Implications.
• Varying mitochondrial phenotypes are present at a young age, are refined by exercise training, and impact the performance of equine athletes.
• Breeding selection, training regimens, and nutritional interventions influence mitochondrial phenotype and, therefore, may be optimized to improve the performance of individual animals for specific sports.
• Novel technologies, such as high-resolution respirometry, are allowing deeper insights into mitochondrial adaptations to interventions such as diet and exercise.
Introduction
Many factors support or restrict equine performance. Energy supply is required for cellular function and thus is an important factor contributing to performance in all disciplines. Energy metabolism can be divided into two primary processes: aerobic and anaerobic metabolism. Aerobic metabolism occurs in the mitochondria, colloquially known as the powerhouses of the cell, and is a primary and efficient source of skeletal muscle energy. Mitochondrial aerobic metabolism begins with the breakdown of carbohydrates, lipids, and proteins within and outside the mitochondria to produce reducing equivalents. In the presence of oxygen, mitochondria reduce these equivalents during the process of oxidative phosphorylation (OXPHOS), establishing a protonmotive force that drives the production of the primary source of cellular energy, adenosine triphosphate (ATP). Conversely, in the absence of oxygen, sugar is converted into energy by anaerobic metabolism, a less efficient form of energy production compared with aerobic metabolism. While each equine sport has specific demands requiring different levels of support from each metabolic system, mitochondrial function is necessary at some level for all horses.
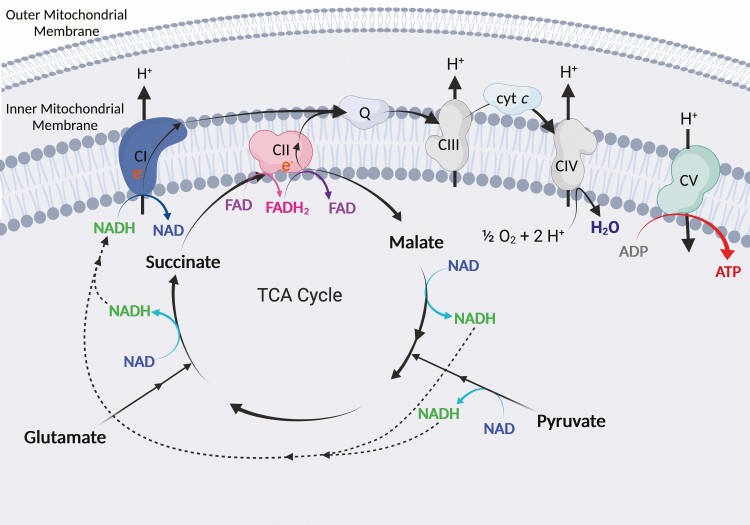
Mitochondria are composed of an inner and outer mitochondrial membrane. The process of OXPHOS involves the transfer of electrons across four main complexes known as the electron transfer system (ETS), more commonly known as the electron transport chain. The ETS is situated within the inner mitochondrial membrane (Figure 1). Beneath the inner mitochondrial membrane, the mitochondrial matrix houses a system of enzymes that conduct a series of chemical reactions known as the tricarboxylic acid (TCA) cycle. This cycle produces the reducing equivalents nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2), which are high-energy electron carriers that deliver electrons to the ETS at complex I (CI) and complex II (CII). NADH fuels CI activity and is derived primarily from pyruvate metabolism, which contributes carbon chains to the TCA cycle, and by malate, glutamate, and isocitrate metabolism in the TCA cycle. CII (also known as succinate dehydrogenase [SDH]) serves dual purposes as a TCA cycle enzyme and an ETS complex, reducing FAD in one subunit to produce FADH2, and subsequently oxidizing FADH2 in another subunit to provide electrons for ETS activity (Figure 1). Electrons from the ETS (CI and CII) and other enzymes converge at the Q-junction (coenzyme Q, also known as ubiquinone) and are transferred to complex III (CIII), cytochrome c (cyt c), and finally to complex IV (CIV, also known as cyt c oxidase). Oxygen is the final electron acceptor and is combined with hydrogen at CIV to form water.
Figure 1.
ETS. NADH and FADH2 are derived from the metabolism of substrates such as pyruvate, malate, glutamate, and succinate through the TCA cycle. NADH and FADH2 deliver electrons to the ETS at CI and CII, respectively. Electrons from CI and CII converge at the Q junction (Q) and are transferred to CIII, then finally to CIV via cyt c. At CIV, oxygen (O2) and hydrogen ions (H+) combine to form water (H2O), the final electron acceptor. Hydrogen ions are pumped from the mitochondrial matrix to the intermembrane space between the inner and outer mitochondrial membranes, creating a proton gradient across the inner mitochondrial membrane. Hydrogen ions flow back to the mitochondrial matrix along the concentration gradient at CV (ATP synthase), producing ATP. Created with BioRender.com.
The transfer of electrons releases energy, which is used by CI, CIII, and CIV to pump hydrogen ions, or protons, from the mitochondrial matrix to the intermembrane space (Figure 1). This creates a concentration gradient with a greater concentration of protons in the intermembrane space than in the mitochondrial matrix. This difference in proton concentration creates a protonmotive force that drives protons through ATP synthase (also known as complex V of the ETS) along the concentration gradient. ATP synthase then phosphorylates adenosine diphosphate (ADP) to ATP. Protons can then be pumped back out to the intermembrane space by the ETS to reinforce the concentration gradient and perpetuate continued OXPHOS.
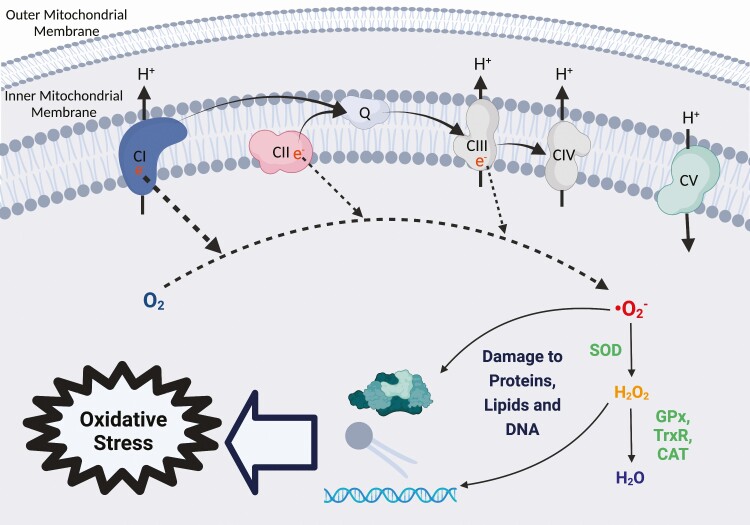
One byproduct of mitochondrial activity is reactive oxygen species (ROS). During the normal mitochondrial activity, electrons traversing the ETS can leak back into the mitochondrial matrix at CI, CII, and CIII. In the mitochondrial matrix, these electrons react with oxygen to form superoxide, a ROS (Figure 2). During exercise and other cellular stressors, ROS production increases and triggers signaling cascades that result in beneficial skeletal muscle adaptations (Niess and Simon, 2007). In healthy muscle, excess superoxide is converted to hydrogen peroxide by the antioxidant enzyme superoxide dismutase. Although hydrogen peroxide is less reactive than superoxide, it is still a ROS and is metabolized to water by the antioxidant enzymes catalase, glutathione peroxidase (GPx), and thioredoxin reductase via thioredoxin peroxidase (Figure 2). Oxidative stress occurs when ROS production overwhelms antioxidant capacity and accumulated ROS react with and damage proteins, lipids, and DNA. Thus, while ROS can play an important role in cellular signaling and adaptation, they can also cause muscle damage if not properly regulated.
Figure 2.
ROS production by the ETS. Electrons leak back into the mitochondrial matrix at CI, CII, and CIII and react with oxygen (O2) to produce superoxide (∙O2−). Superoxide is converted to hydrogen peroxide (H2O2) by the antioxidant superoxide dismutase (SOD), and H2O2 is converted to water (H2O) by antioxidants GPx, thioredoxin reductase (TrxR), and catalase (CAT). Without proper sequestration by antioxidants, ∙O2− and H2O2 may damage proteins, lipids, and DNA, which can cause oxidative stress if left unchecked. Created with BioRender.com.
OXPHOS in the mitochondria is a primary source of energy for skeletal muscle function during exercise. Recently, there has been increasing attention given to the ways that skeletal muscle mitochondrial activity can support or hinder equine performance. This article will review mitochondrial physiology and the relationship of mitochondrial phenotypes to performance in equine athletes. The aims of this review are to 1) outline state-of-the-art techniques in the assessment of mitochondrial function and 2) explore the ways that mitochondrial function has been shown to impact equine performance.
Measurements of Oxidative Capacity
There are several methods of assessing mitochondrial density and function in skeletal muscle that have been used with varying fidelity. Skeletal muscle fiber type can be a rough indication of oxidative capacity because different muscle fiber types often have differing mitochondrial volumes. Muscle fiber type has classically been assigned by immunohistochemical assessment of a combination of myosin ATPase activity to determine twitch speed and SDH (CII) activity to assess oxidative capacity. This method results in the classification of muscle fibers as slow-twitch oxidative fibers (slow, less powerful contractions, high oxidative capacity), fast-twitch highly oxidative fibers (fast, powerful contractions, high oxidative capacity), or fast-twitch lowly oxidative fibers (fast, powerful contractions, low oxidative capacity). However, research in horses suggests that oxidative capacity is not strongly correlated with muscle fiber type when assigned by myosin ATPase activity (López-Rivero et al., 1990). Additionally, the assessment of fiber type by myosin ATPase activity is highly subjective and has thus been largely replaced by immunohistochemical identification of myosin heavy chain (MyHC) isoforms. Myosin heavy chain identification is a more sensitive, less subjective assessment of muscle fiber type that is more indicative of distinct fiber types with distinct oxidative capacities (Rivero et al., 1996). Adult horses express three skeletal muscle MyHC isoforms: MyHC type I (composing slow-twitch highly oxidative fibers), MyHC type IIa (composing fast-twitch highly oxidative fibers), and MyHC type IIx (composing fast-twitch lowly oxidative fibers). Type I and IIa muscle fibers generally have a high density of large mitochondria and are thus described as “oxidative.” Type IIx fibers rely more heavily on anaerobic glycolysis for energy production, have a comparatively smaller mitochondrial network, and are therefore commonly described as “non-oxidative.” While assessment of muscle fiber type provides a rough estimate of mitochondrial capacity, it is a nonspecific measure that does not directly quantify mitochondrial density or capacity.
Measures of mitochondrial volume density
The presence of mitochondrial mass available to perform OXPHOS is influenced by both mitochondrial number and mitochondrial size. Increases in skeletal muscle mitochondrial mass can result from either an increase in the size of existing mitochondria or the manufacture of new mitochondria. Therefore, mitochondrial mass is typically measured in terms of mitochondrial volume density (MitoVD), as it assesses both the number and size of mitochondria. This is especially important as mitochondria are known to fuse together to form highly connected networks in skeletal muscle, presumed to enhance the efficiency of aerobic energy production and communication of mitochondria throughout the myofiber (Glancy et al., 2015).
Transmission electron microscopy (TEM) is the gold standard for determining MitoVD. It involves measurement of mitochondrial area across serial sections of tissue and calculation of the total mitochondrial volume in a sample. However, TEM is technically challenging, time consuming, and expensive. Due to these limitations, other indices of MitoVD are often employed, such as cardiolipin and mitochondrial DNA content, CI, CII (SDH), CIII and CIV protein abundance and activity, and citrate synthase (CS) activity. A study comparing several of these measures found that cardiolipin content, CS activity, and CIV activity were the best indicators of MitoVD in young, healthy human skeletal muscle (Larsen et al., 2012). A follow-up study confirmed the positive relationship between CS activity and MitoVD by TEM both before and after a period of exercise training in healthy men (Meinild Lundby et al., 2018). While this relationship has not been definitively investigated in the horse, CS activity appears to be an accurate representation of MitoVD across species and is, therefore, the most commonly used proxy.
High-resolution respirometry assessment of mitochondrial capacity
Mitochondrial capacity is assessed by respirometry, which measures oxygen consumption that occurs during OXPHOS. Several systems exist to measure oxygen consumption of skeletal muscle samples, including Clark electrode systems, the Seahorse (Agilent Scientific Instruments, Santa Clara, CA, USA), and the Oxygraph 2k (O2k; Oroboros Instruments, Innsbruck, Austria). Clark electrode systems are the classical format to measure oxygen consumption of cells, and they operate in a closed chamber system. Unfortunately, these systems suffer from issues such as signal stability, oxygen consumption of the oxygen sensor, required assumption of linearity of oxygen slope, rapid depletion of oxygen which limits the length and complexity of protocols, oxygen back-diffusion into the chamber, and chemical oxygen consumption (Gnaiger, 2008). Newer methods of assessing mitochondrial capacity have been developed to address and mitigate these limitations. Agilent’s Seahorse system is a newer technology that allows for high-throughput automated measurement of oxygen consumption rate in a transient microchamber in 8- to 96-well plate formats. Limitations of the Agilent Seahorse system include the requirement of single-cell preparations and the capacity to accommodate only 4 titrations per well in a single run. An additional newer technique is high-resolution respirometry (HRR) assessed by an Oroboros O2k, which also utilizes a closed chamber system. Compared with Clark electrode systems, the O2k offers a lower limit of detection of respiratory flux and requires a smaller amount of sample. Additionally, unlike other systems, the Oroboros O2k allows for a larger number of titrations, as well as the use of permeabilized muscle fibers, which maintain cellular context and may more closely represent physiological adaptations to intervention compared with isolated mitochondria preparations. While the Oroboros O2k boasts more sensitive and complex measurements compared with other systems, limitations include the relative expense of the system compared with Clark electrode systems and a lack of automation that makes the system more time consuming and labor intensive compared with the Agilent Seahorse.
HRR in the O2k is performed by measuring oxygen consumption supported by the addition or titration of various substrates, uncouplers, and inhibitors (SUIT). SUIT protocols can be developed to test very specific hypotheses regarding the capacity of different mitochondrial “states” in response to manipulation of substrate availability, chemical uncoupling of mitochondrial processes, and inhibition of enzymes. To achieve this, skeletal muscle fibers are permeabilized or mitochondria are isolated from fibers to allow substrate entry, and samples are then washed to remove substrate molecules (e.g., TCA cycle intermediates and ADP) prior to analysis. Commonly assessed states of equine skeletal muscle and the chemicals used to induce them are outlined below.
In the absence of ADP to support OXPHOS, or using the ATP synthase inhibitor oligomycin, LEAK respiration can be assessed. LEAK respiration is oxygen consumption that occurs independently of ATP synthesis and, therefore, does not result in energy production. LEAK oxygen consumption is caused by proton leak (proton leak from the intermembrane space to the mitochondrial matrix across the inner mitochondrial membrane, diffusion through or around inner mitochondrial membrane proteins, or transport of protons by adenine nucleotide translocase or uncoupling proteins), proton slip (protons slip back to the mitochondrial matrix during pumping at CI, CIII, and CIV), cation cycling (movement of cations such as Na+ and K+ across the inner mitochondrial membrane), and electron leak (electrons leak back into the mitochondrial matrix at CI, CII, and CIII). Thus, LEAK respiration does not support energy production but is a dissipative component of cellular respiration. In vivo, LEAK serves to maintain an appropriate proton gradient across the inner mitochondrial membrane in the absence of ATP synthase activity to prevent the development of an unfavorably high protonmotive force (Gnaiger, 2014).
The addition of ADP stimulates ATP synthase activity in the presence of substrates that can be metabolized to produce NADH and FADH2. OXPHOS capacity in the presence of ADP is assessed by adding saturating concentrations of substrates and metabolites used for OXPHOS, such as TCA cycle intermediates or fatty acids. Commonly used substrates include CI substrates, such as pyruvate, malate, and glutamate, and the CII substrate succinate. Mitochondrial respiration supported by these substrates can be assessed individually or in different combinations to provide valuable insight into factors that drive OXPHOS capacity supported by CI (PCI) and CII (PCII) individually, or together to determine “maximal” OXPHOS capacity (PCI+II).
Chemical uncouplers, such as carbonyl cyanide m-chlorophenyl hydrazone or carbonyl cyanide p-trifluoro-methoxyphenyl hydrazone, can be added to determine the maximal ETS capacity. Uncouplers permeabilize the inner mitochondrial membrane to allow hydrogen ions to flow freely back to the mitochondrial matrix along the concentration gradient. Uncoupled respiration allows for the determination of ETS capacity independent of the limiting rate at which ATP synthase can transport hydrogen ions across the inner mitochondrial membrane (Gnaiger, 2014). ETS capacity with uncouplers is often measured after assessment of OXPHOS capacity to determine ETS with CI (ECI), ETS with CII (ECII), or maximal ETS capacity with CI and CII (ECI+II). The difference between maximal OXPHOS capacity and maximal ETS capacity represents excess ETS capacity. While the precise physiological purpose of this excess ETS capacity is not known, it has been shown to differ between muscle tissue of different species, and in response to different physiological factors. It is important to note that since the chemically induced maximal ETS capacity is not a normal physiological state in vivo, it is possible that differences in this excess ETS capacity could be an analytical artifact, rather than being indicative of consequential physiological differences.
Inhibitors are important tools in HRR that can serve a variety of purposes in SUIT protocols. They can be used to inhibit individual enzymes to determine OXPHOS capacity independent of extraneous enzymatic activity, to improve the description of specific mechanisms, or to improve methodological efficiency and accuracy. For example, rotenone is a commonly used inhibitor of CI (Gnaiger, 2014), which is often included in protocols that assess CI and CII sequentially to determine CII capacity independent of CI. In these protocols, CII substrate is added following the addition of CI substrates to determine OXPHOS capacity supported by CI and CII. Unfortunately, values derived from calculating CII capacity by simple subtraction between CI capacity and CI+II capacity underestimate CII capacity assessed independently of CI substrates. This analytical limitation occurs because both CI and CII substrates are added at saturating nonphysiological concentrations during SUIT protocols, and the increase in flux resulting from sequential addition of CII substrates is limited by the Q-junction’s ability to shuttle both CI and CII electrons. Therefore, rotenone can be used to inhibit CI activity in the presence of CI and CII substrates to provide a more accurate representation of the capacity of CII alone. This prevents the need to conduct an additional HRR experiment, saving appreciable time and minimizing the amount of tissue required for analysis. An additional inhibitor, antimycin A, inhibits CIII and is used as a methodological control to ensure the accuracy of oxidative capacity measures. By inhibiting CIII, the movement of electrons through the ETS ceases, so any residual oxygen consumption in the chamber is non-mitochondrial. By correcting all respiration measurements for this residual oxygen consumption, the accuracy of respiration measures is improved.
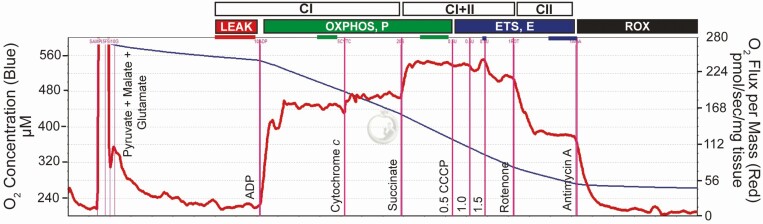
A common concern for ex vivo analyses is the maintenance of sample integrity and quality. In HRR, the assessment of flux in response to exogenous cyt c has traditionally been used as an indicator of outer mitochondrial membrane integrity. During sample preparation for HRR, mechanical or chemical disruption may damage mitochondrial membranes. Cytochrome c is a protein responsible for transferring electrons from CIII to CIV (Figure 1). In mitochondria with an intact outer membrane, the addition of exogenous cyt c results in a negligible increase in oxygen flux because the protein is too large to pass through an intact membrane. However, if the outer membrane is damaged, the protein can associate with the inner mitochondrial membrane, and its addition results in a comparatively large increase in oxygen flux. Therefore, verifying a minimal increase in flux in response to cyt c addition serves as an important step in ensuring the accuracy of data derived from HRR. However, recent data in some elite athletes, including horses and sled dogs, indicate that additional cyt c is required to achieve maximal respiration, and this is unrelated to sample integrity (Laner et al., 2014; Miller et al., 2017; Davis et al., 2021). Instead, the requirement of exogenous cyt c may be a physiological adaptation to cellular stress such as exercise or may indicate that cyt c abundance is a limiting factor to maximal oxidative capacity in some species. The utilization of cyt c as a sample quality measure requires further investigation. Regardless, an example of the data produced from a SUIT protocol assessing LEAK, PCI, PCI+II, ECI+II, and ECII and employing cyt c check for outer membrane integrity and antimycin A addition for correction of background flux is shown in Figure 3.
Figure 3.
Example output (DatLab software by Oroboros, Innsbruck, Austria) from a HRR experiment on the Oxygraph-2k. The y-axes show O2 flux per mass (red line; pmol · s−1 · mg−1) and oxygen (O2) concentration (blue line). The SUIT protocol for this study was as follows: pyruvate + malate + glutamate (LEAK respiration), ADP to determine oxidative capacity (OXPHOS) with CI (PCI), addition of cyt c, succinate to determine OXPHOS with CI and CII (PCI+II), titrations of carbonyl cyanide m-chlorophenyl hydrazone (CCCP) to determine ETS capacity with CI and CII (ECI+II), rotenone to determine ETS capacity with CII (ECII), and antimycin A to determine residual oxygen consumption (ROX).
HRR can not only provide a detailed assessment of whole muscle OXPHOS and ETS capacity but also provides valuable insight into the relative contribution of different complexes to overall capacity and other indices of mitochondrial efficiency. The relative contribution of different states of respiration to ETS capacity can be determined by calculating the flux control ratio (FCR) of each complex individually. The FCR of a given state, such as LEAK, CI capacity, or CII capacity, is calculated by dividing the flux of the state by a reference state, such as maximal ETS capacity. These calculations have the benefit of being independent of MitoVD and provide insight into the way that mitochondria function rather than simply at what rate they can function. This facilitates the determination of which complexes or states most likely contribute to observed differences in mitochondrial capacity. Additionally, the FCR for maximal OXPHOS relative to maximal ETS indicates the degree to which ATP synthase activity limits oxidative capacity and is thus an indication of ATP production efficiency.
When measures of MitoVD and mitochondrial capacity are combined, even more powerful data can be generated. By normalizing integrative (per tissue wet weight) oxidative and electron transfer capacities to MitoVD, intrinsic (per mitochondrial unit) mitochondrial capacities can be calculated. Consideration of integrative and intrinsic mitochondrial capacities together can provide insight into whether differences observed are due to intrinsic differences in mitochondrial function or due to differences in the number or size of mitochondria present.
Many of the described techniques have been used to characterize the impact of mitochondrial capacity on equine performance. These studies provide valuable information regarding beneficial adaptations to exercise training, differences between breeds, changes over the horse’s lifespan, contribution to pathology, and more. Notably, this research highlights the importance and specificity of mitochondrial function for performance in equine athletes.
Relationships between Mitochondria, Exercise, and Performance
As one would expect given the substantial role of mitochondria in energy production, exercise training imparts a myriad of adaptations to skeletal muscle mitochondria in equine athletes. Ten weeks of endurance exercise training enhanced OXPHOS capacity in both the gluteus medius and triceps brachii muscles of mature horses (Votion et al., 2010). This is unsurprising, as endurance-type conditioning programs consisting of long bouts (20 km) of low-intensity exercise (mainly walk and trot) are optimally designed to stimulate mitochondrial adaptations. However, work in young Thoroughbred racehorses also showed that shorter duration (5 to 25 min), high-intensity exercise training increased oxidative capacity (measured as SDH activity; Rivero et al., 2007). These results were supported by reports of increased proportion of oxidative type IIa fibers and skeletal muscle CS activity (Lindholm et al., 1983) as well as increases in genes enriched for CI and CV (Bryan et al., 2017) in response to race training in Thoroughbred horses. Preliminary data generated in our laboratory corroborate these previous reports of training-induced MitoVD increases and confirm that these changes drive increases in integrative CI, maximal OXPHOS, and maximal ETS capacities (Wesolowski et al., 2021).
Beyond overall increases in MitoVD and integrative OXPHOS and ETS capacities, there appear to be complex-specific adaptations to exercise training. Horses trained for competition exhibited almost 2-fold greater maximal OXPHOS and ETS capacities in the triceps brachii muscle compared with overweight horses, but competition trained horses also had a higher FCR for CI compared with overweight, untrained, and trained noncompetitive horses (Votion et al., 2012). This suggests that CI adaptations play a large role in the increase in oxidative capacity observed in response to exercise training. Furthermore, these results highlight the intrinsic exercise-induced adaptations that occur at the organelle level irrespective of volume density. Importantly, horses examined in this study participated in a wide variety of disciplines, including leisure riding, dressage, eventing, jumping, endurance, and racing. It should be noted that the exact nature of the exercise training of the horses was not described and, therefore, may have varied widely between horses and disciplines. However, our laboratory has also noted similar adaptations in young horses entering a training program at relatively low intensities of exercise. Following 9 wk of submaximal exercise, yearling horses had increased CI and maximal OXPHOS and ETS capacities, increased mitochondrial OXPHOS efficiency as indicated by the FCR for maximal oxidative capacity, and decreased contribution of LEAK respiration to maximal ETS capacity in the gluteus medius and triceps brachii muscles (White et al., 2017a).
While exercise training clearly enhances mitochondrial capacity and appears to most strongly impact CI, muscle fiber type and SDH activity have also been shown to differ between breeds in untrained adult horses (Stull and Albert, 1980; López-Rivero et al., 1989). Studies of young, untrained horses of various breeds show dissimilar muscle fiber type percentages (Bechtel and Kline, 1987; Galisteo et al., 1992) and different adaptations to maturation (Bechtel and Kline, 1987). While the aforementioned studies did not examine mitochondrial capacity or density directly, recently published research from our laboratory illustrates breed-specific differences in CS activity, CIV activity, maximal OXPHOS and ETS capacities, CII capacity, and relative contribution of CI to maximal ETS (FCRCI) in weanling racing-bred horses (Latham et al., 2019). These results support the idea that previously reported differences in muscle fiber type and SDH activity are likely accompanied by important differences in mitochondrial phenotype. Taken together, breed-specific differences in these measures suggest a fundamental link between selection for performance traits and mitochondrial phenotype. This link has been observed in a preliminary report of Thoroughbred racehorses, where the sales price was positively correlated with intrinsic (normalized to MitoVD) CIV activity and OXPHOS efficiency in weanlings (Guy et al., 2020a). Altogether, different breeds and even individuals within a breed seem to be selected for specific mitochondrial phenotypes, which presumably support performance in specific disciplines.
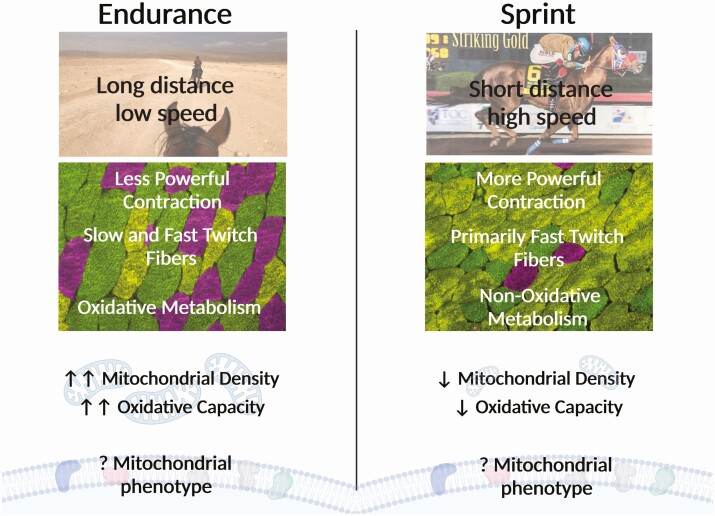
The mitochondrial phenotype required for optimal performance likely varies widely between equine sports and has not been closely studied. However, advantageous muscle fiber type distributions have been reported in a variety of disciplines and may indicate underlying differences in mitochondrial density and phenotype. Unsurprisingly, excellent performers in endurance competitions have a higher percentage and larger size of type I and type IIa oxidative fibers (Rivero et al., 1993). Taken together with what is known about adaptations to exercise training in endurance horses (Votion et al., 2010), these studies suggest that the larger percentage of oxidative muscle fibers necessary to support outstanding performance in equine endurance athletes may be underpinned by greater oxidative capacity and adaptations in CI capacity. Conversely, Quarter Horses with successful racing careers have been shown to have a higher percentage of fast-twitch low oxidative fibers and a lower percentage of slow-twitch oxidative fibers when compared with unsuccessful racing Quarter Horses (Wood et al., 1988). The differences between these seemingly opposite optimal muscle fiber phenotypes likely result from vastly different modes of training and competition exercise intensity, resulting in vastly different contractile and metabolic profile adaptations (Figure 4). Specifically, endurance competitions are characterized by racing over long distances at relatively low speeds, whereas Quarter Horse races are typically performed over short distances at high speeds. Consequently, successful endurance athletes develop a muscle phenotype to support long bouts of low-intensity muscle activity, which can be accomplished by a combination of slow- and fast-twitch muscle fibers but requires significant oxidative capacity to support sustained energy production. In contrast, successful racing Quarter Horses must be able to rapidly generate powerful muscle force that is sustained over a short period of time, which is optimally supported by an abundance of fast-twitch muscle fibers and can be fueled by glycolytic metabolism to meet short-lived energy demands (Figure 4). Thus, it is not surprising that adaptation to these vastly different disciplines results in muscle contractile and metabolic phenotypes that lay on nearly opposite ends of the equine performance spectrum.
Figure 4.
Skeletal muscle and mitochondrial phenotype differences between endurance and sprint sports. Endurance sports consist of long-distance exercise at low speeds. The muscle fiber phenotype is characterized by a greater abundance of type I (slow, pink) and IIa (fast, green) oxidative fibers, which together may provide less contractile power but contain more mitochondria and a higher oxidative capacity. Conversely, sprint sports utilize short-distance exercise at high speeds. The muscle fiber phenotype is characterized by a greater abundance of type IIx (fast non-oxidative, yellow) fibers, which may provide greater contractile power but contain less mitochondria and a lower oxidative capacity. Comparative mitochondrial phenotype related to these two skeletal muscle phenotypes has not yet been fully described. Created with Biorender.com.
More research is needed to determine mechanistic links between detailed measures of mitochondrial capacity and equine performance. Data of this nature could lead to improved recommendations for discipline-specific training programs that optimize performance and prevent injuries that result from fatigue due to suboptimal fitness levels. Recently, preliminary data from our laboratory have shown that race earnings of young Thoroughbreds (2 to 3 yr) are positively correlated with LEAK respiration and negatively correlated with CII capacity that was assessed in the gluteus medius muscle when they were weanlings (Guy et al., 2020b). This is in agreement with earlier research from our laboratory that showed that when compared with Standardbreds, Thoroughbred weanlings had greater LEAK capacity and a greater FCR for CI (Latham et al., 2019). Taken together, this suggests that early phenotypic differences in horses may be predictive of later career success.
In addition to exercise training adaptations and genetic differences between breeds and individuals, nutrition can impact mitochondrial density and function as well as antioxidant capacity. Supplementation of trace minerals that are cofactors of antioxidant enzymes, such as selenium, manganese, copper, and zinc, may contribute to mitochondrial health by preventing oxidative stress. Selenium supplementation has been shown to increase measures of MitoVD in young horses in light exercise training (White et al., 2017b). Further preliminary data from our laboratory indicate that selenium supplementation may support mitochondrial health in cases where vitamin E intake is restricted (Owen, 2019). Similarly, supplementation of a more bioavailable complexed trace mineral mix containing zinc, manganese, and copper resulted in higher GPx activity when compared with inorganic forms of the minerals in young horses in a light exercise training program (Latham et al., 2021). Conjugated linoleic acid (Mrugala et al., 2021), N-acetyl cysteine and coenzyme Q10 (Henry et al., 2021), and other supplements with antioxidant properties have also been shown to impact mitochondrial and antioxidant adaptations. These adaptations to nutritional supplementation provide promising management interventions to optimize mitochondrial function in exercising horses. Future research should focus on whether specialized nutritional interventions may benefit horses with specific mitochondrial phenotypes to improve performance.
Conclusion
Current research suggests that distinct mitochondrial phenotypes are present at a young age and are refined with exercise training. The combination of these and other factors such as nutritional inputs results in a mitochondrial phenotype that may impact the success of competitive equine athletes. Future research should focus on better characterizing breed, lineage, and sport-specific mitochondrial phenotypes. With this information, mitochondrial biology may serve to inform breeding, training, nutrition, and other management practices to optimize performance in equine athletes and decrease the incidence of fatigue-induced injury.
About the Authors
Dr Christine M. Latham, PhD, is a postdoctoral fellow at the University of Kentucky in Lexington, KY, studying skeletal muscle biology in the Department of Athletic Training and Clinical Nutrition. She completed her BS at the University of Florida studying Animal Science with a focus on equine science. She received her master’s degree from the University of Kentucky studying equine nutrition and her PhD from Texas A&M University studying equine nutrition and exercise physiology with a focus on mitochondrial skeletal muscle biology. Her research interests include equine exercise physiology, the physiology of aging, and optimization of skeletal muscle biology to support athletic performance in health and disease.

Chloey P. Guy is a research assistant with Texas A&M Agrilife Research, in Overton, TX. She completed her BS at Judson College in Marion, AL, with a double major in equine science and biology. She received her MS from Texas A&M University, studying equine exercise physiology. Her research interest includes equine nutrition and exercise physiology, with a focus on improving and quantifying equine performance.

Lauren T. Wesolowski is a master’s student at Texas A&M University in College Station, TX, studying equine skeletal muscle adaptations to exercise training and nutritional modifications. She completed her BS at Otterbein University in Columbus, OH, with a major in equine pre-veterinary science. Her research interests include equine skeletal muscle physiology, interventions to mitigate inflammation, and cellular adaptations to heat stress.

Dr Sarah H. White-Springer, PhD, is an Assistant Professor of Equine Physiology at Texas A&M University in College Station, TX. She received her BS in Animal Science with a focus on equine science and a minor in Management and Sales in Agribusiness from the University of Florida in Gainesville, FL. She also received her MS and PhD from the University of Florida majoring in Animal Science with an equine nutrition focus and a PhD minor in Applied Physiology and Kinesiology. Following completion of her PhD, she also completed postdoctoral training at the University of Kentucky in Lexington, KY, investigating human and murine models of muscle disease. Her current research interests include modulation of skeletal muscle metabolism and mitochondrial function to optimize performance in health and disease of equine athletes, beef cattle, swine, sheep, and aging humans.

Contributor Information
Christine M Latham, Department of Animal Science, Texas A&M University and Texas A&M AgriLife Research, College Station, TX, USA.
Chloey P Guy, Department of Animal Science, Texas A&M University and Texas A&M AgriLife Research, College Station, TX, USA.
Lauren T Wesolowski, Department of Animal Science, Texas A&M University and Texas A&M AgriLife Research, College Station, TX, USA.
Sarah H White-Springer, Department of Animal Science, Texas A&M University and Texas A&M AgriLife Research, College Station, TX, USA.
Literature Cited
- Bechtel, P., and Kline K.. . 1987. Muscle fiber type changes in the middle gluteal of quarter and Standardbred horses from birth through one year of age. Comp. Exerc. Physiol. 2:265–270. [Google Scholar]
- Bryan, K., McGivney B.A., Farries G., McGettigan P.A., McGivney C.L., Gough K.F., MacHugh D.E., Katz L.M., and Hill E.W.. . 2017. Equine skeletal muscle adaptations to exercise and training: evidence of differential regulation of autophagosomal and mitochondrial components. BMC Genomics 18(1):1–26. doi: 10.1186/s12864-017-4007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M., Fulton M., and Popken A.. . 2021. Effect of hyperthermia and acidosis on equine skeletal muscle mitochondrial oxygen consumption. Comp. Exerc. Physiol. 17(2):171–179. doi: 10.3920/CEP200041 [DOI] [Google Scholar]
- Galisteo, A.M., Agiüera E., Monterde J.G., and Miró F.. . 1992. Gluteus medius muscle fiber type composition in Young Andalusian and Arabian Horses. J. Equine Vet. Sci. 12(4):254–258. doi: 10.1016/S0737-0806(06)81459-0 [DOI] [Google Scholar]
- Glancy, B., Hartnell L.M., Malide D., Yu Z.X., Combs C.A., Connelly P.S., Subramaniam S., and Balaban R. S. . 2015. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 523(7562):617–620. doi: 10.1038/nature14614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger, E. 2008. Polarographic oxygen sensors, the oxygraph, and high-resolution respirometry to assess mitochondrial function. In: Dykens, J.A., and Will Y., editors. Drug-induced mitochondrial dysfunction. Mitochondr Physiol Network, Innsbruck. p. 325–352. [Google Scholar]
- Gnaiger, E. 2014. Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. Mitochondrial Physiol Network 19.12. Innsbruck: OROBOROS MiPNet Publications. [Google Scholar]
- Guy, C.P., Latham C.M., Owen R.N., Fowler A.L., and White-Springer S.H.. . 2020a. 107 Skeletal muscle mitochondrial parameters correlate with sales price in weanling racing-bred Thoroughbred horses. J. Anim. Sci. 98(Supplement_4):86. doi: 10.1093/jas/skaa278.157 [DOI] [Google Scholar]
- Guy, C.P., Latham C.M., Owen R.N., Fowler A.L., and White-Springer S.H.. . 2020b. 108 President Oral Presentation Pick: select skeletal muscle mitochondrial measures in Thoroughbred weanlings are related to race earnings and sire. J. Anim. Sci. 98(Supplement_4):85. doi: 10.1093/jas/skaa278.155 [DOI] [Google Scholar]
- Henry, M.L., Velez-Irizarry D., Pagan J.D., Sordillo L., Gandy J., and Valberg S.J.. . 2021. The impact of n-acetyl cysteine and coenzyme Q10 supplementation on skeletal muscle antioxidants and proteome in fit Thoroughbred horses. Antioxidants. 10(11): 1739– 1755. doi: 10.3390/antiox10111739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laner, V., Boushel R., Hamilton K., Miller B., Williamson K., Davis M., and Gnaiger E.. . 2014. Cytochrome c flux control factor as a quality criterion in respiratory OXPHOS analysis in canine permeabilized fibres. Mitochondr. Physiol. Network 19.13. [Google Scholar]
- Larsen, S., Nielsen J., Hansen C.N., Nielsen L.B., Wibrand F., Stride N., Schroder H.D., Boushel R., Helge J.W., and Dela F.. . 2012. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 590(14):3349–3360. doi: 10.1113/jphysiol.2012.230185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham, C.M., Dickson E.C., Owen R.N., Larson C.K., and White-Springer S.H.. . 2021. Complexed trace mineral supplementation alters antioxidant activities and expression in response to trailer stress in yearling horses in training. Sci. Rep. 11(1):1–12. doi: 10.1038/s41598-021-86478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham, C.M., Fenger C.K., and White S.H.. . 2019. Rapid Communication: Differential skeletal muscle mitochondrial characteristics of weanling racing-bred horses. J. Anim. Sci. 97(8):3193–3198. doi: 10.1093/jas/skz203 [DOI] [PubMed] [Google Scholar]
- Lindholm, A., Essén-Gustavsson B., McMiken D., Persson S., and Thornton J.. . 1983. Muscle histochemistry and biochemistry of Thoroughbred horses during growth and training. Equine Exerc. Physiol. 7:211–217. [Google Scholar]
- López-Rivero, J.L., Agüera E., Monterde J.G., Rodríguez-Barbudo M.V., and Miró F.. . 1989. Comparative study of muscle fiber type composition in the middle gluteal muscle of Andalusian, Thoroughbred and Arabian horses. J. Equine Vet. Sci. 9(6):337–340. doi: 10.1016/S0737-0806(89)80072-3 [DOI] [Google Scholar]
- López-Rivero, J.L., Agüera E., Rodríguez-Barbudo M.V., Galisteo A.M., and Morales-López J.L.. . 1990. Degree of correspondence between contractile and oxidative capacities in horse muscle fibres: a histochemical study. Histol. Histopathol. 5(1):49–53. [PubMed] [Google Scholar]
- Meinild Lundby, A.K., Jacobs R.A., Gehrig S., de Leur J., Hauser M., Bonne T.C., Fluck D., Dandanell S., Kirk N., Kaech A., . et al. 2018. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiol. 222(1): e12905. doi: 10.1111/apha.12905 [DOI] [PubMed] [Google Scholar]
- Miller, B., Hamilton K., Boushel R., Williamson K., Laner V., Gnaiger E., and Davis M.. . 2017. Mitochondrial respiration in highly aerobic canines in the non-raced state and after a 1600-km sled dog race. PLoS One. 12(4):e0174874. doi: 10.1371/journal.pone.0174874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrugala, D., Leatherwood J.L., Morris E.F., Dickson E.C., Latham C.M., Owen R.N., Beverly M.M., Kelley S.F., and White-Springer S.H.. . 2021. Dietary conjugated linoleic acid supplementation alters skeletal muscle mitochondria and antioxidant status in young horses. J. Anim. Sci. 99(2): 1– 9. doi: 10.1093/jas/skab037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess, A.M., and Simon P.. . 2007. Response and adaptation of skeletal muscle to exercise—the role of reactive oxygen species. Front. Biosci. 12:4826–4838. doi: 10.2741/2431 [DOI] [PubMed] [Google Scholar]
- Owen, R.N. 2019. Effects of decreased dietary vitamin E plus a proprietary antioxidant blend on mitochondria in young performance horses [M.S. thesis]. College Station (TX): Texas A&M University. [Google Scholar]
- Rivero, J.L., Ruz A., Martí-Korff S., Estepa J.C., Aguilera-Tejero E., Werkman J., Sobotta M., and Lindner A.. . 2007. Effects of intensity and duration of exercise on muscular responses to training of thoroughbred racehorses. J. Appl. Physiol. 102(5):1871–1882. doi: 10.1152/japplphysiol.01093.2006 [DOI] [PubMed] [Google Scholar]
- Rivero, J.L., Serrano A.L., Henckel P., and Agüera E.. . 1993. Muscle fiber type composition and fiber size in successfully and unsuccessfully endurance-raced horses. J. Appl. Physiol. 75(4):1758–1766. doi: 10.1152/jappl.1993.75.4.1758 [DOI] [PubMed] [Google Scholar]
- Rivero, J.L., Talmadge R.J., and Edgerton V.R.. . 1996. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in equine skeletal muscle and the influence of training. Anat. Rec. 246(2):195–207. doi: [DOI] [PubMed] [Google Scholar]
- Stull, C.L., and Albert W.W.. . 1980. Comparison of muscle fiber types from 2-year-old fillies of the Belgian, Standardbred, Thoroughbred, Quarter Horse and Welsh breeds. J. Anim. Sci. 51(2):340–343. doi: 10.2527/jas1980.512340x [DOI] [PubMed] [Google Scholar]
- Votion, D.M., Fraipont A., Goachet A.G., Robert C., van Erck E., Amory H., Ceusters J., de la Rebière de Pouyade G., Franck T., Mouithys-Mickalad A., . et al. 2010. Alterations in mitochondrial respiratory function in response to endurance training and endurance racing. Equine Vet. J. Suppl. 42( 38):268–274. doi: 10.1111/j.2042-3306.2010.00271.x [DOI] [PubMed] [Google Scholar]
- Votion, D.M., Gnaiger E., Lemieux H., Mouithys-Mickalad A., and Serteyn D.. . 2012. Physical fitness and mitochondrial respiratory capacity in horse skeletal muscle. PLoS One. 7(4):e34890. doi: 10.1371/journal.pone.0034890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski, L.T., Guy C.P., Simons J.L., Pagan J.D., and White-Springer S.H.. . 2021. Race training improves skeletal muscle mitochondrial volume density, function, and capacity in Thoroughbreds. J. Equine Vet. Sci. 100:103488. doi: 10.1016/j.jevs.2021.103488 [DOI] [Google Scholar]
- White, S.H., Warren L.K., Li C., and Wohlgemuth S.E.. . 2017a. Submaximal exercise training improves mitochondrial efficiency in the gluteus medius but not in the triceps brachii of young equine athletes. Sci. Rep. 7(1):14389. doi: 10.1038/s41598-017-14691-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, S.H., Wohlgemuth S., Li C., and Warren L.K.. . 2017b. Rapid Communication: Dietary selenium improves skeletal muscle mitochondrial biogenesis in young equine athletes. J. Anim. Sci. 95(9):4078–4084. doi: 10.2527/jas2017.1919 [DOI] [PubMed] [Google Scholar]
- Wood, C.H., Ross T.T., Armstrong J.B., and Hall D.C.. . 1988. Variations in muscle fiber composition between successfully and unsuccessfully raced Quarter Horses. J. Equine Vet. Sci. 8(3):217–220. doi: 10.1016/S0737-0806(88)80007-8 [DOI] [Google Scholar]