Abstract
Recombinant Staphylococcus xylosus and Staphylococcus carnosus strains were generated with surface-exposed chimeric proteins containing polyhistidyl peptides designed for binding to divalent metal ions. Surface accessibility of the chimeric surface proteins was demonstrated and the chimeric surface proteins were found to be functional in terms of metal binding, since the recombinant staphylococcal cells were shown to have gained Ni2+- and Cd2+-binding capacity, suggesting that such bacteria could find use in bioremediation of heavy metals. This is, to our knowledge, the first time that recombinant, surface-exposed metal-binding peptides have been expressed on gram-positive bacteria. Potential environmental or biosensor applications for such recombinant staphylococci as biosorbents are discussed.
A rapidly emerging research field involves bacterial surface expression of metal-binding peptides (16, 17, 36, 37, 40) for potential generation of novel biosorbents for removal of toxic metals from wastewater. Bacterial sequestration of toxic metals has previously been investigated using nonengineered bacteria (23), but recombinant DNA technology offers the possibility of improving the metal binding capacity of the bacteria. Such engineered bacteria have in fact been evaluated for removal of Cd2+ from actual factory wastewater (2). Periplasmic expression of a Neurospora crassa metallothionein in Escherichia coli generated cells that were superior to bacteria with cytoplasmic metallothionein localization in terms of metal ion adsorption (29). Surface expression on E. coli cells of yeast or mammalian metallothioneins resulted in recombinant bacteria with increased ability to bind Cd2+ ions (37). Surface expression of hexahistidyl peptides by genetic insertion into the outer membrane protein LamB generated recombinant E. coli cells with improved metalloadsorption capacity (36). Histidine clusters have also been expressed in fimbrial proteins (33), generating bacteria with improved metal binding. Other metal-binding peptides have been expressed in the periplasm (30) or at the cell surface (16, 17, 33) of E. coli, yielding bacteria with enhanced capacity to bind to divalent metal ions.
Attempts to create recombinant bacteria with improved metal-binding capacity have so far been restricted to E. coli. Gram-positive surface display systems have been suggested to exhibit some advantages compared to gram-negative bacteria (21, 38): (i) translocation through only one membrane is required, and (ii) gram-positive bacteria have been shown to be more rigid and therefore less sensitive to shear forces (14, 27) due to the thick cell wall surrounding the cells, and thus they are potentially more suitable for field applications such as bioadsorption. For metal adsorption applications, gram-positive bacteria have the additional advantage of having inherent metal-binding capacity due to the thick peptidoglycan layer (23). Two gram-positive bacteria which have been investigated extensively for various surface display applications are the nonpathogenic bacteria Staphylococcus xylosus and Staphylococcus carnosus (12, 24, 31, 32), which are both used traditionally as starter cultures in meat fermentation applications (11, 18). These staphylococci have been evaluated as live bacterial vaccine delivery vehicles, having foreign antigenic determinants of bacterial, viral, or protozoan origin displayed on their surface (38), but also for the display of single-chain Fv antibody fragments (9) with potential diagnostic applications. Moreover, the two staphylococcal species, and in particular S. carnosus, have been described to have very low extracellular proteolytic activity (6), which should be beneficial since heterologously expressed surface proteins could thereby be stable for extended times postexpression. Here, we have evaluated the S. xylosus and S. carnosus surface display systems for expression of two different polyhistidyl peptides, His3-Glu-His3 and His6. Targeting of the encoded gene products to the cell wall and surface accessibility of the chimeric surface proteins, as well as their functionality in terms of metal binding, have been investigated.
Expression vectors for display of polyhistidyl peptides on staphylococci.
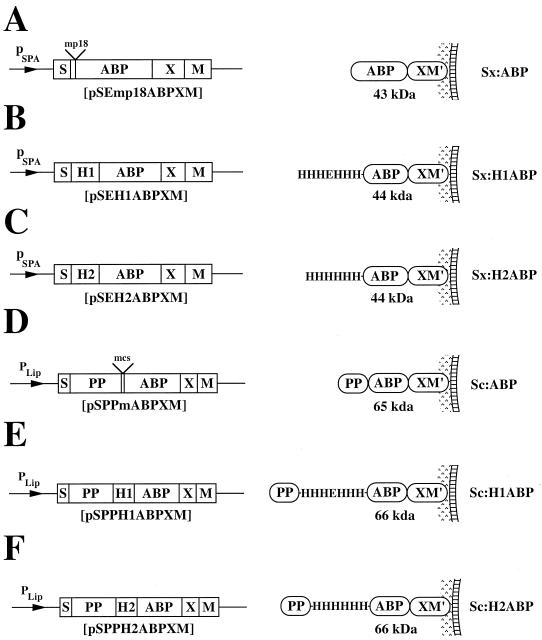
The strains and plasmids used in this study are listed in Table 1. DNA linkers encoding the two different metal-binding peptides His3-Glu-His3 (19), denoted H1, and His6, denoted H2, were constructed by annealing oligonucleotide SP12 (5′-GATCCGGAACACCATCATGAACACCACCATGAC-3′) to SP13 (5′-TCGAGTCATGGTGGTGTTCATGATGGTGTTCCG-3′) and SP14 (5′-GATCCGGAACACCATCATCACCACCATGACGAC-3′) to SP15 (5′-TCG AGTCGTCATGGTGGTGATGATGGTGTTCCG-3′), respectively. The generated linkers were inserted into the BamHI/SalI-restricted shuttle-vectors pSEmp18ABPXM (31) and pSPPmABPXM (32), resulting in expression vectors pSEH1ABPXM, pSEH2ABPXM, pSPPH1ABPXM, and pSPPH2ABPXM (Fig. 1), respectively. Correct nucleotide sequences were verified by solid-phase DNA sequencing (13). Preparation and transformation of protoplasts from S. xylosus and S. carnosus were performed as described by Götz and coworkers (7, 8). The six recombinant staphylococci, for simplicity denoted Sx:ABP, Sx:H1ABP, Sx:H2ABP, Sc:ABP, Sc:H1ABP, and Sc:H2ABP, are schematically depicted in Fig. 1, with their encoded surface proteins illustrated as anchored to the cell wall of S. xylosus or S. carnosus, as appropriate. The S. xylosus expression vectors (Fig. 1A to C) take advantage of the promoter and signal sequence from Staphylococcus aureus protein A (SpA) (43), while the S. carnosus vectors (Fig. 1D to F) utilize the promoter, signal sequence, and propeptide sequence from a Staphylococcus hyicus lipase gene construct (32). Both vector systems contain gene fragments from SpA, comprising X, a charged repetitive region postulated to interact with the peptidoglycan cell wall (10), and M, a region common for many gram-positive cells surface-bound proteins and required for cell surface anchoring (35, 42). The gene encoding an albumin-binding protein (ABP), derived from streptococcal protein G (26, 32), is also present in all expression vectors. The ABP region is expressed as the part of the recombinant receptor closest to the cell wall anchoring motifs. It has been demonstrated to be useful as a reporter peptide in a colorimetric assay to analyze surface accessibility of hybrid surface proteins (32), as an affinity tag for recovery of chimeric surface proteins (32), and as a spacer protein to increase the surface accessibility of displayed proteins (38).
TABLE 1.
Strains and plasmids
| Strain | Plasmid | Reference or source |
|---|---|---|
| S. xylosus KL117 | None | 34 |
| pSEmp18ABPXM | 31 | |
| pSEH1ABPXM | This study | |
| pSEH2ABPXM | This study | |
| S. carnosus TM300 | None | 6 |
| pSPPmABPXM | 32 | |
| pSPPH1ABPXM | This study | |
| pSPPH2ABPXM | This study |
FIG. 1.
Expression cassettes of the different expression vectors designed for surface displays on S. xylosus and S. carnosus shown with their encoded gene products anchored to the cell surface. The names of the constructed expression vectors are given below the expression cassettes, the molecular masses of the encoded surface proteins are indicated, and the abbreviated names of the recombinant staphylococci are shown to the right. Note that the propeptide (PP) from the S. hyicus lipase gene construct is not processed in S. carnosus but is processed in its homologous host, S. hyicus (32). This propeptide has been suggested to be essential for secretion of heterologous gene fusion products from S. carnosus (4) when using the lipase signal peptide for secretion. M′, the processed and covalently anchored form of the M sequence of SpA; pSPA, promoter region from the SpA gene; pLip, S. hyicus promoter region designed for production in S. carnosus (6).
Recovery and characterization of chimeric surface proteins.
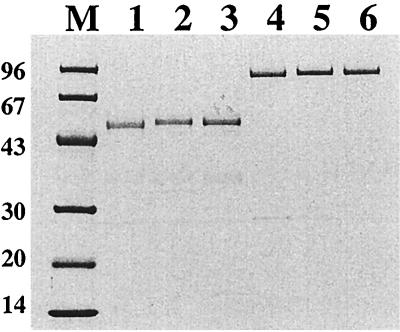
In order to investigate whether the expressed chimeric surface proteins (Fig. 1) were successfully produced and targeted to the cell wall, the recombinant staphylococci were grown to early logarithmic phase and subjected to lysostaphin treatment to release cell wall-bound material, as described earlier in detail (32). The chimeric surface proteins were subsequently recovered by ABP-mediated affinity purification on human serum albumin (HSA) columns (26, 39). The affinity-purified chimeric proteins were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis followed by Coomassie staining of the gel (Fig. 2). As can be seen in Fig. 2, full-length proteins could be recovered from the cell wall fractions of the recombinant staphylococci. The released proteins, and in particular those recovered from S. carnosus, all migrated as somewhat larger than their corresponding theoretical molecular weights (see Fig. 1). This phenomenon has also been observed for similar staphylococcal proteins (1, 9, 32, 41). These results demonstrate that the hybrid receptors were localized to the cell walls of the recombinant S. xylosus and S. carnosus cells. Furthermore, the extracted hybrid surface proteins display serum albumin-binding capacity, since full-length fusion proteins could be recovered by HSA affinity chromatography. However, proper surface accessibility and functionality of the hybrid receptors on intact recombinant staphylococci remained to be demonstrated.
FIG. 2.
Characterization of gene products by SDS-PAGE (10 to 20% polyacrylamide) analysis under reducing conditions. Coomassie brilliant blue was used for staining. The chimeric surface proteins were extracted from the cell wall of the recombinant staphylococci and subsequently subjected to single-step ABP-mediated HSA affinity chromatography. Lane 1, Sx:ABP, lane 2, Sx:H1ABP; lane 3, Sx:H2ABP; lane 4, Sc:ABP; lane 5, Sc:H1ABP; lane 6, Sc:H2ABP; lane M, marker proteins with molecular masses (in kilodaltons).
Surface accessibility of chimeric surface proteins.
The surface accessibility of the displayed chimeric proteins on whole-cell staphylococci was analyzed by a previously described colorimetric assay (32), again by taking advantage of ABP present in the chimeric surface proteins as a reporter peptide. Recombinant and wild-type staphylococci were grown to early logarithmic phase, harvested, and then incubated with biotinylated HSA, followed by an incubation with a streptavidin-alkaline phosphatase conjugate. The presence of surface-displayed ABP-containing surface proteins was detected using a chromogenic substrate. All recombinant staphylococci showed a significant positive response, while wild-type S. xylosus and S. carnosus, as expected, were negative in this assay (data not shown). The chimeric surface proteins were thereby shown to be targeted and anchored, in accessible forms, to the outer surface of the recombinant staphylococci. The magnitudes of the responses were significantly higher for the S. carnosus constructs (data not shown). These findings are in accordance with earlier results, since it has been demonstrated that the S. carnosus system displays approximately 10,000 recombinant surface proteins per bacterial cell while the S. xylosus system generates bacteria with approximately 3,000 surface-exposed proteins (1, 31). Furthermore, the levels of surface expression of chimeric proteins containing the polyhistidyl peptides were found to be of the same magnitude as those constructs encoded by the parental vectors (data not shown), suggesting that the surface density was not reduced by the introduction of the polyhistidyl peptides.
Functional analysis of surface-displayed polyhistidyl peptides.
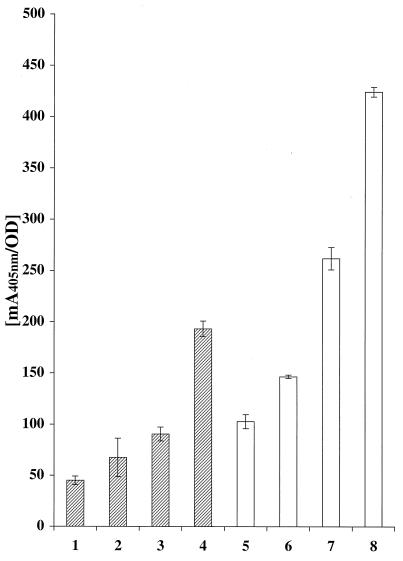
To investigate whether the recombinant staphylococci had obtained an improved ability to bind metal ions, recombinant and wild-type staphylococcal cells were incubated with a nickel-chelating alkaline phosphatase conjugate. This Ni2+-binding assay, based on a previously described method (36), was performed essentially in accordance with the colorimetric assay described above. However, instead of incubating the cells with biotinylated HSA, QIAexpress Ni-nitrilotriacetic acid (NTA)-alkaline phosphatase conjugate (Qiagen, Hilden, Germany) was used. One-milliliter aliquots of cell suspension (A578 = 1), in phosphate-buffered saline (pH 7.5) supplemented with 0.05% Tween 20 (PBST), were incubated for 60 min at room temperature with Ni-NTA-alkaline phosphatase conjugate at a dilution of 1:500. The cells were then washed three times (the two first washes in PBST and the last in substrate buffer) before being resuspended in 1 ml of substrate buffer. Aliquots (100 μl) of each cell type were loaded into a microtiter plate, after which 100 μl of the substrate solution (p-nitrophenyl phosphate) was added. The change in A405 was monitored for 60 min in an ELISA reader (Fig. 3). It was demonstrated that the recombinant S. xylosus cells carrying surface-displayed polyhistidyl peptides, Sx:H1ABP (Fig. 3, bar 3) and Sx:H2ABP (Fig. 3, bar 4), showed higher Ni2+-binding capacities than did wild-type S. xylosus (Fig. 3, bar 1) and Sx:ABP (Fig. 3, bar 2). However, for the S. xylosus cells carrying the discontinuous polyhistidyl peptide, Sx:H1ABP (Fig. 3, bar 3), the increase in Ni2+ binding was not statistically significant compared to Sx:ABP (Fig. 3, bar 2). A similarly improved Ni2+-binding capacity could be observed for the recombinant S. carnosus cells with polyhistidyl peptides exposed on their surface, Sc:H1ABP (Fig. 3, bar 7) and Sc:H2ABP (Fig. 3, bar 8), compared to wild-type S. carnosus (Fig. 3, bar 5) and Sc:ABP (Fig. 3, bar 6). The continuous His6 peptide (H2) seemed in both systems to give a significantly higher metal-binding capacity than the discontinuous His3-Glu-His3-peptide (H1): S. xylosus (Fig. 3, bars 3 and 4) and S. carnosus (Fig. 3, bars 7 and 8), respectively. Furthermore, the results supported the indications from the ABP-based colorimetric assay (see above), suggesting (i) that the chimeric proteins were accessible on the staphylococcal cells and (ii) that S. carnosus cells display a significantly greater number of recombinant proteins at their surfaces.
FIG. 3.
Histogram representation of results from the whole-cell Ni2+-binding assay. Wild-type and recombinant S. xylosus or S. carnosus cells were incubated with nickel-chelated alkaline phosphatase conjugate. Upon addition of substrate, the color response was monitored in five separate samples from each construct. Hatched bars indicate the A405 response for S. xylosus cells: wild type (bar 1), Sx:ABP (bar 2), Sx:H1ABP (bar 3), and Sx:H2ABP (bar 4). Open bars indicate the A405 response for S. carnosus cells: wild type (bar 5), Sc:ABP (bar 6), Sc:H1ABP (bar 7), and Sc:H2ABP (bar 8). Error bars represent standard deviations.
Cd2+ binding.
In order to investigate whether the recombinant staphylococci had gained an increased capability to adsorb certain divalent heavy metal ions, a Cd2+ bioadsorption assay was employed (29, 30). Wild-type and recombinant staphylococci were gown to the same cell densities and tested for the ability to absorb radioactive 109Cd added to the washed cells at a concentration just above Cd2+ concentration acceptable to drinking water (29). Overnight cultures of recombinant and wild-type staphylococci were diluted to an A578 of ≈0.08 in growth medium (containing chloramphenicol when appropriate) and grown at 37°C to an A578 of ≈1. Cell aliquots (10 ml) were withdrawn, and, after sedimentation of the bacterial cells (1,900 × g, 10 min), the cell pellets were washed (5 ml of 0.1 M Trizma base-HCl, pH 7.5) and resuspended in 5 ml of the same buffer. A stock solution of 109Cd was prepared (30) by addition of 0.5 μCi of radioisotopic 109Cd (specific activity, 125 Ci/mmol) (Amersham Pharmacia Biotech, Uppsala, Sweden) to 5 ml of a 20 μM CdCl2 solution, yielding a final specific activity of 5 Ci/mol. To the cell suspension was added 50 μl of the 109Cd stock solution, yielding a final concentration of 0.2 μM. The samples were incubated with end-over-end mixing for 45 min at room temperature. Cells were sedimented (1,900 × g, 10 min), and both cell pellets and supernatants were subjected to radioactivity measurements using a Packard liquid scintillation counter (Packard Instruments, Meriden, Conn.), according to Pazirandeh and coworkers (29, 30) and following the suppliers recommendations. For scintillation counting, 1 ml of cell supernatant was added to 15 ml of Ultima Gold (Packard) scintillation cocktail. The cell pellets were washed (1 M Trizma base-HCl, pH 7.0) and resuspended in 1 ml of 1 M Trizma base-HCl before addition of 15 ml of the scintillation cocktail. Figure 4 shows the residual 109Cd concentrations, expressed as counts per minute, in supernatants of wild-type S. xylosus (Fig. 4, bar 1), Sx:ABP (Fig. 4, bar 2), and Sx:H2ABP (Fig. 4, bar 3) as well as for wild-type S. carnosus (Fig. 4, bar 4), Sc:ABP (Fig. 4, bar 5), and Sc:H2ABP (Fig. 4, bar 6) after incubating the bacterial cells with 109Cd. Each supernatant was analyzed as five separate samples, and the entire experiment was repeated with reproducible results. The results demonstrate that the recombinant staphylococci expressing a continuous hexahistidyl peptide at their surface, Sx:H2ABP (Fig. 4, bar 3) and Sc:H2ABP (Fig. 4, bar 6), had gained a slightly but significantly improved ability to adsorb Cd2+ ions (P values, <0.01 and <0.02, respectively) compared with staphylococci carrying surface proteins with only the ABP moiety, Sx:ABP (Fig. 4, bar 2) and Sc:ABP (Fig. 4, bar 5), respectively. However, the increase in ability to adsorb Cd2+ ions by introduction of a hexahistidyl peptide was only moderate compared to the improved Ni2+ binding, and this is most likely because hexahistidyl peptides are not optimal for Cd2+ binding. Alternative peptides should be considered, as discussed below. Staphylococci carrying the discontinuous polyhistidyl peptide (Sx:H1ABP and Sc:H1ABP) showed intermediate efficiency for bioadsorption (data not shown). A similar pattern was found when analyzing the cell pellets in the liquid scintillation assay, giving the highest residual Cd concentrations in the pellets of the staphylococci carrying the H2 peptide (Sx:H2ABP and Sc:H2ABP), the lowest values for the wild-type bacteria, and intermediate Cd concentrations for the Sx:ABP, Sx:H1ABP, Sc:ABP, and Sc:H1ABP strains (data not shown).
FIG. 4.
Histogram representation of results from the Cd2+ bioadsorption assay. Wild-type and recombinant S. xylosus or S. carnosus cells were incubated with radioactive 109Cd, and residual radioactivity in the cell supernatants, monitored by liquid scintillation, corresponds to the amount of nonadsorbed Cd2+. Each supernatant was analyzed as five separate samples. Hatched bars indicate Cd2+ concentrations for S. xylosus cells: wild type (bar 1), Sx:ABP (bar 2), and Sx:H2ABP (bar 3). Open bars indicate Cd2+ concentrations for S. carnosus cells: wild type (bar 4), Sc:ABP (bar 5), and Sc:H2ABP (bar 6). Error bars represent standard deviations.
Concluding remarks.
We have demonstrated how two different polyhistidyl peptides were expressed in a functional form on the surface of S. xylosus and S. carnosus. SDS-PAGE analysis of recombinant surface proteins, affinity purified from cell wall fractions, verified the cell wall localization of the chimeric proteins, expressed as full-length gene products. Surface accessibility of the chimeric surface proteins was demonstrated for the different recombinant staphylococci by a colorimetric assay based on the ABP. Functionality of the surface-exposed proteins in terms of metal ion binding was demonstrated, since recombinant S. xylosus and S. carnosus cells had gained improved nickel-binding capacity by the introduction of the H1 or H2 peptide into their surface proteins. The continuous H2 peptide was found to be significantly better in Ni2+ binding. When evaluating the staphylococci for the ability to adsorb a divalent heavy metal ion, Cd2+, only slightly improved Cd2+ binding could be demonstrated, suggesting that alternative peptides might need to be investigated in order to generate staphylococci with improved capacity to sequester Cd2+ ions.
A number of different metal-binding peptides have been described in the literature, and, recently, approaches based on combinatorial peptide libraries have allowed the selection of peptides with enhanced specificity for a certain metal, using bacterial (3, 33) or phage (22, 28) display strategies. Bacterial surface display of such peptides could potentially generate tailor-made recombinant bacteria with specific metal-binding abilities, which could thus be envisioned as devices to create selective bioadsorbents or biosensors. Peptides which have been demonstrated to bind metals of significant environmental concern, such as cadmium (16), chromium (3), or mercury (30), would thus be of obvious interest for expression in the staphylococcal surface display systems described here. Alternatively, the presented staphylococcal surface display systems could potentially be used directly for display of combinatorial peptide libraries and subsequent selection through biopanning of peptides specific for a certain metal ion. Also, other protein scaffolds (5, 15, 20, 25) could, if subjected to protein engineering efforts, be envisioned for surface display in order to create bacteria with the capacity for specific metal adsorption.
Taken together, we have demonstrated the possibility of displaying polyhistidyl peptides in a functional form on the surface of two strains of staphylococci, as an initial approach to recruiting surface-engineered gram-positive bacteria for the generation of metal-binding bioadsorbents to be used in environmental or biosensor applications. In order to create staphylococci with specific recognition of certain metal ions, an ion-specific peptide or protein needs to be engineered, for example, by combinatorial library approaches. Nevertheless, this first successful study of how metal-binding peptides have been expressed by recombinant means as surface exposed on food-grade nonpathogenic staphylococci constitutes an important step in this development.
Acknowledgments
We are grateful to Rikard Erlandsson, Mathias Uhlén, Johan Håkans, Lars-Göran Danielsson, and Per-Åke Nygren for valuable discussions.
This work was financially supported in part by the Swedish National Board for Technical and Industrial Development (NUTEK) and in part by the program Cell Factory for Functional Genomics within the Swedish Foundation for Strategic Research.
REFERENCES
- 1.Andréoni C, Goetsch L, Libon C, Samuelson P, Nguyen T N, Robert A, Uhlén M, Binz H, Ståhl S. Flow cytometric quantification of surface-displayed recombinant receptors on staphylococci. BioTechniques. 1997;23:696–704. [PubMed] [Google Scholar]
- 2.Brower J B, Ryan R L, Pazirandeh M. Comparison of ion-exchange resins and biosorbents for the removal of heavy metals from plating factor wastewater. Environ Sci Technol. 1997;31:2910–2914. [Google Scholar]
- 3.Brown S. Metal-recognition by repeating polypeptides. Nat Biotechnol. 1997;15:269–272. doi: 10.1038/nbt0397-269. [DOI] [PubMed] [Google Scholar]
- 4.Demleitner G, Götz F. Evidence for importance of the Staphylococcus hyicus lipase pro-peptide in lipase secretion, stability and activity. FEMS Microbiol Lett. 1994;121:189–197. doi: 10.1111/j.1574-6968.1994.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 5.Dieckmann G R, McRorie D K, Tierney D L, Utschig L M, Singer C P, O'Halloran T V, Penner-Hahn J E, DeGrado W F, Pecoraro V L. De novo design of mercury-binding two- and three-helical bundles. J Am Chem Soc. 1997;119:6195–6196. [Google Scholar]
- 6.Götz F. Staphylococcus carnosus: a new host organism for gene cloning and protein production. J Appl Bacteriol Symp Suppl. 1990;19:49S–53S. doi: 10.1111/j.1365-2672.1990.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 7.Götz F, Ahrne S, Lindberg M. Plasmid transfer and genetic recombination by protoplast fusion in staphylococci. J Bacteriol. 1981;145:74–81. doi: 10.1128/jb.145.1.74-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Götz F, Schumacher B. Improvements of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol Lett. 1987;40:285–288. [Google Scholar]
- 9.Gunneriusson E, Samuelson P, Uhlén M, Nygren P-Å, Ståhl S. Surface display of a functional single-chain Fv antibody on staphylococci. J Bacteriol. 1996;178:1341–1346. doi: 10.1128/jb.178.5.1341-1346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guss B, Uhlén M, Nilsson B, Lindberg M, Sjöquist J, Sjödahl J. Region X, the cell-wall-attachment part of staphylococcal protein A. Eur J Biochem. 1984;138:413–420. doi: 10.1111/j.1432-1033.1984.tb07931.x. [DOI] [PubMed] [Google Scholar]
- 11.Hammes W P, Bosch I, Wolf G. Contribution of Staphylococcus carnosus and Staphylococcus piscifermentans to the fermentation of protein foods. J Appl Bacteriol Symp Suppl. 1995;79:76S–83S. [Google Scholar]
- 12.Hansson M, Ståhl S, Nguyen T N, Bachi T, Robert A, Binz H, Sjölander A, Uhlén M. Expression of recombinant proteins on the surface of the coagulase-negative bacterium Staphylococcus xylosus. J Bacteriol. 1992;174:4239–4245. doi: 10.1128/jb.174.13.4239-4245.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hultman T, Ståhl S, Hornes E, Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989;17:4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelemen M V, Sharpe J E. Controlled cell disruption: a comparison of the forces required to disrupt different micro-organisms. J Cell Sci. 1979;35:431–441. doi: 10.1242/jcs.35.1.431. [DOI] [PubMed] [Google Scholar]
- 15.Klemba M, Gardner K H, Marino S, Clarke N D, Regan L. Novel metal-binding proteins by design. Nat Struct Biol. 1995;2:368–373. doi: 10.1038/nsb0595-368. [DOI] [PubMed] [Google Scholar]
- 16.Kotrba P, Doleckova L, de Lorenzo V, Ruml T. Enhanced bioaccumulation of heavy metal ions by bacterial cells due to surface display of short metal binding peptides. Appl Environ Microbiol. 1999;65:1092–1098. doi: 10.1128/aem.65.3.1092-1098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotrba P, Pospisil P, de Lorenzo V, Ruml T. Enhanced metallosorption of Escherichia coli cells due to surface display of beta- and alpha-domains of mammalian metallothionein as a fusion to LamB protein. J Recept Signal Transduct Res. 1999;19:703–715. doi: 10.3109/10799899909036681. [DOI] [PubMed] [Google Scholar]
- 18.Liepe H-U. Bakterienkulturen und Rohwurst. Forum Mikrobiol. 1982;5:10–15. [Google Scholar]
- 19.Ljungquist C, Breitholtz A, Brink-Nilsson H, Moks T, Uhlén M, Nilsson B. Immobilization and affinity of recombinant proteins using histidine peptide fusions. Eur J Biochem. 1989;186:563–569. doi: 10.1111/j.1432-1033.1989.tb15245.x. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Valentine J S. Engineering metal-binding sites in proteins. Curr Opin Struct Biol. 1997;7:495–500. doi: 10.1016/s0959-440x(97)80112-1. [DOI] [PubMed] [Google Scholar]
- 21.Malik P, Terry T D, Bellintani F, Perham R N. Factors limiting display of foreign peptides on the major coat protein of filamentous bacteriophage capsids and a potential role for leader peptidase. FEBS Lett. 1998;436:263–266. doi: 10.1016/s0014-5793(98)01140-5. [DOI] [PubMed] [Google Scholar]
- 22.Mejàre M, Ljung S, Bülow L. Selection of cadmium specific hexapeptides and their expression as OmpA fusion protein in Escherichia coli. Protein Eng. 1998;11:489–494. doi: 10.1093/protein/11.6.489. [DOI] [PubMed] [Google Scholar]
- 23.Mullen M D, Wolf D C, Ferris F G, Beveridge T J, Fleming C A, Bailey G W. Bacterial sorption of heavy metals. Appl Environ Microbiol. 1989;55:3143–3149. doi: 10.1128/aem.55.12.3143-3149.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen T N, Hansson M, Ståhl S, Bächi T, Robert A, Domzig W, Binz H, Uhlén M. Cell-surface display of heterologous epitopes on Staphylococcus xylosus as a potential delivery system for oral vaccination. Gene. 1993;128:89–94. doi: 10.1016/0378-1119(93)90158-y. [DOI] [PubMed] [Google Scholar]
- 25.Nord, K., E. Gunneriusson, J. Ringdahl, S. Ståhl, M. Uhlén, and P.-Å. Nygren. Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nat. Biotechnol. 15:772–777. [DOI] [PubMed]
- 26.Nygren P-Å, Eliasson M, Palmcrantz E, Abrahmsén L, Uhlén M. Analysis and use of the serum albumin binding domains of streptococcal protein. G. J Mol Recognit. 1988;1:69–74. doi: 10.1002/jmr.300010204. [DOI] [PubMed] [Google Scholar]
- 27.Pagán R, Mañas P, Raso J, Condón S. Bacterial resistance to ultrasonic waves under pressure at nonlethal (manosonication) and lethal (manothermosonication) temperatures. Appl Environ Microbiol. 1999;65:297–300. doi: 10.1128/aem.65.1.297-300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patwardhan A V, Goud G N, Koepsel R R, Ataai M M. Selection of optimum affinity tags from a phage-displayed peptide library. Application to immobilized copper(II) affinity chromatography. J Chromatogr A. 1997;787:91–100. doi: 10.1016/s0021-9673(97)00580-3. [DOI] [PubMed] [Google Scholar]
- 29.Pazirandeh M, Chrisey L A, Mauro J M, Campbell J R, Gaber B P. Expression of the Neurospora crassa metallothionein gene in Escherichia coli and its effect on heavy-metal uptake. Appl Microbiol Biotechnol. 1995;43:1112–1117. doi: 10.1007/BF00166934. [DOI] [PubMed] [Google Scholar]
- 30.Pazirandeh M, Wells B M, Ryan R L. Development of bacterium-based heavy metal biosorbents: enhanced uptake of cadmium and mercury by Escherichia coli expressing a metal binding motif. Appl Environ Microbiol. 1998;64:4068–4072. doi: 10.1128/aem.64.10.4068-4072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert A, Samuelson P, Andréoni C, Bächi T, Uhlén M, Binz H, Nguyen T N, Ståhl S. Surface display on staphylococci: a comparative study. FEBS Lett. 1996;390:327–333. doi: 10.1016/0014-5793(96)00684-9. [DOI] [PubMed] [Google Scholar]
- 32.Samuelson P, Hansson M, Ahlborg N, Andréoni C, Götz F, Bächi T, Nguyen T N, Binz H, Uhlén M, Ståhl S. Cell surface display of recombinant proteins on Staphylococcus carnosus. J Bacteriol. 1995;177:1470–1476. doi: 10.1128/jb.177.6.1470-1476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schembri M A, Kjaergaard K, Klemm P. Bioaccumulation of heavy metals by fimbrial designer adhesins. FEMS Microbiol Lett. 1999;170:363–371. doi: 10.1111/j.1574-6968.1999.tb13396.x. [DOI] [PubMed] [Google Scholar]
- 34.Schleifer K H, Kloos W E. Isolation and characterization of staphylococci from human skin. I. Amended descriptions of Staphylococcus epidermidis and Staphylococcus saprohyticus and descriptions of the three new species Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus. Int J Syst Bacteriol. 1975;35:50–61. [Google Scholar]
- 35.Schneewind O, Fowler A, Faull K F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 36.Sousa C, Cebolla A, de Lorenzo V. Enhanced metalloadsorption of bacterial cells displaying poly-His peptides. Nat Biotechnol. 1996;14:1017–1020. doi: 10.1038/nbt0896-1017. [DOI] [PubMed] [Google Scholar]
- 37.Sousa C, Kotrba P, Ruml T, Cebolla A, De Lorenzo V. Metalloadsorption by Escherichia coli cells displaying yeast and mammalian metallothioneins anchored to the outer membrane protein LamB. J Bacteriol. 1998;180:2280–2284. doi: 10.1128/jb.180.9.2280-2284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ståhl S, Samuelson P, Hansson M, Andréoni C, Goetsch L, Libon C, Liljeqvist S, Gunneriusson E, Binz H, Nguyen T N, Uhlén M. Development of non-pathogenic staphylococci as vaccine delivery vehicles. In: Pozzi G, Wells J, editors. Gram-positive bacteria: vaccine vehicles for mucosal immunization. Georgetown, Tex: Landes Bioscience; 1997. pp. 62–81. [Google Scholar]
- 39.Ståhl S, Sjölander A, Nygren P-Å, Berzins K, Perlmann P, Uhlén M. A dual expression system for the generation, analysis and purification of antibodies to a repeated sequence of the Plasmodium falciparum antigen Pf155/RESA. J Immunol Methods. 1989;124:43–52. doi: 10.1016/0022-1759(89)90184-1. [DOI] [PubMed] [Google Scholar]
- 40.Ståhl S, Uhlén M. Bacterial surface display: trends and progress. Trends Biotechnol. 1997;15:185–192. doi: 10.1016/S0167-7799(97)01034-2. [DOI] [PubMed] [Google Scholar]
- 41.Strauss A, Götz F. In vivo immobilization of enzymatically active polypeptides on the cell surface of Staphylococcus carnosus. Mol Microbiol. 1996;21:491–500. doi: 10.1111/j.1365-2958.1996.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 42.Ton-That H, Faull K F, Schneewind O. Anchor structure of staphylococcal surface proteins. A branched peptide that links the carboxyl terminus of proteins to the cell wall. J Biol Chem. 1997;272:22285–22292. doi: 10.1074/jbc.272.35.22285. [DOI] [PubMed] [Google Scholar]
- 43.Uhlén M, Nilsson B, Guss B, Lindberg M, Gatenbeck S, Philipson L. Gene fusion vectors based on the gene for staphylococcal protein A. Gnee. 1983;23:369–378. doi: 10.1016/0378-1119(83)90025-2. [DOI] [PubMed] [Google Scholar]