Abstract
Polyphosphate accumulation by Paracoccus denitrificans was examined under aerobic, anoxic, and anaerobic conditions. Polyphosphate synthesis by this denitrifier took place with either oxygen or nitrate as the electron acceptor and in the presence of an external carbon source. Cells were capable of poly-β-hydroxybutyrate (PHB) synthesis, but no polyphosphate was produced when PHB-rich cells were incubated under anoxic conditions in the absence of an external carbon source. By comparison of these findings to those with polyphosphate-accumulating organisms thought to be responsible for phosphate removal in activated sludge systems, it is concluded that P. denitrificans is capable of combined phosphate and nitrate removal without the need for alternating anaerobic/aerobic or anaerobic/anoxic switches. Studies on additional denitrifying isolates from a denitrifying fluidized bed reactor suggested that polyphosphate accumulation is widespread among denitrifiers.
Due to environmental problems associated with phosphorus discharge, phosphorus removal has become a standard treatment practice in wastewater purification plants. Enhanced biological phosphorus removal (EBPR) is the most common biological phosphorus removal method. The method is based on enrichment of so-called polyphosphate-accumulating organisms (PAOs) through recycling of the sludge between anaerobic and aerobic zones. Under these conditions, the PAOs release phosphorus in the anaerobic zone and store phosphorus as polyphosphate in the aerobic zone. Phosphorus is subsequently removed from the process stream by harvesting a fraction of the phosphorus-rich bacterial biomass (26).
Recently, it was demonstrated that, not only under aerobic conditions but also under anoxic conditions, i.e., with nitrate as the electron acceptor, some PAOs are capable of polyphosphate accumulation (3, 6, 12, 15). Since attempts to isolate bacteria with all characteristics attributed to PAOs have failed so far, information on these organisms is based on studies with crude sludge samples and enrichment cultures obtained from EBPR plants. On the basis of these studies, the following set of metabolic properties has been attributed to PAOs (22).
Under anaerobic conditions, acetate or other low-molecular-weight organic compounds are converted to polyhydroxyalkanoates (PHA), polyphosphate and glycogen are degraded, and phosphate is released. Under aerobic or anoxic conditions, PHA is converted to glycogen, phosphate is assimilated, and polyphosphate is intracellularly produced. Under the latter conditions, bacterial growth and phosphate uptake are regulated by the energy released from the breakdown of PHA.
We studied the phosphorus dynamics in a prototype treatment system used for removal of organic matter and inorganic nitrogen from recirculating fish culture systems. The system comprised a nitrifying filter for oxidation of ammonia to nitrate, a digestion basin for anaerobic digestion of organic matter, and a denitrifying filter for reduction of nitrate to nitrogen gas (1). It was found that much of the phosphorus in the system was retained in the denitrifying biomass (up to 11% of the bacterial dry weight) within the fluidized bed reactor (2). The fluidized bed reactor, containing the denitrifiers, received a continuous supply of water from the digestion basin, devoid of oxygen and rich in volatile fatty acids and nitrate. Conditions within the reactor were permanently anoxic, as low levels of nitrate were present in the reactor effluent at all times. Upon examination of the phosphate uptake characteristics of the denitrifying bacteria isolated from the fluidized bed reactor, we found that most of these organisms were capable of polyphosphate synthesis (unpublished data). In the present study, results are presented on phosphorus accumulation by one of these isolates (Paracoccus denitrificans).
Organism.
P. denitrificans was isolated from a fluidized-bed reactor used for nitrate removal in intensive fish-culture systems (28). Identification of P. denitrificans was based on the API-NE system, consisting of eight conventional tests and 12 assimilatory tests (23).
Culture conditions.
P. denitrificans cells were cultured in medium containing (per liter) the following components: Na-acetate · 3H2O, 5.67 g; NH4Cl, 1 g; MgSO4 · 7H2O, 0.6 g; KH2PO4, 0.4 g; Na2S2O3 · 5H2O, 0.1 g; CaCl2 · 2H2O, 0.07 g; Tris buffer (hydroxymethyl aminomethane), 12 g; and 2 ml of a trace mineral solution (29). The pH was adjusted to 7.0 with 6 N HCl. Acetate was used as a carbon and electron donor. Studies were conducted with cells harvested during the late log phase of growth (after 4 to 5 days). Cells were washed twice and resuspended in the medium described above. Experiments were conducted in a temperature-controlled (30°C) incubation vessel (300 ml) placed on a magnetic stirrer and fitted with nitrate, pH, and oxygen and temperature electrodes. Cells were incubated either under aerobic conditions, by means of flushing the medium with sterile compressed air, or under anaerobic conditions, obtained by continuous flushing of the vessel with prepurified nitrogen gas. Overpressure within the incubation vessel prevented oxygen penetration, as verified by continuous oxygen monitoring. Anoxic incubation conditions refer to those conditions in which the cells were incubated in the presence of nitrate and the absence of oxygen. Anaerobic conditions refer to conditions in which neither oxygen nor nitrate was present. The experiments were initiated by adding acetate at concentrations as indicated. Periodically, samples were withdrawn for determination of nitrate, nitrite, ammonia, phosphate, acetate, and protein in the medium and for determination of intracellular poly-β-hydroxybutyrate (PHB), total polysaccharides, and total phosphorus contents. Each of the various incubation conditions was examined in triplicate. Results presented are the average values of triplicate runs under each of the conditions tested. Variations between the mean values of each run were less than 10% as determined by the analysis of co-variance.
Analytical procedures.
Total ammonia (NH3 and NH4+) was determined as described by Scheiner (24), nitrite was measured according to Strickland and Parsons (25), nitrate was measured with a specific nitrate electrode (Radiometer, Copenhagen, Denmark) amplified with a pH meter (Radiometer, model PHM92), and phosphate was measured (soluble orthophosphate) according to the method of Golterman et al. (8). Protein was determined according to a modified Lowry procedure (19) with bovine serum albumin as the standard. Oxygen and temperature were measured with a YSI model 57 temperature and oxygen probe (Yellow Springs Instruments, Yellow Springs, Ohio). Total phosphorus (8), polysaccharide (9), and PHB (18) contents of the cells were determined on concentrated cell suspensions (centrifugation at 8,000 × g) and expressed as milligrams per gram of protein. Intracellular polyphosphate bodies were determined by electron microscopy after negative staining of the cells with uranyl acetate (4). Acetate in the medium was determined with a Hewlett Packard (model 5890) gas chromatograph equipped with an 1/8-in.-internal-diameter stainless-steel column (length, 200 cm) packed with 60–80 mesh Chromosorb W. Injector, oven, and flame ionization detector temperatures were set at 170, 140, and 175°C, respectively. Nitrogen was used as the carrier gas at a flow rate of 6 ml/min.
Polyphosphate dynamics during aerobic, anaerobic, and anoxic incubation.
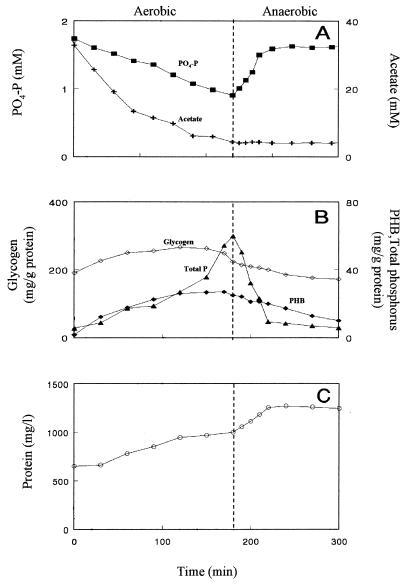
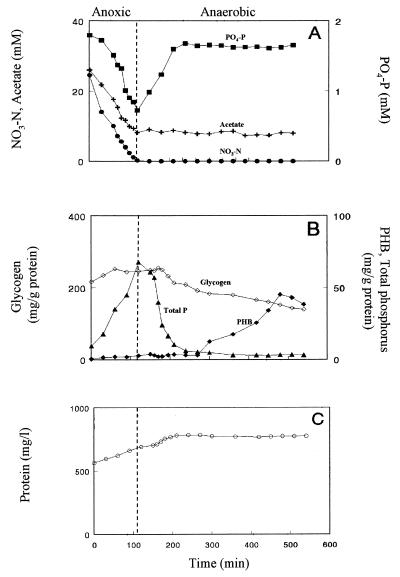
Aerobic incubation of P. denitrificans in the presence of acetate resulted in a decrease of phosphate in the medium (Fig. 1A). Phosphate removal took place as long as acetate was available. In additional experiments it was found that, once acetate was depleted under aerobic conditions, phosphate was released, and it was once more assimilated upon acetate addition (data not shown). During aerobic incubation of the organisms, the PHB content of the cells increased and their total phosphorus content increased while their glycogen content increased during the initial stage of aerobic incubation (Fig. 1B). Protein concentrations increased during aerobic incubation (Fig. 1C). When the cells were switched to anaerobic conditions, their phosphorus content decreased and phosphate was released into the medium. Under these conditions, the PHB and glycogen contents of the cells decreased and acetate was not assimilated (Fig. 1A and B). Growth, as measured by changes in protein concentrations in the medium, ceased with the depletion of the intracellular phosphorus pool (Fig. 1C). Anoxic incubation (with nitrate) of P. denitrificans, preincubated, before initiation of the anoxic incubation, for 12 h without electron donor and acceptor resulted in a decrease of nitrate, acetate, and phosphate concentrations in the medium (Fig. 2A). The cells' total phosphorus content increased and their glycogen content increased during the initial stage of anoxic incubation, while the PHB content of the cells was low throughout the anoxic incubation period (Fig. 2B). Under these conditions, cell growth took place as is evident from the increase in protein concentrations (Fig. 2C). Upon nitrate depletion (t = 110 min), phosphate was released into the medium and no acetate was assimilated. Glycogen and phosphorus contents of the cells decreased, while the PHB content of the cells increased after the depletion of the intracellularly stored phosphorus pool. As is evident from the changes in protein concentrations, cell growth ceased with the depletion of intracellular phosphorus (Fig. 2C). An electron microscopic examination of uranyl acetate-stained cells harvested after 110 min of incubation revealed the presence of a considerable number of polyphosphate bodies (data not shown). It should be noted that higher phosphorus contents were found in cells incubated for longer periods under denitrifying conditions in the presence of phosphate. Batch growth of P. denitrificans under these conditions resulted in a phosphorus content as high as 9% (on a dry cell weight basis) in cells harvested in the late log phase of growth (data not shown).
FIG. 1.
Changes in acetate and inorganic phosphate (A), cellular glycogen, phosphorus, and PHB contents (B), and bacterial protein concentration (C) in the medium during aerobic and anaerobic incubation of P. denitrificans. Oxygen was kept at saturation levels during the aerobic incubation period.
FIG. 2.
Changes in acetate, nitrate, and inorganic phosphate (A), cellular glycogen, phosphorus, and PHB contents (B), and bacterial protein concentration (C) in the medium during anoxic and anaerobic incubation of P. denitrificans.
PHB as an internal carbon and electron donor.
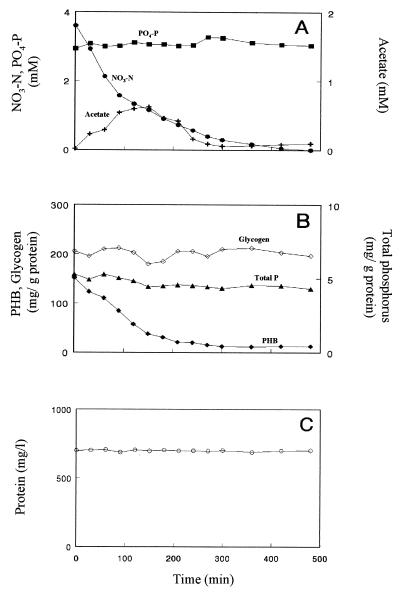
The possible role of PHB as a carbon and energy source was examined by incubation of P. denitrificans cells in medium without an external carbon donor and with nitrate (Fig. 3). PHB-rich cells were obtained by preincubating the cells under anaerobic conditions for 12 h in the presence of acetate. Under these conditions, a decrease in the PHB content of the cells (Fig. 3B) coincided with a release of acetate into the medium and a decrease in nitrate (Fig. 3A). Phosphate was not assimilated (Fig. 3A). During this incubation period, only slight changes were observed in the total phosphorus and glycogen contents of the cells (Fig. 3B). No cell growth was observed under these conditions (Fig. 3C).
FIG. 3.
Changes in acetate, nitrate, and inorganic phosphate (A), cellular glycogen, phosphorus, and PHB contents (B), and bacterial protein concentrations (C) in the medium during anoxic incubation of PHB-rich P. denitrificans in the absence of an external carbon source.
Most studies concerned with biological phosphorus removal have been conducted on crude sludge samples or on enrichment cultures obtained from EBPR plants. The main features of the PAOs thought to underlie phosphorus removal in these plants are: (i) the requirement for alternating anaerobic/ aerobic (anoxic) conditions for phosphorus removal, (ii) the ability to produce PHAs, and (iii) the ability to grow on intracellular PHA in the absence of an external carbon source. Table 1 summarizes the main physiological characteristics of PAOs in comparison to the P. denitrificans isolate examined in this study. In contrast to PAOs, polyphosphate synthesis by P. denitrificans was found to take place only in the presence of an external carbon donor under either aerobic or anoxic conditions. Furthermore, unlike PAOs, P. denitrificans was unable to utilize PHB as an energy source for polyphosphate synthesis. With PHB and without an external carbon source, P. denitrificans was able to reduce nitrate. However, this mode of respiration resulted in no measurable growth. Studies on the effect of the addition of an external carbon source on phosphorus removal and denitrification have resulted in conflicting data. In some studies (16, 30), phosphate uptake by organisms present in activated sludge samples was found to be inhibited under aerobic or anoxic incubation in the presence of carbon. Also, Meinhold et al. (20) reported a significant release of intracellularly stored phosphorus by activated sludge samples upon addition of acetate in the anoxic treatment stage. Hascoet and Florentz (10), however, reported on a simultaneous uptake and release of phosphorus under anoxic conditions in the presence of an external carbon source, while van Niel et al. (27) showed that, in the presence of nitrate, phosphorus release and acetate uptake by activated sludge organisms were severely inhibited. The studies described above, as well as most other studies on denitrification and phosphate removal in wastewater treatment plants (12, 21), are based on the assumption that denitrifying PAOs with the physiological characteristics summarized in Table 1 are the only PAOs present in these environments. The present study demonstrates also that heterotrophic denitrifiers like P. denitrificans exhibit the ability to synthesize polyphosphate. Studies on a number of denitrifying isolates in our laboratory (unpublished data) revealed that this mode of phosphorus accumulation is not restricted to P. denitrificans. In this respect, it is worthwhile to note that one of these isolates, Pseudomonas sp. strain JR12, stored polyphosphate in a mode similar to that of P. denitrificans without being capable of PHB synthesis. This latter finding might serve as additional evidence for the fact that polyphosphate synthesis by these organisms is not dependent on internally stored carbon reserves. Although information on combined polyphosphate accumulation and nitrate reduction in true denitrifiers (not PAOs) is limited to P. denitrificans (7, 13), it seems that, on the basis of results obtained with other denitrifiers in our laboratory, this ability may be a common trait among many denitrifiers. The most striking feature of the mode of phosphate accumulation exhibited by P. denitrificans and other denitrifiers examined in our laboratory is their ability to synthesize polyphosphate without the need for alternating anaerobic/aerobic (anoxic) conditions. Therefore, the significance of this finding is that, contrary to the common assumption among wastewater engineers, biological phosphorus and nitrate removal may be conducted in one treatment step. Indeed, long-term operation of a denitrifying reactor used for nitrate removal from an intensive aquaculture system resulted in a considerable withdrawal of phosphate by the denitrifying organisms in the reactor (2).
TABLE 1.
Physiological characteristics of Paracoccus denitrificans and PAOs
| Organism(s) | Anaerobic metabolism | Aerobic/anoxic metabolism | References |
|---|---|---|---|
| P. denitrificans | Unable to use external carbon source for PHA synthesis; degrades glycogen for PHA synthesis; degrades polyphosphate, releases phosphate; degradation of polyphosphate provides energy for growth | Produces polyphosphate and grows on energy provided by external carbon source; produces glycogen; in absence of external carbon source, cells with PHA do not grow and do not produce polyphosphate | 7, 13, this study |
| PAOs | Use external carbon source for PHA synthesis; release phosphate; degrade polyphosphate; degrade glycogen | Produce polyphosphate by degradation of PHA, produce glycogen; grow in absence of external carbon source on PHA; when present, external carbon source might inhibit polyphosphate synthesis or is used for PHA production but not for growth | 5, 11, 14, 17, 22 |
REFERENCES
- 1.Aboutboul Y, Arbiv R, van Rijn J. Anaerobic treatment of fish culture effluent: volatile fatty acid mediated denitrification. Aquaculture. 1995;133:21–32. [Google Scholar]
- 2.Barak, Y., and J. van Rijn. Biological phosphorus removal in a prototype recirculating aquaculture system. Aquacultural Eng., in press.
- 3.Barker P S, Dold P L. Denitrification behavior in biological excess phosphorus removal activated sludge system. Water Res. 1996;30:769–780. [Google Scholar]
- 4.Clinton J D. Biological techniques in electron microscopy. New York, N.Y: Barnes and Noble; 1971. pp. 146–148. [Google Scholar]
- 5.Comeau Y, Hall K J, Hancock R E W, Oldham W K. Biochemical model for enhanced biological phosphorus removal. Water Res. 1986;20:1511–1521. [Google Scholar]
- 6.Egly T, Zehnder J B. Phosphate and nitrate removal. Curr Opin Biotechnol. 1994;5:275–284. [Google Scholar]
- 7.Ferguson S T, Gadian D G. Evidence from 31P nuclear magnetic resonance that polyphosphate synthesis is a slip reaction in Paracoccus denitrificans. Biochem Soc Trans. 1979;7:176–179. doi: 10.1042/bst0070176. [DOI] [PubMed] [Google Scholar]
- 8.Golterman H L, Clymo R S, Ohnstad M A M. IBP handbook no. 8. 2nd ed. Oxford, United Kingdom: Blackwell Scientific Publications; 1978. Methods for physical and chemical analysis of fresh waters; pp. 111–117. [Google Scholar]
- 9.Hanson R S, Phillips J A. Chemical composition. In: Gerhardt P, editor. Manual of methods for general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. p. 333. [Google Scholar]
- 10.Hascoet M C, Florentz M. Influence of nitrates on biological phosphorus removal from wastewater. Water S A. 1985;11:1–8. [Google Scholar]
- 11.Iwema A, Meunier A. Influence of nitrate on acetic acid induced biological phosphorus removal. Water Sci Technol. 1985;17:289–294. [Google Scholar]
- 12.Jorgensen K S, Pauli A S L. Polyphosphate accumulation among denitrifying bacteria in activated sludge. Anaerobe. 1995;1:161–168. doi: 10.1006/anae.1995.1014. [DOI] [PubMed] [Google Scholar]
- 13.Kaltwasser H, Vogt G, Schlegel H G. Polyphosphatsynthese wahrend der nitrat-atmung von Micrococcus denitrificans Stamm 11. Arch Mikrobiol. 1961;44:259–265. [PubMed] [Google Scholar]
- 14.Kerrn-Jespersen J P, Henze M. Biological phosphorus uptake under anoxic and aerobic conditions. Water Res. 1993;27:617–624. [Google Scholar]
- 15.Kuba T, Smolders G, van Loosdrecht M C M, Heijnen J J. Biological phosphorus removal from wastewater by anaerobic-anoxic sequencing batch reactor. Water Sci Technol. 1993;27:241–252. [Google Scholar]
- 16.Kuba T, Smolders G, Wachtmeister A, van Loosdrecht M C M, Heijnen J J. Effect of nitrate on phosphorus release in biological phosphorus removal system. Water Sci Technol. 1994;30:263–269. [Google Scholar]
- 17.Kuba T, Smolders G, van Loosdrecht M C M, Heijnen J J. A metabolic model for biological phosphorus removal by denitrifying organisms. Biotechnol Bioeng. 1997;52:685–695. doi: 10.1002/(SICI)1097-0290(19961220)52:6<685::AID-BIT6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 18.Law J H, Slepecky R A. Assay of poly-β-hydroxybutyric acid. J Bacteriol. 1961;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markwell M A, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 20.Meinhold J, Filipe D C M, Daiger T G, Isaacs S. Characterisation of the denitrifying fraction of phosphate accumulating organisms in biological phosphate removal. Water Sci Technol. 1999;39:31–42. [Google Scholar]
- 21.Merzouki M, Delgenus P J, Bernet N, Moletta R, Benlemlih M. Polyphosphate accumulating and denitrifying bacteria isolated from anaerobic-anoxic sequencing batch reactors. Curr Microbiol. 1999;38:9–17. doi: 10.1007/pl00006776. [DOI] [PubMed] [Google Scholar]
- 22.Mino T, van Loosdrecht M C M, Heijnen J J. Microbiology and biochemistry of the enhanced biological phosphate removal processes. Water Res. 1998;32:3193–3207. [Google Scholar]
- 23.Peladan F, Monteil H. Identification of Pseudomonas flavobacterium and Alcaligenes sp. with API 20 NE. Syst Pathol Biol. 1988;36:31–36. [PubMed] [Google Scholar]
- 24.Scheiner D. Determinations of ammonia and Kjeldahl nitrogen by the indophenol method. Water Res. 1976;10:31–36. [Google Scholar]
- 25.Strickland J D, Parsons T R. A practical handbook of seawater analysis. Bull Fish Res Board Can. 1968;167:77–80. [Google Scholar]
- 26.Toerien D F, Gerber A, Lotter L H, Cloete T E. Enhanced biological phosphorus removal in activated sludge systems. In: Marshall K C, editor. Advances in microbial ecology. Vol. 11. New York, N.Y: Plenum Press; 1990. pp. 173–219. [Google Scholar]
- 27.van Niel E W J, Appeldoorn K J, Zehnder A J B, Kortslee G J J. Inhibition of anaerobic phosphate release by nitric oxide in activated sludge. Appl Environ Microbiol. 1998;64:2925–2930. doi: 10.1128/aem.64.8.2925-2930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rijn J, Tal Y, Barak Y. Influence of volatile fatty acids on nitrite accumulation by a Pseudomonas stutzeri strain isolated from a denitrifying fluidized bed reactor. Appl Environ Microbiol. 1996;62:2615–2620. doi: 10.1128/aem.62.7.2615-2620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visniac W, Santer M. The thiobacilli. Bacteriol Rev. 1975;21:195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wentzel M C, Dold P L, Ekama G A, Marais G v R. Enhanced polyphosphorus organism cultures in activated sludge systems. Part 3: kinetic model. Water S A. 1989;15:89–102. [Google Scholar]