Abstract
The development of heteronuclear metal complexes as potent anticancer agents has received increasing attention in recent years. In this study, two new heteronuclear Ru(Ⅱ)-Re(Ⅰ) metal complexes, [Ru(bpy)2LRe(CO)3(DIP)](PF6)3 and [Ru(phen)2LRe(CO)3(DIP)](PF6)3 [RuRe-1 and RuRe-2, L = 2-(4-pyridinyl)imidazolio[4,5-f][1,10]phenanthroline, bpy = 2,2′-bipyridine, DIP = 4,7-diphenyl-1,10-phenanthroline, phen = 1,10-phenanthroline], were synthesized and characterized. Cytotoxicity assay shows that RuRe-1 and RuRe-2 exhibit higher anticancer activity than cisplatin, and exist certain selectivity toward human cancer cells over normal cells. The anticancer mechanistic studies reveal that RuRe-1 and RuRe-2 can induce apoptosis through the regulation of cell cycle, depolarization of mitochondrial membrane potential (MMP), elevation of intracellular reactive oxygen species (ROS), and caspase cascade. Moreover, RuRe-1 and RuRe-2 can effectively inhibit cell migration and colony formation. Taken together, heteronuclear Ru(Ⅱ)-Re(Ⅰ) metal complexes possess the prospect of developing new anticancer agents with high efficacy.
Keywords: heteronuclear metal complexes, ruthenium(II) complexes, rhenium(I) complexes, anticancer activity, apoptosis
Introduction
Cancer is a malignant disease that seriously threatens human health and life (Lortet-Tieulent et al., 2020). Although platinum-based drugs show outstanding antitumor activity, they also exhibit strong toxic side effects and drug resistance (Oun et al., 2018). This makes the development of non-platinum drugs particularly important. Ruthenium metal complexes, which offer the advantages of easy cellular uptake (Notaro and Gasser., 2017), good biodistribution (Sun et al., 2021), low toxicity (Thota et al., 2018), induction of cell apoptosis (Galczynska et al., 2020), selective anti-invasion and anti-metastasis activity (Cao et al., 2017), are the most promising non-platinum antitumor drugs. At present, several ruthenium complexes have entered clinical studies, including NAMI-A (Alessio and Messori, 2019), KP1019 (Alessio and Messori, 2018), KP1339 (Heffeter et al., 2013), TLD1433 (McFarland et al., 2020). Ruthenium complexes can exert their anti-tumor activity by interacting with biomolecules such as proteins (Nehru et al., 2020; da Silva et al., 2021), DNA (Gill and Thomas, 2012; Li et al., 2016; Zhang et al., 2018), RNA (Jain et al., 2013; Wang P. et al., 2021), and subcellular organelles (Qiu et al., 2017; Huang et al., 2020; Tan et al., 2021). For example, Ru(II) polypyridine complexes containing the planar ligand DPPZ (dipyrido[3,2-a:2′,3′-c]phenazine) show high insertion affinity with DNA and act as the “light switch” of DNA molecules (Holmlin et al., 1998). By coupling Ru(II)-polypyridyl moiety with a phenanthroline substituted SAHA (suberoylanilide hydroxamic acid) derivative, our group reported three Ru(II)-based histone deacetylases inhibitors that show excellent antitumor activity and histone deacetylase inhibition (Ye et al., 2013). In summary, ruthenium-based metal complexes play an important role in the development of antitumor drugs. Liu’s group reported a series of organometallic half-sandwich Ru(II) complexes bearing aryl-BIAN chelating ligands that can elicit cytotoxicity through lysosome-mediated apoptosis in vitro and suppress tumor growth in vivo (Xu et al., 2020).
In addition to ruthenium complexes, rhenium complexes also exhibit potent anticancer activity and have attracted much attention in metal-based anticancer drugs (Leonidova and Gasser, 2014; Collery et al., 2019; Capper et al., 2020). Rhenium-based compounds exhibit high stability (Kumar et al., 2016), structural diversity (Imstepf et al., 2016), ease of real-time imaging (Palmioli et al., 2017) and lack of off-site toxicity (Capper et al., 2020). Recently, by integrating deferasirox with Re(I) moiety, Mao’s group designed a mitochondria-targeted rhenium(I) complex that disrupts both mitochondrial metabolism and iron homeostasis (Pan et al., 2020). Phosphorescent Re(I) tricarbonyl complexes bearing β-carboline derivatives exhibit pH-dependent phosphorescence that specifically image lysosomes, causing lysosomal dysfunction and impaired lysosomal activity, which in turn leads to autophagy and apoptosis-dependent cell death (He et al., 2019). Our group reported a series of phosphorescent rhenium(I) complexes conjugated with artesunate, showing mitochondrial targeting and dual induction of apoptosis-ferroptosis (Ye et al., 2021). The photodynamic anticancer activity of Re(I) complexes has also been extensively studied (Leonidova et al., 2014; Liew et al., 2020; He et al., 2022).
Heterobimetallic complexes have been explored in the anticancer field in order to associate different metals within a single entity to enhance their activity (Jain, 2019; Johnson et al., 2020; Guedes et al., 2020; Tsolis et al., 2021). Pt(II)-Re(I) complexes synthesized by Paulo’s team showed dual imaging and anticancer properties (Quental et al., 2017). Compared with Pt(II)-Re(I) heteronuclear metal complexes, the antitumor properties of Pt(II)-Ru(II) heteronuclear metal complexes are relatively more studied. Pt(II)-Ru(II) complexes reported by singh’s group could bind to DNA and showed phototoxicity to MCF-7 cells (Singh et al., 2021). Brenda S. J. Winkel’s group reported that the polyazine bridged Pt(II)-Ru(II) complex displayed significant DNA modification, cell growth inhibition, and toxicity towards F98 malignant glioma cells following visible light irradiation (Zhu et al., 2017). Pt(II)-Ru(II) complex designed by Mao and Tan’s team could overcome cisplatin resistance by photodamaging mitochondrial DNA (Zheng et al., 2018). The research on Ru(II)-Re(II) heteronuclear metal complexes mostly focus on catalysis (Coleman et al., 2008; Li et al., 2021). There are few publications reporting the DNA switching (Foxon et al., 2007; Jarman et al., 2019) and pH luminescence switching (Zheng et al., 2014) effects of these complexes.
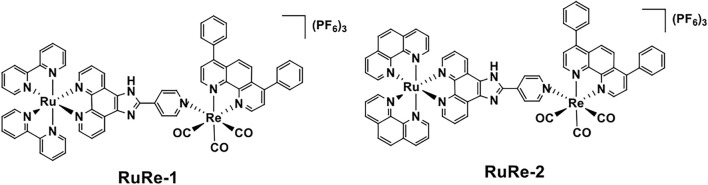
In this context, two heterobimetallic complexes based on Ru(II) and Re(I) units, [Ru(bpy)2LRe(CO)3(DIP)](PF6)3 and [Ru(phen)2LRe(CO)3(DIP)](PF6)3 (RuRe-1 and RuRe-2, L = 2-(4-pyridinyl)imidazolio[4,5-f][1,10]phenanthroline, bpy = 2,2′-bipyridine, DIP = 4,7-diphenyl-1,10-phenanthroline, phen = 1,10-phenanthroline), were designed and synthesized (Scheme 1). We first evaluated their anti-proliferative activities. Then, the anticancer mechanisms of RuRe-1 and RuRe-2 were discussed in detail, including the effects on apoptosis, cell cycle, mitochondrial membrane potential (MMP), reactive oxygen species (ROS), cell migration and colony formation. These findings will contribute to the development of heteronuclear metal complexes in anticancer field.
SCHEME 1.
Chemical structures of RuRe-1 and RuRe-2.
Results and Discussion
Synthesis, Characterization, and Photophysical Properties
The synthetic routes of RuRe-1 and RuRe-2 are shown in Supplementary Figure S1. Firstly, mononuclear complexes Ru-1 (Halpin et al., 2013) and Re-1 (Woźna and Kapturkiewicz, 2015) were synthesized according to literature methods. Ru-2 was prepared following a similar procedure to that of Ru-1, except that cis-[Ru(phen)2Cl2]·2H2O (Sullivan et al., 1978) was used instead of cis-[Ru(bpy)2Cl2]·2H2O (Hartshorn and Barton, 1992). Target complexes RuRe-1 and RuRe-2 could be successfully synthesized through the direct reaction of Ru-1 or Ru-2 with Re-1 in acetone. After most of the solvents were concentrated in vacuum, a red precipitate was obtained by dropwise addition of saturated NH4PF6 aqueous solution, then the crude product was purified by silica gel column chromatography with a mobile phase of acetonitrile: water: saturated potassium nitrate = 100: 9: 1. The products were characterized using ESI-MS, 1H NMR, FT-IR (Supplementary Figures S2–S9) and elemental analysis.
The electronic absorption and emission spectra of mononuclear complexes (Ru-1, Ru-2, Re-1) and heteronuclear RuRe-1 and RuRe-2 were recorded in phosphate buffered saline (PBS), dichloromethane and acetonitrile at 298 K (Supplementary Figure S10 and Supplementary Figure S11). As shown in Supplementary Figure S11A, an intense absorption band at approximately 260–320 nm was observed, which could be assigned to the intraligand transition, and another two less intense absorptions in visible light range at approximately 350–400 nm and 420–500 nm could be ascribed to d(Ru)→L(π*) and d(Re)→L(π*) metal-to-ligand charge-transfer, respectively. Upon excitation at 455 nm, RuRe-1 and RuRe-2 showed similar emission bands, with the maximum emission around 590 nm in PBS (Supplementary Figure S11B). And the emission quantum yields (Ф em) of mononuclear complexes (Ru-1, Ru-2, Re-1) and heteronuclear RuRe-1 and RuRe-2 have also been determined. The Ф em of RuRe-1 and RuRe-2 were similar to those of Ru-1 and Ru-2. The photophysical data were summarized in Supplementary Table S1.
Stability
The stabilities of RuRe-1 and RuRe-2 in PBS and human serum albumin (HSA) were tested through UV–Vis spectroscopy. As shown in Supplementary Figure S12, there was no significant change in the spectral characteristics and absorption peaks of RuRe-1 and RuRe-2 collected at 0, 24, and 48 h, indicating that these complexes are stable under physiological conditions.
Lipophilicity and Cellular Uptake
Drugs exert their effects through pharmacokinetic processes such as absorption, distribution, and metabolism, which are closely related to the n-octanol/water partition coefficient of the drug (log P o/w) (Choi et al., 2012). Herein, the log P o/w of RuRe-1 and RuRe-2 were determined by the shaking flask method to be 0.47 and 2.10, respectively, indicating that these two compounds are hydrophobic and can be well absorbed by cells.
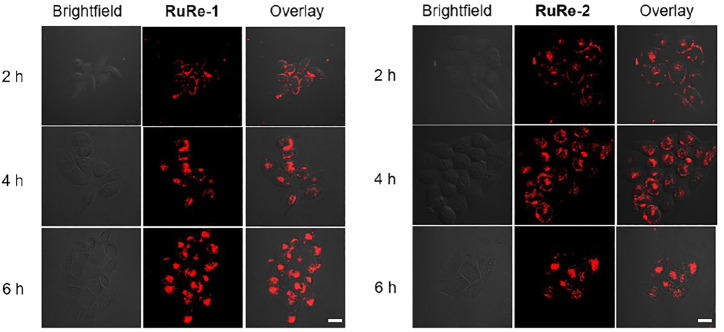
The cellular uptake of RuRe-1 and RuRe-2 was first qualitatively investigated by confocal microscopy. As shown in Figure 1, with the increase of incubation time, both complexes could effectively penetrate into HeLa cells and exhibited bright fluorescence in the cytoplasm. As exogenous elements, ruthenium and rhenium in cells can be quantified by inductively coupled plasma mass spectrometry (ICP-MS). Upon incubation with 10 μM RuRe-1 and RuRe-2 for 6 h, the ratio of intracellular ruthenium and rhenium content was approximately 1:1 (Supplementary Figure S13). This result further confirms the stability of the heteronuclear Ru(Ⅱ)-Re(Ⅰ) complexes under physiological conditions.
FIGURE 1.
Intracellular uptake of RuRe-1 (10 μM) and RuRe-2 (10 μM) measured by confocal microscopy (λ ex = 488 nm, λ em = 590 ± 20 nm). Scale bar: 20 μm.
in vitro Antitumor Activity
The anticancer activities of RuRe-1 and RuRe-2 against four cancer cell lines: HeLa (human cervical cancer cells), HepG2 (human hepatocellular carcinoma), A549 (human lung cancer cells), A549R (cisplatin-resistant A549) and one normal cell line LO2 (human normal liver cells) were determined by 3-(4,5-dimethylthiazole)-2,5-diphenyltetraazolium bromide (MTT) assay, while the mononuclear complexes Ru-1, Ru-2, Re-1 and cisplatin were selected as controls. As shown in Table 1, RuRe-1 and RuRe-2 showed the best anticancer effects on HeLa cells, with IC50 values of 3.1 μM and 2.5 μM, respectively. And the in vitro antiproliferative efficacies of the compounds against HeLa cells were in the following order: RuRe-2 > RuRe-1 > Re-1 > cisplatin > Ru-1 > Ru-2. The mononuclear ruthenium complexes Ru-1 and Ru-2 showed negligible antitumor activity against all cancer cell lines screened. The anticancer activity of the mononuclear rhenium complex Re-1 was between 5.6 μM and 8.0 μM, which contributed the most to the antitumor activities of the heteronuclear Ru(II)-Re(Ⅰ) metal complexes. In addition, RuRe-1 and RuRe-2 displayed approximately 8.7-fold and 5.4-fold greater ability to kill A549R cells than cisplatin, indicating that they can conquer the resistance of cisplatin. Furthermore, the cytotoxicity of RuRe-1 and RuRe-2 against LO2 cells was lower than that of HepG2 cells, revealing their selectivity to cancer cells.
TABLE 1.
IC50 values of tested compounds towards different cell lines a .
| Compounds | IC50 (μM) | ||||
|---|---|---|---|---|---|
| HeLa | HepG2 | A549 | A549R | LO2 | |
| RuRe-1 | 3.1 ± 0.8 | 10.0 ± 0.8 | 11.8 ± 0.4 | 10.4 ± 1.2 | 25.1 ± 0.7 |
| RuRe-2 | 2.5 ± 1.3 | 12.5 ± 0.9 | 11.2 ± 0.9 | 16.7 ± 0.8 | 28.1 ± 1.1 |
| Ru-1 | 39.8 ± 0.6 | >50 | >50 | >50 | >50 |
| Ru-2 | 43.6 ± 1.2 | >50 | >50 | >50 | >50 |
| Re-1 | 7.7 ± 0.8 | 5.6 ± 0.5 | 6.9 ± 0.9 | 8.0 ± 1.1 | 5.8 ± 0.2 |
| Cisplatin | 19.3 ± 0.5 | 23.5 ± 2.0 | 25.9 ± 0.8 | 90.3 ± 0.3 | 22.5 ± 2.0 |
IC50 values are drug concentrations necessary for 50% inhibition of cell viability. The data are presented as mean ± standard deviation (SD) and cell viability is assessed after 48 h of incubation.
Apoptosis Assay
Apoptosis is an evolutionarily conserved form of programmed cell death that is essential for animal development and tissue homeostasis (Obeng, 2020). Apoptosis is characterized by a series of defined biochemical and morphological events, such as activation of caspase family proteases, loss of cell membrane asymmetry accompanied by phosphatidylserine translocation from the inner plasma membrane to the outer cell surface, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation.
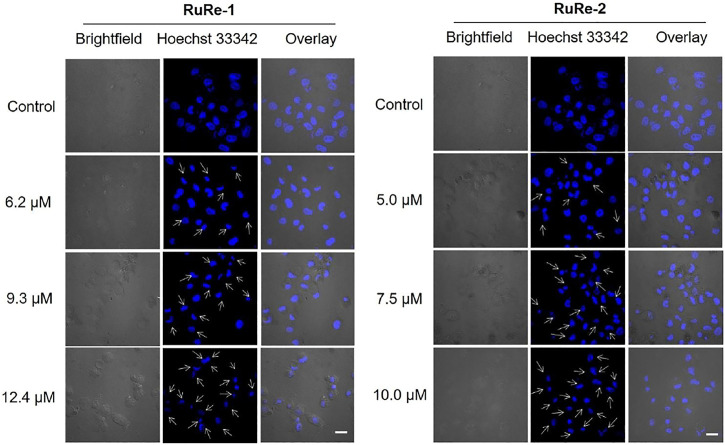
First, changes in cell morphology of HeLa cells induced by RuRe-1 and RuRe-2 were examined with 2'-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi-1H-benzimidazole trihydrochloride (Hoechst 33342) staining. Hoechst 33342 is a blue fluorescent dye that can penetrate cell membranes and is commonly used to stain cell nuclei (Tian et al., 2018). The results were given in Figure 2, the nuclei of the control group showed a regular round shape, while the cells treated with RuRe-1 and RuRe-2 showed typical apoptotic characteristics, in which the nuclei solidified into a homogeneous dense mass and then broke into fragments of different sizes, and the number of cells showing this phenomenon increased with the concentration of the drug administered.
FIGURE 2.
RuRe-1 and RuRe-2 induced HeLa cells apoptosis measured by confocal microscopy with Hoechst 33342 staining (λ ex = 405 nm, λ em = 460 ± 20 nm). The incubation time of the compounds was 24 h. Arrows indicate apoptotic morphological nuclei. Scale bar: 20 μm.
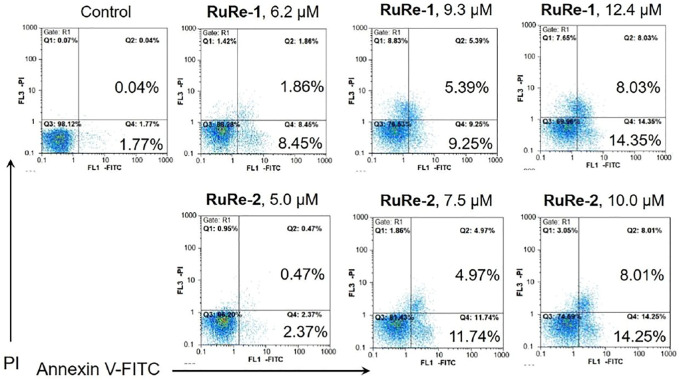
The ability of RuRe-1 and RuRe-2 to induce apoptosis in HeLa cells was further verified using Annexin V-FITC/PI (FITC: fluorescein isothiocyanate; PI: propidium iodide) double-staining. In the early stage of apoptosis, the surface of the cell membrane is broken, at which point the phosphatidylserine on the surface of the apoptotic cell flips from the inner cell membrane to the outer cell membrane, where it can be labelled by Annexin V (Jan and Chaudhry, 2019). The membrane permeability of PI is poor and thus only necrotic cells can be labelled (Zec et al., 2014). As shown in Figure 3, after treatment of cells with RuRe-1 and RuRe-2 for 24 h, the proportion of apoptotic cells (early apoptotic + late apoptotic) increased in a concentration-dependent manner. Specifically, treatment with RuRe-1 (12.4 μM) or RuRe-2 (10.0 μM) significantly increased the percentage of apoptotic cells from 1.81% (control) to 22.38% (RuRe-1) and 22.26% (RuRe-2), respectively. Overall, the results indicate their ability to induce apoptosis in HeLa cells.
FIGURE 3.
RuRe-1 and RuRe-2 induced HeLa cells apoptosis measured by flow cytometry with Annexin V/PI staining. The incubation time of the compounds was 24 h.
Cysteine aspartate proteases play an important role in apoptosis, especially caspase-3 protein, which is one of the most important execution factors in the apoptotic pathway (Wang X. et al., 2021). The poly(ADP-ribose) polymerase (PARP) is associated with DNA repair and guardianship of genetic integrity (Pandey and Black, 2021). After treating HeLa cells with different concentrations of RuRe-1 or RuRe-2 for 24 h, the expression of caspase-3 and the caspase substrate PARP apoptotic protein were detected by western blot. As shown in Figure 4, RuRe-1 and RuRe-2 induced the cleavage of caspase-3 and PARP in a dose-dependent manner. It was further shown that RuRe-1 and RuRe-2 are activators of caspase-3, which can trigger apoptosis in HeLa cells.
FIGURE 4.
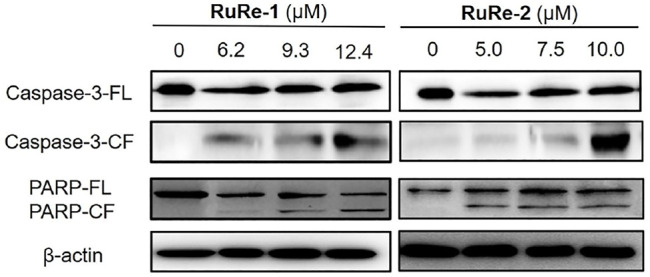
Expression of apoptosis-related protein (caspase-3 and PARP) in HeLa cells treated with RuRe-1 and RuRe-2 for 24 h.
Cell cycle arrest studies provide a good understanding of cell apoptosis induced by metal drugs (Sadoughi et al., 2021). To further clarify the mechanism of apoptosis induced by RuRe-1 and RuRe-2, we analyzed the cell cycle of HeLa cells treated with RuRe-1 and RuRe-2 by flow cytometry with PI staining. The results (Figure 5, Supplementary Figure S14 and Supplementary Table S2) showed that compared with the control group, the content of S-phase cells in the RuRe-1 (12.4 μM) and RuRe-2 (10.0 μM) treated groups increased from 14.4 to 33.8% and 40.0%, respectively. A corresponding decrease in cell content in G1 and G2 phases was observed. The result suggests that RuRe-1 and RuRe-2 may induce cell death by regulating the cell cycle.
FIGURE 5.
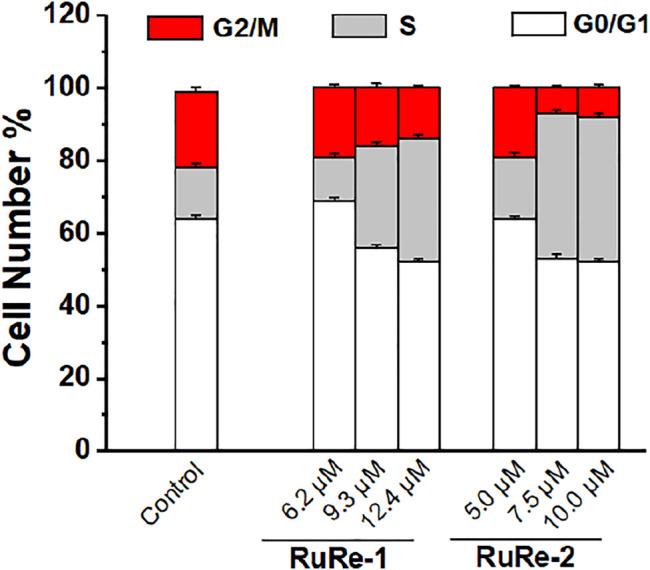
Quantitative cell-cycle distribution data for HeLa cells after treatment with RuRe-1 and RuRe-2 for 24 h.
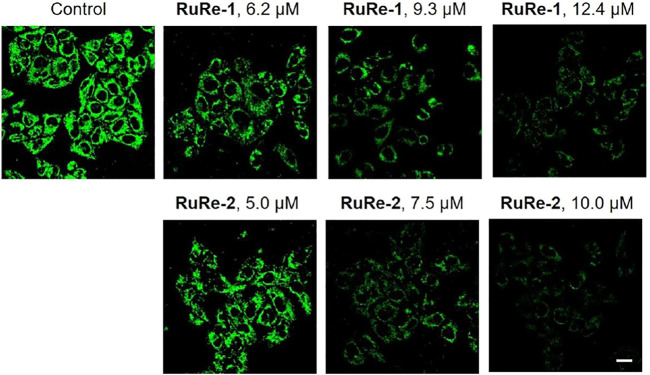
Apoptosis is associated with a decrease in MMP (Wu et al., 2022), so we investigated the effects of RuRe-1 and RuRe-2 on MMP. A decrease in fluorescence intensity of rhodamine 123 (Rh123) can indicate the loss of MMP (Ma et al., 2019). As shown in Figure 6, treated HeLa cells with RuRe-1 and RuRe-2 for 6 h induced the decrease in the green fluorescence intensity of Rh123 in a dose-dependent manner. The decline of MMP further confirmed that RuRe-1 and RuRe-2 could influence mitochondrial function and promote apoptosis.
FIGURE 6.
RuRe-1 and RuRe-2 induced the loss of MMP analyzed by confocal microscopy with Rh123 staining (λ ex = 488 nm, λ em = 530 ± 20 nm). The incubation time of the compounds was 6 h. Scale bar: 20 μm.
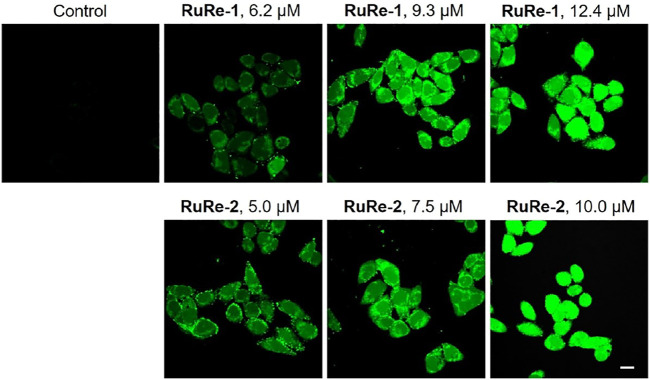
ROS are natural by-product of normal oxygen metabolism and play an important role in cellular signaling transduction and homeostasis in vivo (Casas et al., 2020). It has been shown that the excessive production of ROS may cause oxidative stress and lead to cell death (Jia et al., 2020). Herein, cells were treated with RuRe-1 and RuRe-2 for 6 h and then stained with 2′,7′-dichlorodihydrouorescein diacetate (H2DCFDA). H2DCFDA is non-fluorescent and can be oxidized by intracellular ROS to highly fluorescent 2′,7′-dichlorofluorescein (DCF) (Abdel Hadi et al., 2021). The intensity of green fluorescence can respond to the accumulation of intracellular ROS. As shown in Figure 7, treated HeLa cells with RuRe-1 and RuRe-2 for 6 h induced an increase in intracellular ROS levels in a concentration-dependent manner. It reveals that RuRe-1 and RuRe-2 possess a strong ability to cause cell oxidative stress.
FIGURE 7.
RuRe-1 and RuRe-2 induced the elevation of intracellular ROS levels examined by confocal microscopy with H2DCFDA staining (λ ex = 488 nm, λ em = 530 ± 20 nm). The incubation time of the compounds was 6 h. Scale bar: 20 μm.
Inhibit Cell Migration and Colony Formation
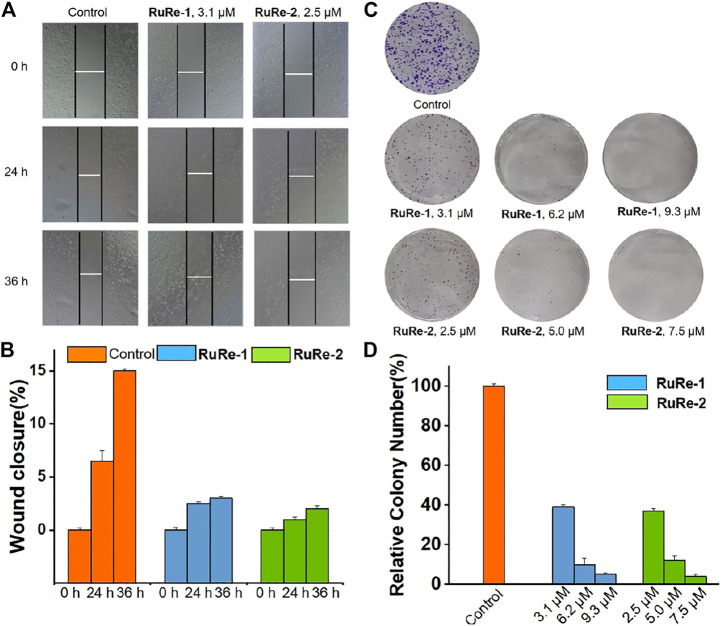
Metastasis is a major obstacle to cancer treatment, which will cause the failure of cancer treatment and the death of patients (Fouani et al., 2017). Cell migration is the main feature of metastasis. Herein, the effects of RuRe-1 and RuRe-2 on inhibiting cell migration were studied through wound healing. As compared to control group, HeLa cells treated with RuRe-1 (3.1 μM) and RuRe-2 (2.5 μM) exhibited a significant time-dependent inhibition of wound healing integrity (Figure 8A). After 36 h cultivated, both RuRe-1 and RuRe-2 inhibited cell migration, and the wound closure rates were 3% and 2%, respectively, which were lower than 15% of the control group (Figure 8B). The results show that these compounds can effectively inhibit cell migration.
FIGURE 8.
(A) Wound healing of HeLa cells after treated with RuRe-1 (3.1 μM) and RuRe-2 (2.5 μM) for 0, 24, and 36 h. (B) Quantitative data of wound-healing. Wound closure (%) = [1−(width at indicated time)/(width at 0 h)] × 100%. (C) Inhibition of colony formation by RuRe-1 and RuRe-2. (D) Quantitative data of colony formation assays.
The consequence of increased local cell migration is distal invasion, and the clonal growth of distal invasive cells will produce a second tumor (Wang et al., 2017). We further investigated the ability of RuRe-1 and RuRe-2 to inhibit the formation of cell colonies by cell colony formation assay. As shown in Figure 8C, the control group formed multiple cell colonies that covered almost the entire study area, while the number of cell colonies in the experimental groups treated with RuRe-1 and RuRe-2 decreased with increasing concentration of the administered drug. Notably, cell populations were almost invisible after treatment with RuRe-1 (9.3 μM) and RuRe-2 (7.5 μM). The quantitative graphs showed that the cell populations at this time were only 4.8% and 3.9% compared to the control group (Figure 8D). The results show that RuRe-1 and RuRe-2 are effective in reducing cell migration and inhibiting colony formation.
Conclusion
In general, two heteronuclear metal complexes, RuRe-1 and RuRe-2, have been synthesized and their antitumor potential has been developed. Confocal microscopy studies have shown that RuRe-1 and RuRe-2 can penetrate cells effectively. Cell viability inhibition assays show that RuRe-1 and RuRe-2 are selective killers of tumor cells and exhibit higher toxicity to A549R cells than cisplatin. The mechanistic studies reveal that RuRe-1 and RuRe-2 induce MMP depolarization, causing damage to the mitochondria, which in turn increases the intracellular ROS levels and further induces apoptosis. Meanwhile, the process of apoptosis is accompanied by the activation of caspases and the arrest of the cell cycle in S phase. Finally, RuRe-1 and RuRe-2 exert potent inhibitory effects on migration and colony formation. Overall, the study of RuRe-1 and RuRe-2 provides a basis for the synthesis of heteronuclear metal complexes and their antitumor activity research.
Materials and Methods
Materials and Instruments
RuCl3·nH2O (J&K), bpy (J&K), phen (J&K), DIP (J&K), Re(CO)5Cl (Sigma Aldrich), silver trifluoromethanesulfonate (Alfa Aesar), 4-pyridinecarboxaldehyde (Alfa Aesar), 5,6-diamino-1,10-phenanthroline (Alfa Aesar), NH4PF6 (Alfa Aesar), 4% paraformaldehyde (Beyotime), crystal violet (Beyotime), Dulbecco’s Modified Eagle Medium (DMEM, Gibco), Roswell Park Memorial Institute 1640 (RPMI 1640, Gibco), Fetal bovine serum (FBS, Gibco), penicillin-streptomycin (Gibco), MTT (J&K), Rh123 (J&K), H2DCFDA (J&K), Hoechst 33342 (J&K), Annexin V-FITC Apoptosis Detection Kit (Beyotime). Primary antibodies against caspase-3 and PARP were purchased from Cell Signaling Technology. RuRe-1, RuRe-2 were dissolved in DMSO just before the experiments, and the concentration of DMSO in biological experiments was 1% (v/v). Cisplatin was dissolved in 0.9% sodium chloride solution just before use.
A LCQ DECA XP spectrometer was used for obtaining ESI-MS spectra. A Bruker Avance 600 spectrometer was used for obtaining 1H NMR spectra. A SpetraMax M2 plate reader was used for determining cell viability. A Nikon A1R/A1 laser-scanning confocal microscope was used for obtaining cell imaging images. A CyFlow Space flow cytometer was used for performing the flow cytometry analysis.
Synthesis of Heteronuclear Ru(Ⅱ)-Re(Ⅰ) Metal Complexes
Mononuclear complexes Ru-1 (Halpin et al., 2013) and Re-1 (Woźna and Kapturkiewicz, 2015) were synthesized according to literature methods. Ru-2 was prepared following a similar procedure to that of Ru-1, except that cis-[Ru(phen)2Cl2]·2H2O (Sullivan et al., 1978) was used instead of cis-[Ru(bpy)2Cl2]·2H2O (Hartshorn and Barton, 1992).
Ru-2: 1H NMR (600 MHz, [D6]DMSO) δ 9.08 (t, J = 14.2 Hz, 1H), 8.87 (d, J = 5.2 Hz, 1H), 8.78 (d, J = 8.2 Hz, 2H), 8.40 (s, 2H), 8.23 (d, J = 5.9 Hz, 1H), 8.14 (d, J = 5.0 Hz, 1H), 8.06 (dd, J = 19.8, 5.0 Hz, 2H), 7.83 (s, 1H), 7.77 (dd, J = 8.2, 5.3 Hz, 2H). ESI-MS (CH3CN): m/z 758.1345 [M-PF6]+, 379.5706 [M-2PF6]2+.
RuRe-1: The synthetic route of RuRe-1 is shown in Scheme 1. A mixture of Ru-1 (0.140 g, 0.138 mmol) and Re-1 (0.131 g, 0.166 mmol) were dissolved in 60 ml acetone. After stirred at 329 K for 24 h under nitrogen, the reaction solution was concentrated to 5 ml. A red precipitate was obtained by dropwise addition of saturated NH4PF6 aqueous solution. Then, the solid was purified by silica gel column chromatography (acetonitrile: water: saturated potassium nitrate, 100:9:1). The PF6 salt of RuRe-1 was again formed by adding saturated NH4PF6 aqueous solution, and then dried under vacuum. Yield: 79% (red solid). 1H NMR (600 MHz, [D6]DMSO) δ 9.88 (d, J = 5.4 Hz, 1H), 8.97–8.71 (m, 4H), 8.31–8.22 (m, 1H), 8.24–8.12 (m, 3H), 8.08 (dd, J = 12.3, 4.7 Hz, 1H), 7.99 (s, 1H), 7.83 (d, J = 5.3 Hz, 2H), 7.77–7.64 (m, 5H), 7.58 (t, J = 4.0 Hz, 2H), 7.32 (t, J = 6.6 Hz, 1H). FT-IR (KBr) νCO/cm−1: 2032.45, 1914.89. ESI-MS (CH3CN): m/z 656.6029 [M-3PF6-H]2+, 603.0723 [M-Ru(bpy)2L-2PF6]+, 355.5717 [M-Re(DIP)(CO)3-3PF6]2+. Elemental analysis: calcd (%) for C65H43F18N11O3P3ReRu: C, 44.66; H, 2.48; N, 8.81; found: C, 44.56; H, 2.63; N, 8.92.
RuRe-2: Complex RuRe-2 was prepared following a similar procedure to that of RuRe-1, except that Ru-2 was used instead of Ru-1. Yield: 82% (red solid). 1H NMR (600 MHz, [D6]DMSO) δ 9.89 (d, J = 5.4 Hz, 1H), 8.89 (s, 1H), 8.81–8.68 (m, 1H), 8.39 (s, 1H), 8.31–8.24 (m, 1H), 8.20–8.12 (m, 1H), 8.08 (dd, J = 19.4, 4.7 Hz, 1H), 7.95 (s, 1H), 7.79–7.64 (m, 1H). FT-IR (KBr) νCO/cm−1: 2031.69, 1918.85. ESI-MS (CH3CN): m/z 680.6032 [M-3PF6-H]2+, 603.0723 [M-Ru(phen)2L-2PF6]+, 379.5722 [M-Re(DIP)(CO)3-3PF6]2+. Elemental analysis: calcd (%) for C69H43F18N11O3P3ReRu: C, 46.14; H, 2.41; N, 8.58; found: C, 46.46; H, 2.66; N, 8.42.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XM, JL, and PY performed the experiments. BH, RL, and RY designed the project. XM and RY wrote the paper.
Funding
We thank the National Natural Science Foundation of China (21967014, 22007042), Applied Basic Research Projects of Yunnan Province (202001AT070036), the Innovative Team of Yunnan Province (No. 2019HC018), High-level Scientific Research Foundation for Talent Introduction of Kunming University of Science and Technology (KKKP201826008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.890925/full#supplementary-material
References
- Abdel Hadi N., Reyes-Castellanos G., Carrier A. (2021). Targeting Redox Metabolism in Pancreatic Cancer. Ijms 22, 1534. 10.3390/ijms22041534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessio E., Messori L. (2018). 5. The Deceptively Similar Ruthenium(iii) Drug Candidates Kp1019 and Nami-A Have Different Actions. What Did We Learn in the Past 30 years? Met. Ions Life Sci. 18, 141–170. 10.1515/9783110470734-01110.1515/9783110470734-005 [DOI] [PubMed] [Google Scholar]
- Alessio E., Messori L. (2019). NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-To-Face: A Case Story in Medicinal Inorganic Chemistry. Molecules 24, 1995. 10.3390/molecules24101995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Wu Q., Zheng W., Li L., Mei W. (2017). Microwave-assisted Synthesis of Polypyridyl Ruthenium(II) Complexes as Potential Tumor-Targeting Inhibitors against the Migration and Invasion of Hela Cells through G2/M Phase Arrest. RSC Adv. 7, 26625–26632. 10.1039/C7RA00658F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capper M. S., Packman H., Rehkämper M. (2020). Rhenium‐Based Complexes and In Vivo Testing: A Brief History. ChemBioChem 21, 2111–2115. 10.1002/cbic.202000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas A. I., Nogales C., Mucke H. A. M., Petraina A., Cuadrado A., Rojo A. I., et al. (2020). On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 72, 801–828. 10.1124/pr.120.019422 [DOI] [PubMed] [Google Scholar]
- Choi A. W.-T., Louie M.-W., Li S. P.-Y., Liu H.-W., Chan B. T.-N., Lam T. C.-Y., et al. (2012). Emissive Behavior, Cytotoxic Activity, Cellular Uptake, and PEGylation Properties of New Luminescent Rhenium(I) Polypyridine Poly(ethylene Glycol) Complexes. Inorg. Chem. 51, 13289–13302. 10.1021/ic301948d [DOI] [PubMed] [Google Scholar]
- Coleman A., Brennan C., Vos J., Pryce M. (2008). Photophysical Properties and Applications of Re(I) and Re(I)-Ru(II) Carbonyl Polypyridyl Complexes. Coord. Chem. Rev. 252, 2585–2595. 10.1016/j.ccr.2008.07.001 [DOI] [Google Scholar]
- Collery P., Desmaele D., Vijaykumar V. (2019). Design of Rhenium Compounds in Targeted Anticancer Therapeutics. Cpd 25, 3306–3322. 10.2174/1381612825666190902161400 [DOI] [PubMed] [Google Scholar]
- da Silva M. M., Ribeiro G. H., de Camargo M. S., Ferreira A. G., Ribeiro L., Barbosa M. I. F., et al. (2021). Ruthenium(II) Diphosphine Complexes with Mercapto Ligands that Inhibit Topoisomerase IB and Suppress Tumor Growth In Vivo . Inorg. Chem. 60, 14174–14189. 10.1021/acs.inorgchem.1c01539 [DOI] [PubMed] [Google Scholar]
- Fouani L., Menezes S. V., Paulson M., Richardson D. R., Kovacevic Z. (2017). Metals and Metastasis: Exploiting the Role of Metals in Cancer Metastasis to Develop Novel Anti-metastatic Agents. Pharmacol. Res. 115, 275–287. 10.1016/j.phrs.2016.12.001 [DOI] [PubMed] [Google Scholar]
- Foxon S. P., Phillips T., Gill M. R., Towrie M., Parker A. W., Webb M., et al. (2007). A Multifunctional Light Switch: DNA Binding and Cleavage Properties of a Heterobimetallic Ruthenium-Rhenium Dipyridophenazine Complex. Angew. Chem. 119, 3760–3762. 10.1002/ange.200604837 [DOI] [PubMed] [Google Scholar]
- Gałczyńska K., Drulis-Kawa Z., Arabski M. (2020). Antitumor Activity of Pt(II), Ru(III) and Cu(II) Complexes. Molecules 25, 3492. 10.3390/molecules25153492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M. R., Thomas J. A. (2012). Ruthenium(II) Polypyridyl Complexes and DNA-From Structural Probes to Cellular Imaging and Therapeutics. Chem. Soc. Rev. 41, 3179–3192. 10.1039/c2cs15299a [DOI] [PubMed] [Google Scholar]
- Guedes A. P. M., Mello-Andrade F., Pires W. C., de Sousa M. A. M., da Silva P. F. F., de Camargo M. S., et al. (2020). Heterobimetallic Ru(II)/Fe(II) Complexes as Potent Anticancer Agents against Breast Cancer Cells, Inducing Apoptosis through Multiple Targets. Metallomics 12, 547–561. 10.1039/c9mt00272c [DOI] [PubMed] [Google Scholar]
- Halpin Y., Logtenberg H., Cleary L., Schenk S., Schulz M., Draksharapu A., et al. (2013). An Electrochemical and Raman Spectroscopy Study of the Surface Behaviour of Mononuclear Ruthenium and Osmium Polypyridyl Complexes Based on Pyridyl‐ and Thiophene‐Based Linkers. Eur. J. Inorg. Chem. 2013, 4291–4299. 10.1002/ejic.201300366 [DOI] [Google Scholar]
- Hartshorn R. M., Barton J. K. (1992). Novel Dipyridophenazine Complexes of Ruthenium(II): Exploring Luminescent Reporters of DNA. J. Am. Chem. Soc. 114, 5919–5925. 10.1021/ja00041a002 [DOI] [Google Scholar]
- He L., Pan Z.-Y., Qin W.-W., Li Y., Tan C.-P., Mao Z.-W. (2019). Impairment of the Autophagy-Related Lysosomal Degradation Pathway by an Anticancer Rhenium(I) Complex. Dalton Trans. 48, 4398–4404. 10.1039/c9dt00322c [DOI] [PubMed] [Google Scholar]
- He S.-F., Liao J.-X., Huang M.-Y., Zhang Y.-Q., Zou Y.-M., Wu C.-L., et al. (2022). Rhenium-guanidine Complex as Photosensitizer: Trigger HeLa Cell Apoptosis through Death Receptor-Mediated, Mitochondria-Mediated and Cell Cycle Arrest Pathways. Metallomics. 10.1093/mtomcs/mfac008 [DOI] [PubMed] [Google Scholar]
- Heffeter P., Atil B., Kryeziu K., Groza D., Koellensperger G., Körner W., et al. (2013). The Ruthenium Compound KP1339 Potentiates the Anticancer Activity of Sorafenib In Vitro and In Vivo . Eur. J. Cancer 49, 3366–3375. 10.1016/j.ejca.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmlin R. E., Stemp E. D. A., Barton J. K., Barton J. K. (1998). Ru(phen)2dppz2+ Luminescence: Dependence on DNA Sequences and Groove-Binding Agents. Inorg. Chem. 37, 29–34. 10.1021/ic970869r [DOI] [PubMed] [Google Scholar]
- Huang C., Li T., Liang J., Huang H., Zhang P., Banerjee S. (2020). Recent Advances in Endoplasmic Reticulum Targeting Metal Complexes. Coord. Chem. Rev. 408, 213178–213193. 10.1016/j.ccr.2020.213178 [DOI] [Google Scholar]
- Imstepf S., Pierroz V., Rubbiani R., Felber M., Fox T., Gasser G., et al. (2016). Organometallic Rhenium Complexes Divert Doxorubicin to the Mitochondria. Angew. Chem. Int. Ed. 55, 2792–2795. 10.1002/anie.201511432 [DOI] [PubMed] [Google Scholar]
- Jain A. (2019). Multifunctional, Heterometallic Ruthenium-Platinum Complexes with Medicinal Applications. Coord. Chem. Rev. 401, 213067. 10.1016/j.ccr.2019.213067 [DOI] [Google Scholar]
- Jain S. S., Anderson C. M., Dirienzo F., Taylor I. R., Jain K., Guha S., et al. (2013). RNA Binding and Inhibition of Primer Extension by a Ru(III)/Pt(II) Metal Complex. Chem. Commun. 49, 5031–5033. 10.1039/c3cc40699g [DOI] [PubMed] [Google Scholar]
- Jan R., Chaudhry G.-E. -S. (2019). Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 9, 205–218. 10.15171/apb.2019.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman P. J., Noakes F., Fairbanks S., Smitten K., Griffiths I. K., Saeed H. K., et al. (2019). Exploring the Cytotoxicity, Uptake, Cellular Response, and Proteomics of Mono- and Dinuclear DNA Light-Switch Complexes. J. Am. Chem. Soc. 141, 2925–2937. 10.1021/jacs.8b09999 [DOI] [PubMed] [Google Scholar]
- Jia P., Dai C., Cao P., Sun D., Ouyang R., Miao Y. (2020). The Role of Reactive Oxygen Species in Tumor Treatment. RSC Adv. 10, 7740–7750. 10.1039/C9RA10539E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A., Marzo I., Gimeno M. C. (2020). Heterobimetallic Propargyl Gold Complexes with π-bound Copper or Silver with Enhanced Anticancer Activity. Dalton Trans. 49, 11736–11742. 10.1039/D0DT02113J [DOI] [PubMed] [Google Scholar]
- Kumar C. A., Nagarajaprakash R., Victoria W., Veena V., Sakthivel N., Manimaran B. (2016). Synthesis, Characterisation and Cytotoxicity Studies of Manganese(I) and Rhenium(I) Based Metallacrown Ethers. Inorg. Chem. Commun. 64, 39–44. 10.1016/j.inoche.2015.12.011 [DOI] [Google Scholar]
- Leonidova A., Gasser G. (2014). Underestimated Potential of Organometallic Rhenium Complexes as Anticancer Agents. ACS Chem. Biol. 9, 2180–2193. 10.1021/cb500528c [DOI] [PubMed] [Google Scholar]
- Leonidova A., Pierroz V., Rubbiani R., Heier J., Ferrari S., Gasser G. (2014). Towards Cancer Cell-specific Phototoxic Organometallic Rhenium(I) Complexes. Dalton Trans. 43, 4287–4294. 10.1039/c3dt51817e [DOI] [PubMed] [Google Scholar]
- Li G., Sun L., Ji L., Chao H. (2016). Ruthenium(II) Complexes with Dppz: From Molecular Photoswitch to Biological Applications. Dalton Trans. 45, 13261–13276. 10.1039/C6DT01624C [DOI] [PubMed] [Google Scholar]
- Li L., Dostagir N. H., Shrotri A., Fukuoka A., Kobayashi H. (2021). Partial Oxidation of Methane to Syngas via Formate Intermediate Found for a Ruthenium-Rhenium Bimetallic Catalyst. ACS Catal. 11, 3782–3789. 10.1021/acscatal.0c05491 [DOI] [Google Scholar]
- Liew H. S., Mai C.-W., Zulkefeli M., Madheswaran T., Kiew L. V., Delsuc N., et al. (2020). Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy. Molecules 25, 4176–4199. 10.3390/molecules25184176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortet‐Tieulent J., Georges D., Bray F., Vaccarella S. (2020). Profiling Global Cancer Incidence and Mortality by Socioeconomic Development. Int. J. Cancer 147, 3029–3036. 10.1002/ijc.33114 [DOI] [PubMed] [Google Scholar]
- Ma D.-L., Wu C., Wu K.-J., Leung C.-H. (2019). Iridium(III) Complexes Targeting Apoptotic Cell Death in Cancer Cells. Molecules 24, 2739. 10.3390/molecules24152739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland S. A., Mandel A., Dumoulin-White R., Gasser G. (2020). Metal-based Photosensitizers for Photodynamic Therapy: the Future of Multimodal Oncology? Curr. Opin. Chem. Biol. 56, 23–27. 10.1016/j.cbpa.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehru S., Veeralakshmi S., Kalaiselvam S., Subin David S. P., Sandhya J., Arunachalam S. (2020). Protein Binding and Antioxidant Studies of Diimine Based Emissive surfactant-Ruthenium(II) Complexes. J. Biomol. Struct. Dyn. 39, 1535–1546. 10.1080/07391102.2020.1733664 [DOI] [PubMed] [Google Scholar]
- Notaro A., Gasser G. (2017). Monomeric and Dimeric Coordinatively Saturated and Substitutionally Inert Ru(II) Polypyridyl Complexes as Anticancer Drug Candidates. Chem. Soc. Rev. 46, 7317–7337. 10.1039/C7CS00356K [DOI] [PubMed] [Google Scholar]
- Obeng E. (2020). Apoptosis (Programmed Cell Death) and its Signals - A Review. Braz. J. Biol. 81, 1133–1143. 10.1590/1519-6984.228437 [DOI] [PubMed] [Google Scholar]
- Oun R., Moussa Y. E., Wheate N. J. (2018). The Side Effects of Platinum-Based Chemotherapy Drugs: a Review for Chemists. Dalton Trans. 47, 6645–6653. 10.1039/c8dt00838h [DOI] [PubMed] [Google Scholar]
- Palmioli A., Aliprandi A., Septiadi D., Mauro M., Bernardi A., De Cola L., et al. (2017). Glyco-functionalized Dinuclear Rhenium(I) Complexes for Cell Imaging. Org. Biomol. Chem. 15, 1686–1699. 10.1039/c6ob02559e [DOI] [PubMed] [Google Scholar]
- Pan Z. Y., Tan C. P., Rao L. S., Zhang H., Zheng Y., Hao L., et al. (2020). Recoding the Cancer Epigenome by Intervening in Metabolism and Iron Homeostasis with Mitochondria‐Targeted Rhenium(I) Complexes. Angew. Chem. Int. Ed. 59, 18755–18762. 10.1002/anie.202008624 [DOI] [PubMed] [Google Scholar]
- Pandey N., Black B. E. (2021). Rapid Detection and Signaling of DNA Damage by PARP-1. Trends Biochem. Sci. 46, 744–757. 10.1016/j.tibs.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu K., Chen Y., Rees T. W., Ji L., Chao H. (2019). Organelle-targeting Metal Complexes: From Molecular Design to Bio-Applications. Coord. Chem. Rev. 378, 66–86. 10.1016/j.ccr.2017.10.022 [DOI] [Google Scholar]
- Quental L., Raposinho P., Mendes F., Santos I., Navarro-Ranninger C., Alvarez-Valdes A., et al. (2017). Combining Imaging and Anticancer Properties with New Heterobimetallic Pt(ii)/M(i) (M = Re, 99mTc) Complexes. Dalton Trans. 46, 14523–14536. 10.1039/c7dt00043j [DOI] [PubMed] [Google Scholar]
- Sadoughi F., Hallajzadeh J., Asemi Z., Mansournia M. A., Alemi F., Yousefi B. (2021). Signaling Pathways Involved in Cell Cycle Arrest during the DNA Breaks. DNA Repair 98, 103047. 10.1016/j.dnarep.2021.103047 [DOI] [PubMed] [Google Scholar]
- Singh S., Varma M., Shravage B., Kulkarni P., Kumbhar A. (2021). Photoactivated Cytotoxicity Induced by Heterobimetallic Ru(II)-Pt(II) Polypyridyl Complexes in MCF-7 Cells. J. Chem. Sci. 133, 89–101. 10.1007/s12039-021-01935-0 [DOI] [Google Scholar]
- Sullivan B. P., Salmon D. J., Meyer T. J. (1978). Mixed Phosphine 2,2'-bipyridine Complexes of Ruthenium. Inorg. Chem. 17, 3334–3341. 10.1021/ic50190a006 [DOI] [Google Scholar]
- Sun Q., Li Y., Shi H., Wang Y., Zhang J., Zhang Q. (2021). Ruthenium Complexes as Promising Candidates against Lung Cancer. Molecules 26, 4389. 10.3390/molecules26154389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.-P., Zhong Y.-M., Ji L.-N., Mao Z.-W. (2021). Phosphorescent Metal Complexes as Theranostic Anticancer Agents: Combining Imaging and Therapy in a Single Molecule. Chem. Sci. 12, 2357–2367. 10.1039/D0SC06885C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thota S., Rodrigues D. A., Crans D. C., Barreiro E. J. (2018). Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 61, 5805–5821. 10.1021/acs.jmedchem.7b01689 [DOI] [PubMed] [Google Scholar]
- Tian Y., Huang Y., Gao P., Chen T. (2018). Nucleus-targeted DNA Tetrahedron as a Nanocarrier of Metal Complexes for Enhanced Glioma Therapy. Chem. Commun. 54, 9394–9397. 10.1039/c8cc04021d [DOI] [PubMed] [Google Scholar]
- Tsolis T., Nikolaou N., Ypsilantis K., Kougioumtzi A., Kordias D., Magklara A., et al. (2021). Synthesis, Characterization, Interactions with 9-MeG and Cytotoxic Activity of Heterobimetallic RuII-PtII Complexes Bridged with 2, 2′-bipyrimidine. J. Inorg. Biochem. 219, 111435. 10.1016/j.jinorgbio.2021.111435 [DOI] [PubMed] [Google Scholar]
- Wang F.-X., Chen M.-H., Lin Y.-N., Zhang H., Tan C.-P., Ji L.-N., et al. (2017). Dual Functions of Cyclometalated Iridium(III) Complexes: Anti-metastasis and Lysosome-Damaged Photodynamic Therapy. ACS Appl. Mater. Interfaces 9, 42471–42481. 10.1021/acsami.7b10258 [DOI] [PubMed] [Google Scholar]
- Wang P., Yang H., Liu C., Qiu M., Ma X., Mao Z., et al. (2021). Recent Advances in the Development of Activatable Multifunctional Probes for In Vivo Imaging of Caspase-3. Chin. Chem. Lett. 32, 168–178. 10.1016/j.cclet.2020.11.056 [DOI] [Google Scholar]
- Wang X., Liu X., Tan L. (2021). Comparative Studies on the Binding Interaction of Two Chiral Ru(II) Polypyridyl Complexes with Triple- and Double-Helical Forms of RNA. J. Inorg. Biochem. 214, 111301–111435. 10.1016/j.jinorgbio.2020.111301 [DOI] [PubMed] [Google Scholar]
- Woźna A., Kapturkiewicz A. (2015). The Luminescence Properties of the Heteroleptic [Re(CO)3(N∩N)Cl] and [Re(CO)3(N∩N)(CH3CN)]+ Complexes in View of the Combined Marcus-Jortner and Mulliken-Hush Formalism. Phys. Chem. Chem. Phys. 17, 30468–30480. 10.1039/c5cp05167c [DOI] [PubMed] [Google Scholar]
- Wu Z., Ho W. S., Lu R. (2022). Targeting Mitochondrial Oxidative Phosphorylation in Glioblastoma Therapy. Neuromol. Med. 24, 18–22. 10.1007/s12017-021-08678-8 [DOI] [PubMed] [Google Scholar]
- Xu Z., Huang J., Kong D., Yang Y., Guo L., Jia X., et al. (2020). Potent Half-Sandwich Ru(Ⅱ) N^N (Aryl-BIAN) Complexes: Lysosome-Mediated Apoptosis, In Vitro and In Vivo Anticancer Activities. Eur. J. Med. Chem. 207, 112763. 10.1016/j.ejmech.2020.112763 [DOI] [PubMed] [Google Scholar]
- Ye R.-R., Chen B.-C., Lu J.-J., Ma X.-R., Li R.-T. (2021). Phosphorescent Rhenium(I) Complexes Conjugated with Artesunate: Mitochondrial Targeting and Apoptosis-Ferroptosis Dual Induction. J. Inorg. Biochem. 223, 111537–111547. 10.1016/j.jinorgbio.2021.111537 [DOI] [PubMed] [Google Scholar]
- Ye R.-R., Ke Z.-F., Tan C.-P., He L., Ji L.-N., Mao Z.-W. (2013). Histone-deacetylase-targeted Fluorescent Ruthenium(II) Polypyridyl Complexes as Potent Anticancer Agents. Chem. Eur. J. 19, 10160–10169. 10.1002/chem.201300814 [DOI] [PubMed] [Google Scholar]
- Zec M., Srdic-Rajic T., Krivokuca A., Jankovic R., Todorovic T., Andelkovic K., et al. (2014). Novel Selenosemicarbazone Metal Complexes Exert Anti-tumor Effect via Alternative, Caspase-independent Necroptotic Cell Death. Mc 10, 759–771. 10.2174/1573406410666140327122009 [DOI] [PubMed] [Google Scholar]
- Zhang S.-Q., Gao L.-H., Zhao H., Wang K.-Z. (2020). Recent Progress in Polynuclear Ruthenium Complex-Based DNA Binders/Structural Probes and Anticancer Agents. Cmc 27, 3735–3752. 10.2174/0929867326666181203143422 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Zhang D. Y., Zhang H., Cao J. J., Tan C. P., Ji L. N., et al. (2018). Photodamaging of Mitochondrial DNA to Overcome Cisplatin Resistance by a Ru II -Pt II Bimetallic Complex. Chem. A Eur. J. 24, 18971–18980. 10.1002/chem.201803630 [DOI] [PubMed] [Google Scholar]
- Zheng Z.-B., Wu Y.-Q., Wang K.-Z., Li F. (2014). pH Luminescence Switching, Dihydrogen Phosphate Sensing, and Cellular Uptake of a Heterobimetallic Ruthenium(II)-Rhenium(I) Complex. Dalton Trans. 43, 3273–3284. 10.1039/c3dt52568f [DOI] [PubMed] [Google Scholar]
- Zhu J., Rodríguez-Corrales J. Á., Prussin R., Zhao Z., Dominijanni A., Hopkins S. L., et al. (2017). Exploring the Activity of a Polyazine Bridged Ru(ii)-Pt(ii) Supramolecule in F98 Rat Malignant Glioma Cells. Chem. Commun. 53, 145–148. 10.1039/c6cc07978d [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.