Psoriasis is a chronic, immune-mediated disease associated with increased risk of cardiovascular disease [1]. Subclinical atherosclerosis, carotid intima–media thickening, arterial stiffness and endothelial dysfunction have been reported in psoriasis [2]. Tumor necrosis factor-alpha (TNF-α) is central to the pathogenesis of psoriasis and contributes to endothelial dysfunction [3]. Circulating progenitor cells (PCs) represent an index of regenerative potential and are stimulated by injury. Low PC counts are predictors of adverse cardiovascular outcomes [4]. PCs are identified by expression of CD34 on mononuclear cells and concomitant expression of vascular endothelial growth factor receptor-2 (VEGFR2) identifies a sub-population enriched for endothelial PCs [5]. Early studies have suggested that psoriasis is associated with decreased PCs [6,7]. Etanercept, a competitive inhibitor of TNF-α, improves arterial stiffness and endothelial dysfunction in rheumatoid arthritis and may decrease cardiovascular disease risk [8]. Herein, we characterized endogenous regenerative capacity as the number of circulating PCs, endothelial function measured as flow-mediated vasodilation, and arterial stiffness in subjects with psoriasis, before and after TNF-α inhibition with etanercept. We hypothesized that subjects with psoriasis will have lower flow-mediated dilation, increased arterial stiffness, and lower PC levels compared to a matched healthy cohort, and that etanercept will improve these indices.
Subjects with psoriasis were consented and enrolled in a double-blind, placebo-controlled, cross-over study to receive subcutaneous injections of either etanercept at 50 mg twice weekly or placebo for 12 weeks. Subjects had determination of the Psoriasis Area and Severity Index, and underwent blood and vascular function testing at baseline and the end of each treatment period. The study was approved by the Emory Institutional Review Board.
Of 34 subjects enrolled, 6 failed screening, 7 withdrew, and 21 completed the study. Adults with psoriasis without recent (<3 months) change in medications were enrolled. Those with uncontrolled cardiovascular disease risk factors, etanercept, infliximab, prednisone or phototherapy in the previous 3 months, and history of tuberculosis infection were excluded. For comparison, a control group of 228 subjects free of acute illness was selected from those recruited in the Emory Predictive Health Initiative after matching for age, gender, race, body mass index and low-density lipoprotein level [9].
Endothelial function was measured as brachial artery flow-mediated dilation and nitroglycerin-mediated dilation at baseline, 12 weeks and 24 weeks and allometrically scaled as previously described [9]. Aortic pulse waveform, augmentation index and pulse wave velocity were assessed using a SphygmoCor device (Atcor Medical, NSW, Australia) as previously described [9]. Cell populations enriched for circulating PCs were enumerated using flow cytometry as CD45med cells co-expressing CD34, CD133, VEGFR2, or CXCR4 and their combination as described previously [10]. Circulating CD45med mononuclear cells enriched for hematopoietic PCs include those expressing CD34 and/or CD133 and/or CXCR4 epitopes. The sub-population of CD34+ cells also co-expressing the VEGFR2+ epitope is considered to be enriched for endothelial PCs.
Normally distributed variables – pulse wave velocity and augmentation index – were analyzed using a linear mixed effects model for repeated measures and non-normally distributed variables (PC counts) using the Mann–Whitney U test or Wilcoxon Signed Rank test. Correlation analyses used Pearson's correlation for normally and Kendall's Tau for non-normally distributed variables.
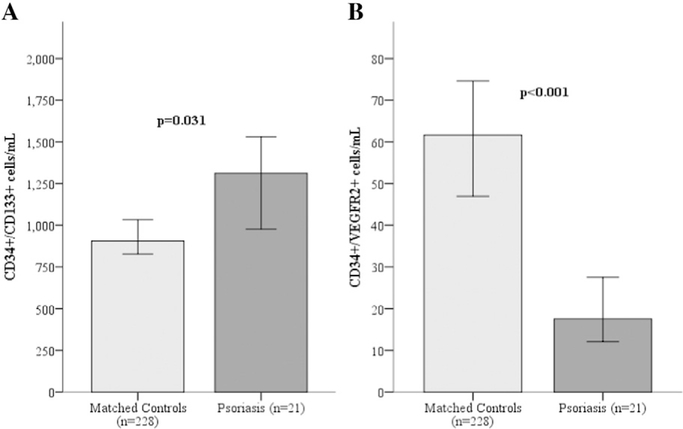
Enrolled subjects were relatively young, bi-racial individuals with mild psoriasis (Table 1). There were no statistically significant differences between subjects with psoriasis and matched controls. Psoriasis severity decreased with etanercept compared to placebo (5.8 (3.9, 11.3 [IQR]) and 2.9 (1.3, 6.2 [IQR]), respectively, p = 0.003). Blood pressure, body mass index, low-density lipoprotein levels and creatinine clearance remained unchanged with etanercept. Cells enriched for hematopoietic PCs (CD34+, CD34+/CD133+, and CD34+/CD133+/CXCR4+) were higher in subjects with psoriasis compared to controls (Fig. 1 and Table 2). In contrast, cells enriched for endothelial PCs (CD34+/VEGFR2+, CD34+/CD133+/VEGFR2+ and CD34+/CXCR4/VEGFR2+) were lower in psoriasis compared to controls. Treatment with etanercept produced an almost two-fold increase in endothelial PCs compared to placebo (Table 2). There was also a trend to a significant negative correlation between the baseline psoriasis severity and CD34+/VEGFR2+ PC counts (−0.28, p = 0.068), suggesting lower counts in those with more severe psoriasis. Flow-mediated dilation, pulse wave velocity and augmentation index were similar in participants with psoriasis compared to controls and remained unchanged with etanercept therapy (Table 2).
Table 1.
Clinical characteristics and demographics.
| Variables | Psoriasis (n = 21) | Control (n = 228) |
|---|---|---|
|
| ||
| Age, years | 42 (12) | 45 (9) |
| Male, n (%) | 10 (44%) | 93 (41%) |
| Body mass index, kg/m2 | 33 (11) | 30 (7) |
| Gender | ||
| White, n (%) | 11 (48) | 145 (64) |
| Black, n (%) | 12 (52) | 83 (36) |
| Clinical characteristics | ||
| Psoriasis area and severity index | 8 (5, 12 [IQR]) | – |
| Systolic blood pressure, mm Hg | 123 (19) | 123 (17) |
| Diastolic blood pressure, mm Hg | 78 (12) | 78 (11) |
| Hypertension, n (%) | 6 (25%) | 69 (30%) |
| Diabetes mellitus, n (%) | 1 (4%) | 18 (8%) |
| Low-density lipoprotein, mg/dL | 115 (45) | 106 (28) |
| High-density lipoprotein, mg/dL | 49 (12) | 60 (17) |
| Triglycerides, mg/dL | 135 (88) | 101 (50) |
| Glucose, mg/dL | 92 (10) | 89 (17) |
| Creatinine, mg/dL | 0.90 (0.19) | 0.85 (0.15) |
Variables are reported as mean (SD) or median (IQR) and n (%) where indicated.
Fig. 1.
Hematopoetic and endothelial progenitor cell counts in subjects with psoriasis and matched controls.
Table 2.
Endothelial function, arterial stiffness, and circulating progenitor cells in subjects with psoriasis and controls.
| Variables | Control | p-Value psoriasis vs. control | Psoriasis |
||
|---|---|---|---|---|---|
| Placebo | Etanercept | p-Value | |||
|
| |||||
| Endothelial function | |||||
| Brachial artery diameter, mm | 3.41 (0.62) | 0.5 | 3.52 (0.86) | 3.49 (0.88) | 0.6 |
| Flow-mediated dilation, % | 6.7 (3.5) | 0.2 | 8.77 (5.99) | 8.28 (5.25) | 0.7 |
| Arterial stiffness | |||||
| Augmentation index at HR 75 bpm, % | 19.9 (14.0) | 0.9 | 19.25 (13.38) | 19.90 (13.97) | 0.9 |
| Pulse wave velocity, m/s | 7.21 (1.20) | 0.7 | 7.24 (1) | 6.97 (1) | 0.7 |
| Hematopoietic progenitor cells | |||||
| CD34+ (cells/mL) | 2000 (1300, 2900) | 0.01 | 2900 (1700, 3700) | 2400 (1700, 3500) | 0.1 |
| CD34+/CD133+ (cells/mL) | 900 (600, 1400) | 0.031 | 1300 (800, 1800) | 1100 (800, 1700) | 0.8 |
| CD34+/CXCR4+ (cells/mL) | 800 (480, 1280) | 0.08 | 990 (660, 1480) | 1110 (610, 1770) | 0.8 |
| CD34+/CD133+/CXCR4+ (cells/mL) | 350 (200, 520) | 0.045 | 420 (280, 730) | 510 (280, 770) | 0.6 |
| Endothelial progenitor cells | |||||
| CD34+/VEGFR2+ (cells/mL) | 62 (26, 147) | <0.001 | 15 (6, 36) | 26 (6, 46) | 0.029 |
| CD34+/CD133+/VEGFR2+ (cells/mL) | 28 (13, 65) | <0.001 | 6 (0, 14) | 12 (6, 19) | 0.6 |
| CD34+/CXCR4+/VEGFR2+ (cells/mL) | 60 (30, 141) | <0.001 | 13 (6, 36) | 26 (6, 41) | 0.012 |
For endothelial function and arterial stiffness values are mean (SD). Progenitor cell counts are reported as median (25th, 75th percentile). p-values <0.05 are in bold.
Chronic inflammatory diseases including psoriasis are associated with cardiovascular disease [1]. We demonstrated that sub-clinical vascular disease including endothelial function and arterial stiffness were not significantly different between subjects with mild psoriasis and matched controls, and treatment with the TNF-α antagonist, etanercept did not affect vascular function. Further, we studied whether regenerative capacity measured as circulating PCs is altered in psoriasis. Compared to a matched control group, subjects with mild psoriasis had higher counts of PCs enriched for hematopoietic progenitors and lower endothelial PC counts. Treatment with etanercept resulted in a two-fold increase in endothelial PCs. Previous studies in subjects with psoriatic arthritis or moderately severe psoriasis have reported the presence of endothelial dysfunction, but findings in mild psoriasis have been contradictory [11]. Three studies have reported increased arterial stiffness in subjects with psoriatic arthritis or moderately severe psoriasis [11]. In comparison, our cohort was relatively young, had mild psoriasis, no arthritis and few risk factors, features that may explain the lack of endothelial dysfunction and arterial stiffness.
Circulating PC levels are modulated with exposure to risk factors and predict cardiovascular events [4]. Two previous studies reported reduced numbers of endothelial PCs (CD34+/VEGFR2+) in psoriasis, a finding confirmed in our study [6,7]. We also found that circulating numbers of hematopoietic PCs were higher in psoriasis compared to controls, a finding we previously also noted in younger individuals exposed to multiple risk factors or injury. Thus, subjects will mild psoriasis appear to have impairment of their circulating endothelial PCs and stimulated numbers of hematopoietic PCs, even in the absence of subclinical signs of vascular dysfunction. Interestingly, treatment with etanercept increased the endothelial PC count indicating that it improved endogenous endothelial regenerative capacity. These findings are consistent with a similar study performed in patients with longstanding rheumatoid arthritis on corticosteroids [12], further reinforcing the hypothesis that TNF-α is the common mediator of PC dysregulation in autoimmune diseases, which is at least partially reversible with TNF-α antagonism. Our findings are limited to subjects with mild psoriasis without known cardiovascular disease which may be the reason for the lack of vascular dysfunction observed.
Funding sources
This study was supported by Amgen Inc. award reference number 20080502 (Thousand Oaks, CA) and by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources
Footnotes
Conflict of interest
None of the authors have conflicts of interest to disclose.
ClinicalTrials.gov IDENTIFIER: NCT01989689, URL: http://clinicaltrials.gov/ct2/show/NCT01989689.
References
- [1].Gaeta M, Castelvecchio S, Ricci C, Pigatto P, Pellissero G, Cappato R, Role of psoriasis as independent predictor of cardiovascular disease: a meta-regression analysis, Int. J. Cardiol. 168 (3) (2013) 2282–2288. [DOI] [PubMed] [Google Scholar]
- [2].Shaharyar S, Warraich H, McEvoy JW, Oni E, Ali SS, Karim A, et al. , Subclinical cardiovascular disease in plaque psoriasis: association or causal link? Atherosclerosis 232 (1) (2014) 72–78. [DOI] [PubMed] [Google Scholar]
- [3].Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, et al. , TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury, Arterioscler. Thromb. Vasc. Biol. 26 (3) (2006) 475–480. [DOI] [PubMed] [Google Scholar]
- [4].Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. , Circulating endothelial progenitor cells and cardiovascular outcomes, N. Engl. J. Med. 353 (10) (2005) 999–1007. [DOI] [PubMed] [Google Scholar]
- [5].Krause DS, Fackler MJ, Civin CI, May WS, CD34: structure, biology, and clinical utility [see comments], Blood 87 (1) (1996) 1–13. [PubMed] [Google Scholar]
- [6].Ablin JN, Goldstein Z, Aloush V, Matz H, Elkayam O, Caspi D, et al. , Normal levels and function of endothelial progenitor cells in patients with psoriatic arthritis, Rheumatol. Int. 29 (3) (2009) 257–262. [DOI] [PubMed] [Google Scholar]
- [7].Batycka-Baran A, Paprocka M, Krawczenko A, Kantor A, Dus D, Szepietowski JC, Reduced number of circulating endothelial progenitor cells (CD133+/KDR+) in patients with plaque psoriasis, Dermatology 225 (1) (2012) 88–92. [DOI] [PubMed] [Google Scholar]
- [8].Maki-Petaja KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, et al. , Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy, Circulation 114 (11) (2006) 1185–1192. [DOI] [PubMed] [Google Scholar]
- [9].Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, et al. , Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans, J. Am. Coll. Cardiol. 58 (2) (2011) 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Al Mheid I, Corrigan F, Shirazi F, Veledar E, Li Q, Alexander WR, et al. , Circadian variation in vascular function and regenerative capacity in healthy humans, J. Am. Heart Assoc. 3 (3) (2014) e000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brezinski EA, Follansbee MR, Armstrong EJ, Armstrong AW, Endothelial dysfunction and the effects of TNF inhibitors on the endothelium in psoriasis and psoriatic arthritis: a systematic review, Curr. Pharm. Des. 20 (4) (2014) 513–528. [DOI] [PubMed] [Google Scholar]
- [12].Spinelli FR, Metere A, Barbati C, Pierdominici M, Iannuccelli C, Lucchino B, et al. , Effect of therapeutic inhibition of TNF on circulating endothelial progenitor cells in patients with rheumatoid arthritis, Mediat. Inflamm. 2013 (2013) 537539. [DOI] [PMC free article] [PubMed] [Google Scholar]