Abstract
Objective
We investigated the relationship between contralesional ankle weakness recovery and the corticospinal tract and corticoreticular tract in stroke patients with complete injuries of the ipsilesional corticospinal tract and corticoreticular tract.
Design
Thirty-six patients with complete injuries of the ipsilesional corticospinal tract and corticoreticular tract were recruited. Medical Research Council and the Functional Ambulation Category were used to determine motor function of ankle dorsiflexor and gait function. Patients were assigned into two groups: group A (poor recovery) and group B (good recovery). Fractional anisotropy, apparent diffusion coefficient, and tract volume were obtained for diffusion tensor imaging parameter.
Results
A total of 58.3% of patients showed good recovery of contralesional ankle dorsiflexor weakness, with remainder having poor recovery. Tract volume of the contralesional corticoreticular tract in group B was higher than that in group A (P < 0.05); no other diffusion tensor imaging parameters were significantly different between two groups. Tract volume of the contralesional corticoreticular tract and corticospinal tract showed strong (r = 0.521) and moderate (r = 0.399) positive correlations with Medical Research Council score of contralesional ankle dorsiflexor, respectively (P < 0.05).
Conclusions
We found that the number of fibers of the contralesional corticospinal tract and corticoreticular tract was closely related to the recovery of contralesional ankle dorsiflexor weakness in stroke patients with complete injuries of the ipsilesional corticospinal tract and corticoreticular tract. Moreover, the contralesional corticoreticular tract had a closer relationship to recovery than the contralesional corticoreticular tract.
Key Words: Motor Recovery, Diffusion Tensor Imaging, Diffusion Tensor Tractography, Stroke, Ankle
What Is Known
The importance of good recovery of weak ankle dorsiflexor (DF) for achieving a good gait pattern without braces in stroke patients and the relationship of motor function with corticospinal tract (CST) and corticoreticular tract (CRT) have been reported. However, very little has been reported about recovery mechanisms of ankle DF weakness.
What Is New
The number of fibers of the contralesional CST and CRT was closely related to the recovery of contralesional ankle DF weakness in stroke patients with complete injuries of the ipsilesional CST and CRT. Moreover, the contralesional CRT had a closer relationship to recovery than the contralesional CRT.
Motor weakness is one of the serious sequelae of stroke, with more than 50% of stroke patients experiencing motor function impairment.1 Stroke patients with motor weakness have their independent activities of daily living hindered, and their quality of life degraded. Especially, severe motor weakness of the ankle dorsiflexor (DF) requires some form of supportive device, such as an ankle-foot orthosis or high-top shoes to improve toe clearance during gait.2 Consequently, good recovery of a weak ankle DF is mandatory for achieving a good gait pattern without braces; in particular, recovery is important for patients with severe motor weakness due to complete injuries of the ipsilesional corticospinal tract (CST) and corticoreticular tract (CRT), which are the major neural tracts for motor function.3,4
Detailed information on motor recovery mechanisms of ankle DF weakness after stroke can be useful when establishing effective rehabilitation strategies. Specifically, recently developed noninvasive brain stimulation therapies such as repetitive transcranial magnetic stimulation or transcranial direct current stimulation can be applied to specific neural tracts to assist in the recovery of contralesional ankle DF weakness.5,6 Several motor recovery mechanisms after stroke have been suggested, including recovery of the injured ipsilesional neural tract, perilesional tract reorganization, activation of the contralesional neural tract, and contributions from secondary motor areas or aberrant neural pathways.7–10 However, most of these studies have focused on recovery mechanisms of hand or entire limb muscle weakness.8,9,11,12 By contrast, very little has been reported about recovery mechanisms of ankle DF weakness.13
In the human brain, the neural tracts for motor function are classified according to the pyramidal tract, which includes the CST, and the nonpyramidal tract, which includes the CRT.14,15 The CST is the major tract for motor function, especially as it is involved in the distal musculature used for fine movements. By contrast, the CRT is mainly involved in motor functions of the trunk and legs.14–16 The recent development of diffusion tensor tractography (DTT), a neural tract imaging system derived from diffusion tensor imaging (DTI) data, has enabled estimation of the CST and CRT via its capacity to produce three-dimensional reconstructions of neural tracts.17,18 A unique advantage of DTT is its ability to reveal changes in neural tracts through configuration analysis and three-dimensional reconstruction of the tracts of interest, such as the CST and CRT.17,18 Changes in the configuration and status of neural tracts can be determined by examining changes in DTT parameters, such as the fractional anisotropy (FA), apparent diffusion coefficient (ADC), and tract volume (TV) parameters.19,20 The FA value indicates the state of white matter organization as it is a measure of the degree of directionality and integrity of white matter microstructures, whereas ADC value represents the magnitude of water diffusion. The TV means the number of voxels within a neural tract, which represents the number of fibers within of a neural tract.19,20
Many studies have used DTT to describe the recovery of injured ipsilesional CSTs or CRTs in stroke patients.9,21–23 However, no study has described the recovery mechanism for contralesional ankle DF weakness in stroke patients with complete injuries of the ipsilesional CST and CRT. However, one study did use DTT to determine that activation of the contralesional CRT was closely associated with gait function in chronic stroke patients with complete injury of the ipsilesional CST.24 Therefore, we hypothesized the contralesional CRT might be related with recovery of contralesional ankle DF weakness in stroke patients with complete injuries of the ipsilesional CST and CRT.
In this study, we used DTT to investigate the relationship between the recovery of contralesional ankle DF weakness and the state of the CST and CRT in stroke patients with complete injuries of the ipsilesional CST and CRT.
METHODS
Subjects
Thirty-six stroke patients (21 male, 15 female; mean age = 60.19 ± 10.50 yrs; range = 32–77 yrs) who had no history of neurological or psychiatric disease and visited the rehabilitation department of a university hospital were enrolled in the study (Table 1). Patients were recruited consecutively according to the following inclusion criteria: (1) first-ever stroke, (2) age at the time of stroke: 20–79 yrs, (3) more than 3 mos after stroke onset, (4) supratentorial stroke confirmed by a neuroradiologist (Fig. 1A), (5) hemiplegia after stroke onset, (6) severe motor weakness of the contralesional ankle DF with a Medical Research Council (MRC) score at onset of 0–1, and (7) complete injuries of the ipsilesional CST and CRT (i.e., complete discontinuation of the ipsilesional CRT and CRT around or below the lesion) observed on DTT. All patients underwent comprehensive rehabilitative therapy, including movement therapies provided by physical and occupational therapists (motor strengthening of the contralesional upper and lower limbs, and exercises for trunk stability and control, static and dynamic balance training on sitting and standing positions; twice/day, 40 mins/time, 5 d/wk), and neuromuscular electrical stimulation for the contralesional finger extensors and ankle DF. This study was conducted retrospectively, and the study protocol was approved by the institutional review board of a university hospital. The participants provided written informed consent.
TABLE 1.
Demographic data of patient groups A and B
| Group A | Group B | P | |
|---|---|---|---|
| Patients, n | 21 | 15 | — |
| Sex, male/female | 12:9 | 9:6 | 0.86 |
| Age, yr | 59.24 ± 12.11 | 61.53 ± 7.93 | 0.62 |
| Lesion side, right/left | 8:13 | 6:9 | 0.91 |
| Stroke type, hemorrhage/infarct | 12:9 | 4:11 | 0.07 |
| Duration from onset to DTI, mos | 12.41 ± 13.02 | 11.52 ± 10.36 | 0.60 |
| MRC, onset | 0.19 ± 0.40 | 0.40 ± 0.51 | 0.17 |
| MRC, chronic | 0.67 ± 0.48 | 3.60 ± 0.99 | <0.01a |
| FAC, onset | 0.19 ± 0.68 | 0.20 ± 0.77 | 0.68 |
| FAC, chronic | 1.95 ± 1.13 | 3.27 ± 1.00 | 0.03a |
| Orthosis,b n | 9 | 1 | — |
Values are presented as mean ± SD.
a Significant difference between groups A and B, P < 0.05.
b Orthosis and high-top shoes for ankle.
FIGURE 1.
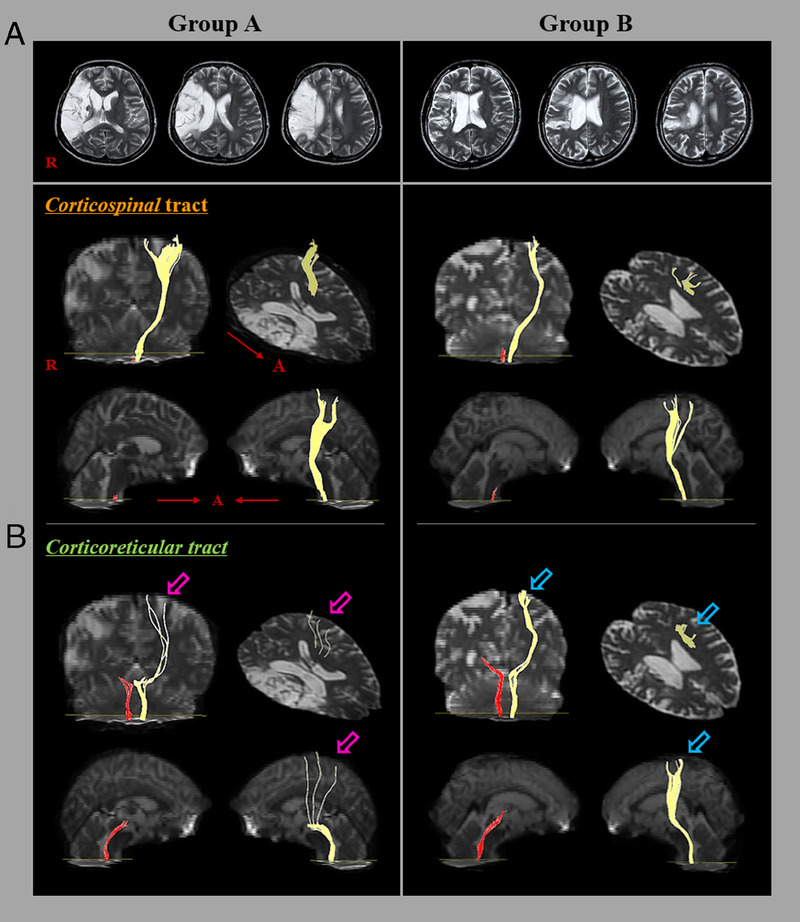
Results of DTT of the CST and CRT in representative patients of group A (32-yr-old woman) and group B (56-yr-old man). A, T2-weighted brain magnetic resonance images were obtained at the time of the DTI. B, Results of DTT of the CST and CRT. The DTT results for the ipsilesional CST and CRT in groups A and B showed tract discontinuations. Moreover, the contralesional CRT in group A (pink arrow) had a lower tract volume than that in group B (blue arrow).
Clinical Evaluation
The motor and gait function were evaluated at stroke onset and at DTI scanning. The MRC was used to measure contralesional ankle DF motor function.25 Higher scores indicate better motor function. Scoring for the MRC was as follows: 0, no ability to contract the muscle; 1, the ability to initiate a feeble contraction or flicker of muscle or tendon movement that is visible or palpable, but the body part does not move; 2, the ability to move the body part into the test position with gravity lessened; 3, the ability to move the body part into the test position and hold against gravity; 4, the ability to move the body part into the test position and hold against gravity and moderate resistance; and 5, the ability to move the body part into the test position and hold against gravity and maximum resistance. The Functional Ambulation Category (FAC), designed to determine the levels of assistance required during a 15-m walk, was used to assess gait function.26 Six categories are included in the FAC: 0, nonambulatory; 1, needs continuous support from one person; 2, needs intermittent support from one person; 3, needs only verbal supervision; 4, help is required on stairs and uneven surfaces; and 5, can walk independently anywhere. The validity and reliability of the MRC and FAC are well established.25,27 We assigned the patients group into two groups based on the MRC score of the contralesional ankle DF at DTI scanning (i.e., chronic stage; more than 3 mos after stroke onset). The enrolled 36 patients were assigned into one of two groups based on the recovery of contralesional ankle DF weakness: group A (MRC < 2; poor recovery, 21 patients) and group B (MRC ≥ 2; good recovery, 15 patients). The demographic characteristics of groups A and B are summarized in Table 1. No significant differences in the sex, age, lesion side, stroke type, duration from the onset, MRC score at onset, and FAC score at onset compositions/distributions were observed between groups A and B (P > 0.05).
Diffusion Tensor Imaging
The DTI data obtained for this study were acquired during the patients’ poststroke chronic stage at an average of 12.04 ± 11.83 mos after onset. The scanning system used a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Ltd, Best, the Netherlands) and provided single-shot echo-planar imaging. For each of the 32 noncollinear diffusion sensitizing gradients, we acquired contiguous slices parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192 matrix; field of view = 240 × 240 mm; repetition time = 10,398 ms; echo time = 72 ms; parallel imaging reduction factor (SENSE factor) = 2; echo planar imaging factor = 59; b = 1000 sec/mm2; number of excitations = 1; slice thickness = 2.5 mm. Before undertaking the fiber tracking, eddy current correction was applied using the FMRIB Software Library to correct for the head motion effect and image distortion. DTI-Studio software (CMRM; Johns Hopkins Medical Institute, Baltimore, MD) was used to evaluate the CST and CRT of each subject. The CST and CRT were reconstructed based on the fibers passing through two regions of interest (ROIs) as visualized on the DTI color map. For CST reconstruction, the seed ROI was placed on the CST portion of the pontomedullary junction, and the target ROI was placed the CST portion of the anterior midpons.18 For CRT reconstruction, the seed ROI was located on the reticular formation of the medulla, and the target ROI was placed at the tegmentum of the midbrain.17 Fiber tracking termination criteria were: FA value <0.2 and an angle of <60 degrees.28 The FA values and TVs for the DTT-reconstructed CST and CRT were obtained for both hemispheres in all patients.
Statistical Analysis
Statistical analysis was performed by using SPSS 21.0 for Windows software (SPSS, Chicago, IL). The Mann-Whitney U test was used to determine the significance of differences in DTT parameter values (FA, ADC, and TV) of the CST and CRT, duration from the onset, and age distribution between groups A and B. The χ2 test was used to examine the differences in sex composition, lesion side, stroke type, MRC score, and FAC score between groups A and B. Spearman correlation analysis was used to detect correlations between the MRC score of the contralesional ankle DF and DTT parameters of the CST and CRT in each of the patient groups. The significance of a relationship was accepted when the P value was <0.05. A correlation coefficient (r value) was interpreted as strong when >0.50, as moderate when between 0.30 and 0.49, and weak when between 0.10 and 0.29.29
RESULTS
Twenty one of the 36 patients (58.3%) showed good recovery (chronic stage MRC ≥ 2) of contralesional ankle DF weakness, whereas 15 patients (41.7%) exhibited poor recovery (chronic stage MRC < 2). A comparison of the FA and ADC values and TVs of the CST and CRT in groups A and B is summarized in Table 2. A significant difference was observed in the TVs of the contralesional CRT between groups A and B (P < 0.05). By contrast, there were no significant differences between the two groups in the FA and ADC values and TVs of the contralesional CST and the FA and ADC value of the contralesional CRT (P > 0.05). Moreover, no significant differences in the FA and ADC values and TVs of the ipsilesional CST and CRT between the group A and B were detected (P > 0.05; Table 2; Fig. 1B).
TABLE 2.
Comparison of DTT parameter results between groups A and B
| Group A | Group B | P | |
|---|---|---|---|
| Ipsilesional CST | |||
| FA | 0.39 ± 0.05 | 0.42 ± 0.07 | 0.15 |
| ADCa | 0.60 ± 0.14 | 0.59 ± 0.12 | 0.89 |
| TVb | 143.71 ± 204.07 | 209.33 ± 216.64 | 0.20 |
| Contralesional CST | |||
| FA | 0.54 ± 0.03 | 0.53 ± 0.03 | 0.14 |
| ADCa | 0.78 ± 0.12 | 0.72 ± 0.07 | 0.13 |
| TVb | 922.52 ± 353.26 | 1113.87 ± 345.05 | 0.09 |
| Ipsilesional CRT | |||
| FA | 0.48 ± 0.04 | 0.48 ± 0.05 | 0.51 |
| ADCa | 0.74 ± 0.15 | 0.70 ± 0.08 | 0.86 |
| TVb | 421.00 ± 342.21 | 643.00 ± 414.02 | 0.69 |
| Contralesional CRT | |||
| FA | 0.48 ± 0.05 | 0.49 ± 0.03 | 0.09 |
| ADCa | 0.71 ± 0.10 | 0.68 ± 0.05 | 0.15 |
| TVb | 395.81 ± 213.28 | 864.60 ± 375.61 | <0.01c |
Values are presented as mean ± SD.
a Unit = 10−3 mm2/sec.
b Unit = mm3.
c Significant difference between groups A and B, P < 0.05.
The correlations between the MRC score of the contralesional ankle DF and DTT parameters of the CST and CRT are shown in Table 3. There was a strong positive correlation between the TV of the contralesional CRT and the MRC score for the contralesional ankle DF (r = 0.521, P < 0.05) and a moderate positive correlation between the TV of the contralesional CST and the MRC score of the contralesional ankle DF (r = 0.399, P < 0.05; Figs. 2, 3). However, no significant correlations were observed between the MRC score of the contralesional ankle DF and the FA or ADC values or TVs of the ipsilesional CST and CRT or the FA or ADC values of the contralesional CST and CRT (P > 0.05).
TABLE 3.
Correlation coefficients between the MRC scores of the contralesional ankle DF and DTT parameter values for the corticospinal and CRTs in groups A and B
| MRC | ||
|---|---|---|
| r | P | |
| Ipsilesional CST | ||
| FA | 0.227 | 0.18 |
| ADCa | −0.059 | 0.73 |
| TVb | 0.173 | 0.31 |
| Contralesional CST | ||
| FA | −0.246 | 0.15 |
| ADCa | −0.321 | 0.06 |
| TVb | 0.399 | 0.02c |
| Ipsilesional CRT | ||
| FA | −0.007 | 0.97 |
| ADCa | −0.148 | 0.39 |
| TVb | 0.256 | 0.13 |
| Contralesional CRT | ||
| FA | 0.167 | 0.33 |
| ADCa | −0.114 | 0.51 |
| TVb | 0.521 | 0.01c |
Values are presented as mean ± SD.
a Unit = 10−3 mm2/sec.
b Unit = mm3.
c Significant correlations between the MRC score and DTT parameters, P < 0.05.
FIGURE 2.
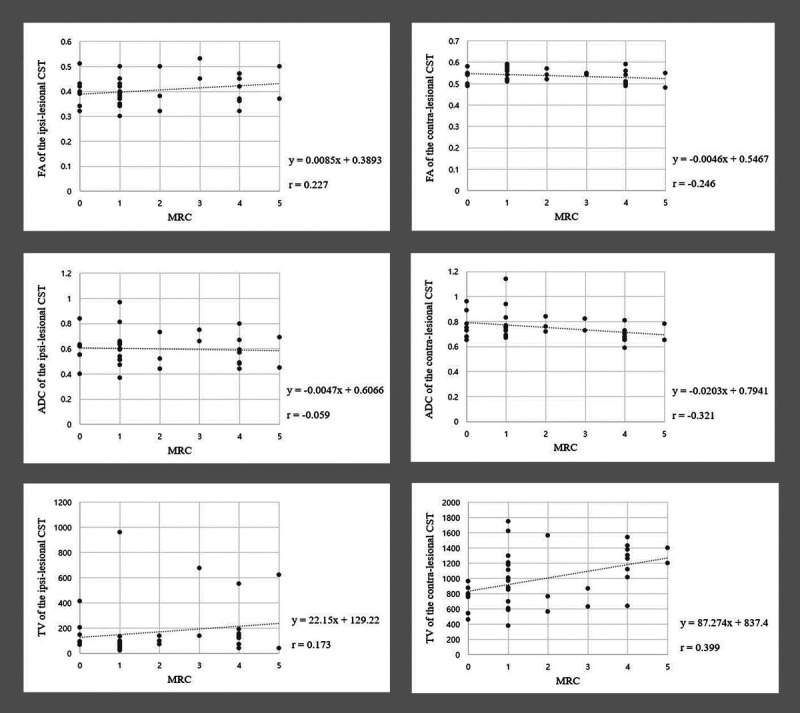
Scatter plots showing the correlation of the MRC scores with DTT parameter values of the CST. The MRC scores show moderate positive correlation with the TV of the contralesional CST (r = 0.399, P < 0.05).
FIGURE 3.
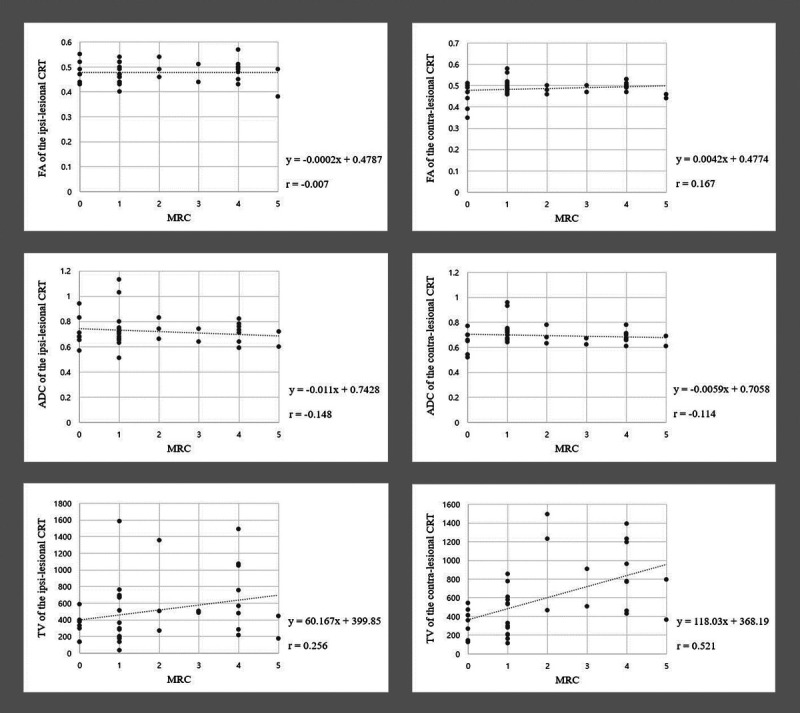
Scatter plots showing the correlation of the MRC scores with DTT parameter values of the CRT. The MRC scores show strong positive correlation with the TV of the contralesional CST (r = 0.521, P < 0.05).
DISCUSSION
In the current study, we used DTT to investigate the relationship between the recovery of contralesional ankle DF weakness with the CST and CRT status in stroke patients with severe motor function weakness due to complete injuries of the ipsilesional CST and CRT. We observed the following. (1) Among the stroke patients who revealed nearly complete weakness of the contralesional ankle DF at stroke onset, 58.3% showed good recovery of contralesional ankle DF function, and the others (41.7%) had poor ankle DF function recovery. Thus, approximately 40% of stroke patients with severe weakness due to severe injuries of the ipsilesional CST and CRT could show poor recovery of the contralesional ankle DF weakness. (2) Comparison of the FA and ADC value and TV parameters of the contralesional neural tracts between the two recovery groups revealed that only the TV of the contralesional CRT in group B was higher than that in group A, with no significant differences in the other DTT parameter values for the other neural tracts. (3) Correlations between MRC score of the contralesional ankle DF and the various DTT parameters were only detected for the TV of the contralesional CRT and CST, strong and moderate positive correlations, respectively.
In many studies, the FA, ADC, and TV results have been used to determine the status of neural tracts in stroke patients.28,30 A relatively high FA value for a neural tract indicates high directionality of the tract, whereas a high ADC value for a neural tract indicates high diffusivity of the tract. High TV for a neural tract indicates a high number of neural fibers in the neural tract.28,30 Therefore, the higher TV of the contralesional CRT in group B compared with group A indicates that the number of neural fibers within the contralesional CRT was higher in patients who showed good recovery of contralesional ankle DF weakness than that in patients with poor weakness recovery. These results suggest that the number of neural fibers in the contralesional CRT seems to be related to the amount of recovery of contralesional ankle DF weakness in stroke patients with complete injuries of the ipsilesional CST and CRT. Our results indirectly support those in a previous study showing that the contralesional CRT contributes to functional gait recovery in stroke patients with complete injury of the ipsilesional CST.24
Our assessment of the correlations between the MRC score of the contralesional ankle DF and DTT parameters revealed positive correlations between the MRC score of the contralesional ankle DF and the TVs of the contralesional CST and CRT (contralesional CST: moderate correlation, contralesional CRT: strong correlation). These results indicate that an increase in the number of neural fibers in the contralesional CST and CRT seems to be related to an increase in the degree of recovery of contralesional ankle DF weakness in stroke patients with complete injuries of the ipsilesional CST and CRT. Interestingly, compared with the contralesional CST, the contralesional CRT seems to be more closely associated with recovery of contralesional ankle DF weakness.
As far as we are aware, only one previous study demonstrated a recovery mechanism for ankle motor weakness in a stroke patient.13 In 2021, Cho and Jang13 used DTT to demonstrate that contralesional ankle DF weakness was recovered through activation of leg somatotopic fibers of the ipsilesional CST and the application of transcranial magnetic stimulation in a chronic patient with middle cerebral artery infarction. Another related study reported recovery of the gait function in patients with complete injury of the ipsilesional CST.24 In 2013, Jang et al.24 used DTT to demonstrate a relationship between the contralesional CRT state and gait function in 54 chronic stroke patients. They found that the TV of the contralesional CRT was closely associated with gait function in chronic stroke patients with complete injury of the ipsilesional CST.24 Nonetheless, to the best of our knowledge, the present study is the first to demonstrate a relationship between contralesional ankle DF weakness and the contralesional CST and CRT status in stroke patients with complete injuries of the ipsilesional CST and CRT.
However, some limitations of this study should be considered. First, we observed complete discontinuations of the ipsilesional CST and CRT around or below the lesion on DTT; however, the discontinuations might not be complete because, throughout the white matter of the brain, DTT may produce false-negative results due to crossing fiber or partial volume effects.31 Small tracts with different directions within a single voxel will not be imaged due secondary to partial volume artifacts. This can arise not only when evaluating small fiber bundle but also when attempting to visualize the beginning of large fiber tracts, such as the subcortical portion of the pyramidal tracts. Thus, the possibility of false-negative effect should be considered.32 Second, because this study was conducted retrospectively, we could not obtain DTT parameter data during the early (acute or subacute) poststroke stage, sufficient clinical data including spasticity. In addition, we could not provide detailed data on rehabilitation interventions and individual activities, which could affect MRC of the ankle DF. Therefore, further prospective studies that assess follow-up DTT data from early to chronic stages should be encouraged. In addition, an investigation into the effects of the application of repetitive transcranial magnetic stimulation or transcranial direct current stimulation on the contralesional CST and CRT on contralesional ankle DF weakness recovery is needed. In addition, the relationship between contralesional ankle DF weakness recovery and neural tract status should be assessed in patients with partial injuries of the ipsilesional CST and CRT.
In conclusion, we found that the number of neural fibers of the contralesional CST and CRT was closely related to the recovery of the contralesional ankle DF weakness in stroke patients with complete injuries of the ipsilesional CST and CRT. Moreover, the contralesional CRT had a closer relationship to such recovery than the contralesional CST. We suggest that our results could be useful during neurorehabilitation planning; for example, repetitive transcranial magnetic stimulation or transcranial direct current stimulation could be directed to the contralesional CRT (primary target) and CST (secondary target) for recovery of contralesional ankle DF weakness in stroke patients with complete injuries of the ipsilesional CST and CRT.
Footnotes
Supported by the National Research Foundation of Korea grant funded by the Korean Government (Ministry of Science, ICT and Future Planning, No. 2021R1A2B5B01001386).
SHJ did the study concept and design, manuscript development, writing, funding, and critical revision of manuscript for intellectual content. MKC did the study concept, design, and critical revision of manuscript for intellectual content.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Contributor Information
Sung Ho Jang, Email: strokerehab@hanmail.net.
Min Kyeong Cho, Email: jmk09609@daum.net.
REFERENCES
- 1.Duncan PW Goldstein LB Matchar D, et al. : Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 1992;23:1084–9 [DOI] [PubMed] [Google Scholar]
- 2.Pongpipatpaiboon K Mukaino M Matsuda F, et al. : The impact of ankle-foot orthoses on toe clearance strategy in hemiparetic gait: a cross-sectional study. J Neuroeng Rehabil 2018;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BR Moon WJ Kim H, et al. : Transcranial magnetic stimulation and diffusion tensor tractography for evaluating ambulation after stroke. J Stroke 2016;18:220–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choo YJ, Kim JH, Chang MC: The prediction of need of using ankle-foot orthoses in stroke patients based on findings of a transcranial magnetic stimulation study. J Integr Neurosci 2021;20:119–23 [DOI] [PubMed] [Google Scholar]
- 5.Adeyemo BO Simis M Macea DD, et al. : Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in non-invasive brain stimulation in stroke. Front Psych 2012;3:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha HG, Kim MK: Effects of strengthening exercise integrated repetitive transcranial magnetic stimulation on motor function recovery in subacute stroke patients: a randomized controlled trial. Technol Health Care 2017;25:521–9 [DOI] [PubMed] [Google Scholar]
- 7.Cramer SC Nelles G Benson RR, et al. : A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 1997;28:2518–27 [DOI] [PubMed] [Google Scholar]
- 8.Jang SH Ahn SH Ha JS, et al. : Peri-infarct reorganization in a patient with corona radiata infarct: a combined study of functional MRI and diffusion tensor image tractography. Restor Neurol Neurosci 2006;24:65–8 [PubMed] [Google Scholar]
- 9.Jang SH Byun WM Han BS, et al. : Recovery of a partially damaged corticospinal tract in a patient with intracerebral hemorrhage: a diffusion tensor image study. Restor Neurol Neurosci 2006;24:25–9 [PubMed] [Google Scholar]
- 10.Jang SH: A review of motor recovery mechanisms in patients with stroke. NeuroRehabilitation 2007;22:253–9 [PubMed] [Google Scholar]
- 11.Cramer SC Moore CI Finklestein SP, et al. : A pilot study of somatotopic mapping after cortical infarct. Stroke 2000;31:668–71 [DOI] [PubMed] [Google Scholar]
- 12.Jang SH Kim K Kim SH, et al. : The relation between motor function of stroke patients and diffusion tensor imaging findings for the corticospinal tract. Neurosci Lett 2014;572:1–6 [DOI] [PubMed] [Google Scholar]
- 13.Cho MJ, Jang SH: Delayed activation of leg somatotopic fibers of an injured corticospinal tract in a patient with cerebral infarction. Neural Regen Res 2022;17:2551–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidoff RA: The pyramidal tract. Neurology 1990;40:332–9 [DOI] [PubMed] [Google Scholar]
- 15.Mendoza JE, Foundas AL: Clinical Neuroanatomy: A Neurobehavioral Approach. New York; London, Springer, 2007 [Google Scholar]
- 16.Jang SH: The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med 2014;46:193–9 [DOI] [PubMed] [Google Scholar]
- 17.Yeo SS Chang MC Kwon YH, et al. : Corticoreticular pathway in the human brain: diffusion tensor tractography study. Neurosci Lett 2012;508:9–12 [DOI] [PubMed] [Google Scholar]
- 18.Jang SH, Kim SY: Injury of the corticospinal tract in patients with mild traumatic brain injury: a diffusion tensor tractography study. J Neurotrauma 2016;33:1790–5 [DOI] [PubMed] [Google Scholar]
- 19.Mori S Crain BJ Chacko VP, et al. : Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–9 [DOI] [PubMed] [Google Scholar]
- 20.Assaf Y, Pasternak O: Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008;34:51–61 [DOI] [PubMed] [Google Scholar]
- 21.Yeo SS, Jang SH: Recovery of an injured corticospinal tract and an injured corticoreticular pathway in a patient with intracerebral hemorrhage. NeuroRehabilitation 2013;32:305–9 [DOI] [PubMed] [Google Scholar]
- 22.Jang SH, Lee J, Lee HD: Peri-infarct reorganization of an injured corticoreticulospinal tract in a patient with cerebral infarct. Int J Stroke 2015;10:E62–3 [DOI] [PubMed] [Google Scholar]
- 23.Jang SH, Chang MC: Recovery of an injured corticoreticulospinal tract in a patient with pontine hemorrhage. Int J Stroke 2016;11:NP18–9 [DOI] [PubMed] [Google Scholar]
- 24.Jang SH Chang CH Lee J, et al. : Functional role of the corticoreticular pathway in chronic stroke patients. Stroke 2013;44:1099–104 [DOI] [PubMed] [Google Scholar]
- 25.Council MR : Aids to the Examination of the Peripheral Nervous System. London, Her Majesty’s Stationery Office, 1976 [Google Scholar]
- 26.Mehrholz J Wagner K Rutte K, et al. : Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil 2007;88:1314–9 [DOI] [PubMed] [Google Scholar]
- 27.Cunha IT Lim PA Henson H, et al. : Performance-based gait tests for acute stroke patients. Am J Phys Med Rehabil 2002;81:848–56 [DOI] [PubMed] [Google Scholar]
- 28.Yoo JS Choi BY Chang CH, et al. : Characteristics of injury of the corticospinal tract and corticoreticular pathway in hemiparetic patients with putaminal hemorrhage. BMC Neurol 2014;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, L. Erlbaum Associates, 1988 [Google Scholar]
- 30.Pagani E Agosta F Rocca MA, et al. : Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage 2008;41:657–67 [DOI] [PubMed] [Google Scholar]
- 31.Yamada K Sakai K Akazawa K, et al. : MR tractography: a review of its clinical applications. Magn Reson Med Sci 2009;8:165–74 [DOI] [PubMed] [Google Scholar]
- 32.Reinges MH Schoth F Coenen VA, et al. : Imaging of postthalamic visual fiber tracts by anisotropic diffusion weighted MRI and diffusion tensor imaging: principles and applications. Eur J Radiol 2004;49:91–104 [DOI] [PubMed] [Google Scholar]


