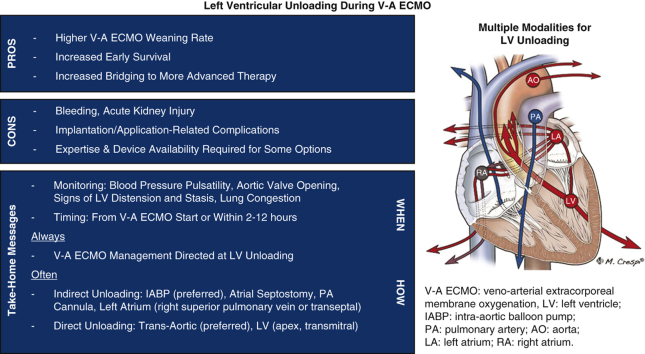
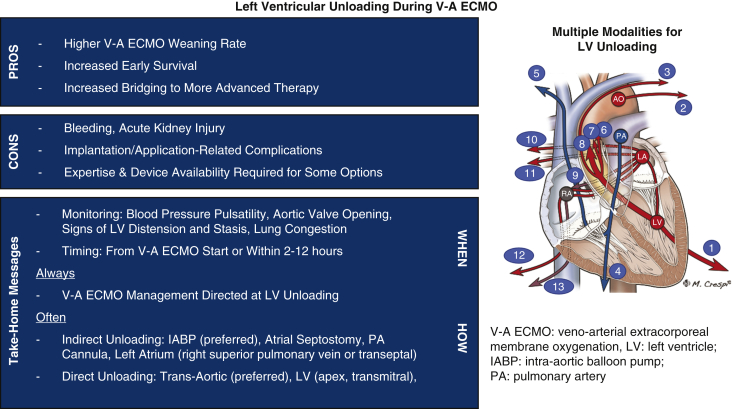
Graphical abstract
Modalities and efficacy (arrow width) of left ventricular venting in extracorporeal membrane oxygenation.
Central Message.
Left ventricular unloading should always be applied during V-A ECMO on the basis of higher rates of weaning, survival, and bridging to advanced therapy despite potential complications.
Although mechanical circulatory support (MCS) has been used to support patients with cardiogenic shock (CS) for many years, recent advances in device technology, together with the lackluster performance of isolated pharmacological therapy, have increased its utilization in this setting.1, 2, 3 Veno-arterial extracorporeal membrane oxygenation (V-A ECMO) has been increasingly implemented, particularly in patients with postcardiotomy CS or cardiac arrest, because V-A ECMO has several advantages over other MCS modalities. Advantages of V-A ECMO include rapid deployment, biventricular support, gas exchange provisions, peripheral and percutaneous approaches for insertion, the ability to provide support for days or weeks, relatively inexpensive disposables for the equipment, and widespread availability with well established programs at most major centers.4 However, despite the established benefits of V-A ECMO, several shortcomings of this technology persist and remain a matter of thorough debate.
One persistent shortcoming of V-A ECMO is that increased left ventricular (LV) afterload is induced by retrograde flow, particularly when V-A ECMO is inserted peripherally.5,6 This retrograde extracorporeal membrane oxygenation (ECMO) flow toward the aortic valve can reduce or impede LV ejection, which then leads to blood stasis and left chamber distension.4, 5, 6, 7, 8 LV afterload always increases during peripheral V-A ECMO but does not lead to overt LV distension or evident left chamber or aortic root blood stasis in most patients.7,8 However, reduced or absent forward blood flow across the aortic valve might occur also due to a mismatch between LV afterload, LV preload, and LV contractility. Some degree of preload is necessary with ECMO support to maintain aortic valve opening. A completely empty ventricle might not eject with normal afterload or adequate contractility, a situation which might be generated by excessive LV drainage. This potential disadvantage of uncontrolled LV unloading underlines the relevance of close monitoring to adequately determine the need, and pros as well as cons of LV decompression during V-A ECMO.
The impelling need and benefits of LV venting in a severely dysfunctional heart that does not generate an effective ejection or that is markedly dilated are fairly well established.9 However, even in the absence of severe LV failure, there is increasing evidence that LV unloading during V-A ECMO is beneficial. Uniform protocols for LV unloading using dedicated devices and procedures failed to provide conclusive evidence, and the ideal timing and modalities for LV unloading remain undefined.7,8
Specific questions to evaluate were as follows: (1) Is LV unloading during V-A ECMO beneficial, even in the absence of overt LV distension, to reduce or avoid further myocardial damage? (2) Is LV unloading instrumental, even in the absence of overt LV distension, for enhanced or quicker LV recovery? (3) Does LV unloading during V-A ECMO affect ECMO weaning, survival, or the ability to bridge the patient to more advanced therapies? (4) Do complications related to LV unloading techniques affect patient outcomes? (5) Which LV unloading strategies are available and are well managed with the local expertise at each center? To address these questions, the pros and cons of LV unloading during V-A ECMO are discussed and recent publications and ongoing research are highlighted, with a specific focus on postcardiotomy V-A ECMO. In addition, our standard practice and future directions for LV unloading during V-A ECMO in patients suffering CS are addressed.
Modalities for LV Unloading
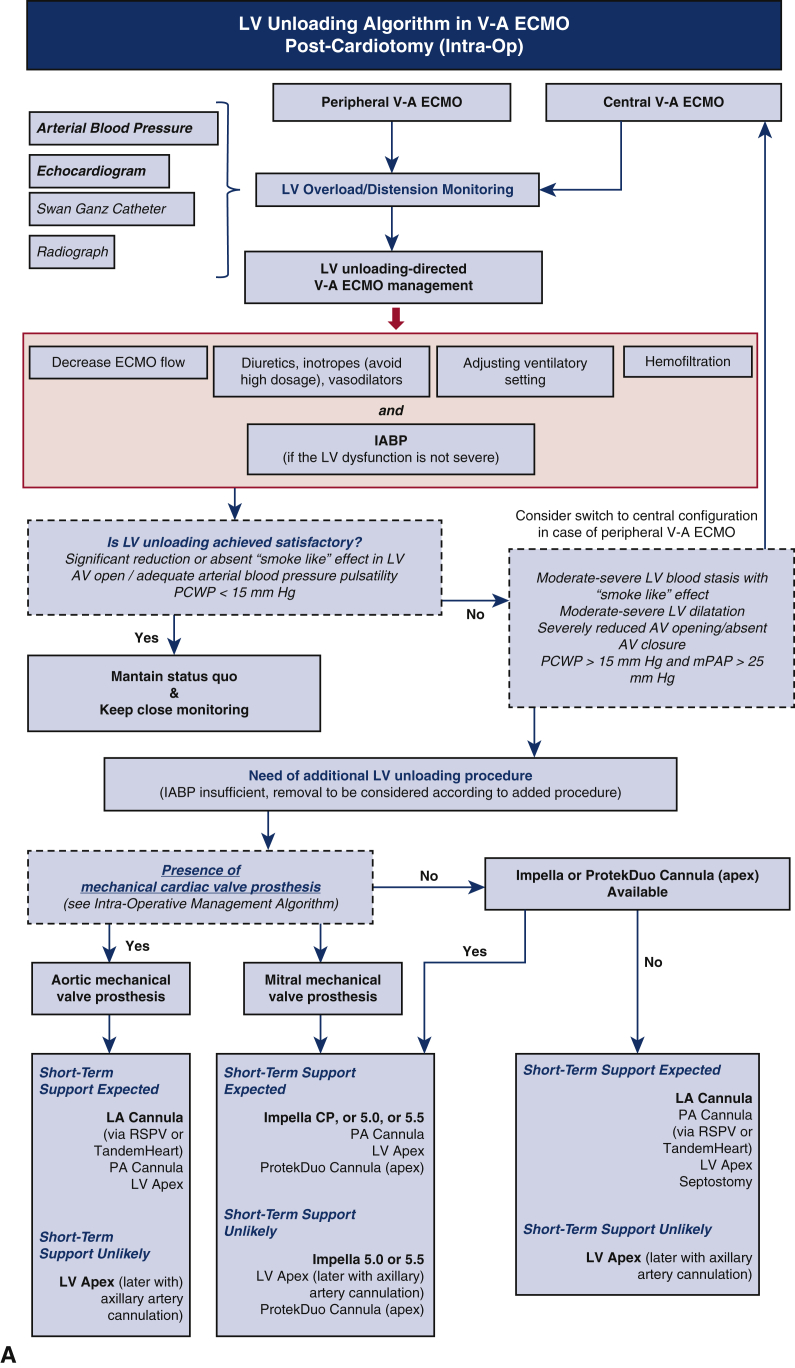
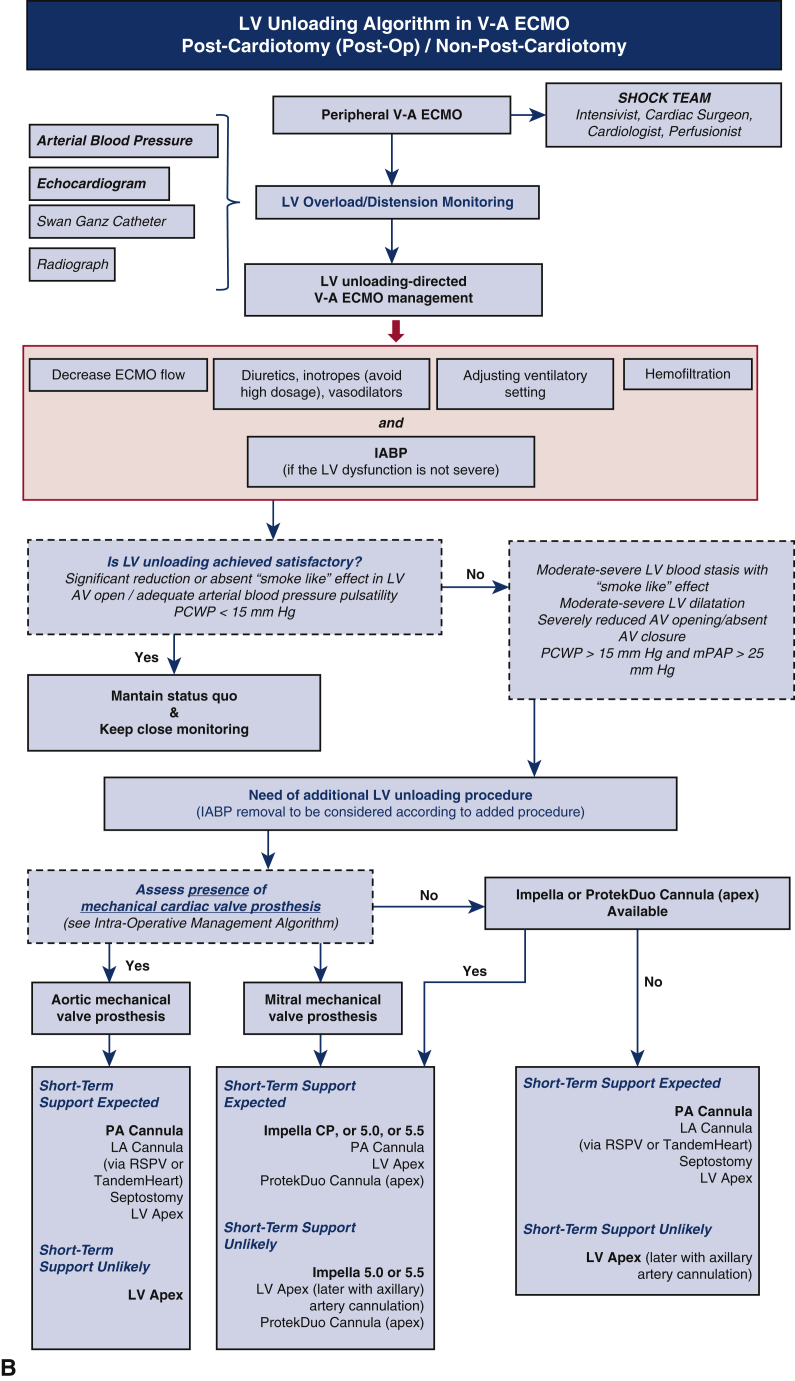
There are several ways to achieve LV unloading during V-A ECMO including noninvasive maneuvers and invasive noncatheter, catheter-based, and device-dependent modalities. Each approach allows for either direct (active) or indirect (passive) unloading. The available modalities differ in terms of access, extent of LV decompression, complexity, cost, and potential complications (Table 1).8,10,11 Thus far, limited comparisons of LV unloading techniques have been published, and more compelling evidence is necessary to determine the superiority or inferiority of any of the various techniques.12, 13, 14 Until such evidence is available, the advantages and disadvantages of each modality should be considered during the decision-making process with attention to potential therapeutic actions after ECMO weaning, patient management, and the presence of mechanical cardiac valves on the left side of the heart (Figure 1).7,8
Table 1.
LV unloading during veno-arterial extracorporeal membrane oxygenation: modalities, advantages, and potential complications
| Procedure/device | Mechanisms of LV unloading | Efficacy of LV unloading∗ | Approach | Cost and complexity of application∗ | Advantages | Disadvantages/complications |
|---|---|---|---|---|---|---|
| Noninvasive maneuvers | ||||||
| Reduced ECMO flow | Enhanced LV ejection/unloading (indirect) | ✓–✓✓ | ✓ |
|
|
|
| Modified ventilator settings (increased PEEP) | Increased right-sided drainage (indirect) | ✓–✓✓ | ✓ |
|
|
|
| Diuretics | Reduced cardiac loading (indirect) | ✓–✓✓ | ✓ |
|
|
|
| Hemofiltration | Reduced cardiac loading (indirect) | ✓✓ | ✓✓ |
|
|
|
| Inotropes | Enhanced LV ejection (indirect) | ✓✓ | ✓ |
|
|
|
| Systemic vasodilation | Enhanced LV ejection (indirect) | ✓–✓✓ | ✓ |
|
|
|
| Invasive maneuvers | ||||||
| Extracardiac procedures | ||||||
| IABP | Reduced LV afterload (enhanced systolic ejection) and reduced LV end-diastolic pressure (enhanced left atrium and pulmonary veins unloading; indirect) | ✓ | Percutaneous, femoral (or surgical in case of specific adverse conditions, like severe peripheral vascular disease requiring an axillary or transaortic implant) | ✓✓ |
|
|
| Transaortic procedures | ||||||
| Percutaneous LV assist devices (Impella 2.5, CP, 5.0, and 5.5) | LV blood suction (direct) | ✓✓✓✓✓ | ✓✓✓✓✓ |
|
|
|
| Percutaneous, femoral | Impella 2.5 and CP | |||||
|
|
|||||
| Surgical, trans-subclavian or axillary, or aortic | Impella 5.0 and 5.5 | |||||
|
|
|||||
| Transaortic catheter | LV blood suction (direct) | ✓✓✓✓✓ | Percutaneous, femoral | ✓✓✓✓ |
|
|
| Transapical dual-lumen cannula (ProtekDuo) | LV unloading (direct) | ✓✓✓✓ |
|
✓✓✓✓ |
|
|
| Transaortic pump (PulseCath i-VAC) | LV blood ejection (direct) | ✓✓✓ | Percutaneous, femoral | ✓✓✓ |
|
|
| LV apex procedures | ||||||
| Transapical or transmitral valve catheter | LV unloading (direct) | ✓✓✓✓✓ |
|
✓✓✓✓ |
|
|
| Trans-septal or biatrial procedures | ||||||
| Percutaneous septostomy usually with ballooning or stenting |
|
✓✓✓ | Percutaneous, femoral (venous access) | ✓✓✓ (For all procedures) |
|
|
| Left atrium procedures | ||||||
| Trans-septal or interatrial groove, or left atrial roof, or right superior pulmonary vein catheter or cannula attached to the ECMO venous return |
|
✓✓✓✓ | Surgical (either via sternotomy or right minithoracotomy) | ✓✓✓✓ |
|
|
| TandemHeart | Left atrium unloading LV unloading and unloading of the pulmonary veins (indirect) |
✓✓✓✓ | Percutaneous, femoral (venous access) or surgical (only arterial access) | ✓✓✓✓✓ |
|
|
| Pulmonary artery procedures | ||||||
| Pulmonary artery cannula surgically or percutaneously placed |
|
✓✓ | Percutaneous (right internal jugular vein) or surgical, sternotomy (direct or through a vascular prosthesis) | ✓✓✓ |
|
|
| Increased systemic venous blood drainage (additional cannulas) | ||||||
| Systemic vein (femoral, jugular, subclavian) or right atrium |
|
✓✓ | Percutaneous (venous access) or sternotomy (central access) | ✓✓✓ |
|
|
Impella devices are from Abiomed; ProtekDuo is from TandemLife/LivaNova; PulseCath i-VAC is from PulseCath BV; and TandemHeart is from LivaNova. Adapted from Lorusso.8LV, Left ventricular; ECMO, extracorporeal membrane oxygenation; PEEP, positive end expiratory pressure; RV, right ventricular; IABP, intra-aortic balloon pump; VSD, ventricular septal defect; VAD, ventricular assist device; ASD, atrial septal defect; VAV, veno-arterial-venous; PA, pulmonary artery.
Grade from least (✓) to most powerful (✓✓✓✓✓).
Figure 1.
A, Algorithm for management of left ventricle (LV) unloading to accompany intraoperative (intra-op) extracorporeal membrane oxygenation (ECMO) insertion post cardiotomy. B, Algorithm for management of LV unloading when ECMO is inserted postoperatively (post-op) or in a non-postcardiotomy setting. Red boxes indicate measures that should be considered in all patients supported by veno-arterial (V-A) ECMO to unload the LV and avoid LV distension and aortic valve dysfunction. Impella CP, 5.0, and 5.5 are from Abiomed; ProtekDuo is from TandemLife/LivaNova; TandemHeart is from LivaNova. IABP, Intra-aortic balloon pump; AV, aortic valve; PCWP, post-capillary wedge pressure; mPAP, mean pulmonary arterial pressure; RSPV, right superior pulmonary vein; PA, pulmonary artery; LA, left atria.
Noninvasive Modalities
Several noninvasive maneuvers can be immediately applied during all ECMO application procedures as tolerated to reduce LV afterload. Noninvasive methods for reducing LV afterload include avoiding high ECMO flow, increasing positive end expiratory pressure slightly, limited vasodilation, and limited administration of inotropic drugs. These prompt actions might prevent or limit the negative effects of increased LV afterload even when an aggressive LV venting procedure has been implemented before ECMO insertion because of intraoperative implant, failure to wean from cardiopulmonary bypass, or the presence of an intra-aortic balloon pump (IABP) or an Impella device (Abiomed).11
Noninvasive maneuvers to decrease LV afterload have potential shortcomings, however, and are not tolerated by or effective in all patients. Limiting ECMO flow might increase the volume flowing into the pulmonary artery bed and, in combination with protective, limited ventilatory settings, can result in hypo-oxygenated blood reaching the left ventricle and aortic arch, generating differential hypoxemia or Harlequin syndrome.4,5 The use of diuretics, hemofiltration, or vasodilators to reduce the LV preload or afterload is rarely effective in the presence of severe CS requiring a high level of ECMO flow. Inotropes have often been suggested to enhance the residual cardiac contractility and promote LV ejection, while also allowing for effective aortic valve opening. However, negative effects of inotropes in patients with an ongoing or recent myocardial injury has been repeatedly demonstrated, suggesting that such agents should be used judiciously, particularly in the presence of ischemia-induced myocardial damage.7,8,11
Invasive Modalities
Invasive catheter-based and device-dependent procedures can achieve effective LV decompression without relying on the residual LV contractile resources. The location of catheter placement determines whether decompression is direct (active) or indirect (passive) and the efficacy of the chosen approach (Table 1).10,12,13 IABP placement is the easiest device-based approach because percutaneous access allows easy and fast insertion and removal. The IABP has repeatedly shown benefits when used in combination with V-A ECMO.15, 16, 17 However, the IABP has limited effects compared with alternative direct and indirect LV unloading approaches, and improved early survival as a result of IABP placement has not been demonstrated.4
Furthermore, the site of blood stasis resulting from LV afterload should be carefully assessed because the location of stasis and additional variables (such as the presence of a mechanical valve prosthesis) will dictate the most appropriate strategy to achieve LV decompression.7,8 Not all forms of LV unloading reduce blood stasis at the aortic root, which is often caused by protracted aortic valve closure or severely reduced valve opening. Additionally, stasis might be exacerbated by several LV unloading procedures including indirect modalities or direct LV unloading without transaortic or aortic access. If there are no contraindications, aortic systems, such as an IABP, or transaortic systems, such as the Impella CP, 5.0, or 5.5, or the PulseCath i-VAC (PulseCath BV), can be used to reduce the risk of complications compared with transapical LV unloading. Impella devices have been increasingly used when invasive, direct LV unloading with a device-based approach is desired. The Impella devices are axial pumps that can be inserted using a percutaneous, transfemoral approach (Impella 2.5 or Impella CP) or surgical access (Impella 5.0 or Impella 5.5), but the presence of a mechanical aortic valve prosthesis is a contraindication for their use.
Safety and Complications
The use of any LV unloading strategy must always be entertained with caution and appropriate knowledge of the functionality of the available procedures and devices, as well as the complications associated with each.7,8 Adverse events related to the application of LV unloading techniques and the potential for maladaptive changes in the pulmonary vasculature and cardiac valve, structural disease, bleeding, thrombosis, and pathophysiological and hemodynamic changes are of paramount importance (Table 1). Complications of LV decompression modalities include protracted aortic valve closure, hemolysis, leg ischemia, bleeding, cardiac chamber perforation, renal replacement therapy, and infection (Table 1). Complications of unloading occur concurrently with the risks imparted by V-A ECMO support.
Current Controversies
Is LV Unloading During V-A ECMO Necessary?
There is significant debate as to whether LV unloading during V-A ECMO is necessary, especially using invasive modalities. Camboni and Schmid18 reported use of a LV venting procedure in only 2% of their patients supported with V-A ECMO and instead preferred to regulate LV afterload using noninvasive methods. In contrast, Truby and colleagues19 reported that 22% of their patients supported with V-A ECMO had subclinical LV distension and 7% had clinical LV distention requiring decompression immediately after ECMO initiation. In total, 16% of the patients in their case series experienced LV distension requiring decompression during V-A ECMO support. Importantly, Weber and colleagues20 showed that 4% of patients who underwent femoral V-A ECMO developed intracardiac or extracardiac thrombi despite receiving adequate anticoagulation, a condition representing the worst scenario linked with protracted LV distension and blood stasis. Although some of the patients with thrombi underwent surgical procedures to remove the clots, none ultimately survived.20 A “smoke-like effect” indicating blood stasis in the left ventricle, left atria, or at the aortic root and pulmonary congestion secondary to protracted aortic valve closure are not infrequent observations in patients supported with V-A ECMO.5,7,8,21 Moreover, an aggressive anticoagulation regimen, which is sometimes suggested when blood stasis occurs during ECMO, is a prohemorrhagic intervention and often predisposes the patient to cerebral hemorrhage or uncontrollable generalized bleeding episodes. We believe the potential for these complications makes LV decompression advisable.
Evidence of the Benefits of LV Unloading
Table 2 includes a summary of several relevant publications, including limited single-center analyses, multi-center studies, and extensive meta-analyses, that specifically addressed the occurrence of LV distension-related events and the effects of LV unloading in patients supported by V-A ECMO. From these studies, the need for and the benefits of LV unloading, particularly if applied early, appear concrete and relevant. Most studies identified advantages of LV decompression during V-A ECMO with increased rates of weaning from ECMO, early survival, and bridging to more advanced therapies.15, 16, 17,19,22, 23, 24, 25, 26 It is worth mentioning, however, that no randomized trials of LV unloading have been published, and patients selected for LV unloading and studied in retrospective analyses were likely at high risk. Furthermore, several different modalities were used for LV unloading in the available studies. Modalities were often mixed within each study and included IABP, Impella, direct LV cannulation, and left atrial venting. Each approach has unique benefits and shortcomings.
Table 2.
Summary of recent publications that specifically addressed the use of left ventricular unloading during V-A ECMO
| First author | Study design | Patients | LV unloading modality | Outcomes | LV unloading technique-related complications | Limitations of the study |
|---|---|---|---|---|---|---|
| Weymann22 | Single-center | 12 | Central cannulation Right superior pulmonary vein |
No control group | NR | Central V-A ECMO (right atrium-aorta) in all patients IABP used in 25% Recovery in only 3 of 12 patients |
| Truby19 | Single-center | 121 | Impella, septostomy | No difference in survival | NR | Classification of LV distension (no LV distension, subclinical LV distension, clinical LV distension) incomplete Data available on 121 of 224 patients |
| Pappalardo23 | Two centers; propensity-matched | 153 (2:1 propensity match analysis, 42 without and 21 with Impella) | Impella | Lower in-hospital mortality (47% vs 80%) Higher bridging rate to recovery or upgraded therapy (68% vs 28%) |
Hemolysis (76% vs 33%) CVVH (48% vs 19%) |
Limited patient cohort Not clear indication for LV unloading |
| Brechot15 | Single-center | 259 (40.1% with LV unloading; 126 patients with propensity match for each group) | IABP | Lower risk for pulmonary edema More d off mechanical ventilation |
NR | No No wedge pressure measurement |
| Chen16 | Single-center | 60 | IABP | Better survival to discharge in patients with concomitant V-A ECMO with IABP vs delayed IABP after ECMO implant | NR | Limited patient cohort Only postcardiotomy shock patients Limb ischemia in 22% not described if distal perfusion- or IABP-related |
| Na24 | Single-center | 50∗ (half prophylactic and half therapeutic) | Trans-septal atrial cannula | Lower mortality rate and higher rate for bridging in the prophylactic group | NA | Only 50 patients of 335 patients in this study |
| Meani17 | Single-center | 10† (single-center) | IABP | Aortic valve opening in 80% of the treated patients with protracted aortic valve closure during peripheral V-A ECMO | None | Limited patient cohort Selection bias possible Hemodynamic effects by LV unloading not evaluated |
| Russo25 | Systematic review, meta-analysis | 3997 (17 observational studies; 42% with LV unloading) |
|
Lower mortality rate (54% vs 65%) | Hemolysis | No RCT No evaluation about effect of LV unloading timing Underpowered for complications analysis Lack of uniform definition about LV distension and stasis among the studies |
| Al-Fares26 | Systematic review, meta-analysis | 7955 (62 observational studies; 3458 with LV unloading) | All modalities | Improved weaning and improved early survival with early LV unloading (<12 h), improved short-term survival More favorable outcome especially in primary graft failure after HTx or ischemic cardiomyopathy |
More time on V-A ECMO and mechanical ventilation Hemolysis |
No evaluation of effect of LV unloading timing Likely under-reporting of adverse events No head-to-head comparison of different venting strategies |
| Kowalewski27 | Systematic review, meta-analysis | 7581 (44.1% with LV unloading) | All modalities | 35% Higher chance of V-A ECMO weaning 12% Risk reduction for in-hospital mortality |
No difference between unloading and no unloading | Lack of RCTs No information about timing of V-A ECMO and LV unloading institution Lack of information about LV unloading escalation (eg, from IABP to Impella, or others) Incomplete information about V-A ECMO weaning strategy |
| Schrage28 | Multi-center, retrospective, propensity-matched | 686 (337 patients with LV unloading; 255 patients matched for each group) | Impella | Lower 30-d mortality rate (HR, 0.79%; 95% CI, 0.63-0.98) | Increased severe bleeding, access site-related ischemia, abdominal compartment syndrome, and renal replacement therapy | No detailed description of LV distension in all patients Incomplete hemodynamic description Incomplete information about V-A ECMO weaning strategy |
Impella is from Abiomed. LV, Left ventricular; NR, not reported; V-A ECMO, veno-arterial extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; CVVH, continuous veno-venous hemofiltration; ECMO, extracorporeal membrane oxygenation; NA, not applicable; RCT, randomized controlled trial; HTx, heart transplant; HR, hazard ratio; CI, confidence interval.
Of 355 patients studied.
Of 182 patients studied.
Timing of LV Unloading
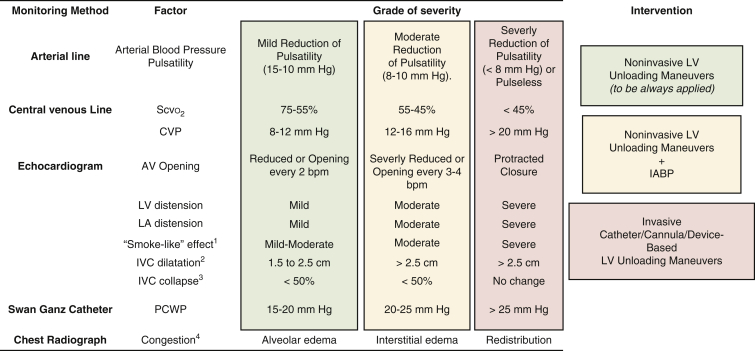
Figure 2 details available diagnostic tools for monitoring LV function during V-A ECMO and recommended algorithms to determine the urgency and preferences of LV unloading measures. Accurate monitoring and earlier LV unloading translates into an increased likelihood of myocardial recovery, faster myocardial recovery, and a better early survival.16,24,26, 27, 28 Indeed, Chen and colleagues16 showed that the concomitant implementation of IABP and ECMO was associated with more favorable survival outcomes than adjunctive support with an unloading system after ECMO insertion. Their study was performed primarily in the intensive care unit in patients affected by postcardiotomy CS. Na and colleagues24 confirmed that favorable outcomes, namely a lower early mortality rate and a higher likelihood of successful bridging to more advanced MCS, occurred more frequently in patients who underwent immediate, prophylactic LV venting compared with patients who were treated with a therapeutic strategy to treat overt LV distension. Al-Fares and colleagues26 conducted an extensive meta-analysis that included almost 8000 patients and showed that an unloading procedure within 12 hours after the start of ECMO was significantly associated with better weaning and early survival compared with LV unloading procedures initiated more than 12 hours after the initiation of ECMO support. Interestingly, there were benefits of LV unloading using an IABP, regardless of timing, and the difference in outcomes was driven mainly by the timing of Impella implementation. Finally, Schrage and colleagues28 recently reported that implantation of the Impella device within 2 hours of ECMO application was associated with a lower mortality risk, regardless of the patient demographic characteristics (ie, older vs younger, pre-cardiac arrest or not). This reduction in mortality risk was no longer observed when a combination of devices was applied more than 2 hours after ECMO application, however, highlighting the influence of early venting as opposed to late venting or no venting.
Figure 2.
Monitoring and determining the urgency of left ventricle (LV) unloading in patients undergoing veno-arterial extracorporeal membrane oxygenation. 1Classification according to Fatkin D, Loupas T, Jacobs N, Feneley MP. Quantification of blood echogenicity: evaluation of a semiquantitative method of grading spontaneous echo contrast. Ultrasound Med Biol. 1995;21:1191-8. 2IVC diameter during inspiration (according to Whitson and Mayo. Crit Care 2016;20:227). 3IVC collapse during expiration (according to Whitson and Mayo. Crit Care 2016;20:227). 4Classified according to Ravin CE. Radiographic analysis of pulmonary vascular distribution: a review. Bull N Y Acad Med. 1983;59:728-43. Scvo2, Central venous blood oxygen saturation; CVP, central venous pressure; AV, aortic valve; LA, left atria; IVC, inferior vena cava; bpm, beats per minute; IABP, intra-aortic balloon pump. Modified with permission from Meani and colleagues.17
In summary, the current evidence, although still limited, supports that LV unloading using noninvasive measures should be immediately instituted when managing V-A ECMO, and LV afterload and function should be continuously monitored. A more aggressive strategy, with a highly effective, direct, or device-based unloading modality, might also be needed and should be instituted either at the time of ECMO insertion or within 2 to 12 hours of the initiation of ECMO support.
LV Unloading and Postcardiotomy Extracorporeal Life Support
LV unloading during V-A ECMO is of particular importance in postcardiotomy patients. Postcardiotomy CS is often characterized by several factors associated with poor outcomes, such as prolonged myocardial ischemic time and edema, complications of the procedure requiring cardiotomy, cardiopulmonary bypass-related inflammatory reactions, preoperative heart dysfunction, and an increased tendency to bleed. Therefore, the potential shortcomings of postcardiotomy ECMO might further exacerbate cardiac compromise and the inability to cope with the increased afterload, particularly in patients with retrograde flow from peripheral V-A ECMO. The decision between taking a central or peripheral approach for V-A ECMO has been recently addressed in 2 meta-analyses.29,30 Although the central approach is well suited for effective right and LV unloading, both meta-analyses showed fewer bleeding complications and lower rates of in-hospital mortality with the peripheral configuration. Thus, peripheral V-A ECMO is currently recommended, and LV unloading might be of the utmost importance to facilitate good outcomes.31 Furthermore, postcardiotomy ECMO might be required under conditions that carry an increased risk of thrombosis due to blood stasis, such as in patients with mechanical prostheses. Nonaggressive procedures that promote LV ejection and IABP use from the start of ECMO are always recommended, particularly in the presence of a mechanical valve prosthesis (Figure 1). When a more aggressive LV unloading approach is needed, the use of techniques with a reduced risk of bleeding is also recommended, such as preferring a left atrial or transaortic approach on the basis of the presence or absence of a mechanical prosthesis. Furthermore, the use of techniques that can simultaneously accomplish LV unloading and support, such as implantation of an IABP or Impella, is preferable postcardiotomy to promote weaning from the device (Figure 1, A).
Recommendations for LV Unloading: a Stepwise Approach
Our policy is that LV unloading should be immediately established after V-A ECMO insertion to prevent LV distension and related complications. We believe that noninvasive maneuvers to enhance LV ejection together with an early implantation of an IABP, should be routinely performed at the start of ECMO (Figure 1). The lowest ECMO flow that provides metabolic/hemodynamic support (as indicated by decreasing lactate levels), light inotrope support, slightly increased positive end-expiratory pressure, and light vasodilation, if afforded by the patient's hemodynamics, should be always immediately instituted. The extent of LV unloading should be continuously and indirectly or directly monitored even with this strategy in place (Figure 2). In patients with persistence of LV distension and blood stasis, more aggressive LV unloading should be pursued using either a direct device-based or direct catheter- or cannula-based strategy. The Impella devices have been the focus of several clinical investigations, and outcomes appear favorable when used in combination with V-A ECMO.4,23 The type of aggressive strategy will depend on the setting (eg, intraoperative, postcardiotomy, or in a non-postcardiotomy patient; central or peripheral ECMO), the cardiac function of the patient (degree of residual contractility), ECMO requirements (high or low flow), and the chances of myocardial recovery (Figure 1). We recommend this approach until conclusive and convincing evidence defining the standard of care is available, because there are clearly advantages of an aggressive approach to LV unloading concurrent with the initiation of ECMO support.
Ongoing Clinical and Preclinical Studies and Future Perspectives
Despite several reviews, meta-analyses, and multicenter experiences providing clinical data as well as modeling and bench simulation studies,7,8,12,13,21,25,26 conclusive evidence on the safety and efficacy of LV unloading is still lacking. Clinical and preclinical investigations are ongoing. Two randomized clinical trials are under way to investigate the effects of implementing LV unloading procedures from the start of ECMO support compared with ECMO support without LV unloading in patients with acute CS. One trial is using the Impella CP for ventricular unloading (ClinicalTrials.gov Identifier: NCT03431467), and the other is using the Impella 5.0 (ClinicalTrials.gov Identifier: NCT04084015). The results will hopefully provide compelling information regarding the potential benefits of such an approach relative to V-A ECMO in isolation.
Conclusions
There is increasing evidence that LV unloading during V-A ECMO, particularly if applied early, might be associated with a higher rate of weaning and improved early survival (Figure 3). However, there is still reluctance to apply LV unloading before LV distension develops because of the potential for complications, the cost, and because some advanced devices that can be used for LV unloading are not ubiquitously available. For the time being, LV unloading with noninvasive approaches should be immediately considered for all patients supported with V-A ECMO, and aggressive, catheter-, or device-based LV unloading modalities should be considered early after the initiation of ECMO support in a patient-tailored way. It is imperative that health care providers who care for V-A ECMO patients know the mechanisms, extent of support, and advantages and disadvantages of LV unloading modalities. Additionally, they should be confident in their ability to perform the necessary procedures and manage LV unloading including monitoring and timing of application. Further research is needed to provide compelling and conclusive evidence defining the timing, best protocol, and balance of additional risks and benefits, while keeping up with technological advances, when considering LV unloading during V-A ECMO support.
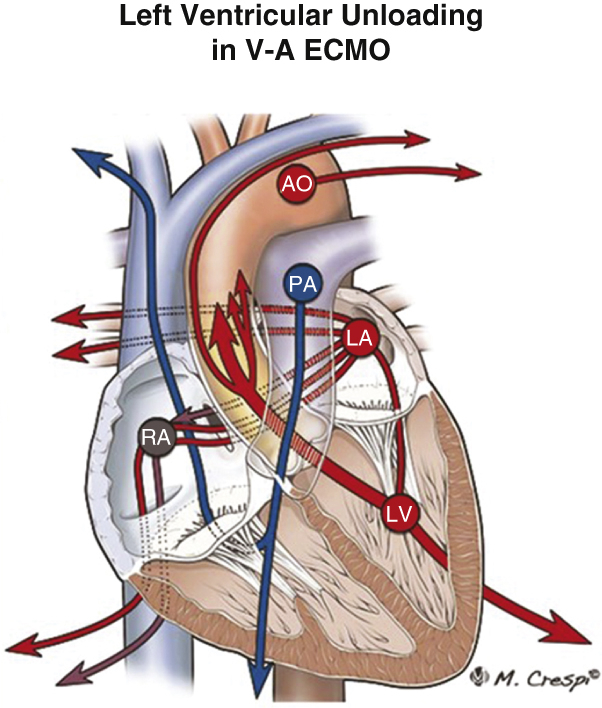
Figure 3.
Modalities for left ventricle (LV) unloading. 1. Single-lumen cannula vent. 2. Intra-aortic balloon pump. 3. Single-lumen catheter. 4. Single-lumen pulmonary artery [PA] cannula (either percutaneous through femoral vein or surgically implanted directly in the pulmonary artery). 5. Single or double-lumen cannula. 6. PulseCath i-VAC pump (PulseCath BV; intraventricular suction and aorta (AO) ascendens ejection). 7. Double-lumen cannula (ProtekDuo [TandemLife/LivaNova] through the ventricular apex; intraventricular suction and AO ascendens ejection). 8. Transaortic axial pump (Impella CP, 5.0, or 5.5; Abiomed; intraventricular suction and AO ascendens ejection, through the femoral or axillary or aorta artery). 9. Septostomy. 10. Left atrial catheter (through the right superior pulmonary vein). 11. Transmitral LV catheter (through the right superior pulmonary vein). 12. Left atrial catheter/cannula (through the interatrial septum and the femoral vein). 13. Left atrial and right atrial catheter/cannula (TandemHeart [LivaNova]; through the interatrial septum and the femoral vein). V-A ECMO, Veno-venous extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LA, left atrium; RA, right atrium. Modified with permission from Kowalewski M, Malvindi PG, Zieliński K, Martucci G, Słomka A, Suwalski P, et al. Left ventricle unloading with veno-arterial extracorporeal membrane oxygenation for cardiogenic shock. Systematic review and meta-analysis. J Clin Med. 2020;9:1039.
Acknowledgments
We thank Shannon Wyszomierski for editing the manuscript.
Footnotes
Disclosures: Dr Lorusso is a consultant for Getinge, LivaNova, and Medtronic, and a member of the Medical Advisory Board of Eurosets. All honoraria are paid to the University to support research activities. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Esposito M.I., Zhang Y., Qiao X., Reyelt L., Paruchuri V., Schnitzler G.R., et al. Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J Am Coll Cardiol. 2018;72:501–514. doi: 10.1016/j.jacc.2018.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa K., Meyns B. Acute mechanical LV unloading in ischemia reperfusion injury. Be prepared. J Am Coll Cardiol. 2018;72:515–517. doi: 10.1016/j.jacc.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiele H., Ohman E.M., de Waha-Thiele S., Zeymer U., Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40:2671–2683. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 4.Abrams D., Combs A., Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63:2769–2778. doi: 10.1016/j.jacc.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Lo Coco V., Lorusso R., Raffa G.M., Malvindi P.G., Pilato M., Martucci G., et al. Clinical complications during veno-arterial extracorporeal membrane oxygenation in post-cardiotomy and non post-cardiotomy shock: still the Achille's heel. J Thorac Dis. 2018;10:6993–7004. doi: 10.21037/jtd.2018.11.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickstein M.L. The Starling relationship and veno-arterial ECMO: ventricular distension explained. ASAIO J. 2018;64:497–501. doi: 10.1097/MAT.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 7.Van Diepen S. Routine unloading in patients treated with extracorporeal membrane oxygenation for cardiogenic shock. Circulation. 2020;142:2107–2109. doi: 10.1161/CIRCULATIONAHA.120.050847. [DOI] [PubMed] [Google Scholar]
- 8.Lorusso R. Are two crutches better than one? The ongoing dilemma of the effects and need of left ventricular unloading during veno-arterial extracorporeal membrane oxygenation. Eur J Heart Fail. 2017;19:413–415. doi: 10.1002/ejhf.695. [DOI] [PubMed] [Google Scholar]
- 9.Kapur N.K., Reyelt L., Swain L., Esposito M., Qiao X., Annamalai S., et al. Mechanical left ventricular unloading to reduce infarct size during acute myocardial infarction: insight from preclinical and clinical studies. J Cardiovasc Transl Res. 2019;12:87–94. doi: 10.1007/s12265-019-09876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meani P., Gelsomino S., Natour E., Johnson D.M., Rocca H.B., Pappalardo F., et al. Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail. 2017;19(Suppl 2):84–91. doi: 10.1002/ejhf.850. [DOI] [PubMed] [Google Scholar]
- 11.Amarelli C., Musumeci F., Loforte A., Montalto A., Di Franco S., Hernandez-Monfort J. In: Advances in Extracorporeal Perfusion Therapies. Firstenberg M.S., editor. IntechOpen Limited; 2018. Flow optimization, management and prevention of left ventricular distension during VA ECMO. [DOI] [Google Scholar]
- 12.Tepper S., Masood M.F., Baltazar Garcia M., Pisani M., Ewald G.A., Lasala J.M., et al. Left ventricular unloading by Impella device versus surgical vent during extracorporeal life support. Ann Thorac Surg. 2017;104:861–867. doi: 10.1016/j.athoracsur.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Stephens A.F., Wanigasekara D., Pellegrino V.A., Burrel A.J.C., Marasco S.F., Kaye D.M., et al. Comparison of circulatory unloading techniques for veno-arterial extracorporeal membrane oxygenation. ASAIO J. 2021;67:623–631. doi: 10.1097/MAT.0000000000001268. [DOI] [PubMed] [Google Scholar]
- 14.Meani P., Mlcek M., Kowalewski M., Raffa G.M., Popkova M., Pilato M., et al. Trans-aortic or pulmonary artery drainage for left ventricular unloading and veno-arterial extracorporeal life support: a porcine cardiogenic shock model. Semin Thorac Cardiovasc Surg. 2021;33:724–732. doi: 10.1053/j.semtcvs.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Brechot N., Demondion P., Sati F., Lebreton G., Pham T., Dalakidis A., et al. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. 2018;7:62–69. doi: 10.1177/2048872617711169. [DOI] [PubMed] [Google Scholar]
- 16.Chen K., Hou J., Tang H., Hu S. Concurrent implantation of intra-aortic balloon pump and extracorporeal membrane oxygenation improved survival of patients with postcardiotomy shock. Artif Organs. 2019;43:142–149. doi: 10.1111/aor.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meani P., Delnoij T., Raffa G.M., Morici N., Viola G., Sacco A., et al. Protracted aortic valve closure during peripheral extracorporeal life support: is the intra-aortic balloon pump an effective solution? Perfusion. 2019;34:35–41. doi: 10.1177/0267659118787426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camboni D., Schmid C. To vent or not on veno-arterial extracorporeal membrane oxygenation, does it improve myocardial recovery and outcome? J Thorac Dis. 2017;9:4915–4918. doi: 10.21037/jtd.2017.11.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truby L.K., Takeda K., Mauro C., Yuzefpolskaya M., Garan A.R., Kirtane A.J., et al. Incidence and implications of left ventricular distension during veno-arterial extracorporeal membrane oxygenation support. ASAIO J. 2017;63:257–265. doi: 10.1097/MAT.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 20.Weber C., Deppe A.C., Sabashnikov A., Slottosch I., Kuhn E., Eghbaldadeh K., et al. Left ventricular thrombus formation in patients undergoing femoral veno-arterial extracorporeal membrane oxygenation. Perfusion. 2018;33:283–288. doi: 10.1177/0267659117745369. [DOI] [PubMed] [Google Scholar]
- 21.Lusenbrink E., Orban M., Kupka D., Scherer C., Hagl C., Zimmer S., et al. Prevention and treatment of pulmonary congestion in patients undergoing veno-arterial extracorporeal membrane oxygenation for cardiogenic shock. Eur Heart J. 2020;41:3753–3761. doi: 10.1093/eurheartj/ehaa547. [DOI] [PubMed] [Google Scholar]
- 22.Weymann A., Schmarck B., Sabashnikov A., Bowles C.T., Raake P., Arif R., et al. Central extracorporeal life support with left ventricular decompression for the treatment of refractory cardiogenic shock and lung failure. J Cardiothorac Surg. 2014;9:60. doi: 10.1186/1749-8090-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappalardo F., Schulte C., Pieri M., Schrage B., Contri R., Soeffker G., et al. Concomitant implantation of Impella((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19:404–412. doi: 10.1002/ejhf.668. [DOI] [PubMed] [Google Scholar]
- 24.Na S.J., Yang J.H., Yang J.H., Sung K., Choi J.O., Hahn J.Y., et al. Left heart decompression at venoarterial extracorporeal membrane oxygenation initiation in cardiogenic shock: prophylactic versus therapeutic strategy. J Thorac Dis. 2019;11:3746–3756. doi: 10.21037/jtd.2019.09.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo J.J., Aleksova N., Pitcher I., Couture E., Parlow S., Faraz M., et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 26.Al-Fares A.A., Randhawa V.K., Englesakis M., McDonald M.A., Nagpal A.D., Estep J.D., et al. Optimal strategy and timing of left ventricular venting during veno-arterial extracorporeal life support for adults in cardiogenic shock. Circ Heart Fail. 2019;12:e006486. doi: 10.1161/CIRCHEARTFAILURE.119.006486. [DOI] [PubMed] [Google Scholar]
- 27.Kowalewski M., Malvindi P.G., Zielinski K., Martucci G., Slomka A., Suwalski P., et al. Left ventricular unloading with veno-arterial extracorporeal membrane oxygenation for cardiogenic shock. Systematic review and meta-analysis. J Clin Med. 2020;9:1039. doi: 10.3390/jcm9041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrage B., Becher P.M., Bernhardt A., Bezerra H., Blankenberg S., Brunner S., et al. Left ventricular unloading is associated with lower mortality in cardiogenic shock patients treated with veno-arterial extracorporeal membrane oxygenation: results from an international, multicentre cohort study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariscalco G., Salsano A., Fiore A., Dalen M., Ruggieri V.G., Saeed D., et al. Peripheral versus central extracorporeal membrane oxygenation for postcardiotomy shock: multicenter registry, systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2020;160:1207–1216. doi: 10.1016/j.jtcvs.2019.10.078. [DOI] [PubMed] [Google Scholar]
- 30.Kowalewski M., Zielinski K., Brodie D., MacLaren G., Whitman G., Raffa G.M., et al. Venoarterial extracorporeal membrane oxygenation for postcardiotomy shock. Analysis of the ELSO Registry. Crit Care Med. 2021;49:1107–1117. doi: 10.1097/CCM.0000000000004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorusso R., Whitman G., Milan M., Raffa G., McMullan D.M., Boeken U., et al. 2020 EACTS/ELSO/STS/AATS expert consensus on post-cardiotomy extracorporeal life support. J Thorac Cardiovasc Surg. 2021;161:1287–1331. doi: 10.1016/j.jtcvs.2020.09.045. [DOI] [PubMed] [Google Scholar]