Abstract
Objectives:
Children receiving prolonged extracorporeal membrane oxygenation (ECMO) support may benefit from tracheostomy during ECMO by facilitating rehabilitation; however the procedure carries risks, especially hemorrhagic complications. Knowledge of tracheostomy practices and outcomes of ECMO-supported children who undergo tracheostomy on ECMO may inform decision-making.
Design:
Retrospective cohort study
Setting:
ECMO centers contributing to the Extracorporeal Life Support Organization (ELSO) Registry
Patients:
Children birth to 18 years who received ECMO support for 7 days or greater for respiratory failure from January 1st 2015 to December 31st 2019.
Interventions:
None
Measurements and Main Results:
3685 children received at least seven days of ECMO support for respiratory failure. The median duration of ECMO support was 13.0 days (IQR 9.3-19.9), and in-hospital mortality was 38.7% (1426/3685). A tracheostomy was placed during ECMO support in 94/3685 (2.6%). Of those who received a tracheostomy on ECMO, the procedure was performed at a median 13.2 days (IQR 6.3-25.9) after initiation of ECMO. Surgical site bleeding was documented in 26% of children who received a tracheostomy (12% after tracheostomy placement). Among children who received a tracheostomy, the median duration of ECMO support was 24.2 days (IQR 13.0-58.7); in-hospital mortality was 30/94 (32%). Those that received a tracheostomy before 14 days on ECMO were older (median age 15.8 years (IQR 4.7-15.5) versus 11.7 years (IQR 11.5-17.3); p-value=0.002) and more likely to have been supported on VV-ECMO (84% vs 52%, p=0.001). Twenty-two percent (11/50) of those who received a tracheostomy before 14 days died in the hospital, compared to 19/44 (43%) of those who received a tracheostomy at 14 days or later (p=0.03).
Conclusions:
Tracheostomies during ECMO were uncommon in children. One in four patients who received a tracheostomy on ECMO had surgical site bleeding. Children who had tracheostomies placed after 14 days were younger and had worse outcomes, potentially representing tracheostomy as a “secondary” strategy for prolonged ECMO support.
Keywords: Tracheostomy; Extracorporeal Membrane Oxygenation; Respiratory Distress Syndrome, Acute; Respiratory Insufficiency; Critical Care Outcomes; Health Services Research
Introduction
The decision to place a tracheostomy in a child with respiratory failure is difficult for both parents and health care teams (1–3). For parents, tracheostomy decisions can be complicated by conflict and regret (4). For healthcare teams, clinical uncertainty and lack of outcome data can lead to variation in tracheostomy recommendations and practices (3). Over 3000 children are supported by Extracorporeal Membrane Oxygenation (ECMO) each year, and half of these cases are for respiratory support (5). In these children with severe lung injury, desicion-making around tracheostomy can be even more difficult. Few studies have evaluated tracheostomy practices or outcomes among ECMO-supported children with respiratory failure, so there is little evidence to inform these discussions.
Proposed benefits to tracheostomy include reduced sedation, improved mobility, and reduced healthcare utilization (6–10). However, these benefits need to be balanced with the risks, including bleeding, airway injuries, and the need for future airway operations (11). Studies evaluating tracheostomy practices in children requiring ECMO support are limited (12, 13). There are currently no consensus recommendations for tracheostomy placement in pediatric patients with respiratory failure. The ELSO registry can facilitate the characterization of tracheostomy practices in children who are supported with ECMO across the over 450 ELSO centers around the world (14). Our aim is to describe the frequency and timing of tracheostomy placement in neonatal and pediatric patients receiving ECMO support for at least seven days. We also aimed to describe the clinical characteristics and clinical outcomes in children who receive a tracheostomy, including bleeding complications.
Methods
Data Source and Population
The ELSO Registry contains clinical and outcome data collected by trained data managers with error and validity checks and full-record validation to ensure completeness (15). This cohort study of anonymized data from the ELSO Registry was determined to be exempt from human subjects review by the University of Michigan Institutional Review Board (HUM00195595).
Patients in the ELSO Registry aged less than 18 years with a primary support indication of respiratory failure from January 1st 2015 to December 31st 2019 were included. We excluded children who were liberated from ECMO support or died before seven days of ECMO support as we considered these children less likely to be considered for a tracheostomy during ECMO. We identified neonates as children aged 28 days or less.
Exposure
Our primary exposure was whether a patient had a tracheostomy procedure on ECMO. Data managers recorded whether patients had a tracheostomy before ECMO cannulation. The ELSO registry advises data managers to enter procedure codes if a procedure was performed during ECMO or directly related to ECMO. Procedure codes were extracted for Current Procedural Terminology (CPT) codes for tracheostomy procedures (31600, 31601, 31603, 31605, 31610). When CPT codes were present, we reported time from ECMO initiation. Patients were classified as having a pre-existing tracheostomy if a procedure was recorded before ECMO initiation. No timing of tracheostomy was available if a procedure code was not available. In the calendar years of 2018 and 2019, data managers also reported whether a patient was extubated, with an option of “N/A Tracheostomy.” For patients on ECMO in these years, we report whether a patient ever had a tracheostomy if they (1) had a pre-existing tracheostomy, (2) had a procedure code for a tracheostomy during ECLS, or (3) a data manager indicated tracheostomy in the extubation field. A priori, we chose to evaluate tracheostomies that were performed before and after 14 days, as 10-14 days is often a cutpoint for “early” vs “late” studies in adults and 14 days was median time to tracheostomy in one pediatric study (16–22).
Outcomes
The primary outcomes were hospital mortality and duration of ECMO support. We also report the hospital length of stay, the incidence of relevant complications during ECMO such as surgical site bleeding, mechanical complications, and neurologic complications. Surgical site bleeding was defined as requiring packed red blood cell transfusion ≥20ml/kg in 24 hours or ≥ three adult units in 24 hours. Mechanical complications included oxygenator failure, pump failure, raceway/tubing rupture, circuit change, cannula problems, heat exchanger malfunction, clots and air emboli. Neurologic complications included brain death, seizures, central nervous system (CNS) ischemia/infarction, hemorrhage, or neurosurgical procedures. Records from 2018 and 2019 contain detail about patient mobilization for children aged 8 years or older. Among runs from the years 2018 and 2019, we reported the proportion of patients who were mobilized on ECMO to a level of “sitting in bed, exercises in bed” or higher.
Statistical Analysis
Our primary analysis compared those that were recorded as having a tracheostomy placed on ECMO vs all others (pre-existing tracheostomy, never received a tracheostomy, tracheostomy during the hospitalization but not associated with the ECMO run). Descriptive statistics were provided as median and interquartile ranges for continuous variables and as count and proportion for categorical variables. Univariable comparisons were conducted via Wilcoxon rank-sum test for continuous variables or Chi-square test for categorical variables. Two-sided p-value is reported for the incidence rate difference for complication rates. Analyses were completed in Stata (16.1, StataCorp LLC, College Station, TX).
Results
Patient Characteristics
We identified 3685 children who were supported on ECMO for at least 7 days for respiratory failure from 2015-2019, 1792 (48.6%) of whom were neonates. In this cohort, in-hospital mortality was 38.7% (1426/3685) and the median duration of ECMO support was 13.0 days (IQR 9.3-19.9). Of those discharged alive, the median duration of ECMO support was 11.5 days (IQR 8.8-16.8) and the hospital length of stay was 54 days (IQR 33-91).
Tracheostomy Practices
94 (2.6%) of children received a tracheostomy while on ECMO support. Over time, tracheostomies during ECMO became slightly more commonly used. In 2015, 1.7% of patients received a tracheostomy, 1.4% in 2016, 2.7% in 2017, 3.5% in 2018, and 3.4% in 2019 (p=0.03).
In the years 2018-2019 during which data managers were asked to record if a patient received a tracheostomy instead of extubation, 1.4% (22/1562) children had a pre-existing tracheostomy and 3.4% (53/1562) received a tracheostomy on ECMO. A tracheostomy was reported as present for 12.8% (200/1562) of children at any point during their hospitalization (the timing of tracheostomy placement was not available if a tracheostomy procedure code was not recorded).
Among ECMO-supported neonates, only one patient received a tracheostomy while on ECMO support. The overall prevalence of tracheostomy among neonates during the hospitalization was 42/661 (6.0%) during 2018/2019. Among children over 28 days of age, a pre-existing tracheostomy was present among 37 (2.0%), and 93 (4.9%) received a tracheostomy while on ECMO. Notably, overall prevalence of tracheostomy during the hospitalization was 158/701 (18.4%) children aged 29 days or older in 2018/2019.
When comparing children who received a tracheostomy on ECMO to all others (including those who died, received a tracheostomy at a unknown time, or never received a tracheostomy), there were several differences (Table 1). Among children outside of the neonatal period, the median age of those who received a tracheostomy was 14.7 years (IQR 8.5-17.1) versus 1.7 years (IQR 0.5-8.1) for all others; p-value<0.001). The duration of ventilation before ECMO cannulation was similar between the two cohorts — median 2 days (IQR 1-5) in those that did not receive a tracheostomy on ECMO vs 2 days (IQR 0-6) in those that did (p=0.67). They were also more likely to be supported on VV-ECMO (56% vs 69%; p-value=0.008).
Table 1.
Characteristics of total cohort of patients who were supported on ECMO ≥7 days and those that received a tracheostomy while on ECMO
| Characteristics | Did not receive tracheostomy on ECMOa | Tracheostomy on ECMO | p-value |
|---|---|---|---|
| N=3591 | n=94 | ||
| Age cohort | <0.001 | ||
| Neonatal, n (%) | 1791 (50%) | 1 (1%) | |
| Pediatric, n (%) | 1800 (50%) | 93 (99%) | |
| Age (years), median (IQR) | 1.7 (0.5-8.1) | 14.7 (8.5-17.1) | <0.001 |
| Sex, n (%) | 0.84 | ||
| Male | 1901 (53%) | 52 (55%) | |
| Female | 1604 (45%) | 42 (45%) | |
| Days of Ventilation before ECMO, median (IQR) | 2 (1-5) | 2 (0-6) | 0.67 |
| Mode, n (%) | <0.001 | ||
| VV | 1275 (36%) | 65 (69%) | |
| VA | 2055 (56%) | 6 (6%) | |
| Conversion | 207 (6%) | 22 (23%) | |
| Other/unknown | 54(2%) | 1 (1%) |
Includes those with pre-existing tracheostomy, those who received a tracheostomy after ECMO run or at an unknown time
VV- Veno-venous VA-Veno-arterial
Outcomes after Tracheostomy
Among the 94 children who received a tracheostomy during ECMO, in-hospital mortality was 32%, similar to those that did not receive a tracheostomy (39%, p=0.17) (Table 2). In 2018 and 2019, mobilization to a level of sitting in bed or higher was achieved in more children who received a tracheostomy: 22% (44/196) of children age 8 years or above who did not receive a tracheostomy on ECMO and 44% (16/36) of those who received a tracheostomy on ECMO (p=0.006). In those who received a tracheostomy, the median duration of ECMO support was 31.0 days (IQR 15-60.1) and among survivors was 24.2 days (IQR 13.0-55.6). After receiving a tracheostomy, the median additional duration of ECMO support was 13.7 days (IQR 6.1-30.9) and among survivors was 11.9 days (IQR 5.4 -24.5).
Table 2:
Clinical Outcomes of all children on ECMO for ≥7 days for respiratory failure and those that received a tracheostomy on ECMO
| Characteristics | Did not receive tracheostomy on ECMOa | ECMO Trach | Pb |
|---|---|---|---|
| (n=3591) | (n=94) | ||
| Hospital Mortality, n (%) | 1426 (39%) | 30 (32%) | 0.17 |
| Surgical Site Bleeding | |||
| Total run, n (%) | 369 (10%) | 24 (26%) | <0.001 |
| Pre-tracheostomy, n (%) | -- | 13 (14%) | |
| Neurologic Complication | |||
| Total run, n (%) | 359 (10%) | 5 (5%) | 0.16 |
| Pre-tracheostomy, n (%) | -- | 4 (4%) | |
| Rate/1000hrs | 0.38 | 0.06 | <0.001 |
| Mechanical Complication | |||
| Total run, n (%) | 1890 (51%) | 63 (67%) | 0.002 |
| Pre-tracheostomy, n (%) | -- | 47 (50%) | |
| Rate/1000hrs | 3.1 | 2.8 | 0.12 |
| Mobilized on ECMO c , n (%) | 44/196 (22%) | 16/36 (44%) | 0.006 |
| ECMO duration in survivors, median (IQR) | 11.5 (8.8-16.8) | 24.2 (13.0-58.7) | <0.001 |
| Length of Stay in survivors, median (IQR) | 54 (33-91) | 62 (43-110) | 0.06 |
Includes those with pre-existing tracheostomy, those who received a tracheostomy after ECMO run or at an unknown time
p-value determined by Wilcoxon rank-sum for continuous variables, chi-squared for categorical variables, incidence rate difference for rates
Only reported in those age 8yrs and older in 2018 and 2019
Complications
Among the 94 patients who received a tracheostomy on ECMO, 24/94 (26%) experienced a bleeding complication. In those that did not receive a tracheostomy on ECMO, 369/3591 (10%) of patients experienced surgical site bleeding. We are unable to determine whether the surgical site bleeding reported is at the site of the tracheostomy, but 13/94 (14%) of the patients with tracheostomy had a report of surgical site bleeding before tracheostomy placement and 12/94 (12%) had surgical site bleeding after tracheostomy. There were similar rates of neurologic complications in those that did not receive a tracheostomy compared to those that did (359/3591 (10%) versus 5/94 (5%), respectively; p-value=0.16). Mechanical complications were more common among children who received a tracheostomy (63/94 (67%) vs 1890/3591 (51%); p<0.01) with many (47/94 of those with a tracheostomy) occurring before tracheostomy placement.
Timing of Tracheostomy
Of those who received a tracheostomy on ECMO, the procedure was performed at a median of 13.2 days (IQR 6.3-25.9) after ECMO initiation. Tracheostomies were performed on the day of ECMO cannulation through day 171 of ECMO support (Figure 1). When a tracheostomy was performed during ECMO, 50/94 (53%) were performed less than 14 days after ECMO cannulation. Those that received a tracheostomy before 14 days were older (median age 15.8 years (IQR 4.7-15.5) versus 11.7 years (IQR 11.5-17.3); p-value=0.002) and more likely to be supported on VV-ECMO (84% vs 52%, p=0.001). The duration of ventilation before ECMO cannulation was similar between the two cohorts — median 2 days (IQR 0-7) in those that received a tracheostomy before 14 days vs 3 days (IQR 0-5) in those that received a tracheostomy on or after 14 days (p=0.77). Twenty-two percent (11/50) of those who received a tracheostomy before 14 days died in the hospital, compared to 19/44 (43%) of those who received a tracheostomy at 14 days or later (p-value=0.03). Those who received a tracheostomy before 14 days had shorter ECMO duration (median 16 days vs 56 days, p-value<0.001) (Table 3).
Figure 1:
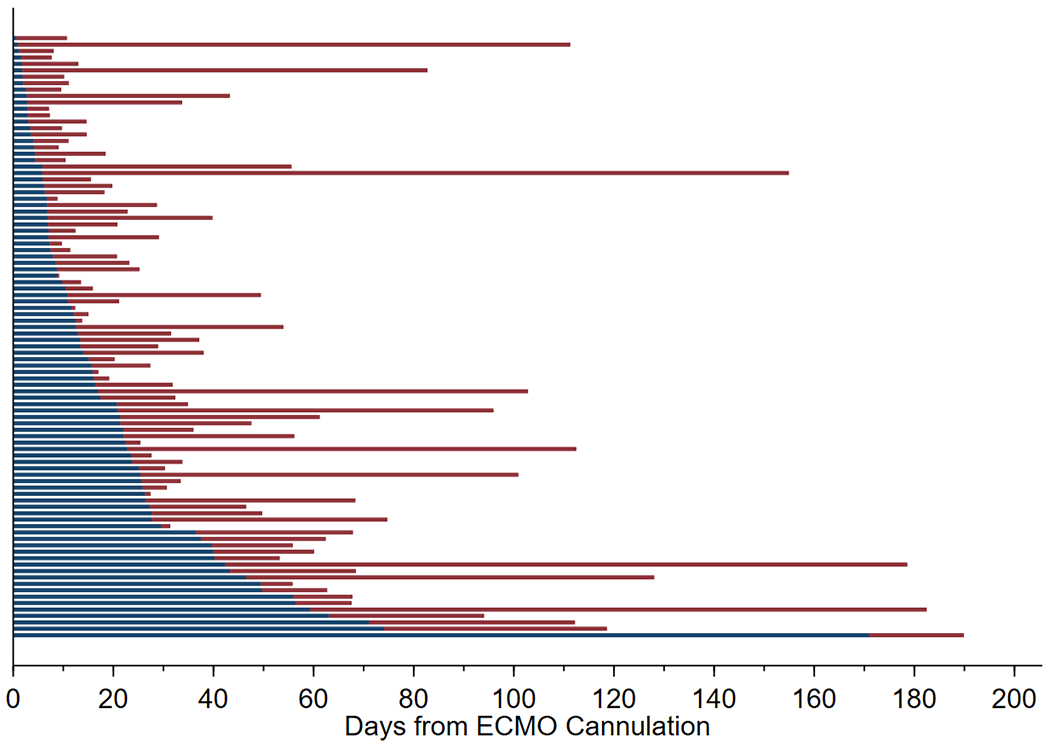
Timing of Tracheostomy placement among patients who received a tracheostomy on ECMO: The ECMO duration of every patient who received a tracheostomy on ECMO is represented by a bar. Blue represents time before tracheostomy placement and red represents ECMO time after tracheostomy placement.
Table 3:
Clinical Characteristics and Outcomes of Children who Received a Tracheostomy on ECMO before and after 14 days
| Characteristics | Trachestomy <14 days | Trachestomy ≥14 days | pa |
|---|---|---|---|
| n=50 | n=44 | ||
| Age cohort | |||
| Neonatal, n (%) | 0 (0%) | 1 (2%) | |
| Pediatric, n (%) | 50 (100%) | 43 (98%) | |
| Age (years), median (IQR) | 16 (12-17) | 12 (5-16) | 0.002 |
| Sex, n (%) | |||
| Female | 18 (36%) | 24 (55%) | 0.07 |
| Male | 32 (64%) | 20 (45%) | |
| Mode, n (%) | 0.01 | ||
| VV | 42 (84%) | 23 (52%) | |
| VA | 2 (4%) | 4 (9%) | |
| Conversion | 6 (12%) | 16 (36%) | |
| Other | 0 (0%) | 1 (2%) | |
| Days of Ventilation before ECMO, median (IQR) | 2 (0-7) | 3 (0-5) | 0.77 |
| Surgical site bleeding, n (%) | 7 (14%) | 17 (39%) | 0.006 |
| ECMO days, median (IQR) | 16 (11-29) | 56 (32-84) | <0.001 |
| ECMO days after tracheostomy, median (IQR) | 11 (5-22) | 18 (9-40) | 0.04 |
| Length of stay, median (IQR) | 49 (29-62) | 80 (56-119) | <0.001 |
| Mobilized on ECMOb , n (%) | 7/24 (29%) | 9/12 (75%) | 0.01 |
| Hospital Mortality, n (%) | 11 (22%) | 19 (43%) | 0.03 |
p-value determined by Wilcoxon rank-sum for continuous variables and chi-squared for categorical variables
Only reported in those age 8yrs and older in 2018 and 2019
VV- Veno-venous VA-Veno-arterial
Discussion
In this analysis of the ELSO Registry, we identified 3685 children who had an ECMO run of at least seven days over five years. During that time, there was a trend towards more patients receiving a tracheostomy during ECMO (1.7% in 2015 to 3.4% in 2019), but overall the practice remains uncommon. Those who received a tracheostomy were older and more likely to be supported by VV ECMO. Among ECMO-supported children older than 28 days, 18% of children had a tracheostomy at some point during the respiratory ECMO hospitalization in 2018/2019.
Tracheostomy placement has the potential to improve outcomes by allowing reduced sedation and facilitating rehabilitation (6, 23). In our study, 44% of patients age 8 and older who received a tracheostomy were mobilized to a level of sitting in bed or higher. In an analysis of the Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) study, children with respiratory failure on ECMO were often deeply sedated and receiving neuromuscular blockade (24). These children subsequently experienced higher rates of withdrawal (24). An “awake” ECMO strategy that prioritizes rehabilitation through extubation or tracheostomy to minimize sedation has been proposed as an option in some patients with prolonged respiratory failure (25–27). While more patients who received a tracheostomy in this cohort were mobilized, it remains unknown whether this mobilization was enhanced by tracheostomy placement and whether it improves longer-term patient-centered outcomes after ECMO.
12% of children who received a tracheostomy had surgical site bleeding after tracheostomy placement, however we are unable to determine if this bleeding is at the tracheostomy site. Of note, the incidence of surgical site bleeding in those that did not receive a tracheostomy was 10% and 14% of children who received a tracheostomy had surgical site bleeding reported before tracheostomy placement. The risks of placing a tracheostomy on ECMO in a child may be different than in adult patients, in whom the practice is more common. In adults and adult-sized children, tracheostomy placement can involve holding anticoagulation surrounding the procedure and percutaneous dilational tracheostomy to minimize bleeding risks (28, 29). In small children however, lower blood flow rates and greater use of VA ECMO for respiratory failure increases the risk for circuit clotting when withholding anticoagulation. Similarly, although there are reports of percutaneous tracheostomy placement in children as young as one month of age, pediatric tracheostomies are rarely performed percutaneously and are more often surgical tracheostomies (21, 30). Of note, patients who had a tracheostomy placed on ECMO had higher rates of mechanical complications than those that did not, however rates normalized by duration of ECMO support were not statistically different.
The placement of a tracheostomy in a patient on ECMO for respiratory support is more common in adults (31). In adults with respiratory failure, early tracheostomy (<7-10 days of mechanical ventilation) has been associated with lower mortality, less risk of ventilator-associated pneumonia, reduced length of mechanical ventilation, and shorter ICU stay (32, 33). In a meta-analysis of retrospective studies of tracheostomy timing in pediatric patients with critical illness, early tracheostomy was associated with fewer days of mechanical ventilation, shorter length of stay, and lower mortality (8). Despite this, pediatric physicians are less likely to recommend tracheostomy in the setting of prolonged mechanical ventilation (34). There were notable differences between patients that received a tracheostomy on ECMO before 14 days and after 14 days from ECMO initiation. The patients who received an early tracheostomy may represent a management strategy of early mobilization and an “awake ECMO” strategy (27, 35). This is supported by the fact that children who received a tracheostomy before 14 days were older (median age 16 vs 12 years) and more likely to be supported on VV ECMO. Children who received a tracheostomy after 14 days were more likely to be converted another mode of ECMO, to have a bleeding complication, and to experience a longer ECMO run. This “late” group could represent patients where tracheostomy was used as a secondary strategy for prolonged ECMO support rather than a primary “early” tracheostomy strategy. Of note, hospital mortality was higher in those that received a tracheostomy after 14 days (43% in the “late” cohort vs 22% in the “early” cohort, p=0.03). These differing approaches to tracheostomy placement may have important implications when determing the risk and benefits of the procedure and should be considered in future studies of tracheostomy placement in children on ECMO.
There are several limitations to this study. We cannot determine the procedural details of each tracheostomy, so it is not possible to evaluate whether a tracheostomy was placed surgically or percutaneously. Bleeding complications are not linked to a specific procedure in the ELSO registry, so we are unable to determine whether surgical site bleeding was secondary to tracheostomy or another surgical procedure. We are also unable to determine length of ventilation and whether patients who received a tracheostomy were liberated from a ventilator before discharge.
Conclusions
Tracheostomies on ECMO for respiratory failure remain rare in children— 2.6% of children on ECMO for ≥ 7 days for respiratory failure received a tracheostomy on ECMO. Further research should focus on identifying patients that may benefit from tracheostomy while on ECMO and surgical approaches to the procedure that can minimize complications.
Research in Context:
There is considerable uncertainty surrounding the decision to place a tracheostomy in a ECMO supported child with respiratory failure. Although tracheostomies are commonly placed in adult supported by ECMO, there is little evidence to support the practice in children.
Potential risks and benefits of a tracheostomy during ECMO include the potential benefit of imporved mobilization and reduce sedative requirements, but it also carres the risk of bleeding with ongoing systemic anticoagulation.
We found that tracheostomies are rarely performed in children during ECMO support. Those that received a tracheostomy were older, more likely to be on VV ECMO support, and more often mobilized.
At the bedside:
Tracheostomies during ECMO support were rarely performed (2.6% in children on ECMO support for ≥7 days).
Those who received a tracheostomy were older and more likely to be supported by venovenous (VV) ECMO.
12% of children who received a tracheostomy had surgical site bleeding after placement, although this study cannot directly attribute bleeding to the tracheostomy placement.
Conflict of Interest:
RPB reports grants from National Institutes of Health (R01 HL153519-ASCEND) outside the submitted work; and I am a member of the Extracorporeal Life Support Organization (ELSO) Steering Committee as the ELSO Registry Chair. GM is a member of the Board of Directors of ELSO
Copyright Form Disclosure:
Dr. MacLaren disclosed that he serves on the Board of Directors of the Extracorporeal Life Support Organization (ELSO). Dr. Friedman disclosed the off-label product use of ECMO. Dr. Barbabaro’s institution received funding from ARDS in Children and ECMO Initiation Strategies Impact on Neuro-Development (ASCEND), the National Heart, Lung, and Blood Institute, the National Institutes of Health (NIH) (R01 HL153519), and the Pediatric Implantable Artificial Lung from the National Institute of Child Health and Human Development (R01 HD015434); he disclosed that he is the ELSO Registry Chair; he received support for article research from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Nageswaran S, Golden SL, Gower WA, King NMP: Caregiver Perceptions about their Decision to Pursue Tracheostomy for Children with Medical Complexity. J Pediatr 2018; 203:354–360.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert LM, Watson AC, Madrigal V, October TW: Discussing Benefits and Risks of Tracheostomy: What Physicians Actually Say. Pediatr Crit Care Med 2017; 18:e592–e597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gower WA, Golden SL, King NMP, Nageswaran S: Decision-Making About Tracheostomy for Children With Medical Complexity: Caregiver and Health Care Provider Perspectives. Acad Pediatr 2020; 20:1094–1100 [DOI] [PubMed] [Google Scholar]
- 4.October TW, Jones AH, Michals HG, et al. : Parental conflict, regret, and short-term impact on quality of life in tracheostomy decision-making. Pediatr Crit Care Med 2020; 21:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaro RP, Paden ML, Guner YS, et al. : Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017; 63:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cagino LM, Kercheval JB, Kenes MT, et al. : Association of Tracheostomy with Changes in Sedation During COVID-19: A Quality Improvement Evaluation at the University of Michigan. Ann Am Thorac Soc. 10.1513/annalsats.202009-1096rl [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holloway AJ, Spaeder MC, Basu S: Association of Timing of Tracheostomy on Clinical Outcomes in PICU Patients. Pediatr Crit Care Med 2015; 16:e52–e58 [DOI] [PubMed] [Google Scholar]
- 8.Alkhatip AAAMM, Younis M, Jamshidi N, et al. : Timing of tracheostomy in pediatric patients: A systematic review and meta-analysis. Crit Care Med 2020; 233–240 [DOI] [PubMed] [Google Scholar]
- 9.Andriolo BN, Andriolo RB, Saconato H, et al. : Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev 2015; 2017: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiChiacchio L, Boulos FM, Brigante F, et al. : Early tracheostomy after initiation of venovenous extracorporeal membrane oxygenation is associated with decreased duration of extracorporeal membrane oxygenation support. Perfusion 2020; 35:509–514 [DOI] [PubMed] [Google Scholar]
- 11.Watters K, O’Neill M, Zhu H, et al. : Two-year mortality, complications, and healthcare use in children with medicaid following tracheostomy. Laryngoscope 2016; 126:2611–2617 [DOI] [PubMed] [Google Scholar]
- 12.Tripathi S, Swayampakula AK, Deshpande GG, et al. : Illustration of the current practice and outcome comparison of early versus late tracheostomy after pediatric ECMO. Int J Artif Organs 2020; 43:726–734 [DOI] [PubMed] [Google Scholar]
- 13.Mallory PP, Barbaro RP, Bembea MM, et al. : Tracheostomy and Long-Term Mechanical Ventilation in Children after Veno-Venous Extracorporeal Membrane Oxygenation. 10.1002/ppul.25546 [DOI] [PubMed]
- 14.Extracorporeal Life Support Organization - ECMO and ECLS > Registry > Statistics. https://www.elso.org/Registry/Statistics.aspx. Accessed 9 Aug 2021
- 15.Lorusso R, Alexander P, Rycus P, Barbaro R: The Extracorporeal Life Support Organization Registry: update and perspectives. Ann Cardiothorac Surg 2019; 8:938–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandian V, Murgu S, Lamb CR: COUNTERPOINT: Tracheostomy in Patients With COVID-19: Should We Do It Before 14 Days? No. Chest 2021; 159:1727–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung NH, Napolitano LM: Tracheostomy : Epidemiology , Indications , Timing , Technique , and Outcomes. 2014; 895–919 [DOI] [PubMed] [Google Scholar]
- 18.Miles BA, Schiff B, Ganly I, et al. : Tracheostomy during SARS-CoV-2 pandemic: Recommendations from the New York Head and Neck Society. Head Neck 2020; 42:1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner MJ, Feller-Kopman D, De Cardenas J: POINT: Tracheostomy in Patients With COVID-19: Should We Do It Before 14 Days? Yes. Chest 2021; 159:1723–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young D, Harrison D a, Cuthbertson BH, et al. : Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA 2013; 309:2121–9 [DOI] [PubMed] [Google Scholar]
- 21.Watters KF: Tracheostomy in Infants and Children. Respir Care 2017; 62:799–825 [DOI] [PubMed] [Google Scholar]
- 22.Wakeham MK, Kuhn EM, Lee KJ, et al. : Use of tracheostomy in the PICU among patients requiring prolonged mechanical ventilation. Intensive Care Med 2014; 40:863–870 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz SP, Bonadonna D, Hartwig MG, Cheifetz IM: Bedside tracheostomy on pediatric icu subjects supported by extracorporeal membrane oxygenation. Respir Care 2017; 62:1447–1455 [DOI] [PubMed] [Google Scholar]
- 24.Schneider JB, Sweberg T, Asaro LA, et al. : Sedation Management in Children Supported on Extracorporeal Membrane Oxygenation for Acute Respiratory Failure. Crit Care Med 2017; 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swol J, Strauch JT, Schildhauer TA: Tracheostomy as a bridge to spontaneous breathing and awake-ECMO in non-transplant surgical patients. Eur J Heart Fail 2017; 19:120–123 [DOI] [PubMed] [Google Scholar]
- 26.Costa J, Dirnberger DR, Froehlich CD, et al. : Awake Neonatal Extracorporeal Membrane Oxygenation. ASAIO J 2020; E70–E73 [DOI] [PubMed] [Google Scholar]
- 27.Langer T, Santini A, Bottino N, et al. : “Awake” extracorporeal membrane oxygenation (ECMO): Pathophysiology, technical considerations, and clinical pioneering. Crit Care 2016; 20:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grewal J, Sutt A-L, Shekar K, Fraser JF: Steps to Enhance Safety of Tracheostomy on ECMO. J Intensive Care Med 2019; 088506661985107 [DOI] [PubMed] [Google Scholar]
- 29.Harris DD, Shafii AE, Baz M, et al. : Increased blood transfusion and its impact in patients having tracheostomy while on extracorporeal membrane oxygenation. Perfus (United Kingdom) 2019; 34:143–146 [DOI] [PubMed] [Google Scholar]
- 30.Gollu G, Ates U, Can OS, et al. : Percutaneous tracheostomy by Griggs technique under rigid bronchoscopic guidance is safe and feasible in children. J Pediatr Surg 2016; 51:1635–1639 [DOI] [PubMed] [Google Scholar]
- 31.Barbaro RP, MacLaren G, Boonstra PS, et al. : Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020; 396:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chorath K, Hoang A, Rajasekaran K, Moreira A: Association of Early vs Late Tracheostomy Placement with Pneumonia and Ventilator Days in Critically Ill Patients: A Meta-analysis. JAMA Otolaryngol - Head Neck Surg 2021; 78229:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andriolo BN, Andriolo RB, Saconato H, et al. : Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 10.1002/14651858.CD007271.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer-Macaulay CB, Dayre McNally J, O’Hearn K, et al. : Factors Impacting Physician Recommendation for Tracheostomy Placement in Pediatric Prolonged Mechanical Ventilation: A Cross-Sectional Survey on Stated Practice. Pediatr Crit Care Med 2019; 20:e423–e431 [DOI] [PubMed] [Google Scholar]
- 35.Abrams D, Javidfar J, Farrand E, et al. : Early mobilization of patients receiving extracorporeal membrane oxygenation: A retrospective cohort study. Crit Care 2014; 18:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


