Abstract
Whole grains are a pivotal food category for the human diet and represent an invaluable source of carbohydrates, proteins, fibers, phytocompunds, minerals, and vitamins. Many studies have shown that the consumption of whole grains is linked to a reduced risk of cancer, cardiovascular diseases, and type 2 diabetes and other chronic diseases. However, several of their positive health effects seem to disappear when grains are consumed in the refined form. Herein we review the available literature on whole grains with a focus on molecular composition and health benefits on many chronic diseases with the aim to offer an updated and pragmatic reference for physicians and nutrition professionals.
Keywords: cereals, grains, fibers, cancer, cardiovascular diseases
Introduction
Grains are a vital food category for many populations of the world and their annual production exceeds 2,700 tons with an alignment of supply and demand (1).
Grains represent an important source of carbohydrates, proteins, fibers, minerals, vitamins, and phytochemicals and their regular consumption appears to be associated with many health benefits (2, 3). Indeed, incidence and mortality of several chronic non-communicable conditions like cancers, type 2 diabetes, and cardiovascular diseases have been shown to be reduced in people regularly eating whole grains (3). However, these health benefits might not be replicated by the consumption of refined grains (RG) which are characterized by lower levels of minerals, vitamins, fibers, and phytochemicals (4). This represents an important topic of public health since in the canonical western diet the vast majority of consumed grains are generally in the refined form (5).
Herein we review the available literature on whole grains (WG) with a focus on their molecular composition and their health benefits with the aim to offer an updated and pragmatic reference for physicians and nutrition professionals.
Composition of Cereal Grains
Cereals are made of carbohydrates for 50–80% of their weight and contain a lower but significant amount of proteins (5–6%), and lipids (1–10%) (Table 1; Figure 1). Whole grains are an important source of mineral salts (1.5–2.5%) (phosphorus, calcium, magnesium, potassium, iron, zinc, copper), and vitamins (thiamine, riboflavin, niacin, pyridoxine, biotin, folic acid, vitamin E, and vitamin A) (25, 26).
Table 1.
Nutrients of cereals and pseudocereals.
| Cereals | Kcal | CHO | Pro | Fat | FTOT | FSOL | Vitamins | Micros | Phytochemicals | Antinutrients |
|---|---|---|---|---|---|---|---|---|---|---|
| Barley (Hordeum vulgare) | 354 | 73.5 | 12.5 | 2.3 | 17.3 | / | Thiamine 0.646 mg | Manganese 1.94 mg | FibersU, anthocyanins (6), polyphenols (7) | Tannins (Proanthocyanidins) (8), phytate (9, 10), oxalates (9) |
| Riboflavin 0.285 mg | Zinc 2.77 mg | |||||||||
| Niacin 4.6 mg | Copper 0.498 mg | |||||||||
| Pantothenic acid 0.282 mg | Iron 3.6 mg | |||||||||
| Vitamin B6 0.318 mg | Phosphorus 264 mg | |||||||||
| Vitamin A 0.007 mg | Magnesium 133 mg | |||||||||
| Calcium 33 mg | ||||||||||
| Potassium 452 mg | ||||||||||
| Sodium 12 mg | ||||||||||
| Buckwheat (Fagopyrum esculentum Moench) | 343 | 71.5 | 13.2 | 3.4 | 10 | / | Thiamine 0.6 mg | Iron 2 mg | FibersU | Tannins (Proanthocyanidins) (8) |
| Niacin 4.4 mg | Phosphorus 330 mg | |||||||||
| Calcium 67 mg | ||||||||||
| Potassium 311 mg | ||||||||||
| Sodium 1 mg | ||||||||||
| Potassium 450 mg | ||||||||||
| Calcium 110 mg | ||||||||||
| Iron 4 mg | ||||||||||
| Emmer (Triticum dicoccum, Triticum spelta) | 353 | 69.3 | 14.6 | 2.4 | 6.5 | 0.96 | Niacin 8.511 mg | Phosphorus 387 mg | Polyphenols (11, 12), fibersU | NA |
| Magnesium 128 mg | ||||||||||
| Calcium 35 mg | ||||||||||
| Potassium 407 mg | ||||||||||
| Zinc 4.79 mg | ||||||||||
| Copper 0.39 mg | ||||||||||
| Manganese 2 mg | ||||||||||
| Iron 1.53 mg | ||||||||||
| Einkorn (Triticum monococcum) | 354 | 62.5 | 13 | 2.9 | 9.8 | / | Thiamine 0 mg | Magnesium 125 mg | Polyphenols (11–13), fibersU [β-glucan, lignin, fructans (13)] | NA |
| Riboflavin 0.212 mg | Calcium 83 mg | |||||||||
| Niacin 4.167 mg | Sodium 0 mg | |||||||||
| Vitamin B6 0.417 mg | Iron 4.5 mg | |||||||||
| Vitamin A 0.062 mg | Phosphorus 417 mg | |||||||||
| Zinc 4.69 mg | ||||||||||
| Manganese 3 mg | ||||||||||
| Fonio (Digitaria exilis) | 378 | 86.67 | 4.44 | 1.11 | 2.2 | / | Iron 1.6 mg | Fibers (14) | Phytate (15) | |
| Kamut (Triticum turgidum subsp. turanicum Jakubz.) | 337 | 70.6 | 14.5 | 2.13 | 11.1 | / | Vitamin A 0.003 mg | FibersU | Phytate (9), oxalates (9) | |
| Thiamine 0.566 mg | Potassium 403 mg | |||||||||
| Riboflavin 0.184 mg | Sodium 5 mg | |||||||||
| Vitamin B6 0.259 mg | Zinc 3.68 mg | |||||||||
| Niacin 6.38 mg | Selenium 0.815mg | |||||||||
| Pantothenic Acid 0.949 mg | Copper 0.506 mg | |||||||||
| Vitamin E (alpha-tocopherol) 0.61 mg | Phosphorus 364 mg | |||||||||
| Vitamin K (phylloquinone) 0.018mg | Magnesium 130 mg | |||||||||
| Manganese 2.74 mg | ||||||||||
| Iron 3.77 mg | ||||||||||
| Calcium 22 mg | ||||||||||
| Maize (Zea mays L.) | 357 | 75.1 | 9.2 | 3.8 | 2 | / | Thiamine 0.36 mg | Phosphorus 256 mg | FibersU (fructans, cellulose, β-glucan, arabinoxylan, lignin) (16), phenolic acid (16), anthocyanins (6), polyphenols (7) | Phytate (10, 17), polyphenol (17) |
| Riboflavin 0.2 mg | Magnesium 120 mg | |||||||||
| Niacin 1.5 mg | Calcium 15 mg | |||||||||
| Vitamin A 0.062 mg | Iron 2.4 mg | |||||||||
| Zinc 2.21 mg | ||||||||||
| Potassium 287 mg | ||||||||||
| Sodium 35 mg | ||||||||||
| Selenium 0.155 mg | ||||||||||
| Copper 0.31 mg | ||||||||||
| Millets (Panicum miliaceum L.) | 378 | 72.8 | 11 | 4.22 | 8.5 | / | Thiamine 0.421 mg | Calcium 8 mg | FibersU, anthocyanins (6), polyphenols (7) | Goitrogens (18, 19), tannin (10, 17), phytate (9, 10, 17), oxalates (9) |
| Riboflavin 0.29 mg | Potassium 195 mg | |||||||||
| Niacin 4.72 mg | Sodium 5 mg | |||||||||
| Pantothenic acid 0.848 mg | Phosphorus 285 mg | |||||||||
| Vitamin B6 0.384 mg | Iron 3.01 mg | |||||||||
| Magnesium 114 mg | ||||||||||
| Zinc 1.68 mg | ||||||||||
| Copper 0.75 mg | ||||||||||
| Manganese 1.63 mg | ||||||||||
| Selenium 0.027 mg | ||||||||||
| Oats (Avena sativa) | 389 | 66.3 | 16.9 | 6.9 | 10.6 | / | Thiamine 0.763 mg | Calcium 54 mg | Saponins (20), fibersU (fructans, cellulose, β-glucan, arabinoxylan, lignin), phenolic acid (16, 20), polyphenols (7) | Oxalate (9, 18), Saponins (steroidal avenacosides accumulating in the leaves and triterpenoid avenacins in the rootsand) (21), phytate (9, 10) |
| Riboflavin 0.139 mg | Potassium 429 mg | |||||||||
| Niacin 0.961 mg | Sodium 2 mg | |||||||||
| Pantothenic acid 1.35 mg | Phosphorus 523 mg | |||||||||
| Vitamin B6 0.119 mg | Iron 4.72 mg | |||||||||
| Magnesium 177 mg | ||||||||||
| Zinc 3.97 mg | ||||||||||
| Copper 0.626 mg | ||||||||||
| Manganese 4.92 mg | ||||||||||
| Rice (Oryza sativa L.) | 334 | 80.4 | 6.7 | 0.4 | 1 | 0.08 | Thiamine 0.11 mg | Calcium 24 mg | FibersU (fructans, cellulose, β-glucan, arabinoxylan, lignin) (16), phenolic acid (16), anthocyanins (6), polyphenols (7) | Phytate (10, 18), tannins (proanthocyanidins) (8) |
| Riboflavin 0.03 mg | Potassium 92 mg | |||||||||
| Niacin 1.3 mg | Sodium 5 mg | |||||||||
| Magnesium 20 mg | ||||||||||
| Phosphorus 94 mg | ||||||||||
| Iron 0.8 mg | ||||||||||
| Copper 0.18 mg | ||||||||||
| Zinc 1.3 mg | ||||||||||
| Selenium 0.01 mg | ||||||||||
| Rye (Secale cereale L.) | 338 | 75.9 | 10.3 | 1.63 | 15.1 | / | Thiamine 0.316 mg | Calcium 24 mg | FibersU (fructans, cellulose, β-glucan, arabinoxylan, lignin) (16), phenolic acid (16), β-caroteneU, Anthocyanins (6) | Phytate (9, 10), oxalates (9) |
| Riboflavin 0.251 mg | Iron 2.63 mg | |||||||||
| Niacin 4.27 mg | Magnesium 110 mg | |||||||||
| Pantothenic acid 1.46 mg | Phosphorus 332 mg | |||||||||
| Vitamin B6 0.294 mg | Potassium 510 mg | |||||||||
| Vitamin A 0.003 mg | Sodium 2 mg | |||||||||
| Vitamin E (alpha-tocopherol) 0.85 mg | Zinc 2.65 mg | |||||||||
| Vitamin K (phylloquinone) 0.059 mg | Copper 0.367 mg | |||||||||
| Manganese 2.58 mg | ||||||||||
| Selenium | ||||||||||
| 0.139 mg | ||||||||||
| Sorghum [Sorghum bicolor (L.) Moench] | 329 | 72.1 | 10.6 | 3.46 | 6.7 | / | Thiamine 0.332 mg | Calcium 13 mg | FibersU, β-caroteneU, anthocyanins (6), polyphenols (7) | Tannins (proanthocyanidins) (8, 17), phytate (9, 10, 17), oxalates (9) |
| Riboflavin 0.096 mg | Iron 3.36 mg | |||||||||
| Niacin 3.69 mg | Magnesium 165 mg | |||||||||
| Pantothenic acid 3.67 mg | Phosphorus 289 mg | |||||||||
| Vitamin B6 0.443 mg | Potassium 363 mg | |||||||||
| Vitamin E (alpha-tocopherol) 0.5 mg | Sodium 2 mg | |||||||||
| Zinc 1.67 mg | ||||||||||
| Copper 0.284 mg | ||||||||||
| Manganese 1.6 mg | ||||||||||
| Selenium | ||||||||||
| 0.122 mg | ||||||||||
| Spelt (Triticum aestivum L. subsp. spelta) | 338 | 70.2 | 14.6 | 2.43 | 10.7 | / | Vitamin K (phylloquinone) 0.036 mg | Copper 0.511 mg | FibersU | Phytate (9), oxalates (9) |
| Vitamin B6 0.23 mg | Calcium 27 mg | |||||||||
| Vitamin E (alpha-tocopherol) 0.79 mg | Iron 4.44 mg | |||||||||
| Thiamine 0.364 mg | Magnesium 136 mg | |||||||||
| Riboflavin 0.113 mg | Manganese 2.98 mg | |||||||||
| Niacin 6.84 mg | Potassium 388 mg | |||||||||
| Pantothenic acid 1.07 mg | Sodium 8 mg | |||||||||
| Phosphorus 401 mg | ||||||||||
| Selenium 0.117 mg | ||||||||||
| Zinc 3.28 mg | ||||||||||
| TeffZ [Eragrostis tef (Zuccagni) Trotter] | 367 | 73.1 | 13.3 | 2.38 | 8 | / | Thiamine 0.39 mg | Calcium 180 mg | FibersU, β-caroteneU | Phytic acid (9), lectins (9), saponins (9), goitrogens (9) |
| Riboflavin 0.27 mg | Potassium 427 mg | |||||||||
| Niacin 3.36 mg | Sodium 12 mg | |||||||||
| Pantothenic acid 0.942 mg | Phosphorus 429 mg | |||||||||
| Vitamin B6 0.482 mg | Iron 7.63 mg | |||||||||
| Vitamin E (alpha-tocopherol) 0.08 mg | Magnesium 184 mg | |||||||||
| Vitamin A 0.003 mg | Zinc 3.63 mg | |||||||||
| Vitamin K (phylloquinone) 0.019 mg | Copper 0.81 mg | |||||||||
| Manganese 9.24 mg | ||||||||||
| Selenium 0.044 mg | ||||||||||
| Triticale (X Triticosecale spp.) | 338 | 73.1 | 13.2 | 1.81 | 14.6 | / | Thiamine 0.378 mg | Calcium 35 mg | Phenolic acids (free and bound) (22), proanthocyanidins (22), lignans (22) | Phytate (23), tannin (23) |
| Riboflavin 0.132 mg | Iron 2.59 mg | |||||||||
| Niacin 2.86 mg | Magnesium 153 mg | |||||||||
| Pantothenic acid 2.17 mg | Phosphorus 321 mg | |||||||||
| Vitamin B-6 0.403 mg | Potassium 466 mg | |||||||||
| Vitamin E (alpha-tocopherol) 0.9 mg | Sodium 2 mg | |||||||||
| Zinc 2.66 mg | ||||||||||
| Copper 0.559 mg | ||||||||||
| Manganese 4.18 mg | ||||||||||
| Wheat (Triticum Aestivum) | 336 | 65.2 | 12.3 | 2.6 | 9.7 | / | Thiamine 0.42 mg | Calcium 35 mg | Fibers (Fructans, cellulose, β-glucan, arabinoxylan, lignin) (16, 24), phenolic acid (16), anthocyanins (6), polyphenols (7) | Lectins (18), oxalate (9, 18), phytate (9, 10, 18), tannin (17) |
| Riboflavin 0.14 mg | Phosphorus 304 mg | |||||||||
| Niacin 5.4 mg | Iron 3.3 mg | |||||||||
| Copper 0.31 mg | ||||||||||
| Zinc 3.1 mg | ||||||||||
| Wheat (Triticum durum) | 332 | 62.5 | 13.0 | 2.9 | 9.8 | / | Thiamine 0.43 mg | Calcium 30 mg | Fibers (fructans, cellulose, β-glucan, arabinoxylan, lignin) (16, 24), phenolic acid (16), anthocyanins (6), polyphenols (7) | Lectins (18), oxalate (9, 18), phytate (9, 10, 18), tannin (17) |
| Riboflavin 0.15 mg | Phosphorus 330 mg | |||||||||
| Niacin 5.7 mg | Iron 3.6 mg | |||||||||
| Vitamin A 0.002 mg | Copper 0.40 mg | |||||||||
| Zinc 2.9 mg | ||||||||||
| Potassium 494 mg | ||||||||||
| Magnesium 160 mg | ||||||||||
| Selenium 0.038 mg | ||||||||||
| Wild rice (Zizania spp.) | 357 | 74.9 | 14.7 | 1.08 | 6.02 | / | Thiamine 0.115 mg | Calcium 21 mg | FibersU, β-caroteneU | Phytate (18) |
| Riboflavin 0.262 mg | Iron 1.96 mg | |||||||||
| Niacin 6.73 mg | Magnesium 177 mg | |||||||||
| Pantothenic acid 1.07 mg | Phosphorus 433 mg | |||||||||
| Vitamin B-6 0.391 mg | Potassium 427 mg | |||||||||
| Vitamin E (alpha-tocopherol) 0.82 mg | Sodium 7 mg | |||||||||
| Vitamin A 0.006 mg | Zinc 5.96 mg | |||||||||
| Vitamin K (phylloquinone) 0.019 mg | Copper 0.524 mg | |||||||||
| Manganese 1.33 mg | ||||||||||
| Selenium 0.028 mg | ||||||||||
| Amaranth | 371 | 65.25 | 13.6 6 | 7.02 | 6.7 | Vitamin C 4.2 mg | Calcium 159 mg | FibersU, β-caroteneU | Oxalate (18), phytic acid (9), lectins (9), saponins (9), goitrogens (9) | |
| (Amaranthus spp.) | Riboflavin 0.2 mg | Copper 0.525 mg | ||||||||
| Niacin 0.923 mg | Iron 7.61 mg | |||||||||
| Thiamin 0.116 mg | Magnesium 248 mg | |||||||||
| Pantothenic acid 1.46 mg | Manganese 3.33 mg | |||||||||
| Vitamin B6 0.591 mg | Phosphorus 557 mg | |||||||||
| Vitamin E (alpha-tocopherol) 1.19 mg | Potassium 508 mg | |||||||||
| Vitamin A 0.001 mg | Sodium 4 mg | |||||||||
| Zinc 2.87 mg | ||||||||||
| Selenium | ||||||||||
| 0.187 mg | ||||||||||
| Buckwheat (Fagopyrum esculentum Moench) | 335 | 70.6 | 12.6 | 3.1 | 10 | / | Riboflavin 0.19mg | Calcium 41 mg | FibersU | Phytic acid (9), lectins (9), saponins (9), goitrogens (9) |
| Niacin 6.15 mg | Copper 0.515 mg | |||||||||
| Thiamin 0.417 mg | Iron 4.06 mg | |||||||||
| Pantothenic acid 0.44 mg | Magnesium 251 mg | |||||||||
| Vitamin B6 0.582 mg | Manganese 2.03 mg | |||||||||
| Vitamin E (alpha-tocopherol) 0.32 mg | Phosphorus 337 mg | |||||||||
| Vitamin K (phylloquinone) 0.007 mg | Potassium 577 mg | |||||||||
| Sodium 11 mg | ||||||||||
| Zinc 3.12 mg | ||||||||||
| Selenium | ||||||||||
| 0.057 mg | ||||||||||
| Quinoa | 368 | 64.2 | 14.1 | 6.07 | 7 | / | Thiamine 0.36 mg | Calcium 47 mg | FibersU, β-caroteneU | Phytate (IP6) (9, 18), saponins (9, 17), lectins (9), goitrogens (9) |
| (Chenopodium quinoa) | Riboflavin 0.318 mg | Iron 4.57 mg | ||||||||
| Niacin 1.52 mg | Magnesium 197 mg | |||||||||
| Pantothenic acid 0.772 mg | Phosphorus 457 mg | |||||||||
| Vitamin B-6 0.487 mg | Potassium 563 mg | |||||||||
| Vitamin A 0.004 mg | Sodium 5 mg | |||||||||
| Vitamin E (alpha-tocopherol) 2.44 mg | Zinc 3.1 mg | |||||||||
| Vitamin K (menaquinone-4) 0.011 mg | Copper 0.59 mg | |||||||||
| Manganese 2.03 mg | ||||||||||
| Selenium 0.085 mg |
CHO, carbohydrates; Fat, lipids; FSOL, soluble fibers; FTOT, total fibers; LA, limiting aminoacid(s); Macros, macronutrients; Micro, micronutrients; NA, not available; Oligos, oligonutrients; Pro, proteins. Nutritional values were founded on USDA Database (https://fdc.nal.usda.gov), CREA Database (https://www.alimentinutrizione.it/sezioni/tabelle-nutrizionali), BDA IEO Database (http://www.bda-ieo.it).
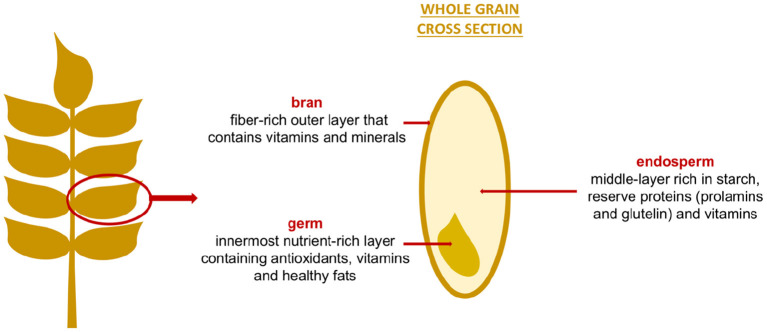
Figure 1.
Structure and composition of grains.
Four different parts are present in cereal grains: bran, endosperm, germ and aleurone layer. The endosperm is rich in starch, and reserve proteins (prolamins and glutelin) while the bran, the aleurone layer, and the germ have more proteins with essential amino acids, vitamins, minerals, fibers, lipids (greater presence in the germ), and bioactive substances (e.g., phenolic acids, flavonoids, alkylresorcinols, avenantramides, tannins, carotenoids, lignans and phytosterols) (25, 26).
Whole grains represent a widely consumed food in many diets and have been linked with healthy effects (see below). These beneficial effects seem to be, at least in part, attributable to the presence of dietary fibers which overall amount and types varies between different cereals. In general, whole grains and pseudo-cereals are rich in both soluble and insoluble fiber; the most abundant ones are: cellulose, arabinoxylan-glucan, xyloglucan, and fructan (27).
Many vitamins are present in cereals: thiamine, riboflavin, niacin, pyridoxine, biotin, folic acid, pantothenic acid, vitamins A, E, and K (28). Since they are especially distributed in the integument and in the germ, the refinement process can remove a significant amount (29).
In addition, the absolute abundance of vitamins and minerals is determined by a myriad of factors, including cultivar (30), soil composition (30), and the degree of refinement (29).
Relevant Phytochemicals in Cereals and Their Effect on Health
In cereals, as other plant food, an equilibrium exists between phytochemicals and antinutrients, which could be two aspects of the same compound. Phytochemicals are bioactive molecules characteristic of plants, and they are crucial for the human health by playing a pleiotropic action. Phytochemicals act as anti-oxidants or might have a role in maintaining DNA repair, controlling cell proliferation, cell differentiation, cancer cells apoptosis and DNA metabolism (16, 20). Examples of phytochemicals in cereal grains include terpenoids, polyphenols, phenolic constituents, alkaloids, carotenoids, phytosterols, saponins, and fibers (20, 24) (Table 2).
Table 2.
Phytochemicals in cereals and their effects on health.
| Phytochemical/antinutrient | Potential effect on health | References |
|---|---|---|
| Flavonoids, isoflavonoids, anthocyanidins | Benefits: anti-free radicals' action in cellular signaling, cancer prevention | (7, 20, 31–35) |
| Harms: platelet aggregation, allergies, inflammation, and hepatotoxins | ||
| Fibers | Benefits: blood cholesterol reduction, prevention of cardiovascular disease, metabolic syndrome, type 2 diabetes and cancer | (16, 20, 24, 36) |
| Lectins | Benefits: possible cancer diagnostic and treatment tools | (9, 18, 37–42) |
| Harms: altered gut function, inflammation, food poisoning by lectin-rich foods, if not prepared correctly | ||
| Oxalates | Harms: probable inhibition of calcium absorption and increased calcium kidney stone formation. | (9, 18, 43) |
| Phytate (IP6) | Benefits: antioxidant, antineoplastic effect, decreasing kidney stone risk, decreasing osteoporosis risk, decreasing dental calculi risk, preventing age-related cardiovascular calcification | (9, 17, 18, 25, 44–52) |
| Harms: chelate calcium, iron and zinc, interfering with their absorption | ||
| Goitrogens | Benefits: antineoplastic effect | (9, 18, 19, 53–55) |
| Harms: altered thyroid function (hypothyroidism and/or goiter); inhibit iodine uptake | ||
| Phytoestrogens | Benefits: reduced menopausal symptoms, reduced risk of cardiovascular disease, obesity, metabolic syndrome, type 2 diabetes, cognitive disorders, several forms of cancer | (18, 56–64) |
| Tannins | Benefits: antioxidant and radical scavenging agents, anticarcinogenic, immunomodulatory, anti-diabetic, anti-obesity and cardioprotective agents | (8, 9, 18, 65–70) |
| Harms: inhibit iron absorption, negatively impact iron stores. | ||
| Saponins | Harms: alteration of intestinal epithelial integrity, alteration of lipids absorption (including vitamins A and E), putative hemolysis. | (9, 21, 71) |
| Proteinase's inhibitors (α-amylase inhibitors, trypsin inhibitors and protease inhibitors) | Benefits: prevention of obesity and type 2 diabetes. | (9, 17, 72, 73) |
| Harms: delay in growth, reduction of protein digestibility, decreased glucose absorption rate. | ||
| Anthocyanins | Benefits: antioxidant and anti-hypertension activity, cancer and metabolic syndrome prevention, glycemic and bodyweight control, neurological, hepatic and retinal protection, hypolipidemic agent, enhancing immune response and anti-aging agent | (6, 74–88) |
Polyphenols represent an heterogeneous group of compounds, constituted by flavonoids and phenolic acids anthocyanins. They have antioxidant and anti-inflammatory activity, but their bio accessibility depends on the type of polyphenol or cereal matrix involved. In the gastrointestinal ambient, polyphenols can potentially change the gut microbiome favorably (7). Anthocyanins are pigments of colored cereals: wheat, rye, millet, barley, rice, maize, and sorghum (6). Several studies, both in vitro and in vivo, demonstrated that anthocyanins have positive health effects (6, 74): antioxidant activity (75, 76), inhibition of cholesterol absorption (77, 78), reduction of starch digestibility (79), neuro-protection (80), anticancer (81) and antimetastatic activity (82), anti-hypertension effect (83), retinal protection (84), body fat reduction (85), hepatoprotection (86), prevention of metabolic syndrome (31, 87), enhancement of the immune response (82), anti-aging effect (88).
Among the several properties of phytochemicals, the beneficial interaction with the intestinal microbiota represents an open area of research (89). Jayachandran et al. (90) explained as the “fiber gap”, i.e. a diet with low consumption of fibers, could interfere with gut microbiota equilibrium reducing its healthy metabolites. Some of its positive effects on human health are due to the gut microbiota production of short chain fatty acids (SCFAs), such as butyrate, acetate, propionate: these molecules act as anti-inflammatory and anti-oxidant agents, insulin sensitivity modulators, epigenetic modulators (89). SCFAs are the result of fibers degradations by gut microbiota, which contribute to digest soluble fibers, which otherwise would not be metabolized in the bowl. So that, gut microbiota homeostasis is linked to dietary fibers variety, which influences SCFAs production (89, 91, 92). Myhrstad and colleagues analyzed the results of 16 trials conducted to investigating the role of dietary fiber in modulating gut microbiota and human metabolic regulation (92). Despite of heterogeneity in the microbiota analyses, the reviewed studies considered the different types of SCFA-producing bacteria (i.e., Bifidobacterium, Ruminococcus, Dorea, ecc.), in particular Prevotella/Bacteroides ratio. Authors reported as gut microbiota rich in Prevotella spp. could be linked to fibers-enrich diet, but its association with improved metabolic regulation is still unclear. Besides, a fibers-enrich diet was associated with increased presence of SCFA-producing bacteria, however changing in gut microbiota not always corresponds to host metabolic changes.
Antinutrients reduce nutrient bioavailability, sometimes causing some adverse interference. In fact, this heterogenous group has an important role in cereals composition: phytates, lectins, saponins, enzyme inhibitors, tannins, goitrogens, oxalates, and phytoestrogens. Among antinutrients, we could include also mycotoxins, in particular aflatoxins, since cereals contamination represents a relevant concern for human health in several countries (93). Moreover, the plant microbiome composition could also impact on the amount and the types of compounds present in the grains; however, the exact role on health has yet to be elucidated (94).
Phytic acid, also known as phytate or myo-inositol hexaphosphate (IP6), represents an energy and antioxidant agent in seed germination (44). IP6 binds mineral cations (Fe3+, Cu2+, Zn2+, and Ca2+) (95), creating insoluble complexes not digestible by human enzymes, with a consequential decreased mineral bioavailability (9, 45). Cereals contain the highest concentrations of phytate, mainly in the outermost layer (18). Despite their antinutrient activity, phytates might be antioxidants chelating excess iron, thereby avoiding the damaging Fenton reactions (46, 47). Phytate could have pleiotropic effect [enhancing immunity, inhibiting inflammatory cascade, decreasing cell proliferation (44, 47), decreasing kidney stone risk (48), osteoporosis risk (25, 49), dental calculi (50), preventing age-related cardiovascular calcification (51, 52)]. However, these observations need to be confirmed by further research.
Lectins, also named hemagglutinins, are a group of carbohydrate/glycoconjugates-binding proteins (96), which reversibly link characteristic carbohydrate portions on cells, getting involved in autoimmune diseases genesis by presenting wrong immune system codes and stimulation of the differentiation of some white blood cells (possibly leading to cancer, but no studies have yet demonstrated lectins to be carcinogenic) (18, 97–99). Due their resistance to host enzyme and bacterial degradation, lectins arrive functionally and immunologically intact into the small intestine (37). In animal models, binding to glycoconjugates and glycan receptors of the enterocytes on their luminal surface, lectins showed to induce alteration of intestinal integrity by compromising nutrient absorption and reducing growth (9, 38–40). However, considering human studies, lectins might have therapeutic effect as nutraceutical agents: their high affinity and specificity to glycans could be used as cancer diagnostic and treatment tools, as adjuvants, together with conventional chemotherapy agents (41, 42).
Saponins, a various family of secondary metabolites produced by oats only among cereals, have a potent antifungal activity (17, 21). Due their ability to bind to cholesterol group in erythrocyte surfaces, saponins could lead to hemolysis in vitro (71) and hinder sterol absorption and activity (including vitamins A and E) (17), however saponins bio accessibility is very low and so these interactions could be considered of uncertain significance in vivo (100). Furthermore, saponins could be inhibitors of digestive enzymes (e.g., trypsin, glucosidase, amylase, lipase, and chymotrypsin) causing alterations of intestinal epithelial cells integrity (17). However, saponins have a strong hypocholesterolemic effect, in presence of cholesterol (9).
In cereals, compounds with antinutrient activities include α-amylase inhibitors, trypsin inhibitors, and protease inhibitors (17). The inhibition of α-amylase function increases time of carbohydrate absorption (72), while in human diets trypsin inhibitors reduce protein digestion and consequent amino acids availability, leading to decreased growth rate and pancreatic hyperplasia (17), like protease inhibitors (9). Nevertheless, several studies demonstrated that enzyme inhibitors, including alpha-amylase, alpha-glucosidase, and lipase inhibitors, might prevent type 2 diabetes and obesity (73).
Tannins are high molecular weight polyphenol compounds which link with carbohydrates and proteins with intra- and inter-molecular hydrogen bonds, acting as antioxidant, anticarcinogenic, immunomodulatory, and cardioprotective agents (8, 9, 65–69). Although positive effect of antioxidants, tannins could prevent dietary minerals absorption, such as zinc, copper and iron absorption (9, 70, 101). They are present in cereal grains, seeds, legumes, fruits, juices, cocoa beans, tea, wines and nuts, representing one of the most plenty metabolites among secondary plant ones (8).
Goitrogens are a heterogenous compounds group which include foods, environmental toxins and drugs (102). They play a role in thyroid function alteration increasing goiter and other thyroid diseases risk. With mastication and ingestion, myrosinase (enzyme produced by human microflora and activated in damaged plant tissue) converts the goitrongen glucosinolates to several other compounds: nitriles, thiocyanates, sulforaphane and isothiocyanates (53). Glucosinolates could have a role in preventing cancer but also in impairing thyroid function (54, 55). Among cereal, only millet contains a goitrogenic compounds: C-glycosylflavones which inhibit thyroid peroxidase (TPO), as shown in in-vitro models (19).
Both plants and mammals produce oxalic acid, or oxalate, in small amounts: plants for several functions (i.e., plant defense, detoxification of heavy metals and calcium regulation) while in mammals it represents a metabolite of ascorbate, hydroxyproline, glyoxylate, and glycine. Oxalate can form soluble (with sodium and potassium) or insoluble (with iron, magnesium, and calcium) salts or esters (18), reducing absorption and probably contributing to calcium oxalate kidney stone formation due to hyperoxaluria (9, 18, 43). Oxalic acid is present in whole grains in smaller amounts than amaranth in which it is more enriched (18). Considering soluble and insoluble oxalate, wheat bran contains more soluble oxalate than whole grains products (44 mg/100 g in whole wheat flour, 113 mg/100 g dry weight vs. 13.8 mg in oats) (103).
Phytoestrogens are polyphenolic compounds derived from plants with peculiar structural analogies to 17-β-estradiol, female main sex hormone (104). They bind to estrogen receptors (ER) with an higher affinities for β receptor rather than α one and a fainter bond than 17-β-estradiol (56). Intestinal microflora converts lignan phytoestrogens, one of the four phenolic phytoestrogens compounds, to the “mammalian lignans,” enterolactone and enterodiol (57, 104). Lignans are extant in a very small amounts (<0.01 mg/100 g) in whole grains, excepted for multigrain bread (105). According to the currently published literature, phytoestrogens could have positive effects on health (i.e., reducing risk of metabolic syndrome, cardiovascular disease, type 2 diabetes, obesity, cognitive disorders, several cancer types, menopausal symptoms). Their role in increasing estrogen-sensitive breast and uterine cancer risk has not been demonstrated, as their role as endocrine disrupts, but last one only in babies and infants because of their underdeveloped digestive tract (18). Petroski and Minich (18) reported how phytoestrogen-rich products could be considered in cancer prevention (i.e., breast, prostate, endometrial, and colorectal cancer).
Farming Methods and Processing and Their Impact on Grains Constituents
Numerous studies have showed that organic cereals, relative to non-organic crops, contain higher levels of some vitamins (e.g., vitamin C) and minerals (e.g., iron, magnesium and phosphorus), and are also low in nitrates and pesticide residues (106) (Table 3). Organic foods provide higher levels of anthocyanins, flavonoids, and carotenoids (106). Moreover, regular consumption of these foods has been supposed to be associated with reduced risks of several diseases, such as cancer (141). From a general perspective, organic farming has some pros- and some contra-. Among the former, it should be highlighted the greater biodiversity and the better health of agricultural soil (142, 143); among the latter the crop yield remains low per area compared to conventional farming (144–146).
Table 3.
Effects of processing on cereals nutrients composition.
| Process | Effect | References |
|---|---|---|
| Organic farming | May increase levels of vitamin C, iron, magnesium, phosphorus, anthocyanins, flavonoids and carotenoids | (107) |
| Refining | Reduction of fiber, vitamins, minerals, and phytonutrients. Refining increases the glycemic index | (29, 108) |
| Soaking | Reduction of phytic acid. Reduction of minerals and extractable proteins from the water | (109) |
| Germination | With germination, depending on the type of cereal, it improves digestion and protein content; increases crude fiber and the amount of sugars. The quantity of vitamins, mineral salts, oxalates, tannins, phytates, flavonoids and phenols could be also modified. | (110–119) |
| Fermentation | Starch hydrolysis. Increases the bioavailability of minerals (e.g., calcium, phosphorus, and iron). Variable effect on the glycemic index | (120–127) |
| Cooking | Water-cooking methods facilitates losses of water-soluble vitamins and minerals; the losses amount varied by the cooking method and the duration. Cooking also increases the glycaemic index and, possibly, the antioxidant activity | (29, 128–140) |
With the increase of the world population, it is necessary to identify alternative strategies to the use of chemical substances to preserve crops, in particular cereals, from diseases caused by phytopathogens, because these substances negatively alter the beneficial microbiota of the soil with repercussions also on the final consumer (147). On the contrary, biocontrol uses soil and beneficial microbes associated with the plants themselves in order to stimulate the microbial population, including antagonists and pathogens, so as to promote plant growth and avoid the use of synthetic chemicals (148). This mechanism is expressed through: indirect antipathogenesis in which microbes improve the availability of soil nutrients or nitrogen fixation, and direct antipathogenesis, in which microbes fight pathogens by competition, by antibiosis, through production of antipathogenic substances, by stimulating plant defenses through induced systemic resistance, by enhancing the soil microbiota (suppression of the soil), by hyperparasitism that is the use of parasitic microbes of the pathogen, by the insertion of genetic sequences with antipathogenic action on transgenic crops (148). However, how these harvest methods could impact on cereals nutrients contents is an area of active research.
Cooking is another aspect that might impact the vitamin and micronutrients content in grains. For example, boiling causes little losses of riboflavin relative to microwave and pressure cooking (29); in pasta it has been reported that niacin losses are higher than thiamine and riboflavin (128). Folic acid losses were lower after boiling and frying compared to microwave and pressure cooking (129, 130) while vitamin B12 losses after boiling are moderate (131). Among methods that could cause vitamin C loss, pressure cooking appears to induce the maximum loss (132, 133). Interestingly, a study carried out on two varieties of rice showed that boiling increases antioxidant activities and the glycaemic index but does not impact on the phenolic content (134). However, cooking temperature is a major factor the losses of carotenoids (135–140).
Beyond farming and cooking methods, refining (i.e., removing the bran and germ) has a pivotal role in vitamin and micronutrients loss (29, 108). The main cereals subject to refining are wheat, rice, corn, barley, oats, rye, and millet. After bran and germ removal, a refined grains are composed mainly by the endosperm and is therefore rich in starch.
Germination is the process that converts seeds into plants; it takes place in favorable environmental conditions, including the presence of water, oxygen, and suitable temperatures (110). The effect of germination on the nutritional value of grains can vary by the compound considered. For example, an increase in phenolic compounds, flavonoids, crude proteins, and antioxidant was described in buckwheat Instead, germination decreases phytic acid content (111, 112). In corn, germination causes an increase in phenols, fats, crude fibers, and total proteins content (113, 114) while in Finger millet it increases the sugars, the digestion of proteins, tannins, phytates, and starch (115). In sorghum and millet there is an increase in crude fiber, minerals and vitamins, increases the digestibility of proteins, sucrose, glucose and fructose; on the other hand, oxalates, tannins and phytates decrease (116–119).
Fermentation is the digestion of food compounds by bacteria and/or yeast and represents one of the most ancestral methods for food processing and preservation. Fermentation could improve the sensory profile of foods favoring their preservation (120). Through this process is possible to activate enzymes such as α-amylase and maltase which hydrolyze starch and break it down into maltodextrins and simple sugars. During fermentation there is a decrease of total carbohydrates because of their metabolization by microorganisms (121). Minerals have a very low bioavailability as they are bound to polysaccharides and phytates of the cell wall; for example potassium is inaccessible to digestive enzymes as long as it is chelated by phytate molecules (122). Through fermentation it is possible to release these minerals and make them readily bioavailable (123). Furthermore, fermentation increases the bioavailability of calcium, phosphorus and iron probably because of degradation of oxalates and phytates (124). Regarding glycaemic index, fermentation has variable effects and different studies have showed discordant results (125–127).
Soaking is a practice that consist in dipping whole grains in water for a variable amount of time before being cooked. Through soaking the endogenous phytases are activated and a significant part of phytic acid is removed. For example, soaking sorghum flour for 24 h leads to acid phytic reduction up to 20% (109). However, while soaking can reduce the contents of antinutrients, it might also facilitate the loss of minerals and extractable proteins from the water.
Overall, since the various cooking methods could impact on the nutrients composition of grains, consumers should be advised to alternate cooking techniques and methods of preparations.
The Effect of Consuming Whole Grains on Obesity
Observational and interventional trials have reported an inverse correlation between whole grain-rich diets and obesity parameters, in contrast to refined grains, which present a lower nutritional quality (27, 149–151). There are different mechanisms through which whole grains can help in regulating the body weight, including the fact that they promote satiety, thus leading to a reduced food consumption (152).
A low glycemic index (GI) diet, typical of those containing whole grains, has been shown to have a higher satiating power than a high GI one, regardless of confounding factors like consistency, flavor or percentage of fiber. A low GI diet is characterized by slow digestion and absorption, thus stimulating gastrointestinal receptors that induce satiety (153).
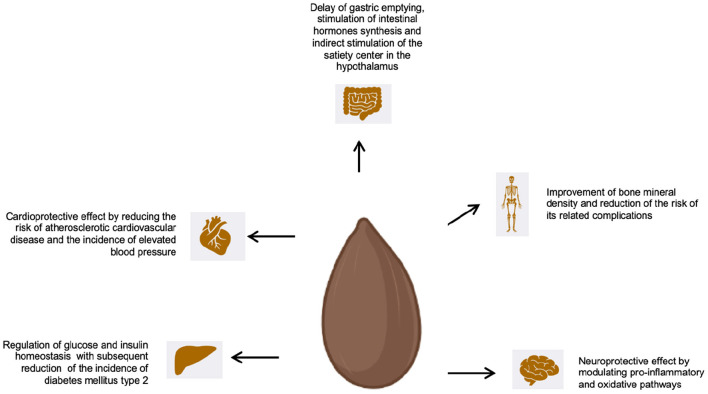
Among the various whole grain components, dietary fibers play a pivotal role in this setting. When ingested, dietary fibers bind water and form a thick agglomerate that can delay gastric emptying and slow down the bowel movements, leading to a reduced glucose absorption. Moreover, dietary fiber can reduce the synthesis of insulin by the pancreas and reduce the risk of hypoglycaemia in the post-absorption setting, thus inducing early satiety and fatty acid catabolism with reduced fat accumulation (154).
Dietary fibers stimulate the synthesis of intestinal hormones, which exert an effect upon satiety and glucose metabolism. For example, cholecystokinin, which is produced in the small intestine, helps in regulating the release of pancreatic hormones and in controlling gastric distention, but it also exerts an effect at the level of the satiety regulation center in the hypothalamus. Finally, the incretins (GIP and GLP-1), which are also produced at the level of the small bowel, stimulate post-prandial insulin synthesis and glucose homeostasis (155).
Moreover, when processed by the gut microbiota, dietary fibers are broken down into short chain fatty acids (SCFAs), which help in controlling body weight by decreasing gastric emptying rate and increasing the satiating effect. In particular, propionic and acetic acid have been found to reduce non-esterified fatty acids plasma concentration, involved in peripheral and hepatic insulin resistance. In addition, propionate may act on glucose and insulin homeostasis and may stimulate GLP-1 release, as suggested in an intervention trial in which a dinner based on non-digestible carbohydrates has been shown to improve glucose response of the subsequent breakfast (156).
Finally, whole grains have been reported to be rich in prebiotics, which can influence gut microbiota composition thus exerting a positive effect over the host body weight control. The gut microbiota has recently emerged among the regulators of body weight. It has been reported that the microbiota of obese mice and human subjects is composed by less Bacteroidetes and more Firmicutes compared to non-obese subjects. The different composition of the bacterial flora may explain the different rate of absorption of ingested foods, thus explaining the role of dysbiosis in increased body weight (157, 158). However, several lines of evidence have shown that microbiota composition could modulate also systemic inflammation, which is an important factor in glycemic resistance, and thermogenesis, which is a contributor of body mass regulation (159).
Whole Grains and Cancer
Cancer is caused by a complex interaction between environment and genetic background (160). However, it has been estimated that up to 40% of cancers could be avoided by a healthier lifestyle, including diet (161). Although diet should be evaluated as the whole foods that are assumed by a person (i.e., the specific diet pattern), several lines of evidence highlight a protective role of WG on cancers, particularly on colorectal cancer (CC).
For example, the umbrella review of Tieri et al. (3) has shown a convincing risk-reducing effect of WG on CC incidence. This data is aligned with several lines of evidence that support a protective role of WG on CC [e.g., data from 2018 World Cancer Research Fund's (WCRF)]. However, this work has also showed a putative risk-increasing effect of WG on prostate cancer. This observation is not supported by the evidence of WCRF. Possible explanations for this discrepancy might stem from the different definitions of WG in the studies included in the analyzes or the increased prostate cancer screening (i.e., PSA testing) in the population consuming high levels of WG that could be composed by more health-conscious men (3).
In the pivotal work of Reynold et al., it has been shown that a diet high in WG and/or fibers has a significant lowering impact on CC incidence and whole cancer mortality (162). In particular, the protective effect on CC and mortality in terms of relative risk is 0.84 (IC 95% 0.78–0.89) and 0.87 (IC 95% 0.79–0.95) for high fiber diet and 0.87 (IC 95% 0.79–0.96) and 0.84 (IC 95% 0.76–0.92) for high WG consumption. Although this study represents solid evidence to recommend regular consumption of grains in their whole form, it is not able to show how they exert their protective role on cancer incidence/mortality. In particular, it seems that a large part of the positive health effect of WG could be linked to their high fiber contents (162, 163), although a protective role of other components (e.g., phytochemicals, antioxidants, minerals) could not be excluded.
A regular consumption of fibers, estimated to be at least 25–29 g per day (162), seems to protect against cancer with at least three different mechanisms. First, since fibers are chemical substances that cannot be metabolized by the gastrointestinal tract, their presence could accelerate the feces' transit time, reducing the exposition of carcinogens to colonocytes (164–166). Second, fibers can be digested by gut microbiota (i.e., lactic-acid-producing bacteria) which can produce lactic acid and short-chain fatty acids (SFAs) (e.g., butyrate, acetate, propionate) as a consequence of their metabolism (167). It has been shown that butyrate can be used directly by colonocytes as a growth factor and as a nutrient (167). However, it might also exert some epigenetic effects, for example stimulating the acetylation of histones (168). It has been postulated that this epigenetic activity might exert a protective role on the neoplastic degeneration of colonocytes (168). Third, fibers may slow down food digestion and increase satiety thus favoring the loss of weight. Since several lines of evidence link visceral fat with insulin resistance, hyperinsulinemia, pro-inflammatory state, and cancer, fibers could indirectly reduce cancer pathogenesis acting of body composition (169–173).
Although the anticancer role of other nutrients present in WG is still debated, it appears plausible that some phytochemicals could be able to impair malignant cell transformations or progression. For example, phytates might chelate various metals, reducing the probability of oxidative damage of normal cells in presence of oxygen (174). A putative anticancer role has been hypothesized also for saponins (175) and some other phytochemicals (164).
Phytoestrogens are phytochemical compounds present in some cereals, and, in the past, it was postulated that they increase the risk of some cancers because of their pro-estrogenic effect (56). However, several lines of clinical evidence support the idea that these compounds can instead reduce the risk of hormonal cancers such as breast cancers (176). Although this observation could appear puzzling, the cancer protective effect might derive from their competing activity with endogenous estrogens and their preferential signaling through estrogen receptors beta, that have shown to impair cancer cells growth (177).
Comprehensively, several lines of evidence highlight that high WG intake reduces CC incidence and overall cancer mortality; fewer solid conclusions can be drawn for the other cancer types. Although the protective role of WG on cancers appears to be linked with their fibers content, the effect of other phytochemicals cannot be excluded.
Whole Grains and Cardiovascular Diseases
Healthy dietary regimens exert a protective role against a number of chronic diseases. In particular, whole-grains cereal products and their components, including cereals fibers and bran, have been consistently found to exert a cardioprotective effect (178–180).
Whole grain and cereals fiber consumption reduces the risk of atherosclerosis and coronary artery disease (CAD) progression. Indeed, a comprehensive analysis that evaluated the association of whole grains consumption and atherosclerotic cardiovascular diseases (CVD) showed that an increased consumption of whole grains significantly reduces the risk of occurrence of CVD (181, 182).
The correlation between whole grain intake and ischemic stroke is less clear. An increased consumption of whole grains has been found to reduce, in a non-significant manner, the risk of stroke when compared to a lower intake. Instead, significant data have been reported for individual whole grain components. In particular, cold breakfast cereals and bran consumption lowers the risk of occurrence of ischemic stroke, while regular popcorn consumption enhances this risk. However, since such a correlation was not reported for light and fat-free popcorn, it could be postulated that several compounds, including trans fat, butter and sodium, could be responsible for this effect (179).
Whole grains have demonstrated to have pleiotropic effects and the cardioprotective action related to whole grain consumption depends upon several mechanisms (183). Whole grains are composed by bran, germ and endosperm. When whole grains are processed to produce flour, the endosperm (full of carbohydrates) is retained, whereas bran and germ, along with their constituents (fiber, vitamins, minerals and anti-oxidants) are eliminated. The cardioprotective benefit obtained through the consumption of whole grains is particularly attributable to the effects upon the lipid metabolism: whole grains components, including soluble fiber and phytosterols, act upon numerous lipid intermediates, determining a less atherogenic lipid profile. Moreover, whole grains constituents, like phytoestrogens and anti-oxidants, exert a beneficial effect upon the vascular endothelium, thus reducing the risk of endothelial dysfunction, that is another major risk factor for atherosclerosis (178, 183). In the CARDIA study, whole grains were inversely associated with cell-adhesion molecules related to vascular dysfunction and CDV. In particular, adiponectin, which is a peptide released from adipocytes, exerts a cardioprotective activity and lower plasmatic concentrations of adiponectin are commonly observed in obese subjects as well as those with CVD and can induce HTN and endothelial malfunctioning (184, 185).
Whole grains, cereal fibers and bran intakes have also been proven to reduce the level of circulating inflammatory markers and to lower incidence of elevated blood pressure, which are both recognized cardiovascular risk factors (186, 187).
Among the various cardiovascular risk factor, the role of dysbiosis has recently emerged. Gut microbiota dysregulation induces bowel inflammation, with consequent increase in the permeability of the gut membrane and thereafter increased release of bacterial components and metabolites that can lead to an augmented risk for CVD. Dietary modifications increasing the daily consumption of whole grains can represent a feasible opportunity in this setting, aiming at restoring the integrity of the gut barrier and at improving the composition of the intestinal flora. Whole grains are in fact rich in prebiotics, which can positively modify the composition of the intestinal flora with beneficial effects upon the host (188, 189).
Whole Grains and Diabetes Mellitus Type 2
Following a regular, balanced diet and practicing moderate physical activity in a regular manner are crucial for both the prevention and management of type 2 diabetes mellitus (T2DM).
Whole grains consumption not only allows to prevent T2DM occurrence, but it also helps in controlling T2DM-related factors, such as overweight and obesity, as it has been reported that a diet rich in whole grains determines a better control of the BMI (121, 179–183).
However, conflicting data exist upon this topic. In fact, a meta-analysis of 26 randomized clinical trials (RCTs) reported a non-significant impact upon body weight control in subjects with an elevated BMI when following a diet rich in whole grains, even though whole grain consumption can actually reduce the body fat percentage, when compared to a diet rich in refined grains (190, 191). If individual constituents are considered, instead, a diet rich in rye helps in controlling and, in particular, in lowering the body weight, differently from refined wheat-based diet (192).
Different pathways can be activated by whole grains and their components (especially dietary fiber), determining an effect upon the glycemic control. Dietary fiber, in particular, increases gastric distention and delays intestinal transit time, thus determining early satiety and leading to increased production of molecules active upon the energetic and glycemic balance. Furthermore, the lower energy density of whole grains reduces the energy intake, which leads to decreased body fat and improved insulin sensitivity. Finally, dietary fiber delays nutrient absorption at the intestinal level, reducing insulin demand and stimulating fat absorption, ultimately reducing the fat storage (191).
Whole grains constituents exert anti-oxidant properties and reduce the synthesis of pro-inflammatory molecules, thereby improving insulin sensitivity and preventing the onset and evolution of T2DM (193).
Whole grains are also rich in vitamins and minerals, which help in improving the glycemic control by both regulating insulin-mediated hepatic glucose uptake (vitamin B complex and magnesium) and regulating the oxidative and inflammatory pathways (vitamin E and zinc). T2D comes with a progressive reduction of intracellular zinc levels and increased urinary excretion: zinc supplementation has been proven to exert anti-oxidant properties and lower the synthesis of inflammatory molecules, such as TNF-alpha and IL-1beta (194–197).
In patients with a diagnosis of diabetes mellitus (DM), glycemic control helps in lowering the risk of T2DM-related micro-/macro- vascular complications. An increased intake of fiber (especially soluble fiber) has been associated with a better glycemic control in diabetic patients. With respect to individual constituents, glycated hemoglobin and fasting plasma glucose have been found to be significantly lower in diabetic patients following a diet including regular oat intake, if compared to dietary regimens rich in other cereals. Moreover, oatmeal intake allows for a better glycemic control, with lower post-prandial glucose and improved insulin activity, when compared with a control meal (191, 198–200).
Whole Grains and Neurodegenerative Diseases
The activation of oxidative and pro-inflammatory pathways, as well as mitochondrial dysfunction, can alter several physiological mechanisms, ultimately leading to different kinds of neurodegenerative disorders. Novel approaches in regulating this mechanisms could be helpful in treating and preventing the onset of these diseases (201, 202).
Whole grains are rich in polyphenols, which are involved in the regulation of several pathways, including the oxidative and inflammatory ones, and they are also involved in the modulation of the host immune response, as suggested by both observational end experimental studies (203–205). Moreover, polyphenols inhibit mitochondrial dysfunction by activating pro-survival molecules, such as Bcl-2 and PERK and by releasing anti-oxidant enzymes, including catalase and superoxide dismutase (206, 207). Polyphenols can help in improving cognitive functioning by blocking the action of acetylcholinesterase and butyrylcholinesterase, thus inducing metal chelation, autophagy regulation and prion elimination (208).
Preclinical data from mice suggest that resveratrol might prevent neuronal loss. SRT501, an oral formulation of resveratrol, stimulates mitochondrial function by activating SIRT1, an NAD+-dependent deacetylase, in autoimmune encephalomyelitis, an animal model of multiple sclerosis (209). Resveratrol is also associated with a neuroprotective effect in 6-hydroxydopamine (6-OHDA)-induced Parkinson's disease rat model, as evidenced by the reduction in DNA condensation and vacuolization of dopaminergic neurons in the substancia nigra. In addition, a lower expression of COX-2 and TNF-α has been shown (210).
Therefore, due to all these beneficial effects, whole grains and their constituents, especially polyphenols, may exert a beneficial, neuroprotective effect, thus representing a feasible therapeutic opportunity in this setting (211).
Whole Grains and Autoimmune Diseases
As already reported, whole grain consumption can regulate the organism's inflammatory state. Gluten is a proteic complex that can be found in different types of cereals and it is composed by glutenins and gliadins. The gliadins present in wheat gluten can induce celiac disease (CD) in genetically susceptible individuals: it is a chronic inflammatory disorder characterized by inflammation of the intestinal mucosa with increased permeability, small intestinal villous atrophy and consequent malabsorption. A similar histopathological pattern has been observed in other autoimmune diseases and in healthy people (212, 213).
The exposure of mononuclear cells to gliadins determines the expression of inflammatory cytokines in both CD and non-CD patients (214). Enterocytes, in response to the interaction between gliadins and CXCR3 (a chemokine receptor), release zonulin, which can alter the integrity of the plasma membrane. It has been reported that gliadin intake can lead to the release of zonulin in both CD and non-CD patients, in both cases leading to an increased intestinal permeability, with an increased amount of microbial and dietary elements that can reach the periphery and interact with the immune system (215). Actually, this increased intestinal permeability has been postulated to be associated with multiple forms of autoimmune diseases in genetically predisposed individuals (216–218).
Besides gliadin, the lectin wheat germ agglutinin (WGA) plays a synergistic role. In rat small bowel, WGA stimulates monocytes and macrophages to produce pro-inflammatory cytokines, after binding to N-glycolylneuraminic acid, a glycocalyx sialic acid; this, subsequently, affects intestinal permeability (219, 220). Human data are lacking, although high concentrations of antibody to WGA have been found in CD-patients (221).
Thereafter, diets rich in cereals seem to exert a detrimental effect upon the insurgence of autoimmune disease, especially CD.
However, a better understanding of the effects of whole grains in this setting is needed and, in particular, it could be interesting to evaluate what could be the consequences of eliminating the cereals from the diet in terms of inflammatory response and intestinal permeability, not only in healthy individuals, but also in those presenting with autoimmune disorders (222).
Whole Grains and Other Chronic Diseases
An increased consumption of whole grains and their constituents, like dietary fiber, can determine a positive outcome upon several gastrointestinal disorders.
In particular, dietary fiber, with its intrinsic characteristics, such as viscosity, solubility and fermentability, can regulate the glycemic and lipidic absorption at the bowel level, it can control the bowel movements and determine an alteration of the intestinal flora (166).
There is evidence supporting the role of dietary fiber in controlling and ameliorating IBS symptoms (mostly in forms of the disease which present with constipation), by regulating the texture and the frequency of the stool output (223, 224).
In IBD, dietary fiber consumption is associated with the production of short-chain fatty acids (especially butyrate), which improve the bowel inflammatory status by up- and down-regulating the anti- and pro-inflammatory cytokines, respectively.
Finally, dietary fiber intake lowers the incidence of diverticular disease by rendering the stools more bulky, attenuating the pressure at the level of the large intestine membrane and thus reducing micro-herniation (166).
Patients with chronic kidney disease (CDK) on hemodialysis are recommended not to include whole grains in their diet, given the high amount of phosphorus contained in these foods. In CKD, phosphorus elimination is impaired, determining secondary hyperparathyroidism and inducing calcium deposits within the blood vessel wall, with consequent higher frequency of occurrence of CVD and negative impact upon survival (225).
As said, whole grains constituents determine a protective effect against a number of different pathologies, such as obesity, insulin resistance, T2DM, CVD and cancer (179, 180). These protective effects can be observed in both subjects with CVD, T2DM and elevated BMI and in healthy individuals; furthermore, it has been postulated that these beneficial effects are present also in patients with kidney disease, especially the ones presenting T2DM, CVD and elevated BMI as comorbidities (225). Whole grain consumption in CKD patients could be therefore reconsidered, but further research is needed to better understand their role in this setting.
Low bone mineral density (BMD) depends upon different non-modifiable and modifiable risk factors, including lifestyle factors. Consequently, it has been postulated that the occurrence of serious complications of low BMD, including fractures, can be avoided through lifestyle changes. The role of diet in ameliorating bone health is well established and dietary regimens including whole grains have been found to determine positive effect upon BMD (226, 227).
Cereals are often part of many people's breakfasts. They are usually considered a healthy food due to the misleading messages that praise their properties, not infrequently found in commercial packages. However, it is important to pay attention to the composition of ingredients which can vary between the different products on the market. In particular, it is necessary to favor those richer in fibers and with low carbohydrate content. In addition, it is important to emphasize the theoretical detrimental effect of phytates in wheat bran that may interfere with absorption of calcium from other foods consumed at the same time (228). For example, having breakfast with wheat bran and milk concomitantly might limit the absorption of calcium with a virtual reduction of the preventive effects in terms of bone health. It should be noted that the same interference with calcium absorption seems attenuated in wheat bran present in bread or other foods, where the cereal and therefore the phytates are present in lower concentrations.
Conclusion
Whole grains represent a pivotal food for a healthy diet (Table 4; Figure 2). Besides their carbohydrates content which makes whole grains a main energetic food, whole grains are also an important source of protein, fibers, phytocompounds, minerals, and vitamins. In particular, it should not underestimated their role as source of fibers, since a regular consumption of fibers has shown to be associated with a lower risk of several chronic diseases. Moreover, the recent scientific progress on microbiota field has allowed to appreciate the crosstalk between fibers and commensal flora to impinge on metabolic disfunction and immune dysregulation. Although the complex interplay between fibers/phytocompounds and microbiota has yet to be elucidated, it might represent the crucial pathway behind the healthy effect of whole grains. Moreover, it could be postulated that the identification of microbiota's produced compounds after ingestion of WG could be used to synthetize new drugs or the new generation of supplements.
Table 4.
Potential effects of whole grains on diseases.
| Disease | Potential effects of cereals | References |
|---|---|---|
| Cancer | Reduced colon cancer incidence and cancer mortality | (162) |
| Atherosclerotic cardiovascular disease | Whole grains reduce the risk of atherosclerotic CVDs | (181, 182) |
| Stroke | Breakfast cereal and bran lower the risk of ischemic stroke; regular popcorn increases the risk of ischemic stroke | (229) |
| Elevated blood pressure | Whole grains lower the incidence of elevated BP | (187) |
| Type 2 diabetes mellitus | Whole grains lower the risk of T2DM and related risk factors | (191, 230) |
| Irritable bowel syndrome | Dietary fiber attenuates IBS symptoms | (223, 224) |
| Inflammatory bowel disease | Dietary fiber reduces intestinal inflammation | (166) |
| Diverticular disease | Dietary fiber reduces the risk of diverticular disease | (166) |
| Chronic kidney disease | Whole grains can exert a beneficial effect in CDK | (225) |
| Low bone mineral density | Whole grains improve BMD and reduce the risk of its related complications | (226, 227) |
| Alzheimer's disease | Acetylcholinesterase inhibition by polyphenols, improving synaptic transmission, amyloid β toxicity reduction | (231) |
| Multiple sclerosis | Neural loss prevention through SIRT1 activation by polyphenols | (232) |
| Parkinson's disease | Antioxidant and antiapoptotic effect by polyphenols | (211) |
| Huntington's disease | Genetic and immunological modulation by poliphenols | (233) |
| Celiac disease | Intestinal mucosal inflammation and increased permeability by gliadin and prolamins | (215) |
Figure 2.
Effect of WG on health and diseases.
In addition, it should be underlined the importance of limiting refined grains since they are a poorer source of fibers, phytocompounds, minerals, and vitamins in comparison to their whole counterpart. As stated by the Dietary American guidelines, whole grains should be consumed on a daily basis and at least the half of the quantity should be in the whole form. Substituting refined cereals with their integral counterparts should be facilitated by educational campaigns.
Comprehensively, while the whole grains positive effects on health are clear, in the future it will be crucial to understand the underlying biological mechanisms that govern their activities.
Author Contributions
MG: conceptualization. MG, GN, RM, LC, and FT: writing and editing. MG and FP: supervision. All authors: methodology and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health (Ricerca Corrente) and by contribution 5 × 1,000 per la Ricerca Sanitaria and by Fondazione Costa—Ivrea (TO).
Conflict of Interest
MG reports advisory board from Novartis, Eli Lilly, Pierre-Fabre, all out-side the submitted work. FP reports receipt of grants/research supports from Astrazeneca, Eisai, Roche, and receipt of honoraria or consultation fees from Amgen, Astrazeneca, Daichii Sankyo, Celgene, Eisai, Eli Lilly, Gilead, GSK, Ipsen, MSD, Novartis, Pierre-Fabre, Pfizer, Roche, Seagen, Takeda, Viatris. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- CAD
coronary artery disease
- CC
colorectal cancer
- CD
celiac disease
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- DM
diabetes mellitus
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- IP6
myo-inositol hexaphosphate
- RCT
randomized clinical trial
- SCFAs
short-chain fatty acids
- T2DM
type 2 diabetes mellitus
- WCRF
World Cancer Research Fund's
- WG
whole grains.
References
- 1.FAO . FAO Cereal Supply and Demand Brief. World Food Situation. Food and Agriculture Organization of the United Nations. Available online at: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed November 14, 2021).
- 2.Zhu Y, Sang S. Phytochemicals in whole grain wheat and their health-promoting effects. Mol Nutr Food Res. (2017) 61:10347–10352. 10.1002/MNFR.201600852 [DOI] [PubMed] [Google Scholar]
- 3.Tieri M, Ghelfi F, Vitale M, Vetrani C, Marventano S, Lafranconi A, et al. Whole grain consumption and human health: an umbrella review of observational studies. Int J Food Sci Nutr. (2020) 71:668–77. 10.1080/09637486.2020.1715354 [DOI] [PubMed] [Google Scholar]
- 4.Willett WC, Liu S. Carbohydrate quality and health: distilling simple truths from complexity. Am J Clin Nutr. (2019) 110:803–4. 10.1093/AJCN/NQZ215 [DOI] [PubMed] [Google Scholar]
- 5.Lang R, Jebb SA. Who consumes whole grains, and how much? Proc Nutr Soc. (2003) 62:123–7. 10.1079/PNS2002219 [DOI] [PubMed] [Google Scholar]
- 6.Zhu F. Anthocyanins in cereals: composition and health effects. Food Res Int. (2018) 109:232–49. 10.1016/J.FOODRES.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 7.Ed Nignpense B, Francis N, Blanchard C, Santhakumar AB. Bioaccessibility and bioactivity of cereal polyphenols: a review. Foods. (2021) 10:1595. 10.3390/FOODS10071595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smeriglio A, Barreca D, Bellocco E, Trombetta D. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol. (2017) 174:1244–62. 10.1111/BPH.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popova A, Mihaylova D. Antinutrients in plant-based foods: a review. Open Biotechnol J. (2019) 13:68–76. 10.2174/1874070701913010068 [DOI] [Google Scholar]
- 10.Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. (2015) 52:676. 10.1007/S13197-013-0978-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serpen A, Gokmen V, Gokmen G, Karagoz A, Karagoz K, Koksel H, Koksel K. Phytochemical quantification and total antioxidant capacities of emmer (Triticum dicoccon Schrank) and einkorn (Triticum monococcum L.) wheat landraces. J Agric Food Chem. (2008) 56:7285–92. 10.1021/JF8010855 [DOI] [PubMed] [Google Scholar]
- 12.Benincasa P, Tosti G, Farneselli M, Maranghi S, Bravi E, Marconi O, et al. Phenolic content and antioxidant activity of einkorn and emmer sprouts and wheatgrass obtained under different radiation wavelengths. Ann Agric Sci. (2020) 65:68–76. 10.1016/J.AOAS.2020.02.001 [DOI] [Google Scholar]
- 13.Hidalgo A, Brandolini A. Nutritional properties of einkorn wheat (Triticum monococcum L.). J Sci Food Agric. (2014) 94:601–12. 10.1002/JSFA.6382 [DOI] [PubMed] [Google Scholar]
- 14.Barikmo I, Ouattara F, Oshaug A. Protein, carbohydrate and fibre in cereals from Mali—how to fit the results in a food compostion table and database. J Food Compos Anal. (2004) 17:291–300. 10.1016/J.JFCA.2004.02.008 [DOI] [Google Scholar]
- 15.Koreissi-Dembélé Y, Fanou-Fogny N, Hulshof PJM, Brouwer ID. Fonio (Digitaria exilis) landraces in Mali: nutrient and phytate content, genetic diversity and effect of processing. J Food Compos Anal. (2013) 29:134–43. 10.1016/J.JFCA.2012.07.010 [DOI] [Google Scholar]
- 16.Bach Knudsen KE, Nørskov NP, Bolvig AK, Hedemann MS, Lærke HN. Dietary fibers and associated phytochemicals in cereals. Mol Nutr Food Res. (2017) 61:1–15. 10.1002/mnfr.201600518 [DOI] [PubMed] [Google Scholar]
- 17.Samtiya M, Aluko RE, Dhewa T. Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod Process Nutr. (2020) 2:1–14. 10.1186/S43014-020-0020-5 [DOI] [Google Scholar]
- 18.Petroski W, Minich DM. Is there such a thing as “anti-nutrients”? A narrative review of perceived problematic plant compounds. Nutrients. (2020) 12:1–32. 10.3390/NU12102929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boncompagni E, Orozco-Arroyo G, Cominelli E, Gangashetty PI, Grando S, Tenutse Kwaku Zu T, et al. Antinutritional factors in pearl millet grains: phytate and goitrogens content variability and molecular characterization of genes involved in their pathways. PLoS ONE. (2018) 13:198394. 10.1371/JOURNAL.PONE.0198394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakur M, Singh K, Khedkar R. Phytochemicals: extraction process, safety assessment, toxicological evaluations, and regulatory issues. Funct Preserv Prop Phytochem. (2020) 2020:341–61. 10.1016/B978-0-12-818593-3.00011-7 [DOI] [Google Scholar]
- 21.Osbourn AE. Saponins in cereals. Phytochemistry. (2003) 62:1–4. 10.1016/S0031-9422(02)00393-X [DOI] [PubMed] [Google Scholar]
- 22.Hosseinian FS, Mazza G. Triticale bran and straw: potential new sources of phenolic acids, proanthocyanidins, and lignans. J Funct Foods. (2009) 1:57–64. 10.1016/J.JFF.2008.09.009 [DOI] [Google Scholar]
- 23.Demissew A, Temesgen K, Meresa A. Evaluation of anti-nutritional factor reduction techniques for triticale improved utilization system in Amhara Region. J Food Process Technol. (2017) 8:681. 10.4172/2157-7110.1000681 [DOI] [Google Scholar]
- 24.Stevenson L, Phillips F, O'sullivan K, Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int J Food Sci Nutr. (2012) 63:1001–13. 10.3109/09637486.2012.687366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Gonzalez AA, Grases F, Marí B, Tomás-Salvá M, Rodriguez A. Urinary phytate concentration and risk of fracture determined by the FRAX index in a group of postmenopausal women. Turkish J Med Sci. (2019) 49:458–63. 10.3906/SAG-1806-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartłomiej S, Justyna RK, Ewa N. Bioactive compounds in cereal grains—occurrence, structure, technological significance and nutritional benefits—a review. Food Sci Technol Int. (2012) 18:559–68. 10.1177/1082013211433079 [DOI] [PubMed] [Google Scholar]
- 27.Nirmala Prasadi VP, Joye IJ. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients. (2020) 12:1–20. 10.3390/nu12103045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acosta-Estrada BA, Gutiérrez-Uribe JA, Serna-Saldivar SO. Minor constituents and phytochemicals of the Kernel. In: Corn: Chemistry and Technology, 3rd Edition (Arnold, ML: AACC International Press; ), p. 369–403. [Google Scholar]
- 29.Garg M, Sharma A, Vats S, Tiwari V, Kumari A, Mishra V, et al. Vitamins in cereals: a critical review of content, health effects, processing losses, bioaccessibility, fortification, and biofortification strategies for their improvement. Front Nutr. (2021) 8:254. 10.3389/fnut.2021.586815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marles RJ. Mineral nutrient composition of vegetables, fruits and grains: the context of reports of apparent historical declines. J Food Compos Anal. (2017) 56:93–103. 10.1016/J.JFCA.2016.11.012 [DOI] [Google Scholar]
- 31.Vitale M, Hanhineva K, Koistinen V, Auriola S, Paananen J, Costabile G, et al. Putative metabolites involved in the beneficial effects of wholegrain cereal: nontargeted metabolite profiling approach. Nutr Metab Cardiovasc Dis. (2021) 31:1156–65. 10.1016/J.NUMECD.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 32.Kaur C, Kapoor HC. Antioxidants in fruits and vegetables—the millennium's health. Int J Food Sci Technol. (2001) 36:703–25. 10.1111/J.1365-2621.2001.00513.X [DOI] [Google Scholar]
- 33.Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. (2005) 45:287–306. 10.1080/1040869059096 [DOI] [PubMed] [Google Scholar]
- 34.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. (2002) 113:71–88. 10.1016/S0002-9343(01)00995-0 [DOI] [PubMed] [Google Scholar]
- 35.Rao S, Santhakumar AB, Chinkwo KA, Vanniasinkam T, Luo J, Blanchard CL. Chemopreventive potential of cereal polyphenols. Nutr Cancer. (2018) 70:913–27. 10.1080/01635581.2018.1491609 [DOI] [PubMed] [Google Scholar]
- 36.Packer L, Weber SU, Rimbach G. Molecular aspects of α-tocotrienol antioxidant action and cell signalling. J Nutr. (2001) 131:369S−373S. 10.1093/JN/131.2.369S [DOI] [PubMed] [Google Scholar]
- 37.De Mejía EG, Prisecaru VI. Lectins as bioactive plant proteins: a potential in cancer treatment. Crit Rev Food Sci Nutr. (2005) 45:425–45. 10.1080/10408390591034445 [DOI] [PubMed] [Google Scholar]
- 38.Alatorre-Cruz JM, Pita-López W, López-Reyes RG, Ferriz-Martínez RA, Cervantes-Jiménez R, de Jesús Guerrero Carrillo M, et al. Effects of intragastrically-administered Tepary bean lectins on digestive and immune organs: preclinical evaluation. Toxicol Rep. (2017) 5:56–64. 10.1016/J.TOXREP.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramadass B, Dokladny K, Moseley PL, Patel YR, Lin HC. Sucrose co-administration reduces the toxic effect of lectin on gut permeability and intestinal bacterial colonization. Dig Dis Sci. (2010) 55:2778–84. 10.1007/S10620-010-1359-2 [DOI] [PubMed] [Google Scholar]
- 40.Gong T, Wang X, Yang Y, Yan Y, Yu C, Zhou R, et al. Plant lectins activate the nlrp3 inflammasome to promote inflammatory disorders. J Immunol. (2017) 198:2082–92. 10.4049/JIMMUNOL.1600145 [DOI] [PubMed] [Google Scholar]
- 41.Bhutia SK, Panda PK, Sinha N, Praharaj PP, Bhol CS, Panigrahi DP, et al. Plant lectins in cancer therapeutics: targeting apoptosis and autophagy-dependent cell death. Pharmacol Res. (2019) 144:8–18. 10.1016/J.PHRS.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 42.Apfelthaler C, Skoll K, Ciola R, Gabor F, Wirth M. A doxorubicin loaded colloidal delivery system for the intravesical therapy of non-muscle invasive bladder cancer using wheat germ agglutinin as targeter. Eur J Pharm Biopharm. (2018) 130:177–84. 10.1016/J.EJPB.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 43.Savage GP, Vanhanen L, Mason SM, Ross AB. Effect of cooking on the soluble and insoluble oxalate content of some New Zealand foods. J Food Compos Anal. (2000) 13:201–6. 10.1006/JFCA.2000.087917135028 [DOI] [Google Scholar]
- 44.Buades Fuster JM, Sanchís Cortés P, Perelló Bestard J, Grases Freixedas F. Plant phosphates, phytate and pathological calcifications in chronic kidney disease. Nefrologia. (2017) 37:20–8. 10.1016/J.NEFRO.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 45.Gibson RS, Raboy V, King JC. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr Rev. (2018) 76:793–804. 10.1093/NUTRIT/NUY028 [DOI] [PubMed] [Google Scholar]
- 46.Silva EO, Bracarense APFRL. Phytic acid: from antinutritional to multiple protection factor of organic systems. J Food Sci. (2016) 81:R1357–62. 10.1111/1750-3841.13320 [DOI] [PubMed] [Google Scholar]
- 47.Vucenik I, Shamsuddin AM. Protection against cancer by dietary IP6 and inositol. Nutr Cancer. (2006) 55:109–25. 10.1207/S15327914NC5502_1 [DOI] [PubMed] [Google Scholar]
- 48.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Arch Intern Med. (2004) 164:885–91. 10.1001/ARCHINTE.164.8.885 [DOI] [PubMed] [Google Scholar]
- 49.Sanchis P, López-González ÁA, Costa-Bauzá A, Busquets-Cortés C, Riutord P, Calvo P, Grases F. Understanding the protective effect of phytate in bone decalcification related-diseases. Nutrients. (2021) 13:2859. 10.3390/NU13082859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grases F, Perelló J, Sanchis P, Isern B, Prieto RM, Costa-Bauzá A, et al. Anticalculus effect of a triclosan mouthwash containing phytate: a double-blind, randomized, three-period crossover trial. J Periodontal Res. (2009) 44:616–21. 10.1111/J.1600-0765.2008.01168.X [DOI] [PubMed] [Google Scholar]
- 51.Grases F, Sanchis P, Perello J, Isern B, Prieto RM, Fernandez-Palomeque C, et al. Phytate reduces age-related cardiovascular calcification. Front Biosci. (2008) 13:7115–22. 10.2741/3214 [DOI] [PubMed] [Google Scholar]
- 52.Fernández-Palomeque C, Grau A, Perelló J, Sanchis P, Isern B, et al. Relationship between urinary level of phytate and valvular calcification in an elderly population: a cross-sectional study. PLoS ONE. (2015) 10:136560. 10.1371/JOURNAL.PONE.0136560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. (2001) 56:5–51. 10.1016/S0031-9422(00)00316-2 [DOI] [PubMed] [Google Scholar]
- 54.Tawfiq N, Heaney RK, Plumb JA, Fenwick GR, Musk SRR, Williamson G. Dietary glucosinolates as blocking agents against carcinogenesis: glucosinolate breakdown products assessed by induction of quinone reductase activity in murine hepa1c1c7 cells. Carcinogenesis. (1995) 16:1191–4. 10.1093/CARCIN/16.5.1191 [DOI] [PubMed] [Google Scholar]
- 55.Willemin ME, Lumen A. Thiocyanate: a review and evaluation of the kinetics and the modes of action for thyroid hormone perturbations. Crit Rev Toxicol. (2017) 47:537–63. 10.1080/10408444.2017.1281590 [DOI] [PubMed] [Google Scholar]
- 56.Mense SM, Hei TK, Ganju RK, Bhat HK. Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environ Health Perspect. (2008) 116:426–33. 10.1289/EHP.10538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dixon RA. Phytoestrogens. Annu Rev Plant Biol. (2004) 55:225–61. 10.1146/ANNUREV.ARPLANT.55.031903.141729 [DOI] [PubMed] [Google Scholar]
- 58.Casini ML, Marelli G, Papaleo E, Ferrari A, D'Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. (2006) 85:972–8. 10.1016/J.FERTNSTERT.2005.09.048 [DOI] [PubMed] [Google Scholar]
- 59.D'Anna R, Cannata ML, Atteritano M, Cancellieri F, Corrado F, Baviera G, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 1-year randomized, double-blind, placebo-controlled study. Menopause. (2007) 14:648–55. 10.1097/01.GME.0000248708.60698.98 [DOI] [PubMed] [Google Scholar]
- 60.Louis XL, Raj P, Chan L, Zieroth S, Netticadan T, Wigle JT. Are the cardioprotective effects of the phytoestrogen resveratrol sex-dependent? Can J Physiol Pharmacol. (2019) 97:503–14. 10.1139/CJPP-2018-0544 [DOI] [PubMed] [Google Scholar]
- 61.Zaw JJT, Howe PRC, Wong RHX. Does phytoestrogen supplementation improve cognition in humans? A systematic review. Ann N Y Acad Sci. (2017) 1403:150–63. 10.1111/NYAS.13459 [DOI] [PubMed] [Google Scholar]
- 62.Soni M, Rahardjo TBW, Soekardi R, Sulistyowati Y, Lestariningsih, Yesufu-Udechuku A, et al. Phytoestrogens and cognitive function: a review. Maturitas. (2014) 77:209–20. 10.1016/J.MATURITAS.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 63.Patisaul HB. Endocrine disruption by dietary phyto-oestrogens: impact on dimorphic sexual systems and behaviours. Proc Nutr Soc. (2017) 76:130–44. 10.1017/S0029665116000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torrens-Mas M, Roca P. Phytoestrogens for cancer prevention and treatment. Biology. (2020) 9:1–19. 10.3390/biology9120427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pizzi A. Tannins: prospectives and actual industrial applications. Biomolecules. (2019) 9:344. 10.3390/BIOM9080344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delimont NM, Haub MD, Lindshield BL. The impact of tannin consumption on iron bioavailability and status: a narrative review. Curr Dev Nutr. (2017) 1:1–12. 10.3945/CDN.116.000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goszcz K, Duthie GG, Stewart D, Leslie SJ, Megson IL. Bioactive polyphenols and cardiovascular disease: chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br J Pharmacol. (2017) 174:1209–25. 10.1111/BPH.13708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodges RE, Minich DM. Modulation of metabolic detoxification pathways using foods and food-derived components: a scientific review with clinical application. J Nutr Metab. (2015) 2015:760689. 10.1155/2015/760689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Del Bo C, Bernardi S, Marino M, Porrini M, Tucci M, Guglielmetti S, et al. Systematic review on polyphenol intake and health outcomes: is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients. (2019) 11:1355. 10.3390/NU11061355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petry N, Egli I, Zeder C, Walczyk T, Hurrell R. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J Nutr. (2010) 140:1977–82. 10.3945/JN.110.125369 [DOI] [PubMed] [Google Scholar]
- 71.Fleck JD, Betti AH, Pereira da Silva F, Troian EA, Olivaro C, Ferreira F, Verza SG. Saponins from Quillaja saponaria and Quillaja brasiliensis: particular chemical characteristics and biological activities. Molecules. (2019) 24:171. 10.3390/MOLECULES24010171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhutkar MA, Bhise SB. In vitro assay of alpha amylase inhibitory activity of some indigenous plants. Int J Chem Sci. (2012) 10:457–62.35057448 [Google Scholar]
- 73.Li L, Tsao R. UF-LC-DAD-MSn for discovering enzyme inhibitors for nutraceuticals and functional foods. J Food Bioact. (2019) 7:27–35. 10.31665/JFB.2019.7195 [DOI] [Google Scholar]
- 74.Petroni K, Pilu R, Tonelli C. Anthocyanins in corn: a wealth of genes for human health. Planta. (2014) 240:901–11. 10.1007/S00425-014-2131-1 [DOI] [PubMed] [Google Scholar]
- 75.Shen Y, Zhang H, Cheng L, Wang L, Qian H, Qi X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. (2016) 194:1003–12. 10.1016/J.FOODCHEM.2015.08.083 [DOI] [PubMed] [Google Scholar]
- 76.Hao J, Zhu H, Zhang Z, Yang S, Li H. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J Cereal Sci. (2015) 64:92–9. 10.1016/J.JCS.2015.05.003 [DOI] [Google Scholar]
- 77.Yao SL, Xu Y, Zhang YY, Lu YH. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. (2013) 4:1602–8. 10.1039/C3FO60196J [DOI] [PubMed] [Google Scholar]
- 78.Um MY, Ahn J, Ha TY. Hypolipidaemic effects of cyanidin 3-glucoside rich extract from black rice through regulating hepatic lipogenic enzyme activities. J Sci Food Agric. (2013) 93:3126–8. 10.1002/JSFA.6070 [DOI] [PubMed] [Google Scholar]
- 79.Lemlioglu-Austin D, Turner ND, McDonough CM, Rooney LW. Effects of sorghum [Sorghum bicolor (L.) moench] crude extracts on starch digestibility, estimated glycemic index (EGI), and resistant starch (RS) contents of porridges. Molecules. (2012) 17:11124–38. 10.3390/MOLECULES170911124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thummayot S, Tocharus C, Pinkaew D, Viwatpinyo K, Sringarm K, Tocharus J. Neuroprotective effect of purple rice extract and its constituent against amyloid beta-induced neuronal cell death in SK-N-SH cells. Neurotoxicology. (2014) 45:149–58. 10.1016/J.NEURO.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 81.Suganyadevi P, Saravanakumar KM, Mohandas S. The antiproliferative activity of 3-deoxyanthocyanins extracted from red sorghum (Sorghum bicolor) bran through P53-dependent and Bcl-2 gene expression in breast cancer cell line. Life Sci. (2013) 92:379–82. 10.1016/J.LFS.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 82.Fan MJ, Yeh PH, Lin JP, Huang AC, Lien JC, Lin HY, et al. Anthocyanins from black rice (Oryza sativa) promote immune responses in leukemia through enhancing phagocytosis of macrophages in vivo. Exp Ther Med. (2017) 14:59–64. 10.3892/ETM.2017.4467/HTML [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee C, Han D, Kim B, Baek N, Baik BK. Antioxidant and anti-hypertensive activity of anthocyanin-rich extracts from hulless pigmented barley cultivars. Int J Food Sci Technol. (2013) 48:984–91. 10.1111/IJFS.12050 [DOI] [Google Scholar]
- 84.Tanaka J, Nakanishi T, Ogawa K, Tsuruma K, Shimazawa M, Shimoda H, et al. Purple rice extract and anthocyanidins of the constituents protect against light-induced retinal damage in vitro and in vivo. J Agric Food Chem. (2011) 59:528–36. 10.1021/JF103186A [DOI] [PubMed] [Google Scholar]
- 85.Kim HW, Lee AY, Yeo SK, Chung H, Lee JH, Hoang MH, et al. Metabolic profiling and biological mechanisms of body fat reduction in mice fed the ethanolic extract of black-colored rice. Food Res Int. (2013) 53:373–90. 10.1016/J.FOODRES.2013.05.001 [DOI] [Google Scholar]
- 86.Jiang X, Guo H, Shen T, Tang X, Yang Y, Ling W. Cyanidin-3-O-β-glucoside purified from black rice protects mice against hepatic fibrosis induced by carbon tetrachloride via inhibiting hepatic stellate cell activation. J Agric Food Chem. (2015) 63:6229–30. 10.1021/ACS.JAFC.5B02181 [DOI] [PubMed] [Google Scholar]
- 87.Guzmán-Gerónimo RI, Alarcón-Zavaleta TM, Oliart-Ros RM, Meza-Alvarado JE, Herrera-Meza S, Chávez-Servia JL. Blue maize extract improves blood pressure, lipid profiles, and adipose tissue in high-sucrose diet-induced metabolic syndrome in rats. J Med Food. (2017) 20:110–5. 10.1089/JMF.2016.0087 [DOI] [PubMed] [Google Scholar]
- 88.Lu X, Zhou Y, Wu T, Hao L. Ameliorative effect of black rice anthocyanin on senescent mice induced by D-galactose. Food Funct. (2014) 5:2892–7. 10.1039/C4FO00391H [DOI] [PubMed] [Google Scholar]
- 89.Mousa WK, Chehadeh F, Husband S. Recent advances in understanding the structure and function of the human microbiome. Front Microbiol. (2022) 13:111. 10.3389/fmicb.2022.825338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jayachandran M, Chung SSM, Xu B. A critical review of the relationship between dietary components, the gut microbe Akkermansia muciniphila, and human health. Crit Rev Food Sci Nutr. (2020) 60:2265–76. 10.1080/10408398.2019.1632789 [DOI] [PubMed] [Google Scholar]
- 91.Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. (2022) 23:1105. 10.3390/ijms23031105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Myhrstad MCW, Tunsjø H, Charnock C, Telle-Hansen VH. Dietary fiber, gut microbiota, and metabolic regulation—current status in human randomized trials. Nutrients. (2020) 12:859. 10.3390/nu12030859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mousavi Khaneghah A, Ismail E, Raeisi S, Fakhri Y. Aflatoxins in cereals: state of the art. J Food Saf. (2018) 38:e12532. 10.1111/jfs.12532 [DOI] [Google Scholar]
- 94.Mousa WK, Schwan AL, Raizada MN. Characterization of antifungal natural products isolated from endophytic fungi of finger millet (Eleusine coracana). Molecules. (2016) 21:1171. 10.3390/molecules21091171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castro-Alba V, Lazarte CE, Bergenståhl B, Granfeldt Y. Phytate, iron, zinc, and calcium content of common Bolivian foods and their estimated mineral bioavailability. Food Sci Nutr. (2019) 7:2854. 10.1002/FSN3.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mishra A, Behura A, Mawatwal S, Kumar A, Naik L, Mohanty SS, et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem Toxicol. (2019) 134:110827. 10.1016/J.FCT.2019.110827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He S, Simpson BK, Sun H, Ngadi MO, Ma Y, Huang T. Phaseolus vulgaris lectins: a systematic review of characteristics and health implications. Crit Rev Food Sci Nutr. (2018) 58:70–83. 10.1080/10408398.2015.1096234 [DOI] [PubMed] [Google Scholar]
- 98.Lam SK, Ng TB. Lectins: production and practical applications. Appl Microbiol Biotechnol. (2011) 89:45–55. 10.1007/S00253-010-2892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carvalho EVMM, Oliveira WF, Coelho LCBB, Correia MTS. Lectins as mitosis stimulating factors: briefly reviewed. Life Sci. (2018) 207:152–7. 10.1016/J.LFS.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 100.Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. (2010) 9:425. 10.1007/S11101-010-9183-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karamać M. Chelation of Cu(II), Zn(II), and Fe(II) by tannin constituents of selected edible nuts. Int J Mol Sci. (2009) 10:5485. 10.3390/IJMS10125485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bajaj JK, Salwan P, Salwan S. Various possible toxicants involved in thyroid dysfunction: a review. J Clin Diagn Res. (2016) 10:FE01. 10.7860/JCDR/2016/15195.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siener R, Hönow R, Voss S, Seidler A, Hesse A. Oxalate content of cereals and cereal products. J Agric Food Chem. (2006) 54:3008–11. 10.1021/JF052776V [DOI] [PubMed] [Google Scholar]
- 104.Rietjens IMCM, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharmacol. (2017) 174:1263–80. 10.1111/BPH.13622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. (2006) 54:184–201. 10.1207/S15327914NC5402_5 [DOI] [PubMed] [Google Scholar]
- 106.Crinnion WJ. Organic foods contain higher levels of certain nutrients, lower levels of pesticides, and may provide health benefits for the consumer. Altern Med Rev. (2010) 15:4–12. [PubMed] [Google Scholar]
- 107.FAO . Cereal Supply and Demand Brief. Geneva: Food and Agriculture Organization of the United Nations; (2022). [Google Scholar]
- 108.Slavin JL, Jacobs D, Marquart L. Grain processing and nutrition. Crit Rev Food Sci Nutr. (2000) 40:309–26. 10.1080/10408690091189176 [DOI] [PubMed] [Google Scholar]
- 109.Mahgoub SEO, Elhag SA. Effect of milling, soaking, malting, heat treatment and fermentation on phytate level of four Sudanese sorghum cultivars. Food Chem. (1998) 61:77–80. 10.1016/S0308-8146(97)00109-X [DOI] [Google Scholar]
- 110.Yang F, Basu TK, Ooraikul B. Studies on germination conditions and antioxidant contents of wheat grain. Int J Food Sci Nutr. (2001) 52:319–30. 10.1080/09637480120057567 [DOI] [PubMed] [Google Scholar]
- 111.Shreeja K, Devi SS, Suneetha WJ, Prabhakar BN. Effect of Germination on nutritional composition of common buckwheat (Fagopyrum esculentum Moench). Int Res J Pure Appl Chem. (2021) 2021:1–7. 10.9734/IRJPAC/2021/V22I130350 [DOI] [Google Scholar]
- 112.Zhang G, Xu Z, Gao Y, Huang X, Zou Y, Yang T. Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat. J Food Sci. (2015) 80:H1111–9. 10.1111/1750-3841.12830 [DOI] [PubMed] [Google Scholar]
- 113.Žilić S, Delić N, Basić Z, Ignjatović-Micić D, Janković M, Vančetović J. Effects of alkaline cooking and sprouting on bioactive compounds, their bioavailability and relation to antioxidant capacity of maize flour. J Food Nutr Res. (2015) 54:155–64. [Google Scholar]
- 114.Ongol MP, Niyonzima E, Gisanura I, Vasanthakaalam H. Effect of germination and fermentation on nutrients in maize flour. Pakistan J Food Sci. (2013) 23:183–8. [Google Scholar]
- 115.Mbithi-Mwikya S, Van Camp J, Yiru Y, Huyghebaert A. Nutrient and antinutrient changes in finger millet (Eleusine coracan) during sprouting. LWT Food Sci Technol. (2000) 33:9–14. 10.1006/fstl.1999.0605 [DOI] [Google Scholar]
- 116.Ogbonna AC, Abuajah CI Ide EO, Udofia US. Effect of malting conditions on the nutritional and anti-nutritional factors of sorghum grist. Ann Univ Dunarea Jos Galati, Fascicle VI Food Technol. (2012) 36:64–72. [Google Scholar]
- 117.Ojha P, Adhikari R, Karki R, Mishra A, Subedi U, Karki TB. Malting and fermentation effects on antinutritional components and functional characteristics of sorghum flour. Food Sci Nutr. (2017) 6:47–53. 10.1002/FSN3.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Onyango CA, Ochanda SO, Mwasaru MA, Ochieng JK, Mathooko FM, Kinyuru JN. Effects of malting and fermentation on anti-nutrient reduction and protein digestibility of red sorghum, white sorghum and pearl millet. J Food Res. (2013) 2:41. 10.5539/jfr.v2n1p41 [DOI] [Google Scholar]
- 119.Traoré T, Mouquet C, Icard-Vernière C, Traoré AS, Trèche S. Changes in nutrient composition, phytate and cyanide contents and α-amylase activity during cereal malting in small production units in Ouagadougou (Burkina faso). Food Chem. (2004) 88:105–14. 10.1016/j.foodchem.2004.01.032 [DOI] [Google Scholar]
- 120.Tsafrakidou P, Michaelidou AM, Biliaderis CG. Fermented cereal-based products: nutritional aspects, possible impact on gut microbiota and health implications. Foods. (2020) 9:734. 10.3390/FOODS9060734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Osman MA. Effect of traditional fermentation process on the nutrient and antinutrient contents of pearl millet during preparation of Lohoh. J Saudi Soc Agric Sci. (2011) 10:1–6. 10.1016/j.jssas.2010.06.001 [DOI] [Google Scholar]
- 122.Torre M, Rodriguez AR, Saura-Calixto F. Effects of dietary fiber and phytic acid on mineral availability. Crit Rev Food Sci Nutr. (1991) 30:1–22. 10.1080/10408399109527539 [DOI] [PubMed] [Google Scholar]
- 123.Lopez Y, Gordon DT, Fields ML. Release of phosphorus from phytate by natural lactic acid fermentation. J Food Sci. (1983) 48:953–4. 10.1111/j.1365-2621.1983.tb14938.x [DOI] [Google Scholar]
- 124.Sripriya G, Antony U, Chandra TS. Changes in carbohydrate, free amino acids, organic acids, phytate and HCl extractability of minerals during germination and fermentation of finger millet (Eleusine coracana). Food Chem. (1997) 58:345–50. 10.1016/S0308-8146(96)00206-3 [DOI] [Google Scholar]
- 125.Ihediohanma NC. Determination of the glycemic indices of three different cassava granules (garri) and the effect of fermentation period on their glycemic responses. Pakistan J Nutr. (2011) 10:6–9. 10.3923/pjn.2011.6.9 [DOI] [Google Scholar]
- 126.Mlotha V, Mwangwela AM, Kasapila W, Siyame EWP, Masamba K. Glycemic responses to maize flour stiff porridges prepared using local recipes in Malawi. Food Sci Nutr. (2016) 4:322–8. 10.1002/fsn3.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scazzina F, Del Rio D, Pellegrini N, Brighenti F. Sourdough bread: starch digestibility and postprandial glycemic response. J Cereal Sci. (2009) 49:419–21. 10.1016/j.jcs.2008.12.00826838096 [DOI] [Google Scholar]
- 128.Ayhan DK, Koksel H. Investigation of the effect of different storage conditions on vitamin content of enriched pasta product. Qual Assur Saf Crop Foods. (2019) 11:701–12. 10.3920/QAS2019.1575 [DOI] [Google Scholar]
- 129.Otemuyiwa IO, Falade OS, Adewusi SRA. Effect of various cooking methods on the proximate composition and nutrient contents of different rice varieties grown in Nigeria. Int Food Res J. (2018) 25:747–54. [Google Scholar]
- 130.Silveira CMM, Moreira AVB, Martino HSD, Gomide RS, Pinheiro SS, Della Lucia CM, Pinheiro-Sant'ana HM. Effect of cooking methods on the stability of thiamin and folic acid in fortified rice. Int J Food Sci Nutr. (2017) 68:179–87. 10.1080/09637486.2016.1226273 [DOI] [PubMed] [Google Scholar]
- 131.Kyritsi A, Tzia C, Karathanos VT. Vitamin fortified rice grain using spraying and soaking methods. LWT Food Sci Technol. (2011) 44:312–20. 10.1016/j.lwt.2010.06.001 [DOI] [Google Scholar]
- 132.Shao Y, Fang C, Zhang H, Shi Y, Hu Z, Zhu Z. Variation of phenolics, tocols, antioxidant activities, and soluble sugar compositions in red and black rice (Oryza sativa L.) during boiling. Cereal Chem. (2017) 94:811–9. 10.1094/CCHEM-04-17-0076-R [DOI] [Google Scholar]
- 133.Geng DH, Zhou S, Wang L, Zhou X, Liu L, Lin Z, et al. Effects of slight milling combined with cellulase enzymatic treatment on the textural and nutritional properties of brown rice noodles. LWT. (2020) 128:109520. 10.1016/j.lwt.2020.109520 [DOI] [Google Scholar]
- 134.Adedayo BC, Adebayo AA, Nwanna EE, Oboh G. Effect of cooking on glycemic index, antioxidant activities, α-amylase, and α-glucosidase inhibitory properties of two rice varieties. Food Sci Nutr. (2018) 6:2301–7. 10.1002/fsn3.806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang S, Ji J, Zhang S, Guan C, Wang G. Effects of three cooking methods on content changes and absorption efficiencies of carotenoids in maize. In: Food and Function. London: The Royal Society of Chemistry, 944–54. [DOI] [PubMed] [Google Scholar]
- 136.Sowa M, Yu J, Palacios-Rojas N, Goltz SR, Howe JA, Davis CR, et al. Retention of carotenoids in biofortified maize flour and β-cryptoxanthin-enhanced eggs after household cooking. ACS Omega. (2017) 2:7320–8. 10.1021/acsomega.7b01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prasanthi PS, Naveena N, Vishnuvardhana Rao M, Bhaskarachary K. Compositional variability of nutrients and phytochemicals in corn after processing. J Food Sci Technol. (2017) 54:1080–90. 10.1007/s13197-017-2547-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pillay K, Siwela M, Derera J, Veldman FJ. Provitamin A carotenoids in biofortified maize and their retention during processing and preparation of South African maize foods. J Food Sci Technol. (2014) 51:634–44. 10.1007/s13197-011-0559-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lozano-Alejo N, Carrillo GV, Pixley K, Palacios-Rojas N. Physical properties and carotenoid content of maize kernels and its nixtamalized snacks. Innov Food Sci Emerg Technol. (2007) 8:385–9. 10.1016/j.ifset.2007.03.015 [DOI] [Google Scholar]
- 140.Pretorius B, Schönfeldt HC. Vitamin A content of fortified maize meal and porridge as purchased and consumed in South Africa. Food Res Int. (2012) 47:128–33. 10.1016/j.foodres.2011.03.033 [DOI] [Google Scholar]
- 141.Kesse-Guyot E, Lairon D, Allès B, Seconda L, Rebouillat P, Brunin J, et al. Key findings of the French bionutrinet project on organic food–based diets: description, determinants, and relationships to health and the environment. Adv Nutr. (2021) 13:208–24. 10.1093/advances/nmab105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reganold JP, Wachter JM. Organic agriculture in the twenty-first century. Nat Plants. (2016) 2:15221. 10.1038/nplants.2015.221 [DOI] [PubMed] [Google Scholar]
- 143.Bengtsson J, Ahnström J, Weibull AC. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J Appl Ecol. (2005) 42:261–9. 10.1111/j.1365-2664.2005.01005.x28488295 [DOI] [Google Scholar]
- 144.Seufert V, Ramankutty N, Foley JA. Comparing the yields of organic and conventional agriculture. Nature. (2012) 485:229–32. 10.1038/nature11069 [DOI] [PubMed] [Google Scholar]
- 145.Ponisio LC, M'gonigle LK, Mace KC, Palomino J, Valpine P De, Kremen C. Diversification practices reduce organic to conventional yield gap. Proc R Soc B Biol Sci. (2015) 282:1396. 10.1098/rspb.2014.1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barbieri P, Pellerin S, Seufert V, Smith L, Ramankutty N, Nesme T. Global option space for organic agriculture is delimited by nitrogen availability. Nat Food. (2021) 2:363–72. 10.1038/s43016-021-00276-y [DOI] [PubMed] [Google Scholar]
- 147.Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. (2019) 393:447–92. 10.1016/S0140-6736(18)31788-4 [DOI] [PubMed] [Google Scholar]
- 148.Mousa WK, Raizada MN. Natural disease control in cereal grains. In: Encyclopedia of Food Grains: Second Edition (New York: Academic Press; ), 257–263. [Google Scholar]
- 149.Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs DR, Spiegelman D, et al. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am J Clin Nutr. (2004) 80:1237–45. 10.1093/ajcn/80.5.1237 [DOI] [PubMed] [Google Scholar]
- 150.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. (2003) 78:920–7. 10.1093/ajcn/78.5.920 [DOI] [PubMed] [Google Scholar]
- 151.McKeown NM, Yoshida M, Shea MK, Jacques PF, Lichtenstein AH, Rogers G, et al. Whole-grain intake and cereal fiber are associated with lower abdominal adiposity in older adults. J Nutr. (2009) 139:1950–5. 10.3945/jn.108.103762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Giacco R, Della Pepa G, Luongo D, Riccardi G. Whole grain intake in relation to body weight: from epidemiological evidence to clinical trials. Nutr Metab Cardiovasc Dis. (2011) 21:901–8. 10.1016/j.numecd.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 153.Bornet FRJ, Jardy-Gennetier AE, Jacquet N, Stowell J. Glycaemic response to foods: Impact on satiety and long-term weight regulation. Appetite. (2007) 49:535–53. 10.1016/j.appet.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 154.Weickert MO, Pfeiffer AFH. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. (2008) 138:439–42. 10.1093/JN/138.3.439 [DOI] [PubMed] [Google Scholar]
- 155.Van Dijk G, Thiele TE. Glucagon-like peptide-1 (7-36) amide: a central regulator of satiety and interoceptive stress. Neuropeptides. (1999) 33:406–14. 10.1054/NPEP.1999.0053 [DOI] [PubMed] [Google Scholar]
- 156.Reimer RA, Mcburney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology. (1996) 137:3948–56. 10.1210/ENDO.137.9.8756571 [DOI] [PubMed] [Google Scholar]
- 157.DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. (2008) 83:460–9. 10.4065/83.4.460 [DOI] [PubMed] [Google Scholar]
- 158.Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C, et al. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr. (2008) 99:110–20. 10.1017/S0007114507793923 [DOI] [PubMed] [Google Scholar]
- 159.Lundgren P, Thaiss CA. The microbiome-adipose tissue axis in systemic metabolism. Am J Physiol Gastrointest Liver Physiol. (2020) 318:G717–24. 10.1152/ajpgi.00304.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Blackadar CB. Historical review of the causes of cancer. World J Clin Oncol. (2016) 7:54. 10.5306/WJCO.V7.I1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brennan P, Smith GD. Identifying novel causes of cancers to enhance cancer prevention: new strategies are needed. J Natl Cancer Inst. (2021) 114:353–60. 10.1093/JNCI/DJAB204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. (2019) 393:434–45. 10.1016/S0140-6736(18)31809-9 [DOI] [PubMed] [Google Scholar]
- 163.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum NN, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. (2017) 46:1029–56. 10.1093/IJE/DYW319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miller PE, Snyder DC. Phytochemicals and cancer risk: a review of the epidemiological evidence. Nutr Clin Pract. (2012) 27:599–612. 10.1177/0884533612456043 [DOI] [PubMed] [Google Scholar]
- 165.Bultman SJ. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol Nutr Food Res. (2017) 61:1500902. 10.1002/MNFR.201500902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gill SK, Rossi M, Bajka B, Whelan K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. (2021) 18:101–16. 10.1038/S41575-020-00375-4 [DOI] [PubMed] [Google Scholar]
- 167.Gong L, Cao W, Chi H, Wang J, Zhang H, Liu J, et al. Whole cereal grains and potential health effects: involvement of the gut microbiota. Food Res Int. (2018) 103:84–102. 10.1016/J.FOODRES.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 168.Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, et al. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. (2009) 682:39–53. 10.1016/J.MRREV.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 169.Tahergorabi Z, Khazaei M, Moodi M, Chamani E. From obesity to cancer: a review on proposed mechanisms. Cell Biochem Funct. (2016) 34:533–45. 10.1002/CBF.3229 [DOI] [PubMed] [Google Scholar]
- 170.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. (2012) 85:1–10. 10.1259/BJR/38447238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hervik AK, Svihus B. The role of fiber in energy balance. J Nutr Metab. (2019) 2019:4983657. 10.1155/2019/4983657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol. (2014) 6:41. 10.4251/WJGO.V6.I2.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McRae MP. The benefits of dietary fiber intake on reducing the risk of cancer: an umbrella review of meta-analyses. J Chiropr Med. (2018) 17:90–6. 10.1016/J.JCM.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Graf E, Eaton JW. Suppression of colonic cancer by dietary phytic acid. Nutr Cancer. (1993) 19:11–19. 10.1080/01635589309514232 [DOI] [PubMed] [Google Scholar]
- 175.Koczurkiewicz P, Czyz J, Podolak I, Wójcik K, Galanty A, Janeczko Z, et al. Multidirectional effects of triterpene saponins on cancer cells—mini-review of in vitro studies. Acta Biochim Pol. (2015) 62:383–93. 10.18388/ABP.2015_1089 [DOI] [PubMed] [Google Scholar]
- 176.AICR WCRF . Diet, Nutrition, Physical Activity and Breast Cancer. London: WCRF; (2018). [Google Scholar]
- 177.Basu P, Maier C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed Pharmacother. (2018) 107:1648–66. 10.1016/j.biopha.2018.08.100 [DOI] [PubMed] [Google Scholar]
- 178.Barrett EM, Batterham MJ, Ray S, Beck EJ. Whole grain, bran and cereal fibre consumption and CVD: a systematic review. Br J Nutr. (2019) 121:914–37. 10.1017/S000711451900031X [DOI] [PubMed] [Google Scholar]
- 179.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. (2016) 353:1–14. 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. (2010) 23:65–134. 10.1017/S0954422410000041 [DOI] [PubMed] [Google Scholar]
- 181.Erkkilä AT, Herrington DM, Mozaffarian D, Lichtenstein AH. Cereal fiber and whole-grain intake are associated with reduced progression of coronary-artery atherosclerosis in postmenopausal women with coronary artery disease. Am Heart J. (2005) 150:94–101. 10.1016/j.ahj.2004.08.013 [DOI] [PubMed] [Google Scholar]
- 182.Anderson JW. Whole grains protect against atherosclerotic cardiovascular disease. Proc Nutr Soc. (2003) 62:135–42. 10.1079/pns2002222 [DOI] [PubMed] [Google Scholar]
- 183.Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. (2008) 18:283–90. 10.1016/j.numecd.2006.12.008 [DOI] [PubMed] [Google Scholar]
- 184.Kirwan JP, Malin SK, Scelsi AR, Kullman EL, Navaneethan SD, Pagadala MR, et al. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. J Nutr. (2016) 146:2244–51. 10.3945/jn.116.230508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sijtsma FPC, Meyer KA, Steffen LM, Van Horn L, Shikany JM, Odegaard AO, et al. Diet quality and markers of endothelial function: the CARDIA study. Nutr Metab Cardiovasc Dis. (2014) 24:632–8. 10.1016/j.numecd.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Qi L, Van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. (2006) 29:207–11. 10.2337/diacare.29.02.06.dc05-1903 [DOI] [PubMed] [Google Scholar]
- 187.Steffen LM, Kroenke CH Yu X, Pereira MA, Slattery ML, Van Horn L, Gross MD, et al. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. (2005) 82:1169–77. 10.1093/ajcn/82.6.1169 [DOI] [PubMed] [Google Scholar]
- 188.Ma J, Li H. The role of gut microbiota in atherosclerosis and hypertension. Front Pharmacol. (2018) 9:1–14. 10.3389/fphar.2018.01082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Novakovic M, Rout A, Kingsley T, Kirchoff R, Singh A, Verma V, et al. Role of gut microbiota in cardiovascular diseases. World J Cardiol. (2020) 12:110–22. 10.4330/wjc.v12.i4.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. (2013) 98:872–84. 10.3945/ajcn.113.064659 [DOI] [PubMed] [Google Scholar]
- 191.Della Pepa G, Vetrani C, Vitale M, Riccardi G. Wholegrain intake and risk of type 2 diabetes: evidence from epidemiological and intervention studies. Nutrients. (2018) 10:1288. 10.3390/nu10091288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Suhr J, Vuholm S, Iversen KN, Landberg R, Kristensen M. Wholegrain rye, but not wholegrain wheat, lowers body weight and fat mass compared with refined wheat: a 6-week randomized study. Eur J Clin Nutr. (2017) 71:959–67. 10.1038/ejcn.2017.12 [DOI] [PubMed] [Google Scholar]
- 193.Singh A, Sharma S. Bioactive components and functional properties of biologically activated cereal grains: a bibliographic review. Crit Rev Food Sci Nutr. (2017) 57:3051–71. 10.1080/10408398.2015.1085828 [DOI] [PubMed] [Google Scholar]
- 194.Via M. The Malnutrition of obesity: micronutrient deficiencies that promote diabetes. ISRN Endocrinol. (2012) 2012:1–8. 10.5402/2012/103472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Badawi A. Vitamins D, C, and E in the prevention of type 2 diabetes mellitus: modulation of inflammation and oxidative stress. Biol Targets Ther. (2011) 7:S14417. 10.2147/BTT.S14417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Barbagallo M. Magnesium and type 2 diabetes. World J Diabetes. (2015) 6:1152. 10.4239/wjd.v6.i10.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Heer M, Egert S. Nutrients other than carbohydrates: their effects on glucose homeostasis in humans. Diabetes Metab Res Rev. (2015) 31:14–35. 10.1002/dmrr.2533 [DOI] [PubMed] [Google Scholar]
- 198.Hou Q, Li Y, Li L, Cheng G, Sun X, Li S, et al. The metabolic effects of oats intake in patients with type 2 diabetes: a systematic review and meta-analysis. Nutrients. (2015) 7:10369–87. 10.3390/nu7125536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shen X, Zhao T, Zhou Y, Shi X, Zou Y, Zhao G. Effect of oat β-glucan intake on glycaemic control and insulin sensitivity of diabetic patients: a meta-analysis of randomized controlled trials. Nutrients. (2016) 8:39. 10.3390/nu8010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li X, Cai X, Ma X, Jing L, Gu J, Bao L, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. (2016) 8:549. 10.3390/nu8090549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hung CW, Chen YC, Hsieh WL, Chiou SH, Kao CL. Ageing and neurodegenerative diseases. Ageing Res Rev. (2010) 9 Suppl 1:S36. 10.1016/J.ARR.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 202.Jones QRD, Warford J, Rupasinghe HPV, Robertson GS. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol Sci. (2012) 33:602–10. 10.1016/J.TIPS.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 203.Basli A, Soulet S, Chaher N, Mérillon JM, Chibane M, Monti JP, Richard T. Wine polyphenols: potential agents in neuroprotection. Oxid Med Cell Longev. (2012) 2012:805762. 10.1155/2012/805762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Akhlaghi M, Bandy B. Preconditioning and acute effects of flavonoids in protecting cardiomyocytes from oxidative cell death. Oxid Med Cell Longev. (2012) 2012:782321. 10.1155/2012/782321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jung M, Triebel S, Anke T, Richling E, Erkel G. Influence of apple polyphenols on inflammatory gene expression. Mol Nutr Food Res. (2009) 53:1263–80. 10.1002/MNFR.200800575 [DOI] [PubMed] [Google Scholar]
- 206.Martín S, González-Burgos E, Carretero ME, Gómez-Serranillos MP. Neuroprotective properties of Spanish red wine and its isolated polyphenols on astrocytes. Food Chem. (2011) 128:40–8. 10.1016/J.FOODCHEM.2011.02.074 [DOI] [PubMed] [Google Scholar]
- 207.Nones J, De Sampaio e Spohr TCL, Gomes FCA. Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem Res. (2011) 36:1776–84. 10.1007/S11064-011-0493-3 [DOI] [PubMed] [Google Scholar]
- 208.Xiao J, Chen X, Zhang L, Talbot SG Li GC, Xu M. Investigation of the mechanism of enhanced effect of EGCG on huperzine A's inhibition of acetylcholinesterase activity in rats by a multispectroscopic method. J Agric Food Chem. (2008) 56:910–5. 10.1021/JF073036K [DOI] [PubMed] [Google Scholar]
- 209.Nimmagadda VK, Bever CT, Vattikunta NR, Talat S, Ahmad V, Nagalla NK, et al. Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 Targets. J Immunol. (2013) 190:4595–607. 10.4049/jimmunol.1202584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jin F, Wu Q, Lu YF, Gong QH, Shi JS. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's disease in rats. Eur J Pharmacol. (2008) 600:78–82. 10.1016/j.ejphar.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 211.Bhullar KS, Rupasinghe HPV. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid Med Cell Longev. (2013) 2013:891748. 10.1155/2013/891748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shewry PR. Wheat. J Exp Bot. (2009) 60:1537–53. 10.1093/JXB/ERP058 [DOI] [PubMed] [Google Scholar]
- 213.Neuhausen SL, Steele L, Ryan S, Mousavi M, Pinto M, Osann KE, et al. Co-occurrence of celiac disease and other autoimmune diseases in celiacs and their first-degree relatives. J Autoimmun. (2008) 31:160–5. 10.1016/J.JAUT.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Harris KM, Fasano A, Mann DL. Cutting edge: IL-1 controls the IL-23 response induced by gliadin, the etiologic agent in celiac disease. J Immunol. (2008) 181:4457–60. 10.4049/JIMMUNOL.181.7.4457 [DOI] [PubMed] [Google Scholar]
- 215.Drago S, El Asmar R, Di Pierro M, Clemente MG, Tripathi A, Sapone A, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. (2006) 41:408–19. 10.1080/00365520500235334 [DOI] [PubMed] [Google Scholar]
- 216.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. (2012) 42:71–8. 10.1007/S12016-011-8291-X [DOI] [PubMed] [Google Scholar]
- 217.Cordain L, Toohey L, Smith MJ, Hickey MS. Modulation of immune function by dietary lectins in rheumatoid arthritis. Br J Nutr. (2000) 83:207–17. 10.1017/S0007114500000271 [DOI] [PubMed] [Google Scholar]
- 218.Secondulfo M, Iafusco D, Carratù R, deMagistris L, Sapone A, Generoso M, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis. (2004) 36:35–45. 10.1016/J.DLD.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 219.Shaw L, Yousefi S, Dennis JW, Schauer R. CMP-N-acetylneuraminic acid hydroxylase activity determines the wheat germ agglutinin-binding phenotype in two mutants of the lymphoma cell line MDAY-D2. Glycoconj J. (1991) 8:434–41. 10.1007/BF00731295 [DOI] [PubMed] [Google Scholar]
- 220.Schumacher U, Von Armansperg NG, Kreipe H, Welsch U. Lectin binding and uptake in human (Myelo)monocytic cell lines: HEL60 and U937. Ultrastruct Pathol. (1996) 20:463–71. 10.3109/01913129609016350 [DOI] [PubMed] [Google Scholar]
- 221.Sollid LM, Kolberg J, Scott H, Ek J, Fausa O, Brandtzaeg P. Antibodies to wheat germ agglutinin in coeliac disease. Clin Exp Immunol. (1986) 63:95–100. [PMC free article] [PubMed] [Google Scholar]
- 222.de Punder K, Pruimboom L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients. (2013) 5:771–87. 10.3390/NU5030771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rao SSC Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. (2015) 41:1256–70. 10.1111/apt.13167 [DOI] [PubMed] [Google Scholar]
- 224.Nagarajan N, Morden A, Bischof D, King EA, Kosztowski M, Wick EC, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome. Eur J Gastroenterol Hepatol. (2015) 27:1002–10. 10.1097/MEG.0000000000000425 [DOI] [PubMed] [Google Scholar]
- 225.Williams C, Ronco C, Kotanko P. Whole grains in the renal diet - is it time to reevaluate their role? Blood Purif. (2013) 36:210–4. 10.1159/000356683 [DOI] [PubMed] [Google Scholar]
- 226.Denova-Gutiérrez E, Méndez-Sánchez L, Muñoz-Aguirre P, Tucker K, Clark P. Dietary patterns, bone mineral density, and risk of fractures: a systematic review and meta-analysis. Nutrients. (2018) 10:1922. 10.3390/nu10121922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shin S, Sung J, Joung H. A fruit, milk and whole grain dietary pattern is positively associated with bone mineral density in Korean healthy adults. Eur J Clin Nutr. (2015) 69:442–8. 10.1038/ejcn.2014.231 [DOI] [PubMed] [Google Scholar]
- 228.Shkembi B, Huppertz T. Calcium absorption from food products : food matrix effects. Nutrients. (2022) 14:180. 10.3390/nu14010180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Juan J, Liu G, Willett WC, Hu FB, Rexrode KM, Sun Q. Whole grain consumption and risk of ischemic stroke results from 2 prospective cohort studies. Stroke. (2017) 48:3203–9. 10.1161/STROKEAHA.117.018979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.De Munter JSL, Hu FB, Spiegelman D, Franz M, Van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. (2007) 4:1385–95. 10.1371/journal.pmed.0040261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Swardfager W, Lanctt K, Rothenburg L, Wong A, Cappell J, Herrmann N, et al. meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry. (2010) 68:930–41. 10.1016/J.BIOPSYCH.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 232.Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuro-Ophthalmol. (2010) 30:328. 10.1097/WNO.0B013E3181F7F833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Lawrence Marsh J, et al. activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. (2011) 20:261–70. 10.1093/HMG/DDQ460 [DOI] [PMC free article] [PubMed] [Google Scholar]