Abstract
We reported previously on the function of acyl coenzyme A (acyl-CoA) oxidase isozymes in the yeast Yarrowia lipolytica by investigating strains disrupted in one or several acyl-CoA oxidase-encoding genes (POX1 through POX5) (H. Wang et al., J. Bacteriol. 181:5140–5148, 1999). Here, these mutants were studied for lactone production. Monodisrupted strains produced similar levels of lactone as the wild-type strain (50 mg/liter) except for Δpox3, which produced 220 mg of γ-decalactone per liter after 24 h. The Δpox2 Δpox3 double-disrupted strain, although slightly affected in growth, produced about 150 mg of lactone per liter, indicating that Aox2p was not essential for the biotransformation. The Δpox2 Δpox3 Δpox5 triple-disrupted strain produced and consumed lactone very slowly. On the contrary, the Δpox2 Δpox3 Δpox4 Δpox5 multidisrupted strain did not grow or biotransform methyl ricinoleate into γ-decalactone, demonstrating that Aox4p is essential for the biotransformation.
The biotransformation of fatty acids into γ-decalactone, a compound with a peach-like aroma, has led to numerous patents. Most processes are based on the use of yeast strains to transform ricinoleic acid or its derivatives into lactones, as reviewed by Endrizzi et al. (4). The development of such processes initially involves optimizing physical and chemical conditions for the biotransformation or screening to find new organisms that produce γ-decalactone. This stage of development is continuing, but finding ways to increase lactone production is increasingly demanding improvements in our knowledge of the relevant cellular mechanisms. Endrizzi et al. (2) showed that the β-oxidation pathway is involved in the biotransformation, and studies of intermediates have intensified (9, 10, 14, 20). Féron et al. (6, 7) have focused on the toxicity of lactone for the yeast that produces it, and Endrizzi-Joran (5) has focused on the reconsumption of lactone. Pagot (15) has been looking for critical steps and has found two potentially rate-limiting steps: the entry of fatty acids into peroxisomes, which is at least partially carnitine dependent (16), and the first and limiting (13, 18) reaction of peroxisomal β-oxidation (17), involving acyl coenzyme A (acyl-CoA) thioester oxidation by an acyl-CoA oxidase (Aox) to yield trans-2-enoyl-CoA derivatives. All these studies have increased our understanding of the mechanisms involved but have not substantially increased lactone production.
In Yarrowia lipolytica, acyl-CoA oxidases are encoded by five genes (POX1 through POX5). An investigation of the function of the Aox isozymes demonstrated a chain length specificity for Aox2p (long chain) and Aox3p (short chain). Aox2p and Aox3p together account for 70 to 80% of global Aox activity (22). Pagot et al. (17) disrupted POX1 (encoding Aox1p) and observed higher levels of β-oxidation and higher Aox activities but lower levels of lactone production, thereby demonstrating that Aox plays a fundamental role in lactone production. So far, no technological applications have resulted from the research of yeast acyl-CoA oxidases apart from that of Picataggio et al. (19), who blocked β-oxidation by sequential disruption of Aox genes in Candida tropicalis to redirect fatty acids to the ω-oxidation pathway, leading to the production of long-chain dicarboxylic acids.
We decided to focus on this step with the goal of significantly increasing lactone production. We used the set of disrupted strains for the POX genes described previously (22), with the aim of determining their role in lactone production. We found that Aox composition affects lactone production, either positively or negatively. Particularly, mutants disrupted for the POX3 gene encoding the short-chain-specific enzyme (Aox3p) had increased lactone productions.
The Y. lipolytica strains used in this study are derived from the wild-type Y. lipolytica W29 (ATCC 20460). Their constructions have been described elsewhere (21, 22). All strains were cultured for 48 h on malt extract agar (Difco) at 27°C and used to inoculate a 500-ml baffled Erlenmeyer flask containing 200 ml of glucose medium (15 g of glucose/liter, 2.5 g of ammonium chloride/liter, 0.1 g of yeast extract/liter, 2.1 g of monopotassium phosphate/liter, 4.51 g of disodium phosphate/liter, 0.2 g of magnesium sulfate/liter, 0.1 g of sodium chloride/liter, 9.14 mg of iron (II) sulfate heptahydrate/liter, 0.5 mg of zinc chloride/liter, 1.56 mg of copper sulfate/liter) to an optical density at 600 nm (OD600) of 0.25 (6 × 106 cells/ml). Flasks were shaken at 140 rpm for 18 h until the cultures reached the late logarithmic growth phase. Cells were harvested (10,000 × g, 5 min), washed twice with phosphate buffer (50 mM, pH 7.4), and resuspended in MD medium (6.7 g of yeast nitrogen base/liter, 5 g of ammonium chloride/liter, 1 g of methyl decanoate/liter) (OD600 = 0.25) for growth experiments or in MR medium (MD medium with 0.2 g of Tween 80/liter and with 1 g of methyl decanoate/liter replaced with 5 g of methyl ricinoleate/liter [Dubois, Boulogne, France]) (OD600 = 0.25) for growth and lactone production kinetic experiments.
Cells grown in MR or MD medium were counted in a Malassez cell. Slide Write+ (Advanced Graphics Software Inc., Carlsbad, Calif.) was used to fit curves and to calculate the parameters corresponding to the Gompertz model as modified by Zwietering et al. (23): Xmax, the maximal amount of biomass; μmax, the maximal growth rate (h−1); and λ, the lag phase (h).
For γ-decalactone extraction and analysis, 1.5 ml was removed and centrifuged. The supernatant (both aqueous and oil phases) was mixed, and its pH was lowered to 2 with HCl (6 N). The internal standard (γ-undecalactone) was added, and the mixture was extracted with 1.5 ml of diethyl ether in 4-ml glass vials by shaking for 1.5 min. The ether phase was then analyzed in an HP6890 gas chromatograph with an HP- INNOWax capillary column (30.0 m by 320 μm by 0.25 μm) using N2 as a carrier gas at a linear flow rate of 4.3 ml/min. The split injector (split ratio, 7.1:1) temperature was set to 250°C, and that of the flame ionization detector to 300°C. The oven temperature was programmed to increase from 60 to 145°C at a rate of 5°C/min and then at a rate of 2°C/min to 180°C.
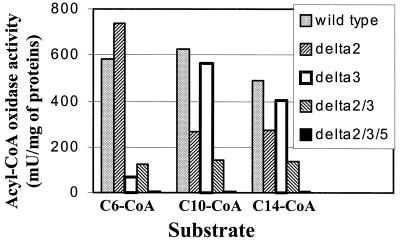
Contributions of each Aox isozyme to the global acyl-CoA oxidase activity can be deduced from Fig. 1, which represents the effect of gene disruption on the activity of the remaining enzymes in the cell extracts depending on substrate chain length (21, 22). As shown in Table 1, a POX monodisruption had no significant effect on the growth characteristics of the mutant strains. Consistent with Aox2 and Aox3 playing the major roles, the growth of the double-disrupted strain, Δpox2 Δpox3, was altered, with a lower μmax (0.07 h−1 compared to 0.15 h−1 for the wild type) and a longer λ (3.7 h, compared to 2.2 h). However, there were no significant differences in Xmax (results not shown) except for Δpox2 Δpox3 Δpox4 Δpox5 cells, which did not grow at all due to the lack of activity of the enzyme encoded by POX1 in those conditions.
FIG. 1.
Acyl-CoA oxidase activity profiles of mutant strains as a function of substrate chain length. Activity was measured independently with C6-CoA, C10-CoA, and C14-CoA substrates and was standardized for protein concentration (21). The strains presented here are the wild type, Δpox2, Δpox3, Δpox2 Δpox3, and Δpox2 Δpox3 Δpox5.
TABLE 1.
Growth in MR medium and γ-decalactone production kinetics for various mutant strains and the wild typea
| Strain | μmax (h−1) | λ (h) | (dP/dt)max (mg/liter/h) | qpmax (mg/106 cells/h) |
|---|---|---|---|---|
| Wild type | 0.15 ± 0.01 | 2.2 ± 0.4 | 6.1 | 0.34 |
| Δpox2 | 0.18 ± 0.01 | 2.7 ± 0.2 | 13.5 | 0.66 |
| Δpox3 | 0.15 ± 0.02 | 1.3 ± 0.5 | 31.1 | 1.30 |
| Δpox5 | 0.15 ± 0.01 | 2.9 ± 0.5 | 12.0 | 0.62 |
| Δpox2 Δpox3 | 0.07 ± 0.01 | 3.7 ± 0.4 | 9.4 | 0.48 |
| Δpox3 Δpox5 | 0.16 ± 0.01 | 1.4 ± 0.5 | 22.0 | 1.22 |
| Δpox2 Δpox3 Δpox5 | ND | ND | 7.1 | 0.67 |
| Δpox2 Δpox3 Δpox4 Δpox5 | 0 | ∞ | 0 | 0 |
Maximal growth rate (μmax) and lag phase (λ) were calculated with the Gompertz parameters. Maximal rate of production [(dP/dt)max] and maximal rate of specific production (qpmax) were determined from the production curves shown in Fig. 2. ND, not determined.
To determine whether particular Aox isozymes were involved in C10 consumption, we studied the growth of various mutants on the C10 substrate methyl decanoate instead of the toxic γ-decalactone (Table 2). μmax was significantly higher for Δpox2 and lower for Δpox3 than for the wild type. At least one of the two enzymes (Aox2p and Aox3p) must be present if the yeast is to grow in MD medium, as the double-disrupted strain, Δpox2 Δpox3, cannot grow on this substrate.
TABLE 2.
Growth kinetics of various mutant strains and the wild type in methyl decanoate
| Strain | Xmax (cells/ml−1) | μmax (h−1) |
|---|---|---|
| Wild type | 9.0 × 107 | 0.16 |
| Δpox2 | 10.8 × 107 | 0.23 |
| Δpox3 | 4.0 × 107 | 0.10 |
| Δpox2 Δpox3 | 6.0 × 106 (=X0) | 0 |
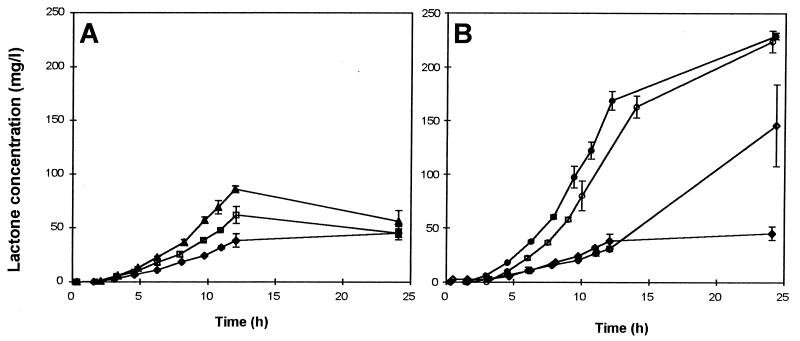
Figure 2 shows lactone production kinetics of various strains for the first 24 h of culture in MR medium. We can distinguish the mutants with Δpox3 (Δpox3 and Δpox3 Δpox5) and Δpox2 Δpox3 disruptions (Fig. 2B) from those with another disruption (Δpox2, Δpox5) or no disruption (wild type) (Fig. 2A). The strains with POX2 and POX3 produced γ-decalactone in the first 12 h, and thereafter, the reconsumption rate was at least equal to the production rate. For strains containing the POX3 deletion, lactone was produced during the first 24 h of culture to higher levels. The rate of productivity for Δpox3 and Δpox3 Δpox5 in the first 12 h was significantly higher (more than 12.5 mg/liter/h) than that of all the other strains (between 3 and 7 mg/liter/h). However, strains with Δpox2 Δpox3 disruptions produced lactone more slowly, with maximal production occurring, as for the maximal biomass production, between 12 and 24 h.
FIG. 2.
Lactone production by the various strains. (A) Monodisrupted strains other than Δpox3: Δpox2 (▴); Δpox5 (□), and wild type (⧫). (B) Δpox3 strains: Δpox3 (●); Δpox2 Δpox3 (◊); Δpox3 Δpox5 (○), and wild type (⧫). Lactone concentration was determined in the culture medium after extraction.
Table 1 shows the maximal rate of lactone production [(dP/dt)max] and maximal rate of specific production [qpmax = (dP/dt X)max]. Δpox2 Δpox3 Δpox4 Δpox5 cells did not grow or produce any lactone. The rates of production (6.1 mg/liter/h) and specific production were the lowest in wild-type cells. The strain with Δpox2 Δpox3 simultaneously disrupted had a slightly higher rate of lactone production (9.4 mg/liter/h). Higher rates (twice that of the wild type) were obtained with Δpox2 and Δpox5 strains, and the highest rate was obtained with the Δpox3-disrupted strains, which had rates of production four to five times that of the wild type (22.0 mg/liter/h for Δpox3 Δpox5 and 31.1 mg/liter/h for Δpox3). The qpmax for Δpox3 strains was also three to four times higher than that of the wild type: 1.3 mg/106 cells/h for Δpox3 and 1.22 mg/106 cells/h for Δpox3 Δpox5. For the double-disrupted strain Δpox2 Δpox3, qpmax was only slightly higher than that of the wild type: 0.48 mg/106 cells/h instead of 0.34 mg/106 cells/h, but the period of (dP/dt X)max was much longer.
Aox2 and Aox3 combined provide the main Aox activity (70 to 80% of the total) (Fig. 1), but the other three Aox proteins are involved in β-oxidation. For some gene disruptions, cells compensate for the lack of the corresponding Aox, most probably through regulation of the other POX gene(s), resulting in global Aox activity higher than that of the wild type (22). One of the genes causing an enhanced Aox activity (POX1) was not functional or not expressed in our conditions, but its disruption affected not only the level of Aox activity but also the lactone productivity of the mutant strain (17). The double disruption of POX2 and POX3 clearly shows that at least one other gene product is involved in the production pathway. Aox5p may be functional, as the triple-disrupted strain (Δpox2 Δpox3 Δpox5) had no detectable activity but grew and biotransformed methyl ricinoleate into γ-decalactone. Another gene product, Aox4p, is functional in Yarrowia cells because the Δpox2 Δpox3 Δpox4 Δpox5 mutant did not grow or produce lactone, contrary to Δpox2 Δpox3 Δpox5. Methyl ricinoleate biotransformation in yeast utilizes the peroxisomal β-oxidation pathway (2). The search for intermediates established that ricinoleyl-CoA is shortened by two carbons four times, resulting in the accumulation of γ-decalactone (9, 14, 20). The precursor of this lactone is 4-hydroxydecanoyl-CoA, or 4-hydroxydecanoic acid (6), which can be oxidized in one cycle, leading to 2-hydroxyoctanoic acid (14). Alternatively, it may be oxidized in half a cycle, yielding 3,4-dihydroxydecanoic acid and 3-hydroxy-γ-decalactone (9). 4-Hydroxydecanoic acid and its CoA form give rise easily to the more stable lactone form, accounting for the accumulation of γ-decalactone (9, 14). However, a decrease in lactone concentration has often been observed because some yeasts are able to reconsume this metabolic product (3, 8, 14). One mechanism which could be implicated in the reconsumption is ω-oxidation followed by the β-oxidation of the carbon chain extremity (5), but other possibilities include delactonization by a lactonase producing a hydroxy acid which is fed into the β-oxidation pathway (1, 11, 12).
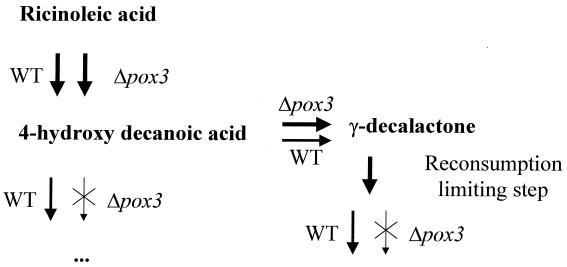
The significant increase in the lactone production of Δpox3 strains most likely results from two effects, as schematically presented in Fig. 3. Reconsumption is particularly evident because for POX3-possessing strains, lactone concentration in the culture medium drastically decreases after 12 h. Presumably, disrupting the short-chain-specific Aox gene slows down β-oxidation cycles at the C10 level as the growth of Δpox3 strains is greatly affected on C10 substrate. This will increase the size of the 4-hydroxydecanoic acid pool and therefore slightly increase γ-decalactone production, and it will also stop any β-oxidation following the possible ω-oxidation or delactonization and, hence, will lower the rate of decalactone disappearance. The initial step of lactone reconsumption may involve an ω-oxidation reaction or opening of the lactone ring, and the Aox step only affects the fluxes. We added γ-decalactone to the medium in the presence of the wild type, Δpox2, Δpox3, and Δpox2 Δpox3 and monitored its disappearance. We found no significant differences between the strains in 4 h of reaction (results not shown).
FIG. 3.
Schematic representation of the role of Aox3p in the pathway of production of γ-decalactone.
This study, showing a fivefold increase in the production rate, demonstrates that mastering the control of Aox, particularly lowering the impact of short-chain-specific Aox, is a promising way to increase lactone production. However, it will still be necessary to overcome lactone toxicity and to inhibit reconsumption.
REFERENCES
- 1.Barton P, Page M I. The esterase catalysed resolution of lactones and spirodilactone. J Chem Soc Perkin Trans I. 1993;2:2317–2318. [Google Scholar]
- 2.Endrizzi A, Awadé A C, Belin J-M. Presumptive involvement of methyl ricinoleate beta-oxidation in the production of gamma-decalactone by the yeast Pichia guilliermondii. FEMS Microbiol Lett. 1993;114:153–160. [Google Scholar]
- 3.Endrizzi A, Belin J-M. Bioconversion of methyl ricinoleate to 4-hydroxy-decanoic acid and to gamma-decalactone by yeasts of the genus Candida. J Basic Microbiol. 1995;35:285–292. doi: 10.1002/jobm.3620350503. [DOI] [PubMed] [Google Scholar]
- 4.Endrizzi A, Pagot Y, Le Clainche A, Nicaud J-M, Belin J-M. Production of lactones and peroxisomal beta-oxidation in yeasts. Crit Rev Biotechnol. 1996;16:301–329. doi: 10.3109/07388559609147424. [DOI] [PubMed] [Google Scholar]
- 5.Endrizzi-Joran A. Thèse de Doctorat. Dijon, France: Université de Bourgogne; 1994. [Google Scholar]
- 6.Féron G, Dufossé L, Mauvais G, Bonnarme P, Spinnler H E. Fatty acid accumulation in the yeast Sporidiobolus salmonicolor during batch production of gamma-decalactone. FEMS Microbiol Lett. 1997;149:17–24. doi: 10.1111/j.1574-6968.1997.tb10302.x. [DOI] [PubMed] [Google Scholar]
- 7.Féron G, Dufossé L, Pierard E, Bonnarme P, Le Queré J L, Spinnler H E. Production, identification, and toxicity of gamma-decalactone and 4-hydroxydecanoic acid from Sporidiobolus spp. Appl Environ Microbiol. 1996;62:2826–2831. doi: 10.1128/aem.62.8.2826-2831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuganti C, Grasselli P, Barbeni M. Biogeneration of aromas: gamma and delta lactones from C6 to C12. NATO ASI Ser Ser A. 1991;207:1–17. [Google Scholar]
- 9.Gatfield I L, Güntert M, Sommer H, Werkhoff P. Some aspects of the microbiological production of flavor-active lactones with particular reference to gamma-decalactone. Chem Mikrobiol Technol Lebensm. 1993;15:165–170. [Google Scholar]
- 10.Haffner T, Tressl R. Biosynthesis of (R)-gamma-decanolactone in the yeast Sporobolomyces odorus. J Agric Food Chem. 1996;44:1218–1223. [Google Scholar]
- 11.Khalameyzer V, Fischer I, Bornscheuer U T, Altenbuchner J. Screening, nucleotide sequence, and biochemical characterization of an esterase from Pseudomonas fluorescens with high activity towards lactones. Appl Environ Microbiol. 1999;65:477–482. doi: 10.1128/aem.65.2.477-482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C H, Endo T, Yonehara H. Studies on mikamycin B lactonase V. Metabolic control in mikamycin B fermentation. J Antibiot. 1988;41:73–80. doi: 10.7164/antibiotics.41.73. [DOI] [PubMed] [Google Scholar]
- 13.Lazarow P B, De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci USA. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okui S, Uchiyama M, Mizugaki M. Metabolism of hydroxy fatty acids. 2. Intermediates of the oxidative breakdown of ricinoleic acid by genus Candida. J Biochem. 1963;54:536–540. doi: 10.1093/oxfordjournals.jbchem.a127827. [DOI] [PubMed] [Google Scholar]
- 15.Pagot Y. Thèse de Doctorat. Dijon, France: Université de Bourgogne; 1997. [Google Scholar]
- 16.Pagot Y, Belin J-M. Involvement of carnitine acyltransferases in peroxisomal fatty acid metabolism by the yeast Pichia guilliermondii. Appl Environ Microbiol. 1996;62:3864–3867. doi: 10.1128/aem.62.10.3864-3867.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagot Y, Le Clainche A, Nicaud J-M, Waché Y, Belin J-M. Peroxisomal beta-oxidation activities and gamma-decalactone production by the yeast Yarrowia lipolytica. Appl Microbiol Biotechnol. 1998;49:295–300. doi: 10.1007/s002530051172. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen J I, Eggersten G, Hellman U, Anderson U, Björkhem I. Molecular cloning and expression of cDNA encoding 3alpha, 7alpha, 12alpha-trihydroxy-5beta-cholestanoyl-CoA oxidase from rabbit liver. J Biol Chem. 1997;272:18481–18489. doi: 10.1074/jbc.272.29.18481. [DOI] [PubMed] [Google Scholar]
- 19.Picataggio S, Rohrer T, Deanda K, Lanning D, Reynolds R, Mielenz J, Eirich L D. Metabolic engineering of Candida tropicalis for the production of long chain dicarboxylic acids. Bio/Technology. 1992;10:894–898. doi: 10.1038/nbt0892-894. [DOI] [PubMed] [Google Scholar]
- 20.Spinnler H E, Ginies C, Khan J A, Vulfson E N. Analysis of metabolic pathways by the growth of cells in the presence of organic solvents. Proc Natl Acad Sci USA. 1996;93:3373–3376. doi: 10.1073/pnas.93.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Le Clainche A, Le Dall M-T, Waché Y, Pagot Y, Belin J M, Gaillardin C, Nicaud J M. Cloning and characterization of the peroxisomal acyl CoA oxidase ACO3 gene from the alkane-utilizing yeast Yarrowia lipolytica. Yeast. 1998;14:1373–1386. doi: 10.1002/(SICI)1097-0061(199811)14:15<1373::AID-YEA332>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang H J, Le Dall M-T, Waché Y, Laroche C, Belin J-M, Gaillardin C, Nicaud J-M. Evaluation of acyl Coenzyme A oxidase (Aox) isozyme function in the n-alkane-assimilating yeast Yarrowia lipolytica. J Bacteriol. 1999;181:5140–5148. doi: 10.1128/jb.181.17.5140-5148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwietering M H, Jongenburger I, Rombouts F M, Van't Riet K. Modelling of the bacterial growth curve. Appl Environ Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]