink J, Plastina P, Vincken JP, Poland M, Attya M, Balvers M, et al. The Ethanolamide Metabolite of DHA, Docosahexaenoylethanolamine, Shows Immunomodulating Effects in Mouse Peritoneal and RAW264.7 Macrophages: Evidence for a New Link Between Fish Oil and Inflammation. Br J Nutr (2011) 105:1798–807. doi: 10.1017/S0007114510005635 [DOI] [PubMed] [Google Scholar]
- 176. An WS, Kim HJ, Cho KH, Vaziri ND. Omega-3 Fatty Acid Supplementation Attenuates Oxidative Stress, Inflammation, and Tubulointerstitial Fibrosis in the Remnant Kidney. Am J Physiol Renal Physiol (2009) 297:F895–903. doi: 10.1152/ajprenal.00217.2009 [DOI] [PubMed] [Google Scholar]
- 177. Metro D, Cernaro V, Papa M, Benvenga S. Marked Improvement of Thyroid Function and Autoimmunity by Aloe Barbadensis Miller Juice in Patients With Subclinical Hypothyroidism. J Clin Transl Endocrinol (2018) 11:18–25. doi: 10.1016/j.jcte.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ahmad M, Nangyal H, Sherwani S, Islam Z, Shah S. Effect of Heat Stress on Fatty Acids Profiles of Aloe Vera and Bryophyllum Pinnatum Leaves. World Appl Sci J (2013) 28:1592–6. doi: 10.5829/idosi.wasj.2013.28.11.14128 [DOI] [Google Scholar]
- 179. Woeller CF, Woodroof A, Cottler PS, Pollock SJ, Haidaris CG, Phipps RP. In Vitro Characterization of Variable Porosity Wound Dressing With Anti-Scar Properties. Eplasty (2018) 18:e21. [PMC free article] [PubMed] [Google Scholar]
- 180. Misawa E, Tanaka M, Nomaguchi K, Nabeshima K, Yamada M, Toida T, et al. Oral Ingestion of Aloe Vera Phytosterols Alters Hepatic Gene Expression Profiles and Ameliorates Obesity-Associated Metabolic Disorders in Zucker Diabetic Fatty Rats. J Agric Food Chem (2012) 60:2799–806. doi: 10.1021/jf204465j [DOI] [PubMed] [Google Scholar]
- 181. Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules (2020) 25:1324. doi: 10.3390/molecules25061324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Klaikeaw N, Wongphoom J, Werawatganon D, Chayanupatkul M, Siriviriyakul P. Anti-Inflammatory and Anti-Oxidant Effects of Aloe Vera in Rats With non-Alcoholic Steatohepatitis. World J Hepatol (2020) 12:363–77. doi: 10.4254/wjh.v12.i7.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Seo JM, Cheng S, Soliman N, Nabi Z, Pan L. The Blend of Taurine and Aloe Vera Extract Boosts Action Against Skin Irritation: In Vitro and Clinical Evaluations. J Cosmet Sci (2018) 69:213–28. [PubMed] [Google Scholar]
- 184. Na HS, Song YR, Kim S, Heo JY, Chung HY, Chung J. Aloin Inhibits Interleukin (IL)-1β-Stimulated IL-8 Production in KB Cells. J Periodontol (2016) 87:e108–115. doi: 10.1902/jop.2016.150447 [DOI] [PubMed] [Google Scholar]
- 185. Radha MH, Laxmipriya NP. Evaluation of Biological Properties and Clinical Effectiveness of Aloe Vera: A Systematic Review. J Tradit Complement Med (2014) 5:21–6. doi: 10.1016/j.jtcme.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Budai MM, Varga A, Milesz S, Tőzsér J, Benkő S. Aloe Vera Downregulates LPS-Induced Inflammatory Cytokine Production and Expression of NLRP3 Inflammasome in Human Macrophages. Mol Immunol (2013) 56:471–9. doi: 10.1016/j.molimm.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 187. Shin E, Shim KS, Kong H, Lee S, Shin S, Kwon J, et al. Dietary Aloe Improves Insulin Sensitivity via the Suppression of Obesity-Induced Inflammation in Obese Mice. Immune Netw (2011) 11:59–67. doi: 10.4110/in.2011.11.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Langmead L, Makins RJ, Rampton DS. Anti-Inflammatory Effects of Aloe Vera Gel in Human Colorectal Mucosa. vitro. Aliment Pharmacol Ther (2004) 19:521–7. doi: 10.1111/j.1365-2036.2004.01874.x [DOI] [PubMed] [Google Scholar]
- 189. ANSA . L'Italia Prima Nell'ue Per Gli Acquisti Di Nutraceutical (2022). Available at: https://www.ansa.it/cibus_2018/notizie/salute_benessere/2018/05/07/italia-prima-in-ue-acquisti-nutraceutica_3db89be3-de8d-4920-88b1-7d5d22f61e05.html (Accessed January 19, 2022).
- 190. FEDERSALUS . 5th Industry Survey. The Italian Food Supplement Supply Chain 2019-2020 (2022). Available at: https://www.federsalus.it/wp-content/uploads/2020/06/Report_5th-Industry-survey-2.pdf (Accessed January 19, 2022).
- 191. Wootan GD, Phillips MB. Detox Diets for Dummies. Hoboken, NJ, (USA: Wiley Publishing, Inc; (2010). 88 p. [Google Scholar]
- 192. STATISTA . Total U.S. Dietary Supplements Market Size From 2016 to 2024 (2022). Available at: https://www.statista.com/statistics/828481/total-dietary-supplements-market-size-in-the-us/ (Accessed January 19, 2022).
- 193. GLOBE NEWS WIRE . Dietary Supplements Market Worth USD 117.92 Billion by 2027 (2022). Available at: https://www.globenewswire.com/news-release/2021/10/22/2319238/0/en/Dietary-Supplements-Market-Worth-USD-117-92-Billion-by-2027-Fortune-Business-Insights.html (Accessed January 19, 2022).
- 194. GRAND VIEW RESEARCH . Omega 3 Market Size (2022). Available at: https://www.grandviewresearch.com/industry-analysis/omega-3-market (Accessed January 19, 2022).
- 195. MONDOR INTELLIGENCE . Omega-3 Products Market (2022). Available at: https://www.mordorintelligence.com/industry-reports/omega-3-product-market (Accessed January 19, 2022).
- 196. ALLIED MARKET RESEARCH . Omega-3 Market (2022). Available at: https://www.alliedmarketresearch.com/omega-3-market (Accessed January 19, 2022).
- 197. FORTUNE BUSINESS INSIGHTS . Food Additives & Ingredients - Omega-3 Fatty Acids Market (2022). Available at: https://www.fortunebusinessinsights.com/industry-reports/omega-3-fatty-acids-market-100248 (Accessed January 19, 2022).
- 198. AOCS . Omega-3 Fatty Acids: $13 Billion Global Market (2022). Available at: https://www.aocs.org/stay-informed/inform-magazine/featured-articles/omega-3-fatty-acids-13-billion-global-market-october-2011?SSO=True (Accessed January 19, 2022).
- 199. BUSINESS WIRE . The European Omega-3 Products Market to 2024 - $14.6 Billion Opportunity Analysis Featuring Unilever, Amway Corporation and Nestle (2022). Available at: https://www.businesswire.com/news/home/20190523005353/en/The-European-Omega-3-Products-Market-to-2024—14.6-Billion-Opportunity-Analysis-Featuring-Unilever-Amway-Corporation-and-Nestle—ResearchAndMarkets.com (Accessed January 19, 2022).
- 200. FRIEND OF THE SEA . Sustainable Omega 3 Consumption: A Positive Trend Set to Increase (2022). Available at: https://friendofthesea.org/it/sustainable-omega-3-consumption-a-positive-trend-set-to-increase/(Accessed January 19, 2022).
- 201. Hinriksdottir HH, Jonsdottir VL, Sveinsdottir K, Martinsdottir E, Ramel A. Bioavailability of Long-Chain N-3 Fatty Acids From Enriched Meals and From Microencapsulated Powder. Eur J Clin Nutr (2015) 69:344–8. doi: 10.1038/ejcn.2014.250 [DOI] [PubMed] [Google Scholar]
- 202. Yurko-Mauro K, Kralovec J, Bailey-Hall E, Smeberg V, Stark JG, Salem N, Jr. Similar Eicosapentaenoic Acid and Docosahexaenoic Acid Plasma Levels Achieved With Fish Oil or Krill Oil in a Randomized Double-Blind Four-Week Bioavailability Study. Lipids Health Dis (2015) 14:99. doi: 10.1186/s12944-015-0109-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ramprasath VR, Eyal I, Zchut S, Jones PJ. Enhanced Increase of Omega-3 Index in Healthy Individuals With Response to 4-Week N-3 Fatty Acid Supplementation From Krill Oil Versus Fish Oil. Lipids Health Dis (2013) 12:178. doi: 10.1186/1476-511X-12-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ganesan B, Brothersen C, McMahon DJ. Fortification of Foods With Omega-3 Polyunsaturated Fatty Acids. Crit Rev Food Sci Nutr (2014) 54:98–114. doi: 10.1080/10408398.2011.578221 [DOI] [PubMed] [Google Scholar]
- 205. Metcalf RG, James MJ, Mantzioris E, Cleland LG. A Practical Approach to Increasing Intakes of N-3 Polyunsaturated Fatty Acids: Use of Novel Foods Enriched With N-3 Fats. Eur J Clin Nutr (2003) 57:1605–12. doi: 10.1038/sj.ejcn.1601731 [DOI] [PubMed] [Google Scholar]
- 206. Benvenga S, Trimarchi F. Changed Presentation of Hashimoto's Thyroiditis in North-Eastern Sicily and Calabria (Southern Italy) Based on a 31-Year Experience. Thyroid (2008) 18:429–41. doi: 10.1089/thy.2007.0234 [DOI] [PubMed] [Google Scholar]
- 207. Rizzo M, Rossi RT, Bonaffini O, Scisca C, Altavilla G, Calbo L, et al. Increased Annual Frequency of Hashimoto's Thyroiditis Between Years 1988 and 2007 at a Cytological Unit of Sicily. Ann Endocrinol (Paris) (2010) 71:525–34. doi: 10.1016/j.ando.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 208. Caturegli P, De Remigis A, Chuang K, Dembele M, Iwama A, Iwama S. Hashimoto's Thyroiditis: Celebrating the Centennial Through the Lens of the Johns Hopkins Hospital Surgical Pathology Records. Thyroid (2013) 23:142–50. doi: 10.1089/thy.2012.0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid Cancer Incidence Trends by Histology in 25 Countries: A Population-Based Study. Lancet Diabetes Endocrinol (2021) 9:225–34. doi: 10.1016/S2213-8587(21)00027-9 [DOI] [PubMed] [Google Scholar]
- 210. Kitahara CM, Sosa JA. Understanding the Ever-Changing Incidence of Thyroid Cancer. Nat Rev Endocrinol (2020) 16:617–8. doi: 10.1038/s41574-020-00414-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cheng F, Xiao J, Shao C, Huang F, Wang L, Ju Y, et al. Burden of Thyroid Cancer From 1990 to 2019 and Projections of Incidence and Mortality Until 2039 in China: Findings From Global Burden of Disease Study. Front Endocrinol (Lausanne) (2021) 12:738213. doi: 10.3389/fendo.2021.738213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317:1338–48. doi: 10.1001/jama.2017.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. J Cancer Epidemiol (2013) 2013:965212. doi: 10.1155/2013/965212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Park HT, Cho GJ, Ahn KH, Shin JH, Hong SC, Kim T, et al. Thyroid Stimulating Hormone is Associated With Metabolic Syndrome in Euthyroid Postmenopausal Women. Maturitas (2009) 62:301–5. doi: 10.1016/j.maturitas.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 215. He J, Lai Y, Yang J, Yao Y, Li Y, Teng W, et al. The Relationship Between Thyroid Function and Metabolic Syndrome and Its Components: A Cross-Sectional Study in a Chinese Population. Front Endocrinol (Lausanne) (2021) 12:661160. doi: 10.3389/fendo.2021.661160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Kalra S, Aggarwal S, Khandelwal D. Thyroid Dysfunction and Dysmetabolic Syndrome: The Need for Enhanced Thyrovigilance Strategies. Int J Endocrinol (2021) 2021:9641846. doi: 10.1155/2021/9641846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Tang K, Zhang Q, Peng NC, Zhang M, Xu SJ, Li H, et al. Epidemiology of Metabolic Syndrome and its Components in Chinese Patients With a Range of Thyroid-Stimulating Hormone Concentrations. J Int Med Res (2020) 48:300060520966878. doi: 10.1177/0300060520966878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Morini E, Catalano A, Lasco A, Morabito N, Benvenga S. In Thyroxine-Replaced Hypothyroid Postmenopausal Women Under Simultaneous Calcium Supplementation, Switch to Oral Liquid or Softgel Capsule L-Thyroxine Ensures Lower Serum TSH Levels and Favorable Effects on Blood Pressure, Total Cholesterolemia and Glycemia. Endocrine (2019) 65:569–79. doi: 10.1007/s12020-019-01979-w [DOI] [PubMed] [Google Scholar]
- 219. Deshmukh V, Farishta F, Bhole M. Thyroid Dysfunction in Patients With Metabolic Syndrome: A Cross-Sectional, Epidemiological, Pan-India Study. Int J Endocrinol (2018) 2018:2930251. doi: 10.1155/2018/2930251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zhou YC, Fang WH, Kao TW, Wang CC, Chang YW, Peng TC, et al. Exploring the Association Between Thyroid- Stimulating Hormone and Metabolic Syndrome: A Large Population-Based Study. PloS One (2018) 13:e0199209. doi: 10.1371/journal.pone.0199209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Janovsky CCPS, Cesena FH, Valente VAT, Conceição RDO, Santos RD, Bittencourt MS. Association Between Thyroid-Stimulating Hormone Levels and Non-Alcoholic Fatty Liver Disease Is Not Independent From Metabolic Syndrome Criteria. Eur Thyroid J (2018) 7:302–7. doi: 10.1159/000492324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical Hypothyroidism is an Independent Risk Factor for Atherosclerosis and Myocardial Infarction in Elderly Women: The Rotterdam Study. Ann Intern Med (2000) 132:270–8. doi: 10.7326/0003-4819-132-4-200002150-00004 [DOI] [PubMed] [Google Scholar]
- 223. Chaker L, Baumgartner C, den Elzen WP, Ikram MA, Blum MR, Collet TH, et al. Subclinical Hypothyroidism and the Risk of Stroke Events and Fatal Stroke: An Individual Participant Data Analysis. J Clin Endocrinol Metab (2015) 100:2181–1291. doi: 10.1210/jc.2015-1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical Hypothyroidism and the Risk of Coronary Heart Disease and Mortality. JAMA (2010) 304:1365–74. doi: 10.1001/jama.2010.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Ashizawa K, Imaizumi M, Usa T, Tominaga T, Sera N, Hida A, et al. Metabolic Cardiovascular Disease Risk Factors and Their Clustering in Subclinical Hypothyroidism. Clin Endocrinol (Oxf) (2010) 72:689–95. doi: 10.1111/j.1365-2265.2009.03697.x [DOI] [PubMed] [Google Scholar]
- 226. Singh S, Duggal J, Molnar J, Maldonado F, Barsano CP, Arora R. Impact of Subclinical Thyroid Disorders on Coronary Heart Disease, Cardiovascular and All-Cause Mortality: A Meta-Analysis. Int J Cardiol (2008) 125:41–8. doi: 10.1016/j.ijcard.2007.02.027 [DOI] [PubMed] [Google Scholar]
- 227. Siemińska L, Wojciechowska C, Walczak K, Borowski A, Marek B, Nowak M, et al. Associations Between Metabolic Syndrome, Serum Thyrotropin, and Thyroid Antibodies Status in Postmenopausal Women, and the Role of Interleukin-6. Endokrynol Pol (2015) 66:394–403. doi: 10.5603/EP.2015.0049 [DOI] [PubMed] [Google Scholar]
- 228. Cengiz H, Demirci T, Varim C, Tamer A. The Effect of Thyroid Autoimmunity on Dyslipidemia in Patients With Euthyroid Hashimoto Thyroiditis. Pak J Med Sci (2021) 37:1365–70. doi: 10.12669/pjms.37.5.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Wu Y, Shi X, Tang X, Li Y, Tong N, Wang G, et al. The Correlation Between Metabolic Disorders And Tpoab/Tgab: A Cross-Sectional Population-Based Study. Endocr Pract (2020) 26:869–82. doi: 10.4158/EP-2020-0008 [DOI] [PubMed] [Google Scholar]
- 230. Chen Y, Zhu C, Chen Y, Wang N, Li Q, Han B, et al. Are Thyroid Autoimmune Diseases Associated With Cardiometabolic Risks in a Population With Normal Thyroid-Stimulating Hormone? Mediators Inflammation (2018) 2018:1856137. doi: 10.1155/2018/1856137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Fallahi P, Ferrari SM, Ruffilli I, Elia G, Biricotti M, Vita R, et al. The Association of Other Autoimmune Diseases in Patients With Autoimmune Thyroiditis: Review of the Literature and Report of a Large Series of Patients. Autoimmun Rev (2016) 15:1125–8. doi: 10.1016/j.autrev.2016.09.009 [DOI] [PubMed] [Google Scholar]