Abstract
In this narrative review, we summarise the evidence for and against the glycaemic legacy effect from the long-term follow-up of major diabetes trials and observational cohort studies. We provide a summary of the pathophysiological basis for the legacy effect and discuss some translational research. Results from trials of early diabetes and observational cohort studies suggest that a long-term effect of early glycaemic control exists; however, long-term follow-up from trials in participants with established diabetes is not supportive. Additionally, findings for the legacy effect are more conclusive for microvascular complications than macrovascular events. Overall, these results suggest that the glycaemic legacy effect is a long-term benefit (or risk) conferred to individuals in the early stages of diabetes and which is muted over time as individuals’ vasculature changes and they develop complications from diabetes.
Keywords: Cardiovascular disease, Diabetes mellitus, Diabetic complications, Diabetic nephropathies, Diabetic retinopathy, Glycaemic control, Review
Introduction
In clinical practice, it is common to assume that the major effect of a treatment is an immediate change to a clinical outcome, especially since evidence for this is widespread. For example, glucose-lowering medications reduce blood sugar levels within hours of ingestion or administration. However, in complex chronic diseases such as diabetes, decades-old literature has established that there are long-term benefits of previous periods of euglycaemia and long-term harms associated with previous periods of hyperglycaemia, a phenomenon known as the legacy effect, or metabolic memory. In this review, we will summarise the evidence for this legacy effect of glycaemic control, starting with a review of the observational evidence from randomised trials, real-world cohorts and other studies, and ending with a brief review of the pathophysiological basis underlying the legacy effect.
RCTs: observational follow-up
Every major diabetes trial has investigated the presence of the legacy effect in the post-trial observational follow-up period. Because of their strong initial study design and successful longitudinal follow-up these studies provide the strongest evidence for and against the legacy effect of glycaemic control in diabetes. We will review the three major trials involving participants with recently diagnosed diabetes, all of which favour a legacy effect, and then we will review the three major trials involving participants with established diabetes that provide mixed evidence for the legacy effect. A summary of these findings can be found in Table 1.
Table 1.
Summary of results from RCTs and follow-up studies
| Study | Study duration (years) | No. of participants | Mean HbA1c at end of trial | Study findings | Follow-up study period post-trial (years) | Significant legacy effect finding |
|---|---|---|---|---|---|---|
| DCCT | 6.5 | 1441 with recently diagnosed T1D | Intensive: 7.4% (57 mmol/mol) Control: 9.1% (76 mmol/mol) |
Decreased rates of microvascular disease in intensive arm No difference in macrovascular disease |
17–30 | Decreased rates of microvascular disease and macrovascular disease in intensive control arm at follow-up |
| UKPDS | 5 | 4209 with newly diagnosed T2D | Intensive: 7.0% (53 mmol/mol) Control: 7.9% (63 mmol/mol) |
Decreased risk of microvascular disease in intensive arm Non-significant reduction in macrovascular disease |
10 | Decreased rates of microvascular disease, reduction in MI and mortality in intensive control arm at follow-up |
| Steno-2 | 7.8 | 160 with T2D and microalbuminuria | Intensive: 7.9% (63 mmol/mol) Control: 9.0% (75 mmol/mol) |
Decreased rates of microvascular disease in intensive arm | 5.5 | Decreased rates of microvascular disease, lower rates of CV events, CV mortality and all-cause mortality in intensive control arm at follow-up |
| ADVANCE and ADVANCE-ON | 5 | 11,140 with pre-existing diabetes (mean duration 8 years) |
Intensive: 6.5% (48 mmol/mol) Control: 7.3% (56 mmol/mol) |
Decreased rates of microvascular disease in intensive arm No significant difference in macrovascular disease |
5.4 | Lower rates of ESRD in intensive control arm at follow-up |
| ACCORD and ACCORDION | 3.5 | 10,251 with pre-existing diabetes (mean duration 10 years) |
Intensive: 6.4% (46 mmol/mol) Control: 7.5% (78 mmol/mol) |
Increased mortality in intensive control arm Decreased rates of non-fatal CV events |
4 | No effect on primary outcome of CV events Decreased rates of retinopathy in intensive control group |
| VADT | 5.6 | 1791 with pre-existing diabetes (mean duration 11.5 years) |
Intensive: 6.9% (52 mmol/mol) Control: 8.4% (68 mmol/mol) |
No significant difference in macrovascular events between arms | 10–15 | Reduction in macrovascular events in intensive control arm at 10 years follow-up but effect was lost at 15 years |
| ADN CKD 3–4 | 2 | 120 with pre-existing diabetes and advanced nephropathy (mean duration 15 years) | Intensive: 7.3% (56 mmol/mol) Control: 8.3% (67 mmol/mol) |
Decreased rate of progression to ESRD in intensive arm | 2 | No significant difference in progression to ESRD between arms at follow-up |
Study duration is presented as mean, except for ADVANCE (median), ACCORD (study stopped at 3.5 years due to increased mortality in intensive control group) and ADN CKD 3–4 (median 2 years) ADVANCE-ON, ADVANCE Observational Study; CV, cardiovascular; MI, myocardial infarction; T1D, type 1 diabetes; T2D, type 2
Early diabetes
The first compelling data for the legacy effect emerged from the long-term follow-up of the DCCT, conducted from 1983 to 1989, known as the Epidemiology of Diabetes Interventions and Complications (EDIC) study. The trial randomised 1441 participants with newly or recently diagnosed type 1 diabetes to receive either intensive glycaemic control (i.e. external insulin pump or three insulin injections daily) or standard glycaemic control (i.e. two insulin injections daily) [1]. The investigators originally found that intensive control significantly decreased rates of microvascular complications but no significant difference in macrovascular disease [1]. After decades of follow-up, which was completed by 97% of the original participants, despite the HbA1c levels converging in the two study arms 1 year after the study conclusion, the intensive control arm continued to have a significantly lower rate of microvascular complications. Furthermore, a new significant decrease in macrovascular complications emerged (relative risk reduction [RRR] 42% [95% CI 9, 63] for CVD; p = 0.02) [2–4]. This effect was seen at 17 years of follow-up and persisted for at least 30 years (RRR 30% [95% CI 7, 48] for CVD; p = 0.02), while the HbA1c remained the same in both groups [5].
Similar results were found in the type 2 diabetes population in the pivotal UK Prospective Diabetes Study (UKPDS). Individuals with newly diagnosed type 2 diabetes were randomised to undergo intensive vs conventional glycaemic control (N = 4209; median age 53 years) and after the trial ended there was a 25% (95% CI 7, 40; p = 0.0099) RRR in microvascular complications and a 16% difference in macrovascular complications (approaching statistical significance, p = 0.052) [6]. Similar to the DCCT, differences in glycaemic control were lost at 1 year after the study conclusion. However, a 24% (p = 0.001) reduction in microvascular events persisted in the intensive control arm for 10 years after the study ended, by which time a 15% reduction in myocardial infarction (p = 0.01) and 13% reduction in mortality (p = 0.007) had emerged [7].
Lastly, in the Steno-2 trial, 160 participants with type 2 diabetes and microalbuminuria (mean age 55 years) were randomised to undergo intensive vs conventional glycaemic control and were followed for a total of a mean 13.3 years [8]. Again, despite convergence in glycaemic control after the end of the study, participants in the intensive control arm had a lower risk of cardiovascular events (HR 0.41 [95% CI 0.25, 0.67]; p = 0.02), cardiovascular mortality (HR 0.43 [95% CI 0.19, 0.94; p = 0.04) and all-cause mortality (HR 0.54 [95% CI 0.32, 0.89]; p = 0.02) [8].
Established diabetes
The evidence for the legacy effect becomes less clear for individuals with established diabetes on examination of data from the major diabetes trials that compared intensive with standard glycaemic control. The results most consistent with the early diabetes trials came from the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. In this trial, 11,140 participants (mean age 66 years) with pre-existing diabetes (mean duration 8 years) were randomised to undergo intensive glycaemic control with a goal HbA1c of 48 mmol/mol (6.5%) vs standard glycaemic control. This study found significant reductions in microvascular disease (HR 0.86 [95% CI 0.77, 0.97]; p = 0.01), mostly due to a difference in nephropathy rates (HR 0.79 [95% CI 0.66, 0.97]; p = 0.006) [9]. The reduced rate of end-stage renal disease (ESRD) was maintained in the post-trial follow-up (HR 0.54 [95% CI 0.34, 0.85]; p = 0.007), again despite convergence of HbA1c levels after the study period ended [10]. However, there are several caveats to these findings that may diminish their significance (e.g. the post-trial follow-up study did not measure nephropathy, which would have provided important process measure data to explain results). Additionally, there were few ESRD events and no difference in renal death, raising the possibility that the difference may be related to survivorship bias.
In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, findings were different from those of other studies. In this trial, 10,251 participants (mean age 62.2 years; median diabetes duration 10 years) were randomised to undergo either intensive glycaemic control (target HbA1c 42 mmol/mol [6.0%]) or standard glycaemic control (HbA1c 53–63 mmol/mol [7.0–7.9%]) [11]. The study was stopped after 3.5 years due to a significant increase in the rate of death in the intensive arm. In observational follow-up, intensive glycaemic control did not have an effect on the primary outcome of cardiovascular events (HR 0.95 [95% CI 0.87, 1.04]; p = 0.27) [12, 13]. However, in a subgroup analysis (the ACCORD Follow-on [ACCORDION] eye study), participants in the intensive glycaemic control group displayed a 45% decreased progression of retinopathy (adjusted OR 0.42 [95% CI 0.28, 0.63]; p < 0.0001), suggesting a possible legacy effect on microvascular outcomes [13]. Importantly, the participants in the ACCORDION eye study had to have survived the 4 year follow-up period and were slightly younger, with a shorter duration of diabetes and lower baseline HbA1c, than the average participant in the ACCORD trial.
Another conflicting result came from follow-up of the Veterans Affair Diabetes Trial (VADT). In observational studies following the VADT, which randomised 1791 individuals with longstanding diabetes to undergo intensive vs conventional glycaemic control, a significant reduction in macrovascular events (HR 0.83 [95% CI 0.70, 0.99]; p = 0.04) was seen in the intensive-control arm in an analysis performed after about 10 years of follow-up [14]. However, at the 15 year follow-up, when data were adjusted for the most recent HbA1c values, no significant reduction in macrovascular complications (HR 0.91 [95% CI 0.78, 1.06]; p = 0.23) was evident [15]. Unfortunately, results from the VADT trial on the long-term effects of intensive glycaemic control on microvascular diabetic complications have not been published.
Lastly, a smaller trial (Advanced Diabetic Nephropathy [ADN] CKD 3–4 Trial) enrolled 120 participants (mean age 57.5 years; mean diabetes duration 15 years) with advanced diabetic nephropathy and randomised them to undergo intensive control (integrated intensive diabetes and renal care with behavioural/dietary and pharmacological interventions) vs standard care [16]. After 2 years of follow-up, participants in the intensive control arm showed less progression towards ESRD (HR 0.13 [95% CI 0.02, 0.54]). About 60% of these participants were then followed after the trial to monitor for a legacy effect of diabetes control. The trial did not find a significant difference in outcomes in the follow-up period (23.7% advanced to ESRD in the intervention group vs 20.6% in the control group) [17].
In summary, the results from the observational follow-up of the clinical trials suggest that the duration of diabetes may modify the legacy effect, with longer durations of diabetes dampening any effects. Additionally, the long-term effects of glycaemic control likely have a greater effect on microvascular complications than macrovascular complications.
Real-world observational cohort studies
More evidence to elucidate the concept of the legacy effect comes from retrospective real-world cohort studies. These studies have been completed after major trials to examine whether the legacy effect exists in cohorts outside of clinical trial populations and to examine the extent of this effect on long-term microvascular and macrovascular outcomes.
Several studies have been conducted using data from the Kaiser Permanente Northern California (KPNC) Diabetes Registry. In a cohort study using KPNC data, following 34,737 individuals with newly diagnosed diabetes (mean age 56.8 years) for a mean of 13 years, participant outcomes were correlated to HbA1c values over each year of the study. The study found that when compared with an HbA1c <48 mmol/mol (6.5%), higher HbA1c values during the first year after diabetes diagnosis were associated with an increased future risk of microvascular diabetic complications and mortality, even when corrected for HbA1c values after the first year [18]. Additionally, longer periods of early exposure to an HbA1c >64 mmol/mol (8.0%) were associated with increased microvascular events and increased mortality [18]. These findings suggest that very early glycaemic control after a diagnosis of diabetes can have long-term effects on complications up to 10 years from diagnosis and echo the results of the DCCT and UKPDS trials.
In another longitudinal study using KPNC data, 28,016 individuals with newly diagnosed diabetes (mean age 56.2 years) were found to fit into several different HbA1c trajectories over the course of 10 years [19]. After adjustment for demographic factors, the study found that individuals whose HbA1c was initially high and then later decreased had a 27% higher mortality rate (HR 1.27 [95% CI 1.03, 1.58]) and a 28% higher rate of microvascular disease (HR 1.28 [95% CI 1.08, 1.53]) when compared with individuals who had a low stable HbA1c trajectory [19]. These findings again demonstrated the important influence of early glycaemic control on future outcomes.
Similar results for the legacy effect were seen in a Japanese study of 1547 individuals (mean age 56 years) with diabetes (mean duration 5.9 years) followed up to 22 years [20]. Even with this long follow-up time, baseline HbA1c was found to be a significant predictor of microvascular diabetes complications. The investigators used a moving mean analysis of HbA1c values over the course of 22 years to determine that the duration of the legacy effect for this population appeared to be 14–19 years, with a greater effect seen at ≤10 years. The greatest effects were seen for diabetic retinopathy outcomes, followed by diabetic kidney disease, and the least effect was seen for CVD.
Systematic review evidence
One systematic review has attempted to summarise evidence for the legacy effect of glycaemic control in diabetes, specifically regarding cardiovascular outcomes [21]. In a review of seven RCTs, all of which are discussed above, data from a total of 40,346 participants were analysed. The review found that intensive glycaemic control correlated with significantly decreased risk of cardiovascular events (OR 0.86 [95% CI 0.77, 0.96]; p = 0.007). These findings were more pronounced in individuals with shorter duration of diabetes (<10 years) (OR 0.73 [95% CI 0.56, 0.94]; p = 0.01) and no pre-existing CVD (OR 0.64 [95% CI 0.48, 0.86]; p = 0.003), supporting the hypothesis that individuals with early diabetes are more likely to benefit from the legacy effect of intensive glycaemic control. However, when the authors of this systematic review examined post-trial observational studies, there appeared to be no evidence of a protective legacy effect on CVD (OR 0.99 [95% CI 0.92, 1.06]; p = 0.81). A review of observational studies carried about by the authors showed that in real-life populations there is some evidence for a legacy effect, although these studies are limited by their observational nature [21].
Other legacy effects in individuals with diabetes
While discussing the legacy effect of glycaemic control, it is important to reflect that the concept of long-term benefits conferred from early intensive treatment is not unique to glycaemic control. For example, a follow-up study of the ACCORD trial examined individuals with diabetes and dyslipidaemia who had received fibrate therapy in addition to statin therapy during the study period [22]. Though few of the participants continued fibrate therapy after the study ended, those in the treatment group continued to display lower rates of cardiovascular outcomes (HR 0.65 [95% CI 0.45, 0.94]; p = 0.02) even 5 years after the end of the trial. Additionally, a retrospective study examining outcomes in individuals with type 2 diabetes who underwent bariatric surgery found that the individuals whose diabetes went into remission after surgery had improved long-term microvascular outcomes even after they experienced a relapse of diabetes [23]. This points to long-term benefits of even a brief period of glycaemic control. Lastly, an observational follow-up of an RCT found that participants with diabetes who were treated with olmesartan had a significantly decreased rate of microvascular and macrovascular complications, even 3 years after they stopped using the medication [24]. All of these examples illustrate the lasting effects that various therapies can have on long-term diabetic outcomes.
Pathophysiological mechanisms
While the pathophysiological mechanisms underlying the legacy effect are not completely understood, several reviews have well summarised the evidence to date on the extended effects of hyperglycaemia at the cellular level (see Fig. 1) [25–27]. Currently, two major hypotheses have emerged. One important factor in the cellular impact of hyperglycaemia seems to be epigenetics. Elevated blood glucose has been shown to lead to epigenetic modifications of the endothelium through histone modification, DNA methylation and non-coding RNAs. These modifications to the endothelium are believed to drive changes in the microvasculature, leading to microvascular and macrovascular diabetes-related diseases [25]. The intracellular production of superoxide anions also appears to contribute to the lasting effects of hyperglycaemia. Increased glucose levels in cells leads to overproduction of superoxide anions in the mito-chondria, which results in downstream effects including the formation of AGEs, which have been associated with diabetes complications. The overproduction of superoxide anions continues even after blood glucose levels normalise, possibly explaining the lasting legacy effects seen after diabetes control is established [26]. Hyperglycaemia can also promote the formation of AGEs independently of superoxide anions. In addition, transient hyperglycaemia can induce the accumulation of senes-cent cells [28]. All of these mechanisms, including histone modification, DNA methylation, RNA alteration and AGE formation, converge on the activation of proinflammatory pathways, provid-ing a rationale for the chronic low-grade inflammatory status of type 2 diabetes of long duration [29]. Moreover, some particular aspects of glycaemic control may be involved in the phenomenon of metabolic memory [30].
Fig. 1.
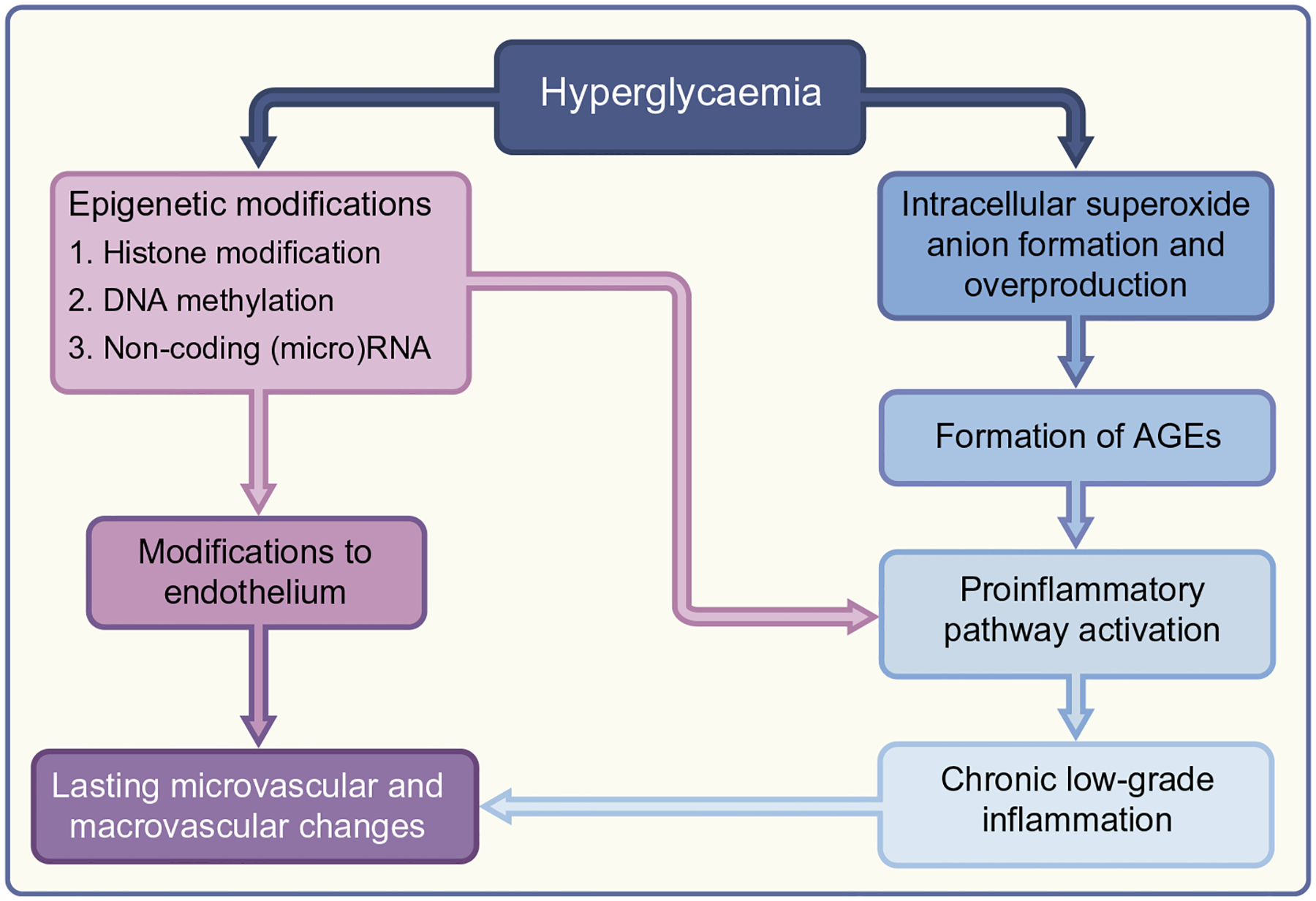
Pathophysiological mechanisms underlying the legacy effect.
Evidence for the legacy effect comes from both animal and human studies. In a landmark study, hyperglycaemia was induced in dogs at various levels in order to examine its effect on retinopathy [31]. The dogs that underwent induced hyperglycaemia exhibited higher rates of retinopathy, even if they had only experienced a short period of hyperglycaemia followed by euglycaemia. This study suggested that early and even brief hyperglycaemia could have long-term effects on vascular disease progression. In human studies, AGEs, in particular, have been closely tied to diabetic complications. In a follow-up study of the DCCT, higher levels of AGEs in skin biopsies were significantly correlated with the development of retinopathy and nephropathy [32, 33].
Conclusion
This review sought to summarise the evidence for the glycaemic legacy effect, the long-term effects of early glycaemic control in diabetes. The data seem to support more clearly the concept of a legacy effect for individuals with early diabetes. Evidence from the DCCT, UKPDS, Steno-2 and the KPNC observational cohort studies consistently demonstrate this conclusion for microvascular disease, and the DCCT, UKPDS and Steno-2 support this also for macrovascular disease. Results from trials involving individuals with established type 2 diabetes have been more mixed in regard to the legacy effect. There was consistency that there may be a glycaemic legacy effect for microvascular complications even in the ADVANCE and ACCORD long-term follow-up, but not the ADN CKD 3–4 trial. In addition, evidence for an effect on macrovascular disease was not present in ADVANCE, ACCORD or the VADT. Another complicating factor regarding the legacy effect and macrovascular complications is that glycaemic control has a small effect on macrovascular complications at best and since the time period during which DCCT and UKPDS were conducted new strategies have emerged that have a greater impact on the management of macrovascular diseases (e.g. tobacco cessation, statin therapy).
Overall, these results suggest that the glycaemic legacy effect is a long-term benefit (or risk) conferred to individuals with early diabetes that is muted over time as the individuals’ vasculature changes and they develop complications from diabetes.
Supplementary Material
Funding
Work in NL’s laboratory is supported by the National Institute on Minority Health and Health Disparities (R01 MD013420) and the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK092949). The funders had no role in the design and conduct of the review or the content and preparation of the manuscript.
Abbreviations
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ACCORDION
ACCORD Follow-on
- ADN
Advanced Diabetic Nephropathy
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- ESRD
End-stage renal disease
- KPNC
Kaiser Permanente Northern California
- RRR
Relative risk reduction
- UKPDS
UK Prospective Diabetes Study
- VADT
Veterans Affair Diabetes Trial
Footnotes
Supplementary Information The online version contains a slide of the figure for download available at https://doi.org/10.1007/s00125-021-05539-8.
Authors’ relationships and activities The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
References
- 1.The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329(14):977–986. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group (2009) Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Arch Intern Med 169(14):1307–1316. 10.1001/archinternmed.2009.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer IH, Rue TC, Cleary PA et al. (2011) Long-term Renal Outcomes of Patients With Type 1 Diabetes Mellitus and Microalbuminuria: An Analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Cohort. Arch Intern Med 171(5):412–420. 10.1001/archinternmed.2011.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY et al. (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353(25):2643–2653. 10.1056/NEJMoa052187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group (2016) Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care 39(5):686–693. 10.2337/dc15-1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352(9131):837–853. 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359(15):1577–1589. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 8.Gaede P, Lund-Andersen H, Parving HH, Pedersen O (2008) Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358(6):580–591. 10.1056/NEJMoa0706245 [DOI] [PubMed] [Google Scholar]
- 9.Patel A, MacMahon S, Chalmers J et al. (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358(24):2560–2572. 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 10.Wong MG, Perkovic V, Chalmers J et al. (2016) Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care 39(5):694–700. 10.2337/dc15-2322 [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Miller ME, Byington RP et al. (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358(24): 2545–2559. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Genuth S et al. (2011) Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 364(9):818–828. 10.1056/NEJMoa1006524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group (2016) Persistent Effects of Intensive Glycemic Control on Retinopathy in Type 2 Diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study. Diabetes Care 39(7):1089–1100. 10.2337/dc16-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayward RA, Reaven PD, Wiitala WL et al. (2015) Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 372(23):2197–2206. 10.1056/NEJMoa1414266 [DOI] [PubMed] [Google Scholar]
- 15.Reaven PD, Emanuele NV, Wiitala WL et al. (2019) Intensive Glucose Control in Patients with Type 2 Diabetes — 15-Year Follow-up. N Engl J Med 380(23):2215–2224. 10.1056/NEJMoa1806802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogelfeld L, Hart P, Miernik J et al. (2017) Combined diabetes-renal multifactorial intervention in patients with advanced diabetic nephropathy: Proof-of-concept. J Diabetes Complicat 31(3):624–630. 10.1016/j.jdiacomp.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 17.Kuzhively J, Tahsin B, Hart P, Fogelfeld L (2018) Legacy effect in combined diabetic-renal multifactorial intervention in patients with advanced diabetic nephropathy. J Diabetes Complicat 32(5):474–479. 10.1016/j.jdiacomp.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 18.Laiteerapong N, Ham SA, Gao Y et al. (2019) The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care 42(3):416–426. 10.2337/dc17-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laiteerapong N, Karter AJ, Moffet HH et al. (2017) Ten-year hemo-globin A1c trajectories and outcomes in type 2 diabetes mellitus: The Diabetes & Aging Study. J Diabetes Complicat 31(1):94–100. 10.1016/j.jdiacomp.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takao T, Matsuyama Y, Suka M, Yanagisawa H, Kasuga M (2019) Analysis of the duration and extent of the legacy effect in patients with type 2 diabetes: A real-world longitudinal study. J Diabetes Complicat 33(8):516–522. 10.1016/j.jdiacomp.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 21.Prattichizzo F, de Candia P, De Nigris V, Nicolucci A, Ceriello A (2020) Legacy effect of intensive glucose control on major adverse cardiovascular outcome: Systematic review and meta-analyses of trials according to different scenarios. Metabolism 110:154308. 10.1016/j.metabol.2020.154308 [DOI] [PubMed] [Google Scholar]
- 22.Zhu L, Hayen A, Bell KJL (2020) Legacy effect of fibrate add-on therapy in diabetic patients with dyslipidemia: a secondary analysis of the ACCORDION study. Cardiovasc Diabetol 19(1):28. 10.1186/s12933-020-01002-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman KJ, Haneuse S, Johnson E et al. (2016) Long-term Microvascular Disease Outcomes in Patients With Type 2 Diabetes After Bariatric Surgery: Evidence for the Legacy Effect of Surgery. Diabetes Care 39(8):1400–1407. 10.2337/dc16-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menne J, Ritz E, Ruilope LM, Chatzikyrkou C, Viberti G, Haller H (2014) The Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) observational follow-up study: benefits of RAS blockade with olmesartan treatment are sustained after study discontinuation. J Am Heart Assoc 3(2): e000810. 10.1161/jaha.114.000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin J, Wang X, Zhi X, Meng D (2019) Epigenetic regulation in diabetic vascular complications. J Mol Endocrinol 63(4):R103–R115. 10.1530/JME-19-0170 [DOI] [PubMed] [Google Scholar]
- 26.Ceriello A (2009) Hypothesis: the “metabolic memory”, the new challenge of diabetes. Diabetes Res Clin Pract 86(Suppl 1):S2–S6. 10.1016/S0168-8227(09)70002-6 [DOI] [PubMed] [Google Scholar]
- 27.Ceriello A, Ihnat MA, Thorpe JE (2009) Clinical review 2: The “metabolic memory”: is more than just tight glucose control neces-sary to prevent diabetic complications? J Clin Endocrinol Metab 94(2):410–415. 10.1210/jc.2008-1824 [DOI] [PubMed] [Google Scholar]
- 28.Prattichizzo F, Giuliani A, De Nigris V et al. (2016) Extracellular microRNAs and endothelial hyperglycaemic memory: a therapeutic opportunity? Diabetes Obes Metab 18(9):855–867. 10.1111/dom.12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sies H (2017) Hydrogen peroxide as a central redox signaling mole-cule in physiological oxidative stress: Oxidative eustress. Redox Biol 11:613–619. 10.1016/j.redox.2016.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceriello A, Nicolucci A (2019) Intensive Glucose Control and Type 2 Diabetes - 15 Years On. N Engl J Med 381(13):1292–1293. 10.1056/NEJMc1909041 [DOI] [PubMed] [Google Scholar]
- 31.Engerman RL, Kern TS (1987) Progression of incipient diabetic retinopathy during good glycemic control. Diabetes 36(7):808–812. 10.2337/diab.36.7.808 [DOI] [PubMed] [Google Scholar]
- 32.Genuth S, Sun W, Cleary P et al. (2005) Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 54(11):3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genuth S, Sun W, Cleary P et al. (2015) Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes 64(1):266–278. 10.2337/db14-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


