SUMMARY
A cohort study was performed to investigate cryptosporidial prevalence and species distribution in 13 organic and 13 conventional dairy herds. Faecal samples were collected from 221 calves and 259 cows. Management routines were recorded at farm inspection and through a questionnaire. Samples were concentrated using sodium chloride flotation and cryptosporidial oocysts were detected by epifluorescence microscopy. Molecular analysis was used to determine species and subtypes. A multivariable model for factors associated with calves being Cryptosporidium spp. positive was built. Cryptosporidium spp.-positive animals were identified in all herds. Prevalences were similar in organic and conventional calves (44·7% vs. 52·3%), as well as in cows (3·1% vs. 3·8%), P > 0·05. Cryptosporidium bovis, C. ryanae and C. parvum were identified. C. ryanae was identified in a calf younger than the described prepatent period. The multivariable model included four significant variables; calf age, cleanliness of bedding, cleaning routines for group pens and farmers' attitudes towards biosecurity.
Key words: Cattle, Cryptosporidium, epidemiology, zoonoses
INTRODUCTION
Parasites of the protozoan genus Cryptosporidium spp. are common in cattle throughout the world (summarized in table 6 of Hamnes et al. [1]). Cryptosporidia have recently been shown to be ubiquitous also in Swedish dairy herds, with 96% and 100% herd prevalence in two studies [2, 3]. Four species are considered to commonly infect cattle. Cryptosporidium parvum is the main species in young calves and is a cause of neonatal diarrhoea. C. bovis, C. ryanae and C. andersoni are more common in weaned calves and older cattle, with different prevalences and age distribution reported [4–7]. All four species have been identified in Swedish dairy cattle, but the dominant species in preweaned calves (age ⩽2 months) was C. bovis with 74% prevalence, whereas C. parvum was only detected in 20% of preweaned calves [8]. C. bovis was also the main species in young stock and cows, whereas C. ryanae and C. andersoni was only detected in a few animals [8].
Organic farming has been associated with risk for parasite infections such as lungworm [9] but the association of organic farming and Cryptosporidium spp. infection has to our knowledge not been thoroughly investigated. Organic herds in Sweden are required to follow the regulations of the Swedish Organization for Organic Farming (KRAV [10]) in addition to the EU organic legislation. Regulations include double withdrawal times for milk and meat after antibiotic use [10, 11], and a restriction in which disinfectants are allowed [11]. The Swedish animal welfare legislation is strict, and as an effect, alternative medicine is not encouraged for organic herds [12]. Thus, antibiotic treatment strategies might not differ in Swedish organic and conventional herds. Concerning parasites, it is a requirement that a veterinarian identifies a clinical parasite problem in order for an organic farmer to use antiparasitic drugs, whereas a conventional farmer can use antiparasitic drugs prophylactically. Because internal cryptosporidial stages are resistant to antibiotics and antiparasitic drugs, and oocysts are resistant to many disinfectants, less use of such compounds should not be an explanation for a potentially higher infection risk. However, other management differences, such as keeping organic calves with the dam for 3 days after birth, could affect infection pressure and thus prevalence.
A recent study investigated management factors associated with Cryptosporidium spp. infection in sampled cattle [2]. For organic cows, the odds ratio (OR) of being infected was 4·0 compared to conventional cows. Because few organic cows (n = 30) were sampled compared to conventional cows (n = 219), this could have produced a skewed association. In addition, organic cows were sampled closer to parturition, which could be an explanation if a periparturient rise in oocyst shedding is present [13, 14] and thus organic management might not be the true association. However, such an association was also shown by Maddox-Hyttel et al. [15], with a higher OR for high shedding rates in organic calves compared to conventional calves. DNA analysis was performed on samples from Silverlås et al. [2], but differences in species distribution in organic and conventional farms were not investigated due to the small number of samples from organically farmed animals [16].
This cohort study was performed to investigate whether overall Cryptosporidium spp. prevalence and species distribution differ between organic and conventional dairy herds at herd, calf and cow level. Another aim was to investigate whether differences in management routines could affect the risk of infection in preweaned calves.
METHODS
Study design
Forty herds that participated in a study about possible health differences in conventional and organic herds [17] were contacted by letter and asked to participate in this cohort study. Of these, we received agreement to participate for 13 conventional and 13 organic, four declined and 10 were excluded for technical or time-limiting reasons. All herds were enrolled in the Swedish Official Milk Recording Scheme (SOMRS) and had at least 40 milking cows per year. Herds were located in mid-south Sweden (Fig. 1); Uppland (n = 10), Södermanland (n = 4), Östergötland (n = 11) and Småland (n = 1).
Fig. 1.
Map of Sweden showing the location of the organic and conventional herds included in the study.
Mean herd size was 72·4 (range 41–111) cows in organic herds and 69·5 (range 41–131) cows in conventional herds. Mean milk production during 2009–2010 was 8772 kg (range 7296–12 030) in organic herds and 9258 kg (range 7767–10 766) in conventional herds. In comparison, mean values for all organic herds (n = 351) in SOMRS was 66·2 cows and 8380 kg milk during 2009–2010. Conventional herds (n = 3948) in SOMRS had an average of 63·9 cows and a milk yield of 9299 kg during the same period (N.-E. Larsson, Swedish Dairy Association, personal communication).
Observations on calf management strategies were recorded on a farm inspection form with pre-printed observation points and scoring categories (see online Supplementary Appendix S1). A questionnaire (Supplementary Appendix S2) about biosecurity and calf management was completed by the farmers during the visit. The study was approved by the Ethical Committee for Animal Experimentation in Uppsala, Sweden (C240/9).
Samplings
Samplings were conducted during one indoor season (October 2009 to February 2010). In each herd, 10 cows and 10 preweaned calves (age ⩽2 months/62 days) were to be sampled at one occasion. Cows were selected so that the whole cow-year would be represented: 0–6 weeks in milk (n = 3), 6–24 weeks in milk (n = 3), 24–42 weeks in milk (n = 2) and dry cows (n = 2). If more than 10 calves were present, calves were chosen so that an even distribution in the 2-month age interval would be achieved. If less than 10 calves were present, all calves were sampled.
Rectal faecal samples were collected in individual plastic pots using disposable latex gloves. Samples were cooled within an hour from sampling and kept at 4–8 °C until processed within a week from collection. Data recorded for individual animals at sampling was faecal consistency (all), age and pen type (calves) and lactation stage (cows). Body condition and cleanliness of animal was assessed as overall percent of clean animals and animals in optimum body condition for the whole herd. Standardized five grade assessment scales were used [18, 19]. Animals were considered to be in optimum body condition at grades 3–3·5, and to be clean at grades 1–2. For cows, the SOMRS database was used to obtain recorded dates for last calving before the visit, as well as the next calving date for cows that were pregnant at the visit. The post-visit information from SOMRS was retrieved in May 2010.
Detection of Cryptosporidium spp. oocysts and species and subtype determination
One gram of each faecal sample was cleaned and concentrated by a saturated sodium chloride flotation method, stained with monoclonal anti-Cryptosporidium antibodies (CryptoCel IF test kit (CelLabs, Australia), and analysed for Cryptosporidium spp. oocysts by epifluorescence microscopy as described previously [2]. A 60 μl subsample of the final 1·5 ml volume was used, and an animal was considered Cryptosporidium spp. positive if at least one oocyst was detected. The estimated shedding in oocysts per gram (OPG) is twice the total oocyst count of a concentrated sample due to a 50% loss during processing [20]. Concentrated samples were stored at 4–8 °C.
Concentrated calf samples containing in total ⩾250 oocysts were analysed to determine species. Calf samples with fewer oocysts were used if no samples with ⩾250 oocysts were present in a positive herd. All cow samples were analysed. DNA was extracted using a combined freeze-thawing and QIAamp DNA stool mini kit (Qiagen, USA) protocol. A nested PCR protocol for partial amplification of a ∼800 bp fragment of the 18S rRNA gene, and sequencing of PCR products in both directions was used for species determination. Samples containing C. parvum were subtyped through partial amplification (∼800 bp) of the 60 kDa glycoprotein (gp60) gene using a nested PCR protocol and sequencing in both directions. These methods have been described previously [16].
Statistical analysis
Statistical analysis was performed using Stata 11 (1984–2009, StataCorp LP, USA). Statistics were from herd, calf and cow level, using χ2 test, Fisher's exact test (F) or the Mann–Whitney test (MW) as appropriate. Some categorical variables were re-grouped to reduce number of levels and increase degrees of freedom (d.f.). Regrouping was done based on assessment of, e.g. a low, medium or high percentage of animals with diarrhoea, or to produce an approximately equal number of animals in each level. A multivariable random-effects logistic model for factors associated with being Cryptosporidium spp. positive was done for calves, using the xtlogit command. Animal-level variables (age, pen type, faecal consistency), herd-level variables from the farm inspection (n = 41) and the questionnaire (n = 29) were investigated. Variables with P ⩽ 0·2 in univariable logistic regression were considered for further analysis. A causal web was created to identify potential intervening factors, and variables deemed as intervening were not considered for further modelling. In addition, collinearity of the P ⩽ 0·2 variables was investigated using Spearman's rank correlations test. If correlated ⩾60%, one of the correlated variables was chosen to be included in the multivariable analysis. The variable ‘Organic’ was included in all steps of multivariable modelling even if not significant at P ⩽ 0·2 in the univariable model, and eliminated at the end to check for effects on the model. Modelling was done manually, both by backwards elimination of non-significant variables and by forward selection. For each eliminated or entered variable, confounding was assessed by comparing the coefficient change of included variables. Confounding was considered as present if a coefficient changed by >25%. The eliminated or entered variable was then retained in the model even in case of P > 0·05, and the selection process continued. Because observations were nested within herds, herd was used as a random effect to account for non-independence of observations. Two-way interactions were investigated once a main-effects model had been achieved.
RESULTS
Sampled calves
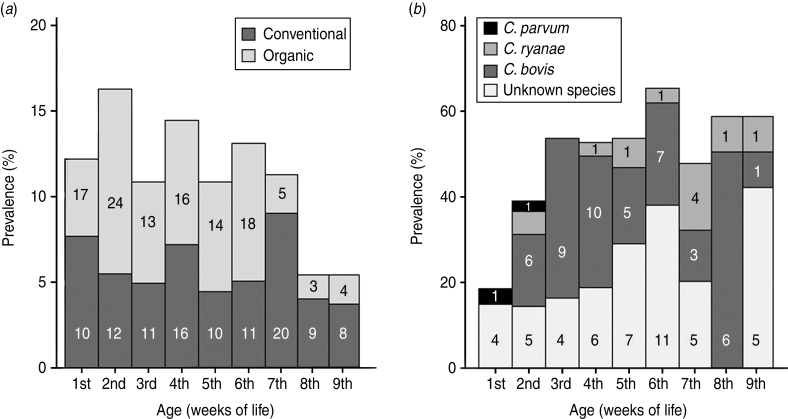
A total of 114 organic and 107 conventional calves were sampled. The number of sampled calves per herd varied from five to 10. The age of sampled calves was 2–65 days (median 26 days). The age distribution of sampled organic and conventional calves is given in Figure 2a. Organic calves were significantly younger than conventional calves, median age 23 vs. 32 days (MW = 3·6, P < 0·001). Sampled calves were more often kept in single pens in conventional herds (χ2= 12·8, d.f. = 1, P < 0·001). C. parvum-like oocysts were detected in 107 (48·6%) of the calves. No C. andersoni oocysts were detected. Prevalence peaked in the 6th week of life with 65·5% calves positive. There was no prevalence difference between organic and conventional calves, 44·7% vs. 52·3% (χ2 = 1·3, d.f. = 1, P > 0·05). Cryptosporidium spp.-negative calves were significantly younger than positive calves (median 22·5 days vs. 30 days; MW = −3·2, P < 0·01). This age difference was also seen in organic herds [median 18 days (n = 63) vs. 27 days (n = 51); MW = −2·7, P < 0·01)], whereas there was no significant age difference in conventional negative and positive calves [median 26 days (n = 51) vs. 36 days (n = 56); MW= −1·5, P > 0·05)]. Shedding rates were 50 to >8 million OPG. There was no association between infection and loose faecal consistency (51·4% prevalence in non-diarrhoeal calves vs. 37·0% prevalence in diarrhoeal calves; χ2 = 3·1, d.f. = 1, P > 0·05) or Cryptosporidium spp. shedding rates and loose faecal consistency (0 to >8 million OPG in non-diarrhoeal calves vs. 0–1 million OPG in diarrhoeal calves; MW = 1·4, P > 0·105). Calves in single and group pens were equally infected (χ2 = 1·8, d.f. = 1, P > 0·05).
Fig. 2.
(a) Age distribution in the 221 sampled preweaned calves, separated by management system, and (b) Cryptosporidium spp. prevalence in sampled calves, separated into the different species determined or samples with unknown species. Numbers within each field represent the number of calves in each specific category. The two calves aged 64 and 65 days are included in the 9th week of life category.
Sampled cows
A total of 129 organic and 130 conventional cows were sampled. Cows were in 1st to 8th parity, with the majority (n = 183) being in the 1st or 2nd parity. For 22 cows that had passed half a parity (182·5 days based on one calving/year), data on next calving were not recorded in the SOMRS database, and last calving date was used to identify how close to calving they were sampled (183–344 days postpartum).
Nine (3·5%) cows, four (3·1%) organic and five (3·8%) conventional, were positive for C. parvum-like oocysts (P > 0·05). No C. andersoni oocysts were detected. Shedding rates were 50 (n = 7) and 150 (n = 2) OPG. Cryptosporidium spp.-positive cows were sampled 11 days prepartum to 187 days postpartum (median 71·6 days) whereas Cryptosporidium spp.-negative cows were sampled 62 days prepartum to 344 days postpartum (median 81·4 days), P > 0·05. Three Cryptosporidium spp.-positive cows were sampled in the 1st parity, three in the 4th parity, and one positive cow each was sampled in the 2nd, 3rd and 5th parity.
Cryptosporidium spp. and subtype analysis
Two cow samples and 72 calf samples, containing 25 oocysts to >4 million oocysts, were used for DNA analysis. Species was successfully determined in 61 (82·4%, 60 calves and one cow) samples.
The most common species in calf samples was C. bovis (78·3%, n = 47), followed by C. ryanae (18·3%, n = 11) and C. parvum (3·3%, n = 2). Shedding rates were 300 to >8 million OPG for C. bovis, 100–835 000 OPG for C. ryanae and 750–500 000 OPG for C. parvum. C. bovis was detected from age 9 days, and the C. parvum-positive calves were aged 6 and 12 days. C. ryanae was detected from age 8 days in a calf shedding 100 000 OPG. Age-related overall prevalence and species distribution is given in Figure 2b. C. parvum subtypes were IIaA16G1R1 and IIaA13R1. C. bovis was identified in 22 conventional and 25 organic calves, whereas C. ryanae was only identified in conventional calves and C. parvum only in organic calves. The cow sample contained C. bovis.
Multivariable model for presence of Cryptosporidium spp. in calves
Two calves were aged >2 months (64 and 65 days) but were kept in the dataset. They were from the same organic herd and one was positive and one was negative for Cryptosporidium spp. Univariable models returned 14 variables with P ⩽ 0·20 (Table 1). Seven of these variables were excluded from multivariable modelling. No collinearity (⩾60%) was identified. Still, because both ‘Number of cows’ and ‘Number of preweaned calves at sampling’ are estimates of herd size, ‘Number of calves at sampling’ was chosen for modelling and ‘Number of cows’ was excluded. The causal web identified ‘Calf cryptosporidial prevalence’ and ‘Percent dirty animals’ as potential intervening factors. ‘Percent animals with diarrhoea’ could be an intervening factor or an effect of Cryptosporidium spp. infection. In addition, the significant level of that variable was the ‘no data’ level and the variable was not used in modelling. For the variables ‘Homogenous density in group pens’ and ‘Separate area for feeding’, the significant level was the ‘no data’ level and these variables were not used in modelling. The questionnaire variable ‘Preweaned calf management’ (P = 0·07, Table 1) was supposed to have identical answers to the farm inspection variable ‘Placing of preweaned calves’ (P = 0·24) (Supplementary Appendix S1). Still, these two variables were only 49% correlated, and it was decided not to use the questionnaire variable in modelling although it had P < 0·2.
Table 1.
Distribution and odds ratios of variables with P ⩽ 0·20 in univariable random logistic regression models of factors associated with being Cryptosporidium spp. positive in 221 calves from 13 organic and 13 conventional herds
| Variable | Number of observations | OR (95% CI) | P | |
|---|---|---|---|---|
| Organic | Conventional | |||
| Calf level | ||||
| Age, days* | 2–65 | 3–60 | 1·03 (1·01–1·05) | 0·003 |
| Herd level | ||||
| Number of cows* | 41–111 | 41–131 | 0·99 (0·98–1·00) | 0·108 |
| Calf cryptosporidial prevalence (%) | 0·000 | |||
| <33 | 30 | 42 | 1 | |
| 33–67 | 57 | 35 | 3·10 (1·57–6·12) | |
| >67 | 27 | 30 | 12·14 (5·25–28·04) | |
| Number of preweaned calves at sampling | 0·089 | |||
| <11 | 28 | 38 | 1 | |
| 11–20 | 66 | 50 | 1·14 (0·51–2·57) | |
| >20 | 20 | 19 | 0·35 (0·11–1·10) | |
| Farm inspection | ||||
| Number of fattening calves per feeding trough | 0·144 | |||
| 1 | 64 | 59 | 1 | |
| >1 | 40 | 39 | 2·23 (1·00–4·97) | |
| No data | 10 | 9 | 1·23 (0·31–4·94) | |
| Homogenous density in group pens | 0·139† | |||
| Yes | 84 | 88 | 1 | |
| No | 10 | 9 | 1·53 (0·39–5·97) | |
| No data | 20 | 10 | 4·17 (0·81–21·52) | |
| Cleanliness of bedding/pen floors (% surface that is clean) | 0·017 | |||
| <20 | 7 | 14 | 1 | |
| 20–50 | 19 | 26 | 0·15 (0·04–0·66) | |
| >50 | 88 | 39 | 0·15 (0·04–0·57) | |
| High variability between pens | 0 | 28 | 0·39 (0·08–1·88) | |
| Separate area for feeding | 0·158† | |||
| Yes | 102 | 91 | 1 | |
| No | 5 | 9 | 1·55 (0·35–6·86) | |
| No data | 7 | 7 | 6·83 (0·81–57·6) | |
| Animals with diarrhoea (%)‡§ | 0·197† | |||
| 0 | 14 | 44 | 1 | |
| 1–10 | 32 | 22 | 1·46 (0·54–3·92) | |
| 11–29 | 40 | 7 | 0·69 (0·24–1·97) | |
| 30–50 | 18 | 26 | 0·89 (0·31–2·54) | |
| No data | 10 | 8 | 4·43 (0·93–21·01) | |
| Dirty animals (%)‡§ | 0·093 | |||
| <20 | 64 | 16 | 1 | |
| 20–40 | 50 | 37 | 2·26 (0·97–5·30) | |
| >40 | 0 | 54 | 2·53 (0·95–6·70) | |
| Questionnaire | ||||
| Bought livestock last 2 years | 0·090 | |||
| No | 59 | 29 | 1 | |
| Yes | 55 | 78 | 0·50 (0·23–1·11) | |
| Attitude towards biosecurity | 0·069 | |||
| Important | 51 | 72 | 1 | |
| Very important | 63 | 35 | 0·49 (0·23–1·06) | |
| Preweaned calf management | 0·063 | |||
| Single pens | 0 | 24 | 1 | |
| Single and group pens | 61 | 74 | 0·94 (0·32–2·81) | |
| Group pens | 28 | 0 | 0·31 (0·07–1·35) | |
| Group pen with nursing cow | 25 | 9 | 2·11 (0·55–8·10) | |
| Cleaning of group pens | 0·131 | |||
| Daily | 28 | 15 | 1 | |
| Once per week | 30 | 8 | 1·34 (0·41–4·36) | |
| Every 2 weeks | 15 | 37 | 3·87 (1·29–11·57) | |
| Less often than every 2 weeks | 41 | 22 | 2·48 (0·88–7·03) | |
| No group pens | 0 | 25 | 2·00 (0·54–7·37) | |
OR, Odds ratio; CI, confidence interval.
Continuous variable, range.
‘No data’ level is the ‘significant’ one.
Includes all sampled animals within a farm.
Point 29a in Supplementary Appendix S1, categorized to produce an approximately equal number of animals in each category.
Forward and backward modelling resulted in the same main-effects model (Table 2). No significant two-way interactions were detected. ‘Organic’ was not significant but was kept in the model as a confounder because it affected two levels of the estimates of ‘Cleanliness of bedding’ with 24% and 44%, respectively. The final model also included four significant variables and the slightly non-significant variable ‘Bought livestock last 2 years’ (P = 0·05). When this variable was removed, the OR of ‘Organic’ changed by 42%, from 1·78 (P = 0·22) to 2·53 (P = 0·03). Thus, the variable was controlled for as a confounder to ‘Organic’.
Table 2.
Final random logistic regression model* of factors associated with being Cryptosporidium spp. positive in 221 calves from 13 organic and 13 conventional herds
| Variable | OR (95% CI) | P |
|---|---|---|
| Organic | ||
| No | 1 | 0·219 |
| Yes | 1·78 (0·71–4·46) | |
| Age, days | 1·03 (1·01–1·06) | 0·001 |
| Cleanliness of bedding/pen floors (% of surface that is clean) | ||
| <20 | 1 | 0·001 † |
| 20–50 | 0·08 (0·01–0·21) | 0·000 |
| >50 | 0·29 (0·05–0·94) | 0·040 |
| High variability between pens | 0·26 (0·03–0·86) | 0·033 |
| Bought livestock last 2 years | ||
| No | 1 | 0·052 |
| Yes | 0·49 (0·24–1·01) | |
| Attitude towards biosecurity | ||
| Important | 1 | 0·001 |
| Very important | 0·21 (0·08–0·54) | |
| Cleaning of group pens | ||
| Daily | 1 | 0·003 † |
| Once per week | 1·72 (0·58–5·09) | 0·326 |
| Every 2 weeks | 8·06 (2·56–25·33) | 0·000 |
| Less often than every 2 weeks | 0·63 (0·18–2·17) | 0·465 |
| No group pens | 2·13 (0·60–7·56) | 0·243 |
OR, Odds ratio; CI, confidence interval.
Wald χ2 = 36·89, P < 0·001, goodness of fit at 8 d.f., χ2 = 3·90, P = 0·87.
Overall P value of multi-level variable.
DISCUSSION
Sampled animals, Cryptosporidium spp. prevalence and species distribution
Cryptosporidium spp. prevalence was lower than in our previous studies, where prevalences were 52% vs. 66% for calves and 6% vs. 14% for cows [2, 3]. Samplings were conducted during the same time of the year in all three studies, and thus season should not affect prevalence. In both previous studies [2, 3] samplings were conducted during two consecutive winters, and we found a prevalence difference between the two years in one study [2]. We suggested that this could possibly be due to different weather conditions. The winter of 2009–2010, when the present study was conducted, was cold with a permanent covering of snow from the beginning of December until March. These weather conditions had not been present in many years, and it is possible that the cold weather had a dampening effect on Cryptosporidium spp. infection pressures compared to the winters of 2005–2008. Calves were targeted at the same age interval (age ⩽2 months) in the present study as in the previous ones, and median age was about the same (24 and 24·5 days, C. Silverlås, unpublished data). Thus, age differences would not cause the lower calf prevalence in the present study. A periparturient rise in oocyst shedding has been shown [13, 14], whereas others have not shown this connection [21]. For cows, one explanation for a lower prevalence could be that in contrast to our two previous studies, periparturient cows were not targeted here. An increase in shedding would benefit detection of infected animals because cows usually shed low oocyst levels [13, 22, 23]. We previously argued that the higher OR of infection for organic cows in one study could be due to the fact that they were accidentally sampled closer to parturition than conventional ones, rather than being from organic herds [2]. The results of the present study seem to contradict that argument, because Cryptosporidium spp.-infected cows were not closer to parturition compared to negative ones, and all positive samples contained few oocysts. However, because of the small number of positive cows in both the present study and our previous ones [2, 3], conclusions cannot be made with certainty.
Cryptosporidium spp. prevalence in calves peaked later than in our previous studies (peaks in 3rd to 5th week of life [2, 3]). This could be due to a lower infection pressure, because a lower infection pressure could extend the time from birth to infection.
The lack of an association of high shedding rates and diarrhoea could be due to the high prevalence of the non-pathogenic species C. bovis that can be shed in high numbers. In this study, 6/8 samples with at least 500 000 OPG contained C. bovis. This lack of association was also identified in our previous studies [3, 16].
Our results indicate that organic calves become infected earlier than conventional calves because prevalences were similar but organic calves were younger than conventional calves. This could be an effect of earlier grouping in organic herds, as indicated by the fact that calves were more commonly kept in single pens in conventional herds. Swedish organic herds are bound by the EU legislation to keep calves in single pens for no longer than 1 week, except in sporadic cases [10]. According to the KRAV legislation [10] at the time the present study was performed, calves were required be kept with the dam for 3 days postpartum. Thus, calves aged >10 days should be kept in group pens. The oldest conventional calf in a single pen was aged 60 days.
As in our previous studies, C. bovis was the main species in calves, and the low presence of C. parvum in the present study further supports our previous results that C. parvum is not a dominant species in preweaned Swedish dairy calves. The two C. parvum subtypes identified both belonged to zoonotic allele family IIa. Subtype IIaA16G1R1 has previously been identified in calves and humans in Sweden, whereas IIaA13R1 has not been identified in either species [3, 16] (Insulander et al., unpublished observations). Whereas C. bovis was evenly distributed between organic and conventional calves, all C. ryanae samples came from conventional calves, and the C. parvum samples were from organic calves. We have previously identified these species in both organic and conventional herds, most samples being from conventional herds for the latter two species [3]. Because of the low total number of samples from both studies ([16], present study), n = 21 for C. ryanae and n = 17 for C. parvum, we cannot conclude that either of these two species is more common in one of the systems.
We have shown previously [16] that the prepatent period of C. bovis is shorter (7 days) than what has been shown by experimental infection (10 days [24]). Here, we show that C. ryanae has a shorter prepatent period than the 11–12 days described previously [25], because we could identify this species in an 8-day-old calf with high shedding rates, which shows that infection was manifest. The two calves experimentally infected with C. ryanae [25] were aged 17–18 days when challenged, and although these calves Cryptosporidium-naive and colostrum deprived, it is possible that neonatal calves are more susceptible to infection and have a shorter prepatent period than older calves. In addition, the infection dose of our calf as well as the dose of the experimentally infected animals is unknown [25]. A higher infection dose in our calf could produce a shorter prepatent period, because shedding rates could pass the detection level earlier than at a lower dose. It is also possible that the detection method we used could detect lower shedding rates than the method used by Fayer and colleagues [24, 25] enabling earlier detection of infection.
Multivariable model
It was interesting to see that the univariable OR of ‘Organic’ (0·72, P = 0·42) changed towards a risk factor during multivariable modelling, with an OR of 1·78 (P = 0·22) in the final model. The univariable estimate is in line with the apparently insignificant lower prevalence in organic calves. Obviously management factors that differ between organic and conventional herds will affect the estimate. Indeed, when ‘Attitude towards biosecurity’ was entered in forward modelling, the ‘Organic’ OR switched from 1·68 (P = 0·21) to 2·53 (P = 0·03), and when ‘Bought livestock last 2 years’ was entered, the OR decreased and became non-significant again. Thus, biosecurity attitudes and routines seem to differ with management and somehow counteract each other. It could be argued that farmers believing biosecurity to be ‘very important’ would be less prone to purchase new animals. On the other hand, biosecurity is very important once new animals are introduced into a herd. Seven organic farmers believed biosecurity to be ‘very important’, compared to four conventional farmers, and thus it seems strange that the OR for ‘Organic’ increased when this variable was entered into the model. It is possible that this variable actually measured something else that farmers with the same attitude had in common, and that was a potential risk factor not observed through the other questions or the farm inspection. Fewer organic farmers (six, compared to nine conventional) had bought livestock, and so the OR decrease of ‘Organic’ when ‘Bought livestock last 2 years’ was entered is in line with the data distribution.
Whereas confounding factors should be controlled for in a model, intervening factors should not be included. Intervening factors are factors that appear in time between the variable of interest and the outcome, and that hide the effect of other factors so that they appear non-significant. ‘Calf cryptosporidial prevalence’ was suspected to be an intervening factor because it would be affected by all management factors important for Cryptosporidium spp. infection pressure. Indeed, when entered into a model including all variables used for multivariable modelling, ‘Calf cryptosporidial prevalence’ became the only significant factor. Thus, this variable was not used for further modelling. ‘Percent dirty animals’ was an overall estimate for all sampled animals in a herd and was identified as a possible intervening factor to ‘Cleanliness of bedding’ that was registered for calves. When tested it generated no spurious effects, but was non-significant and could be dropped from modelling. The reason for excluding variables where the ‘no data’ level was significant is that we cannot interpret how the effects of these variables are mediated and if the estimates are true or simply exist because all data are not present.
‘Preweaned calf management’ (Questionnaire, Supplementary Appendix S2) and ‘Placing of preweaned calves’ (Farm inspection form, Supplementary Appendix S1) were included to cross-check the farmers' opinion of their management with the situation observed at farm inspection. Because the information from the questionnaire was not congruent with the situation observed, it was decided to drop this variable from modelling, and ‘Placing of preweaned calves’ could not be used because of P > 0·2. It could be argued that in that case we should not rely on any of the questionnaire answers, because these measure farmers' opinions, which would bias the data if the opinions are not congruent with the true situation. Still, many of the questions (such as frequency of cleaning) cannot be observed at a single visit, and questionnaire data thus is an extra information source.
Methodological considerations
Although not tested statistically, mean herd size for organic and conventional herds in this study seemed to be slightly larger than mean herd size for all organic and conventional herds in SOMRS. Milk yield in organic herds in our study seemed to be slightly higher than average milk yield in organic herds, whereas conventional herds were close to the overall mean. Still, the differences were not extreme, and herds were considered representative of an average Swedish organic or conventional dairy herd.
It would have been optimal to increase the sample size to at least the 40 original herds in order to make more comparisons at herd level. However, because herds were selected on the basis of joint enrolment in a previous comparative study, lost herds were not replaced.
Because of the low prevalence, no multivariable model was built for the association of Cryptosporidium spp. infection in cows with management factors.
The decision to prioritize samples with ⩾250 oocysts for DNA analysis was based on previous results [8]. Indeed, when samples with <250 oocysts were used, DNA analysis failed in 7/10 (70%) of samples. Successful analysis was achieved in three samples containing 50, 75 and 150 oocysts, respectively. In contrast, DNA analysis only failed in 6/64 (9·4%) of samples with ⩾250 oocysts (range 250–3500). The sample with 3500 oocysts was PCR positive, but the band was not of the expected size (only 5–600 bp) and the sample was not sequenced.
CONCLUSIONS
This study shows that Cryptosporidium spp. prevalence does not differ between Swedish organic and conventional herds. We have shown that the prepatent period of C. ryanae in naive calves is shorter than previously described. Cryptosporidium spp. infection pressure is affected by management differences that could be due to either management system or simply different thinking with regard to biosecurity (independent of system).
Supplementary Material
Supplementary information supplied by authors.
Supplementary information supplied by authors.
ACKNOWLEDGEMENTS
The project was funded by the Swedish University of Agricultural Sciences. Isabel Blanco-Penedo was in receipt of a fellowship from the Spanish Foundation of Pedro Barrié de la Maza. The authors thank Professor Camilla Björkman, for encouraging us to perform this study, and for valuable comments on the manuscript, the farmers who participated in this study and Mia Nordquist who helped during samplings. We also thank Professor Ulf Emanuelson for epidemiological advice and Katarina Näslund for skilful work on the molecular analysis.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268812000830.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Hamnes IS, Gjerde B, Robertson L. Prevalence of Giardia and Cryptosporidium in dairy calves in three areas of Norway. Veterinary Parasitology 2006; 140: 204–216. [DOI] [PubMed] [Google Scholar]
- 2.Silverlås C, et al. Prevalence and associated management factors of Cryptosporidium shedding in 50 Swedish dairy herds. Preventive Veterinary Medicine 2009; 90: 242–253. [DOI] [PubMed] [Google Scholar]
- 3.Silverlås C, et al. Cryptosporidium infection in herds with and without calf diarrhoeal problems. Parasitology Research 2010; 107: 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fayer R, Santin M, Trout JM. Prevalence of cryptosporidium species and genotypes in mature dairy cattle on farms in eastern United States compared with younger cattle from the same locations. Veterinary Parasitology 2007; 145: 260–266. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, et al. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Veterinary Parasitology 2007; 144: 1–9. [DOI] [PubMed] [Google Scholar]
- 6.Kvac M, Vitovec J. Prevalence and pathogenicity of Cryptosporidium andersoni in one herd of beef cattle. Journal of Veterinary Medicine, Series B 2003; 50: 451–457. [DOI] [PubMed] [Google Scholar]
- 7.Langkjaer RB, et al. Molecular and phylogenetic characterization of cryptosporidium and giardia from pigs and cattle in Denmark. Parasitology 2006; 89: 1–12. [DOI] [PubMed] [Google Scholar]
- 8.Silverlås C.Cryptosporidium infection in dairy cattle – prevalence, species distribution and associated management routines (dissertation). Uppsala, Sweden. Swedish University of Agricultural Sciences, 2010, 77 pp (http://pub.epsilon.slu.se/2219/1/silverlas_c_100129.pdf). [Google Scholar]
- 9.Höglund J, Svensson C, Hessle A. A field survey on the status of internal parasites in calves on organic dairy farms in southwestern Sweden. Veterinary Parasitology 2001; 99: 113–128. [DOI] [PubMed] [Google Scholar]
- 10.KRAV legislation 2011. Uppsala, Sweden: KRAV Incorporated Association, 2011. (www.krav.se/Documents/Regler/utgavor/KRAVsreglerUtgavaJanuari2011.pdf) [in Swedish: ]. [Google Scholar]
- 11.Anon. Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No. 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control. Official Journal of the European Union 2008; OJ L 250: 1–84. [Google Scholar]
- 12.Fall N.Health and reproduction in organic and conventional Swedish dairy cows (dissertation). Uppsala, Sweden. Swedish University of Agricultural Sciences, 2009, 57 pp (http://pub.epsilon.slu.se/2069/1/Thesis.Nils.Fall.Korrrigerad.pdf). [Google Scholar]
- 13.Faubert GM, Litvinsky Y. Natural transmission of Cryptosporidium parvum between dams and calves on a dairy farm. Journal of Parasitology 1999; 86: 495–500. [DOI] [PubMed] [Google Scholar]
- 14.Ralston BJ, McAllister TA, Olson ME. Prevalence and infection pattern of naturally acquired giardiasis and cryptosporidiosis in range beef calves and their dams. Veterinary Parasitology 2003; 114: 113–122. [DOI] [PubMed] [Google Scholar]
- 15.Maddox-Hyttel C, et al. Cryptosporidium and giardia in different age groups if Danish cattle and pigs – occurrence and management associated risk factors. Veterinary Parasitology 2006; 141: 48–59. [DOI] [PubMed] [Google Scholar]
- 16.Silverlås C, et al. Molecular characterisation of cryptosporidium isolates from Swedish dairy cattle in relation to age, diarrhoea and region. Veterinary Parasitology 2010; 169: 289–295. [DOI] [PubMed] [Google Scholar]
- 17.Fall N, et al. An observational study on early-lactation metabolic profiles in Swedish organically and conventionally managed dairy cows. Journal of Dairy Science 2008; 91: 3983–3992. [DOI] [PubMed] [Google Scholar]
- 18.Wildman EE, et al. A dairy cow body condition scoring system and its relationship to selected production characteristics. Journal of Dairy Science 1982; 65: 495–501. [Google Scholar]
- 19.Cook NB, Reinemann DJ.A tool box for assessing cow, udder and teat hygiene. Published on the web page of the University of Wisconsin, 2007. (http://fyi.uwex.edu/uwmril/whats-new/). Accessed 25 September 2009
- 20.Andersson S.Cryptosporidium infection in cattle – evaluation of a new method for detection of subclinical infection (master of veterinary medicine thesis) [in Swedish, abstract in English]. Uppsala, Sweden. Swedish University of Agricultural Sciences, 2004, 22 pp (http://ex-epsilon.slu.se:8080/archive/00000081/01/04.89_Tryckfil.pdf). [Google Scholar]
- 21.Atwill ER, et al. Evaluation of periparturient dairy cows and contact surfaces as a reservoir of Cryptosporidium parvum for calfhood infection. American Journal of Veterinary Research 1998; 59: 1116–1121. [PubMed] [Google Scholar]
- 22.Scott CA, et al. An epidemiological study of Cryptosporidium parvum in two herds of adult beef cattle. Veterinary Parasitology 1995; 57: 277–288. [DOI] [PubMed] [Google Scholar]
- 23.Sturdee AP, et al. Long-term study of cryptosporidium prevalence on a lowland farm in the United Kingdom. Veterinary Parasitology 2003; 116: 97–113. [DOI] [PubMed] [Google Scholar]
- 24.Fayer R, Santín M, Xiao L. Cryptosporidium bovis n.sp. (Apicomplexa: Cryptosporidiidae) in cattle (Bos taurus). Journal of Parasitology 2005; 91: 624–629. [DOI] [PubMed] [Google Scholar]
- 25.Fayer R, Santin M, Trout JM. Cryptosporidium ryanae n.sp. (Apicomplexa: Cryptosporidiidae) in cattle (Bos taurus). Veterinary Parasitology 2008; 156: 191–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information supplied by authors.
Supplementary information supplied by authors.
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268812000830.
click here to view supplementary material