Abstract
Background and Objectives
The onset of action for high-efficacy immunotherapies in multiple sclerosis (MS) is an important parameter. This study (MAGNIFY-MS) evaluates the onset of action of cladribine tablets by observing changes in combined unique active (CUA) MRI lesion counts during the first 6 months of treatment in patients with highly active relapsing MS.
Methods
MRI was performed at screening, baseline, and at months 1, 2, 3, and 6 after initiating treatment with cladribine tablets 3.5 mg/kg. CUA lesion counts, defined as the sum of T1 gadolinium-enhancing (Gd+) lesions and new or enlarging active T2 lesions (without T1 Gd+), were compared between postbaseline and the baseline period and standardized to the period length and the number of MRIs performed.
Results
Included in this analysis were 270 patients who received ≥1 dose of cladribine tablets. After treatment initiation, significant reductions in mean CUA lesion counts were observed from month 1 onward compared with the baseline period (−1.193 between month 1 and month 6, −1.500 between month 2 and month 6, and −1.692 between month 3 and month 6; all p < 0.0001). Mean T1 Gd+ lesion counts were decreased from month 2 onward compared with baseline (−0.857 at month 2, −1.355 at month 3, and −1.449 at month 6; all p < 0.0001), whereas the proportion of patients without any CUA lesions increased from 52.0% between month 1 and month 6 to 80.5% between month 3 and month 6.
Discussion
Findings suggest an early onset of action for cladribine tablets, with an increasing reduction in active MRI lesions over time.
Trial Registration Information
NCT03364036; Date registered: December 06, 2017.
Classification of Evidence
Using frequent MRI assessments of the brain over the first 6 months of the MAGNIFY-MS study (NCT03364036), we aimed to determine the onset of action of cladribine tablets 3.5 mg/kg in adult patients with highly active relapsing MS. This study provides Class IV evidence that, in such patients, treatment with cladribine tablets is associated with an early onset of action with reductions in active MRI lesion counts from month 2 (day 60) onward, with an increasing reduction in such lesions over time.
Multiple sclerosis (MS) is a chronic, inflammatory, and neurodegenerative disease of the CNS. A key tool used in the diagnosis and monitoring of MS is MRI, in which T1 gadolinium-enhancing (Gd+) lesions and new or enlarging T2 lesions are routinely evaluated. MRI is also a useful tool in determining the onset of action of disease-modifying therapies (DMTs), a parameter that is considered important for highly effective DMTs.
Cladribine tablets have been approved in the European Union for the treatment of highly active relapsing MS. This disease course typically manifests with rapid and early onset of disability often with high relapse rates and early motor, cerebellar, and/or cognitive dysfunction.1,2 Approximately 4%–32% of clinical trial populations are thought to experience this highly active disease course,1-6 although it should be noted that these studies used different definitions as to what constitutes highly active MS. The early initiation of highly effective DMT is particularly important in this group of patients to prevent disability accrual.7-9
The placebo-controlled CLARITY study demonstrated high efficacy of cladribine tablets on clinical and MRI outcomes over 96 weeks,10,11 and indicated an early clinical effect within the first 6 months.12 Because the first MRI assessment in CLARITY was conducted 6 months after the initiation of treatment, the question of whether the treatment effect of cladribine tablets starts earlier remained unanswered.
Using frequent MRI assessments of the brain over the first 6 months of the MAGNIFY-MS study (NCT03364036), our primary research question concerned the onset of action of cladribine tablets 3.5 mg/kg in patients with highly active relapsing MS. We also report a subgroup analysis of patients with high relapse activity, prior DMT use, or combined unique active (CUA) lesion counts >0 at baseline.
Methods
Study Design and Participants
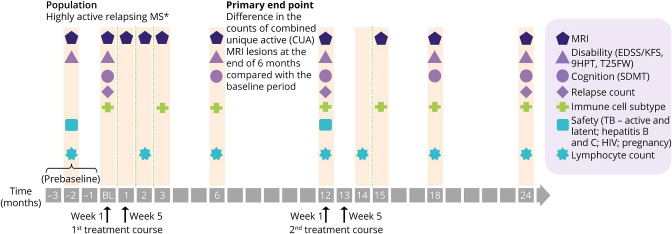
MAGNIFY-MS was a 2-year, phase IV, open-label, single-arm study (Figure 1), in which eligible patients were scheduled to receive cladribine tablets 10 mg (MAVENCLAD, Merck B.V., Amsterdam, The Netherlands; 3.5 mg/kg cumulative dose over 2 years) with 2 weeks of active treatment per annual course. Patients included in the present analysis were planned to receive the first week of treatment starting at baseline, and the second week of treatment during week 5 (month 1). The interim analysis presented here included all data reported up to the 6-month study assessment; the cutoff date for the last patient was on January 23, 2020.
Figure 1. Study Design.
Second treatment course may be delayed for some patients, according to the lymphocyte count. *Highly active relapsing MS was defined as one relapse in the previous year and at least one T1 Gd+ lesion, or 9 or more T2 lesions while on therapy with other DMTs; or 2 or more relapses in the previous year whether on DMT or not. 9HPT = 9-hole peg test; CUA = combined unique active; EDSS = Expanded Disability Status Scale; KFS = Kurtzke Functional System; MS = multiple sclerosis; SDMT = Symbol Digit Modalities Test; T25FW = timed 25-foot walk; TB = tuberculosis.
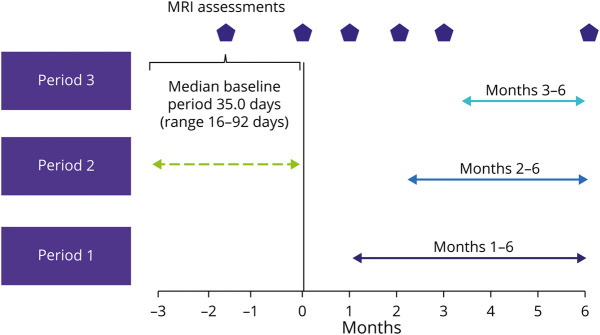
The screening visit of MAGNIFY-MS, at which patients provided informed consent and undertook a clinical MRI assessment, was planned to be conducted 3 months before the baseline visit that was conducted within 7 days of the scheduled start date. A minimum gap of 4 weeks was required between MRI assessment at the screening visit and the next assessment at the baseline visit. Further visits at months 1 (day 30), 2 (day 60), 3 (day 90), and 6 (day 180) after initiating treatment with cladribine tablets had a time window of ±7 days from the scheduled date of visit (Figure 2).
Figure 2. Definition of Periods Used for Analysis in the MAGNIFY-MS Study.
The baseline period was defined as screening to baseline and had a median duration of 35.0 days.
Patients were planned to be included if they were aged ≥18 years, with a diagnosis of highly active relapsing MS and an Expanded Disability Status Scale (EDSS) score of ≤5. Enrollment occurred at 50 centers in 14 countries between May 28, 2018, and April 23, 2019. In this study, highly active relapsing MS was defined as one relapse in the previous year and at least one T1 Gd+ lesion, or 9 or more T2 lesions while on therapy with other DMTs; or 2 or more relapses in the previous year whether on DMT or not. Exclusion criteria included: a lymphocyte count not within normal laboratory limits; presence of signs of progressive multifocal leukoencephalopathy; positive tests for HIV, hepatitis B or C, or active/latent tuberculosis; active malignancy; allergy or hypersensitivity to Gd and/or any other contraindication to perform MRI; and previous exposure to alemtuzumab, fingolimod, mitoxantrone, natalizumab, or ocrelizumab.
Efficacy End Points
The MRI-based end point of the primary analysis was the difference in CUA lesion counts during postbaseline periods (period 1: month 1 visit–month 6 visit, period 2: month 2 visit–month 6 visit, and period 3: month 3 visit–month 6 visit) compared with the baseline period (Screening Visit–Baseline Visit). CUA lesions were defined as the combination of the T1 Gd+ lesions and new or enlarging active T2 lesions (without T1 Gd+) for the respective period and were standardized to one scan (for T1 Gd+) or 1 month (for active T2). As per the MRI protocol, new or enlarging active T2 lesions without T1 Gd+ activity were determined from a comparison of the prior T2-weighted sequence with reference to the T1 Gd-enhanced sequence. As part of the analysis, colocalizing 3-dimensional lesions were counted as one lesion. If a new or enlarging T2 hyperintense lesion on a T2-weighted sequence had a corresponding T1 hyperintense signal on a T1-Gd enhanced sequence at the same time point, the lesion would be counted as a T1 Gd+ hyperintense lesion without also counting it as a new or enlarging T2 lesion.
The statistical analysis also investigated the components of the CUA lesion count (i.e., the standardized T1 Gd+ lesion count calculated as the mean across all scans of a period to account for a different number of scans during a period and the active T2 lesion count standardized to 1 month to account for different period lengths) and the lesion count at each visit. In addition, the proportion of patients CUA lesion free by period and T1 Gd+ lesion free by visit is presented.
Assessment of Relapses and Safety
During the study, qualifying relapses were defined as a neurologic abnormality (either newly appearing or reappearing) separated by at least 30 days from onset of a preceding clinical event and lasting for at least 24 hours; absence of fever (>37.5°C/99.5°F) or known infection; and objective neurologic impairment, correlating with the patient's reported symptoms, defined as either an increase in at least one of the functional system scores of the EDSS or an increase of the total EDSS score. We report occurrences of treatment-emergent adverse events (TEAEs), defined as all adverse events with an onset date after the initiation of treatment with cladribine tablets.
Statistical Analysis
All statistical analyses were performed on the full analysis set, which was defined as all patients who received ≥1 dose of cladribine tablets (full analysis set/safety set). This approach follows the intent-to-treat principle. All patients who were intended to be treated actually received at least one dose of cladribine tablets.
The 3 hypotheses to determine whether CUA lesion counts were reduced after treatment with cladribine tablets were tested separately for each of the 3 predefined postbaseline periods (Figure 2). Type I error inflation due to multiple testing was controlled using a sequential testing procedure starting the test procedure with the test for period 3, followed by period 2, and finally period 1. The hypothesis for each period was rejected at a significance level of 0.025 (one sided) if the previous hypothesis could also be rejected. The change in CUA lesion count was analyzed by applying a mixed-effects linear model accounting for within pooled center correlation and adjusted for CUA lesion count during the baseline period, age, and baseline EDSS score (≤3, >3). The primary analysis was evaluated for robustness by applying a wide range of alternative statistical methods, including nonparametric tests and generalized linear models assuming negative binomial distribution.
Other MRI parameters were analyzed in an explorative way similar to that for the primary end point. For MRI parameters analyzed by visit, a model for repeated measures was applied taking into account within-patient correlations.
To evaluate consistency of results across special patient populations, the following subgroups were defined: patients with high relapse activity (defined as 2 or more relapses in the previous year) vs those without, prior DMT use (yes/no), and a subset of patients with CUA lesion count >0 during the baseline period.
For each patient, the cutoff date for this interim analysis was defined by that individual patient's month 6 visit date. The redacted study protocol and statistical analysis plan for MAGNIFY-MS have been published on clinicaltrials.gov (NCT03364036).13
Sample Size and Statistical Power Considerations
Based on the CLARITY trial, the estimated treatment effect concerning the number of CUA lesions at month 6 was approximately 1.3 vs placebo. As the primary end point for the MAGNIFY-MS trial is comparison of CUA lesions between the baseline and postbaseline periods, but with a single-arm design, a more conservative treatment effect of 0.8 lesions (and SD of 4.0) was assumed for the sample size calculation in the absence of a comparator group. A normal approximation to the distribution of the primary end point was also assumed. On the basis of such assumptions, a sample size of 265 patients was required to achieve 90% power for a paired t-test with a type I error rate of 0.05.
Standard Protocol Approvals, Registrations, and Patient Consents
Ethical approval for the MAGNIFY-MS study was obtained from independent ethics committees at each local trial site, and the study was performed in line with the principles of the Declaration of Helsinki. All participants provided written informed consent before participation in the study.
Data Availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data-sharing portal for the healthcare business of Merck KGaA, Darmstadt, Germany merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When the healthcare business of Merck KGaA, Darmstadt, Germany has a coresearch, codevelopment, or comarketing or copromotion agreement or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany will endeavor to gain agreement to share data in response to requests.
Results
A total of 313 patients were screened for enrollment into the MAGNIFY-MS study, and 270 of these patients initiated treatment with cladribine tablets 3.5 mg/kg (43 patients were screening failures). A mean (SD) age of 37.7 (±9.75) years and a median EDSS score of 2.0 at baseline were observed, and patients were predominantly female (66.7%). Patient demographics and characteristics are shown in Table 1.
Table 1.
Patient Demographics and Characteristics
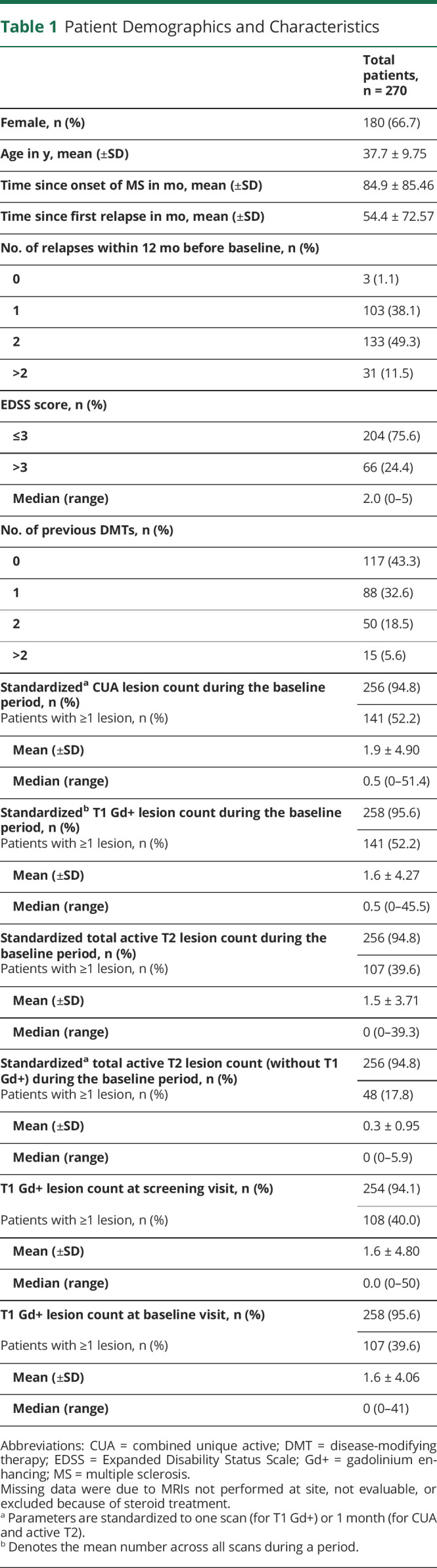
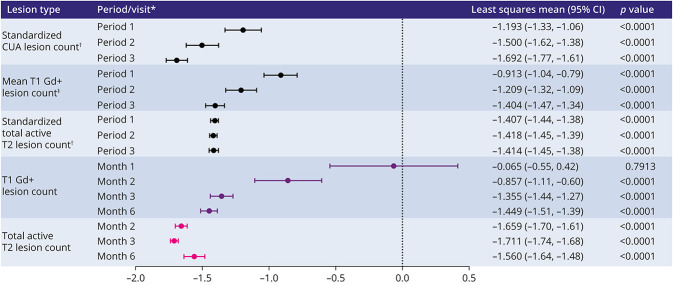
During the baseline period, 52.2% (141/270) of patients had CUA lesions present on their MRI scans; 52.2% (141/270) had T1 Gd+ lesions present, and 17.8% (48/270) had active T2 lesions without the presence of T1 Gd+ lesions (eFigure 1, links.lww.com/NXI/A723). After treatment initiation, significant reductions in the mean CUA lesion count were observed from month 1 onward compared with the baseline period: −1.193 between month 1 and month 6; −1.500 between month 2 and month 6, and −1.692 between month 3 and month 6; all p < 0.0001 (Figure 3). Results from the primary analysis were confirmed by nonparametric methods, as well as methods assuming negative binomial distribution, and are thus considered robust to potential deviations from the normal distribution.
Figure 3. Change in MRI Lesion Counts From the Baseline/Baseline Period.
*Postbaseline periods are compared with the baseline period, and postbaseline visits are compared with the baseline visit. Period 1 (month 1 visit–month 6 visit); period 2 (month 2 visit–month 6 visit); period 3 (month 3 visit–month 6 visit). Models by period: mixed-effects linear model fitted for each period separately. Models by visit: mixed linear model for repeated measures taking within-subject correlation into account. †Parameters are standardized to 1 month. ‡Data are for means within a period. CI = confidence interval; CUA = combined unique active; Gd+ = gadolinium enhancing.
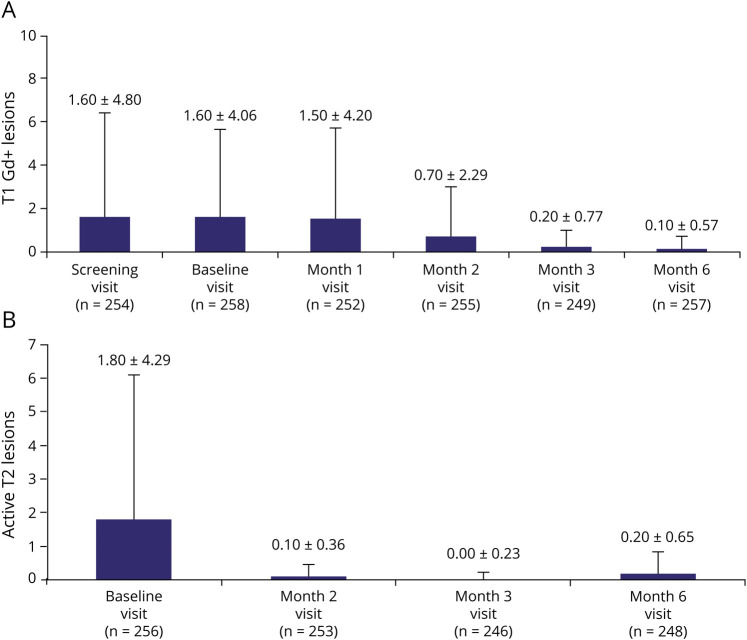
Likewise, standardized T1 Gd+ and total active T2 lesion counts were reduced during all 3 periods. Results also showed that T1 Gd+ and total active T2 lesion counts were reduced between month 2 and month 6 after treatment initiation (Figure 4, A and B). The proportion of patients without any CUA lesions increased from 52.0% during period 1 to 80.5% during period 3 (eFigure 2a, links.lww.com/NXI/A723), and the proportion of patients without any T1 Gd+ lesions increased to 91.4% by month 6 (eFigure 2b, links.lww.com/NXI/A723).
Figure 4. (A) T1 Gd+ Lesion Count and (B) Active T2 Lesion Count* by Visit.
Values indicate the mean +SD for each visit. *Active T2 lesion count (by visit), i.e., the new or enlarging T2 lesions compared with the previous visit. Because of the way in which the data were derived for the active T2 lesions, it was not possible to provide month 1 data. Gd+, gadolinium enhancing.
Subgroup analyses of patients with and without high relapse activity, with and without prior DMT use, and a subset with CUA lesion count >0 during the baseline period, found significant reductions in CUA lesion counts in all subgroups/subsets and for all periods (eTable 1, links.lww.com/NXI/A723). Prior DMTs are listed in eTable 2, links.lww.com/NXI/A723.
After initiating treatment, a total of 13 patients experienced at least one qualifying relapse during the 6-month study period; all were treated with steroids, and 2 patients were hospitalized. For all reported relapses (qualifying and nonqualifying), 21 patients experienced at least one relapse; 18 patients were treated with steroids, and 3 patients were hospitalized.
In all, 70.7% (191/270) of patients experienced a TEAE (eTable 3, links.lww.com/NXI/A723), with 47.4% (128/270) of patients having encountered mild events. Treatment-related TEAEs were experienced by 33.0% (89/270) of patients, with 23.3% (63/270) of patients having encountered mild events. No new safety findings were observed.
Classification of Evidence
Using frequent MRI assessments of the brain over the first 6 months of the MAGNIFY-MS study (NCT03364036), we aimed to determine the onset of action of cladribine tablets 3.5 mg/kg in adult patients with highly active relapsing MS. This study provides Class IV evidence that, in such patients, treatment with cladribine tablets is associated with an early onset of action with reductions in active MRI lesion counts from month 2 (day 60) onward, with an increasing reduction in such lesions over time.
Discussion
The data presented here suggest an early treatment effect of cladribine tablets on MRI-detectable disease activity that can be observed from 1 month after treatment initiation. Reductions in CUA lesion counts increased over time, irrespective of patients' baseline relapse activity or prior DMT use.
MRI is an essential tool in the diagnosis and monitoring of MS, which led to its incorporation into the McDonald criteria in 2001 and its subsequent revisions.14 It is well established that MRI is a more sensitive technique to measure disease activity compared with other disease-related indices, such as relapse rates.15 T1 Gd+ and active T2 lesions are the most commonly used MRI parameters to assess disease activity.15,16
The previously reported MRI results from the CLARITY study underpinned the clinical benefits of treatment with cladribine tablets in patients with relapsing MS and showed a significant reduction in the presence of lesions during the 96-week trial.10,11 The MRI findings from our preplanned analysis of the MAGNIFY-MS study therefore provide key information about the onset of the disease-modifying activity of cladribine tablets. Based on CUA or T1 Gd+ lesions, a significant reduction in lesion activity was observed from month 1 (day 30) after treatment initiation with a more pronounced effect apparent during subsequent months. Using active T2 lesions, the onset of disease-modifying activity was detectable from month 2 (day 60) after treatment initiation. It is well established that T1 Gd+ lesions are the most sensitive conventional MRI index of disease activity, and therefore, it is not surprising that the readouts on T2-weighted scans were also significant from month 2 after treatment initiation.17
Our results corroborate and supersede a recently published post hoc analysis of the ORACLE-MS study of cladribine tablets in patients with a first episode of demyelination (clinically isolated syndrome).18 In that study, significant effects on MRI lesion counts were detected at week 13 and, thus, significantly earlier than in CLARITY, where the first follow-up scans were scheduled at 6 months.12 However, detection of yet earlier effects was precluded by the study design of ORACLE-MS where follow-up MRI was scheduled no earlier than week 13.
The onset of action of cladribine tablets reported here maps onto key elements of the adaptive immune cell response in the peripheral blood, where CD19+ B cells are reduced by approximately 90% within 4–8 weeks after initiation of treatment.19 Although the recovery of the overall CD19+ B-cell pool is driven by naive and activated phenotypes, levels of memory B cells can remain reduced for at least 12 months.20,21 In contrast to the rapid reduction of B cells, cladribine tablets have a slower and more moderate effect on the reduction of CD4+ and CD8+ T cells, whereby nadir is reached at 3–6 months after treatment initiation.21 These findings underpin, at least in part, the long-term effect of cladribine tablets on disease control in patients with highly active relapsing MS. An ongoing analysis of immune cell subset dynamics in the MAGNIFY-MS population will further explore the potential mechanistic basis of the early onset of action of cladribine tablets.21
Previous studies determining the effect on T1 Gd+ and active T2 lesions for current DMTs (including cladribine tablets, fingolimod, and natalizumab) have been comparable, showing reductions in T1 Gd+ lesion counts of between 85% and 92% over 2-year study periods.10,11,22-25 Results for T2 lesion reduction in these articles are more varied, with reductions of 51% observed with fingolimod22 compared with 83% with natalizumab23,24 and 61%–73% with cladribine tablets.10,11 However, in most of the abovementioned studies, the first MRI scans were conducted at 6 months or later after the start of treatment, with the exception of natalizumab25 and further studies investigating fingolimod26 and ocrelizumab27 where MRI was conducted before 6 months. This current lack of literature underlines the importance of the MAGNIFY-MS study in which MRI findings from the first 6 months after treatment were evaluated. The main limitation of MAGNIFY-MS is that this is a single-arm study without a control group. Therefore, results for each period were compared with the baseline period rather than a placebo group, as was generally the case in the aforementioned studies. As a result, regression to mean may contribute to the reduction in active MRI lesions after initiation of treatment. It should also be noted that direct comparisons of MRI efficacy outcomes across studies should be made with caution because of differing assessment schedules and baseline patient characteristics.
In conclusion, this 6-month primary analysis of the MAGNIFY-MS study indicates that treatment with cladribine tablets 3.5 mg/kg is associated with an early onset of action detected on MRI. Significant reductions were observed in CUA lesion counts from month 1 (day 30) after initiation of treatment and irrespective of patients' baseline relapse activity or prior DMT use. No new safety findings were observed. An additional follow-up analysis for the MAGNIFY-MS study is planned at 2 years, when all patients will have completed the study.
Acknowledgment
The authors thank patients and their families, investigators, coinvestigators, and the study teams at each of the participating centers and at the healthcare business of Merck KGaA, Darmstadt, Germany. Medical writing assistance, in terms of a contribution to drafting the manuscript and peer review responses through the collation of information and relevant documentation under the full guidance of the listed authors and harmonizing author comments via an online collaboration tool, was provided by Claire Mwape of inScience Communications, Springer Healthcare Ltd, UK, and supported by the healthcare business of Merck KGaA, Darmstadt, Germany.
Glossary
- CUA
combined unique active
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- Gd+
gadolinium enhancing
- MS
multiple sclerosis
- TEAE
treatment-emergent adverse event
Appendix. Authors
Contributor Information
Frederik Barkhof, Email: f.barkhof@vumc.nl.
Xavier Montalban, Email: xavier.montalban@cem-cat.org.
Anat Achiron, Email: anat.achiron@sheba.health.gov.il.
Tobias Derfuss, Email: tobias.derfuss@usb.ch.
Andrew Chan, Email: andrew.chan@insel.ch.
Suzanne Hodgkinson, Email: s.hodgkinson@unsw.edu.au.
Alexandre Prat, Email: a.prat@umontreal.ca.
Letizia Leocani, Email: leocani.letizia@hsr.it.
Klaus Schmierer, Email: k.schmierer@qmul.ac.uk.
Finn Sellebjerg, Email: sellebjerg@dadlnet.dk.
Patrick Vermersch, Email: patrick.vermersch@univ-lille.fr.
Heinz Wiendl, Email: heinz.wiendl@ukmuenster.de.
Birgit Keller, Email: birgit.keller@merckgroup.com.
Sanjeev Roy, Email: sanjeev.roy@merckgroup.com.
Study Funding
This study was supported by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).
Disclosure
NDS is a consultant for Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; has grants or grants pending from FISM and Novartis; is on the speakers' bureaus of Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; and has received travel funds from the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva. FB is supported by the NIHR Biomedical Research Centre at UCLH and is a consultant to Biogen, Combinostics, IXICO, the healthcare business of Merck KGaA (Darmstadt, Germany), and Roche. XM has received speaking honoraria and travel expenses for participation in scientific meetings and has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with AbbVie, Actelion (Janssen/J&J), Alexion, Bayer, Biogen, Celgene (BMS), EMD Serono, Immunic, Janssen Pharmaceuticals, MedDay, the healthcare business of Merck KGaA (Darmstadt, Germany), Mylan, NervGen, Novartis, Roche, Sanofi, and Teva. AA has received honoraria or consulting fees from Bayer, Biogen, Celgene (BMS), the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, and Sanofi and research support from Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Roche, and Sanofi. TD serves on scientific advisory boards for Bayer, Actelion (Janssen/J&J), Biogen, Celgene (BMS), GeNeuro, MedDay, the healthcare business of Merck KGaA (Darmstadt, Germany), Mitsubishi Pharma, Novartis Pharmaceuticals, Roche, and Sanofi; has received funding for travel and/or speaker honoraria from Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, and Sanofi; and receives research support from Biogen, the European Union, Novartis Pharma, Roche, the Swiss MS Society, and the Swiss National Foundation. AC has received speakers'/board honoraria from Actelion (Janssen/J&J), Almirall, Bayer, Biogen, Celgene (BMS), the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva, all for hospital research funds. He received research support from Biogen, Sanofi, and UCB, the European Union, and the Swiss National Foundation. He serves as associate editor of the European Journal of Neurology, on the editorial board for Clinical and Translational Neuroscience, and as topic editor for the Journal of International Medical Research. SH serves on advisory boards for Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, and Sanofi. She has received money for travel and speaker honoraria from Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, and Sanofi. AP has received honoraria and operating grants from pharmaceutical companies. LL has received honoraria for consulting services or speaking activities from Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, and Roche and research support from Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), and Novartis. KS has received research support from Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), and Novartis; speaking honoraria from, and/or served in an advisory role for, Amgen-Gensenta, Biogen, EMD Serono, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; and remuneration for teaching activities from AcadeMe, Medscape, and the Neurology Academy. FS has served on scientific advisory boards, been on the steering committees of clinical trials, served as a consultant, received support for congress participation, received speaker honoraria, or received research support for his laboratory from Biogen, Celgene (BMS), EMD Serono, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva. PV has received honoraria or consulting fees from AB Science, Biogen, Celgene (BMS), Imcyse, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva and research support from Novartis, Roche, and Sanofi. HW is member of scientific advisory boards/steering committees for Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva. He received speaker honoraria and travel support from Bayer, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, the healthcare business of Merck KGaA (Darmstadt, Germany), OmniaMed, Novartis, Sanofi, and Teva. He received compensation as a consultant from Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, OmniaMed, Roche, and Sanofi. He has received research support from Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Sanofi, and Teva, as well as German Ministry for Education and Research (BMBF), German Research Foundation (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Münster, and RE Children's Foundation. BK is an employee of the healthcare business of Merck KGaA, Darmstadt, Germany. SR is an employee of Ares Trading S.A., Eysins, Switzerland (an affiliate of Merck KGaA, Darmstadt, Germany). Go to Neurology.org/NN for full disclosures.
References
- 1.Iacobaeus E, Arrambide G, Amato MP, et al. Aggressive multiple sclerosis (1): towards a definition of the phenotype. Mult Scler. 2020;26(9):1352458520925369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrambide G, Iacobaeus E, Amato MP, et al. Aggressive multiple sclerosis (2): Treatment. Mult Scler. 2020;26(9):1352458520924595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol. 2009;256(3):405-415. [DOI] [PubMed] [Google Scholar]
- 4.Giovannoni G, Soelberg Sorensen P, Cook S, et al. Efficacy of cladribine tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: a post hoc analysis of the CLARITY study. Mult Scler. 2019;25(6):819-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derfuss T, Bergvall NK, Sfikas N, Tomic DL. Efficacy of fingolimod in patients with highly active relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2015;31(9):1687-1691. [DOI] [PubMed] [Google Scholar]
- 6.Devonshire V, Havrdova E, Radue EW, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 2012;11(5):420-428. [DOI] [PubMed] [Google Scholar]
- 7.Hirst CL, Pace A, Pickersgill TP, et al. Campath 1-H treatment in patients with aggressive relapsing remitting multiple sclerosis. J Neurol. 2008;255(2):231-238. [DOI] [PubMed] [Google Scholar]
- 8.He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307-316. [DOI] [PubMed] [Google Scholar]
- 9.Ziemssen T, Derfuss T, de Stefano N, et al. Optimizing treatment success in multiple sclerosis. J Neurol. 2016;263(6):1053-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011;10(4):329-337. [DOI] [PubMed] [Google Scholar]
- 11.Comi G, Cook SD, Giovannoni G, et al. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J Neurol. 2013;260(4):1136-1146. [DOI] [PubMed] [Google Scholar]
- 12.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426. [DOI] [PubMed] [Google Scholar]
- 13.Clinicaltrials.gov. Identifier NCT03364036, Evaluation of the Onset of Action in Highly Active MS (MAGNIFY); Accessed December 6, 2017. clinicaltrials.gov/ct2/show/NCT03364036. [Google Scholar]
- 14.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. [DOI] [PubMed] [Google Scholar]
- 15.Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25(suppl 2):157-165. [DOI] [PubMed] [Google Scholar]
- 16.Oreja-Guevara C. Overview of magnetic resonance imaging for management of relapsing−remitting multiple sclerosis in everyday practice. Eur J Neurol. 2015;22(S2):22-27. [DOI] [PubMed] [Google Scholar]
- 17.Miller DH, Barkhof F, Nauta JJ. Gadolinium enhancement increases the sensitivity of MRI in detecting disease activity in multiple sclerosis. Brain. 1993;116(pt 5):1077-1094. [DOI] [PubMed] [Google Scholar]
- 18.Freedman MS, Coyle PK, Comi G, et al. . Early MRI outcomes in participants with a first clinical demyelinating event at risk of multiple sclerosis in the ORACLE-MS study. Mult Scler J Exp Transl Clin. 2021;7(1):2055217321990852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol Neuroinflamm. 2017;4(4):e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceronie B, Jacobs BM, Baker D, et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol. 2018;265(5):1199-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiendl H, Schmierer K, Hodgkinson S, et al. . Characterization of peripheral immune cell dynamics and repopulation patterns in the first 12 months of cladribine tablets treatment: MAGNIFY-MS study (2235). Neurol. 2021;96(15 suppl):2235. [Google Scholar]
- 22.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387-401. [DOI] [PubMed] [Google Scholar]
- 23.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899-910. [DOI] [PubMed] [Google Scholar]
- 24.Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68(17):1390-1401. [DOI] [PubMed] [Google Scholar]
- 25.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15-23. [DOI] [PubMed] [Google Scholar]
- 26.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124-1140. [DOI] [PubMed] [Google Scholar]
- 27.Barkhof F, Kappos L, Wolinsky JS, et al. Onset of clinical and MRI efficacy of ocrelizumab in relapsing multiple sclerosis. Neurology. 2019;93(19):e1778–e1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data-sharing portal for the healthcare business of Merck KGaA, Darmstadt, Germany merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When the healthcare business of Merck KGaA, Darmstadt, Germany has a coresearch, codevelopment, or comarketing or copromotion agreement or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany will endeavor to gain agreement to share data in response to requests.