Abstract
Background
COVID-19 disproportionately impacted patients with cancer as a result of direct infection, and delays in diagnosis and therapy. Oncological clinical trials are resource-intensive endeavors that could be particularly susceptible to disruption by the pandemic, but few studies have evaluated the impact of the pandemic on clinical trial conduct.
Patients and methods
This prospective, multicenter study assesses the impact of the pandemic on therapeutic clinical trials at two large academic centers in the Northeastern United States between December 2019 and June 2021. The primary objective was to assess the enrollment on, accrual to, and activation of oncology therapeutic clinical trials during the pandemic using an institution-wide cohort of (i) new patient accruals to oncological trials, (ii) a manually curated cohort of patients with cancer, and (ii) a dataset of new trial activations.
Results
The institution-wide cohort included 4756 new patients enrolled to clinical trials from December 2019 to June 2021. A major decrease in the numbers of new patient accruals (−46%) was seen early in the pandemic, followed by a progressive recovery and return to higher-than-normal levels (+2.6%). A similar pattern (from −23.6% to +30.4%) was observed among 467 newly activated trials from June 2019 to June 2021. A more pronounced decline in new accruals was seen among academically sponsored trials (versus industry sponsored trials) (P < 0.05). In the manually curated cohort, which included 2361 patients with cancer, non-white patients tended to be more likely taken off trial in the early pandemic period (adjusted odds ratio: 2.60; 95% confidence interval 1.00-6.63), and substantial pandemic-related deviations were recorded.
Conclusions
Substantial disruptions in clinical trial activities were observed early during the pandemic, with a gradual recovery during ensuing time periods, both from an enrollment and an activation standpoint. The observed decline was more prominent among academically sponsored trials, and racial disparities were seen among people taken off trial.
Key words: clinical trials, COVID-19, cancer
Introduction
The novel SARS-CoV-2 and the associated clinical disease COVID-19 has caused significant morbidity and mortality in the USA, with >50 million cases of the disease and >800 thousand deaths reported as of December 2021.1 Patients with cancer have been disproportionately affected by the pandemic due to delays in new diagnoses,2 , 3 widespread disruption of routine oncologic care,4 , 5 and severe outcomes when they develop COVID-19.6 , 7
Compared with standard of care therapies, clinical trials may be more susceptible to care disruption due to the additional resources required. Treating patients on interventional studies requires a large team (clinical, regulatory, and administrative) and often mandates additional investigations (such as radiological studies or tissue biopsy) and frequent clinical visits. Despite these challenges, the maintenance of clinical trials is imperative to advancing cancer care. In addition, clinical trials allow patients with cancer to access novel and potentially life-prolonging therapies. To ensure the continuity of clinical trials within the USA, a framework for the conduct of clinical trials during the pandemic was issued by the US Food and Drug Administration in August 2021,8 including guidance regarding how trial sponsors could manage trial deviations and amendments that are inherent to the COVID-19 pandemic.
This report from the COVID-19 and Cancer Outcomes Study (CCOS), a multicenter, prospective cohort study of patients with cancer, aims to assess the impact of the pandemic on therapeutic clinical trials at two large academic centers in the Northeastern United States.
Methods
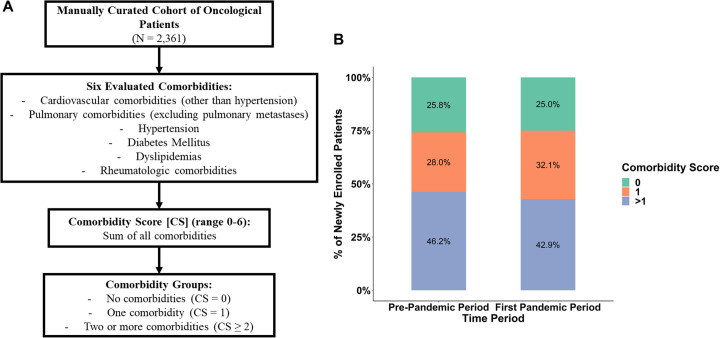
We evaluated clinical trial activities at two large cancer centers [Dana-Farber Cancer Institute, Boston, MA (DFCI) and the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai (ISMMS)] using two types of cohorts: an institution-wide cohort of all new patient accruals to oncological trials (hereafter called institution-wide cohort), and a smaller manually curated cohort of patients (hereafter called manually curated cohort) linked to an electronic data capture platform (REDCap database)9 with a larger set of variables. The institution-wide cohort included all new patient accruals to oncological clinical trials at DFCI and MSSM from December 2019 to June 2021 and captured general patient- and trial-related characteristics. The manually curated cohort consisted of adult patients with a current or past history of malignancy who had an outpatient medical oncology visit on the index week (2-6 March 2020) at DFCI or MSSM. Patient-, cancer-, and treatment-related variables were captured in the manually curated cohort, both during the 3 months before the index week (referred to as the baseline period) and the following 3 months (first pandemic period). Among patient-related variables in the manually curated cohort, six different types of comorbidities were evaluated, and a comorbidity score was calculated for each patient (Supplementary Figure S1A, available at https://doi.org/10.1016/j.annonc.2022.04.071). Additionally, we evaluated new oncological clinical trial activations at DFCI from June 2019 to June 2021 (i.e. trial activation dataset). In the institution-wide cohort of new patient accruals and the trial activation dataset, periods were defined as 3-month intervals. The study was approved by the institutional review boards of DFCI and MSSM.
The primary objective was to assess the enrollment on and accrual to oncology therapeutic clinical trials and to evaluate changes in oncological trials activation during the different time periods from December 2019 to June 2021. Secondary objectives included assessing changes in patient- and trial-related characteristics with respect to study accrual (both in the institution-wide and manually curated cohorts), and analyzing the incidence of trial deviations and serious adverse events (SAEs) and their attribution as pandemic-related or not, defined as a diagnosis of, trial deviation from, or adverse event secondary to COVID-19, or changes in assessments to limit patient health care exposures (in the manually curated cohort only). The percent of change in new patient accruals during each pandemic period compared with the immediate pre-pandemic period (December 2019 to March 2020) and the percent of change in new clinical trial activations during each pandemic period compared with the average of the three pre-pandemic periods (from June to December 2019) were calculated as: (Nperiod−Ncontrol)/Ncontrol. Multivariable logistic regression models evaluated the correlates of trial recruitment in the manually curated cohort, with list-wise deletions for missing data. Self-reported race and ethnicity (non-Hispanic white or non-white), and cancer center were included as independent variables. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were reported. Fisher’s exact test was used to compare the percentage of racial groups and trial categories (i.e. sponsor type) at the institution-wide level during the time periods included, and analyze the percentage of deviations and SAEs between the baseline and pandemic periods in the manually curated cohort. All tests were two-tailed and considered statistically significant for P < 0.05. The Mann–Whitney U test was used to perform pairwise comparisons of age between all time periods (institution-wide cohort). Median follow-up was determined using the inverse Kaplan–Meier method. Adjustment for multiple comparison (when applicable) was carried out using the Benjamini–Hochberg method. A P value (or q value, when applicable) of <0.05 was considered to be statistically significant. All analyses were done in the R statistical environment (v3.6.1).
Results
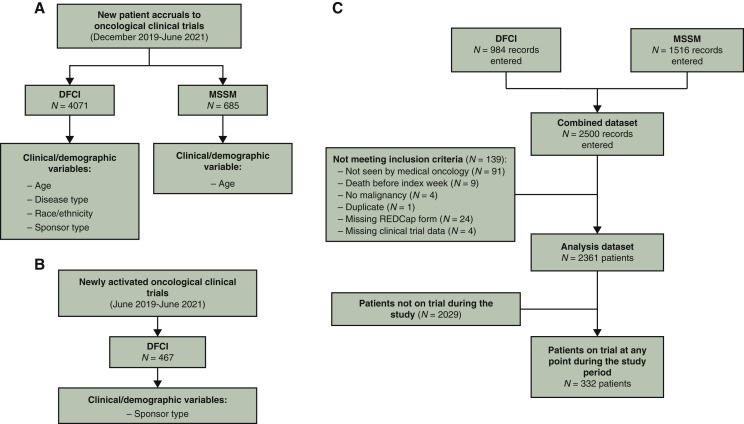
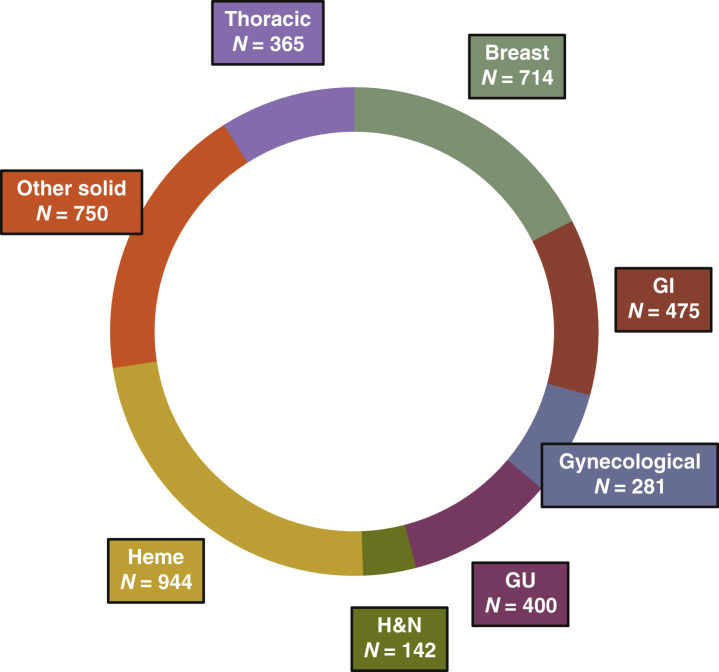
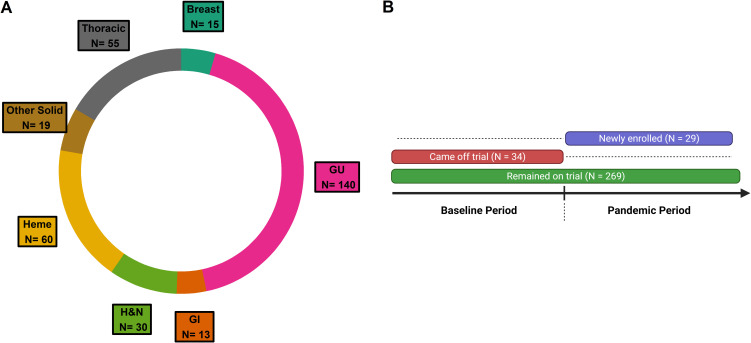
The institution-wide cohort had a total of 4756 newly enrolled patients with a specific set of variables available for each cancer center (i.e. DFCI and MSSM) (Figure 1 A). The mean age in the institution-wide cohort was 58.2 years [standard deviation (SD) 16.1 years]. Among newly enrolled patients from DFCI (n = 4071), 3579 (87.9%) self-identified as non-Hispanic white, 118 (2.9%) as non-Hispanic black, 123 (3.0%) as Hispanic or Latino, and 141 (3.5%) as Asian. All major cancer types were fairly represented among newly enrolled patients across DFCI from December 2019 to June 2021, as shown in Figure 2 . The trial activation dataset had a total of 467 new trial activations from June 2019 to June 2021 at DFCI (Figure 1B).
Figure 1.
(A) Flowchart representing the number of new patient accruals to oncological trials in the institution-wide cohort from DFCI and MSSM, with available patient- and trial-related variables. (B) Flowchart representing the number of newly activated trials included in the clinical trial dataset from DFCI. (C) Flowchart representing the number of patients included from DFCI and MSSM in the manually curated cohort, with numbers of patients excluded with exclusion criteria, patients included in the analysis, and the number of patients who were on trial at any point during the study.
DFCI, Dana-Farber Cancer Institute; MSSM, Mount Sinai School of Medicine.
Figure 2.
Donut plot representing the distribution by cancer type of all patients newly enrolled to oncological clinical trials across all DFCI (n = 4071).
GI, gastrointestinal; GU, genitourinary; H&N, head and neck; Heme, Hematologic.
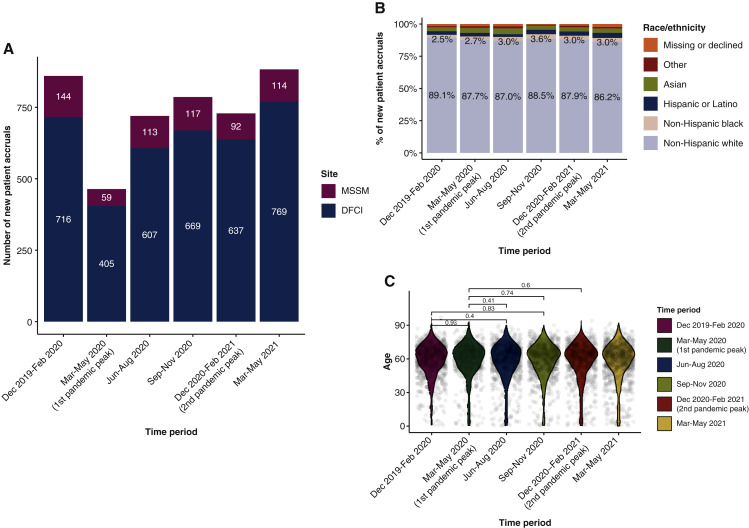
Compared with the immediate pre-pandemic period (December 2019 to February 2020), there was a considerable decrease (−46%) in the numbers of newly enrolled patients early in the pandemic (March to May 2020), followed by a progressive recovery during the subsequent periods and a return to normal levels from March to May 2021 (+2.7% compared with the pre-pandemic period), with a similar pattern seen at both cancer centers (Figure 3 A). There were no statistically significant shifts in the racial distribution of newly enrolled patients at DFCI between all the time periods evaluated (pairwise Fisher’s exact test: q > 0.05; Figure 3B). No significant age differences were identified between the different time periods (pairwise Mann–Whitney U test: q > 0.05; Figure 3C).
Figure 3.
(A) Bar plot representing the number of newly enrolled patients to oncological clinical trials at the institution-wide level at DFCI and MSSM, across the different time periods (3-month intervals). (B) Racial distribution of newly enrolled patients to oncological clinical trials in the institution-wide cohort at DFCI during the studied time periods. (C) Age distribution among newly enrolled patients at DFCI (institution-wide level) during the different time periods, with pairwise comparisons (using Mann–Whitney U test). Only selected pairwise comparisons are shown in the figure, though all pairwise comparisons had P > 0.05.
DFCI, Dana-Farber Cancer Institute; MSSM, Mount Sinai School of Medicine.
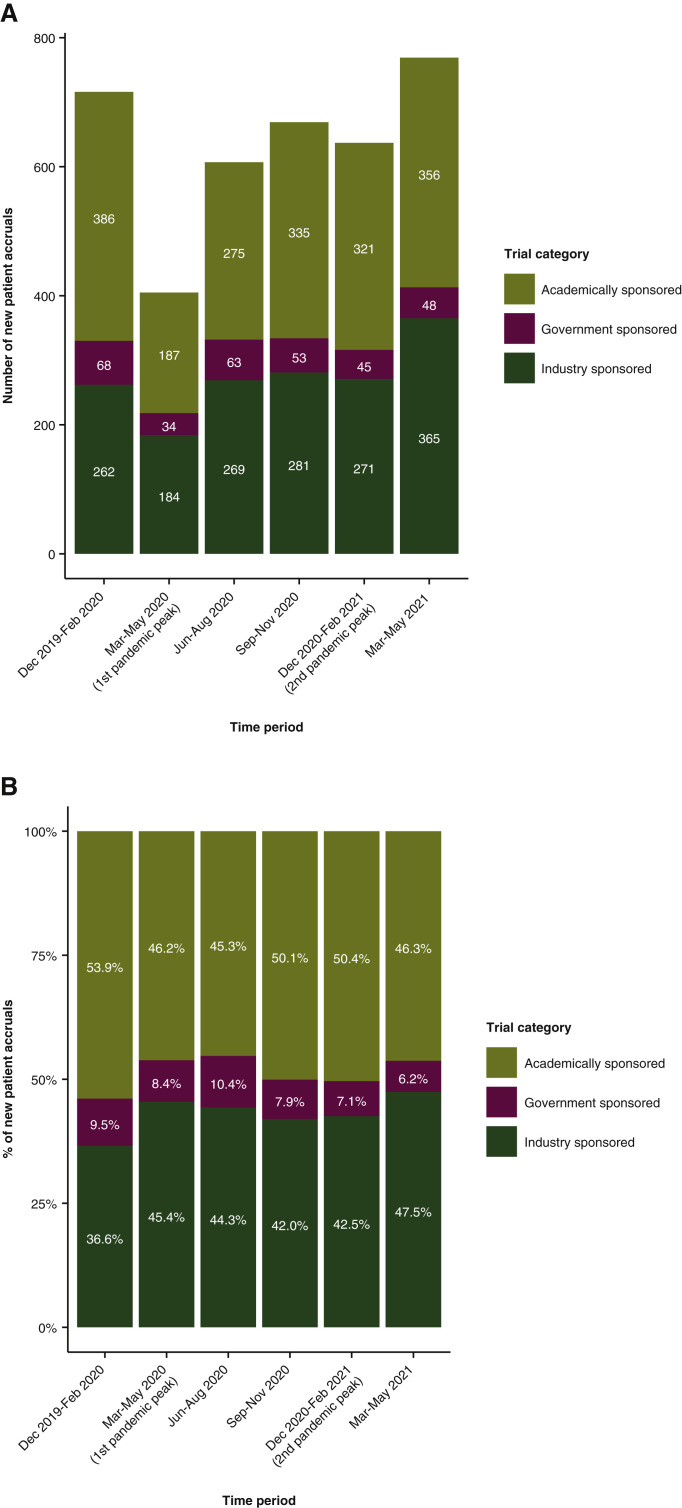
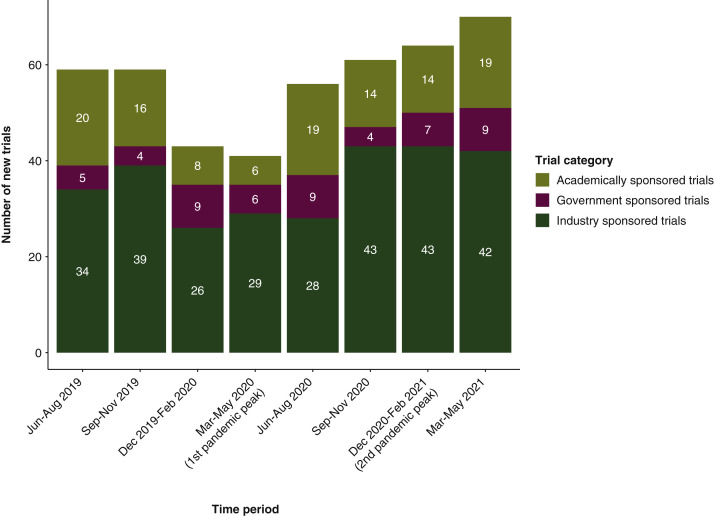
When evaluating clinical trials according to sponsor type (i.e. academic versus industry versus government sponsored trials) among newly enrolled patients at DFCI (n = 4071), there was a statistically significant increase in the proportion of new patients enrolled to industry sponsored trials between the pre-pandemic period (December 2019 to February 2020) and three of the pandemic periods [March to May 2020 (q = 0.04), June to August 2020 (q = 0.04), and March to May 2021 (q = 0.001)] (Figure 4A and B). This contrasted with a significant decrease in the proportion of new patients enrolled to academically sponsored trials between the pre-pandemic period and two pandemic periods [June to August 2020 (q = 0.03) and March to May 2021 (q = 0.04)] (Figure 4A and B).
Figure 4.
Bar plot representing (A) the numbers and (B) the percentages of newly enrolled patients at the institution-wide level at DFCI according to trial categories (i.e. sponsor type).
DFCI, Dana-Farber Cancer Institute.
Similar to what was seen in new patient accruals, the numbers of newly activated oncological trials at DFCI (n = 467) experienced a major decline (−23.6%) early in the pandemic (i.e. March to May 2020), followed by a gradual increase during later periods, eventually reaching levels higher than before the pandemic (+30.4%) in the most recent period (March to May 2021) (Figure 5 ). Moreover, this pattern of evolution appeared to be consistent across all major trial sponsor categories (Figure 5).
Figure 5.
Bar plot representing the numbers of new activated trials across DFCI during the different time periods from June 2019 to May 2021.
DFCI, Dana-Farber Cancer Institute.
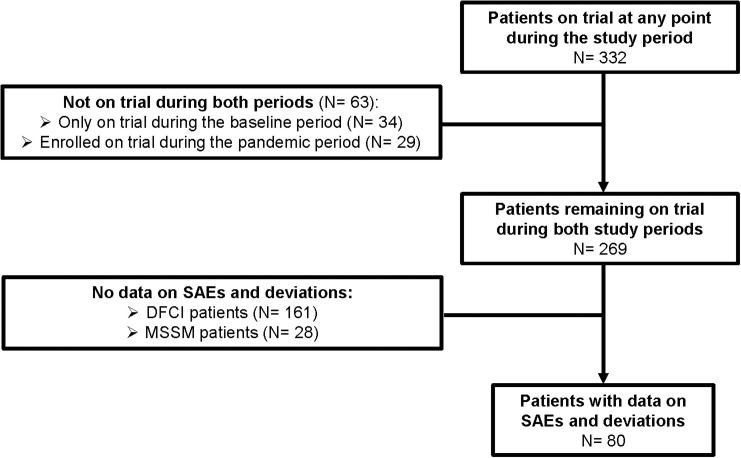
Of 2500 patients with available data in the manually curated cohort, 2361 patients with therapeutic clinical trial data were eligible for analysis (Figure 1C). The mean age in the manually curated cohort was 64.2 years (SD 13.1 years) and 1086 patients (46.0%) were female. With respect to racial and ethnic distribution, 1342 (63.8%) self-identified as non-Hispanic white, 299 (14.2%) were non-Hispanic black, 297 (14.1%) were Hispanic, and 167 (7.9%) were other non-white. At a median follow-up of 84 days (95% CI 82-84 days), 128 (5.5%) patients were diagnosed with COVID-19; 122 infections were confirmed with a PCR or antibody test (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2022.04.071). Three hundred and thirty-two patients were on a clinical trial at any time point ( Figure 1C), encompassing at varying proportions all major cancer types (Supplementary Figure S2A, available at https://doi.org/10.1016/j.annonc.2022.04.071). Two hundred and sixty-nine patients were on trial during the baseline period (pre-pandemic period: December 2019 to March 2020) and remained on trial during the first pandemic period (March to June 2020). Thirty-four patients who were on trial during the baseline period came off trial during the first pandemic period, the majority [18 (52.9%)] as a result of disease progression. No patients came off trial secondary to developing COVID-19 or as a result of other pandemic-related disruptions (Supplementary Figure S2B, available at https://doi.org/10.1016/j.annonc.2022.04.071). Among the patients in the manually curated cohort (n = 2361), only 29 (14 DFCI, 15 MSSM) patients were newly enrolled during the pandemic period, compared with 97 (58 DFCI, 39 MSSM) during the prior 3 months.
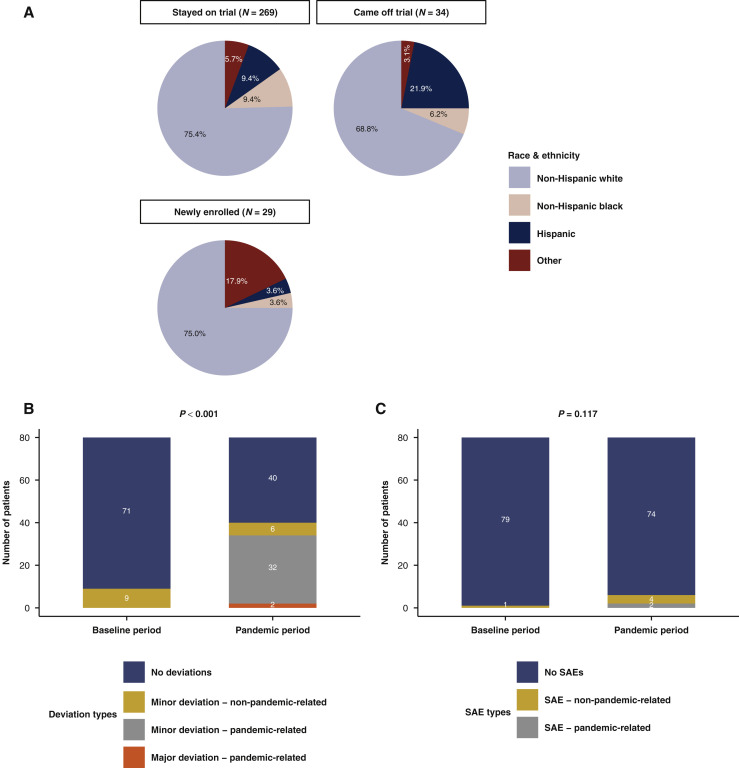
Of the patients who remained on trial during both time periods (i.e. from December 2019 to June 2020), 75.4% were non-Hispanic white. Of the patients newly enrolled into trials during the first pandemic period, 75.0% were non-Hispanic white, while 68.8% of the patients coming off trial during the same period were non-Hispanic white (Figure 6 A). Among patients on trials during the baseline period, non-white patients tended to be more likely to be taken off trial during the first pandemic period compared with white patients (aOR 2.60; 95% CI 1.00-6.63; controlling for cancer center). Patients enrolled into clinical trials during the early pandemic were similar in terms of race/ethnicity to patients who were on trials at baseline and remained so during the pandemic period. Additionally, no significant changes were observed in the distribution of enrolled patients according to comorbidity scores between the baseline and first pandemic periods (Supplementary Figure S1B, available at https://doi.org/10.1016/j.annonc.2022.04.071).
Figure 6.
(A) Pie charts representing the breakdown of race and ethnicity by clinical trial participation group in the manually curated cohort (n = 332). (B) Stacked bar plot representing clinical trial deviations during the baseline and pandemic periods (P value from Fisher’s exact test for deviation versus no deviation reported) in the manually curated cohort (n = 332). (C) Stacked bar plot representing clinical trial serious adverse events during the baseline and pandemic periods (P value from Fisher’s exact test for deviation versus no deviation reported) in the manually curated cohort (n = 332). The baseline period was defined as the pre-pandemic period from December 2019 to March 2020. The pandemic period was defined as the first peak of the pandemic of the Northeastern United States from March to June 2020.
SAE, serious adverse event.
Secondary objectives included assessing the incidence of trial deviations and SAEs and their attribution as pandemic-related, defined as a diagnosis of, trial deviation from, or adverse event secondary to COVID-19, or changes in patient assessments to limit patient and health care worker exposures. A total of 80 of 108 patients from MSSM on trial during both periods had data on deviations and SAEs available (Supplementary Figure S3, available at https://doi.org/10.1016/j.annonc.2022.04.071). During the pandemic period, 40 patients had trial deviations recorded (32 minor and pandemic-related; 2 major and pandemic-related; and 6 minor and non-pandemic-related), which was significantly increased compared with 9 trial deviations for the same patients during the baseline period (P < 0.001; all minor and non-pandemic-related; Figure 6B). SAEs were not more frequent (P = 0.117) during the pandemic period (six SAEs, two of which were pandemic-related) compared with the baseline period (one non-pandemic-related SAE; Figure 6C).
Discussion
During the first peak of the pandemic in the Northeastern United States, significant disruptions to clinical trial conduct were observed at two large academic cancer centers, with a substantial decrease in the numbers of new enrolled patients and newly activated oncological trials. This major decline likely reflects the strain imposed on the health care system during the pandemic as resources were diverted towards immediate hospital and patient needs. Afterwards, a progressive recovery was identified during subsequent periods, both in patient accruals and trial activations, eventually reaching normal levels in most recent times at the two institutions despite the ongoing nature of the pandemic. Hence, while the COVID-19 pandemic resulted in a major alteration of clinical trial organization, academic institutions were able to respond to these unprecedented circumstances, progressively adapting their systems accordingly. This is further attested by a lower decrease in the numbers of newly enrolled patients during the second and most severe peak of the pandemic (from December 2020 to February 2021), compared with the early pandemic period (March to May 2020).
Despite the disruption in clinical trial activities, no major changes were seen in the racial distribution and age of newly enrolled patients during the different pandemic periods, compared with pre-pandemic times. With respect to study sponsor type and new patient accruals, however, there appeared to be a shift towards a higher proportion of industry sponsored trials and a lower proportion of academically sponsored trials. This perhaps shows a better adaptation among industry sponsored clinical activities. Also, academic trials can be more resource-intensive endeavors, often requiring research biopsies and more visits, which could have limited accrual.
Although the majority of patients previously enrolled in clinical trials remained on trial during the first pandemic period in the manually curated cohort, non-white patients were more likely than white patients to be taken off trial during the first pandemic period. The differences in clinical trial enrollment during the pandemic based on race warrants further investigation, though this finding aligns with prior studies suggesting significant racial disparities in care during this time.4 , 10 In addition, more deviations were recorded during the pandemic period and were predominantly pandemic-related, leading to increased administrative workload in logging deviations and communicating with the trial sponsor.
Limitations of this study include the inability to include an external cohort from another academic institution or cooperative group to further validate our current findings, the moderate sample size of the manually curated cohort (n = 332 patients), and differential ability to trace changes in broader study accrual at the two sites.
The lessons learned during this challenging time may improve patient clinical trial access and protocol adaptability in the long term. Many studies with strict time points for clinical examination, blood tests, and imaging were forced to deviate from protocol due to the pandemic, as was observed in our study, further building on previous evidence reported at DFCI early in the pandemic.11 Although the impact of these changes on results remains unknown at this time, this flexibility has been viewed favorably by patients and researchers.12 , 13 In the future, it is likely that trials could be designed with more flexible timelines and reduced data collection requirements without negative consequences. Furthermore, efforts to sustain clinical trial enrollment and safely adapt research practices during the pandemic through the use of telehealth, remote monitoring, and shipment of therapeutic agents11 , 14 , 15 are crucial to both ensuring patient access to novel agents and to advancing clinical science. Measures including increased patient, physician, and trial sponsor acceptance of telehealth and postal delivery of oral experimental medications may decrease geographic barriers to clinical trial enrollment and allow greater flexibility in the setting of future disruptions.
Acknowledgments
Funding
None declared.
Disclosure
The authors report the following conflicts of interest: ZB non-financial support, Bristol Myers Squibb (BMS); research support, Genentech/imCore. Honoraria from UpToDate. CL research support, Genentech/imCore. AS educational travel support, Pfizer and Astellas. MMA research support, BMS Lilly, AstraZeneca, and Genentech; consulting/advisory role, BMS, Lilly, AstraZeneca, Genentech, Merck, Achilles, and Abbvie. RH consulting/advisory role, BMS, Merck, Pfizer, Genetech, AstraZeneca, and GSK; research grant/funding, Merck, BMS, Pfizer, Genentech, GSK, and AstraZeneca. ST institutional research funding from AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Exelixis, BMS, Eisai, Nanostring, Cyclacel, Odonate, and Seattle Genetics; has served as an advisor/consultant to AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, BMS, Eisai, Nanostring, Puma, Sanofi, Celldex, Paxman, Puma, Silverback Therapeutics, G1 Therapeutics, AbbVie, Athenex, OncoPep, Outcomes4Me, Kyowa Kirin Pharmaceuticals, Daiichi-Sankyo, and Samsung Bioepsis Inc. MDG reports: Stock, Rappta Therapeutics; consulting/advisory role, BioMotiv, Janssen, Dendreon, Merck, GlaxoSmithKline, Lilly, Astellas, Genentech, BMS, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Alleron Therapeutics, Dracen, Inovio Pharmaceuticals, Numab, Dragonfly Therapeutics; institutional research funding, Janssen, Dendreon, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, Genentech/Roche. TKC research support, AstraZeneca, Alexion, Bayer, BMS/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, Takeda; honoraria, AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc. (Healthcare Communications Company with several brands such as OnClive, PeerView and PER), Research to Practice, L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, NEJM, Lancet Oncology, Heron Therapeutics, Lilly Oncology; consulting or advisory role, AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Heron Therapeutics, Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, Pionyr, Tempest, Lilly Ventures; Stocks: Pionyr, Osel, and Tempest; leadership role, Director of Genitourinary Oncology Division at Dana-Farber and past President of Medical Staff at Dana-Farber, member of NCCN Kidney panel and the GU Steering Committee, past chairman of the Kidney Cancer Association Medical and Scientific Steering Committee, KidneyCan Advisory board, Kidney cancer Research Summit co-chair (2019-); patents, royalties or other intellectual properties related to checkpoint inhibitors biomarkers and ctDNA (no royalties made as to date); travel, accommodations, expenses, in relation to consulting, advisory roles, or honoraria; medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies (ClinicalThinking, Envision Pharma Group, Fishawack Group of Companies, Health Interactions, Parexel, Oxford PharmaGenesis, and others). TKC’s institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter. TKC has mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components. DD Consulting/Advisory role, Mirati, Ipsen, Boehringer Ingelheim, Atheneum Partners, Boston Healthcare Associates, Dedham Group, Guidepoint Global Advisors; travel expenses, Ipsen. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary Figure S1.
Supplementary Figure S2.
Supplementary Figure S3.
References
- 1.The New York Times Coronavirus World Map: Tracking the Global Outbreak – The New York Times. New York Times. 2020 [Google Scholar]
- 2.Bakouny Z., Paciotti M., Schmidt A., et al. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):2020–2022. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labaki C., Bakouny Z., Schmidt A., et al. Recovery of cancer screening tests and possible associated disparities after the first peak of the COVID-19 pandemic. Cancer Cell. 2021;39(8):1042–1044. doi: 10.1016/j.ccell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt A.L., Bakouny Z., Bhalla S., et al. Cancer care disparities during the COVID-19 pandemic: COVID-19 and cancer outcomes study. Cancer Cell. 2020;38(6):769–770. doi: 10.1016/j.ccell.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrag D., Hershman D.L., Basch E. Oncology practice during the COVID-19 pandemic. J Am Med Assoc. 2020;323(20):2005–2006. doi: 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- 6.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakouny Z., Hawley J.E., Choueiri T.K., et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA Guidance on Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency | FDA. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency. Accessed August 15, 2021.
- 9.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q.Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7:220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolaney S.M., Lydon C.A., Li T., et al. The impact of COVID-19 on clinical trial execution at the Dana-Farber Cancer Institute. J Natl Cancer Inst. 2021;113(11):1453–1459. doi: 10.1093/jnci/djaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber D.E., Sheffield T.Y., Beg M.S., et al. Experience, perceptions, and recommendations concerning COVID-19-related clinical research adjustments. J Natl Compr Canc Netw. 2020:1–8. doi: 10.6004/jnccn.2020.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabhan C., Choueiri T.K., Mato A.R. Rethinking clinical trials reform during the COVID-19 pandemic. JAMA Oncol. 2020;6(9):1327–1329. doi: 10.1001/jamaoncol.2020.3142. [DOI] [PubMed] [Google Scholar]
- 14.Galsky M.D., Shahin M., Jia R., et al. Telemedicine-enabled clinical trial of metformin in patients with prostate cancer. JCO Clin Cancer Inform. 2017;1(1):1–10. doi: 10.1200/CCI.17.00044. [DOI] [PubMed] [Google Scholar]
- 15.FDA. Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency Guidance for Industry, Investigators, and Institutional Review Boards Preface Public Comment, 2020. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency. Accessed August 15, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.