ABSTRACT
Background: Posttraumatic stress symptoms (PTSS) include a constellation of physical and emotional profiles that youth exposed to trauma may experience. An estimated 20% of youth are exposed to trauma, and in refugee populations, up to 54% experience posttraumatic stress. Given the physical and mental health consequences associated with trauma exposure and subsequent psychopathology, identifying biomarkers of symptom severity is a top research priority.
Objective: Previous research in adults found that skin conductance responses to trauma interview predicted current and future PTSS. We extended this method to refugee youth exposed to civilian war trauma and forced migration, to examine associations between PTSS and skin conductance in this uniquely vulnerable child and adolescent population.
Methods: 86 refugee youth ages 7–17 years completed a trauma interview and assessment of self-reported PTSS. The mobile eSense app on a iPad was used to obtain continuous recordings of skin conductance level (SCL) during a trauma interview (trauma SCL). Skin conductance response (SCR) was calculated by subtracting the baseline SCL from the maximum amplitude of the trauma SCL.
Results: SCL during trauma was significantly greater than baseline SCL, Trauma exposure was significantly associated with SCR to trauma interview, R2 = .084, p = .042. SCR to trauma interview was positively correlated with reexperiencing (R2 = .127, p = .028), and hyperarousal symptoms (R2 = .123, p = .048).
Conclusions: The present study provides evidence for feasibility of SCR to trauma interview as a candidate biomarker of PTSS in youth. This is the first study to look at SCR to trauma interview in youth resettled as refugees and is part of the limited but growing body of research to look at biomarkers in refugee cohorts more broadly. As the number of forcibly displaced persons surges, early detection and prevention of trauma-related psychology is becoming more important than ever.
HIGHLIGHTS
Using the mobile eSense app, we demonstrate that skin conductance is measurable in a variety of research settings and that skin conductance response may be a biological indicator of trauma and related psychopathology – namely re-experiencing symptoms – in youth resettled as refugees.
KEYWORDS: Trauma, PTSD, skin conductance response, refugee health, child and adolescent
GRAPHICAL ABSTRACT

Abstract
Antecedentes:
Los síntomas del estrés postraumático (PTSS por sus siglas en inglés) incluyen una constelación de perfiles físicos y emocionales que pueden experimentar los jóvenes expuestos a un trauma. Se estima que un 20% de los jóvenes están expuestos a un trauma y en la población de refugiados hasta el 54% experimenta estrés postraumático. Dadas las consecuencias para la salud física y mental asociadas con la exposición a un trauma y la subsecuente psicopatología, la identificación de biomarcadores de la gravedad de los síntomas es una de las principales prioridades de investigación.
Objetivo:
Investigaciones previas en adultos encontraron que las respuestas de conductancia de la piel a la entrevista de trauma predijeron los PTSS actuales y futuros. Extendimos este método a refugiados jóvenes expuestos al trauma de la guerra civil y migración forzada, para examinar las asociaciones entre PTSS y conductancia de la piel en esta población de niños y adolescentes especialmente vulnerable.
Método:
86 jóvenes refugiados, de 7 a 17 años completaron una entrevista de trauma y una evaluación de PTSS autoinformado. Se utilizó la aplicación móvil eSense en un iPad para obtener registros continuos del nivel de conductancia de la piel (SCL por sus siglas en inglés) durante una entrevista de trauma (SCL trauma). La respuesta de conductancia de la piel (SCR) se calculó restando el SCL a nivel basal de la amplitud máxima del SCL trauma.
Resultados:
La SCL durante el trauma fue significativamente mayor que la SCL basal, la exposición a trauma se asoció significativamente con la SCR a la entrevista de trauma, R2 = .084, p = .042. La SCR a la entrevista de trauma se correlaciono positivamente con reexperimentación (R2 = .127, p = .028), y síntomas de hiperalerta (R2 = .123,p = .048).
Conclusiones:
El presente estudio proporciona evidencia de la viabilidad de la SCR a la entrevista de trauma como un biomarcador candidato de PTSS en jóvenes. Este es el primer estudio que analiza la SCR a la entrevista de trauma en jóvenes reasentados como refugiados y es parte del cuerpo de investigación limitado pero creciente para observar biomarcadores en cohortes de refugiados de manera más amplia. A medida que aumenta el número de personas desplazadas por la fuerza, la detección temprana y la prevención de la psicología relacionada con el trauma se vuelven más importantes que nunca.
PALABRAS CLAVE: Trauma, TEPT, respuesta de conductancia de la piel, salud de los refugiados, niño y adolescente
Abstract
背景:
创伤后应激症状 (PTSS) 包括遭受创伤的青少年可能经历的一系列身体和情绪特征。估计有 20% 的青年遭受过创伤,而在难民人口中,高达 54% 的人经历过创伤后应激。鉴于与创伤暴露和后续精神病相关的身心健康后果,识别症状严重程度的生物标志物是研究的重中之重。
目的:
先前对成人的研究发现,对创伤访谈的皮肤电导反应可以预测当前和未来的 PTSS。我们将这种方法扩展到暴露于平民战争创伤和被迫移民的难民青年,以考查 PTSS 与这个独特的易感儿童和青少年群体皮肤电导之间的关联。
方法:
86 名 7–17 岁的难民青年完成了创伤访谈和自我报告的 PTSS 评估。 iPad 上的移动 eSense 应用程序用于在创伤访谈期间获取皮肤电导水平 (SCL) (创伤 SCL) 的连续记录。通过从创伤 SCL 的最大幅度减去基线 SCL 来计算皮肤电导反应 (SCR)。
结果:
创伤期间SCL显著大于基线 SCL,创伤暴露与创伤访谈SCR显著相关,R2 = .084,p = .042。创伤访谈SCR 与再体验 (R2 = .127, p = .028) 和高唤起症状 (R2 = .123, p = .048) 呈正相关。
结论:
本研究为将创伤访谈SCR 作为青年 PTSS 候选生物标志物的可行性提供了证据。这是第一项在作为难民重新定居的青年中对创伤访谈SCR的研究,并且是有限但不断增长的旨在更广泛研究难民群体中生物标志物的研究的一部分。随着被迫流离失所者人数的激增,早期发现和预防创伤相关心理正在变得比以往任何时候都更加重要。
关键词 : 创伤, PTSD, 皮肤电导反应, 难民健康, 儿童和青少年
1. Introduction
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines trauma as actual or threatened death, serious illness/injury, or sexual violence (American Psychiatric Association, 2013). Trauma can be experienced directly or as part of one’s occupation, witnessed, or learned about. Trauma exposure confers risk for developing posttraumatic stress disorder (PTSD), which is defined by intrusive/reexperiencing, avoidance, negative cognition and mood, and arousal/reactivity (hyperarousal) symptoms. Traumatic experiences are common in childhood, 1 in 5 youth experience trauma (Saunders & Adams, 2014). However, not everyone exposed to trauma develops PTSD – of the 20% of youth exposed, only about 5% develop PTSD (Merikangas et al., 2010) although this rate may be higher in higher-risk populations, including youth who resettle as refugees (rates of PTSD range from 9% to 54%) (Grasser et al., 2021; Perreira & Ornelas, 2013; Solberg et al., 2020). Furthermore, there is heterogeneity in symptom presentation (Galatzer-Levy & Bryant, 2013). Additionally, children with subthreshold symptoms of PTSD may still be significantly distressed and experience functional impairment (Carrion, Weems, Ray, & Reiss, 2002).
The heterogeneity of PTSD and the continuum across which persons may experience posttraumatic stress symptoms (PTSS) has motivated the query for possible biomarkers related to posttraumatic stress. Biomarkers are measurable characteristics reflecting biological function or dysfunction, therapeutic response, and/or natural disease progression (Group et al., 2001). The goal of biomarkers research is to define objective signs and states that can be observed and measured accurately and reproducibly (Michopoulos, Norrholm, & Jovanovic, 2015). Biomarkers may reflect genetic or physiological characteristics that may put an individual at risk for a disease state when confronted with certain environmental exposures, or may reflect ways in which certain exposures have affected physiology and may contribute to disease (Mayeux, 2004; McKeever & Huff, 2003). Currently, there are no validated and reliable biomarkers for any psychiatric condition (Casey et al., 2013; Macaluso & Preskorn, 2012), and diagnoses are made based solely on clinical interviews (Casey et al., 2013; Kupfer & Regier, 2011). However, with growing widespread research in this field (Kalia & Costa e Silva, 2015), biomarkers may be leveraged to predict which individuals are at greater risk for developing PTSD in the aftermath of trauma and provide an objective method for identifying those with the disorder as well as measuring treatment efficacy (Macaluso & Preskorn, 2012). There are over 600,000 ‘ways’ to have PTSD given the 20 DSM-defined symptoms across 4 domains and an additional dissociative subtype (Galatzer-Levy & Bryant, 2013). Biological diatheses (McKeever & Huff, 2003) and the differential ways in which trauma can impact the nervous system (Lanius, Bluhm, Lanius, & Pain, 2006) may contribute to such heterogeneity in posttraumatic stress. Indeed, a recent neuroimaging study found heterogenous stress responses in the immediate aftermath of trauma exposure (Stevens et al., 2021). These brain-based biotypes support the idea that there may be biological phenotypes related to risk, resilience, and differential presentation of posttraumatic stress (Stevens et al., 2021). This heterogeneity in response to trauma exposure and disease expression may require fine-tuned intervention, and biomarkers of specific symptoms could define specific therapeutic targets.
When an individual is exposed to a stressful, dangerous, and/or traumatic event as well as reminders of such events, neurotransmitter and hormonal signalling cascades trigger relevant physiological and behavioural responses, including modulation of sweat secretion (Shibasaki & Crandall, 2010). A component of such sympathetic responses is increased perspiration (Seligowski, Harnett, Merker, & Ressler, 2020; Van der Kolk, 2015), and skin conductance is a biological indicator of arousal/reactivity (Christopoulos, Uy, & Yap, 2019). Amygdala signalling to the hypothalamus in response to salient stimuli or trauma-related cues triggers a cascade of defensive responses, including thermoregulatory responses mediated by the preoptic region of the hypothalamus (Shibasaki & Crandall, 2010). Direct amygdala to brainstem signalling also occurs (Shibasaki & Crandall, 2010). Resultant downstream cholinergic and adrenergic signalling at the eccrine glands stimulates sweat secretion. Sweat gland activity increases with the stress response and serves as a measure of sympathetic activation (Harker, 2013). Increased moisture due to sweat secretion can be detected as a change in conductance and electrical potentials using electrodes. Prior studies have reported alterations in autonomic function, including decreased resting parasympathetic nervous system (PNS) activity and heightened reactivity of the sympathetic nervous system (SNS) during mental stress in PTSD (Buckley & Kaloupek, 2001; Park et al., 2017; Pole, 2007), including elevated sweating, or skin conductance. Here, elevated skin conductance as a marker of increased autonomic reactivity, or arousal, may reflect a predisposing physiological phenotype that increases risk for posttraumatic stress symptoms when confronted with trauma. Alternatively, elevated skin conductance as a marker of autonomic reactivity may reflect effects of trauma exposure – particularly chronic and severe trauma exposure, as in civilian war trauma – on the nervous system. Here, persistent activation of the SNS as described above may contribute to persistent increased skin conductance and confer greater reactivity to novel stressors or reminders thereof following exposure.
Elevated skin conductance response (SCR) to stimulation is a sensitive measure of hyperarousal and predictive of PTSD when using script-driven imagery (Pineles et al., 2013), a method in which individuals listen to trauma-related scripts. Trauma-related scripts may be individualized to one’s own experiences; standardized traumatic scripts may also be used for comparison, in addition to personalized and standardized positive and neutral scripts (Hoge et al., 2012; Pitman, Orr, Forgue, de Jong, & Claiborn, 1987). These protocols have indicated that physiological arousal was specifically associated with individualized trauma-related scripts compared to the standardized trauma scripts in individuals with PTSD (Orr, Metzger, & Pitman, 2002; Pitman et al., 1987). Additionally, neutral and positive scripts have not been shown to generate physiological responses in neither individuals with nor without PTSD (Orr et al., 2002; Pitman et al., 1987). Resultantly, elevated SCR to trauma reminders could serve as a candidate biomarker of PTSD and its specific symptoms. Recent studies have built on these methods using the mobile eSense application, to improve accessibility and cost-effectiveness for clinical and research settings. Using this technology, previous research found that SCRs elicited by a standardized trauma interview – in which participants described a traumatic event during the eSense recording – were positively associated with severity of posttraumatic stress symptoms and significantly predicted presence of the disorder 6 months later (Hinrichs et al., 2017; Hinrichs et al., 2019). Increased SCR to trauma interview is emerging as a candidate biomarker of PTSD and its specific symptoms. Mobile collection methods further the utility of this biomarker, especially in settings where clinicians may be unavailable for full diagnostic interviews and mental health screenings, in the immediate aftermath of trauma, and in challenging and dynamic research contexts. One such example is with persons who are resettling as refugees in host countries who may have encountered various stressors such as war, natural disaster, and forced migration, where data must be collected in homes rather than laboratories.
Ongoing conflicts in Syria and Iraq have exposed millions of youth to trauma and caused many to be displaced. Such youth have experienced potential separation from friends and family members, death of loved ones and strangers alike, loss of belongs, lack of basic living needs, and additional stressors in resettlement camps. Following resettlement, youth may in turn experience new stressors – e.g. being considered a minority group in a host country, discrimination and bullying, language barriers, and sociocultural transitions. As a result, youth who resettle as refugees experience higher rates of PTSD compared to the general United States population (Grasser et al., 2021; Javanbakht, Rosenberg, Haddad, & Arfken, 2018). This underscores a need for objective assessment of trauma-related psychopathology and pathophysiology in youth who have resettled as refugees. Such objective assessments could be feasible for use in not only research labs, but also in real-world settings where they may have the greatest utility and clinical impact (Łoś & Waszkiewicz, 2021) – including resettlement agency offices, medical clinics, community centres, and even patient homes with appropriate consultation and permissions (Hiba Abu Suhaiban, Grasser, & Javanbakht, 2019). Therefore, the present study aimed to investigate the feasibility of collecting SCR during a trauma interview using the mobile eSense system in a cohort of youth ages 7–17 who resettled in the US as refugees of Syria and Iraqi. SCR has previously been used to monitor threat and safety learning in infants (Ingram & Fitzgerald, 1974), healthy non-trauma exposed children and adolescents (Michalska et al., 2016; Miller & Shields, 1980; Morrow, Boring, Keough, & Haesly, 1969; Neumann, Waters, & Westbury, 2008), and youth exposed to maltreatment (physical, and sexual abuse, as well as domestic violence) (McLaughlin et al., 2016). However, this is one of the first studies to use SCR in tandem with a trauma interview and as a biomarker for PTSS in a child and adolescent sample (Wiltshire et al., 2022). We also examined SCR as part of a Research Domain Criteria (RDoC) approach to investigate pathophysiology of mental illness, specifically the negative valence domain construct of arousal, using psychophysiological and self-report data as units of analysis (Cuthbert & Insel, 2013; Cuthbert & Kozak, 2013; Morris & Cuthbert, 2012). We hypothesized that severity of PTSS would be positively correlated with SCR to trauma interview. In addition, we examined association with the specific symptom clusters of re-experiencing and hyperarousal, as those have been previously linked with physiological responses in PTSD (Jovanovic et al., 2010; Norrholm et al., 2015, 2011). Our present study leverages data from a largely underrepresented group in mental health – youth of Middle Eastern/North African (MENA) ethnicity – and provides recommendations for future application of this method.
2. Methods
2.1. Participants
86 youth (36 female, meanage = 12.419, +/− 2.737 SD, range = 7-17) were recruited from an existing cohort of persons resettled as refugees of Syria and Iraq in Southeast Michigan (Grasser et al., 2021; Javanbakht et al., 2018). Participants were initially recruited as families at the Arab American and Chaldean Council Clinic, where all refugees resettling from Middle Eastern nations, received mandatory health screenings within one month of migration to the U. S. Inclusion criteria were as follows: (1) males and females between the ages of 7and 17, (2) refugee status, originating from Syria or Iraq, and (3) willing and able to give oral assent (ages 7–12) or written assent (ages 13–17) with parent/guardian willing and able to give written informed consent. Exclusion criteria were as follows: (1) inability or unwillingness to consent, (2) wardens of the court, and (3) current diagnosis of autism or psychosis spectrum disorders. Siblings were permitted to participate in the study. All study procedures described herein were approved by the Institutional Review Board at Wayne State University (IRB#012416B3F).
2.2. Self-report measures
All self-report assessment measures were administered by bilingual clinicians and research assistants and were available in both English and Arabic. Questionnaires were translated from English to Arabic by a native speaker, back-translated to English by a separate Arabic speaker to ensure consistency and accuracy in translations, and finally certified and approved by external reviews. Internal consistency was assessed with Cronbach’s alpha and included for each measure below.
Trauma exposures were assessed using a modified version of the Harvard Trauma Questionnaire (HTQ), which queries traumatic events more specific to refugee and non-Western cohorts (Mollica et al., 1992; Rasmussen, Verkuilen, Ho, & Fan, 2015). The modified version of the HTQ included 21 items, to which participants would respond ‘yes’ or ‘no’ as to whether they had experienced the event, regardless of frequency. The item responses were then summed to provide the total number of unique traumatic events youth had been exposed to, with a possible range of 0–21. The sections from the original HTQ on head injury, trauma symptoms, and torture history were not included in this modified version – no participants reported head injury at initial screening, and the sections on torture history were inappropriate for use in children and adolescents. The UCLA PTSD Reaction Index was used as a measure of trauma symptoms. The modified version of the measure is available in the Supplementary Materials, with a list of the traumatic events that were excluded in the modification. Cronbach’s alpha for the 21-item HTQ trauma inventory in this sample was 0.887.
The severity of PTSS was assessed using the UCLA PTSD Reaction Index for Children and Adolescents (UCLA PTSD RI) for DSM 5 (Kaplow et al., 2020). The UCLA PTSD RI is a 31-item questionnaire to which participants indicate how much they have been bothered by particular symptoms within the last month, ranging from none of the time (0) to most of the time (4). The measure also generates subscales for re-experiencing, avoidance, negative cognitions, and hyperarousal symptoms. The UCLA PTSD RI has been recommended for use with refugee populations, including those with non-Western backgrounds (Miller, Brown, Shramko, & Svetaz, 2019), and has been found to be reliable in MENA refugee populations (Grasser et al., 2021; Javanbakht et al., 2018). Cronbach’s alpha in this sample for the full measure was 0.933. For re-experiencing symptoms, Cronbach’s alpha was 0.826; for hyperarousal symptoms, alpha was 0.734. Given that these are the symptom clusters most strongly linked to the neurobiological pathways associated with PTSS and related to the biomarker of interest, we made a priori hypotheses about these two symptom clusters and did not do exploratory analyses for the others to avoid Type I error.
2.3. Skin conductance response to trauma interview
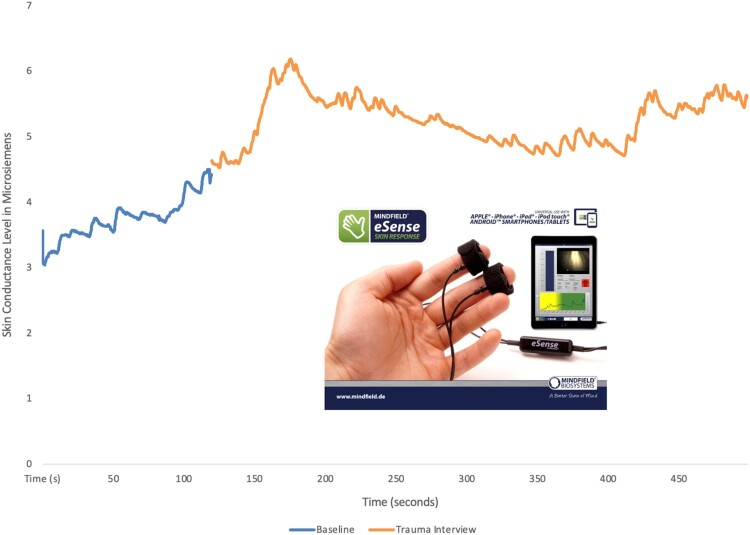
Data were collected either in participants’ homes or in a university lab setting, to minimize barriers to research participation. The procedures were administered the same way in both settings. skin conductance level (SCL) was measured using the eSense app (Mindfield Biosystems, Inc., Berlin, Germany) on an iPad. Two Ag/AgCl electrodes were placed on the index and middle fingers of the non-dominant hand. Isotonic paste was added to electrodes prior to attachment to the middle phalanges of the fingers to increase signal quality and ensure contact with skin. A two-minute baseline was obtained at rest, during which time the participant was seated in a quiet room refraining from talking or engaging with any stimuli. This was followed by a measurement of SCL while the individual was administered the HTQ interview. Throughout the recordings, participants remained seated and still with their hands resting on a table to reduce movement. An example recording from a single participant session is depicted in Figure 1, with an inset image of the data collection software. Data were exported to Excel for initial processing of raw data and scored values were imported to R for statistical analysis. No artifacts were identified in the dataset, and no additional filtering was applied to raw values. As in prior studies, baseline SCL was calculated as the average of the last 30 s of the two-minute baseline recording taken at rest. Trauma SCL was calculated as the average of full trauma interview. SCR was calculated as the baseline SCL subtracted from the peak skin conductance value during the trauma interview. Trauma SCL reflected the continuous level of electrodermal activity throughout the entire interview. SCR reflected the peak response during this trauma interview (maximum amplitude) from which the baseline SCL was subtracted to normalize across participants.
Figure 1.
Mobile continuous recording of electrodermal activity using the Mindfield eSense set-up from n = 1 participant. This set-up requires one set of electrodes which are placed on the middle phalanges of the index and middle finger. The electrodes connect to any smart device running the eSense app. A two-minute baseline is first obtained, followed by the trauma interview. The baseline recording and trauma recording from the entire duration of the HTQ administration (trauma interview) is depicted.
2.4. Data analysis
All analyses were performed in R statistical package version 4.0.3. Data were screened for potential artifacts in skin conductance recordings; we did not identify any artifacts and there were no instances of measurements in which a recording was unable to be obtained. Data were then screened for univariate outliers based on median absolute deviation (Kwak & Kim, 2017; Leys, Ley, Klein, Bernard, & Licata, 2013); outliers for baseline SCL (n = 5), trauma SCL (n = 3) trauma exposure (n = 3), SCR (n = 6), reexperiencing symptoms (n = 8), and hyperarousal symptoms (n = 2) were identified and corrected using Winsorization (Dixon, 1960; Kotz, Read, Balakrishnan, Vidakovic, & Johnson, 2006). Missing data were identified for baseline SCL (4.7% missing), trauma SCL (4.7% missing), SCR (5.8% missing), total PTSD symptoms (17% missing), reexperiencing symptoms (13% missing), and hyperarousal symptoms (9.3% missing). Little’s MCAR indicated that data were missing completely at random, p = .634. Patterns of missing data were also inspected, and no significant patterns of concern emerged. Missing data were imputed using multiple imputation with 8 iterations, given that no variables exceeded 20% missingness and the sample size. Multilevel models were fit to accommodate the nested structure of this dataset. In cases where there were repeated measures (i.e. comparing skin conductance level from baseline to trauma interview), data were nested by individuals, as 2 measurements were obtained – one for baseline and one for trauma SCL – and individuals were nested by family – for 172 total observations across 32 groups. In cases where there were not repeated measures (i.e. assessing relations between variables), data were nested by families only for 86 total observations across 32 groups. Random intercepts, fixed slopes models were selected, as we did not have any reason to hypothesize that patterns would differ by family. This was confirmed by ANOVAs and model fit indices (AIC, BIC) indicating that random intercepts, fixed slopes models were of better fit than random intercepts, random slopes. Sex and location of assessment were included as covariates in the models. Models were screened to ensure that the assumptions of the model were met – namely the assumption of normality of the residuals. Adjusted alpha for four models (p < .0125) was based on the Bonferroni correction for multiple comparisons.
3. Results
3.1. Demographic characteristics
Data were collected from 86 youth (36 female) from 32 families with an average of 2.69 (range: 1-6) participating youth per family. Most of the participants had at least 1 sibling in the study; only 4 participants did not have any siblings in the study sample. 31 individuals were assessed in their homes and 55 at the lab. The number of unique traumatic exposures per individual ranged from 0 to 15. Participants were between the ages of 0 and 9 (Mage = 3.43, SD±2.57) at the time of trauma exposure; ages 7–17 (Mage = 12.42, SD±2.74) at the time of assessment. Descriptive statistics are shown in Table 1.
Table 1.
Demographic characteristics and descriptive statistics of sample, n = 86 (36F, 50M).
| Variable | M | Range | SD |
|---|---|---|---|
| Age | 12.42 | 7–17 | 2.74 |
| Number of Traumas | 4.53 | 0–15 | 4.25 |
| Total PTSS | 22.38 | 0–75 | 18.99 |
| Re-experiencing Sxs | 3.47 | 0–11 | 3.36 |
| Hyperarousal Sxs | 5.72 | 0–18 | 4.59 |
| Baseline SCL(µS) | 8.52 | 1.69–19.47 | 4.94 |
| Trauma SCL (µS) | 9.60 | 1.48–22.97 | 5.26 |
| SCR to Trauma Interview (µS) | 3.02 | −1.67–8.62 | 2.64 |
| Interview Length (Minutes) | 5.85 | 1.98–14.27 | 2.05 |
Note: Trauma exposure was assessed using the Harvard Trauma Questionnaire (HTQ). Posttraumatic stress symptoms (PTSS) were assessed using the child/adolescent self-report version of the UCLA PTSD RI. M is used to represent mean; SD is used to represent standard deviation; SCL stands for skin conductance level; SCR stands for skin conductance response. Sxs is used for symptoms.
Age was significantly associated with number of traumatic events endorsed on the HTQ, R2 = .28, p < .001. Older age was associated with more trauma exposure; however, age was not associated with severity of posttraumatic stress symptoms, all p values > .10. The number of traumatic events endorsed on the HTQ was positively correlated with posttraumatic stress symptoms, R2 = .27, p < .001. Age was not associated with baseline SCL (p = .58) or SCR to trauma interview (p = .29), and neither baseline SCL nor SCR differed by gender (t = 1.09, p = .28 and t = −1.24, p = .22 respectively). Neither age (t = .64, p = .52) nor trauma exposure (t = -.12, p = .91) significantly differed between girls and boys. Girls had higher re-experiencing symptoms than boys (Mgirls = 4.29, Mboys = 2.88; t = −1.97, p = .05); total PTSS (t = −1.30, p = .20) and hyperarousal symptoms (t = −1.14, p = .26) did not differ. While baseline and trauma SCL were higher in the group sampled at home compared to the group sampled in the lab (t = −2.57, p = .013 and t = −2.74, p = .008 respectively), SCR did not differ based on location (t = −1.20, p = .23). There was no significant effect of interview time on trauma SCL (p = .20) nor SCR, (p = .90).
3.2. Feasibility of measuring skin conductance responses in a sample of youth resettled as refugees
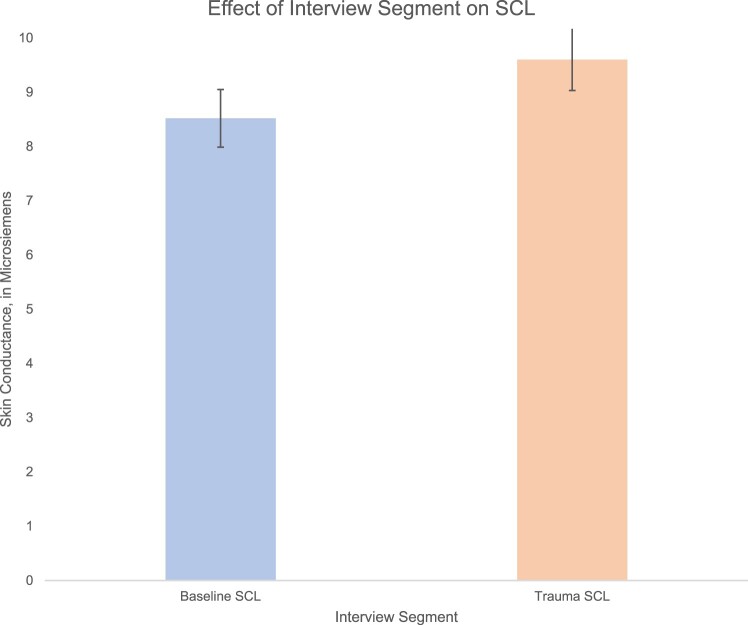
To determine whether the trauma interview evoked changes in skin conductance, we compared baseline and trauma SCL. A random intercepts fixed slopes MLM with skin conductance level as the dependent variable and interview segment as the predictor. The MLM indicated a significant effect of phase on skin conductance, B = 1.08, t(139) = 5.72, p < .001, R2 = .16 (moderate effect), ICC = .49. Skin conductance levels during the trauma interview (M = 9.52, SD = 5.27) were greater than during baseline (M = 8.43, SD = 4.86) by an average of 1.08 µS, see Figure 2.
Figure 2.
A multilevel model indicated a significant effect of interview phase on skin conductance level, such that skin conductance level was greater during the trauma interview compared to baseline (F = 32.29, p < .001, = .275). Error bars are based on standard error of the mean.
3.3. Skin conductance, trauma exposure, and psychopathology
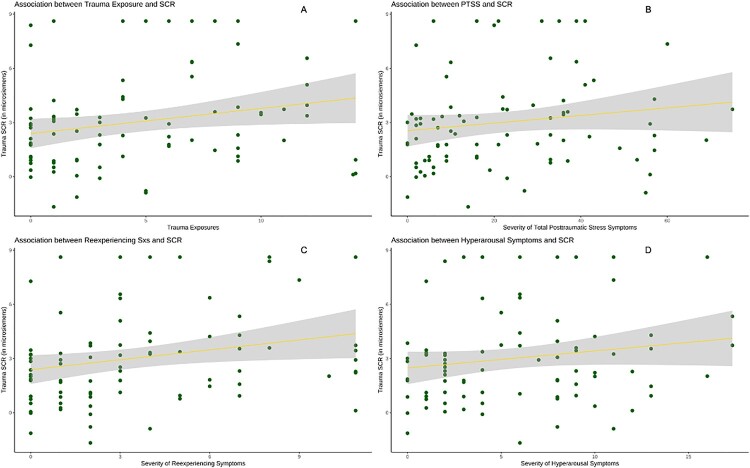
We then examined whether SCR to trauma interview (difference between SCL during trauma and baseline SCL) was significantly associated with trauma exposure and PTSS. A multi-level model (MLM) in which participants’ data was nested within families was used to account for shared variance across siblings in the dataset, controlling for the effects of sex and location. MLMs indicated that trauma exposure was directly correlated with SCR (Figure 3a), see Table 2. SCR was positively associated with re-experiencing (Figure 3c) and hyperarousal symptoms (Figure 3d), but not total posttraumatic stress symptoms (Figure 3b), see Table 3.
Figure 3.
Increasing number of trauma exposures is associated with increased skin conductance response to trauma interview (trauma SCR) in youth resettled as refugees of Syria (A), and severity of reexperiencing (C) and hyperarousal symptoms (D) is associated with SCR. The relation between SCR and total PTSS was not significant (B). Shading reflects standard error.
Table 2.
Effect of trauma exposure on skin conductance response.
| Predictors | Estimates | 95% CI | p | |
|---|---|---|---|---|
| LL | UL | |||
| Intercept | 3.25 | 1.99 | 4.51 | <.001 |
| Sex (Ref = 0; Female) | −0.68 | −1.78 | 0.43 | .235 |
| Location (Ref = 0; Home) | −0.71 | −1.84 | 0.42 | .223 |
| Trauma Exposure | 0.14 | 0.01 | 0.27 | .042 |
Note: R2 = .084, ICC < .001. The estimate for trauma exposure reflect the degree of change in SCR per exposure. Results did not survive Bonferroni correction for multiple comparisons at p < .0125.
Table 3.
Effects of SCR on total PTSS (R2 = .095, ICC = .13), reexperiencing (R2 = .127, ICC = .03), and hyperarousal symptoms (R2 = .123, ICC = .20).
| Total PTSS | Reexperiencing | Hyperarousal | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | 95% CI | p | Estimates | 95% CI | p | Estimates | 95% CI | p | |||
| LL | UL | LL | UL | LL | UL | |||||||
| Intercept | 15.25 | 5.22 | 25.28 | .004 | 2.36 | 0.64 | 4.08 | .010 | 3.50 | 1.08 | 5.92 | .007 |
| Sex (Ref = 0; Female) | −4.28 | −12.06 | 3.49 | .285 | −1.20 | −2.58 | 0.18 | .094 | −0.84 | −2.67 | 0.99 | .372 |
| Location (Ref = 0; Home) | 8.39 | −0.46 | 17.25 | .069 | 1.38 | −0.06 | 2.82 | .066 | 2.31 | 0.10 | 4.51 | .046 |
| SCR | 1.22 | −0.24 | 2.69 | .108 | 0.30 | 0.04 | 0.65 | .028 | 0.36 | 0.01 | 0.70 | .048 |
Note: Results did not survive Bonferroni correction for multiple comparisons at p < .0125. Estimates for sex and location reflect the mean difference in the outcome variable between groups (female/male sex and home/lab assessment location). The estimates for SCR reflect the degree of change in the outcome variable for every one unit change (1 µS) in SCR.
4. Discussion
The present study examined skin conductance in response to a trauma interview in order to assess associations between autonomic function and trauma and stress in youth resettled as refugees. We also aimed to test the utility of a novel method for measuring skin conductance using an app on an iPad in this unique population. Our results indicated that mobile SCL eSense data collection is easy, low-cost, and feasible in a variety of environments – not just emergency rooms, clinical settings, and research labs as has been previously shown (Hinrichs et al., 2017; Hinrichs et al., 2019), but also natural settings like homes. We found that a higher degree of trauma exposure was associated with greater SCR to trauma interview indicating potential SNS hyperactivation to stress and threat in youth at risk for psychopathology. This finding is supported by the strong positive association between re-experiencing symptom severity and SCR to trauma interview. Such autonomic hyperactivation may be an early risk factor for future mental and physical health problems, and youth who experience heightened SCR may benefit from mindfulness-based stress reduction and creative arts/movement therapies (Feen-Calligan et al., 2020; Grasser & Javanbakht, 2021; Grasser & Jovanovic, 2021; Grasser, Al-Saghir, Wanna, Spinei, & Javanbakht, 2019). The association between trauma exposure and SCR to trauma interview replicates that from another child and adolescent sample from a different demographic background – Black Americans – further supporting the notion that trauma may affect autonomic reactivity in highly exposed youth, however, causal conclusions cannot be made from either dataset (Wiltshire et al., 2022).
In contrast to previous findings from adults exposed to trauma, (Hinrichs et al., 2017; Hinrichs et al., 2019), our data did not indicate a significant association between total PTSS and SCR to trauma interview. Future studies in larger samples may be better powered to detect a significant relation replicating the findings in adults, given the small effect we observed in this sample. Alternatively, this method may show specificity towards posttraumatic stress symptoms related to autonomic reactivity – namely reexperiencing symptoms, as well as hyperarousal symptoms. As this is the first application of the mobile eSense method in refugee youth, these data are promising and merit further inquiry.
Looking at specific symptom clusters, we initially found an association between SCR to trauma interview and re-experiencing symptoms, as well as hyperarousal symptoms, with a stronger effect observed for re-experiencing symptoms. Given the nature of the trauma narrative, it is not surprising that those with greater re-experiencing symptoms had higher SCR throughout the trauma interview. Recalling traumatic memories in the interview setting may model a re-experiencing event; therefore, greater severity of re-experiencing symptoms may be associated with reminders of the trauma and predict physiological arousal in this context. Whereas re-experiencing symptoms may predict task-specific autonomic arousal, hyperarousal symptoms may be linked to more tonic autonomic states. In adults, salivary alpha amylase and heart rate variability have also been associated with reexperiencing symptoms (Rombold et al., 2016a; 2016b; Rombold-Bruehl et al., 2017; Schultebraucks et al., 2019); more broadly, startle response and fear-potentiated startle have been correlated with trauma-related psychopathology (Grasser & Jovanovic, 2021), and other physiological responses of the autonomic nervous system – including respiratory rate and blood pressure (Michopoulos et al., 2015) – may constitute candidate biomarkers of PTSS. Future research that investigates these and other biomarkers alongside SCR to trauma interview may provide convergent validity (Krabbe, 2017) for SCR to trauma interview as a biomarker of reexperiencing symptoms and for SCR to trauma interview as a biomarker of PTSS more broadly.
The focus on biomarkers research has been motivated by the need to rapidly and objectively identify individuals in need of clinical care, both for early intervention and in cases where resources may be limited. This is of particular importance in psychiatry, as the mental health provider shortage affects 124 million Americans (Weiner, 2018). Importantly, the aim of biomarkers research and application to clinical practice is not to replace psychometrics and structured clinical interviews (Łoś & Waszkiewicz, 2021). Rather, biomarkers could add to evaluation strategies and may enhance detection – particularly prodromal detection – of psychiatric illness that may, in part, arise from alteration or dysfunction of the nervous system that can be detected through biomarkers (Kalia & Costa e Silva, 2015). Additionally, with substantive validation and replication, biomarkers may be more rapidly obtained than full clinical interviews to provide an initial assessment of which individuals require further follow-up with clinicians in situations where there is limited clinician availability (Kalia & Costa e Silva, 2015) and when even standardized measures fail to identify some disorders in up to 50% of affected individuals (Eack, Greeno, & Lee, 2006). Objective biomarkers could not only help us deliver care to those who need it most, but also identify what treatments may be of best fit for patients with greater specificity than diagnoses alone as there is heterogeneity within psychiatric disease states (Galatzer-Levy & Bryant, 2013; Malhi, 2014; Woods et al., 2012), track treatment efficacy, understand underlying mechanisms of interventions, and define new therapeutic targets (Lydiard & Nemeroff, 2019; Łoś & Waszkiewicz, 2021; Scarr et al., 2015). Yet despite the growing number of biomarkers studies, the field remains inconclusive. Major limitations in this area of research have included small sample size and unmeasured or unmodeled variables that may account for variation in findings – including biological sex, geographic ancestry, and environmental exposures. As a result, there are currently no existing biomarkers for any psychiatric condition, including anxiety and PTSD (Prasadt, Chaichi, Parker Kelley, Fancis, & Ranjan Gartia, 2019). The ability to identify individuals who may be at risk for a trauma-related disorder like PTSD with greater sensitivity and earlier detection than psychological assessments and clinical interviews alone (Mayeux, 2004) would be of great benefit so that treatment could be delivered early on after trauma exposure or during development, which may enhance treatment outcomes or even prevent the development of psychiatric disease (H. Abu Suhaiban et al., 2019; Prasadt et al., 2019). Despite this, there are limited biomarkers studies in youth, although the field continues to grow. The present study provides evidence for feasibility of SCR to trauma interview as a candidate biomarker of autonomic alterations related to reexperiencing and hyperarousal symptoms. The developmental sample described herein provides the unique and important opportunity to assess a candidate biomarker of autonomic functioning linked to trauma-related psychopathology in youth. This is the first study to look at SCR to trauma interview in youth resettled as refugees and is part of the limited but growing body of research to look at biomarkers in refugee cohorts more broadly. We found that not only were symptoms associated with SCR, but also cumulative trauma exposure. Given that trauma exposure was highly correlated with symptoms, and the associations between symptoms and SCR were small, the present data are unable to disentangle whether SCR to trauma interview may be uniquely associated with symptoms above and beyond that of trauma exposure, or whether the elevation in SCR to trauma interview may possibly be, in part, affected by trauma exposure. This elevation in autonomic functioning associated with higher trauma exposure may precede trauma-related psychopathology, however, this has yet to be measured.
Of great community and public health significance, the method used in this study can be used in multiple settings – not only labs and clinics, but also homes and other community sites. Our data indicated that regardless of variation due to location of assessment, the main effects remain significant. When persons resettling as refugees first arrive in the United States, a mandatory health screening is conducted within one month of arrival. While some clinics will conduct mental health screenings, this is not a required component of the screening, and full clinical interviews are not possible given time constraints and lack of access to mental health clinicians. Here, rapidly obtained, objective biomarkers may provide a time-sensitive and cost-effective option to identify those in need of further screening and additional mental health resources. Objective assessments may be especially important when working with immigrant and refugee communities, where verbal presentation of symptoms may differ based on cultural norms and language may be a barrier. Additionally, screening for trauma exposure and autonomic reactivity in child and adolescent medical settings will provide an opportunity for early detection, intervention, and treatment (Gilgoff, Singh, Koita, Gentile, & Marques, 2020).
4.1. Limitations
The major limitation of this study is the small sample size, as we have discussed above. Notably, while our data provided evidence for small to moderate effects, our results did not survive correction for multiple comparison and therefore results should be interpreted with caution. Another limitation of this study is the nature of self-report questionnaires and the lack of clinical interviews. The present data were collected distally from the initial warzone trauma exposure and at least one year into the resettlement period. Therefore, acute symptoms and psychophysiological changes may have subsided, potentially indicating some level of recovery from stress exposure in this sample, although this was not directly measured. At the same time, aligned with clinical observations, our findings suggest that solely being away from the warzone even after several years does not completely resolve all symptoms. Given the single timepoint data collection, we are unable to resolve whether elevated SCR is a predisposing risk factor for developing PTSS/PTSD once one has experienced a traumatic event (McKeever & Huff, 2003), or if elevated SCR results from trauma exposure, particularly cumulative trauma exposure. Regardless, elevated SCR to trauma interview may still be a meaningful indicator of PTSS. Finally, due to the limitation of single timepoint data collection, we are unable to measure whether SCL and SCR habituate upon repeated measurement using this method. This is an outstanding research question that future studies may seek to resolve, especially if this method is to be used to track longitudinal course of illness or treatment efficacy.
5. Conclusions
We found that SCL measured using mobile eSense was feasible for use in multiple settings, and trauma interviews were effective in evoking measurable elevations in SCL in youth resettled as refugees in the US. Greater trauma exposure may drive greater autonomic reactivity in youth, potentially putting them at risk for future mental and physical health conditions. As such, youth should be screened not only for trauma exposure but also the impact of trauma exposure on psychophysiological markers to better inform care and prioritization of resources. The SCR to trauma interview may provide indications of trauma-related psychopathology – specifically re-experiencing and hyperarousal symptoms – and this study was the first to use this method in this especially vulnerable population. Larger future studies can build on this initial evidence to further query SCR to trauma interview as a biomarker of autonomic functioning related to posttraumatic stress in youth. Detecting and treating PTSS in refugee populations with scarce mental health resources is extremely important given the current global climate. Detection and risk assessment are essential first steps, and subsequently must be the development of culturally appropriate interventions that can be deployed to areas of conflict (Sijbrandij et al., 2017) and resettlement (Acarturk et al., 2022; de Graaff et al., 2020; Feen-Calligan et al., 2020; Grasser et al., 2019; Grasser & Javanbakht, 2021; Purgato et al., 2019; Purgato et al., 2021; Tol et al., 2018). As the needs for trauma-informed services are likely to become overwhelming in many areas – on top of recent increased burden on healthcare services – early detection of, and intervention for, trauma-related psychopathology is critical.
Supplementary Material
Acknowledgements
The authors would like to thank the participants who shared their stories and contributed their time as part of this research. The authors would also like to thank the members of the Stress, Trauma, and Anxiety Research Clinic who assisted with data collection, namely Ragda Izar (MD; Wayne State University), Raya Nashef (BS; Wayne State University), Noor Abou Rass (Wayne State University), and Ayat Abed-Ali (Wayne State University), and our protocol coordinator Kathleen Gorski (LMSW; Wayne State University). Finally, the authors would like to thank our community partner, the Arab American Chaldean Council.
Funding Statement
This work is supported by the National Institute of Mental Health [grant number F31MH120927; PI: Grasser] and the National Institute of Child Health and Development [grant number R01HD099178; PI: Javanbakht].
Data availability
Wayne State University requires data use agreements to be drafted and approved prior to any data sharing. If investigators are interested in accessing data, agreements will need to be drafted and approved between institutions and investigators in order to protect these data. Therefore, data cannot be made publicly available at this time, however, data can be made available by request to the authors and institutional approval with an appropriate data use agreement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abu Suhaiban, H., Grasser, L. R., & Javanbakht, A. (2019). Mental health of refugees and torture survivors: A critical review of prevalence, predictors, and integrated care. International Journal of Environmental Research and Public Health, 16(13), doi: 10.3390/ijerph16132309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acarturk, C., Uygun, E., Ilkkursun, Z., Yurtbakan, T., Kurt, G., Adam-Troian, J., … Kiselev, N. (2022). Group problem management plus (PM+) to decrease psychological distress among Syrian refugees in Turkey: A pilot randomised controlled trial. BMC Psychiatry, 22(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed). Washington, DC: American Psychiatric Association. [Google Scholar]
- Buckley, T. C., & Kaloupek, D. G. (2001). A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic Medicine, 63(4), 585–594. doi: 10.1097/00006842-200107000-00011 [DOI] [PubMed] [Google Scholar]
- Carrion, V. G., Weems, C. F., Ray, R., & Reiss, A. L. (2002). Toward an empirical definition of pediatric PTSD: The phenomenology of PTSD symptoms in youth. Journal of the American Academy of Child & Adolescent Psychiatry, 41(2), 166–173. [DOI] [PubMed] [Google Scholar]
- Casey, B., Craddock, N., Cuthbert, B. N., Hyman, S. E., Lee, F. S., & Ressler, K. J. (2013). DSM-5 and RDoC: Progress in psychiatry research? Nature Reviews Neuroscience, 14(11), 810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos, G. I., Uy, M. A., & Yap, W. J. (2019). The body and the brain: Measuring skin conductance responses to understand the emotional experience. Organizational Research Methods, 22(1), 394–420. doi: 10.1177/1094428116681073 [DOI] [Google Scholar]
- Cuthbert, B. N., & Insel, T. R. (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine, 11, 126. doi: 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert, B. N., & Kozak, M. J. (2013). Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology, 122(3), 928–937. doi: 10.1037/a0034028 [DOI] [PubMed] [Google Scholar]
- de Graaff, A. M., Cuijpers, P., Acarturk, C., Bryant, R., Burchert, S., Fuhr, D. C., … Sijbrandij, M. (2020). Effectiveness of a peer-refugee delivered psychological intervention to reduce psychological distress among adult Syrian refugees in the Netherlands: Study protocol. European Journal of Psychotraumatology, 11(1), 1694347. doi: 10.1080/20008198.2019.1694347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, W. J. (1960). Simplified estimation from censored normal samples. The Annals of Mathematical Statistics, 31(2), 385–391. [Google Scholar]
- Eack, S. M., Greeno, C. G., & Lee, B.-J. (2006). Limitations of the patient health questionnaire in identifying anxiety and depression: Many cases are undetected. Research on Social Work Practice, 16(6), 625–631. doi: 10.1177/1049731506291582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feen-Calligan, H., Grasser, L. R., Debryn, J., Nasser, S., Jackson, C., Seguin, D., & Javanbakht, A. (2020). Art therapy with Syrian refugee youth in the United States: An intervention study. The Arts in Psychotherapy, 69, 101665. [Google Scholar]
- Galatzer-Levy, I. R., & Bryant, R. A. (2013). 636,120 ways to have posttraumatic stress disorder. Perspectives on Psychological Science, 8(6), 651–662. doi: 10.1177/1745691613504115 [DOI] [PubMed] [Google Scholar]
- Gilgoff, R., Singh, L., Koita, K., Gentile, B., & Marques, S. S. (2020). Adverse childhood experiences, outcomes, and interventions. Pediatric Clinics of North America, 67(2), 259–273. doi: 10.1016/j.pcl.2019.12.001 [DOI] [PubMed] [Google Scholar]
- Grasser, L. R., Al-Saghir, H., Wanna, C., Spinei, J., & Javanbakht, A. (2019). Moving through the trauma: Dance/movement therapy as a somatic-based intervention for addressing trauma and stress among Syrian refugee children. Journal of the American Academy of Child and Adolescent Psychiatry, 58(11), 1124–1126. [DOI] [PubMed] [Google Scholar]
- Grasser, L. R., Haddad, L., Manji, S., Assari, S., Arfken, C., & Javanbakht, A. (2021). Trauma-related psychopathology in Iraqi refugee youth resettled in the United States, and comparison with an ethnically similar refugee sample: A cross-sectional study [brief research report]. Frontiers in Psychology, 12(704), 574368. doi: 10.3389/fpsyg.2021.574368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser, L. R., & Javanbakht, A. (2021). Virtual arts and movement therapies for youth in the era of COVID-19. Journal of the American Academy of Child and Adolescent Psychiatry, 60(11), 1334–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser, L. R., & Jovanovic, T. (2021). Safety learning during development: Implications for development of psychopathology. Behavioural Brain Research, 408, 113297. doi: 10.1016/j.bbr.2021.113297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, B. D. W., Atkinson Jr, A. J., Colburn, W. A., DeGruttola, V. G., DeMets, D. L., Downing, G. J., … Schooley, R. T. (2001). Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics, 69(3), 89–95. [DOI] [PubMed] [Google Scholar]
- Harker, M. (2013). Psychological sweating: A systematic review focused on aetiology and cutaneous response. Skin Pharmacology and Physiology, 26(2), 92–100. [DOI] [PubMed] [Google Scholar]
- Hinrichs, R., Michopoulos, V., Winters, S., Rothbaum, A. O., Rothbaum, B. O., Ressler, K. J., & Jovanovic, T. (2017). Mobile assessment of heightened skin conductance in posttraumatic stress disorder. Depression and Anxiety, 34(6), 502–507. doi: 10.1002/da.22610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs, R., van Rooij, S. J., Michopoulos, V., Schultebraucks, K., Winters, S., Maples-Keller, J., … Jovanovic, T. (2019). Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress (Thousand Oaks), 3), doi: 10.1177/2470547019844441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge, E. A., Worthington, J. J., Nagurney, J. T., Chang, Y., Kay, E. B., Feterowski, C. M., … Pitman, R. K. (2012). Effect of acute posttrauma propranolol on PTSD outcome and physiological responses during script-driven imagery. CNS Neuroscience & Therapeutics, 18(1), 21–27. doi: 10.1111/j.1755-5949.2010.00227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram, E., & Fitzgerald, H. E. (1974). Individual differences in infant orienting and autonomic conditioning. Developmental Psychobiology, 7(4), 359–367. doi: 10.1002/dev.420070413 [DOI] [PubMed] [Google Scholar]
- Javanbakht, A., Rosenberg, D., Haddad, L., & Arfken, C. L. (2018). Mental health in Syrian refugee children resettling in the United States: War trauma, migration, and the role of parental stress. Journal of the American Academy of Child & Adolescent Psychiatry, 57(3), 209–211 e202. doi: 10.1016/j.jaac.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Jovanovic, T., Norrholm, S. D., Blanding, N. Q., Davis, M., Duncan, E., Bradley, B., & Ressler, K. J. (2010). Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety, 27(3), 244–251. doi: 10.1002/da.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia, M., & Costa e Silva, J. (2015). Biomarkers of psychiatric diseases: Current status and future prospects. Metabolism, 64(3, Supplement 1), S11–S15. doi: 10.1016/j.metabol.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Kaplow, J. B., Rolon-Arroyo, B., Layne, C. M., Rooney, E., Oosterhoff, B., Hill, R., … Pynoos, R. S. (2020). Validation of the UCLA PTSD Reaction Index for DSM-5: A developmentally informed assessment tool for youth. Journal of the American Academy of Child & Adolescent Psychiatry, 59(1), 186–194. doi: 10.1016/j.jaac.2018.10.019 [DOI] [PubMed] [Google Scholar]
- Kotz, S., Read, C., Balakrishnan, N., Vidakovic, B., & Johnson, N. L. (2006). Stratified multistage sampling encyclopedia of statistical sciences. Hoboken, NJ: John Wiley. [Google Scholar]
- Krabbe, P. F. M. (2017). Chapter 7 - validity. In Krabbe P. F. M. (Ed.), The measurement of health and health status (pp. 113–134). Academic Press. doi: 10.1016/B978-0-12-801504-9.00007-6 [DOI] [Google Scholar]
- Kupfer, D. J., & Regier, D. A. (2011). Neuroscience, clinical evidence, and the future of psychiatric classification in DSM-5. American Journal of Psychiatry, 168(7), 672–674. [DOI] [PubMed] [Google Scholar]
- Kwak, S. K., & Kim, J. H. (2017). Statistical data preparation: Management of missing values and outliers. Korean Journal of Anesthesiology, 70(4), 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A., Bluhm, R., Lanius, U., & Pain, C. (2006). A review of neuroimaging studies in PTSD: Heterogeneity of response to symptom provocation. Journal of Psychiatric Research, 40(8), 709–729. doi: 10.1016/j.jpsychires.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Leys, C., Ley, C., Klein, O., Bernard, P., & Licata, L. (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology, 49(4), 764–766. doi: 10.1016/j.jesp.2013.03.013 [DOI] [Google Scholar]
- Lydiard, J., & Nemeroff, C. B. (2019). Biomarker-guided tailored therapy. Advances in Experimental Medicine and Biology, 1192, 199–224. doi: 10.1007/978-981-32-9721-0_10 [DOI] [PubMed] [Google Scholar]
- Łoś, K., & Waszkiewicz, N. (2021). Biological markers in anxiety disorders. Journal of Clinical Medicine, 10(8), doi: 10.3390/jcm10081744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso, M., & Preskorn, S. H. (2012). How biomarkers will change psychiatry: From clinical trials to practice. Part I: Introduction. Journal of Psychiatric Practice®, 18(2), 118–121. [DOI] [PubMed] [Google Scholar]
- Malhi, G. S. (2014). ICD Future (Vol. 48, pp. 107-109). London: Sage. [DOI] [PubMed] [Google Scholar]
- Mayeux, R. (2004). Biomarkers: Potential uses and limitations. NeuroRx: The Journal of the American Society for Experimental NeuroTherapeutics, 1(2), 182–188. doi: 10.1602/neurorx.1.2.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever, V. M., & Huff, M. E. (2003). A diathesis-stress model of posttraumatic stress disorder: Ecological, biological, and residual stress pathways. Review of General Psychology, 7(3), 237–250. doi: 10.1037/1089-2680.7.3.237 [DOI] [Google Scholar]
- McLaughlin, K. A., Sheridan, M. A., Gold, A. L., Duys, A., Lambert, H. K., Peverill, M., … Pine, D. S. (2016). Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology, 41(8), 1956–1964. doi: 10.1038/npp.2015.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas, K. R., He, J. P., Burstein, M., Swanson, S. A., Avenevoli, S., Cui, L., … Swendsen, J. (2010). Lifetime prevalence of mental disorders in U.S. Adolescents: Results from the national comorbidity survey replication–adolescent supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989. doi: 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska, K. J., Shechner, T., Hong, M., Britton, J. C., Leibenluft, E., Pine, D. S., & Fox, N. A. (2016). A developmental analysis of threat/safety learning and extinction recall during middle childhood. Journal of Experimental Child Psychology, 146, 95–105. doi: 10.1016/j.jecp.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos, V., Norrholm, S. D., & Jovanovic, T. (2015). Diagnostic biomarkers for posttraumatic stress disorder: Promising horizons from translational neuroscience research. Biological Psychiatry, 78(5), 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. M., & Shields, S. A. (1980). Skin conductance response as a measure of adolescents’ emotional reactivity. Psychological Reports, 46, 587–590. [Google Scholar]
- Miller, K. K., Brown, C. R., Shramko, M., & Svetaz, M. V. (2019). Applying trauma-informed practices to the care of refugee and immigrant youth: 10 clinical pearls. Children (Basel), 6(8), doi: 10.3390/children6080094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica, R. F., Caspi-Yavin, Y., Bollini, P., Truong, T., Tor, S., & Lavelle, J. (1992). The Harvard trauma questionnaire. Validating a cross-cultural instrument for measuring torture, trauma, and posttraumatic stress disorder in Indochinese refugees. Journal of Nervous and Mental Disease, 180(2), 111–116. https://www.ncbi.nlm.nih.gov/pubmed/1737972. [PubMed] [Google Scholar]
- Morris, S. E., & Cuthbert, B. N. (2012). Research domain criteria: Cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience, 14(1), 29–37. doi: 10.31887/DCNS.2012.14.1/smorris [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow, M. C., Boring, F. W., Keough, T. E., & Haesly, R. R. (1969). Differential GSR conditioning as a function of age. Developmental Psychology, 1(4), 299–302. doi: 10.1037/h0027688 [DOI] [Google Scholar]
- Neumann, D. L., Waters, A. M., & Westbury, H. R. (2008). The use of an unpleasant sound as the unconditional stimulus in aversive pavlovian conditioning experiements that involve children and adolescent participants. Behavior Research Methods, 40(2), 622–625. [DOI] [PubMed] [Google Scholar]
- Norrholm, S. D., Glover, E. M., Stevens, J. S., Fani, N., Galatzer-Levy, I. R., Bradley, B., … Jovanovic, T. (2015). Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. International Journal of Psychophysiology, 98(2 Pt 2), 270–275. doi: 10.1016/j.ijpsycho.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm, S. D., Jovanovic, T., Olin, I. W., Sands, L. A., Karapanou, I., Bradley, B., & Ressler, K. J. (2011). Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biological Psychiatry, 69(6), 556–563. doi: 10.1016/j.biopsych.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, S. P., Metzger, L. J., & Pitman, R. K. (2002). Psychophysiology of post-traumatic stress disorder. The Psychiatric Clinics of North America, 25(2), 271–293. doi: 10.1016/s0193-953x(01)00007-7 [DOI] [PubMed] [Google Scholar]
- Park, J., Marvar, P. J., Liao, P., Kankam, M. L., Norrholm, S. D., Downey, R. M., … Rothbaum, B. O. (2017). Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. Journal of Physiology, 595(14), 4893–4908. doi: 10.1113/jp274269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreira, K. M., & Ornelas, I. (2013). Painful passages: Traumatic experiences and post-traumatic stress among Immigrant latino adolescents and their primary caregivers. The international Migration Review, 47(4), doi: 10.1111/imre.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles, S. L., Suvak, M. K., Liverant, G. I., Gregor, K., Wisco, B. E., Pitman, R. K., & Orr, S. P. (2013). Psychophysiologic reactivity, subjective distress, and their associations with PTSD diagnosis. Journal of Abnormal Psychology, 122(3), 635–644. doi: 10.1037/a0033942 [DOI] [PubMed] [Google Scholar]
- Pitman, R. K., Orr, S. P., Forgue, D. F., de Jong, J. B., & Claiborn, J. M. (1987). Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry, 44(11), 970–975. doi: 10.1001/archpsyc.1987.01800230050009 [DOI] [PubMed] [Google Scholar]
- Pole, N. (2007). The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin, 133(5), 725–746. doi: 10.1037/0033-2909.133.5.725 [DOI] [PubMed] [Google Scholar]
- Prasadt, A., Chaichi, A., Parker Kelley, D., Fancis, J., & Ranjan Gartia, M. (2019). Current and future functional imaging techniques for posttraumatic stress disorder. RSC Advances, 9, 24568–24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgato, M., Carswell, K., Acarturk, C., Au, T., Akbai, S., Anttila, M., … Bird, M. (2019). Effectiveness and cost-effectiveness of self-help plus (SH+) for preventing mental disorders in refugees and asylum seekers in Europe and Turkey: Study protocols for two randomised controlled trials. BMJ Open, 9(5), e030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgato, M., Carswell, K., Tedeschi, F., Acarturk, C., Anttila, M., Au, T., … Churchill, R. (2021). Effectiveness of self-help plus in preventing mental disorders in refugees and asylum seekers in Western Europe: A multinational randomized controlled trial. Psychotherapy and Psychosomatics, 90(6), 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, A., Verkuilen, J., Ho, E., & Fan, Y. (2015). Posttraumatic stress disorder among refugees: Measurement invariance of Harvard Trauma Questionnaire scores across global regions and response patterns. Psychological Assessment, 27(4), 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombold, F., Wingenfeld, K., Renneberg, B., Hellmann-Regen, J., Otte, C., & Roepke, S. (2016a). Influence of the noradrenergic system on the formation of intrusive memories in women: An experimental approach with a trauma film paradigm. Psychological Medicine, 46(12), 2523–2534. [DOI] [PubMed] [Google Scholar]
- Rombold, F., Wingenfeld, K., Renneberg, B., Schwarzkopf, F., Hellmann-Regen, J., Otte, C., & Roepke, S. (2016b). Impact of exogenous cortisol on the formation of intrusive memories in healthy women. Journal of Psychiatric Research, 83, 71–78. [DOI] [PubMed] [Google Scholar]
- Rombold-Bruehl, F., Otte, C., Renneberg, B., Schmied, A., Zimmermann-Viehoff, F., Wingenfeld, K., & Roepke, S. (2017). Lower heart rate variability at baseline is associated with more consecutive intrusive memories in an experimental distressing film paradigm. The World Journal of Biological Psychiatry, 20(8), 662–667. [DOI] [PubMed] [Google Scholar]
- Saunders, B. E., & Adams, Z. W. (2014). Epidemiology of traumatic experiences in childhood. Child and Adolescent Psychiatric Clinics of North America, 23(2), 167–vii. doi: 10.1016/j.chc.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr, E., Millan, M. J., Bahn, S., Bertolino, A., Turck, C. W., Kapur, S., … Dean, B. (2015). Biomarkers for psychiatry: The journey from fantasy to fact, a report of the 2013 CINP think tank. The International Journal of Neuropsychopharmacology, 18(10), doi: 10.1093/ijnp/pyv042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultebraucks, K., Rombold-Bruehl, F., Wingenfeld, K., Hellmann-Regen, J., Otte, C., & Roepke, S. (2019). Heightened biological stress response during exposure to a trauma film predicts an increase in intrusive memories. Journal of Abnormal Psychology, 128(7), 645. [DOI] [PubMed] [Google Scholar]
- Seligowski, A. V., Harnett, N. G., Merker, J. B., & Ressler, K. J. (2020). Nervous and endocrine system dysfunction in posttraumatic stress disorder: An overview and consideration of sex as a biological variable. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(4), 381–391. doi: 10.1016/j.bpsc.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki, M., & Crandall, C. G. (2010). Mechanisms and controllers of eccrine sweating in humans. Front Biosci (Schol Ed), 2, 685–696. doi: 10.2741/s94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij, M., Acarturk, C., Bird, M., Bryant, R. A., Burchert, S., Carswell, K., … El Chammay, R. (2017). Strengthening mental health care systems for Syrian refugees in Europe and the Middle East: Integrating scalable psychological interventions in eight countries. European Journal of Psychotraumatology, 8(sup2), 1388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg, Ø, Nissen, A., Vaez, M., Cauley, P., Eriksson, A. K., & Saboonchi, F. (2020). Children at risk: A nation-wide, cross-sectional study examining post-traumatic stress symptoms in refugee minors from Syria, Iraq and Afghanistan resettled in Sweden between 2014 and 2018. Conflict and Health, 14, 67. doi: 10.1186/s13031-020-00311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, J. S., Harnett, N. G., Lebois, L. A. M., van Rooij, S. J. H., Ely, T. D., Roeckner, A., … Ressler, K. J. (2021). Brain-based biotypes of psychiatric vulnerability in the acute aftermath of trauma. American Journal of Psychiatry, 178(11), 1037–1049. doi: 10.1176/appi.ajp.2021.20101526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol, W., Augustinavicius, J., Carswell, K., Brown, F., Adaku, A., Leku, M., García-Moreno, C., Ventevogel, P., White, R., & Van Ommeren, M. (2018). Translation, adaptation, and pilot of a guided self-help intervention to reduce psychological distress in South Sudanese refugees in Uganda. Global Mental Health, 5, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kolk, B. A. (2015). The body keeps the score: Brain, mind, and body in the healing of trauma. London: Penguin Books. [Google Scholar]
- Weiner, S. (2018). Addressing the escalating psychiatrist shortage. February 12. Washington, DC: Association of American Medical Colleges. [Google Scholar]
- Wiltshire, C. N., Wanna, C. P., Stenson, A. F., Minton, S. T., Reda, M. H., Davie, W. M., … Jovanovic, T. (2022). Associations between children's trauma-related sequelae and skin conductance captured through mobile technology. Behaviour Research and Therapy, doi: 10.1016/j.brat.2022.104036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A. G., Sokolowska, I., Taurines, R., Gerlach, M., Dudley, E., Thome, J., & Darie, C. C. (2012). Potential biomarkers in psychiatry: Focus on the cholesterol system. Journal of Cellular and Molecular Medicine, 16(6), 1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Wayne State University requires data use agreements to be drafted and approved prior to any data sharing. If investigators are interested in accessing data, agreements will need to be drafted and approved between institutions and investigators in order to protect these data. Therefore, data cannot be made publicly available at this time, however, data can be made available by request to the authors and institutional approval with an appropriate data use agreement.