Abstract
Circadian clocks are biological timing mechanisms that generate 24-h rhythms of physiology and behavior, exemplified by cycles of sleep/wake, hormone release, and metabolism. The adaptive value of clocks is evident when internal body clocks and daily environmental cycles are mismatched, such as in the case of shift work and jet lag or even mistimed eating, all of which are associated with physiological disruption and disease. Studies with animal and human models have also unraveled an important role of functional circadian clocks in modulating cellular and organismal responses to physiological cues (ex., food intake, exercise), pathological insults (e.g. virus and parasite infections), and medical interventions (e.g. medication). With growing knowledge of the molecular and cellular mechanisms underlying circadian physiology and pathophysiology, it is becoming possible to target circadian rhythms for disease prevention and treatment. In this review, we discuss recent advances in circadian research and the potential for therapeutic applications that take patient circadian rhythms into account in treating disease.
Keywords: circadian clock, circadian disruption, chronobiology, chronopathology, chronotherapy
The 24-h environmental cycle caused by the daily rotation of the earth led nearly all organisms to evolve endogenous cycles that occur with a period of about 24 h and are called circadian rhythms (from the Latin phrase circa diem meaning about a day) (Dunlap, 1999). This internal timing system allows organisms to anticipate cyclic changes of light, food availability and predation risk, and provides temporal segregation of physiological processes that may be incompatible with each other or with an environmental signal. For instance, temporal compartmentalization of biochemical, metabolic, and redox processes within cells (Bass and Lazar, 2016) may help single-cell organisms time cell division so as to escape the DNA damaging effects of ultra-violet light and ionizing radiation associated with sunlight (Chaix et al., 2016). In multicellular organisms including humans, cellular oscillators in the brain and peripheral tissues interconnect to form a circadian network that coordinates rhythms in physiology and behaviors such as sleep-wake, body temperature, blood pressure, hormone production, neural, and immune systems (Patke et al., 2020).
Modern lifestyles often lack regular patterns of working, eating, and sleeping. The subsequent rhythm disruptions increase susceptibility to cardio-metabolic, digestive, immune, neuropsychiatric disorders, as well as cancers, reflecting the adaptive benefits of circadian rhythms (Masri and Sassone-Corsi, 2018; Scheiermann et al., 2018; Chellappa et al., 2019; Logan and McClung, 2019; Segers and Depoortere, 2021). Studies with animal models containing genetic mutations of clock genes, or animals exposed to circadian desynchrony regimens, reinforce the causal relationship between circadian disturbances and pathology (Mattis and Sehgal, 2016; Aiello et al., 2020; Hadadi et al., 2020; Shafi et al., 2021). In addition to the pathological impact of circadian disruption, symptoms of several medical conditions, including neurological (e.g. epilepsy), cardiovascular (e.g. high-blood pressure, myocardial infarction, stroke), and inflammatory disorders (e.g. asthma, sepsis, rheumatoid arthritis), show time of day variations (Scheiermann et al., 2018; Chellappa et al., 2019; Crnko et al., 2019; Karoly et al., 2021). The mechanisms, in some cases, may be due to rhythmic expression of disease genes and in other cases due to cycling in other aspects of physiology. Moreover, responses to physiological and pathological stimuli such as diet, exercise, and pathogenic infections often show circadian rhythms (Gabriel and Zierath, 2019; Diallo et al., 2020; Lewis et al., 2020).
Given the links between circadian dysregulation and pathology, investigators have sought ways to enhance circadian rhythms for disease treatment, by targeting the circadian clock with non-pharmaceutical interventions such as bright light, time-restricted feeding, or exercise. The fact that target genes of many medications are expressed rhythmically also provides a molecular rationale for timed drug treatment to increase effectiveness and reduce toxicity (R. Zhang et al., 2014; Mure et al., 2018; Ruben et al., 2018). These drugs may directly target clock genes or molecules that cycle under the control of the clock—“output molecules” (Tognini et al., 2018; Hawley et al., 2020; Miller and Hirota, 2020; Borrmann et al., 2021; Rogers et al., 2021). On the other hand, some drugs can alter or disrupt circadian rhythms. Pharmacologically induced changes in circadian behavior can be traced back over a thousand years to the legendary Ethiopian goatherder Khaldi. Khaldi noticed that his goats stayed up at night dancing after they ate the berries of a local tree. He shared this information with a monk, who decided to make a brew from the berries to stay awake during morning prayers. The tree, Coffea arabica, is now one of the most widely cultivated crops and the brew, coffee, is consumed daily by more than two billion people.
In this review, we describe recent advances in chronobiology with a focus on disease models that may have potential for circadian rhythm-based therapies.
MECHANISMS OF CIRCADIAN PHYSIOLOGY
The basic architecture of the circadian clock across organisms consists of a cell-autonomous autoregulatory feedback loop (Chaix et al., 2016; Johnson et al., 2017). In eukaryotes, dedicated positive and negative clock regulators form an interlocked transcriptional translational feed-back loop (TTFL) to create a cell-autonomous oscillator that drives rhythmic expression of output genes involved in metabolic, biosynthetic, signal transduction, and cell cycle pathways (Patke et al., 2020). In mammals, BMAL1 and CLOCK genes form a transcription activator complex that cyclically drives transcription of its own repressors, Period (PER)/Cryptochrome (CRY). The core oscillator is complemented by a second stabilizing loop in which periodic expression of BMAL1 is maintained by the REV-ERBα/β repressor and RORα/β/γ activator proteins (Takahashi, 2017). Besides the core regulatory loops, multiple levels of epigenetic, posttranscriptional, and posttranslational regulation by various kinases and phosphatases, ubiquitin-proteasome pathway components, nuclear-cytoplasmic transporters, non-coding RNAs, and chromatin remodelers, contribute to the molecular clock (J. Lee et al., 2008; Y. Lee et al., 2010, 2015a, 2015b, 2019b; Anafi et al., 2014; Korge et al., 2018; Koronowski and Sassone-Corsi, 2021).
The clock generates rhythms by coordinating temporal programs via multiple clock-output genes. Large-scale genomic studies found that, although the identity of genes expressed rhythmically varies from tissue to tissue, ~50% of mammalian genes show circadian regulation in at least one tissue (R. Zhang et al., 2014; Mure et al., 2018; Ruben et al., 2018). In addition, multi scale-omics studies demonstrated circadian regulation of the epigenome, the metabolome, the proteome/phosphoproteome, and the microbiome (Thaiss et al., 2014; R. Zhang et al., 2014; Chiang et al., 2017; Robles et al., 2017; Solanas et al., 2017; J. Wang et al., 2017; Y. Wang et al., 2018; Dyar et al., 2018; Mure et al., 2018; Noya et al., 2019; Malik et al., 2020). Notably, these studies reveal that oscillations of proteins or metabolites often exhibit different patterns relative to transcript rhythms in a given tissue (e.g. hippocampus, liver), and oscillations at all levels can be reprogramed by circadian rhythm disturbances caused by sleep deprivation, jet lag, high-fat diet, and aging.
A central pacemaker in the brain, housed in the hypothalamic suprachiasmatic nucleus (SCN), synchronizes clocks in brain and peripheral organ systems to create a body-wide circadian network. In particular, the SCN is critical for synchronizing organismal clocks to light as it receives information about light from the retina via the retinohypothalamic tract (RHT) (Koronowski and Sassone-Corsi, 2021). In a hierarchical organization model, the SCN central clock coordinates the circadian phases of extra-SCN clocks in other brain regions via rhythmic release of neurotransmitters and neuropeptides and of peripheral tissues via systemic hormonal secretion and neural innervation (Gizowski et al., 2016; R. M. Buijs et al., 2019; Collins et al., 2020; Paul et al., 2020). For example, via the sympathetic nervous system, the SCN coordinates the rhythmic secretion of the night hormone melatonin in the pineal gland and the morning stress hormone glucocorticoid in the adrenal glands (R. M. Buijs and Kalsbeek, 2001; Ishida et al., 2005; Russell and Lightman, 2019). In addition, the SCN clock regulates the autonomic nervous system to control peripheral clock functions in the heart, kidney, pancreas, lung, intestine, and thyroid glands (F. N. Buijs et al., 2016; Tahara and Shibata, 2016; Firsov and Bonny, 2018; Challet, 2019; Ikegami et al., 2019; Stenvers et al., 2019).
Beyond photic entrainment, multiple non-photic physiological and environmental cues (e.g. temperature, food intake, exercise, gut microbial products, redox cofactors, metal ions, and pathogenic infections), can regulate and/or set the timing of non-SCN brain and peripheral clocks (Son et al., 2008; BanoOtalora and Piggins, 2017; Ehlen et al., 2017; Myung et al., 2018; Hasegawa et al., 2019; Myung et al., 2019; Finger and Kramer, 2021; Segers and Depoortere, 2021; Sinturel et al., 2021; Van Drunen and Eckel-Mahan, 2021). The peripheral clocks, in turn, interact with and influence each other via neural and immunometabolic circuits, resulting in multi-mode regulation of tightly-coupled body clocks that leads to overt rhythms of physiology.
CIRCADIAN RHYTHMS AND CHRONOTHERAPY
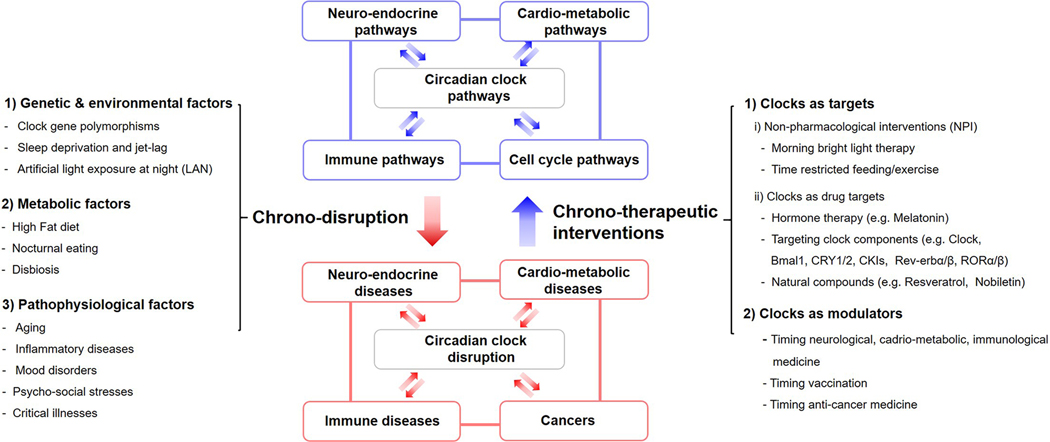
The broad impact of circadian rhythms on physiology underscores the importance of translating mechanistic insights into clinical practice through two major approaches. The first is to increase awareness of how rhythm disturbances by physiological and environmental factors impact physiology and increase susceptibility to disease (Figure 1). Circadian disruptions such as surgical ablation of the SCN clock, whole-body knockout of core clock genes (ex., Bmal1, Per1/2, Cry1/2, Rev-erbα/β) or experimental jet lag (chronic phase shift by altered light schedules) increase the risk of sleep, mood, cardio-metabolic, immune, endocrine, digestive, reproductive, premature aging, neurodegenerative and neoplastic disorders (Kondratov et al., 2006; Alvarez et al., 2008; Musiek et al., 2013; Papagiannakopoulos et al., 2016; Firsov and Bonny, 2018; Stenvers et al., 2019; Bishehsari et al., 2020; Walker et al., 2020b; Segers and Depoortere, 2021). Resonant with these, several human and translational studies have reported adverse health consequences as a result of polymorphisms in clock genes, sleep deprivation, night shiftwork, and artificial light at night exposure (Buxton et al., 2012; West et al., 2017; Tahkamo et al., 2019; Walker et al., 2020a).
Figure 1.
Circadian-disruptive factors and chronotherapeutic interventions in physiology and disease. Abbreviations: CRY = cryptochrome; ROR = Receptor-related orphan receptor. Reciprocal interactions of circadian clocks with other physiological pathways constitute systemic circadian physiology. Multiple factors that disrupt circadian rhythms can increase the risk of disease onset and severity, which in turn feeds forward to cause more chrono-disruption. Conversely, several types of chrono-therapeutic interventions can be implemented to enhance or restore circadian rhythms and thereby reduce disease progression and improve the response to treatment; CKI = casein kinase 1
The second area of medicine that should be developed, based on circadian insights, is a therapeutic approach that takes circadian regulation into account (Figure 1). In this review, we classify chronotherapeutic approaches into two categories: (1) Clocks as targets; non-pharmaceutical or pharmacological interventions to manipulate circadian rhythms or circadian components; (2) Clocks as modulators: timing of medicine to the endogenous rhythm established by clocks in order to improve efficacy and reduce side effects. These concepts would not be exclusive of each other but can be applied in a combinatorial manner. For example, non-pharmaceutical interventions can strengthen the compromised or disordered rhythms to improve the effects of clock-targeted or circadian-modulated drugs.
CLOCKS AS TARGETS
As mentioned above, the circadian system composed of the brain and peripheral clocks can be synchronized through a wide variety of environmental and systemic cues such as light, feeding, hormones, and exercise. Exploiting such rhythm synchronizers can enhance body clock functions, thereby improving circadian rhythms and perhaps reducing susceptibility to diseases that are particularly sensitive to internal desynchrony (Figure 1). Along with system-level interventions, the identification of disease-relevant effects of specific clock components has prompted efforts to develop small-molecule compounds targeting these components (Miller and Hirota, 2020). This effort has been facilitated by chemical and structural biology approaches such as the characterization of X-ray crystal structures of clock protein-compound complexes (Miller and Hirota, 2020). In the following sections, we describe therapeutic applications of non-pharmacological and pharmacological interventions targeting circadian clocks in disease settings.
Enhancing Circadian Rhythms to Improve Health
Circadian rhythms can be enhanced through non-invasive interventions such as bright light or scheduled meal times, making them an ideal target for improving basic health and fitness, in other words, preventive medicine.
Chrono-Phototherapy.
Light is the most well-established means to entrain circadian rhythms, although no specific treatment or light device is FDA approved. Morning bright light exposure, has been widely touted as a way to treat sleep disorders (e.g. advanced or delayed sleep phase syndromes), neuropsychiatric disorders (e.g. autism spectrum disorder, attention deficit hyperactivity disorder, seasonal affective disorder, dementia), associated with genetically, environmentally, and pathologically perturbed circadian rhythms (Lyketsos et al., 1999; Lieverse et al., 2011; Vallee et al., 2020). Randomized, double-blind, parallel-arm, placebo-controlled studies showed that light therapy improves mood and insulin sensitivity in patients with major depression and type 2 diabetes (Brouwer et al., 2015, 2019). Concomitantly, bright light therapy is emerging as a chronotherapeutic tool to alleviate motor disorders, sleep/wake alterations, anxiety, and depression in patients with neurodegenerative disease (Fifel and Videnovic, 2019).
Light also regulates melatonin levels, and some studies show that melatonin inhibits the growth of tumors. Although melatonin is suppressed by light, daytime blue light enhances the effect of nighttime melatonin in inhibiting the growth of prostate, liver, and breast cancers (Mao et al., 2012; Dauchy et al., 2015, 2016, 2018). These correlate with previous reports that melatonin depletion by light exposure late at night stimulates the growth of multiple human cancer xenografts, increasing drug resistance (Blask et al., 2002, 2005; Wu et al., 2011; Dauchy et al., 2014; Xiang et al., 2015). Other experimental human studies with a simulated night shift model show that bright light induces complete and rapid adjustment of peripheral clocks, suggesting phototherapy as a potential non-pharmacological intervention to counteract the deleterious effects of shift work or jet lag (Cuesta et al., 2017; Kervezee et al., 2019). Overall, these studies suggest that targeting the neuro-hormonal axis with light could have benefits in disease prevention and treatment.
Chrono-Diet.
Restricting food intake to a specific daily interval synchronizes some peripheral clocks and has become a popular way to improve metabolic health. The health benefits of clock-modulating diets have been increasingly recognized as has the dynamic cross-talk between circadian clocks and metabolic pathways (Marcheva et al., 2013; Reinke and Asher, 2019). The molecular circadian clock directly or indirectly synchronizes diverse metabolic processes such as gluconeogenesis, mitochondrial metabolism, and lipogenesis (Marcheva et al., 2013). This involves circadian regulation of uptake, synthesis, and break-down of nutrients (e.g. glucose, amino acids, lipids) via rhythmically controlled expression and activity of transporters or enzymes. Reciprocally, the clock receives input from nutrient signaling pathways (e.g. NAD +-dependent sirtuins), which function as rheostats to coordinate metabolic processes with daily cycles of sleep/wake and fasting/feeding (Marcheva et al., 2013; Reinke and Asher, 2019).
Time-restricted feeding (TRF) and metabolic health.
Typically, animals have a circadian rhythm of feeding, but when provided with food ad libitum, feeding rhythms are blunted. Nonetheless, they can be enhanced by time-restricted feeding (TRF) protocols, which synchronize clocks in metabolic tissues (Lewis et al., 2020) and improve health (Mattson, 2019; Lundell et al., 2020). Using rodent models, Panda and colleagues showed that a TRF regimen that limits food availability to 8–9 h in the active phase improves internal clock rhythms and metabolic health indices compromised by high-fat diets, thus relieving or reversing metabolic conditions associated with obesity, diabetes mellitus type 2, hyperlipidemia, fatty liver disease, and inflammation (Hatori et al., 2012; Chaix et al., 2014). Another mouse study showed that restoring peripheral clock rhythmicity and synchrony by TRF normalizes body weight and glucose metabolism in SCN-specific Bmal1 deficient mice in constant darkness (Kolbe et al., 2019).
Corresponding to the animal study results, early time-restricted feeding, in which food intake was limited to 6-h window each day with an 18 h daily fast, improved insulin sensitivity, blood pressure, and oxidative stress without weight loss in prediabetic men (Sutton et al., 2018). Another study with overweight/ obese men showed that short-term TRF (8 h day/d) improves the rhythm of serum and muscle metabolites as well as of genes controlling amino acid transport, without perturbing core clock gene expression (Lundell et al., 2020). In line with this, short-term TRF improved nocturnal and postprandial glycemic control in overweight/obese sedentary males, and also improved feelings of well-being (Parr et al., 2020). As with morning light therapy for inherent short or long sleepers, timed metabolic interventions can relieve the pathological impact of some metabolic disorders.
TRF and the gut microbiome.
Interestingly, TRF appears to exert health benefits, in part, by influencing diurnal gut-microbiome activity. The composition of the microbiome cycles over the course of a circadian day and impaired cycling in a mouse model of diet-induced obesity is rescued by TRF, presumably through a decrease in amounts of obesogenic microflora relative to obesity-protective microflora (Zarrinpar et al., 2014). This was supported by the finding that TRF modulates the circadian rhythm of gut microbiota and hepatic lipid metabolism, reducing the detrimental effects of a high-fat diet (Ye et al., 2020). TRF also significantly enhanced gut microbial richness (Prevotellaceae and Bacteroideaceae) in healthy humans, improving serum lipid and liver profiles (Zeb et al., 2020).
Clock-enhancing diets and aging.
TRF and other dietary interventions have the potential to counteract aging via circadian rhythm enhancement., A fly model revealed that TRF attenuates age-related cardiac decline in Drosophila (Gill et al., 2015). Moreover, short-term intermittent fasting in young flies extended lifespan by improving gut health and resistance to oxidative stress (Catterson et al., 2018). Interestingly, even caloric restriction (CR), which is a well-known lifespan-extending dietary intervention, improves circadian cycling in flies (Katewa et al., 2016).
In mice as in flies, circadian molecular and metabolic profiles altered by aging are reversed by CR (Liang et al., 2018). Solanas and colleagues showed that age-associated rewiring of the oscillatory diurnal transcriptome in adult stem cells, such as the switch from genes involved in homeostasis to those involved in tissue-specific stresses (e.g. DNA damage, inefficient autophagy), was prevented by long-term CR (Solanas et al., 2017). In a parallel study, CR rescued the aging-dependent decline in the global rhythms of transcription and protein acetylation in the liver, improved NAD + availability, enhanced SIRT1 activity, and increased levels of acetate and acetyl-coA, all indicative of enhanced longevity (Sato et al., 2017). Consistent with a role of NAD in circadian heath and aging, Levine and colleagues recently reported that NAD + repletion to youthful levels with the NAD + precursor nicotinamide riboside (NR) restored transcriptional, metabolic, and behavioral rhythms in old mice, through inhibition of the clock repressor PER2 (Levine et al., 2020). Interestingly, elevated acetyl-CoA levels in mice treated with candidate drugs against Alzheimer’s disease reduced many aspects of brain aging such as memory deficits (Currais et al., 2019). Thus, CR or CR mimetics (CRMs) are potent dietary options to counter aging-associated circadian pathologies and/or accelerated aging linked to circadian disruption (Madeo et al., 2019). Notably, molecules that mediate the effects of CR on lifespan, such as insulin/IGF-1, SIRT1, NAMPT, AMPK, PGC-1α, mTOR, GSK3β, and FGF21, are also affected by the timing of food availability (López-Otín et al., 2016). Thus mechanisms underlying effects of TRF and CR may, at least in part, be similar (López-Otín et al., 2016). Indeed, use of an automated feeder system to accurately control duration, amount, and timing of food access showed that CR alters behavioral rhythms and food intake patterns to match that of a temporal restriction paradigm (Acosta-Rodríguez et al., 2017). The authors recommended that circadian timing of food availability be considered in determining longevity effects of dietary restriction regimens (Acosta-Rodríguez et al., 2017).
Clock-enhancing diets and cancer.
In addition to anti-aging benefits, preclinical evidence indicates that CR may have anticancer effects by reducing tumor progression, enhancing the death of cancer cells, and increasing the effectiveness and tolerability of chemoand radiotherapies (Alidadi et al., 2020). Nonetheless, it is increasingly recognized that chronic CR often has detrimental effects on tumor development and chemotherapy, possibly by negatively affecting the immune system, wound healing, and other important functions (Brandhorst and Longo, 2016). Instead, intermittent fasting (IF), a diet-based therapy that alternates between fasting and free feeding/eating for a period of time, reportedly inhibits tumor growth and improves antitumor immune responses in preclinical and clinical studies (X. Zhao et al., 2021). Furthermore, IF can increase cancer sensitivity to chemotherapy and radiotherapy and reduce the side effects of traditional anticancer treatments (X. Zhao et al., 2021). Together these findings holds promise for well-designed dietary intervention tailored to the host’s circadian rhythms as a potential therapeutic regimen to counter cancer.
Chrono-Exercise
Chrono-exercise and aging.
As with TRF, exercise is gaining attention as a potential chrono-therapeutic intervention in the prevention and treatment of multiple diseases (Gabriel and Zierath, 2019). In earlier animal studies, exercise was shown to have positive health effects by strengthening circadian rhythms. For example, Schroeder and colleagues showed that scheduled exercise during the late-night improved many of the rhythmic deficits, including gene expression changes, observed in mice deficient in the peptide vasoactive intestinal polypeptide (Schroeder et al., 2012). Furthermore, aged mice housed with a running wheel showed stronger circadian rhythms in locomotor activity, faster recovery of internal synchrony following a phase advance of 8 h, and increased amplitude of the firing rate rhythm in the SCN, when compared to aged mice housed without a running wheel (Leise et al., 2013). Together with clinical evidence on the protective effect of exercise against multiple morbidities, particularly associated with aging (Duggal et al., 2018; de Souza Teixeira et al., 2020), these results provide a rationale for scheduled exercise as a potential tool to counteract genetic, environmental, and pathophysiological disruptions of circadian rhythms.
Chrono-exercise and metabolic health.
Skeletal muscle has an intrinsic circadian clock that is critical for regulating metabolic activity. Indeed, multiple metabolic indices in the muscle, such as glucose tolerance, insulin sensitivity, and muscle oxidative capacity, show circadian oscillations (Qian and Scheer, 2016; van Moorsel et al., 2016) and these are influenced by the timing of exercise (Erickson et al., 2021). Ezagouri et al showed that both mice and humans exhibit differences in exercise capacity between the early and late part of their active phase, with exercise inducing a time-of-day-dependent transcriptomic and metabolic pattern in the mouse skeletal muscle (Ezagouri et al., 2019). ZMP, an endogenous AMPK activator, is induced by exercise in a time-dependent manner to regulate key steps in glycolytic and fatty acid oxidation pathways, which may in turn enhance exercise capacity (Ezagouri et al., 2019). Sato and colleagues showed that exercise in the evening (beginning of active phase), rather than in the morning (beginning of rest phase), resulted in higher utilization of carbohydrates and ketone bodies, together with degradation of lipids and amino acids in mice (Sato et al., 2019). Exercise selectively activated HIF1α, a central regulator of glycolysis during hypoxia, in a time-dependent manner (Sato et al., 2019).
Resonant with these animal studies, time of day-dependent efficacy of exercise is observed in humans, particularly in metabolically compromised people. A randomized crossover trial with type 2 diabetes men (45–68 years of age) showed that intensive exercise training in the afternoon improved blood glucose levels whereas morning training had an acute, deleterious effect, increasing blood glucose (Savikj et al., 2019). Similarly, another study with adult males (58 ± 7 years) at risk for or diagnosed with type 2 diabetes reported that afternoon (15:00 and 18:00 p.m.) exercise training improved insulin-stimulated glucose disposal, reductions in fat mass and percentage, exercise performance, and basal hepatic glucose output more profoundly than morning exercise (08:00 and 10:00 a.m.) (Mancilla et al., 2021). Interestingly, exercise performed by overweight/obese men before versus after nutrient intake (i.e., in the fasted state) increased skeletal muscle and whole-body lipid utilization and reduced the postprandial increase of insulin in the blood (Edinburgh et al., 2020).
Though no parallel studies with female subjects are reported yet, analysis of 7157 Women’s Health Study participants using accelerometers shows that women engaging in less physical activity before noon have a higher odds ratio for obesity compared to women who are more physically active in the morning (Chomistek et al., 2016). Besides nutrition and sex, personal chronotype appears to influence the time-dependent efficacy of exercise. Among sedentary young men and women, both morning and evening exercise induced phase advances for late chronotypes (people who have a preference for the evening), but only morning exercise advanced early chronotypes (morning people), with evening exercise actually causing a phase delay (Thomas et al., 2020). The authors suggested that late chronotypes benefit from phase advances induced by exercise in the morning or evening, but evening exercise may exacerbate circadian misalignment in early chronotypes (Thomas et al., 2020).
Circadian Components as Drug Targets
Modulating Melatonin.
Diet and exercise timing are examples of non-pharmacological interventions, but several pharmacological agents can target circadian clocks and have been tested in models of neuropsychiatric, metabolic, and neoplastic disorders. The oldest known example is melatonin, which is synthesized and released by the pineal gland at night under control of the SCN, and is thought to be an important signal for synchronizing circadian rhythms and inducing sleep. The extent to which melatonin contributes to circadian synchrony across the organism is debatable, although melatonin receptors are found in the SCN as well in sites of non-SCN clocks in the brain and periphery (fetal adrenal gland, pancreas, liver, kidney, heart, lung, fat, gut, etc.). Multiple melatonin and melatonin agonist administration regimens were developed for the treatment of circadian and sleep disorders (Pevet and Challet, 2011), initially for sleep disruptions caused by shift work or jet lag (Walker et al., 2020b). In a recent systematic review and meta-analysis, melatonin and its analogs (ramelteon, agomelatine, TIK-301, Neu- P11, and tasimelteon) were found to resynchronize circadian rhythms and alleviate depression (Olie and Kasper, 2007; Satyanarayanan et al., 2018).
The use of melatonin analogs to treat circadian disruptions represents the most successful example of circadian medicine. Blind people often have trouble maintaining circadian rhythms, a condition known as Non-24. In 2014, the FDA approved treatment of blind people with tasimelteon, which is marketed by Vanda Pharmaceuticals under the brand name Hetlioz. Ramelteon is also an FDA-approved melatonin agonist, but its approval is as a sedative and not specifically for non-24. Though melatonin cycling is under control of the clock and not actually a component of the core clock, its successful targeting demonstrates that circadian output pathways are druggable, and may actually be better targets than core clock components.
Besides its neuromodulatory functions, melatonin is implicated in antioxidant activity, bone formation, reproduction, cardiovascular function, and immune regulation and has therapeutic benefits for gastrointestinal, inflammatory, cardiovascular diseases, as well as cancers (Najafi et al., 2019; Tarocco et al., 2019). Melatonin has an antagonistic relationship with insulin, and melatonin treatment improves glucose homeostasis, energy balance, and overall health in diabetes mellitus (Wajid et al., 2020). Murine models suggest that exogenous melatonin prevents high-fat diet (HFD)-induced obesity symptoms, via improvement in liver steatosis, low-grade inflammation, insulin resistance, lipid dysmetabolism, and diurnal rhythms of gut microbiota diversity and composition (P. Xu et al., 2017; Yin et al., 2018, 2020). With the established efficacy of melatonin and absence of long-term toxicity, melatonin therapy promises to be one of the safest chronobiotic strategies to treat circadian rhythm-associated diseases.
Modulating Casein Kinases (CKs).
In recent years, identification of small-molecule modulators of circadian rhythms that target core or noncore clock proteins has expanded options for treating circadian-related disorders (Ribeiro et al., 2021). As in the case of most pharmacological agents, however, one cannot exclude the possibility that the small molecules have additional non-circadian targets that contribute to their therapeutic effects.
Familial advanced sleep phase syndrome is caused by a T44A mutation in CK1δ that causes hypophosphorylation of PER2 and shortens circadian period (Y. Xu et al., 2005). Thus, CK1δ, despite being a ubiquitous protein required for many functions, is a component of the circadian clock. Meng et al, showed that pharmacological inhibition of CK1δ by PF-670462, a selective CK1δ inhibitor, restores robust and persistent circadian rhythms in Vipr2−/− mice that have disrupted rhythms due to loss of an SCN signal, vasoactive intestinal peptide receptor type 2 (Meng et al., 2010). Furthermore, administration of a CK1δ/ε inhibitor (PF-5006739) induces rapid and robust circadian rhythms in ex vivo mouse tissues and improves glucose tolerance in both diet-induced and genetic mouse models of obesity (Cunningham et al., 2016).
Modulating REV-ERBs.
Pharmacological targeting of REV-ERBs may have therapeutic potential in a wide range of neuropsychiatric, metabolic and immune disorders and cancers. Compounds thought to be REV-ERB agonists, such as SR9009 and SR9011, induce wakefulness, suppress sleep, regulate emotional behavior in mice, and reduce anxiety-like behavior (Banerjee et al., 2014). Conversely, a REV-ERBα antagonist (SR8278) triggers hyper-dysfunction of midbrain dopaminergic (DAergic) neurons and mania-like behavior in mice. SR9009 and SR9011 also increased energy expenditure and decreased fat mass, plasma triglycerides (TGs), and cholesterol when used in obese mice, while improving mitochondrial respiration and exercise capacity in normal mice (Solt et al., 2012; Woldt et al., 2013). While the specificity of SR9009 is questionable (see below), a different REV-ERB agonist GSK4112, and its analogs, also exhibit an immune-suppressive effect by inhibiting pro-inflammatory IL-6 expression in human cells and Th17 cell differentiation, which has beneficial consequences for autoimmune and inflammatory diseases (Gibbs et al., 2012; Trump et al., 2013). SR9009 and SR9011 have also been credited with anticancer properties in brain, leukemia, breast, colon, glioma, melanoma, and small-cell lung cancer, through effects on a range of cellular processes including autophagy, lipogenesis, ROS levels, and cell proliferation (De Mei et al., 2015; Sulli et al., 2018; Dong et al., 2019; Wagner et al., 2019a; Shen et al., 2020), but these effects should be regarded with caution as SR9009 also produces responses in REV-ERBα/β double-deletion knockouts (Dierickx et al., 2019). Thus, targets other than REV-ERBα/β contribute to effects of SR9009.
Modulating RORs.
Retinoic acid receptor-related orphan receptors (RORs) are positive transcriptional regulators of Bmal1 expression and several isoforms of these are potential candidates to treat disease. For example, pharmacological repression of RORα with SR3335, a RORα-selective inverse agonist, suppresses gluconeogenesis and lowers glucose plasma levels in a diet-induced obesity mouse model (Kumar et al., 2011). SR1555, another inverse agonist modulator of RORγ, reduces food intake, fat mass and body weight, and improves insulin sensitivity in obese diabetic mice, while increasing thermogenic expression and fatty acid oxidation (M. R. Chang et al., 2015). These findings suggest the potential utility of ROR modulators as anti-diabetic and anti-obesity agents. On the other hand, multiple selective RORγ Inhibitors reduce T-helper cell 17 (Th17) responses as well as production of the proinflammatory cytokine IL-17, indicating the therapeutic potential of these molecules for autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, psoriasis, and inflammatory bowel disease (Cyr et al., 2016; Pandya et al., 2018; Cherney et al., 2020; Harcken et al., 2021; Meijer et al., 2021). Pharmacological inhibition of RORγ also exerts potent anti-tumor activity in in vitro and/or in vivo models of castration-resistant prostate cancer (J. Wang et al., 2016), pancreatic adenocarcinoma (Lytle et al., 2019), and triple-negative breast cancer (Cai et al., 2019). However, effects of RORγ, particularly in the case of autoimmune disorders, are likely mediated through non-circadian mechanisms.
Modulating CRYs.
CRY modulators are increasingly implicated in metabolic disease and cancer treatment. Hirota and colleagues first reported that KL001, a CRY stabilizer identified via cell-based high-throughput circadian assays, lengthens circadian period in a variety of cells and tissues, and blocks glucagon-dependent induction of gluconeogenesis in cultured hepatocytes (Hirota et al., 2012). Consistent with these observations, a bioavailable derivative of KL001 exhibits antihyperglycemic activity in diabetic mice (Humphries et al., 2018). Furthermore, two novel CRY1 and CRY2 selective stabilizers, KL101 and TH301, respectively, enhance brown adipose tissue differentiation in culture (Miller et al., 2020).
Notably, the CRY stabilizer KL001 was shown to exhibit anti-tumor activity, such that it impaired self-renewal, cell migration and proliferation, as well as increased apoptosis in patient-derived glioblastoma stem cells (Dong et al., 2019). However, another study showed that KL001 promoted cancer cell migration, but had no effect on cell proliferation or colony formation in U2OS cells (Lin et al., 2020). Adding to these discrepant findings, a CRY1/2 inhibitor, KS15, reduced MCF-7 cell growth and increased chemosensitivity of human breast cancer cells, possibly via the elevation of PER2, a tumor suppressor clock protein (Chun et al., 2014, 2015; Jang et al., 2018). Overall, the effects of CRY modulators on tumors appear to involve divergent mechanisms that are cancer cell type-specific.
Chrono-Modulation With Natural Compounds.
Besides synthetic compounds, some natural chemicals modulate circadian clock function. Resveratrol, a potent natural activator of the NAD + dependent deacetylase SIRT1 that targets PER2, restores rhythms in clock gene expression and ameliorates the cycling of lipid metabolism in HFD-induced obese mice (Sun et al., 2015). In addition to its anti-obesity and anti-diabetic properties, experimental and clinical evidence suggests that resveratrol, as well as other synthetic small-molecule SIRT1 activators, have the potential to prevent or treat aging-related disorders such as inflammation, cardiovascular and neurodegenerative diseases, and some cancers (Hubbard and Sinclair, 2014).
Likewise, nobiletin (NOB), a dietary flavonoid found in citrus fruits, was recently characterized as a RORα and RORγ agonist that enhances circadian rhythms and protects against metabolic syndrome (Mulvihill et al., 2011; He et al., 2016; Shinozaki et al., 2017; Nohara et al., 2019b). Furthermore, NOB promotes healthy aging by fortifying mitochondrial respiration in skeletal muscle (Nohara et al., 2019a). Besides the metabolic benefits, NOB treatment reduces rearing and anxiety-related behavior of Cry1/2 clock mutant mice (Sanghani et al., 2020). NOB also has anti-tumor properties in that it induces apoptosis and cell cycle arrest, suppresses migration and invasion, inhibits many oncogenic drivers, upregulates tumor suppressors, and increases sensitivity of several cancer cell types to chemotherapy (Ashrafizadeh et al., 2020; Lellupitiyage Don et al., 2020). In addition to these compounds, several other natural phytochemicals (e.g. caffeine, catechin, quercetin, kaempferol, myricetin) may target circadian rhythms to promote their anti-diabetes, anti-obesity, antioxidant, anti-inflammatory, anti-cancer, and anti-aging benefits (T. Xu and Lu, 2019; Song et al., 2021).
CLOCKS AS MODULATORS
Most medicines are administered without regard to the time of administration. However, body-wide circadian transcriptome studies reveal that most drug targets are expressed rhythmically in one or other tissue (R. Zhang et al., 2014; Mure et al., 2018; Ruben et al., 2018). This target cycling suggests that timing matters for optimal efficacy of therapeutic drugs (R. Zhang et al., 2014; Peeples, 2018; Ruben et al., 2019c). But timing to the cycle of the target is not sufficient for determining optimal timing because the pharmacological effects of a drug are also determined by absorption, distribution, metabolism and excretion, all of which may be regulated by peripheral and central circadian clocks (Ballesta et al., 2017; Bicker et al., 2020). In addition, toxic effects of drugs are often dose-limiting and off-target mediators of drug toxicity may not cycle the same way as the primary drug target (Anafi et al., 2017).
Based upon the factors above, properties and pharmacology of some drugs may make them more “clockable.” We suggest that drugs and targets with the properties listed below are candidates for circadian medicine.
Cycling target: A strongly cycling target suggests that drugs may be delivered when the target peaks (e.g. see below HMG-coA reductase). Alternatively, rate limiting metabolic or transporter molecules may cycle.
Cycling physiology or pathology: Even though the target may not cycle, the relevant physiology may have a circadian cycle. For instance, caffeine is consumed in the morning to stay awake and sleeping pills are taken in the evening. Pathological symptoms may also manifest at a specific time of day, e.g. fever spikes at night or allergies in the morning.
Short half-life: If drugs are to be timed, they must have circadian peaks in their bioavailability, ideally a half-life of fewer than 6 h (R. Zhang et al., 2014; Ruben et al., 2019a). Some drugs, such as antibodies have half-lives of weeks, so administering them at different times of the day is not likely to affect their action. It is thought that compared with hydrophilic drugs, lipophilic (hydrophobic compounds) drugs exhibit more dependence on circadian phase, probably due to fast intra-cellular distribution (Selfridge et al., 2016). Also, these drugs tend to be substrates of efflux transporters, which display a circadian rhythm of activity in the blood-brain barrier (BBB) and perhaps also elsewhere (see below) (R. Zhang et al., 2014; Ruben et al., 2019a).
Cycling non-specific target: Optimal timing would be when a target with undesirable consequences is at a low point.
We describe below the growing application of circadian medicine to the treatment of disease, with a particular emphasis on the timed therapy of cancer.
Timing Medicine Targeted to the nervous System
In the brain, multiple key steps in intercellular signaling from transmitter synthesis to degradation are time-of-day regulated. Other fundamental processes show daily variation, including endocytosis/exocytosis, waste removal, regulation of synaptic strength and the permeability of the brain and cerebrospinal fluid to blood (Ruben et al., 2019b). Rodent models show that antidepressants are more effective at specific times of day (e.g. milnacipran, fluoxetine, imipramine, venlafaxine, or bupropion) (Kawai et al., 2018a, 2018b, 2019). There does not appear to be a common mode of circadian regulation as dosing time dependency is seen with drugs that have different modes of action in serotonergic, noradrenergic, and dopaminergic neurons. Moreover, drugs show different chrono-pharmacological profiles (Kawai et al., 2018a, 2018b, 2019). The action of neuropsychiatric drugs (eg, lithium, selective serotonin reuptake inhibitors [SSRIs]) is also affected by polymorphisms in circadian clock genes, chronotype, and cellular circadian rhythms, supporting a general role of circadian biology in neuropsychiatric disorders (Rybakowski et al., 2014; McCarthy et al., 2019).
On the other hand, a high evening dose of antiepileptic drugs (e.g. valproic acid, phenytoin and carbamazepine, levetiracetam, oxcarbazepine, clobazam) improves drug responses and tolerance in both juvenile and adult patients with predominantly nighttime and early-morning seizures (Yegnanarayan et al., 2006; Guilhoto et al., 2011; Thome-Souza et al., 2016) (Figure 2). The mechanisms underlying the timely effect of anti-epileptics may involve circadian regulation of permeability through the blood-brain barrier. Fly and mouse studies revealed that the BBB harbors an evolutionarily conserved molecular circadian clock that regulates the activity of efflux transporters that pump out many drugs; indeed, the BBB clock drives time-of-day-dependent responses to an anti-epileptic drug, dilantin/phenytoin, in a fly seizure model (S. L. Zhang et al., 2018b, 2021a). This suggests a novel approach to chronotherapy based on temporal BBB permeability, which may provide therapeutic windows for drugs targeted to the brain.
Figure 2.
Timing of disease symptoms and therapeutic interventions. Abbreviations: NSAIDS = nonsteroidal anti-inflammatory drugs; ACE = angiotensin-converting enzyme; BCG = Bacillus calmette–Guérin; TRF = Time-restricted feeding. The timing of therapeutic interventions is mostly associated with the daily susceptibility of diseases. a higher evening antiepileptic drug dose can reduce nocturnal and early-morning seizures. Night time administration of most anti-hypertensive and anti-inflammatory drugs effectively reduces the early morning severity of the cardio-metabolic and auto-immune disease symptoms, via preemptive suppression of the disease-relevant enzymes or proinflammatory cytokine activities that rise during sleep. On the other hand, morning vaccinations appear to induce better innate and adaptive immune responses. Morning bright light therapy improves circadian rhythms, sleep and, mood disorders. daytime TRf or exercise in the late afternoon can improve circadian rhythms and metabolic symptoms, particularly in patients with diabetes and obesity. Tnf-α = Tumor necrosis factor alpha IL-6 = Interleukin 6
Timing cardio-Vascular and Metabolic Treatments
Many parameters that contribute to cardiovascular physiology, including endothelial properties, thrombus formation, blood pressure, heart rate, cholesterol metabolism, and renal function show circadian variation (McLoughlin et al., 2015; Douma and Gumz, 2018; Thosar et al., 2018; Bartman and Eckle, 2019; Crnko et al., 2019; D. Zhang and Pollock, 2020; Han et al., 2021; L. Zhang and Jain, 2021). Study of these is now accompanied by the identification of cycles in the relevant molecular and cellular processes (Bartman and Eckle, 2019; Crnko et al., 2019; L. Zhang and Jain, 2021).
Not surprisingly, there have been efforts to improve therapeutic efficacy for vascular diseases and metabolic disorders by timing medications.
An early example is simvastatin (SV), one of the most widely prescribed statin drugs for the treatment of hypercholesterolemia, hyperlipidemia, and coronary artery disease. SV is a short-acting inhibitor of hydroxymethylglutaryl coenzyme A reductase (Hmgcr), a rate-limiting enzyme in cholesterol synthesis. Hmgcr is rhythmically expressed in rat and mouse liver with expression peaking at night (Hamprecht et al., 1969; Li et al., 2020b). Several clinical trials have reported that evening administration of SV is more effective than morning administration. It is now approved for treating hyperlipidemia, with a recommendation for it to be taken “once a day in the evening.”(Saito et al., 1991; Wallace et al., 2003; Ruben et al., 2019c). More recently, a study with high-fat diet-fed obese mice confirmed that simvastatin administered at zeitgeber time 13 (ZT13), compared to ZT1, was more effective at decreasing serum levels of total cholesterol, triglycerides, non-esterified free fatty acids and low-density lipoproteins cholesterol involved in atherosclerosis, as well as improving liver pathology (Li et al., 2020a). While the similar timing of efficacy in rodents and humans may present a conundrum, given that rodents are nocturnal and humans are diurnal, a high fat diet reportedly shifts the phase of activity/feeding in mice (Kohsaka et al., 2007).
Similarly, administering blood pressure medication at night may be more effective because blood pressure rises in the morning (Figure 2). Several classes of anti-hypertension and vasodilatory drugs such as calcium channel blockers, β-adrenoceptor antagonists, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers, are more effective at curbing the morning rise of high blood pressure and heart rate when administered at night (Bowles et al., 2018; Hermida et al., 2010, 2017, 2020; Bowles et al., 2018). Additionally nocturnal hypertension is also detrimental for the heart and this can be reduced by taking anti-hypertension medications at night (Verdecchia et al., 1990; Qiu et al., 2005; Hermida et al., 2008a). In patients with chronic kidney disease ingestion of one or more hypertension drugs in the evening reduces blood pressure during sleep and converts the 24 h non-dipper blood pressure profile into a dipper pattern (Hermida et al., 2013). In line with this, a large-scale observational cohort study with hospital patients showed that the clinical response to hydralazine, an acute antihypertensive, is dosing time-dependent and greatest at night (Ruben et al., 2019a). Notably, application of a cyclic ordering by periodic structure (CYCLOPS) algorithm to human gene expression revealed that several drug-metabolizing enzymes, transporters, and drug targets cycle in the cardiovascular system (Ruben et al., 2018). Particularly, several L-type Ca2+ channel subunits, critical for cardiac polarization and targets of commonly prescribed antihypertensives, cycle in the human heart and vasculature at a population scale (Ruben et al., 2018). Consistent with these results, Ca2+ channel blockers such as nifedipine exhibit improved efficacy (e.g. reduced blood pressure and edema) when taken before bedtime (Hermida et al., 2008b).
Circadian regulation is increasingly implicated in the timing of heart attacks, which are most likely to occur in the morning ~6 AM to 12 PM (Muller et al., 1985; Culić, 2007; Martino and Young, 2015; Thosar et al., 2018). In addition to the temporal control of blood pressure, the morning peak of such cardiovascular events is influenced by circadian clock control of platelet activation (Scheer et al., 2011). As in the case of the anti-hypertensives, most anti-thrombosis drugs are more effective when taken at bedtime, likely because bedtime administration improves platelet inhibition during the morning hours (Bonten et al., 2014; Buurma et al., 2019). For example, a clinical trial with the oral anticoagulant drug rivaroxaban showed that, relative to morning ingestion, evening intake was associated with lower thrombin levels and lower probability of thromboembolic events during the vulnerable morning time (Brunner-Ziegler et al., 2016). Similarly, a low dose of aspirin administered in the evening reduces COX-1-dependent platelet function in the morning in healthy patients (Miciak-Ławicka et al., 2018). Also, a more recent randomized, controlled trial in a high-risk group of patients with arterial hypertension showed that, compared with morning use of aspirin (75 mg per day), the same dose administered in the evening resulted in a significant drop in blood pressure and an improvement of the diurnal profile of blood pressure (Krasińska et al., 2021). Timed treatment for cardiovascular pathology can also help to reduce target organ damage. Remodeling of organs such as the heart occurs at night, so delivering therapies (e.g. anti-hypertension drugs) at this time can optimize effects, and reduce organ damage (Martino et al., 2011)
Even the severity of ischemia and reperfusion injury imposed during cardiac surgery has been linked to the molecular circadian clock (Durgan et al., 2010). A clinical study of patients undergoing aortic valve replacement surgery indicated lower perioperative myocardial injury and postoperative morbidity following afternoon, relative to morning, procedures (Montaigne et al., 2018). Further ex-vivo analysis of human myocardium revealed that an intrinsic morning-afternoon variation in hypoxia-reoxygenation tolerance is associated with varying levels of the nuclear receptor Rev-Erbα, which is highest in the morning (Montaigne et al., 2018). In support of a causal relationship, Rev-Erbα gene deletion or antagonist treatment in a mouse model of hypoxia-reoxygenation myocardial injury reduced injury at the sleep-to-wake transition (Montaigne et al., 2018). This suggests that perioperative myocardial injury is transcriptionally regulated by the circadian clock, with Rev-Erbα antagonism emerging as a pharmacological strategy for cardioprotection.
Timing Medicine Targeted to the Immune System
Inflammation shows a circadian cycle with a nightly surge of the cytokines TNF-α and IL-6 (Buttgereit et al., 2015; Cutolo, 2019) (Figure 2). In targeting this surge for treatment of auto-immune diseases, clinical studies found that glucocorticoids (e.g. prednisone, a synthetic human cortisol), nonsteroidal anti-inflammatory drugs (e.g. ketoprofen, indomethacin, aceclofenac, and lornoxicam), and disease-modifying anti-rheumatic drugs (e.g. methotrexate) are more efficacious at improving morning auto-immune symptoms (e.g. asthma, rheumatoid arthritis) when administered at bedtime rather than during the day (Buttgereit et al., 2015; Cutolo, 2019). The bedtime administration may reduce or blunt the effects of the nocturnal rise in pro-inflammatory immune activity.
Timing cOVId-19 Medication
The COVID-19 pandemic of 2020 and 2021 also underscored circadian elements in infectious diseases. One television personality who chronicled his infection live commented that the beast (fever spikes) came out at night. Often the most severe response to COVID-19 infection is a hyper-immune response that manifests as acute respiratory distress syndrome. Notably, the synthetic glucocorticoid dexamethasone, reduced the death rate of critically ill Covid-19 patients by about one-third, perhaps by suppressing hyperactive immune responses in the lung (Group et al., 2021). Considering the success of treating chronic autoimmune diseases with steroid chronotherapy, it is reasonable to speculate that nighttime glucocorticoid delivery would be more effective at reducing severe immunopathological consequences (ex., cytokine storm) of covid.
Beyond the possible benefit of chronotherapy for treating the inflammatory component of COVID-19, viral infection may also cycle, suggesting other chronopharmacological interventions. As discussed earlier, time-series analysis revealed that a high proportion of the best-selling drugs used in the treatment of cardio-metabolic diseases, inflammatory diseases, mental health disorders, and cancer, directly target the products of circadian-regulated genes (R. Zhang et al., 2014; Mure et al., 2018; Ruben et al., 2018). A similar comparative pharmacogenomic analysis of the SARS-CoV-2 interacting host factors revealed that expression profiles of most genes targeted by existing FDA-approved drugs or by candidate clinical and preclinical compounds also exhibit robust 24 h oscillation in at least one organ or tissue (Ray and Reddy, 2020). Cycling genes include human angiotensin-converting enzyme (ACE2), a receptor that mediates entry of SARS-CoV-2 (Bhalla et al., 2020), and is expressed rhythmically in the lung in humans (Anafi et al., 2017), baboons (Mure et al., 2018) and mice (Z. Zhang et al., 2019). The ACE protein levels also exhibit robust time of day expression, coinciding with SARS-CoV entry and infection rates in human epithelial lung cells (Zhuang et al., 2021). Given that multiple existing ACE2 inhibitors are currently prioritized for COVID-19 management, these results hold promise for chrono-therapeutic application of repurposed cardiovascular drugs to improve anti-viral therapy (Lopes et al., 2020).
Timing Vaccination
Mirroring the circadian control of host immune responses to pathogens, emerging evidence suggests that timing vaccinations may boost their efficacy. For instance, adrenergic innervation of lymph nodes (B cells, CD4+, and CD8+ T cells), through β2-adrenergic receptors, modulates the diurnal variation of adaptive immune cell numbers in the circulation and in lymph nodes. This correlates with the consequent magnitude of the antibody response upon immunization (Suzuki et al., 2016). Besides the diurnal variation in the humoral immune response, the response of CD8 T cells to vaccination with dendritic cells loaded with an antigen follows a 24 h rhythm, with higher T cell activation upon vaccination in the daytime compared to the nighttime (Nobis et al., 2019). The early daytime immune response to vaccination was dampened or abolished upon deletion of Bmal1 in dendritic cells or CD8 T cells, suggesting that the cell-intrinsic clock modulates the time-of-day dependence of vaccination efficacy (Nobis et al., 2019). In an early study, an attenuated Venezuaelan equine encephalomyelitis vaccine administered at 8 am resulted in peak antibody titers 4 days earlier than the peak in those vaccinated at 8 pm (Feigin et al., 1967).
There are mixed results in human studies that address variations of vaccine efficacy with time of day. In a study of healthy young university student volunteers, a group of 30 (13 male/17 female) subjects who received the hepatitis B vaccine in the morning (08:00–08:30) did not differ in their response from a group of 33 (14 male/19 female) subjects who were injected in the evening (17:30–18:00), arguing that the immune response to the hepatitis B vaccination is not influenced by circadian timing (Karabay et al., 2008). On the other hand, a study of 164 men and women found that men, not women, mounted a higher antibody response to both hepatitis A and influenza A when vaccinated in the morning (Phillips et al., 2008). Similarly, a subsequent randomized trial study reported that morning vaccination enhances antibody response over afternoon vaccination in older adults, suggesting potential benefits of timed-vaccination strategies to counter age-related decline of antibody production (Long et al., 2016). However, a report of higher influenza A antibody titers in aged (but not younger) individuals following afternoon, versus morning, vaccination was attributed to the timing of sample collections, rather than vaccination timing (Kurupati et al., 2017).
Beyond the vaccine-specific antibody responses, a more recent study with healthy volunteers, using the anti-tuberculosis vaccine bacillus Calmette-Guérin (BCG), found that BCG vaccination in the morning elicited a stronger nonspecific trained innate immune response, as well as Mycobacterium Tuberculosis specific adaptive responses, compared with evening vaccination (de Bree et al., 2020). Concomitantly, morning vaccination with BCG upregulated clock genes Bmal1 and Clock in circulating monocytes, with more accessible chromatin in genes important for the mTOR pathway, which is crucial for the induction of trained immunity (de Bree et al., 2020). Overall, there is reason to believe that the circadian system affects responses to vaccination. However, more research is needed with studies controlled for sex and the timing of blood collection.
Chrono-Chemotherapy
Host-Tissue Tolerance May Vary With Time of Day.
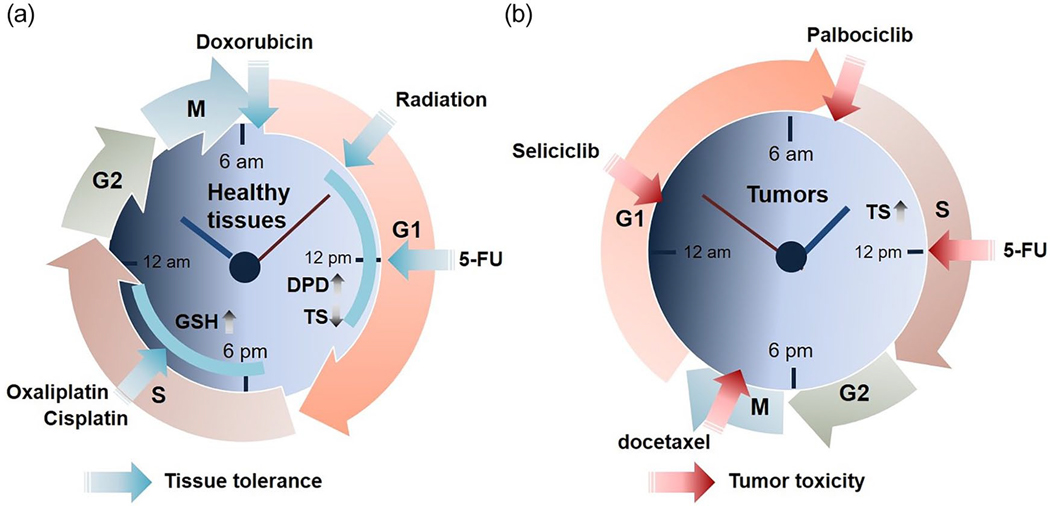
The earliest examples of chronotherapy are from the treatment of cancer, and circadian timing is now increasingly recognized as an important parameter to optimize therapy (Sulli et al., 2019). Anti-cancer drugs are usually cytotoxic to normal tissues as well as malignant cells, so the goal is not just to improve efficacy but also reduce toxic side effects such as host tissue damage and immunological dysfunction. Timing cancer therapy may achieve an optimal balance between drug tolerance and efficacy (Figure 3). In earlier work, the efficacy of over 30 chemotherapy drugs was shown to vary by over 50% based on administration time (Levi, 2001; Innominato et al., 2014). Some of the most commonly used anticancer drugs (e.g. cisplatin, doxorubicin, cyclophosphamide) exhibit differential toxicities depending on the time of administration (Cardoso et al., 1978; Hrushesky, 1985). For example, chronochemotherapy experiments with rat models for cancer found maximal benefit and minimal toxic effects when cisplatin was administered in the middle or later part of the active phase while doxorubicin was most effective when administered near the end of the daily resting phase (Hrushesky et al., 1982; Levi et al., 1982a, 1982b; Sothern et al., 1989). In patients with advanced ovarian cancer, administration of doxorubicin in the morning (e.g. 6 am) and cisplatin in the evening (e.g. at 1800 before or after doxorubicin) caused fewer complications and renal toxicity than administration of doxorubicin in the evening and cisplatin in the morning (Hrushesky, 1985; Sothern et al., 1989; Levi et al., 1990). Likewise, chronotherapy trials of patients with metastatic colorectal cancer showed that timed combination therapy with irinotecan, oxaliplatin, 5-fluorouracil (5-FU), and leucovorin resulted in delayed time to progression and increased overall survival with increased tolerance and safety, compared to routine chemotherapy (Levi et al., 1997; Gholam et al., 2006; Innominato et al., 2020b).
Figure 3.
Chronotherapy for the treatment of cancer. Chronotherapy with anti-cancer medicine or regimen has been proposed to improve host tolerance and safety (a) and tumor cytotoxicity (b). (a) Tissue tolerance to timed anti-cancer therapy. The anti-phasic peak and trough level of DPD, an elimination enzyme of 5-FU, and TS, have been associated with the reduced toxicity of circadian-timed 5-FU treatment. Also, daily variations of GSH, a potent anti-oxidant, have been used as a host tolerance biomarker for the use of platinum-based anti-cancer drugs (e.g. oxaliplatin, cisplatin). Doxorubicin and radiation are generally more effective, with fewer side effects, in the morning. (b) Tumor toxicity in response to timed anti-tumor agents. Several anti-cancer drugs target phases of the tumor cell cycle including G1 phase (Seliciclib), G-S phase-transition (Palbocicilib), S phase (5-FU), and M phase (docetaxel). Notably, some tumor tissues display anti-phasic cell cycle rhythms to host ones, highlighting the potential efficacy of timed chemotherapy to coincide with high tumor cell vulnerability and low toxicity to normal tissue. Abbreviations: M = mitosis; G = Cell growth; DPD = dihydropyrimidine dehydrogenase; GSH = glutathione; TS = thymidine synthase; 5-FU = 5-fluorouracil; S = DNA synthesis.
Several potential mechanisms may underlie the benefits of daily timing for reducing toxicity of anticancer drugs (Levi, 1994). Computational analysis of experimental and clinical results revealed that timing a drug (e.g. 5-FU, oxaliplatin) appropriately can produce minimal cytotoxicity in one cell population, e.g. normal cells, and at the same time display high cytotoxicity toward a different cell population, e.g. tumor cells (Altinok et al., 2009). This underscores a potential advantage of chronotherapy in simultaneously improving chrono-tolerance and chrono-efficacy. At the molecular level, the rhythmic response and toxicity of 5-FU are dependent on circadian oscillations in thymidylate synthase (TS) activity, its molecular target, and dihydropyrimidine dehydrogenase activity (DPD), the rate-limiting enzyme responsible for the elimination of 5-FU (Harris et al., 1990; Johnston et al., 1995; Lincoln et al., 2000; Salonga et al., 2000; Pullarkat et al., 2001). In accordance with this, a recent clinical study found that the circadian oscillation of DPD, with peak activity at 4:00 p.m. and trough at 4:00 a.m., modulates the time-dependent bioavailability and efficacy of 5-FU (Jacobs et al., 2016). On the other hand, glutathione, an anti-oxidant tripeptide involved in drug detoxification, showed a daily fluctuation with peak concentrations in the afternoon (~4 pm), thus better-reducing toxicity caused by platinum drugs (e.g. cisplatin, oxaliplatin) at this time (Zeng et al., 2005). Knowledge of these circadian mechanisms has been widely leveraged to design and implement recent clinical trials, such as chrono-modulated chemotherapy with 5-FU or cisplatin plus radiotherapy for nasopharyngeal carcinoma (Gou et al., 2018; P. X. Zhang et al., 2018a). Importantly, recent analysis of a randomized trial dataset shows that irinotecan administration in the morning for males and in the afternoon for females reduces toxicity, suggesting that gender should be taken into account when timing drug administration (Innominato et al., 2020a).
Cancer Chemotherapy Can Be Targeted to Cell Cycle Rhythms in Tumors.
Besides host circadian rhythms, daily dynamics in tumors can be targeted for optimal treatment (Figure 3b). For instance, a circadian rhythm in the cell cycle was first reported in human mammary cell cancer biopsy samples in 1966 (Garcia-Sainz and Halberg, 1966). Subsequent clinical studies showed time-of-day-dependent peaks of cancer cell mitosis in the tumors of most patients with differing circadian patterns in cancers of different origins (Klevecz et al., 1987; Smaaland et al., 1991; Hrushesky et al., 1998). A flow cytometry study with ovarian cancer patients revealed that tumor cell proliferation, determined by the percentage of cells in S (DNA synthesis) phase, exhibits a highly significant 24 h rhythm with a peak in mid- to late morning, nearly 12 h out of phase with non-tumor cell proliferation in normal tissues (Klevecz et al., 1987). Since most chemotherapy targets the cell cycle, chemotherapy can be timed to coincide with high tumor cell vulnerability and low toxicity to normal tissue (Klevecz et al., 1987). Supporting this approach, later preclinical and clinical cancer studies reported that cell cycle phase-specific drugs like cisplatin or 5-fluorouracil (5-FU) (S phase-specific), docetaxel (M phase-specific) and seliciclib (G1 phase-specific) have maximal efficacy and minimal toxicity at specific times of the day (Tampellini et al., 1998; Iurisci et al., 2006, 2009; Bernard et al., 2010). More recently, palbociclib (PD-0332991), a selective inhibitor of cyclin-dependent kinase (CDK) 4/6 responsible for G1/S cell cycle progression, was found to reduce growth of cultured cells and mouse tumors in a time-of-day-specific manner (Y. Lee et al., 2019a) (Figure 3b).
Circadian Regulation of the Tumor Cell Cycle Can Affect Cancer Chemotherapy.
Molecular connections between the circadian clock and the cell cycle continue to be revealed (Gaucher et al., 2018; Farshadi et al., 2020). In fact, most genes regulating critical steps in the cell cycle are controlled by the circadian clock. Key genes that exhibit circadian cycles of gene expression in a CLOCK/BMAL1 dependent manner include c-MYC and cyclin D1, which induce entry to S phase, and Wee1 that gates entry to M phase (Fu et al., 2002; Matsuo et al., 2003; Granda et al., 2005; Gaucher et al., 2018; Y. Lee et al., 2019a). Circadian regulation also extends to additional cell cycle regulators and checkpoint controllers (e.g. p21, p16/INK4a, p27, and p57), as well as cell cycle signaling pathway factors (e.g. mitogen-activated protein kinase, Wnt/β-Catenin, transforming growth factor), (Sotak et al., 2014). Moreover, DNA damage response, DNA repair, and apoptotic signaling pathways, which are closely associated with cell cycle checkpoints, are directly controlled by the circadian clock (Antoch et al., 2005; Gauger and Sancar, 2005; Gorbacheva et al., 2005; Kang et al., 2009, 2010; Sancar et al., 2010; Gaddameedhi et al., 2012).
Animal and cellular studies show that clock gene perturbations alter rhythmic mitosis and cell proliferation, as well as tumor growth, suggesting circadian coordination of the cell cycle in tumors (Wood et al., 2008; Taniguchi et al., 2009; X. Yang et al., 2009a, 2009b; Kang et al., 2010; N. Zhao et al., 2013; Puram et al., 2016; Kiessling et al., 2017; W. H. Chang and Lai, 2019; Dong et al., 2019; Shafi et al., 2021). In addition, enhancing intratumor circadian rhythms with clock synchronizing agents (e.g dexamethasone, forskolin, heat shock) results in fewer cells in S phase and more in the G1 phase and inhibits tumor growth (Kiessling and Cermakian, 2017). This suggests the tumor-suppressive function of the circadian clock is via induction of cell cycle arrest. On the other hand, some clock genes have been associated with increased tumor growth, although likely not through the cell cycle (see below).
Time-of-day-dependent anticancer efficacy has now been linked to the expression of specific circadian clock genes. The DNA alkylator temozolomide and topoisomerase I inhibitor irinotecan showed rhythmic toxicity in glioblastoma and colorectal cancer cells, respectively, with maximum drug sensitivity occurring near the peak of Bmal1 expression (Ballesta et al., 2011; Dulong et al., 2015; Slat et al., 2017). Cytotoxic rhythms were ablated when Bmal1 was silenced, suggesting a direct role of the clock (Ballesta et al., 2011; Dulong et al., 2015; Slat et al., 2017). Elevated Bmal1 reportedly increases the sensitivity of colorectal cancer and tongue squamous cell carcinoma cells to oxaliplatin and paclitaxel (Zeng et al., 2014; Tang et al., 2017). As with Bmal1, Per2 is an effective circadian modulator of DNA damaging agents (e.g. oxaliplatin), whose efficacy on human oral squamous cell carcinoma tumor grafts can be greatly boosted with timely administration at the peak of PER2 expression (Tang et al., 2019). Mechanistically, PER2 periodically suppresses proliferating cell nuclear antigen (PCNA) transcription which, in turn, impedes the drug-induced DNA repair mechanism, thus increasing sensitivity to the drug (Tang et al., 2019). In addition to chemicals targeting DNA repair, tumor xenograft models revealed that other synthetic (e.g. SR9009, Bortezomib, aldehyde dehydrogenase inhibitor) or natural (e.g. Curcumin) compounds exert time of day-dependent anti-tumor activity via circadian metabolic factors in glioblastoma, liver, and breast cancer cells (Sarma et al., 2016; Matsunaga et al., 2018; Wagner et al., 2019a, 2019b). Anti-cancer compounds that have time-of-day-specific activity were also identified systematically through a cell-based screen, which showed that cytotoxicity of multiple drugs is abrogated in Bmal1 knock out cancer cells (Y. Lee et al., 2021). Bmal1 ablation also affected growth of a melanoma tumor graft model, as well as time of day sensitivity to HSP90 inhibitors in this model (Y. Lee et al., 2021). Interestingly, rhythmic actions of HSP90 inhibitors require not only intact circadian rhythms but also a synchronzied cell cycle, suggesting that circadian regulation of cellular dynamics in tumors can dictate the time of day specificity of anti-cancer drugs (Y. Lee et al., 2021).
Radiation Therapy to Treat Cancer May Also Benefit From Consideration of Time-of-Day.
Besides drug-based chronotherapy, there is increasing, but limited, evidence that radiation, the standard treatment for the majority of brain and peripheral tumors, may be more effective at specific times of day (Mazzola et al., 2021). According to a recent review, radiation therapy given in the morning significantly reduced symptom burden caused by an adenocarcinoma, with minimal effects on healthy mucosal tissue and skin, (Shuboni-Mulligan et al., 2019) (Figure 3a). Gender also appears to affect temporal responses to radiotherapy because female patients with painful bone metastases, but not males, exhibited a significantly better response in the 11:00 am-2:00 pm interval relative to other time windows (Chan et al., 2017). Given circadian regulation of the DNA damage response and repair machinery, as discussed above, it is expected that optimal timing of radiotherapy, as well as genotoxic anti-cancer agents, presents a good option to minimize negative side effects of treatment.
The Circadian Clock Can Be Targeted for Cancer Immunotherapy.
In the last decade, there has been a paradigm shift on the role of the immune system in cancer therapy, with several new therapies that are now FDA-approved to enhance and even engineer the immune system to attack tumors. Some of these new therapies may benefit from chronotherapy because the tumor molecular clock influences the tumor microenvironment and immune cell infiltration (Cash et al., 2021; Z. Zhang et al., 2021b). In multiple cancer types, core circadian clock gene expression has also been linked to tumor antigen processing/presentation, tumor immunogenicity, and human leukocyte antigen (HLA) phenotype, though whether these pathways are upregulated or downregulated depends on the tumor type (Liu et al., 2019). A comprehensive analysis of 340 metastatic melanomas from The Cancer Genome Atlas data set revealed that higher BMAL1 is associated with higher T-lymphocyte infiltration as well as higher expression of the immune checkpoint surface molecule programmed cell death protein 1 [PD-1] and its tumor ligand [PD-L1]). This was correlated with a better objective response to antiPD1 immunotherapy, which activates T cells, and improved overall patient survival (de Assis et al., 2018). Likewise, multi-omics analysis showed that the infiltration level of CD8 T cells is positively correlated with CLOCK and BMAL1 expression levels in lung adenocarcinomas and lung squamous cell carcinomas (Y. Yang et al., 2019). Supporting a beneficial effect of tumor clock-dependent immune responses, multiple ROR agonists (e.g. LYC-55716), which are activators of BMAL1 transcription, are now in preclinical and clinical trials, showing good tolerability, safety, and pharmacokinetics in single or combined treatment with existing immune-therapeutic agents (Cash et al., 2021). On the other hand, a recent study with patient-derived glioblastoma stem cells (GSC) revealed that CLOCK and its heterodimeric partner, BMAL1, enhance GSC self-renewal via transcriptional upregulation of olfactomedin-like protein 3 (OLFML3), a proangiogenic chemokine that recruits immune-suppressive microglia into the tumor microenvironment (Chen et al., 2020). This also corresponds with a recent prognostic model suggesting that circadian clock genes promote glioma progression by affecting tumor immune infiltration and tumor cell proliferation (Z. Wang et al., 2021).
Although chronochemotherapy shows promise, mostly in preclinical studies, chronotype, gender, age, disease status, and genetic differences in rhythm status can contribute to pharmacodynamic variability among cancer patients, posing a daunting challenge in cancer therapy (Ozturk et al., 2017; Pariollaud and Lamia, 2020). Moreover, environmental or physiological perturbation of circadian rhythms such as shift work, abnormal sleep timing, irregular psychosociological stresses, and illness, can cause interindividual variability in both cancer growth and the response to cancer therapy (Y. Lee et al., 2019a; Walker et al., 2020a; Koritala et al., 2021). Circadian disruption may also be related to the chronic sleep loss and depression suffered by many cancer patients following diagnosis and treatment (Innominato et al., 2014; Telias and Wilcox, 2019). Thus, training or enhancing the body clock with scheduled light exposure, mealtimes, or exercise, alongside a carefully timed chemotherapy regimen, may improve patient well-being as well as antitumor treatment.
CONCLUSION
In the past ~25 years, extensive research in chronobiology has expanded our understanding of the functional role and mechanism of the circadian clockwork in human health and disease. Knowledge of the interactions across clocks in the body and between clocks and almost all physiological systems has not only elucidated circadian physiology and pathophysiology but also led to some successful therapeutic recommendations. Despite substantial progress in this area, several questions remain. (1) Are the therapeutic benefits of the extant small molecules targeting clock components mediated entirely by those components? Many drugs have multiple targets, so it is important to confirm that relevant effects are mediated by the clock molecule in question; this can be done by delivering the drug to cell or animals that lack that clock molecule. Using this approach, SR9009 was found to have REV-ERB-independent effects on cell proliferation and metabolism (Dierickx et al., 2019). On the other hand, as most cell signaling pathways targeted by therapeutic compounds cross-talk with the molecular clockwork, it is plausible that drug treatment impacts circadian physiology even if clock molecules are not the relevant direct target. In support of this, several anti-cancer or anti-epileptic drugs disrupt rhythms in patients, causing sleep and mood disorders, which in turn affect recovery (Ozturk et al., 2017; Griggs et al., 2018; Jin et al., 2020). (2) Does a therapeutic targeting a clock molecule actually act through a circadian pathway/mechanism? This is analogous to the question of whether phenotypes of clock gene knockouts reflect circadian function, or result from clock-independent effects of those genes on transcription. A circadian mechanism would be implicated for a therapeutic if other clock components could be manipulated to produce the same effect. In addition, rigorous studies may be able to establish that the relevant biomarkers and physiological effects are circadianly regulated. (3) How generalizable is the application of circadian medicine? Individual variability in circadian rhythms, caused by genetics or environmental factors (e.g. chronotype, lifestyle, illness, age), could have significant effects on treatment, suggesting chronotherapy may need to be individualized for patients. With the growing development of chrono-therapeutic tools and strategies, it may be reasonable to expect that integrative chronotherapy will improve the prevention and treatment of multiple disorders.
ACKNOWLEDGMENTS
The work was supported by the Elson S. Floyd College of Medicine to Y.L., the NIH (R37-NS-048471) and Howard Hughes Medical Institute (HHMI) to A.S., and Children’s Tumor Foundation (2019-05-004) and DOD (NF180079) and NIH (R01HL134923) to J.M.F.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Acosta-Rodríguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, and Takahashi JS (2017) Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab 26:267–277.e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello I, Fedele MLM, Roman F, Marpegan L, Caldart C, Chiesa JJ, Golombek DA, Finkielstein CV, and Paladino N (2020) Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci Adv 6:eaaz4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alidadi M, Banach M, Guest PC, Bo S, Jamialahmadi T, and Sahebkar A (2020) The effect of caloric restriction and fasting on cancer. Semin Cancer Biol 73:30–44. [DOI] [PubMed] [Google Scholar]
- Altinok A, Levi F, and Goldbeter A (2009) Identifying mechanisms of chronotolerance and chronoefficacy for the anticancer drugs 5-fluorouracil and oxaliplatin by computational modeling. Eur J Pharm Sci 36:20–38. [DOI] [PubMed] [Google Scholar]
- Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, and Sehgal A (2008) The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms 23:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anafi RC, Francey LJ, Hogenesch JB, and Kim J (2017) CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci U S A 114: 5312–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anafi RC, Lee Y, Sato TK, Venkataraman A, Ramanathan C, Kavakli IH, Hughes ME, Baggs JE, Growe J, Liu AC, et al. (2014) Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol 12:e1001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoch MP, Kondratov RV, and Takahashi JS (2005) Circadian clock genes as modulators of sensitivity to genotoxic stress. Cell Cycle 4:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafizadeh M, Zarrabi A, Saberifar S, Hashemi F, Hushmandi K, Hashemi F, Moghadam ER, Mohammadinejad R, Najafi M, and Garg M (2020) Nobiletin in cancer therapy: how this plant derived-natural compound targets various oncogene and oncosuppressor pathways. Biomedicines 8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta A, Dulong S, Abbara C, Cohen B, Okyar A, Clairambault J, and Levi F (2011) A combined experimental and mathematical approach for molecular-based optimization of irinotecan circadian delivery. PLoS Comp Biol 7:e1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta A, Innominato PF, Dallmann R, Rand DA, and Levi FA (2017) Systems chronotherapeutics. Pharmacol Rev 69:161–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Wang Y, Solt LA, Griffett K, Kazantzis M, Amador A, El-Gendy BM, Huitron-Resendiz S, Roberts AJ, Shin Y, et al. (2014) Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat Commun 5:5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano-Otalora B and Piggins HD (2017) Contributions of the lateral habenula to circadian timekeeping. Pharmacol Biochem Behav 162:46–54. [DOI] [PubMed] [Google Scholar]
- Bartman CM and Eckle T (2019) Circadian-hypoxia link and its potential for treatment of cardiovascular disease. Curr Pharm Des 25:1075–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J and Lazar MA (2016) Circadian time signatures of fitness and disease. Science 354:994–999. [DOI] [PubMed] [Google Scholar]
- Bernard S, Cajavec Bernard B, Levi F, and Herzel H (2010) Tumor growth rate determines the timing of optimal chronomodulated treatment schedules. PLoS Comp Biol 6:e1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla V, Blish CA, and South AM (2020) A historical perspective on ACE2 in the COVID-19 era. J Hum Hypertens 1–5. Epub ahead of print 14 December. doi: 10.1038/s41371020-00459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker J, Alves G, Falcao A, and Fortuna A (2020) Timing in drug absorption and disposition: the past, present, and future of chronopharmacokinetics. Br J Pharmacol 177:2215–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishehsari F, Voigt RM, and Keshavarzian A (2020) Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol 16:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, et al. (2005) Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65:11174–11184. [DOI] [PubMed] [Google Scholar]
- Blask DE, Dauchy RT, Sauer LA, Krause JA, and Brainard GC (2002) Light during darkness, melatonin suppression and cancer progression. Neuro Endocrinol Lett 23:52–56. [PubMed] [Google Scholar]
- Bonten TN, Saris A, van Oostrom MJ, Snoep JD, Rosendaal FR, Zwaginga J, Eikenboom J, van der Meer PF, and van der Bom JG (2014) Effect of aspirin intake at bedtime versus on awakening on circadian rhythm of platelet reactivity. A randomised cross-over trial. Thromb Haemost 112:1209–1218. [DOI] [PubMed] [Google Scholar]
- Borrmann H, McKeating JA, and Zhuang X (2021) The circadian clock and viral infections. J Biol Rhythms 36: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles NP, Thosar SS, Herzig MX, and Shea SA (2018) Chronotherapy for hypertension. Curr Hypertens Rep 20:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S and Longo VD (2016) Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res 207:241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A, van Raalte DH, Diamant M, Rutters F, van Someren EJ, Snoek FJ, Beekman AT, and Bremmer MA (2015) Light therapy for better mood and insulin sensitivity in patients with major depression and type 2 diabetes: a randomised, double-blind, parallel-arm trial. BMC Psychiatry 15:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A, van Raalte DH, Nguyen HT, Rutters F, van de Ven PM, Elders PJM, Moll AC, Van Someren EJW, Snoek FJ, Beekman ATF, et al. (2019) Effects of light therapy on mood and insulin sensitivity in patients with type 2 diabetes and depression: results from a randomized placebo-controlled trial. Diabetes Care 42:529–538. [DOI] [PubMed] [Google Scholar]
- Brunner-Ziegler S, Jilma B, Schorgenhofer C, Winkler F, Jilma-Stohlawetz P, Koppensteiner R, Quehenberger P, Seger C, Weigel G, Griesmacher A, et al. (2016) Comparison between the impact of morning and evening doses of rivaroxaban on the circadian endogenous coagulation rhythm in healthy subjects. J Thromb Haemost 14:316–323. [DOI] [PubMed] [Google Scholar]
- Buijs FN, León-Mercado L, Guzmán-Ruiz M, Guerrero-Vargas NN, Romo-Nava F, and Buijs RM (2016) The circadian system: a regulatory feedback network of periphery and brain. Physiology (Bethesda) 31:170–181. [DOI] [PubMed] [Google Scholar]
- Buijs RM and Kalsbeek A (2001) Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2:521–526. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Guzman Ruiz MA, Mendez Hernandez R, and Rodriguez Cortes B (2019) The suprachiasmatic nucleus; a responsive clock regulating homeostasis by daily changing the setpoints of physiological parameters. Auton Neurosci 218:43–50. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Smolen JS, Coogan AN, and Cajochen C (2015) Clocking in: chronobiology in rheumatoid arthritis. Nat Rev Rheumatol 11:349–356. [DOI] [PubMed] [Google Scholar]
- Buurma M, van Diemen JJK, Thijs A, Numans ME, and Bonten TN (2019) Circadian rhythm of cardiovascular disease: the potential of chronotherapy with aspirin. Front Cardiovasc Med 6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, and Shea SA (2012) Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Trans Med 4:129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Wang J, Gao B, Li J, Wu F, Zou JX, Xu J, Jiang Y, Zou H, Huang Z, et al. (2019) RORgamma is a targetable master regulator of cholesterol biosynthesis in a cancer subtype. Nat Commun 10:4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso SS, Avery T, Venditti JM, and Goldin A (1978) Circadian dependence of host and tumor responses to cyclophosphamide in mice. Euro J Cancer 14:949–954. [DOI] [PubMed] [Google Scholar]
- Cash E, Sephton S, Woolley C, Elbehi AM, I AR, EkineAfolabi B, and Kok VC (2021) The role of the circadian clock in cancer hallmark acquisition and immune-based cancer therapeutics. J Exp Clin Cancer Res 40:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterson JH, Khericha M, Dyson MC, Vincent AJ, Callard R, Haveron SM, Rajasingam A, Ahmad M, and Partridge L (2018) Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr Biol 28:1714–1724.e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, and Panda S (2016) The circadian coordination of cell biology. J Cell Biol 215:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, and Panda S (2014) Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20:991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E (2019) The circadian regulation of food intake. Nat Rev Endocrinol 15:393–405. [DOI] [PubMed] [Google Scholar]
- Chan S, Zhang L, Rowbottom L, McDonald R, Bjarnason GA, Tsao M, Barnes E, Danjoux C, Popovic M, Lam H, et al. (2017) Effects of circadian rhythms and treatment times on the response of radiotherapy for painful bone metastases. Ann Palliat Med 6:14–25. [DOI] [PubMed] [Google Scholar]
- Chang MR, He Y, Khan TM, Kuruvilla DS, Garcia-Ordonez R, Corzo CA, Unger TJ, White DW, Khan S, Lin L, et al. (2015) Antiobesity effect of a small molecule repressor of RORgamma. Mol Pharmacol 88:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH and Lai AG (2019) Timing gone awry: distinct tumour suppressive and oncogenic roles of the circadian clock and crosstalk with hypoxia signalling in diverse malignancies. J Trans Med 17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Vujovic N, Williams JS, and Scheer FAJL (2019) Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab 30:767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hsu WH, Chang A, Tan Z, Lan Z, Zhou A, Spring DJ, Lang FF, Wang YA, and DePinho RA (2020) Circadian regulator CLOCK recruits immune-suppressive microglia into the GBM tumor microenvironment. Cancer Discov 10:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney RJ, Cornelius LAM, Srivastava A, Weigelt CA, Marcoux D, Duan JJ, Shi Q, Batt DG, Liu Q, Yip S, et al. (2020) Discovery of BMS-986251: a clinically viable, potent, and selective RORgammat inverse agonist. ACS Med Chem Lett 11:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CK, Xu B, Mehta N, Mayne J, Sun WY, Cheng K, Ning Z, Dong J, Zou H, Cheng HM, et al. (2017) Phosphoproteome profiling reveals circadian clock regulation of posttranslational modifications in the murine hippocampus. Front Neurol 8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomistek AK, Shiroma EJ, and Lee IM (2016) The relationship between time of day of physical activity and obesity in older women. J Phys Act Health 13: 416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SK, Chung S, Kim HD, Lee JH, Jang J, Kim J, Kim D, Son GH, Oh YJ, Suh YG, et al. (2015) A synthetic cryptochrome inhibitor induces anti-proliferative effects and increases chemosensitivity in human breast cancer cells. Biochem Biophys Res Commun 467:441–446. [DOI] [PubMed] [Google Scholar]
- Chun SK, Jang J, Chung S, Yun H, Kim NJ, Jung JW, Son GH, Suh YG, and Kim K (2014) Identification and validation of cryptochrome inhibitors that modulate the molecular circadian clock. ACS Chem Biol 9:703–710. [DOI] [PubMed] [Google Scholar]
- Collins B, Pierre-Ferrer S, Muheim C, Lukacsovich D, Cai Y, Spinnler A, Herrera CG, Wen S, Winterer J, Belle MDC, et al. (2020) Circadian VIPergic neurons of the suprachiasmatic nuclei sculpt the sleep-wake cycle. Neuron 108:486–499.e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnko S, Du Pre BC, Sluijter JPG, and Van Laake LW (2019) Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol 16:437–447. [DOI] [PubMed] [Google Scholar]
- Cuesta M, Boudreau P, Cermakian N, and Boivin DB (2017) Rapid resetting of human peripheral clocks by phototherapy during simulated night shift work. Sci Rep 7:16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culić V (2007) Acute risk factors for myocardial infarction. Int J Cardiol 117:260–269. [DOI] [PubMed] [Google Scholar]
- Cunningham PS, Ahern SA, Smith LC, da Silva Santos CS, Wager TT, and Bechtold DA (2016) Targeting of the circadian clock via CK1delta/epsilon to improve glucose homeostasis in obesity. Sci Rep 6:29983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, Huang L, Goldberg J, Petrascheck M, Ates G, Pinto-Duarte A, Shokhirev MN, Schubert D, and Maher P (2019) Elevating acetyl-CoA levels reduces aspects of brain aging. eLife 8:e47866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M (2019) Circadian rhythms and rheumatoid arthritis. Joint Bone Spine 86:327–333. [DOI] [PubMed] [Google Scholar]
- Cyr P, Bronner SM, and Crawford JJ (2016) Recent progress on nuclear receptor RORgamma modulators. Bioorganic Med Chem Lett 26:4387–4393. [DOI] [PubMed] [Google Scholar]
- Dauchy RT, Hoffman AE, Wren-Dail MA, Hanifin JP, Warfield B, Brainard GC, Xiang S, Yuan L, Hill SM, Belancio VP, et al. (2015) Daytime blue light enhances the nighttime circadian melatonin inhibition of human prostate cancer growth. Comp Med 65:473–485. [PMC free article] [PubMed] [Google Scholar]
- Dauchy RT, Wren-Dail MA, Dupepe LM, Hill SM, Xiang S, Anbalagan M, Belancio VP, Dauchy EM, and Blask DE (2018) Effect of daytime blue-enriched LED light on the nighttime circadian melatonin inhibition of hepatoma 7288CTC Warburg effect and progression. Comp Med 68:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy RT, Wren-Dail MA, Hoffman AE, Hanifin JP, Warfield B, Brainard GC, Hill SM, Belancio VP, Dauchy EM, and Blask DE (2016) Effects of daytime exposure to light from blue-enriched light-emitting diodes on the nighttime melatonin amplitude and circadian regulation of rodent metabolism and physiology. Comp Med 66:373–383. [PMC free article] [PubMed] [Google Scholar]
- Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, et al. (2014) Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res 74:4099–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis LVM, Kinker GS, Moraes MN, Markus RP, Fernandes PA, and Castrucci AML (2018) Expression of the circadian clock gene BMAL1 positively correlates with antitumor immunity and patient survival in metastatic melanoma. Front Oncol 8:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bree LCJ, Mourits VP, Koeken VA, Moorlag SJ, Janssen R, Folkman L, Barreca D, Krausgruber T, Fife-Gernedl V, Novakovic B, et al. (2020) Circadian rhythm influences induction of trained immunity by BCG vaccination. J Clin Invest 130:5603–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mei C, Ercolani L, Parodi C, Veronesi M, Lo Vecchio C, Bottegoni G, Torrente E, Scarpelli R, Marotta R, Ruffili R, et al. (2015) Dual inhibition of REV-ERBbeta and autophagy as a novel pharmacological approach to induce cytotoxicity in cancer cells. Oncogene 34:2597–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Teixeira AA, Lira FS, and Rosa-Neto JC (2020) Aging with rhythmicity. Is it possible? Physical exercise as a pacemaker. Life Sci 261:118453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo AB, Coiffard B, Leone M, Mezouar S, and Mege JL (2020) For whom the clock ticks: clinical chronobiology for infectious diseases. Front Immunol 11:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierickx P, Emmett MJ, Jiang C, Uehara K, Liu M, Adlanmerini M, and Lazar MA (2019) SR9009 has REV-ERB-independent effects on cell proliferation and metabolism. Proc Natl Acad Sci U S A 116:12147–12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z, Prager BC, Wang X, Kim LJY, Morton AR, et al. (2019) Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov 9:1556–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma LG and Gumz ML (2018) Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 119:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal NA, Pollock RD, Lazarus NR, Harridge S, and Lord JM (2018) Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 17:e12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulong S, Ballesta A, Okyar A, and Levi F (2015) Identification of circadian determinants of cancer chronotherapy through in vitro chronopharmacology and mathematical modeling. Mol Cancer Ther 14:2154–2164. [DOI] [PubMed] [Google Scholar]
- Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96:271–290. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, and Young ME (2010) Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res 106:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, Jastroch M, Schneider S, de Mateo S, Cervantes M, et al. (2018) Atlas of circadian metabolism reveals systemwide coordination and communication between clocks. Cell 174:1571–1585.e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinburgh RM, Bradley HE, Abdullah NF, Robinson SL, Chrzanowski-Smith OJ, Walhin JP, Joanisse S, Manolopoulos KN, Philp A, Hengist A, et al. (2020) Lipid metabolism links nutrient-exercise timing to insulin sensitivity in men classified as overweight or obese. J Clin Endocrinol Metab 105:660–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen JC, Brager AJ, Baggs J, Pinckney L, Gray CL, DeBruyne JP, Esser KA, Takahashi JS, and Paul KN (2017) Bmal1 function in skeletal muscle regulates sleep. eLife 6:e26557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson ML, Esser KA, Kraus WE, Buford TW, and Redman LM (2021) A role for exercise to counter skeletal muscle clock disruption. Exerc Sport Sci Rev 49:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezagouri S, Zwighaft Z, Sobel J, Baillieul S, Doutreleau S, Ladeuix B, Golik M, Verges S, and Asher G (2019) Physiological and molecular dissection of daily variance in exercise capacity. Cell Metab 30:78–91.e74. [DOI] [PubMed] [Google Scholar]
- Farshadi E, van der Horst GTJ, and Chaves I (2020) molecular links between the circadian clock and the cell cycle. J Mol Biol 432:3515–3524. [DOI] [PubMed] [Google Scholar]
- Feigin RD, Jaeger RF, McKinney RW, and Alevizatos AC (1967) Live, attenuated Venezuelan equine encephalomyelitis virus vaccine. II. Whole-blood amino-acid and fluorescent-antibody studies following immunization. Am J Trop Med Hyg 16:769–777. [PubMed] [Google Scholar]
- Fifel K and Videnovic A (2019) Chronotherapies for Parkinson’s disease. Prog Neurobiol 174:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger AM and Kramer A (2021) Peripheral clocks tick independently of their master. Genes Dev 35:304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firsov D and Bonny O (2018) Circadian rhythms and the kidney. Nat Rev Nephrol 14:626–635. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, and Lee C (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111:41–50. [DOI] [PubMed] [Google Scholar]
- Gabriel BM and Zierath JR (2019) Circadian rhythms and exercise: re-setting the clock in metabolic disease. Nat Rev Endocrinol 15:197–206. [DOI] [PubMed] [Google Scholar]
- Gaddameedhi S, Reardon JT, Ye R, Ozturk N, and Sancar A (2012) Effect of circadian clock mutations on DNA damage response in mammalian cells. Cell Cycle 11:3481–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sainz M and Halberg F (1966) Mitotic rhythms in human cancer, reevaluated by electronic computer programs. Evidence for chronopathology. J Natl Cancer Inst 37:279–292. [DOI] [PubMed] [Google Scholar]
- Gaucher J, Montellier E, and Sassone-Corsi P (2018) Molecular cogs: interplay between circadian clock and cell cycle. Trends Cell Biol. 28:368–379. [DOI] [PubMed] [Google Scholar]
- Gauger MA and Sancar A (2005) Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res 65:6828–6834. [DOI] [PubMed] [Google Scholar]
- Gholam D, Giacchetti S, Brezault-Bonnet C, Bouchahda M, Hauteville D, Adam R, Ducot B, Ghemard O, Kustlinger F, Jasmin C, et al. (2006) Chronomodulated irinotecan, oxaliplatin, and leucovorin-modulated 5-Fluorouracil as ambulatory salvage therapy in patients with irinotecan- and oxaliplatin-resistant metastatic colorectal cancer. Oncologist 11:1072–1080. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, et al. (2012) The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A 109:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC, and Panda S (2015) Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347:1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizowski C, Zaelzer C, and Bourque CW (2016) Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature 537:685–688. [DOI] [PubMed] [Google Scholar]
- Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, Takahashi JS, and Antoch MP (2005) Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A 102:3407–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou XX, Jin F, Wu WL, Long JH, Li YY, Gong XY, Chen GY, Chen XX, and Liu LN (2018) Induction chronomodulated chemotherapy plus radiotherapy for nasopharyngeal carcinoma: a phase II prospective randomized study. J Cancer Res Ther 14:1613–1619. [DOI] [PubMed] [Google Scholar]
- Granda TG, Liu XH, Smaaland R, Cermakian N, Filipski E, Sassone-Corsi P, and Levi F (2005) Circadian regulation of cell cycle and apoptosis proteins in mouse bone marrow and tumor. FASEB J 19:304–306. [DOI] [PubMed] [Google Scholar]
- Griggs CA, Malm SW, Jaime-Frias R, and Smith CL (2018) Valproic acid disrupts the oscillatory expression of core circadian rhythm transcription factors. Toxicol Appl Pharmacol 339:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, et al. (2021) Dexamethasone in hospitalized patients with covid-19. New Eng J Med 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhoto LM, Loddenkemper T, Vendrame M, Bergin A, Bourgeois BF, and Kothare SV (2011) Higher evening antiepileptic drug dose for nocturnal and early-morning seizures. Epilepsy Behav 20:334–337. [DOI] [PubMed] [Google Scholar]
- Hadadi E, Taylor W, Li XM, Aslan Y, Villote M, Riviere J, Duvallet G, Auriau C, Dulong S, Raymond-Letron I, et al. (2020) Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat Commun 11:3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamprecht B, Nussler C, and Lynen F (1969) Rhythmic changes of hydroxymethylglutaryl coenzyme a reductase activity in livers of fed and fasted rats. FEBS Lett 4:117–121. [DOI] [PubMed] [Google Scholar]
- Han Q, Bagi Z, and Daniel Rudic R (2021) Review: circadian clocks and rhythms in the vascular tree. Curr Opin Pharmacol 59:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcken C, Csengery J, Turner M, Wu L, Liang S, Sibley R, Brunette S, Labadia M, Hoyt K, Wayne A, et al. (2021) Discovery of a series of pyrazinone RORgamma antagonists and identification of the clinical candidate BI 730357. ACS Med Chem Lett 12:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BE, Song R, Soong SJ, and Diasio RB (1990) Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res 50:197–201. [PubMed] [Google Scholar]
- Hasegawa S, Fukushima H, Hosoda H, Serita T, Ishikawa R, Rokukawa T, Kawahara-Miki R, Zhang Y, Ohta M, Okada S, et al. (2019) Hippocampal clock regulates memory retrieval via Dopamine and PKA-induced GluA1 phosphorylation. Nat Commun 10:5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15:848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA, Sassone-Corsi P, and Zierath JR (2020) Chrononutrition for the prevention and treatment of obesity and type 2 diabetes: from mice to men. Diabetologia 63:2253–2259. [DOI] [PubMed] [Google Scholar]
- He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, et al. (2016) The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab 23:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Fernández JR, and Calvo C (2008a) Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 51:69–76. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Fernandez JR, Mojon A, Crespo JJ, Rios MT, and Smolensky MH (2017) Bedtime blood pressure chronotherapy significantly improves hypertension management. Heart Fail Clin 13:759–773. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojón A, and Fernández JR (2008b) Chronotherapy with nifedipine GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. Am J Hypertens 21:948–954. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojon A, and Fernandez JR (2010) Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int 27:1629–1651. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Smolensky MH, Mojon A, Fernandez JR, Crespo JJ, Moya A, Rios MT, and Portaluppi F (2013) Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat Rev Nephrol 9:358–368. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Crespo JJ, Dominguez-Sardina M, Otero A, Moya A, Rios MT, Sineiro E, Castineira MC, Callejas PA, Pousa L, et al. (2020) Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J 41:4565–4576. [DOI] [PubMed] [Google Scholar]
- Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, et al. (2012) Identification of small molecule activators of cryptochrome. Science 337:1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrushesky WJ (1985) Circadian timing of cancer chemotherapy. Science 228:73–75. [DOI] [PubMed] [Google Scholar]
- Hrushesky WJ, Lannin D, and Haus E (1998) Evidence for an ontogenetic basis for circadian coordination of cancer cell proliferation. J Natl Cancer Inst 90:1480–1484. [DOI] [PubMed] [Google Scholar]
- Hrushesky WJ, Levi FA, Halberg F, and Kennedy BJ (1982) Circadian stage dependence of cis-diamminedichloroplatinum lethal toxicity in rats. Cancer Res 42:945–949. [PubMed] [Google Scholar]
- Hubbard BP and Sinclair DA (2014) Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 35:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries PS, Bersot R, Kincaid J, Mabery E, McCluskie K, Park T, Renner T, Riegler E, Steinfeld T, Turtle ED, et al. (2018) Carbazole-containing amides and ureas: discovery of cryptochrome modulators as antihyperglycemic agents. Bioorganic Med Chem Lett 28:293–297. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Refetoff S, Van Cauter E, and Yoshimura T (2019) Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol 15:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innominato PF, Ballesta A, Huang Q, Focan C, Chollet P, Karaboue A, Giacchetti S, Bouchahda M, Adam R, Garufi C, et al. (2020a) Sex-dependent least toxic timing of irinotecan combined with chronomodulated chemotherapy for metastatic colorectal cancer: randomized multicenter EORTC 05011 trial. Cancer Med 9: 4148–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innominato PF, Karaboue A, Focan C, Chollet P, Giacchetti S, Bouchahda M, Ulusakarya A, Torsello A, Adam R, Levi FA, et al. (2020b) Efficacy and safety of chronomodulated irinotecan, oxaliplatin, 5-fluorouracil and leucovorin combination as first- or second-line treatment against metastatic colorectal cancer: results from the International EORTC 05011 Trial. Int J Cancer 148:2512–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, and Levi FA (2014) The circadian timing system in clinical oncology. Ann Med 46:191–207. [DOI] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, and Okamura H (2005) Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2:297–307. [DOI] [PubMed] [Google Scholar]
- Iurisci I, Filipski E, Reinhardt J, Bach S, Gianella-Borradori A, Iacobelli S, Meijer L, and Levi F (2006) Improved tumor control through circadian clock induction by Seliciclib, a cyclin-dependent kinase inhibitor. Cancer Res 66:10720–10728. [DOI] [PubMed] [Google Scholar]
- Iurisci I, Filipski E, Sallam H, Harper F, Guettier C, Maire I, Hassan M, Iacobelli S, and Levi F (2009) Liver circadian clock, a pharmacologic target of cyclin-dependent kinase inhibitor seliciclib. Chronobiol Int 26:1169–1188. [DOI] [PubMed] [Google Scholar]
- Jacobs BA, Deenen MJ, Pluim D, van Hasselt JG, Krahenbuhl MD, van Geel RM, de Vries N, Rosing H, Meulendijks D, Burylo AM, et al. (2016) Pronounced between-subject and circadian variability in thymidylate synthase and dihydropyrimidine dehydrogenase enzyme activity in human volunteers. Br J Clin Pharmacol 82:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J, Chung S, Choi Y, Lim HY, Son Y, Chun SK, Son GH, Kim K, Suh YG, and Jung JW (2018) The cryptochrome inhibitor KS15 enhances E-box-mediated transcription by disrupting the feedback action of a circadian transcription-repressor complex. Life Sci 200:49–55. [DOI] [PubMed] [Google Scholar]
- Jin B, Aung T, Geng Y, and Wang S (2020) Epilepsy and its interaction with sleep and circadian rhythm. Front Neurol 11:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Zhao C, Xu Y, and Mori T (2017) Timing the day: what makes bacterial clocks tick? Nat Rev Microbiol 15:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PG, Lenz HJ, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, and Leichman L (1995) Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res 55:1407–1412. [PubMed] [Google Scholar]
- Kang TH, Lindsey-Boltz LA, Reardon JT, and Sancar A (2010) Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci U S A 107:4890–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Reardon JT, Kemp M, and Sancar A (2009) Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci U S A 106:2864–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabay O, Temel A, Koker AG, Tokel M, Ceyhan M, and Kocoglu E (2008) Influence of circadian rhythm on the efficacy of the hepatitis B vaccination. Vaccine 26:1143–1144. [DOI] [PubMed] [Google Scholar]
- Karoly PJ, Rao VR, Gregg NM, Worrell GA, Bernard C, Cook MJ, and Baud MO (2021) Cycles in epilepsy. Nat Rev Neurol 17:267–284. [DOI] [PubMed] [Google Scholar]
- Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, Zheng X, Hall D, Davis S, Nelson CS, Brem RB, et al. (2016) Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in drosophila. Cell Metab 23:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Iwadate R, Ishibashi T, Kudo N, Kawashima Y, and Mitsumoto A (2019) Antidepressants with different mechanisms of action show different chronopharmacological profiles in the tail suspension test in mice. Chronobiol Int 36:1194–1207. [DOI] [PubMed] [Google Scholar]
- Kawai H, Kodaira N, Tanaka C, Ishibashi T, Kudo N, Kawashima Y, and Mitsumoto A (2018a) Time of administration of acute or chronic doses of imipramine affects its antidepressant action in rats. J Circadian Rhythms 16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Machida M, Ishibashi T, Kudo N, Kawashima Y, and Mitsumoto A (2018b) Chronopharmacological analysis of antidepressant activity of a dual-action serotonin noradrenaline reuptake inhibitor (SNRI), milnacipran, in rats. Biol Pharm Bull 41:213–219. [DOI] [PubMed] [Google Scholar]
- Kervezee L, Cuesta M, Cermakian N, and Boivin DB (2019) The phase-shifting effect of bright light exposure on circadian rhythmicity in the human transcriptome. J Biol Rhythms 34:84–97. [DOI] [PubMed] [Google Scholar]
- Kiessling S and Cermakian N (2017) The tumor circadian clock: a new target for cancer therapy? Future Oncol 13:2607–2610. [DOI] [PubMed] [Google Scholar]
- Kiessling S, Beaulieu-Laroche L, Blum ID, Landgraf D, Welsh DK, Storch KF, Labrecque N, and Cermakian N (2017) Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biology 15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevecz RR, Shymko RM, Blumenfeld D, and Braly PS (1987) Circadian gating of S phase in human ovarian cancer. Cancer Res 47:6267–6271. [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, and Bass J (2007) High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6:414–421. [DOI] [PubMed] [Google Scholar]
- Kolbe I, Leinweber B, Brandenburger M, and Oster H (2019) Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol Metab 30:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, and Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge S, Maier B, Bruning F, Ehrhardt L, Korte T, Mann M, Herrmann A, Robles MS, and Kramer A (2018) The non-classical nuclear import carrier Transportin 1 modulates circadian rhythms through its effect on PER1 nuclear localization. PLoS Genet 14:e1007189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koritala BSC, Porter KI, Arshad OA, Gajula RP, Mitchell HD, Arman T, Manjanatha MG, Teeguarden J, Van Dongen HPA, McDermott JE, et al. (2021) Night shift schedule causes circadian dysregulation of DNA repair genes and elevated DNA damage in humans. J Pineal Res 70:e12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronowski KB and Sassone-Corsi P (2021) Communicating clocks shape circadian homeostasis. Science 371:eabd0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasińska B, Paluszkiewicz L, Miciak-Ławicka E, Krasinski M, Rzymski P, Tykarski A, and Krasiński Z (2021) The impact of acetylsalicylic acid dosed at bedtime on circadian rhythms of blood pressure in the high-risk group of cardiovascular patients-a randomized, controlled trial. Eur J Clin Pharmacol 77:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Kojetin DJ, Solt LA, Kumar KG, Nuhant P, Duckett DR, Cameron MD, Butler AA, Roush WR, Griffin PR, et al. (2011) Identification of SR3335 (ML176): a synthetic RORalpha selective inverse agonist. ACS Chem Biol 6:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurupati RK, Kossenkoff A, Kannan S, Haut LH, Doyle S, Yin X, Schmader KE, Liu Q, Showe L, and Ertl HCJ (2017) The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine 35:3700–3708. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee Y, Lee MJ, Park E, Kang SH, Chung CH, Lee KH, and Kim K (2008) Dual modification of BMAL1 by SUMO2/3 and ubiquitin promotes circadian activation of the CLOCK/BMAL1 complex. Mol Cell Biol 28:6056–6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Chun SK, and Kim K (2015a) Sumoylation controls CLOCK-BMAL1-mediated clock resetting via CBP recruitment in nuclear transcriptional foci. Biochim Biophys Acta 1853:2697–2708. [DOI] [PubMed] [Google Scholar]
- Lee Y, Fong SY, Shon J, Zhang SL, Brooks R, Lahens NF, Chen D, Dang CV, Field JM, and Sehgal A (2021) Time-of-day specificity of anticancer drugs may be mediated by circadian regulation of the cell cycle. Sci Adv 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Jang AR, Francey LJ, Sehgal A, and Hogenesch JB (2015b) KPNB1 mediates PER/CRY nuclear translocation and circadian clock function. eLife 4:e08647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lahens NF, Zhang S, Bedont J, Field JM, and Sehgal A (2019a) G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol 17:e3000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee J, Kwon I, Nakajima Y, Ohmiya Y, Son GH, Lee KH, and Kim K (2010) Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J Cell Sci 123:3547–3557. [DOI] [PubMed] [Google Scholar]
- Lee Y, Shen Y, Francey LJ, Ramanathan C, Sehgal A, Liu AC, and Hogenesch JB (2019b) The NRON complex controls circadian clock function through regulated PER and CRY nuclear translocation. Sci Rep 9:11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leise TL, Harrington ME, Molyneux PC, Song I, Queenan H, Zimmerman E, Lall GS, and Biello SM (2013) Voluntary exercise can strengthen the circadian system in aged mice. Age 35:2137–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lellupitiyage Don SS, Robertson KL, Lin HH, Labriola C, Harrington ME, Taylor SR, and Farkas ME (2020) Nobiletin affects circadian rhythms and oncogenic characteristics in a cell-dependent manner. PLoS ONE 15:e0236315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi FA (1994) Chronotherapy of cancer: biological basis and clinical application. Pathol Biol 42:338–341. [PubMed] [Google Scholar]
- Levi FA (2001) Circadian chronotherapy for human cancers. Lancet Oncol 2:307–315. [DOI] [PubMed] [Google Scholar]
- Levi FA, Benavides M, Chevelle C, Le Saunier F, Bailleul F, Misset JL, Regensberg C, Vannetzel JM, Reinberg A, and Mathe G (1990) Chemotherapy of advanced ovarian cancer with 4’-O-tetrahydropyranyl doxorubicin and cisplatin: a randomized phase II trial with an evaluation of circadian timing and dose-intensity. J Clin Oncol 8:705–714. [DOI] [PubMed] [Google Scholar]
- Levi FA, Hrushesky WJ, Blomquist CH, Lakatua DJ, Haus E, Halberg F, and Kennedy BJ (1982a) Reduction of cis-diamminedichloroplatinum nephrotoxicity in rats by optimal circadian drug timing. Cancer Res 42:950–955. [PubMed] [Google Scholar]
- Levi FA, Hrushesky WJ, Halberg F, Langevin TR, Haus E, and Kennedy BJ (1982b) Lethal nephrotoxicity and hematologic toxicity of cis-diamminedichloroplatinum ameliorated by optimal circadian timing and hydration. Eur J Cancer Clin Oncol 18:471–477. [DOI] [PubMed] [Google Scholar]
- Levi FA, Zidani R, and Misset JL (1997) Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350:681–686. [DOI] [PubMed] [Google Scholar]
- Levine DC, Hong H, Weidemann BJ, Ramsey KM, Affinati AH, Schmidt MS, Cedernaes J, Omura C, Braun R, Lee C, et al. (2020) NAD(+) controls circadian reprogramming through PER2 nuclear translocation to counter aging. Molecular Cell 78:835–849.e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P, Oster H, Korf HW, Foster RG, and Erren TC (2020) Food as a circadian time cue: evidence from human studies. Nat Rev Endocrinol 16:213–223. [DOI] [PubMed] [Google Scholar]
- Li H, Rabearivony A, Zhang W, Chen S, An X, and Liu C (2020a) Chronopharmacology of simvastatin on hyperlipidaemia in high-fat diet-fed obese mice. J Cell Mol Med 24:11024–11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang S, Zhang W, Chen S, Rabearivony A, Shi Y, Liu J, Corton CJ, and Liu C (2020b) Endogenous circadian time genes expressions in the liver of mice under constant darkness. BMC Genom 21:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Liu C, Lu M, Dong Q, Wang Z, Wang Z, Xiong W, Zhang N, Zhou J, Liu Q, et al. (2018) Calorie restriction is the most reasonable anti-ageing intervention: a meta-analysis of survival curves. Sci Rep 8:5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, and Hoogendijk WJ (2011) Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry 68:61–70. [DOI] [PubMed] [Google Scholar]
- Lin HH, Robertson KL, Bisbee HA, and Farkas ME (2020) Oncogenic and circadian effects of small molecules directly and indirectly targeting the core circadian clock. Integr Cancer Ther 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln DW II, Hrushesky WJ, and Wood PA (2000) Circadian organization of thymidylate synthase activity in normal tissues: a possible basis for 5-fluorouracil chronotherapeutic advantage. Int J Cancer 88:479–485. [PubMed] [Google Scholar]
- Liu Z, Yu K, Zheng J, Lin H, Zhao Q, Zhang X, Feng W, Wang L, Xu J, Xie D, et al. (2019) Dysregulation, functional implications, and prognostic ability of the circadian clock across cancers. Cancer Med 8:1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW and McClung CA (2019) Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci 20:49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, and Phillips AC (2016) Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34:2679–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Feldman A, D’Andrea Saba Arruda G, de Souza AS, de Albuquerque DC, Mazza L, Santos MF, et al. (2020) Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): the BRACE CORONA Trial. Am Heart J 226:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Galluzzi L, Freije JMP, Madeo F, and Kroemer G (2016) Metabolic control of longevity. Cell 166:802–821. [DOI] [PubMed] [Google Scholar]
- Lundell LS, Parr EB, Devlin BL, Ingerslev LR, Altintas A, Sato S, Sassone-Corsi P, Barres R, Zierath JR, and Hawley JA (2020) Time-restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression. Nat Commun 11:4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Lindell Veiel L, Baker A, and Steele C (1999) A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry 14:520–525. [PubMed] [Google Scholar]
- Lytle NK, Ferguson LP, Rajbhandari N, Gilroy K, Fox RG, Deshpande A, Schurch CM, Hamilton M, Robertson N, Lin W, et al. (2019) A multiscale map of the stem cell state in pancreatic adenocarcinoma. Cell 177:572–586.e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Carmona-Gutierrez D, Hofer SJ, and Kroemer G (2019) Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab 29:592–610. [DOI] [PubMed] [Google Scholar]
- Malik DM, Paschos GK, Sehgal A, and Weljie AM (2020) Circadian and sleep metabolomics across species. J Mol Biol 432:3578–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla R, Brouwers B, Schrauwen-Hinderling VB, Hesselink MKC, Hoeks J, and Schrauwen P (2021) Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol Rep 8:e14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Dauchy RT, Blask DE, Slakey LM, Xiang S, Yuan L, Dauchy EM, Shan B, Brainard GC, Hanifin JP, et al. (2012) Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3beta. Mol Endocrinol 26:1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, and Bass J (2013) Circadian clocks and metabolism. Handb Exp Pharmacol 217:127–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino TA and Young ME (2015) Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology. J Biol Rhythms 30:183–205. [DOI] [PubMed] [Google Scholar]
- Martino TA, Tata N, Simpson JA, Vanderlaan R, Dawood F, Kabir MG, Khaper N, Cifelli C, Podobed P, Liu PP, et al. (2011) The primary benefits of angiotensin-converting enzyme inhibition on cardiac remodeling occur during sleep time in murine pressure overload hypertrophy. J Am Coll Cardiol 57:2020–2028. [DOI] [PubMed] [Google Scholar]
- Masri S and Sassone-Corsi P (2018) The emerging link between cancer, metabolism, and circadian rhythms. Nat Med 24:1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga N, Ogino T, Hara Y, Tanaka T, Koyanagi S, and Ohdo S (2018) Optimized dosing schedule based on circadian dynamics of mouse breast cancer stem cells improves the antitumor effects of aldehyde dehydrogenase inhibitor. Cancer Res 78:3698–3708. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, and Okamura H (2003) Control mechanism of the circadian clock for timing of cell division in vivo. Science 302:255–259. [DOI] [PubMed] [Google Scholar]
- Mattis J and Sehgal A (2016) Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol Metab 27:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP (2019) An evolutionary perspective on why food overconsumption impairs cognition. Trends Cogn Sci 23:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola R, Cuccia F, Bertani A, Tubin S, Conaldi PG, Corradini S, Tolia M, Guba M, and Alongi F (2021) The role of radiotherapy in patients with solid tumours after solid organ transplantation: a systematic review. Lancet Oncol 22:e93–e104. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Wei H, Nievergelt CM, Stautland A, Maihofer AX, Welsh DK, Shilling P, Alda M, Alliey-Rodriguez N, Anand A, et al. (2019) Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology 44:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin SC, Haines P, and FitzGerald GA (2015) Clocks and cardiovascular function. Methods Enzymol 552:211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer FA, Oerlemans GJM, and Brunsveld L (2021) Orthosteric and allosteric dual targeting of the nuclear receptor RORgammat with a bitopic ligand. ACS Chem Biol 16:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, Maywood ES, Bechtold DA, Lu WQ, Li J, Gibbs JE, Dupre SM, Chesham JE, Rajamohan F, Knafels J, et al. (2010) Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci U S A 107:15240–15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miciak-Ławicka E, Begier-Krasińska B, Tykarski A, and Krasiński Z (2018) Does the timing of aspirin administration influence its antiplatelet effect: review of literature on chronotherapy. Kardiochir Torakochirurgia Pol 15:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S and Hirota T (2020) Pharmacological interventions to circadian clocks and their molecular bases. J Mol Biol 432:3498–3514. [DOI] [PubMed] [Google Scholar]
- Miller S, Son YL, Aikawa Y, Makino E, Nagai Y, Srivastava A, Oshima T, Sugiyama A, Hara A, Abe K, et al. (2020) Isoform-selective regulation of mammalian cryptochromes. Nat Chem Biol 16:676–685. [DOI] [PubMed] [Google Scholar]
- Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, et al. (2018) Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 391:59–69. [DOI] [PubMed] [Google Scholar]
- Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, and Robertson T (1985) Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 313:1315–1322. [DOI] [PubMed] [Google Scholar]
- Mulvihill EE, Assini JM, Lee JK, Allister EM, Sutherland BG, Koppes JB, Sawyez CG, Edwards JY, Telford DE, Charbonneau A, et al. (2011) Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 60:1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, et al. (2018) Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359:eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, et al. (2013) Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 123:5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung J, Schmal C, Hong S, Tsukizawa Y, Rose P, Zhang Y, Holtzman MJ, De Schutter E, Herzel H, Bordyugov G, et al. (2018) The choroid plexus is an important circadian clock component. Nat Commun 9:1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung J, Wu MY, Lee CY, Rahim AR, Truong VH, Wu D, Piggins HD, and Wu MS (2019) The kidney clock contributes to timekeeping by the master circadian clock. Int J Mol Sci 20:2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Salehi E, Farhood B, Nashtaei MS, Hashemi Goradel N, Khanlarkhani N, Namjoo Z, and Mortezaee K (2019) Adjuvant chemotherapy with melatonin for targeting human cancers: a review. J Cell Physiol 234:2356–2372. [DOI] [PubMed] [Google Scholar]
- Nobis CC, Dubeau Laramée G, Kervezee L, Maurice De Sousa D, Labrecque N, and Cermakian N (2019) The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc Natl Acad Sci U S A 116:20077–20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Mallampalli V, Nemkov T, Wirianto M, Yang J, Ye Y, Sun Y, Han L, Esser KA, Mileykovskaya E, et al. (2019a) Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat Commun 10:3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Nemkov T, D’Alessandro A, Yoo SH, and Chen Z (2019b) Coordinate regulation of cholesterol and bile acid metabolism by the clock modifier nobiletin in metabolically challenged old mice. Int J Mol Sci 20:4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noya SB, Colameo D, Bruning F, Spinnler A, Mircsof D, Opitz L, Mann M, Tyagarajan SK, Robles MS, and Brown SA (2019) The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 366:eaav2642. [DOI] [PubMed] [Google Scholar]
- Olie JP and Kasper S (2007) Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol 10:661–673. [DOI] [PubMed] [Google Scholar]
- Ozturk N, Ozturk D, Kavakli IH, and Okyar A (2017) Molecular aspects of circadian pharmacology and relevance for cancer chronotherapy. Int J Mol Sci 18:2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya VB, Kumar S, Sachchidanand Sharma R, and Desai RC (2018) Combating autoimmune diseases with retinoic acid receptor-related orphan receptor-gamma (RORgamma or RORc) inhibitors: hits and misses. J Med Chem 61:10976–10995. [DOI] [PubMed] [Google Scholar]
- Papagiannakopoulos T, Bauer MR, Davidson SM, Heimann M, Subbaraj L, Bhutkar A, Bartlebaugh J, Vander Heiden MG, and Jacks T (2016) Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab 24:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariollaud M and Lamia KA (2020) Cancer in the fourth dimension: what is the impact of circadian disruption? Cancer Discov 10:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr EB, Devlin BL, Radford BE, and Hawley JA (2020) A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients 12:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patke A, Young MW, and Axelrod S (2020) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21:67–84. [DOI] [PubMed] [Google Scholar]
- Paul S, Hanna L, Harding C, Hayter EA, Walmsley L, Bechtold DA, and Brown TM (2020) Output from VIP cells of the mammalian central clock regulates daily physiological rhythms. Nat Commun 11:1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples L (2018) Medicine’s secret ingredient: it’s in the timing. Nature 556:290–292. [DOI] [PubMed] [Google Scholar]
- Pevet P and Challet E (2011) Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol 105:170–182. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Gallagher S, Carroll D, and Drayson M (2008) Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology 45:663–666. [DOI] [PubMed] [Google Scholar]
- Pullarkat ST, Stoehlmacher J, Ghaderi V, Xiong YP, Ingles SA, Sherrod A, Warren R, Tsao-Wei D, Groshen S, and Lenz HJ (2001) Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J 1:65–70. [DOI] [PubMed] [Google Scholar]
- Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, Tothova Z, Tejero H, Heckl D, Jaras M, et al. (2016) Core circadian clock genes regulate leukemia stem cells in AML. Cell 165:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J and Scheer F (2016) Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab 27:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YG, Zhu JH, Tao QM, Zheng P, Chen JZ, Hu SJ, Zhang FR, Zheng LR, Zhao LL, and Yao XY (2005) Captopril administered at night restores the diurnal blood pressure rhythm in adequately controlled, nondipping hypertensives. Cardiovasc Drugs Ther 19:189–195. [DOI] [PubMed] [Google Scholar]
- Ray S and Reddy AB (2020) COVID-19 management in light of the circadian clock. Nat Rev Mol Cell Biol 21:494–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke H and Asher G (2019) Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol 20:227–241. [DOI] [PubMed] [Google Scholar]
- Ribeiro RFN, Cavadas C, and Silva MMC (2021) Small-molecule modulators of the circadian clock: pharmacological potentials in circadian-related diseases. Drug Discov Today 26:1620–1641. [DOI] [PubMed] [Google Scholar]
- Robles MS, Humphrey SJ, and Mann M (2017) Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab 25:118–127. [DOI] [PubMed] [Google Scholar]
- Rogers VE, Mowbray C, Rahmaty Z, and Hinds PS (2021) A morning bright light therapy intervention to improve circadian health in adolescent cancer survivors: methods and preliminary feasibility. J Pediatr Oncol Nurs 38:70–81. [DOI] [PubMed] [Google Scholar]
- Ruben MD, Francey LJ, Guo Y, Wu G, Cooper EB, Shah AS, Hogenesch JB, and Smith DF (2019a) A large-scale study reveals 24-h operational rhythms in hospital treatment. Proc Natl Acad Sci U S A 116:20953–20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben MD, Hogenesch JB, and Smith DF (2019b) Sleep and circadian medicine: time of day in the neurologic clinic. Neurol Clin 37:615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben MD, Smith DF, FitzGerald GA, and Hogenesch JB (2019c) Dosing time matters. Science 365:547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben MD, Wu G, Smith DF, Schmidt RE, Francey LJ, Lee YY, Anafi RC, and Hogenesch JB (2018) A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med 10:eaat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G and Lightman S (2019) The human stress response. Nat Rev Endocrinol 15:525–534. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Dmitrzak-Weglar M, Kliwicki S, and Hauser J (2014) Polymorphism of circadian clock genes and prophylactic lithium response. Bipolar Disord 16:151–158. [DOI] [PubMed] [Google Scholar]
- Saito Y, Yoshida S, Nakaya N, Hata Y, and Goto Y (1991) Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study. Arterioscler Thromb 11:816–826. [DOI] [PubMed] [Google Scholar]
- Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB, et al. (2000) Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res 6:1322–1327. [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, and Ozturk N (2010) Circadian clock control of the cellular response to DNA damage. FEBS Lett 584:2618–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghani HR, Jagannath A, Humberstone T, Ebrahimjee F, Thomas JM, Churchill GC, Cipriani A, Attenburrow MJ, Perestenko OV, Cowley SA, et al. (2020) Patient fibroblast circadian rhythms predict lithium sensitivity in bipolar disorder. Mol Psychiatry 2020 May 13. doi: 10.1038/s41380-020-0769-6. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma A, Sharma VP, Sarkar AB, Sekar MC, Samuel K, and Geusz ME (2016) The circadian clock modulates anti-cancer properties of curcumin. BMC Cancer 16:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Basse AL, Schonke M, Chen S, Samad M, Altintas A, Laker RC, Dalbram E, Barres R, Baldi P, et al. (2019) Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab 30:92–110.e114. [DOI] [PubMed] [Google Scholar]
- Sato S, Solanas G, Peixoto FO, Bee L, Symeonidi A, Schmidt MS, Brenner C, Masri S, Benitah SA, and Sassone-Corsi P (2017) Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 170:664–677.e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayanan SK, Su H, Lin YW, and Su KP (2018) Circadian rhythm and melatonin in the treatment of depression. Curr Pharm Des 24:2549–2555. [DOI] [PubMed] [Google Scholar]
- Savikj M, Gabriel BM, Alm PS, Smith J, Caidahl K, Bjornholm M, Fritz T, Krook A, Zierath JR, and Wallberg-Henriksson H (2019) Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia 62:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Michelson AD, Frelinger AL, Evoniuk H, Kelly EE, McCarthy M, Doamekpor LA, Barnard MR, and Shea SA (2011) The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS ONE 6:e24549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Gibbs J, Ince L, and Loudon A (2018) Clocking in to immunity. Nat Rev Immunol 18:423–437. [DOI] [PubMed] [Google Scholar]
- Schroeder AM, Truong D, Loh DH, Jordan MC, Roos KP, and Colwell CS (2012) Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol 590:6213–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers A and Depoortere I (2021) Circadian clocks in the digestive system. Nat Rev Gastroenterol Hepatol 18:239–251. [DOI] [PubMed] [Google Scholar]
- Selfridge JM, Gotoh T, Schiffhauer S, Liu J, Stauffer PE, Li A, Capelluto DG, and Finkielstein CV (2016) Chronotherapy: intuitive, sound, founded. . .but not broadly applied. Drugs 76:1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi AA, McNair CM, McCann JJ, Alshalalfa M, Shostak A, Severson TM, Zhu Y, Bergman A, Gordon N, Mandigo AC, et al. (2021) The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat Commun 12:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Zhang W, Ye W, Wang H, Zhang Q, Shen J, Hong Q, Li X, Wen G, Wei T, et al. (2020) SR9009 induces a REV-ERB dependent anti-small-cell lung cancer effect through inhibition of autophagy. Theranostics 10:4466–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki A, Misawa K, Ikeda Y, Haraguchi A, Kamagata M, Tahara Y, and Shibata S (2017) Potent effects of flavonoid nobiletin on amplitude, period, and phase of the circadian clock rhythm in PER2::LUCIFERASE mouse embryonic fibroblasts. PLoS ONE 12:e0170904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuboni-Mulligan DD, Breton G, Smart D, Gilbert M, and Armstrong TS (2019) Radiation chronotherapy-clinical impact of treatment time-of-day: a systematic review. J Neuro-Oncol 145:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinturel F, Gos P, Petrenko V, Hagedorn C, Kreppel F, Storch KF, Knutti D, Liani A, Weitz C, Emmenegger Y, et al. (2021) Circadian hepatocyte clocks keep synchrony in the absence of a master pacemaker in the suprachiasmatic nucleus or other extrahepatic clocks. Genes Dev 35:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slat EA, Sponagel J, Marpegan L, Simon T, Kfoury N, Kim A, Binz A, Herzog ED, and Rubin JB (2017) Cell-intrinsic, bmal1-dependent circadian regulation of temozolomide sensitivity in glioblastoma. J Biol Rhythms 32:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaaland R, Laerum OD, Lote K, Sletvold O, Sothern RB, and Bjerknes R (1991) DNA synthesis in human bone marrow is circadian stage dependent. Blood 77:2603–2611. [PubMed] [Google Scholar]
- Solanas G, Peixoto FO, Perdiguero E, Jardi M, Ruiz-Bonilla V, Datta D, Symeonidi A, Castellanos A, Welz PS, Caballero JM, et al. (2017) Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 170:678–692.e620. [DOI] [PubMed] [Google Scholar]
- Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, et al. (2012) Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, Lee HW, Choi S, Sun W, Kim H, et al. (2008) Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A 105:20970–20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Yang CS, Zhang X, and Wang Y (2021) The relationship between host circadian rhythms and intestinal microbiota: a new cue to improve health by tea polyphenols. Crit Rev Food Sci Nutr 61:139–148. [DOI] [PubMed] [Google Scholar]
- Sotak M, Sumova A, and Pacha J (2014) Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med 46:221–232. [DOI] [PubMed] [Google Scholar]
- Sothern RB, Levi F, Haus E, Halberg F, and Hrushesky WJ (1989) Control of a murine plasmacytoma with doxorubicin-cisplatin: dependence on circadian stage of treatment. J Natl Cancer Inst 81:135–145. [DOI] [PubMed] [Google Scholar]
- Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, and Kalsbeek A (2019) Circadian clocks and insulin resistance. Nat Rev Endocrinol 15:75–89. [DOI] [PubMed] [Google Scholar]
- Sulli G, Lam MTY, and Panda S (2019) Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer 5:475–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, Plikus MV, Verma IM, and Panda S (2018) Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang Y, Song Y, Cheng XR, Xia S, Rahman MR, Shi Y, and Le G (2015) Resveratrol restores the circadian rhythmic disorder of lipid metabolism induced by high-fat diet in mice. Biochem Biophys Res Commun 458:86–91. [DOI] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, and Peterson CM (2018) Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27:1212–1221.e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Hayano Y, Nakai A, Furuta F, and Noda M (2016) Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med 213:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y and Shibata S (2016) Circadian rhythms of liver physiology and disease: experimental and clinical evidence. Nat Rev Gastroenterol Hepatol 13:217–226. [DOI] [PubMed] [Google Scholar]
- Tahkamo L, Partonen T, and Pesonen AK (2019) Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int 36:151–170. [DOI] [PubMed] [Google Scholar]
- Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat Rev Gen 18:164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini M, Filipski E, Liu XH, Lemaigre G, Li XM, Vrignaud P, Francois E, Bissery MC, and Levi F (1998) Docetaxel chronopharmacology in mice. Cancer Res 58:3896–3904. [PubMed] [Google Scholar]
- Tang Q, Cheng B, Xie M, Chen Y, Zhao J, Zhou X, and Chen L (2017) Circadian clock gene bmal1 inhibits tumorigenesis and increases paclitaxel sensitivity in tongue squamous cell carcinoma. Cancer Res 77:532–544. [DOI] [PubMed] [Google Scholar]
- Tang Q, Xie M, Yu S, Zhou X, Xie Y, Chen G, Guo F, and Chen L (2019) Periodic oxaliplatin administration in synergy with PER2-mediated PCNA transcription repression promotes chronochemotherapeutic efficacy of OSCC. Adv Sci 6:1900667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Fernandez AF, Setien F, Ropero S, Ballestar E, Villanueva A, Yamamoto H, Imai K, Shinomura Y, and Esteller M (2009) Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res 69:8447–8454. [DOI] [PubMed] [Google Scholar]
- Tarocco A, Caroccia N, Morciano G, Wieckowski MR, Ancora G, Garani G, and Pinton P (2019) Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis 10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telias I and Wilcox ME (2019) Sleep and circadian rhythm in critical illness. Crit Care 23:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, et al. (2014) Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159:514–529. [DOI] [PubMed] [Google Scholar]
- Thomas JM, Kern PA, Bush HM, McQuerry KJ, Black WS, Clasey JL, and Pendergast JS (2020) Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 5:e134270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome-Souza S, Klehm J, Jackson M, Kadish NE, Manganaro S, Fernandez IS, and Loddenkemper T (2016) Clobazam higher-evening differential dosing as an add-on therapy in refractory epilepsy. Seizure 40:1–6. [DOI] [PubMed] [Google Scholar]
- Thosar SS, Butler MP, and Shea SA (2018) Role of the circadian system in cardiovascular disease. J Clin Invest 128:2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognini P, Murakami M, and Sassone-Corsi P (2018) Interplay between microbes and the circadian clock. Cold Spring Harb Perspect Biol 10:a028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump RP, Bresciani S, Cooper AW, Tellam JP, Wojno J, Blaikley J, Orband-Miller LA, Kashatus JA, Boudjelal M, Dawson HC, et al. (2013) Optimized chemical probes for REV-ERBalpha. J Med Chem 56:4729–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee A, Lecarpentier Y, Guillevin R, and Vallee JN (2020) The influence of circadian rhythms and aerobic glycolysis in autism spectrum disorder. Trans Psychiatry 10:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Drunen R and Eckel-Mahan K (2021) Circadian rhythms of the hypothalamus: from function to physiology. Clocks Sleep 3:189–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Moorsel D, Hansen J, Havekes B, Scheer F, Jorgensen JA, Hoeks J, Schrauwen-Hinderling VB, Duez H, Lefebvre P, Schaper NC, et al. (2016) Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab 5:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, and Porcellati C (1990) Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 81:528–536. [DOI] [PubMed] [Google Scholar]
- Wagner PM, Monjes NM, and Guido ME (2019a) Chemotherapeutic effect of SR9009, a REV-ERB agonist, on the human glioblastoma T98G cells. ASN Neuro 11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PM, Sosa Alderete LG, Gorne LD, Gaveglio V, Salvador G, Pasquare S, and Guido ME (2019b) Proliferative glioblastoma cancer cells exhibit persisting temporal control of metabolism and display differential temporal drug susceptibility in chemotherapy. Molecular Neurobiology 56:1276–1292. [DOI] [PubMed] [Google Scholar]
- Wajid F, Poolacherla R, Mim FK, Bangash A, and Rutkofsky IH (2020) Therapeutic potential of melatonin as a chronobiotic and cytoprotective agent in diabetes mellitus. J Diabetes Metab Disord 19:1797–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH II, Bumgarner JR, Walton JC, Liu JA, Melendez-Fernandez OH, Nelson RJ, and DeVries AC (2020a) Light pollution and cancer. Int J Mol Sci 21:9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH II, Walton JC, DeVries AC, and Nelson RJ (2020b) Circadian rhythm disruption and mental health. Trans Psychiatry 10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A, Chinn D, and Rubin G (2003) Taking Simvastatin in the morning compared with in the evening: randomised controlled trial. BMJ 327:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mauvoisin D, Martin E, Atger F, Galindo AN, Dayon L, Sizzano F, Palini A, Kussmann M, Waridel P, et al. (2017) Nuclear proteomics uncovers diurnal regulatory landscapes in mouse liver. Cell Metab 25:102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zou JX, Xue X, Cai D, Zhang Y, Duan Z, Xiang Q, Yang JC, Louie MC, Borowsky AD, et al. (2016) ROR-gamma drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat Med 22:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Song L, Liu M, Ge R, Zhou Q, Liu W, Li R, Qie J, Zhen B, Wang Y, et al. (2018) A proteomics landscape of circadian clock in mouse liver. Nat Commun 9:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Su G, Dai Z, Meng M, Zhang H, Fan F, Liu Z, Zhang L, Weygant N, He F, et al. (2021) Circadian clock genes promote glioma progression by affecting tumour immune infiltration and tumour cell proliferation. Cell Prolif 54:e12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AC, Smith L, Ray DW, Loudon ASI, Brown TM, and Bechtold DA (2017) Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat Commun 8:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, et al. (2013) Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 19:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PA, Yang X, Taber A, Oh EY, Ansell C, Ayers SE, Al-Assaad Z, Carnevale K, Berger FG, Pena MM, et al. (2008) Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol Cancer Res 6:1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Dauchy RT, Tirrell PC, Wu SS, Lynch DT, Jitawatanarat P, Burrington CM, Dauchy EM, Blask DE, and Greene MW (2011) Light at night activates IGF-1R/PDK1 signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Res 71:2622–2631. [DOI] [PubMed] [Google Scholar]
- Xiang S, Dauchy RT, Hauch A, Mao L, Yuan L, Wren MA, Belancio VP, Mondal D, Frasch T, Blask DE, et al. (2015) Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J Pineal Res 59:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Wang J, Hong F, Wang S, Jin X, Xue T, Jia L, and Zhai Y (2017) Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res 62. J. Pineal Res 62:e12399. [DOI] [PubMed] [Google Scholar]
- Xu T and Lu B (2019) The effects of phytochemicals on circadian rhythm and related diseases. Crit Rev Food Sci Nutr 59:882–892. [DOI] [PubMed] [Google Scholar]
- Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptacek LJ, and Fu YH (2005) Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 434:640–644. [DOI] [PubMed] [Google Scholar]
- Yang X, Wood PA, Ansell CM, Quiton DF, Oh EY, Du-Quiton J, and Hrushesky WJ (2009a) The circadian clock gene Per1 suppresses cancer cell proliferation and tumor growth at specific times of day. Chronobiol Int 26:1323–1339. [DOI] [PubMed] [Google Scholar]
- Yang X, Wood PA, Oh EY, Du-Quiton J, Ansell CM, and Hrushesky WJ (2009b) Down regulation of circadian clock gene Period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Res Treat 117:423–431. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yuan G, Xie H, Wei T, Zhu D, Cui J, Liu X, Shen R, Zhu Y, and Yang X (2019) Circadian clock associates with tumor microenvironment in thoracic cancers. Aging 11:11814–11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Xu H, Xie Z, Wang L, Sun Y, Yang H, Hu D, and Mao Y (2020) Time-restricted feeding reduces the detrimental effects of a high-fat diet, possibly by modulating the circadian rhythm of hepatic lipid metabolism and gut microbiota. Front Nutr 7:596285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegnanarayan R, Mahesh SD, and Sangle S (2006) Chronotherapeutic dose schedule of phenytoin and carbamazepine in epileptic patients. Chronobiol Int 23:1035–1046. [DOI] [PubMed] [Google Scholar]
- Yin J, Li Y, Han H, Chen S, Gao J, Liu G, Wu X, Deng J, Yu Q, Huang X, et al. (2018) Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res 65:e12524. [DOI] [PubMed] [Google Scholar]
- Yin J, Li Y, Han H, Ma J, Liu G, Wu X, Huang X, Fang R, Baba K, Bin P, et al. (2020) Administration of exogenous melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. mSystems 5. mSystems. 5(3):e00002–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Yooseph S, and Panda S (2014) Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab 20:1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeb F, Wu X, Chen L, Fatima S, Haq IU, Chen A, Majeed F, Feng Q, and Li M (2020) Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr 123:1216–1226. [DOI] [PubMed] [Google Scholar]
- Zeng ZL, Luo HY, Yang J, Wu WJ, Chen DL, Huang P, and Xu RH (2014) Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin Cancer Res 20:1042–1052. [DOI] [PubMed] [Google Scholar]
- Zeng ZL, Sun J, Guo L, Li S, Wu MW, Qiu F, Jiang WQ, Levi F, and Xian LJ (2005) Circadian rhythm in dihydropyrimidine dehydrogenase activity and reduced glutathione content in peripheral blood of nasopharyngeal carcinoma patients. Chronobiol Int 22:741–754. [DOI] [PubMed] [Google Scholar]
- Zhang D and Pollock DM (2020) Diurnal regulation of renal electrolyte excretion: the role of paracrine factors. Annu Rev Physiol 82:343–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L and Jain MK (2021) Circadian regulation of cardiac metabolism. J Clin Invest 131:148276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PX, Jin F, Li ZL, Wu WL, Li YY, Long JH, Chen GY, Chen XX, Gan JY, Gong XY, et al. (2018a) A randomized phase II trial of induction chemotherapy followed by cisplatin chronotherapy versus constant rate delivery combined with radiotherapy. Chronobiol Int 35:240–248. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, and Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Lahens NF, Yue Z, Arnold DM, Pakstis PP, Schwarz JE, and Sehgal A (2021a) A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat Commun 12:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yue Z, Arnold DM, Artiushin G, and Sehgal A (2018b) A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173:130–139.e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hunter L, Wu G, Maidstone R, Mizoro Y, Vonslow R, Fife M, Hopwood T, Begley N, Saer B, et al. (2019) Genome-wide effect of pulmonary airway epithelial cell-specific Bmal1 deletion. FASEB J 33:6226–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zeng P, Gao W, Zhou Q, Feng T, and Tian X (2021b) Circadian clock: a regulator of the immunity in cancer. Cell Commun Signal 19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Yang K, Yang G, Chen D, Tang H, Zhao D, and Zhao C (2013) Aberrant expression of clock gene period1 and its correlations with the growth, proliferation and metastasis of buccal squamous cell carcinoma. PLoS ONE 8:e55894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yang J, Huang R, Guo M, Zhou Y, and Xu L (2021) The role and its mechanism of intermittent fasting in tumors: friend or foe? Cancer Biol Med 18:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Tsukuda S, Wrensch F, Wing PA, Borrmann H, Harris JM, Morgan SB, Mailly L, Thakur N, Conceicao C, et al. (2021) Circadian regulation of SARS-CoV-2 infection in lung epithelial cells. bioRxiv. doi: 10.1101/2021.03.20.436163. [DOI] [Google Scholar]