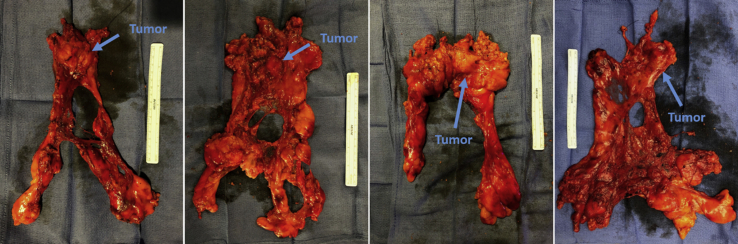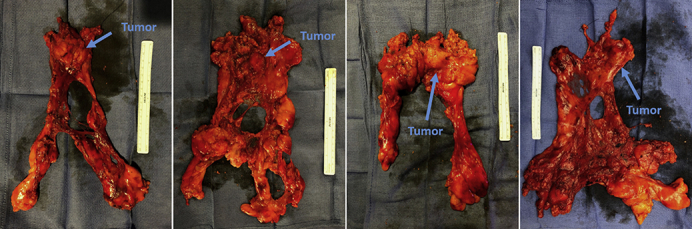
Representative specimens.
Central Message.
The robotic approach to thymectomy using bilateral simultaneous thoracoscopy provides excellent visualization to allow safe and complete dissection around critical structures.
Myasthenia gravis (MG) is a chronic autoimmune disorder caused by antibodies against the nicotinic acetylcholine receptors at the neuromuscular junction. This results in skeletal muscle weakness in the ocular, respiratory, bulbar, and limb distributions. Its prevalence is approximately 250 cases per 1 million people. It is medically treated primarily with acetylcholinesterase inhibitors and steroids. Immunosuppressive therapy, plasmapheresis, and/or intravenous immunoglobulin may also be indicated in advanced cases. MG may occur with or without the presence of thymoma and is a recognized paraneoplastic syndrome of thymoma affecting approximately one third of patients with thymoma. On the contrary, about 10% of patients with primary MG will have an associated thymoma, and its incidence increases with age.1 Primary surgery is indicated in most instances of early-stage thymoma and those cases associated with any type of MG.2 In the case of nonthymomatous MG, current evidence and guidelines have recently strengthened the indications for surgical over medical therapy alone in the majority of cases and recommend thymectomy for patients who have positive acetylcholine receptor antibodies, general type MG, short disease duration (<5 years), age younger than 65 years old, and/or symptoms not controlled with medication.1,3,4 Before surgery, patients should generally be well-recovered from acute exacerbations of MG or crisis, minimized on their steroid dose as feasible, and should be maintained on their cholinesterase inhibitors (pyridostigmine) up to the day of surgery. If indicated for advanced cases, we prefer plasmapheresis and/or immunoglobulin therapy to have been performed within one week of surgery when feasible.
Indication
Our patient is a 63-year-old man with a history of generalized MG (primarily with occulo-bulbar and limb-girdle symptoms) on prednisone and pyridostigmine, presenting with a well-circumscribed 3-cm anterior mediastinal mass suspicious for noninvasive thymoma.
Procedure
It must be understood that several viable alternatives to this procedure using different numbers and configurations of port sites and combinations of instrumentation and energy devices are described both anecdotally and formally in the literature by other groups and/or surgeons. The following descriptions and accompanying Video 1 simply represent our approach to robotic-assisted thymectomy, which has evolved over time (and likely will continue to evolve). It does not implicitly and/or overtly intend to invalidate or diminish the numerous described variations in the literature, which are beyond the scope of this article to fully enumerate.5 All patients featured within the accompanying Video 1 signed informed consent allowing the use of operative procedure videos for creation of anonymized academic and educational material.
We use the da Vinci Xi robotic platforms (Intuitive Surgical) with a 30° robotic thoracoscope. We also use an additional standard 5-mm 30° camera for simultaneous contralateral thoracoscopy during the operation using dedicated software on the robotic platform (TilePro; Intuitive Surgical).6
The operation can generally be approached from either the left or right chest. If present, the predominant laterality of the tumor often dictates the side of approach to ensure clear visualization of the ipsilateral phrenic nerve along the tumor and to address potential pulmonary involvement or adhesions. Our preferred default approach (central tumors or nonthymomatous MG) is from the left chest.7 This is due to the more variable course of the phrenic nerve in the left superior mediastinum and the greater propensity for close apposition of pericardial fat to the phrenic nerve at the costophrenic insertion, which may greatly obscure its course even under direct vision. Combined with the relatively less-variable position of the right phrenic nerve along the lateral aspect of the superior vena cava, and the greater propensity for separation of the nerve from the thymus/pericardial fat from the middle mediastinum to the costophrenic insertion, the course of the right phrenic nerve is more predictable and can be more confidently approached from the contralateral chest compared with the left. In contradistinction, in patients with significant mediastinal adiposity combined with left cardiac hypertrophy and/or a smaller thoracic cavity, visualization and working space may be more restricted, although increased surgeon's experience compensates for this to a great degree. Of note, it is critical to avoid disruption of the tumor and subsequent consequences of tumor spillage and pleural dissemination, and a strict minimal or “no-touch” technique with little direct manipulation of the tumor is advocated at all times.
The patient is placed under general anesthesia with a single lumen endotracheal tube and a bifurcated dual-sided bronchial blocker (Rusch EZ-Blocker; Teleflex) is placed to isolate the left lung. Alternatively, a standard double-lumen tube may be placed. While lung isolation is preferred for our procedures, intermittent periods of apnea combined with CO2 insufflation during right- or left-sided dissection is also a workable strategy. Although infrequent, hypotension may occur with CO2 insufflation, and communication with the anesthesia team regarding such episodes should be clearly maintained.
Step 1: Patient Positioning and Port Placement
The patient is placed in the right hemilateral position with the left chest up and left shoulder retracted/dropped slightly posterior such that the patient's forearm is parallel to the level of operating table, as depicted in Video 1. An 8-mm camera port is placed at the level of the fifth intercostal space in approximately the anterior axillary line (Arm 3). Of note, it is critical not to attempt placement of the inferior most ports first and before insufflation of the chest to avoid inadvertent direct cardiac and/or subdiaphragmatic visceral injury. If this must be placed first, a standard direct cut-down technique is highly recommended. Two additional 8-mm working ports are placed at approximately the level of the third intercostal space anterior axillary line (Arm 4) and sixth intercostal space mid clavicular line (Arm 2) for the “right” and “left” working hands. Care should be taken to place Arm 2 (“left” hand) relatively lateral on the chest wall to access the inferior most aspect of the ipsilateral anterior costophrenic fat. A robotic 12-mm port is placed for bedside assistance (using a 5- to 8-mm cap), and as a potential secondary robotic camera or instrument site (with a 12-mm cap and 8-mm robotic instrument reducer) if needed to optimize visualization and/or instrument collisions during portions of the operation (in particular for caudal views of the mediastinum. This port is placed slightly more caudal and between Arms 2 and 3 at approximately the seventh or eighth intercostal space. Insufflation with CO2 between 4 and 8 mm Hg is used to create significantly more working space in the thoracic cavity. A bipolar grasper (Fenestrated Bipolar Grasper; Intuitive Surgical) is used in Arm 2 and robotic ultrasonic dissector (Harmonic Scalpel, Ethicon) in Arm 4. Alternatively, a curved bipolar instrument (Maryland Bipolar Dissector; Intuitive Surgical) may also be used in Arm 4 if preferred.
Step 2: Mobilization of the Left Phrenic Nerve (LPN) and Bilateral Costophrenic/Diaphragmatic Dissection
The left phrenic nerve (LPN) is identified as it runs across the pericardium. Dissection of the mediastinal pleura is initiated just anterior to the LPN. This plane is opened from the diaphragmatic recess to the junction of the LPN, left internal mammary artery (LIMA), and innominate vein. All associated fatty and thymic tissues medial to the LPN are mobilized over the bilateral diaphragmatic surfaces, the majority of the pericardium, aortopulmonary window, and off the posterior table of the sternum just medial to the LIMA and right internal mammary artery (RIMA).
Step 3: Mobilization of the Left and Right Thymic Horns
Incision of the cervical pleura is started at from the junction of the LIMA and LPN. It is carried medially to the mid-sternal line or medial border of the RIMA (the contralateral pleura may be opened during this portion of the operation), and then extended toward the innominate vein and superior vena cava confluence on the right side. The innominate vein is identified just medial and cranial to the “junction” of the LPN and LIMA, and exposed. The thymic tissues are retracted caudally and into the contralateral chest to allow for better exposure of the thymic horns above the innominate vein. The investing fascia of the horn is incised and the fatty tissue of the superior poles retracted caudally and “drawn out” with gentle tension and dissection. The thyrothymic ligament is ligated to release the thymic horns from their superior attachments. Vasculature may be ligated with the harmonic scalpel, with or without clips (Small Clip Applier; Intuitive Surgical), per the surgeon's discretion.
Step 4: Innominate Vein Dissection and Identification of Thymic Veins
The dissection is then carried medially along the innominate vein, with care to clearly identify and expose thymic tributary veins from the innominate vein. These may be singular or multiple, and range from small to robust. While these may often be ligated and divided with the ultrasonic dissector alone, robotically applied clips may be preferred per surgeon preference (Small Metal Clip Applier or Medium Large Hemo-Lock Applier; Intuitive Surgical) and can be applied with precision directly by the console operator. Standard thoracoscopic clips may be manually applied by the assistant as well.
Step 5: Contralateral Simultaneous Thoracoscopy
To prepare for right-sided dissection, a 5-mm 30° thoracoscopic camera is introduced into the right chest under direct vision approximately at the level of the inframammary fold in the midclavicular line. Dedicated software on the robotic console allows for multiple video feeds into the surgeon's console, allowing for simultaneous bilateral thoracoscopy (TilePro; Intuitive Surgical).
Step 6: Identification and Mobilization of Right Phrenic Nerve (RPN)
Contralateral lung isolation is established after re-expansion of the left lung. Care must be taken to temporarily release CO2 insufflation and allow complete/unrestricted expansion of the left lung before isolating the right to avoid severe hypoxia, which can be sudden and dramatic. Alternatively, if visualization is relatively clear, intermittent apnea may suffice. The contralateral RPN is identified and visualized concurrently by the surgeon along with the view from the robotic camera. It is consistently found along the lateral aspect of the superior vena cava, although visualization can be challenging without attention to meticulous maintenance of hemostasis, or in patients with copious fat (potentially due to long term steroid treatment), or if the dissection undermines the nerve before establishing direct visualization. If not already performed, the right mediastinal pleura is opened just medial to the RIMA. Dissection is carried caudally to the level of the previously completed dissection along the pericardium and contralateral diaphragm. An avascular tissue plane overlying the right heart often clearly separates the pericardial and thymic fat from the more posterior and hilar course of the RPN, allowing for a greater margin of safe dissection. All fatty tissues medial to the RPN are thus mobilized under direct vision and removed en bloc with the specimen.
Step 7: Specimen Removal
A 12-mm or 15-mm endoscopic specimen bag is introduced into the chest cavity through the 12-mm assistant port, or directly through the incision if a 15 mm device is used. The mass is placed above the heart in the anterior mediastinum and “dropped” into the opened bag within the left chest. The instruments are removed and robotic platform undocked. Smaller tumors can often be removed with only modest expansion of the assistant port. Larger tumors may require moderate expansion and potential “shingling” of a small portion of rib to remove without disrupting integrity of the specimen. Regardless, extension of this incision beyond 4 to 5 cm is rarely needed. The specimen is palpated for gross margins, oriented with marking sutures, and sent for pathologic specimen. Frozen section to assess margins and/or diagnosis is performed at the discretion of the surgeon. Representative specimens are shown in Figure 1. A single small chest tube may be left in the ipsilateral chest or across the mediastinum. A small “pigtail” type catheter may also be placed and left through the right 5-mm port site per the surgeon's preference. Tubes are often removed the morning after surgery, and patients discharged on postoperative day 1or 2.
Figure 1.
Representative specimens of complete thymectomy with thymoma via robotic approach. Sutures mark the right and left thymic horns.
Discussion
Thymectomy is a standard part of the armamentarium to treat MG. Minimally invasive approaches have emerged to become more prevalent in the last decade as compared to the longtime standard of open sternotomy. Minimally invasive thymectomy constitutes a broad category of operations, including variations of transcervical, transthoracic and subxiphoid approaches, video-assisted thoracoscopic surgery (VATS) (unilateral and bilateral), and robotic technology. However, important principles of the operation have not changed, including preservation of both phrenic nerves and complete phrenic-to-phrenic exenteration of all potential thymic bearing and antibody producing tissues within the anterior mediastinum from the neck to the diaphragm. It is important to note that little to no high-level studies directly comparing different operative approaches, and/or definitively determining superiority of one approach over another, currently exists. With that said, several studies have demonstrated benefits of minimally invasive approaches including decreased blood loss and shorter hospitalization, with similar outcomes to that of open sternotomy.5,8 Studies have found comparable overall morbidity between robotic and VATS approaches, and with some suggesting improved complete remission rates of MG with robotic operations.9 Advantages of the robot approach including increased degrees of instrument articulation, finer instrument control, and improved 3-dimensional visualization, may help with the complete removal of all thymic tissues. However, even amongst robotic surgeons, there is significant debate over the optimal operative approach, including some debate regarding the necessity to perform formal bilateral VATS in all patients. In the case of unilateral robotic thymectomy, considerations may include location of the thymic lobes within the mediastinum, safety of trocar introduction, and overall visualization. Importantly, there are few data to support a clear advantage of one approach over another, and the surgeon should proceed at their discretion with whatever procedure is felt necessary to accomplish the goals of the operation within the safety and quality considerations outlined.5
As mentioned, proponents of a left-sided approach cite a decreased chance of nerve injury, given increased variation of the LPN. In addition, often there is little-to-no thymic tissue “beneath” (posterior to) the RPN, such that avoidance of injury is more likely even when visualization from the contralateral left side is difficult. Other arguments for a left-sided approach include the dissection of the aortopulmonary window, a common site for ectopic thymic tissue, as well as the potential superior ability to visualize and dissect out upper thymic poles between the innominate vein and aortic arch. Furthermore, often the bulk of the left thymic lobes is larger and the LPN more involved with the thymic tissues, so direct visualization is important, especially given the relative protuberance of the left sided heart.10,11 In the case of a right-sided approach, proponents argue that introduction of trocars is safer given absence of the cardiac apex, and that identification and visualization of the innominate and superior vena cava confluence is superior.12 While bilateral robotic and/or combined hybrid VATS/robotic approaches have also been advocated to directly visualize both phrenic nerves and pericardial fat basins from their respective ipsilateral sides, there may be significant barriers to adoption, given time needed for additional contralateral trocar placement, potential patient repositioning, possible redocking of the instrument arms (although this has simplified with subsequent generations of the robotic platforms), and increased patient pain.13
In our robotic-assisted experience using bilateral simultaneous thoracoscopy with a single 5-mm contralateral port, we believe the benefits of both left- and right-sided approaches are realized without several of the aforementioned disadvantages. In conclusion, the operative steps outlined in this report describe an innovative approach to robotic thymectomy that takes into consideration direct visualization of both phrenic nerves simultaneously and allows for safe and efficacious en bloc removal of all potentially thymic bearing tissues for patients with MG, with or without associated thymoma.
Acknowledgments
The authors acknowledge Mr Matthew Vercauteren PA-C for video editing assistance, and Ms Kathy Lovas for editorial assistance.
Footnotes
Disclosures: Dr Sarkaria has received compensation for education, speaking, and/or consulting from Intuitive Surgical, Auris Medical, AMSI, and Cambridge Medical Robotics. Dr Luketich has received honoraria for consulting and speaking from Intuitive Surgical. He is also a stockholder with Intuitive Surgical. Dr Su reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Video available at: https://www.jtcvs.org/article/S2666-2507(22)00180-8/fulltext.
References
- 1.Gilhus N.E. Myasthenia gravis. N Engl J Med. 2016;375:2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 2.Davenport E., Malthaner R.A. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg. 2008;86:673–684. doi: 10.1016/j.athoracsur.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe G.I., Kaminski H.J., Aban I.B., Minisman G., Kuo H.C., Marx A., et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375:511–522. doi: 10.1056/NEJMoa1602489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okusanya O.T., Hess N., Christie N., Luketich J.D., Sarkaria I.S. Improved outcomes with surgery vs. medical therapy in non-thymomatous myesthenia gravis: a perspective on the results of a randomized trial. Ann Transl Med. 2016;4:526. doi: 10.21037/atm.2016.12.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess N.R., Sarkaria I.S., Pennathur A., Levy R.M., Christie N.A., Luketich J.D. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg. 2016;5:1–9. doi: 10.3978/j.issn.2225-319X.2016.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess N.R., Baker N., Levy R.M., Pennathur A., Christie N.A., Luketich J.D., et al. Robotic assisted minimally invasive thymectomy with simultaneous bilateral thoracoscopy and contralateral phrenic nerve visualization. J Thorac Dis. 2020;12:114–122. doi: 10.21037/jtd.2020.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilshire C.L., Blitz S.L., Fuller C.C., Rückert J.C., Li F., Cerfolio R.J., et al. Minimally invasive thymectomy for myasthenia gravis favours left-sided approach and low severity class. Eur J Cardiothorac Surg. 2021;60:898–905. doi: 10.1093/ejcts/ezab014. [DOI] [PubMed] [Google Scholar]
- 8.Friedant A.J., Handorf E.A., Su S., Scott W.J. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta-analysis. J Thorac Oncol. 2016;11:30–38. doi: 10.1016/j.jtho.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruckert J.C., Swierzy M., Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg. 2011;141:673–677. doi: 10.1016/j.jtcvs.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Rückert J.C., Ismail M., Swierzy M., Sobel H., Rogalla P., Meisel A., et al. Thoracoscopic thymectomy with the da Vinci Robotic System for myasthenia gravis. Ann NY Acad Sci. 2008;1132:329–335. doi: 10.1196/annals.1405.013. [DOI] [PubMed] [Google Scholar]
- 11.Elsayed H.H., Gamal M., Raslan S., Abdel Hamid H. Video-assisted thoracoscopic thymectomy for non-thymomatous myasthenia gravis: a right-sided or left-sided approach? Interact Cardiovasc Thorac Surg. 2017;25:651–653. doi: 10.1093/icvts/ivx136. [DOI] [PubMed] [Google Scholar]
- 12.Cerfolio R.J., Bryant A.S., Minnich D.J. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg. 2011;91:1729–1736. doi: 10.1016/j.athoracsur.2011.01.104. discussion 1736-7. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi K., Fukui T., Nakamura S., Taniguchi T., Yokoi K. A bilateral approach to extended thymectomy using the da Vinci Surgical System for patients with myasthenia gravis. Surg Today. 2018;48:195–199. doi: 10.1007/s00595-017-1567-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video available at: https://www.jtcvs.org/article/S2666-2507(22)00180-8/fulltext.