Abstract
CD8+ T cells are critical mediators of cytotoxic effector function in infection, cancer, and autoimmunity. In cancer and chronic viral infection, CD8+ T cells undergo a progressive loss of cytokine production and cytotoxicity, a state termed T cell exhaustion. In autoimmunity, autoreactive CD8+ T cells retain the capacity to effectively mediate the destruction of host tissues. Although the clinical outcome differs in each context, CD8+ T cells are chronically exposed to antigen in all three. These chronically stimulated CD8+ T cells share some common phenotypic features, as well as transcriptional and epigenetic programming, across disease contexts. A better understanding of these CD8+ T cell states may reveal novel strategies to augment clearance of chronic viral infection and cancer, and mitigate self-reactivity leading to tissue damage in autoimmunity.
Introduction
CD8+ T cells are often described as existing at opposite ends of a spectrum of functionality in the context of chronic viral infection and cancer versus autoimmunity (Figure 1). In autoimmunity, CD8+ T cells overcome numerous tolerance mechanisms, including thymic selection and T cell activation requirements, to exert inappropriate effector function and cause damage to self tissue. In contrast, CD8+ T cells in chronic infection are exposed to appropriate activating cues (signal 1: antigen/T cell receptor (TCR), signal 2: costimulation, and signal 3: inflammatory cytokines). While CD8+ T cells exhibit similar profiles of transcriptional activation at the early phases of both acute and chronic infection at an early timepoint,1 responding CD8+ T cells in chronic infection quickly begin to exhibit insufficient effector function and are unable to clear the infection. This state of relative dysfunction, termed “exhaustion,” is also observed in the tumor microenvironment (TME). Blockade or deficiency of immune checkpoints like PD-1 ameliorates aspects of T cell exhaustion; these same perturbations can precipitate or exacerbate autoimmunity.
Figure 1: “Opposite ends of the spectrum” framework of CD8+ T cells in autoimmunity versus chronic viral infection.
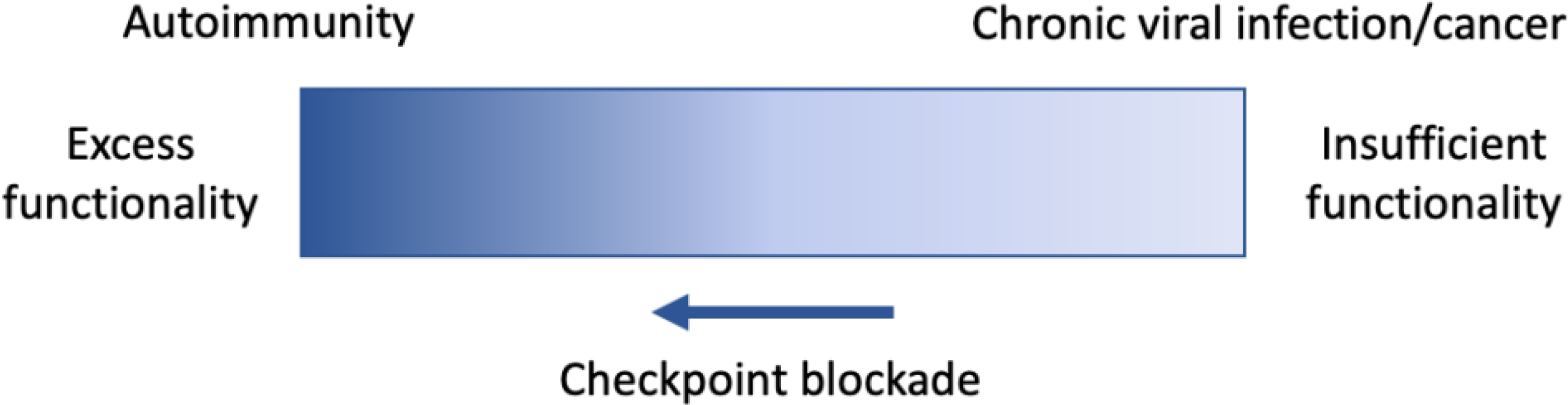
CD8+ T cells in autoimmunity (left) exhibit excessive and inappropriate effector functionality. In some autoimmune diseases, CD8+ T cells cause extensive damage of self-tissue by overcoming numerous tolerance mechanisms that normally prevent and/or halt this reaction in homeostasis. In contrast, CD8+ T cells in chronic viral infection and cancer (right) become “exhausted” and exhibit reduced effector functionality, as compared to effector function elicited by acute infection. This dysfunction contributes to viral persistence and continued tumor growth. Blockade of immune checkpoints such as co-inhibitory receptors PD-1 and CTLA-4 can augment effector functionality of CD8+ T cells in both contexts, leading to better control of chronic viral infection/cancer and exacerbated autoimmunity.
Yet, the settings of chronic infection, cancer, and autoimmunity share a key defining feature: chronic antigen exposure. New research has begun to define phenotypic similarities between CD8+ T cells in exhausted and autoimmune contexts, including functional, transcriptional, and epigenetic features. This may seem counterintuitive, given the “opposite ends of the spectrum” framework of CD8+ T cell state in exhaustion versus autoimmunity, as outlined in Figure 1. However, the perturbation of some exhaustion-associated features has recently revealed that “dysfunctionality” or “hyporesponsiveness” may in fact be integral for CD8+ T cell persistence in a chronic stimulatory context—thus providing a rationale for finding these shared features in chronic viral infection, cancer, and autoimmunity. CD8+ T cells in each of these contexts have been extensively discussed in previous reviews2–8; here we will focus on salient aspects of CD8+ T cell dysfunction most relevant for highlighting areas of similarity and distinction. A better understanding of CD8+ T cell states in these contexts may reveal new ways to alleviate T cell exhaustion and enhance CD8+ T cell functions in cancer and chronic viral infection, as well as strategies to induce or augment exhaustion-like features to treat autoimmunity.
TCR specificity and affinity
In autoimmunity, chronic infection, and cancer, chronic TCR signaling is a critical signal in the development and maintenance of the associated CD8+ T cell state. While chronic TCR signaling is a common feature across these contexts, the target varies: foreign antigens are targeted in chronic viral infection, while T cell responses are directed towards self-antigens in autoimmune disease—and a combination of both neoantigens and self-antigens may be targeted on tumors.
In cancer and chronic viral infection, chronic TCR signaling in the context of inflammatory signals is a critical driver of T cell dysfunction: tumor antigen-specific CD8+ T cells are significantly more exhausted (by reduced cytokine production, high co-inhibitory receptor expression, and high TOX expression) than co-transferred non-reactive cells in mouse studies9–11. In mice infected with a chronic strain of lymphocytic choriomeningitis virus (LCMV Clone 13), significantly reducing the load of a specific antigen without altering the overall viral load is sufficient to mitigate the development of certain aspects of exhaustion (chronic high PD-1 expression, low TNF production)12. There is also evidence that persistent antigen recognition plays an important role in the maintenance of exhausted cells. When exhausted cells from day 30 of LCMV Clone 13 infection are transferred into mice previously infected with acute LCMV Armstrong (cleared of antigen), they persist relatively poorly compared to transfer into chronically infected mice13. Additionally, in mice infected with LCMV Clone 13, blockade of homeostatic/memory maintenance signals IL-7 and IL-15 does not affect antigen-specific cell persistence; this may instead be supported by stimulation from residual antigen14. Furthermore, an epitope mutation in the chronic viral infection simian immunodeficiency virus (SIV) leads to the loss of responding CD8+ T cell clones15. However, a recent study of HCV-specific CD8+ T cells from patients treated with direct-acting antiviral (DAA) therapy identifies some HCV-specific cells that do persist in a setting of undetectable viral load (with a bias towards the progenitor-exhausted subset, discussed in more detail below)16. The role of IL-7 and IL-15 signaling, or very low level antigen, in the maintenance of these cells remains to be determined.
The prevalence of specific CD8+ T cell clones changes during the progression of chronic viral infection and autoimmunity, driven by stages of antigen-driven expansion and subsequent suppression or attrition. In chronic viral infection, high affinity T cells (e.g. D(b)NP396-404-specific T cells) respond to early dominant epitopes, but are extensively deleted by day 25, to a greater extent than lower affinity T cells (e.g. D(b)GP33-41, D(b)GP276-286-specific T cells)17–19. This pattern of overactivation leading to cell death could potentially function as a mechanism to prevent excessive damage to the host. At the same time, LCMV Clone 13 engineered to express a lower-affinity GP33 variant antigen generates an antigen-specific CD8+ T cell population with reduced exhaustion-associated features, suggesting that T cell exhaustion may be best elicited by antigens of intermediate affinity12.
To maintain homeostasis, high affinity autoreactive CD8+ T cells that escape central tolerance normally undergo clonal deletion in the periphery, in a process known as cross-tolerization20,21. In autoimmunity, CD8+ T cells undergo antigen-driven expansion into oligoclonal populations that have been implicated in the pathogenesis of multiple autoimmune diseases; for the purposes of this review, we will focus on multiple sclerosis (MS)22,23, Type 1 Diabetes (T1D)24,25, and vitiligo26–30. In the nonobese diabetic (NOD) model of T1D, high affinity CD8+ T cells mediate extensive tissue damage at the later stages of disease. At 3 weeks of age, NOD mice are disease-free and few low-affinity IGRP206–214–specific CD8+ T cells infiltrate the pancreas. By 10–15 weeks of age, the pancreata of NOD mice exhibit overt insulitis and are heavily infiltrated with high-affinity NRP-V7-specific CD8+ T cells31–33.
While a substantial amount of work has explored the evolution of high- versus low-affinity neoantigen-MHC interactions in cancer, less is known about the impact of differential peptide-MHC-TCR affinity. A recent study demonstrated that high affinity T cell clones can exert superior effector function in a mouse model of concurrent cancer and autoimmunity, using shared tumor/self-antigen34. Additionally, work from human clinical trials suggests that TCR clonality may have an important role in the response to checkpoint blockade therapies. CTLA-4 blockade has been shown to augment the repertoire diversity of tumor-infiltrating lymphocytes (TILs)35,36. For PD-1 blockade, early work suggested that response was associated with higher intratumor clonality37. More recent studies have demonstrated that PD-1 blockade drives the recruitment and expansion of novel TCR clones in the tumor microenvironment (TME), termed “clonal replacement”38. These findings suggest that tumor-specific T cells in the TME may possess limited reinvigoration ability, and that response to PD-1 blockade may be due to T cell clones recruited to the tumor. Indeed, TCR sequencing paired with single-cell RNA-sequencing has revealed that expanded clonotypes in the tumor tend to be more exhausted/dysfunctional38,39.
Functional profile
In an acute viral infection, CD8+ T cells are critical mediators of a protective immune response. Activated CD8+ T cells exert effector function through direct cytotoxicity (release of granzyme B and perforin) and cytokine production (effector cytokines like IFNγ and TNF, as well as proliferation-promoting cytokines like IL-2). After clearance of the infection, the responding CD8+ T cell population contracts, with a subset persisting as memory cells that are primed to regain effector function. Chronic viral infection, cancer, and autoimmunity all elicit aspects of CD8+ T cell functionality; exhausted CD8+ T cells in the former two contexts show clear deficits, while the relative effector and memory functionality of CD8+ T cells in autoimmunity is less clear.
Compared to CD8+ T cells generated in response to acute infection such as the Armstrong strain of LCMV, exhausted antigen-specific CD8+ T cells generated by LCMV Clone 13 are characterized by poor proliferation and persistence, in addition to reduced cytotoxicity and cytokine production (less IFNγ, minimal TNF, and almost absent IL-2)17,18,40,41. Exhausted CD8+ T cells also are distinguished by persistent high expression of multiple co-inhibitory receptors such as PD-1, LAG3, 2B4, TIM3, and CTLA-442–45; this is distinct from the transient expression of co-inhibitory receptors elicited by an acute infection. These key features have been recapitulated in tumor-specific CD8+ T cells isolated from the TME46–50. T cell dysfunction begins to develop early in chronic viral infection and cancer, and progressively becomes more severe. By day 8, responding CD8+ T cells in mice infected with LCMV Clone 13 are less cytotoxic than those from LCMV Armstrong17 and scRNA-Seq segregates T cells from mice infected with Armstrong or Clone 13 into distinct clusters1. Cytokine production (IFNγ, TNF) is further reduced in CD8+ T cells by day 30 of LCMV Clone 13 compared to day 717. Eventually, exhausted T cells lose the capacity to regain functionality: CD8+ T cells isolated from early LCMV Clone 13 infection13 or tumors51 are better able to respond after transfer to an antigen-matched acute infection, compared to cells transferred at a later time point.
Despite their relatively poor functionality compared to memory and effector cells, exhausted CD8+ T cells still play a critical role in chronic infection and cancer. In a non-human primate model of HIV infection, depletion of CD8+ T cells leads to an increase in plasma viremia, even at 1–3 years of infection52,53. Similarly, infection of β−2-microglobulin deficient mice (which lack CD8+ T cells) results in higher LCMV Clone 13 viral titers54. In cancer, enhanced tumor growth is seen with CD8 depletion in mouse tumor models, and in human patients, CD8+ T cell infiltration correlates positively with prognosis (disease-free survival, overall survival) in multiple tumor types55,56. Furthermore, studies of the TME suggest that features of exhaustion are predictive of relatively more functionality (compared to non-exhausted, likely non-responding cells). In human tumors, tumor infiltrating lymphocytes (TILs) expressing markers of exhaustion (PD-1, LAG3, TIM3) are more likely to express IFNγ47. By scRNA-Seq, a “dysfunctional” cluster of TILs from human melanoma can be defined, characterized by high expression of exhaustion-associated markers TIGIT, PDCD1, and LAG3; tumors with a higher proportion of these “dysfunctional” CD8+ T cells are associated with greater TIL ex vivo tumor reactivity and greater clonality39. While exhausted CD8+ T cells have reduced function compared to those elicited by an acute infection, these data suggest that it is specifically the exhausted CD8+ T cells that are exerting residual control over tumor growth.
In contrast to the limited pathological effect of responding CD8+ T cells on tissues in chronic infection and cancer, T cells effectively damage host tissues in various autoimmune diseases. Pathogenic CD8+ T cells in these contexts are primarily effector, effector memory, and resident memory cells, and are critical for disease initiation and progression. The prevalence of CD69+CD103+ resident memory T cells in the epidermis is positively correlated with active vitiligo57,58 and these cells can recruit cytotoxic effector T cells from the circulation important for sustained disease59. In T1D patients, 50–60% of antigen-specific CD8+ T cells that infiltrate the exocrine pancreas are of a memory (CD45RO+) phenotype60,61. In MS, both effector and effector memory CD45RA+/−CCR7− CD8+ T cells are associated with active disease62–64. Consistently across these autoimmune diseases, pathogenic CD8+ T cells express high levels of effector molecules (granzyme B, perforin, IFNγ, TNF) in vivo or upon ex vivo restimulation. This is apparent in autoreactive CD8+ T cells that mediate vitiligo28,58,65, NOD diabetes25,32,66, MS64, or central nervous system (CNS) inflammation in mouse models22,67,68. Encephalitogenic and diabetogenic CD8+ T cells also upregulate a combination of co-inhibitory receptors, including LAG3, TIM3, and PD-122,61,67–70. In contrast, pathogenic T cells in vitiligo express relatively low levels of PD-1 and LAG328,58,65. Whereas exhausted T cells in chronic viral infection are independent of IL-7/IL-15 for persistence, pathogenic effector/memory T cells in autoimmune diabetes can be inhibited by blockade of IL-771,72 or IL-1573. IL-15 blockade also can inhibit vitiligo28 and IL-15 has been implicated in promoting CD8+ T cell effector function in MS74,75.
Despite their maintenance of effector function, there is some evidence that autoreactive CD8+ T cells also exhibit features of exhaustion in autoimmunity. Transcriptional profiling has identified a subset of CD8+ T cells that infiltrate the pancreatic islets of T1D patients and aged NOD mice with aspects of T cell exhaustion; these exhausted-like CD8+ T cells expand as mice age and progress towards diabetes while a cytotoxic effector subset does not69,70. The prevalence and importance of exhausted T cells in autoimmunity remains unclear—however, exhausted autoreactive T cells could represent a suppressed but not halted autoimmune disease process. Activated islet-specific CD8+ memory T cells were prevalent in subjects with T1D who experienced rapid loss of the antigen C-peptide; in contrast, slow disease progression was associated with an exhaustion-like profile, with expression of multiple inhibitory receptors, limited cytokine production, and reduced proliferative capacity69. A transcriptional profile of T cell exhaustion also correlated with improved prognosis in patients with vasculitis, Crohn’s disease, and Systemic Lupus Erythematosus (SLE)76,77. This relationship between exhaustion-associated properties in autoreactive CD8+ T cells and the rate of autoimmune disease progression make these phenotypes attractive putative biomarkers of disease trajectory and potential targets for therapeutic intervention.
Heterogeneity within dysfunctional T cell populations
With the expansion of single-cell profiling technologies in immunology, from flow cytometry to single-cell RNA-sequencing and beyond, the fields of exhaustion and autoimmunity are now equipped to better understand the diversity of CD8+ T cell states across these contexts. In chronic viral infection and cancer, it has recently been appreciated that there are multiple subsets of exhausted cells with distinct functionalities (reviewed in more detail here7,78,79). Several exhausted subset frameworks have been described39,80–82; here we focus on one framework with substantial coherence across cancer and chronic viral infection in both mice and humans. A similar dissection of CD8+ T cell heterogeneity in autoimmunity is underway, with some clear parallels to exhaustion-defined subsets.
A “progenitor” exhausted subset was first described in LCMV Clone 13 based on intermediate PD-1 expression and high CD44 expression; this was associated with improved persistence, and more expansion in response to anti-PD-L1 treatment83 (Table 1). This subset has also been identified in the TME11,84. The key feature of progenitor-exhausted cells is their self-renewal capacity85; they can also differentiate into a “terminal” exhausted subset in an antigen-dependent process11,84. The transcription factor TCF1 (encoded by the gene TCF7), is required for progenitor-exhausted CD8+ T cells. TCF1 has also been shown to play an important role in central memory86,87 and stem cell memory CD8+ T cells88, suggesting it may function to promote self-renewal and persistence. This subset plays an integral role in control of infection or tumor: TCF1-deficient mice exhibit higher viral titers and accelerated tumor growth, while transferring progenitor-exhausted cells (compared to terminal-exhausted cells) enhances tumor control11,84,89,90. Importantly, this stem-like population provides the proliferative burst after PD-1 pathway blockade during chronic viral infection11,83–85. While a “progenitor exhausted-like” subset of human memory CD8+ T cells has recently been identified91, it is important to note that progenitor-exhausted CD8+ T cells are distinct from memory cells: they express high levels of the exhaustion-associated transcription factor TOX92, and are more similar to terminal-exhausted cells than memory cells by chromatin accessibility profiling11.
Table 1:
Heterogeneity in exhausted CD8+ T cells in chronic viral infection and cancer.
| Disease | Chronic Viral Infection | Cancer | ||
|---|---|---|---|---|
| Model | LCMV Clone 13 | HCV, HIV | Various | Various |
| Species | Mouse | Human | Mouse | Human |
| Target antigens | Viral antigens | Viral antigens | Model antigen (e.g. OVA), neoantigen & self-antigen | Neoantigen & self-antigen |
| Key markers | Progenitor: TCF1, Slamf6, CXCR5 Transitory/intermediate: TIM3, CX3CR1 Terminal: TIM3, CD10180,85,89,93,94 |
Progenitor: Tcf1+, CD127+ Terminal: Tcf1−, CD127−16,177,178 |
Progenitor: TCF1, Slamf6 Terminal: TIM311,84 | Progenitor: TCF1, CCR7, CXCR5 Terminal: CD39, TIM11,84,116,179,180 |
| Functional properties | Progenitor: self-renewal, greatest persistence, differentiate into terminal populations Transitory: greatest proliferation, differentiate into terminal exhausted85,89,93 |
Progenitor: greater persistence after loss of antigen stimulation (DAA therapy), higher percentage associated with greater ex vivo expansion capacity16,178 | Progenitor: self-renewal, greater persistence, greater proliferative capacity Terminal: greater proliferation, greater apoptosis11,84 |
Progenitor: greater persistence in vivo (in an ACT product) and ex vivo, give rise to terminal exhausted ex vivo179–181 |
| Co-inhibitory receptor expression | Progenitor: least expression of PD-1, TIM3, LAG3, CD160, TIGIT Transitory/intermediate: intermediate expression of PD-1, TIGIT, high expression of TIM3 Terminal: greatest expression of PD-1, LAG3, CD160, TIGIT, high expression of TIM380,85,89,93 |
Terminal: greater expression of PD-1, CD39, 2B4, TIGIT16,177 | Terminal: greater expression of PD-1, TIM3, CD244, CD39, CD101, CTLA-411,84 | Terminal: greater expression of PD-1, TIM3, CD39, Lag3, CTLA-484,116,180 |
| CD8+ T cell response to PD-1 pathway blockade | Progenitor: respond to checkpoint blockade through enhanced proliferation and differentiation to transitory, terminal exhausted83,85,89 | ? | Progenitor: required for response to checkpoint blockade, respond through enhanced proliferation and differentiation to terminal exhausted11,81 | More progenitor-exhausted cells: greater response to checkpoint blockade and PFS11,116 |
| Cytokine production | Progenitor: greatest IFNγ, TNF, IL-2 Transitory/intermediate: intermediate IFNγ Terminal: least IFNγ85,93,94 |
More progenitor-exhausted cells: greater polycytokine co-production (TNF and IFNγ)177 | Progenitor: greater TNF, greater polycytokine co-production Terminal: greater IFNγ11,84 | Progenitor: greater TNF, IL-2, polycytokine co-production Terminal: greater IFNγ180 |
| Cytotoxicity | Progenitor: least Gzmb Transitory/intermediate: greatest Gzmb Terminal: intermediate Gzmb85,89,93,94 |
Terminal: greater expression of Gzmb177,178 | Terminal: greater Gzmb expression, ex vivo target killing capacity11,84 | Terminal: greater expression of Gzmb116 |
ACT, adoptive cell therapy; DAA, direct-acting antiviral; Gzmb, Granzyme B; PFS, progression-free survival.
The second major subset, terminal-exhausted cells, has more direct cytotoxic function11, but persists poorly and does not proliferate in response to PD-1 blockade nor tumor vaccination84. Within this TIM3+ subset, additional subpopulations have recently been described: CX3CR1+ “transitory” cells with greater proliferation, cytokine production and cytotoxic potential, which can develop into CD101+ “terminal” cells with the least functionality and poorest survival93,94. This evolving subset landscape highlights the complex role that exhausted CD8+ T cells play: balancing effector function with stem-like function for long-term pathogen/tumor control.
Subsets of stem-like autoreactive CD8+ T cells that share some features with progenitor-exhausted T cells have been identified in autoimmunity, but there is no current consensus on markers to define these populations. In NOD mice, scRNA-Seq analysis revealed terminal effector-like (Pdcd1hiLag3hi) and stem-like (Tcf7hiToxhi) CD8+ T cell populations, both of which showed differential expression of an exhaustion-associated gene signature by GSEA70. There is also evidence of non-exhausted stem-like T cells within autoimmune effector and memory effector CD8+ T cell populations. Using dextramers for various beta-islet antigens, antigen-specific CD8+ T cells can be identified in the blood of both healthy individuals and patients with T1D. In patients with T1D, these autoreactive CD8+ T cells are primarily a stem-like effector memory phenotype characterized by the expression of CD95 (CD45RA+CD45RO−CCR7+CD95+), while the majority of beta-islet-specific T cells remain naive (CD45RA+CD45RO−CCR7+CD95−) in the blood of healthy individuals24,95,96. Considering autoimmune-related conditions more broadly, non-naive but stem-like CD8+ T cells (CD45RO−CD45RA+CCR7+CD27+CD95+) are elevated in the blood of patients with aplastic anemia, autoimmune uveitis, and sickle cell disease97. These findings suggest that stem-like subsets of autoreactive CD8+ T cells present in the blood may be important for the sustained production of more terminal populations that mediate cytotoxic destruction in autoimmune organs. Further studies are required to elucidate the significance of stem-like subsets of CD8+ T cells in autoimmune disease and the potential for durable therapeutic strategies targeting this population.
PD-1/PD-L1 pathway
The PD-1/PD-L1 pathway is a critical immunoregulatory pathway initially described in the context of autoimmunity98–100 that is also important in chronic viral infection and cancer. The PD-1 co-inhibitory receptor can be expressed on various hematopoietic cells including activated CD8+ T cells. Binding of PD-1 to its ligands, PD-L1 or PD-L2, reduces downstream TCR and CD28 signaling and effector T cell functionality101. Physiologically, this pathway negatively regulates T cell activation, mediates T cell tolerance and controls resolution of inflammation. PD-L1 expression by host tissues is a protective mechanism against damage directed towards healthy tissues. For example, PD-L1 is upregulated by beta-islets in the context of T1D102, as well as by astrocytes and microglia/macrophages in the brains of MS patients103,104. This pathway’s critical importance is apparent in PD-1/PD-L1 deficient mice, which develop severe spontaneous autoimmunity on autoimmune-prone backgrounds (NOD, MRL)105,106. In addition to protecting against autoimmunity due to its effects on autoreactive CD8+ T cells107, the PD-1 pathway is critical for preventing excess damage in the context of chronic viral infection: LCMV Clone 13 infection is lethal in PD-1-pathway deficient mice at doses that are non-lethal for wildtype mice43. This is due to unchecked perforin-mediated destruction of endothelial cells leading to circulatory collapse108.
Whereas PD-1 is transiently expressed on CD8+ T cells during the early response to acute viral infection and downregulated upon viral clearance, chronic infection and cancer cause high, sustained PD-1 expression on exhausted CD8+ T cells43. Early in chronic infection, expression is highest on TCF1+ progenitor-exhausted CD8+ T cells109, while later, expression becomes highest on TIM3+ cells11,85, particularly on the CD101+ terminal-exhausted subset93. These temporal changes in relative PD-1-expression indicate that PD-1 may have distinct roles at various stages of the development of T cell exhaustion.
High levels of PD-1 expression contribute to the characteristic dysfunction seen in exhausted CD8+ T cells. PD-1 negatively regulates proliferation and effector function (cytokine production, cytotoxicity) at the functional and transcriptional level43,110. PD-1 blockade can transiently ameliorate these functional deficits43,110, but it does not enhance memory potential of the exhausted CD8+ T cells110. Interestingly, while PD-1 deficient CD8+ T cells show enhanced proliferation early during the course of chronic viral infection, this effect is accompanied by increased apoptosis and more profound deficits in cytokine production111, illustrating a critical role for PD-1 in the maintenance of T cell functionality and persistence. During acute infection, PD-1 also plays a role in proper memory formation112,113, highlighting the importance of early PD-1 signals in the long-term maintenance of T cell populations.
Therapeutic modulation of PD-1 was first demonstrated in the context of LCMV Clone 13: blockade of the PD-1 pathway resulted in lower viral titers across tissues43. Antibody-based PD-1 pathway blockade is now an FDA-approved therapy for many tumor types114 and has a demonstrated positive effect on numerous clinical indicators, particularly long-term survival115. Murine tumor models have demonstrated that PD-1 blockade specifically promotes proliferation and differentiation from progenitor-exhausted to terminal-exhausted T cells11. Correspondingly, in human tumors, a higher proportion of progenitor-exhausted cells was associated with a greater likelihood of response to checkpoint blockade116. While PD-1 blockade therapies have been successful in multiple tumor types, immune-mediated side-effects, termed immune-related adverse events (irAEs), can limit their efficacy. There are many unresolved questions about how irAEs arise and their connection to classical autoimmunity (reviewed in Box 3).
Box 3: The role of CD8+ T cells in immune-related adverse events (irAEs).
Autoimmune-prone mouse models clearly demonstrate that disruption of the PD-1/PD-L1 and/or CTLA-4 pathways can promote the development of various autoimmune diseases such as spontaneous lymphoproliferative disorder153, autoimmune diabetes105,154, peripheral neuropathy155, and myocarditis105,156,157. Thus it is not surprising that checkpoint blockade therapy in cancer patients can lead to autoimmune conditions known as “immune-related adverse events” (irAEs). There is immense diversity in the clinical presentation of irAEs, ranging from mild dermatological and gastrointestinal events to serious and life-threatening pulmonary, endocrine, cardiovascular, and neurological events with time of presentation from immediately after treatment to years later158. Even when mild or moderate, irAEs may require immune suppression and/or culminate in treatment discontinuation. Patients treated with PD-1/PD-L1 blockade tend to develop different irAEs than those treated with CTLA-4 blockade, while combination therapy increases both the incidence and severity of irAEs compared to either therapy alone159–161.
CD8+ T cells are present in the lymphocytic infiltrates of affected organs in patients with checkpoint blockade-induced pneumonitis162, dermatological conditions163,164, myocarditis165, and colitis166,167. A recent study used high-dimensional flow cytometry and scRNA-seq to characterize checkpoint inhibitor-induced colitis in patients. TCR sequencing revealed that a significant fraction of colitis-associated CD8+ T cells originated from tissue-resident memory cells, explaining the frequently early onset of colitis following the start of checkpoint therapy. Colitis was associated with major changes in T cell and myeloid populations, in particular a highly proliferative and cytotoxic CD8+ T cell population that expresses PDCD1, LAG3, HAVCR2 (TIM3), and CTLA4167.
Multiple mechanisms may contribute to the generation of irAEs, including a pre-existing susceptibility to autoimmunity, aberrant presentation of self-antigen in the tumor microenvironment, and generation of a new, cross-reactive repertoire to self post-therapy. Such autoreactive T cells may also be exerting anti-tumor effects directly towards self-antigens expressed by the tumor. Studies in mouse models of vitiligo and melanoma show that autoreactive CD8+ T cells towards shared self/tumor antigens such as gp100, MART1, or tyrosinase can also be found in the TME168 and clonal expansion of these populations can confer protection against the development of cancer65,169–172. irAEs have been correlated with improved durable therapeutic responses to checkpoint blockade towards tumors with low mutational burden and few neoantigens172–174, but further work is needed to understand if this association has predictive value. Vitiligo and paraneoplastic neurological syndromes are thought to occur largely due to CD8+ T cell responses towards self-antigens common to both the tumor and affected organ65,168,170,175,176. This mechanism is supported by findings that high affinity TCR transgenic CD8+ T cells are capable of mediating both anti-tumor effects and autoimmunity in mouse models exhibiting shared tumor and self-antigens34,169,177.
While autoreactive CD8+ T cells likely have a significant role in eliciting irAEs, the mechanisms leading to irAE development remain elusive. A deeper understanding of the pathogenesis of irAEs may allow us to promote effective anti-tumor immunity without concomitant autoimmunity.
Given the role of the PD-1/PD-L1 pathway in mediating T cell tolerance, the induction of tolerance through administration of checkpoint agonists is currently being investigated for the treatment of autoimmune disease. PD-1 agonism with PD-L1 has shown efficacy in a variety of preclinical animal models, including colitis mediated by dextran sulfate sodium (DSS) or adoptive T cell transfer117, psoriasis118, EAE119, lupus120, and collagen-induced arthritis121.
Regulation of T cell dysfunction by TOX
While many transcription factors play important roles in CD8+ T cell biology, TOX has recently become of particular interest to the field of exhaustion. TOX is highly expressed by exhausted CD8+ T cells in chronic infection and cancer, particularly at later time points, but largely absent from naive CD8+ T cells and acute infection-induced CD8+ T cell populations (effector and memory)9,92,122,123. Overexpression of TOX is sufficient to recapitulate a significant fraction of exhaustion-associated gene expression changes, both in naive CD8+ T cells and in fibroblasts9,122. Most strikingly, TOX deficient T cells are unable to persist in chronic infection and tumor models9,122, suggesting that exhaustion is an adaptation for T cells to survive in an environment of chronic antigen stimulation.
In the context of autoimmunity, TOX may play a role in controlling the pathogenicity and maintenance of self-reactive CD8+ T cells. In murine models of CNS autoimmunity, TOX expression is increased in encephalitogenic CD8+ T cells, and TOX-deficient T cells exhibit reduced encephalitogenic potential, cytokine production, and persistence67,68. TOX is also expressed by a subset of T cells in the pancreas of aged NOD mice, and the TOX locus shows increased chromatin accessibility within a subset of beta-islet-cell specific CD8+ T cells from humans with T1D70,96. Further work is needed to understand the signals driving TOX expression in autoimmunity and the disease-modifying impact that TOX+ subsets confer. It remains unclear whether TOX (1) promotes T cell persistence and maintenance of cytotoxic function, or (2) promotes the development of exhaustion to reduce cytotoxicity and maintain tolerance.
Epigenetic regulation of CD8+ T cell dysfunction
Along with the expression of transcription factors, a cell regulates when and how much of a gene transcript is expressed through epigenetic regulatory mechanisms. There are many types of epigenetic regulation, but thus far CD8+ T cells in exhaustion and autoimmunity have primarily been characterized in terms of chromatin accessibility, DNA methylation, and histone modifications that affect the rate of transcription of genes.
Recent advances in epigenomic profiling have revealed that exhausted CD8+ T cells in chronic infection and cancer have a distinct epigenetic landscape compared to other CD8+ T cell states. In both LCMV Clone 13 and tumor contexts, progenitor- and terminal-exhausted subsets show significant chromatin accessibility differences from naive, effector, and memory populations11,51,110,124,125. These findings are conserved in human disease contexts: exhaustion-associated chromatin accessible regions (ChARs) from human CD8+ T cells in HIV and HCV significantly overlap with orthologous regions from T cells isolated from LCMV Clone 13124. This distinct epigenetic profile develops early: exhausted versus effector CD8+ T cell chromatin accessibility patterns diverge by day 7 of LCMV Clone 13 infection110,124, as do H3K27 acetylation patterns1, a histone modification marking active promoters/enhancers. These epigenetic changes have transcriptional consequences: multiple regions near the Tox locus show significant increases in chromatin accessibility as well as H3K27 acetylation, consistent with increased Tox expression specifically in exhausted cells.
DNA methylation profiling also reveals distinctions between exhausted CD8+ cells and naive, effector, or memory T cells. While naive CD8+ T cells are highly methylated, corresponding to a state of relative transcriptional quiescence, many regions become demethylated in the effector state and remain demethylated in memory CD8+ T cells. In contrast, a significant portion of effector demethylated regions become remethylated in exhausted cells, which could represent a mechanism to reduce gene expression and consequent effector function in chronic stimulation contexts126. Interestingly, the inverse of this pattern occurs at regulatory regions near the PD-1 locus (CR-B, CR-C); these regions become remethylated in memory T cells, but remain demethylated in exhausted T cells from LCMV Clone 13, consistent with high sustained PD-1 expression. This pattern is also seen at orthologous loci in human CD8+ T cells when comparing cells in chronic viral infections (Epstein-Barr virus and cytomegalovirus-specific) to memory cells (Yellow fever virus-specific)127.
Recent studies suggest that both encephalitogenic and diabetogenic CD8+ T cells also exhibit distinct epigenetic profiles compared to naive, effector, or memory T cells. Beta-islet-specific CD8+ T cells were tetramer-sorted from the blood of T1D patients for whole-genome methylation analysis. These profiles were compared with the methylation profiles of sorted naive (CCR7+CD45RO−CD45RA+CD95−), stem-cell memory (CCR7+CD45RO−CD95+), central memory (CCR7+CD45RO+), and effector memory (CCR7−CD45RO+) from T1D patients and healthy controls, as well as exhausted tetramer+ T cells from the blood of HIV patients. The methylation profile of tetramer+ T1D T cells most closely resembled stem-cell memory cells, with both naive-associated and effector-associated features including demethylation at PRF1, GZMK, IFNG, and TBX21. Single-cell ATAC-Seq analysis confirmed that this hybrid epigenetic profile coexists in a subset of T cells. In the NOD T1D mouse model, the methylation profile of beta-islet-specific CD8+ T cells varied between tissue compartments: the epigenetic profile of tetramer+ T cells in the pancreas was distinct from effector-memory T cells and most comparable to exhausted P14 T cells isolated from mice with LCMV Clone 13 infection, while splenic tetramer+ cells shared epigenetic characteristics of both effector-memory and central-memory T cells96. This location-based difference mirrors a bias seen in exhausted subsets from chronic viral infection: progenitor-exhausted cells (which relatively enrich for memory signatures) tend to reside in lymphoid organs, while terminal-exhausted cells tend to reside in non-lymphoid tissues85. Chromatin accessibility profiling has also revealed epigenetic features of exhaustion in encephalitogenic CD8+ T cells in a murine model of CNS autoimmunity. In mice expressing the LCMV glycoprotein in myelin-forming CNS cells (MOG-GP mice), infection with an attenuated LCMV variant initiates antigen-specific CD8+ T cell-mediated neurologic disease, whereas in WT mice, it causes a transient choriomeningitis that resolves by day 10. At day 21 post-infection, antigen-specific CD8+ T cells from the CNS of autoimmune MOG-GP mice, compared to matched cells from infected wild-type mice, exhibited increased chromatin accessibility at a majority of exhaustion-associated ChARs68. These data suggest that autoreactive CD8+ T cells exist in a stem-like state in the blood and lymphoid organs, but develop epigenetic features of exhaustion upon infiltration into the target organ.
Beyond deepening our understanding of CD8+ T cell state and heterogeneity, epigenetic profiling also has the potential to reveal mechanisms of existing therapies, as well as offer new therapeutic strategies. Despite its important clinical utility, PD-1 blockade does not alter the exhausted epigenetic profile of T cells, as demonstrated by chromatin accessibility profiling10,11,110 and methylation profiling126. This finding reinforces our understanding of the mechanism of action of PD-1 blockade as promoting proliferation and differentiation from progenitor- to terminal-exhausted. Reversing exhaustion-associated epigenetic changes may represent a new class of therapeutics to augment T cell functionality in cancer and chronic viral infection. Consistent with this concept, genetic or pharmacologic inhibition of exhaustion-associated enhanced remethylation can increase CD8+ T cell numbers as well as effector function. This treatment synergizes with anti-PD-L1 blockade to further improve control of LCMV Clone 13 infection and tumor growth126.
Conclusion and future perspectives
In an acute infection, a productive CD8+ T cell response is generated, characterized by appropriate effector function (leading to clearance of the pathogen) followed by relative CD8+ T cell quiescence in the memory state, with minimal damage to self-tissue. In comparison, CD8+ T cells develop dysfunctional states in response to chronic antigen stimulation in the context of cancer, chronic infection, and autoimmunity. Autoimmunity and chronic viral infection/cancer are traditionally exemplified as the pathological consequences at opposite ends of a spectrum of CD8+ T cell functionality (Figure 1). Despite the distinct consequences of reduced T cell effector function in chronic infection and cancer compared to sustained effector function in the context of autoimmunity, CD8+ T cells exhibit some key similarities across these conditions (Figure 2). Notably, both mouse and human CD8+ T cells in these different contexts share some molecular programming at both the transcriptional and epigenetic levels. Recent work suggests that exhaustion-like states may be found in an even broader set of pathological states, including infections like SARS-CoV2128.
Figure 2: Temporal changes of CD8+ T cells in cancer, chronic viral infection, and autoimmunity.
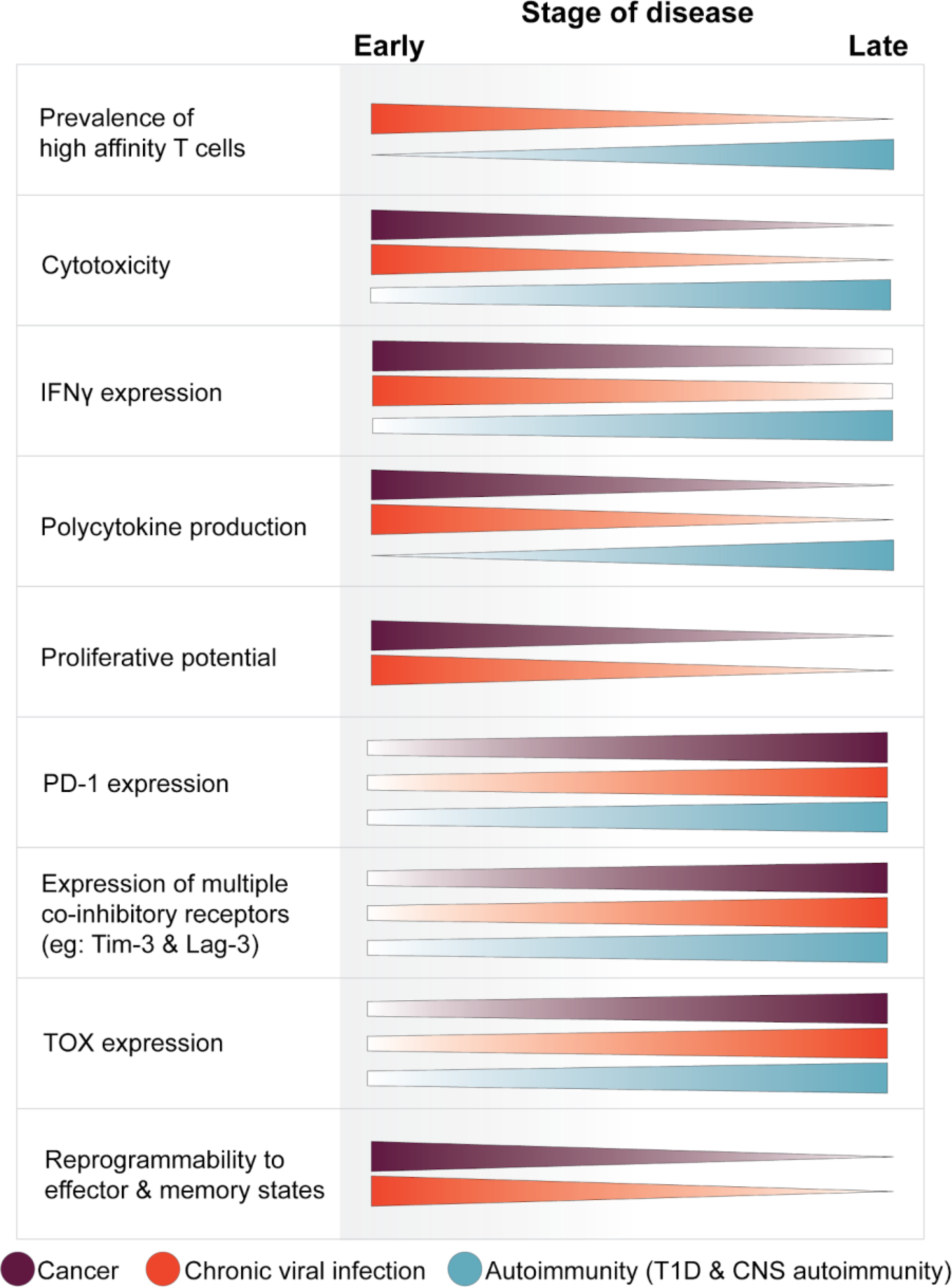
Antigen-specific CD8+ T cells progressively develop distinct phenotypic changes in the context of chronic stimulation in disease. The phenotypic changes described for bulk autoreactive CD8+ T cells are generalized from various studies of diabetogenic and encephalitogenic T cells within the affected organ of mouse models. The changes in T cell properties are described as disease progresses from an early to late time point, corresponding to approximately: day 7 and day 30–60 of LCMV Clone 13 infection; TILs in newly developing tumors compared to TILs in late-stage tumors; early NOD insulitis at 3–4 weeks of age to overt diabetes at 10–12 weeks; and early day 7 of CNS autoimmunity in MOG-GP mice compared to day 28. Changes are described for cancer (purple), chronic viral infection (orange), and autoimmunity (cyan).
While much has been learned about the dysfunctional states in chronic infection, cancer, and autoimmunity, there are still critical gaps in our knowledge. TCR affinity and self-antigen specific T cell responses are well studied in the context of autoimmunity, but have been less well studied in the context of cancer, with most work focused on neoantigen responses. Self-antigen responses may have important potential relevance to checkpoint-therapy induced irAEs. Moreover, our understanding of the function of non-specific “bystander” CD8+ T cells in contexts of chronic antigen stimulation is limited. Intriguingly, “bystander” CD8+ T cells that do not recognize the relevant self-antigen have the potential to inhibit an autoimmune response129. Although non-reactive bystander cells have also been identified in tumors10,130,131, their role in anti-tumor responses is unknown.
Likewise, while exhausted subsets have been defined epigenetically, transcriptionally, and functionally in cancer and chronic viral infection, CD8+ T cell heterogeneity on these three levels remains incompletely explored in autoimmunity. Further research using clonotype, epigenetic, and single-cell analyses is needed to define the presence and significance of exhausted and stem-like T cell subsets in a broader set of autoimmune diseases. It is not yet clear whether exhausted autoreactive CD8+ T cells in T1D remain important effectors of tissue destruction, or whether a progenitor-exhausted subset might be responsible for the sustained production of terminal-exhausted effectors. Alternatively, exhausted autoreactive CD8+ T cells might represent a minority population that does not effectively mediate tissue destruction in the autoimmune pancreas. If so, it could be desirable to induce a more wide-spread, profound, and durable state of exhaustion with strategies such as PD-1 agonism to ameliorate autoimmune disease.
In contexts of exhaustion, we are still working to disentangle what constitutes features of dysfunction versus preservation of residual function. Exhausted CD8+ T cells in chronic infection and cancer are regulated by a set of complex mechanisms intended to maintain homeostasis, which includes protection against pathogens and cancer as well as protection from aberrant self-destruction. Genetic deletion of dysfunction-associated molecules PD-1 and TOX in exhausted contexts results in a transient increase in proliferation/effector function, but dramatically reduces T cell persistence. Conversely, loss of effector-associated transcription factors (IRF4) promotes the maintenance of T cell functionality and persistence at the cost of reduced cell numbers132. These findings suggest that T cell exhaustion may represent a trade-off between proliferation and cellular persistence/maintenance of function in a chronic immunostimulatory environment.
In all three contexts, epigenetics is an area that requires a deeper understanding of the biology and consequent therapeutic implications. As we come to appreciate the importance of multiple subsets with distinct roles in development and maintenance of exhaustion and autoimmune disease processes, more work is also needed to understand the epigenetic landscape of dysfunction over time and at the single-cell level. Such epigenetic profiling studies may provide mechanistic insight into the capabilities of exhausted and autoreactive T cell subpopulations. Because epigenetic modulation provides finer tuning of gene expression, modulating dysfunction-associated epigenetic features may permit the amelioration of dysfunctional aspects without perturbing appropriate effector and memory functionality. More broadly, further investigation into the similarities and differences in CD8+ T cell fate decisions and dysfunction across autoimmunity, chronic infection, and cancer will provide critical insight into the remarkably diverse roles of T cells in health and disease, and reveal new therapeutic strategies.
Box 1: CD4+ T cell help.
CD4+ T cells can “help” CD8+ T cells by upregulating B7 costimulatory molecule expression on APCs to augment T cell activation, and by secreting multiple immunoregulatory cytokines including IL-2, which supports CD8+ T cell proliferation. In the context of chronic inflammation, CD4+ T cells play a critical role in modulating T cell dysfunction.
In autoimmunity, CD4+ T cells are considered the primary mediators of disease. In T cells from a varied set of human autoimmune diseases, a transcriptional signature of CD4+ T cell help was inversely correlated with clinical outcome, suggesting an important pathogenic role. This transcriptional signature of CD4+ T cell help was also inversely correlated with a signature of CD8+ T cell exhaustion77. Indeed, CD4+ T cell depletion in the setting of chronic viral infection results in more severe CD8+ T cell dysfunctionality (including reduced proliferation, cytokine production, and cytotoxicity), corresponding to extended viral persistence18,133. The CX3CR1+ CD8+ T cell population, which relatively maintains effector function in exhaustion, is particularly reduced upon depletion of CD4+ T cells in LCMV Clone 13 infection94 without significant depletion of the TCF1+ progenitor-exhausted population94,134. CD4+ T cell help can also play an important role in supporting CD8+ T cell driven anti-tumor immunity. In one assessment of tumor vaccination strategies, inclusion of CD4 epitopes presented on MHC Class II improved CD8+ T cell recruitment to the microenvironment; this effect appeared to be mediated by CD4+ modulation of dendritic cells135. Similarly, enforced cancer cell expression of an MHC Class II predicted epitope enhanced the number and functionality of CD8+ T cells responding to a co-expressed MHC Class I predicted epitope136.
Together, these findings highlight the critical role of CD4+ T cells in supporting CD8+ T cell functionality across chronic infection, cancer, and autoimmunity.
Box 2: Dysfunction of CD4+ T cells in chronic disease.
T cell dysfunction is not limited to CD8+ T cells—various subsets of CD4+ T cells (both FoxP3− conventional and FoxP3+ regulatory) develop altered functional capacity in the context of chronic viral infection, cancer, and autoimmunity. Conventional CD4+FoxP3− T cells (Tcon) subsets, including Th1, Th2, and Th17, secrete cytokines that support the activation and maintenance of CD8+ T cells (see box 1: CD4+ T cell help) and B cell production of antibodies. Tcon are also critical for the formation of tertiary lymphoid structures associated with sustained T cell responses in chronic infection, cancer, and autoimmunity137. Similar to CD8+ T cells, Tcon in chronic infection and cancer can develop features of T cell exhaustion, including upregulation of multiple co-inhibitory receptors such as Pdcd1, Havcr2, and Ctla4 and poor cytokine production138–140 resulting from chronic antigen stimulation141. This may be beneficial in autoimmunity, as a transcriptional signature of Tcon exhaustion is positively correlated with reduced severity of lupus nephritis142, T1D, and optic neuritis76. A subset of IFNγlow TCF1+ colitogenic Tcon with enhanced survival capacity and a gene signature comparable to progenitor-exhausted CD8+ T cells may be responsible for the chronicity of disease143. Stem-like features in Tcon are associated with expression of TCF1 which may serve as a marker of progenitor-exhausted Tcon similarly to progenitor-exhausted CD8+ T cells143,144.
CD4+ FoxP3+ Tregs have a critical role in the maintenance of peripheral tolerance by suppressing effector responses towards host tissues. Dysfunctional Tregs have been described with an IFNγ+ Th1-like phenotype and reduced suppressive capacity despite maintaining high FoxP3 expression145–147, and have been identified in autoimmunity147,148, chronic viral infection (HCV)149,150, and cancer145,146. These aberrant Tregs show canonical features of T cell exhaustion including impaired proliferative capacity151 and enhanced co-inhibitory receptor expression (PDCD1, HAVCR2, LAG3)145,147. In particular, the PD-1/PD-L1 pathway has an important role in the progressive development of Treg dysfunction. In patients with chronic HCV infection, a higher fraction of intrahepatic PD-1hi Tregs was correlated with lower viral burden and greater hepatic damage, consistent with reduced immune suppression150. In patients with glioblastoma multiforme (GBM), compared to Tregs in the peripheral blood, tumor-resident Tregs enriched for a transcriptional signature of exhaustion. Circulating PD-1hi Tregs also displayed reduced suppressive capacity, a transcriptional profile of exhaustion, and produced IFNγ; treatment with PD-1 blockade skewed circulating Tregs towards the exhausted IFNγ+ phenotype145. Mice with PD-1 deficient Tregs are protected from both EAE and T1D due to their enhanced suppressive capacity152. These studies indicate that PD-1 on Tregs acts to constrain their suppressive capacity and may promote a dysfunctional phenotype characterized by a transcriptional profile of exhaustion and an IFNγ+ Th1-like phenotype.
In summary, like CD8+ T cells, CD4+ T cell subsets also exhibit altered phenotypes including features of exhaustion across cancer, chronic infection, and autoimmunity. Further integration of our knowledge of CD8+ and CD4+ T cell dysfunction will allow us to better understand the complex mechanisms defining T cell states in both homeostasis and disease.
Funding
A.H.S. received funding from the National Institutes of Health, grants P01 AI56299, P01AI039671, and P01 AI108545. S.A.W. received funding from the National Institutes of Health, award T32GM007753.
Footnotes
Competing interests
A.H.S. has patents/pending royalties on the PD-1 pathway from Roche and Novartis. A.H.S. is on advisory boards for Surface Oncology, Elstar, SQZ Biotechnologies, Elpiscience, Selecta, Bicara and Monopteros, GlaxoSmithKline and Janssen, and consults for Novartis. A.H.S. has received research funding from Novartis, Roche, UCB, Ipsen, Quark, Merck and AbbVie unrelated to this project.
References
- 1.Yao C et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat. Immunol. 20, 890–901 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liblau RS, Wong FS, Mars LT & Santamaria P Autoreactive CD8 T cells in organ-specific autoimmunity: emerging targets for therapeutic intervention. Immunity 17, 1–6 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Sinha S, Boyden AW, Itani FR, Crawford MP & Karandikar NJ CD8(+) T-Cells as Immune Regulators of Multiple Sclerosis. Front. Immunol. 6, 619 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne KT & Turk MJ New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget 2, 684–694 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pugliese A Autoreactive T cells in type 1 diabetes. J. Clin. Invest. 127, 2881–2891 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wherry EJ T cell exhaustion. Nat. Immunol. 12, 492–499 (2011). [DOI] [PubMed] [Google Scholar]
- 7.McLane LM, Abdel-Hakeem MS & Wherry EJ CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 37, 457–495 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto M et al. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu. Rev. Med. 69, 301–318 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Scott AC et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mognol GP et al. Exhaustion-associated regulatory regions in CD8 tumor-infiltrating T cells. Proc. Natl. Acad. Sci. U. S. A. 114, E2776–E2785 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller BC et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utzschneider DT et al. High antigen levels induce an exhausted phenotype in a chronic infection without impairing T cell expansion and survival. J. Exp. Med. 213, 1819–1834 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelosanto JM, Blackburn SD, Crawford A & Wherry EJ Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86, 8161–8170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin H, Blackburn SD, Blattman JN & John Wherry E Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. Journal of Experimental Medicine vol. 204 941–949 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price DA et al. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity 21, 793–803 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Wieland D et al. TCF1+ hepatitis C virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat. Commun. 8, 15050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R & Ahmed R Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zajac AJ et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grayson JM, Weant AE, Holbrook BC & Hildeman D Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J. Virol. 80, 8627–8638 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath WR, Kurts C, Miller JF & Carbone FR Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J. Exp. Med. 187, 1549–1553 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redmond WL & Sherman LA Peripheral tolerance of CD8 T lymphocytes. Immunity 22, 275–284 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Wagner CA, Roqué PJ, Mileur TR, Liggitt D & Goverman JM Myelin-specific CD8+ T cells exacerbate brain inflammation in CNS autoimmunity. J. Clin. Invest. 130, 203–213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babbe H, Roers A, Waisman A & Lassmann H Clonal Expansions of Cd8+ T Cells Dominate the T Cell Infiltrate in Active Multiple Sclerosis Lesions as Shown by Micromanipulation and Single Cell Polymerase …. The Journal of (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skowera A et al. β-Cell–Specific CD8 T Cell Phenotype in Type 1 Diabetes Reflects Chronic Autoantigen Exposure. Diabetes 64, 916–925 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiLorenzo TP et al. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor α chain gene rearrangement. Proceedings of the National Academy of Sciences 95, 12538–12543 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittet MJ et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J. Exp. Med. 190, 705–715 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogg GS, Rod Dunbar P, Romero P, Chen JL & Cerundolo V High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J. Exp. Med. 188, 1203–1208 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richmond JM, Strassner JP & Zapata L Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Boorn JG et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J. Invest. Dermatol. 129, 2220–2232 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Le Gal F-A et al. Direct Evidence to Support the Role of Antigen-Specific CD8+ T Cells in Melanoma-Associated Vitiligo. J. Invest. Dermatol. 117, 1464–1470 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Han B et al. Developmental control of CD8+ T cell–avidity maturation in autoimmune diabetes. J. Clin. Invest. 115, 1879–1887 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amrani A et al. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406, 739–742 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Garyu JW et al. Characterization of Diabetogenic CD8+ T Cells: IMMUNE THERAPY WITH METABOLIC BLOCKADE. The Journal of biological chemistry vol. 291 11230–11240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller AM, Bahmanof M, Zehn D, Cohen EEW & Schoenberger SP Leveraging TCR Affinity in Adoptive Immunotherapy against Shared Tumor/Self-Antigens. Cancer Immunol Res 7, 40–49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forde PM et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 378, 1976–1986 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert L et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin. Cancer Res. 20, 2424–2432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tumeh PC et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yost KE et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 176, 775–789.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskophidis D, Lechner F, Pircher H & Zinkernagel RM Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362, 758–761 (1993). [DOI] [PubMed] [Google Scholar]
- 41.Gallimore A et al. Induction and Exhaustion of Lymphocytic Choriomeningitis Virus–specific Cytotoxic T Lymphocytes Visualized Using Soluble Tetrameric Major Histocompatibility Complex Class I–Peptide Complexes. J. Exp. Med. 187, 1383–1393 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry EJ et al. Molecular Signature of CD8 T Cell Exhaustion during Chronic Viral Infection. Immunity vol. 27 824 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Barber DL et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Blackburn SD et al. Coregulation of CD8 T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunology vol. 10 29–37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford A & Wherry EJ The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr. Opin. Immunol. 21, 179–186 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baitsch L et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gros A et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gubin MM et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fourcade J et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen–specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 207, 2175–2186 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Y, Li Y & Zhu B T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 6, e1792 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schietinger A et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 45, 389–401 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin X et al. Dramatic Rise in Plasma Viremia after CD8 T Cell Depletion in Simian Immunodeficiency Virus–infected Macaques. Journal of Experimental Medicine vol. 189 991–998 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitz JE et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Matloubian M, Concepcion RJ & Ahmed R CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galon J et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Pagès F et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29, 1093–1102 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Boniface K et al. Vitiligo Skin Is Imprinted with Resident Memory CD8 T Cells Expressing CXCR3. J. Invest. Dermatol. 138, 355–364 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Cheuk S et al. CD49a Expression Defines Tissue-Resident CD8 T Cells Poised for Cytotoxic Function in Human Skin. Immunity 46, 287–300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richmond JM et al. Resident Memory and Recirculating Memory T Cells Cooperate to Maintain Disease in a Mouse Model of Vitiligo. J. Invest. Dermatol. 139, 769–778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bender C, Rodriguez-Calvo T, Amirian N, Coppieters KT & von Herrath MG The healthy exocrine pancreas contains preproinsulin-specific CD8 T cells that attack islets in type 1 diabetes. Sci Adv 6, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Culina S et al. Islet-reactive CD8 T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Science Immunology vol. 3 eaao4013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jilek S et al. CSF enrichment of highly differentiated CD8+ T cells in early multiple sclerosis. Clin. Immunol. 123, 105–113 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Ifergan I et al. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on α4 integrin. Brain 134, 3560–3577 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malmeström C et al. Relapses in multiple sclerosis are associated with increased CD8+ T-cell mediated cytotoxicity in CSF. J. Neuroimmunol. 196, 159–165 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Malik BT et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Jersey J et al. β cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice. Proceedings of the National Academy of Sciences 104, 1295–1300 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Page N et al. Expression of the DNA-Binding Factor TOX Promotes the Encephalitogenic Potential of Microbe-Induced Autoreactive CD8+ T Cells. Immunity 50, 763 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Page N et al. Persistence of self-reactive CD8+ T cells in the CNS requires TOX-dependent chromatin remodeling. Nat. Commun. 12, 1009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiedeman AE et al. Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J. Clin. Invest. 130, 480–490 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zakharov PN, Hu H, Wan X & Unanue ER Single-cell RNA sequencing of murine islets shows high cellular complexity at all stages of autoimmune diabetes. J. Exp. Med. 217, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Penaranda C et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc. Natl. Acad. Sci. U. S. A. 109, 12668–12673 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee L-F et al. Anti–IL-7 receptor-α reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc. Natl. Acad. Sci. U. S. A. 109, 12674–12679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bobbala D et al. Interleukin-15 plays an essential role in the pathogenesis of autoimmune diabetes in the NOD mouse. Diabetologia 55, 3010–3020 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Saikali P, Antel JP, Pittet CL, Newcombe J & Arbour N Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J. Immunol. 185, 5693–5703 (2010). [DOI] [PubMed] [Google Scholar]
- 75.Kivisäkk P et al. IL-15 mRNA expression is up-regulated in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis (MS). Clin. Exp. Immunol. 111, 193 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKinney EF et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat. Med. 16, 586–91, 1p following 591 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McKinney EF, Lee JC, Jayne DRW, Lyons PA & Smith KGC T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Philip M & Schietinger A Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr. Opin. Immunol. 58, 98–103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Leun AM, Thommen DS & Schumacher TN CD8+ T cell states in human cancer: insights from single-cell analysis. Nat. Rev. Cancer 20, 218–232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beltra J-C et al. Developmental Relationships of Four Exhausted CD8+ T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity 52, 825–841.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kurtulus S et al. Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1-CD8+ Tumor-Infiltrating T Cells. Immunity 50, 181–194.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamauchi T et al. CX3CR1−CD8+ T cells are critical in antitumor efficacy but functionally suppressed in the tumor microenvironment. JCI Insight 5, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blackburn SD, Shin H, Freeman GJ & Wherry EJ Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 105, 15016–15021 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siddiqui I et al. Intratumoral Tcf1+PD-1+CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 50, 195–211.e10 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Im SJ et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou X et al. Differentiation and Persistence of Memory CD8+ T Cells Depend on T Cell Factor 1. Immunity 33, 229–240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeannet G et al. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl. Acad. Sci. U. S. A. 107, 9777–9782 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gattinoni L et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Utzschneider DT et al. T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity 45, 415–427 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Wu T et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galletti G et al. Two subsets of stem-like CD8+ memory T cell progenitors with distinct fate commitments in humans. Nat. Immunol. 21, 1552–1562 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alfei F et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Hudson WH et al. Proliferating Transitory T Cells with an Effector-like Transcriptional Signature Emerge from PD-1 Stem-like CD8 T Cells during Chronic Infection. Immunity 51, 1043–1058.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zander R et al. CD4+ T Cell Help Is Required for the Formation of a Cytolytic CD8+ T Cell Subset that Protects against Chronic Infection and Cancer. Immunity vol. 51 1028–1042.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vignali D et al. Detection and Characterization of CD8+ Autoreactive Memory Stem T Cells in Patients With Type 1 Diabetes. Diabetes 67, 936–945 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Abdelsamed HA et al. Beta cell-specific CD8+ T cells maintain stem cell memory-associated epigenetic programs during type 1 diabetes. Nat. Immunol. 21, 578–587 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hosokawa K et al. Memory Stem T Cells in Autoimmune Disease: High Frequency of Circulating CD8+ Memory Stem Cells in Acquired Aplastic Anemia. J. Immunol. 196, 1568–1578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Freeman GJ et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishimura H, Nose M, Hiai H, Minato N & Honjo T Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999). [DOI] [PubMed] [Google Scholar]
- 100.Probst HC, McCoy K, Okazaki T, Honjo T & van den Broek M Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 6, 280–286 (2005). [DOI] [PubMed] [Google Scholar]
- 101.Sharpe AH & Pauken KE The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Pauken KE, Jenkins MK, Azuma M & Fife BT PD-1, but Not PD-L1, Expressed by Islet-Reactive CD4+ T Cells Suppresses Infiltration of the Pancreas During Type 1 Diabetes. Diabetes 62, 2859–2869 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trabattoni D et al. Costimulatory pathways in multiple sclerosis: distinctive expression of PD-1 and PD-L1 in patients with different patterns of disease. J. Immunol. 183, 4984–4993 (2009). [DOI] [PubMed] [Google Scholar]
- 104.Pittet CL, Newcombe J, Antel JP & Arbour N The majority of infiltrating CD8 T lymphocytes in multiple sclerosis lesions is insensitive to enhanced PD-L1 levels on CNS cells. Glia 59, 841–856 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lucas JA et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J. Immunol. 181, 2513–2521 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J et al. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc. Natl. Acad. Sci. U. S. A. 102, 11823–11828 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keir ME, Freeman GJ & Sharpe AH PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179, 5064–5070 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Frebel H et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209, 2485–2499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Z et al. TCF-1-Centered Transcriptional Network Drives an Effector versus Exhausted CD8 T Cell-Fate Decision. Immunity 51, 840–855.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pauken KE et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Odorizzi PM, Pauken KE, Paley MA, Sharpe A & Wherry EJ Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pauken KE et al. The PD-1 Pathway Regulates Development and Function of Memory CD8+ T Cells following Respiratory Viral Infection. Cell Rep. 31, 107827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johnnidis JB et al. Inhibitory signaling sustains a distinct early memory CD8+ T cell precursor that is resistant to DNA damage. Sci Immunol 6, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Waldman AD, Fritz JM & Lenardo MJ A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larkin J et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 381, 1535–1546 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Sade-Feldman M et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175, 998–1013.e20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song M-Y et al. Protective effects of Fc-fused PD-L1 on two different animal models of colitis. Gut 64, 260–271 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Kim JH et al. Programmed cell death ligand 1 alleviates psoriatic inflammation by suppressing IL-17A production from programmed cell death 1–high T cells. J. Allergy Clin. Immunol. 137, 1466–1476.e3 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Hirata S, Senju S & Matsuyoshi H Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein …. The Journal of (2005). [DOI] [PubMed] [Google Scholar]
- 120.Ding H et al. Delivering PD-1 inhibitory signal concomitant with blocking ICOS co-stimulation suppresses lupus-like syndrome in autoimmune BXSB mice. Clin. Immunol. 118, 258–267 (2006). [DOI] [PubMed] [Google Scholar]
- 121.Raptopoulou AP et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis & Rheumatism 62, 1870–1880 (2010). [DOI] [PubMed] [Google Scholar]
- 122.Khan O et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571, 211–218 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X et al. TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma. J. Hepatol. 71, 731–741 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Sen DR et al. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jadhav RR et al. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc. Natl. Acad. Sci. U. S. A. 116, 14113–14118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ghoneim HE et al. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell 170, 142–157.e19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Youngblood B et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity 35, 400–412 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kusnadi A et al. Severely ill COVID-19 patients display impaired exhaustion features in SARS-CoV-2-reactive CD8+ T cells. Sci Immunol 6, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Christoffersson G, Chodaczek G, Ratliff SS, Coppieters K & von Herrath MG Suppression of diabetes by accumulation of non–islet-specific CD8+ effector T cells in pancreatic islets. Science Immunology 3, (2018). [DOI] [PubMed] [Google Scholar]
- 130.Simoni Y et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018). [DOI] [PubMed] [Google Scholar]
- 131.Scheper W et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 25, 89–94 (2019). [DOI] [PubMed] [Google Scholar]
- 132.Man K et al. Transcription Factor IRF4 Promotes CD8+ T Cell Exhaustion and Limits the Development of Memory-like T Cells during Chronic Infection. Immunity 47, 1129–1141.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Aubert RD et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 108, 21182–21187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kanev K et al. Proliferation-competent Tcf1+ CD8 T cells in dysfunctional populations are CD4 T cell help independent. Proc. Natl. Acad. Sci. U. S. A. 116, 20070–20076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahrends T et al. CD4+ T Cell Help Confers a Cytotoxic T Cell Effector Program Including Coinhibitory Receptor Downregulation and Increased Tissue Invasiveness. Immunity 47, 848–861.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Alspach E et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574, 696–701 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pitzalis C, Jones GW, Bombardieri M & Jones SA Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 14, 447–462 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Guo X et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 24, 978–985 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Crawford A et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hwang S, Cobb DA, Bhadra R, Youngblood B & Khan IA Blimp-1–mediated CD4 T cell exhaustion causes CD8 T cell dysfunction during chronic toxoplasmosis. Journal of Experimental Medicine vol. 213 1799–1818 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Han S, Asoyan A, Rabenstein H, Nakano N & Obst R Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc. Natl. Acad. Sci. U. S. A. 107, 20453–20458 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen P-M et al. Kidney tissue hypoxia dictates T cell-mediated injury in murine lupus nephritis. Sci. Transl. Med. 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shin B et al. Effector CD4 T cells with progenitor potential mediate chronic intestinal inflammation. J. Exp. Med. 215, 1803–1812 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nish SA et al. CD4+ T cell effector commitment coupled to self-renewal by asymmetric cell divisions. J. Exp. Med. 214, 39–47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lowther DE et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saito T et al. Two FOXP3 CD4 T cell subpopulations distinctly control the prognosis of colorectal cancers. Nature Medicine vol. 22 679–684 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Dominguez-Villar M, Baecher-Allan CM & Hafler DA Identification of T helper type 1–like, Foxp3 regulatory T cells in human autoimmune disease. Nature Medicine vol. 17 673–675 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McClymont SA et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 186, 3918–3926 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li S et al. Analysis of FOXP3+ regulatory T cells that display apparent viral antigen specificity during chronic hepatitis C virus infection. PLoS Pathog. 5, e1000707 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franceschini D et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J. Clin. Invest. 119, 551–564 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Carbone F et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 20, 69–74 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Tan CL et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J. Exp. Med. 218, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khattri R, Auger JA, Griffin MD, Sharpe AH & Bluestone JA Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J. Immunol. 162, 5784–5791 (1999). [PubMed] [Google Scholar]
- 154.Ansari MJI et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198, 63–69 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yoshida T, Jiang F, Honjo T & Okazaki T PD-1 deficiency reveals various tissue-specific autoimmunity by H-2b and dose-dependent requirement of H-2g7 for diabetes in NOD mice. Proc. Natl. Acad. Sci. U. S. A. 105, 3533–3538 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wei SC et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov. (2020) doi: 10.1158/2159-8290.CD-20-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int. Immunol. 22, 443–452 (2010). [DOI] [PubMed] [Google Scholar]
- 158.Xing P et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer 7, 341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boutros C et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016). [DOI] [PubMed] [Google Scholar]
- 160.Wei SC et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 170, 1120–1133.e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Das R et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J. Immunol. 194, 950–959 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.John S et al. Progressive hypoventilation due to mixed CD8 and CD4 lymphocytic polymyositis following tremelimumab - durvalumab treatment. J Immunother Cancer 5, 54 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jaber SH et al. Skin Reactions in a Subset of Patients With Stage IV Melanoma Treated With Anti–Cytotoxic T-Lymphocyte Antigen 4 Monoclonal Antibody as a Single Agent. Archives of Dermatology vol. 142 (2006). [DOI] [PubMed] [Google Scholar]
- 164.Hodi FS et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. U. S. A. 100, 4712–4717 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Johnson DB et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 375, 1749–1755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Coutzac C et al. Colon Immune-Related Adverse Events: Anti-CTLA-4 and Anti-PD-1 Blockade Induce Distinct Immunopathological Entities. J. Crohns. Colitis 11, 1238–1246 (2017). [DOI] [PubMed] [Google Scholar]
- 167.Luoma AM et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 182, 655–671.e22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Palermo B et al. Qualitative difference between the cytotoxic T lymphocyte responses to melanocyte antigens in melanoma and vitiligo. Eur. J. Immunol. 35, 3153–3162 (2005). [DOI] [PubMed] [Google Scholar]
- 169.Byrne KT et al. Autoimmune melanocyte destruction is required for robust CD8+ memory T cell responses to mouse melanoma. J. Clin. Invest. 121, 1797–1809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Becker JC, Guldberg P, Zeuthen J, Bröcker EB & Straten PT Accumulation of identical T cells in melanoma and vitiligo-like leukoderma. J. Invest. Dermatol. 113, 1033–1038 (1999). [DOI] [PubMed] [Google Scholar]
- 171.Dougan SK et al. Transnuclear TRP1-specific CD8 T cells with high or low affinity TCRs show equivalent antitumor activity. Cancer Immunol Res 1, 99–111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lo JA et al. Epitope spreading toward wild-type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci. Transl. Med. 13, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Attia P et al. Autoimmunity Correlates With Tumor Regression in Patients With Metastatic Melanoma Treated With Anti–Cytotoxic T-Lymphocyte Antigen-4. Journal of Clinical Oncology vol. 23 6043–6053 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dick J et al. Use of LDH and autoimmune side effects to predict response to ipilimumab treatment. Immunotherapy 8, 1033–1044 (2016). [DOI] [PubMed] [Google Scholar]
- 175.Dalmau J, Furneaux HM, Cordon-Cardo C & Posner JB The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am. J. Pathol. 141, 881–886 (1992). [PMC free article] [PubMed] [Google Scholar]
- 176.Rosenfeld MR, Eichen JG, Wade DF, Posner JB & Dalmau J Molecular and clinical diversity in paraneoplastic immunity to Ma proteins. Annals of Neurology vol. 50 339–348 (2001). [PubMed] [Google Scholar]
- 177.Gebauer C et al. CD4 and CD8 T cells are both needed to induce paraneoplastic neurological disease in a mouse model. OncoImmunology vol. 6 e1260212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hensel N et al. Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat. Immunol. 1–11 (2021). [DOI] [PubMed] [Google Scholar]
- 179.Rutishauser RL et al. TCF-1 regulates the stem-like memory potential of HIV-specific CD8+ T cells in elite controllers. Cold Spring Harbor Laboratory 2020.01.07.894535 (2020) doi: 10.1101/2020.01.07.894535. [DOI] [Google Scholar]
- 180.Krishna S et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 370, 1328–1334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brummelman J et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J. Exp. Med. 215, 2520–2535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jansen CS et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


