Abstract
Background
This study aimed to estimate potential undetected cancers over the first 2 years of the COVID-19 pandemic in Catalonia.
Methods
Cancer incidence was compared between pre-pandemic (2019) and pandemic (March 2020-January 2022) periods in the Catalan Pathology Registry (CPR) according to sex, age, and tumor site. The correlation between cancer diagnosis and COVID-19 health care workload was also evaluated by means of the Pearson's correlation coefficient (R). The expected incident cancers (E) during the pandemic were estimated by applying 2019 CPR cancer incidence specific rates by sex and 5-year age groups to the 2020 and 2021 Catalan population pyramids. CPR incident cancers were considered observed (O). Standardized incidence ratios (SIR) and 95% confidence intervals (CIs) were calculated using the O/E ratio.
Results
After two pandemic years, cancer diagnosis decreased by 12% (SIR 0.88, 95% CI 0.87-0.89), or ∼7700 undetected cancers (13 000 with nonmelanoma skin cancer). Without nonmelanoma skin cancer, 72% of the cancer underdiagnosis was generated in 2020. Diagnoses decreased more in men (whole pandemic −14%; 2020 −21%; 2021 −8%) than in women (−9%, −19%, −3%, respectively), dropping significantly overall in all pandemic waves but the fifth (first −37%, second −16%, third −8%, fourth −6%, fifth −2%, sixth −6%), and across all adult age groups. In the first wave, CPR cancer diagnosis was inversely correlated with COVID-19 caseload in primary care (R −0.91, 95% CI −0.97 to −0.75) and occupancy in conventional hospital wards (R −0.91, 95% CI −0.99 to −0.48) and intensive care (R −0.91, 95% CI 95% −0.98 to −0.70).
Conclusions
Our study evaluated the overall pandemic impact on cancer diagnosis on a large scale and with minimal selection bias, showing that as of February 2022, cancer detection in Catalonia had not yet recovered to pre-pandemic levels. Pending cancer incidence data from population-based cancer registries, early CPR data could inform the development of Spanish cancer control plans.
Key words: COVID-19, SARS-CoV-2, pandemic, cancer diagnosis, undetected cancers
Highlights
-
•
Pathological cancer diagnoses fell by 12% over the two pandemic years in Catalonia.
-
•
There may still be 7700 individuals (13 000 including nonmelanoma skin cancer) undetected cancers in Catalonia.
-
•
Pending data from population-based cancer registries, real time pathology data can assess national cancer control plans.
Introduction
Cancer is one of the leading causes of disease worldwide. At least 19 million new cancers were expected in 2020,1 although this estimate did not take into account the impact of the COVID-19 pandemic and the major challenges it has entailed for protecting public health and maintaining routine medical care. In the first pandemic wave, health systems were subjected to sudden and overwhelming pressure.2,3 In cancer, this meant delaying diagnoses4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 and altering treatment regimens.30, 31,42, 43, 44, 45, 46, 47 Subsequent pandemic waves, including the one linked to the omicron variant, have triggered new health crises.48 Worldwide, uneven implementation of testing, contact tracing, social restrictions, health system capacity, and COVID-19 vaccine coverage has driven the inequitable impact of the pandemic between countries.3 Four large, nationwide European studies have consistently reported decreases in the overall cancer diagnosis during the first pandemic wave,4, 5, 6, 7, 8 and two have described an incomplete recovery after the first pandemic year.5,7
Following the first confirmed COVID-19 case in Spain on 31 January 2020, the country became one of the hardest hit in Europe during the spring of 2020.49 A strict nationwide lockdown was in effect from 14 March to 11 May 2020, with restrictions gradually lifting to June 20. During the lockdown, telemedicine was expanded, cancer protocols modified, and organized cancer screening programs and non-essential surgery temporarily suspended.37 In the five successive waves (with the last beginning in November 2021), less restrictive measures were established based on health care pressure and vaccination coverage in each Spanish region. Single-center and multicenter hospital-based studies have estimated an overall decrease in cancer diagnosis of 21% to 37% in the first wave,33, 34,36, 37 and 17% in the first pandemic year.35 The heterogeneous methods and hospital biases inherent to this type of study, however, make it difficult to precisely estimate potential undetected cancers during the pandemic.
In Catalonia, a region in northeast Spain with 7.7 million inhabitants, the first case of COVID-19 was confirmed on 25 February 2020. During the first wave, the lockdown was the same as in the rest of Spain, but in the following waves, social restrictions have been tailored to regional epidemiological indicators.50 As elsewhere, studies evaluating the impact of the pandemic on cancer diagnosis in Catalonia have described heterogeneous methods and results. One large population-based study carried out in primary care estimated an overall 34% drop in cancer diagnoses in patients older than 14 years up to September 2020.38 Meanwhile, a study based on two hospital pathology laboratories reported a decrease of 6% to 20% until December 2020.40
Our study assessed incident cancers from January 2019 to January 2022 in the Catalan Pathology Registry (CPR), which covers ∼90% of all pathological diagnoses in the Catalan public health system (71 high-tech and general hospitals, plus 212 primary care centers that have generated pathological specimens). We aimed to estimate the potential number of undetected cancers in Catalonia according to sex, age, tumor site, pandemic waves, and COVID-19 health care workload over two pandemic years.
Material and methods
Since 2015, pathology laboratories have generated structured reports on all specimens originating in publicly funded hospitals and primary care centers, resulting in the identification of >610 000 neoplastic specimens in the CPR. Before the pandemic, an average of 3500 specimens coded with SNOMED CT terminology were sent to CPR every working day (around 900 000 specimens/year).51 Specimens include cytologies, biopsies, and necropsies. To ensure cancer identification according to international rules of population-based cancer registries,52, 53 neoplastic diagnoses are systematically assigned to the International Classification of Diseases for Oncology, 2nd revision (ICD-O-3.2)54 Neoplastic reports include topography, histology, and tumor behaviors.
The present study included all incident malignancies registered from January 2019 to January 2022, along with benign and uncertain cases in the nervous system (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100486). Pseudonymized specimen data were pooled and ordered chronologically by patient. If a patient had different neoplastic morphologies in a single organ, multiple tumors were considered, and topography, morphology, and the date of first neoplastic specimen were collected for each tumor. If specimens came from different organs and with different morphologies, multiple tumors were also considered, and the topography (in this case different), morphology, and date of first neoplastic specimen were recorded for each tumor.53 Date of cancer incidence was defined as the date of the first neoplastic specimen. Finally, after identifying all cancers, previously validated algorithms were applied based on the date of cancer incidence, location, and morphology of tumors to exclude prevalent tumors and ensure that only annual incident cancers from January 2019 to January 2022 were included in the study.55
Incident cancers were classified according to ICD-O-3.2 topography: lip, oral cavity, pharynx (C00-C14); esophagus (C15); stomach (C16); colorectal, anus (C18-C21); liver, gallbladder, extrahepatic bile ducts (C22-C24); pancreas (C25); larynx (C32); lung (C34), thymus, heart, mediastinum, pleura (C37-C39); bones (C40-C41); soft tissues, peritoneum, retroperitoneum (C47-C49); breast (C50); vulva, vagina (C51-C52); cervix (C53); endometrium (C54); ovary (C56.9); prostate (C61.9); testis (C62); kidney (C64.9); urinary tract (C66.9-C68.0); brain, meninges, spinal cord (C70-C72); thyroid (C73.9); other tumor sites (C17 small intestine, C58.9 placenta, C60 penis, C69 eye, C74.9-C75 endocrine glands); and unknown primary site (C26.9, C39.9, C57.9, C63.9, C68.9, C76, C80.9). Skin melanoma was identified by topography (C44) and specific ICD-O-3.2 morphologies (M87203-M87803). The rest of the skin tumors were considered nonmelanoma skin cancer (mostly basal and squamous cell cancers). Lymphoid and myeloid malignancies were identified by topography (C42, C77) and morphology (lymphoid: M95903-M97383, M97493-M97691, M98113-M98373, M99403, M99483, M99703-M99713, M97283-M99713; myeloid: M97403-M97423, M98053-M98093, M98403-M99313, M99453-M99463, M99503-M99683, M-99753-M96703).54
Age at diagnosis was determined according to the cancer incidence date and categorized in three age groups (0-49; 50-69, ≥70 years). The 50-69-year age group coincided with the target population for population-based colorectal and breast cancer screening programs in Catalonia. The study period was divided into two intervals: (i) a pre-pandemic validation set (1 January-31 December 2019) and (ii) the pandemic as analysis set (14 March 2020-31 January 2022). The pandemic period was then divided into seven cycles according to the six Catalan pandemic waves50: (i) first: 14 March-20 June 2020; (ii) summer 2020, 21 June-30 September 2020; (iii) second: 1 October-23 December 2020; (iv) third: 24 December 2020-25 March 2021; (v) fourth: 26 March-1 July 2021; (vi) fifth: 2 July-31 October 2021; and (vii) sixth: 1 November 2021-31 January 2022. The pre-pandemic period (2019) was likewise segmented into seven analogous cycles.
Data for primary care caseload (14 March 2020-31 January 2022) and occupancy in conventional hospital wards (1 January 2020-31 January 2022) and intensive care units (ICUs: 1 April 2020-31 January 2022) due to COVID-19 were supplied by the Catalan Health Department’s Agency for Healthcare Quality and Evaluation.50 COVID-19 cases were confirmed by PCR/rapid antigen test; in patients with more than one positive test, we included the first one. In the first wave, COVID-19 diagnosis was mainly based on PCR. ICD-10 codes used to identify COVID-19 cases in the Catalan hospital census register were: B97.29, B34.2 (before 1 July 2020), B97.21, J12.81 and U07.1 (from 1 July 2020).50
To analyze data, CPR cancer diagnoses were aggregated weekly to avoid the effect of weekends and holidays. Likewise, mean weekly COVID-19 occupancy in ambulatory and inpatient settings during the pandemic was calculated. The temporal association between cancer diagnoses and health facility workload was investigated by means of a dynamic correlation analysis, assuming a window size of 13 weeks.56 The linear correlation between weekly CPR incident cancers and health care workload due to COVID-19 during each pandemic wave was calculated using Pearson’s correlation coefficient and its 95% confidence intervals (95% CI).57 To estimate CPR cancer underdiagnosis, excess deaths that occurred in the pandemic were taken into account.2,58,59 Expected incident cancers (E) in the CPR during the pandemic were estimated by applying 2019 cancer incidence specific rates by sex and 5-year age groups to the updated 2020 and 2021 Catalan population pyramids for each tumor site.60 CPR incident cancers during the pandemic were considered observed cancers (O). Standardized incidence ratios (SIR) with their 95% CIs were calculated using the ratio O/E,61 with values of 1 indicating that observed and expected cancers were equal before and during the pandemic, and therefore there were no differences in cancer diagnosis between pre-pandemic and pandemic periods. SIRs below and above 1 indicate, respectively, underdiagnosis and overdiagnosis of cancer. The value obtained by subtracting the SIR value from the unit was interpreted as the percentage variation of underdiagnosis or overdiagnosis of cancer. If the 95% CI did not include the value 1, it was considered statistically significant. Negative differences between O and E cancers were considered an estimate of the undetected cancers in the pandemic.
Specific statistical analyses were carried out for pediatric tumors (0-14 years) according to the International Classification of Childhood Cancer.62 Statistical analysis and representation of the results using a color-blind friendly palette63 was carried out using R statistical software.64
The study was approved by the Research Ethics Committee of the Bellvitge University Hospital (PR264/21). Due to its retrospective design and the use of pseudonymized data, it did not require consent to participate.
Results
At two years from the start of the COVID-19 pandemic, cancer diagnosis had decreased by 12% (7700 incident cancers) in the CPR (SIR 0.88; 95% CI 0.87-0.89) compared with those expected based on the pre-pandemic period. If nonmelanoma skin cancer is included, the decrease is estimated at 14% (SIR 0.86, 95% CI 0.85-0.86); 13 000 undetected cancers (Table 1). From now on, to ease reading, we will omit information on nonmelanoma skin cancer as it can be found in the figures and tables of this paper and in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100486. Altogether, ∼72.2% of the cancer underdiagnosis was generated in 2020 (Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2022.100486). This pattern was similar in both sexes, but the decline was more evident in men (whole pandemic, 2020, 2021 men: −14%, −21%, −8%; women: −9%, −19%, −3%, respectively; Figure 1, Supplementary Tables S3-S5, available at https://doi.org/10.1016/j.esmoop.2022.100486).
Table 1.
Estimated undetected cancers during the COVID-19 pandemic in Catalonia. Comparison of cancer incidence in the Catalan Pathology Registry (CPR) between pre-pandemic (2019) and pandemic (March 2020-January 2022) periods by age and tumor site (both sexes)
| 0-49 |
50-69 |
>=70 |
All ages |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor site | O | E | Dif | SIR (95% CI) | O | E | Dif | SIR (95% CI) | O | E | Dif | SIR (95% CI) | O | E | Dif | SIR (95% CI) |
| Lip, oral cavity, pharynx (COO-C14) | 182 | 223 | −41 | 0.82 (0.70-0.94) | 843 | 1069 | −226 | 0.79 (0.74-0.84) | 868 | 1032 | −164 | 0.84 (0.79-0.90) | 1893 | 2324 | −431 | 0.81 (0.78-0.85) |
| Esophagus (C15) | 58 | 41 | 17 | 1.42 (1.08-1.83) | 330 | 356 | −26 | 0.93 (0.83-1.03) | 294 | 341 | −47 | 0.86 (0.77-0.97) | 682 | 738 | −56 | 0.92 (0.86-1.00) |
| Stomach (C16) | 110 | 159 | −49 | 0.69 (0.57-0.84) | 583 | 720 | −137 | 0.81 (0.75-0.88) | 859 | 1101 | −242 | 0.78 (0.73-0.83) | 1552 | 1980 | −428 | 0.78 (0.75-0.82) |
| Colon, rectum, anus (C17-C21) | 427 | 504 | −77 | 0.85 (0.77-0.93) | 2993 | 3710 | −717 | 0.81 (0.78-0.84) | 4299 | 4827 | −528 | 0.89 (0.86-0.92) | 7719 | 9041 | −1322 | 0.85 (0.83-0.87) |
| Liver, gallbladder, ext. bile ducts (C22-C24) | 246 | 251 | −5 | 0.98 (0.86-1.11) | 1393 | 1447 | −54 | 0.96 (0.91-1.01) | 1356 | 1551 | −195 | 0.87 (0.83-0.92) | 2995 | 3249 | −254 | 0.92 (0.89-0.96) |
| Pancreas (C25) | 65 | 59 | 6 | 1.10 (0.85-1.40) | 412 | 398 | 14 | 1.03 (0.94-1.14) | 449 | 452 | −3 | 0.99 (0.90-1.09) | 926 | 910 | 16 | 1.02 (0.95-1.09) |
| Larynx (C32) | 24 | 49 | −25 | 0.49 (0.31-0.73) | 420 | 486 | −66 | 0.86 (0.78-0.95) | 273 | 340 | −67 | 0.80 (0.71-0.90) | 717 | 875 | −158 | 0.82 (0.76-0.88) |
| Lung (C34) | 264 | 317 | −53 | 0.83 (0.74-0.94) | 2627 | 2975 | −348 | 0.88 (0.85-0.92) | 2470 | 2789 | −319 | 0.89 (0.85-0.92) | 5361 | 6080 | −719 | 0.88 (0.86-0.91) |
| Thymus, heart, mediast., pleura (C37-C39) | 12 | 20 | −8 | 0.59 (0.30-1.03) | 48 | 46 | 2 | 1.04 (0.76-1.37) | 80 | 115 | −35 | 0.69 (0.55-0.86) | 140 | 182 | −42 | 0.77 (0.65-0.91) |
| Bones (C40-C41) | 37 | 62 | −25 | 0.60 (0.42-0.83) | 33 | 41 | −8 | 0.80 (0.55-1.13) | 23 | 10 | 13 | 2.37 (1.50-3.56) | 93 | 113 | −20 | 0.83 (0.67-1.01) |
| Soft tissues (C47-C49) | 166 | 163 | 3 | 1.02 (0.87-1.19) | 251 | 308 | −57 | 0.81 (0.72-0.92) | 177 | 218 | −41 | 0.81 (0.70-0.94) | 594 | 690 | −96 | 0.86 (0.79-0.93) |
| Skin, melanoma (C44) | 315 | 344 | −29 | 0.91 (0.82-1.02) | 493 | 566 | −73 | 0.87 (0.80-0.95) | 562 | 653 | −91 | 0.86 (0.79-0.93) | 1370 | 1564 | −194 | 0.88 (0.83-0.92) |
| Skin, nonmelanoma (C44) | 1677 | 1857 | −180 | 0.90 (0.86-0.95) | 5749 | 7124 | −1375 | 0.81 (0.79-0.83) | 14 058 | 17 917 | −3859 | 0.78 (0.77-0.80) | 21 484 | 26 898 | −5414 | 0.80 (0.79-0.81) |
| Breast (C50) | 1808 | 1993 | −185 | 0.91 (0.87-0.95) | 3869 | 4278 | −409 | 0.90 (0.88-0.93) | 2676 | 2841 | −165 | 0.94 (0.91-0.98) | 8353 | 9112 | −759 | 0.92 (0.90-0.94) |
| Vulva, vagina (C51-C52) | 45 | 31 | 14 | 1.46 (1.07-1.96) | 102 | 146 | −44 | 0.70 (0.57-0.85) | 239 | 251 | −12 | 0.95 (0.84-1.08) | 386 | 427 | −41 | 0.90 (0.82-1.00) |
| Cervix (C53) | 208 | 247 | −39 | 0.84 (0.73-0.97) | 187 | 243 | −56 | 0.77 (0.66-0.89) | 151 | 127 | 24 | 1.19 (1.00-1.39) | 546 | 617 | −71 | 0.89 (0.81-0.96) |
| Endometrium (C54) | 182 | 163 | 19 | 1.12 (0.96-1.29) | 807 | 874 | −67 | 0.92 (0.86-0.99) | 632 | 605 | 27 | 1.04 (0.96-1.13) | 1621 | 1642 | −21 | 0.99 (0.94-1.04) |
| Ovary (C56) | 153 | 162 | −9 | 0.95 (0.80-1.11) | 306 | 259 | 47 | 1.18 (1.05-1.32) | 150 | 137 | 13 | 1.10 (0.93-1.29) | 609 | 558 | 51 | 1.09 (1.01-1.18) |
| Prostate (C61.9) | 53 | 61 | −8 | 0.87 (0.65-1.14) | 2533 | 2908 | −375 | 0.87 (0.84-0.91) | 3280 | 3849 | −569 | 0.85 (0.82-0.88) | 5866 | 6818 | −952 | 0.86 (0.84-0.88) |
| Testis (C62) | 208 | 259 | −51 | 0.80 (0.70-0.92) | 45 | 52 | −7 | 0.87 (0.64-1.17) | 19 | 12 | 7 | 1.64 (0.99-2.57) | 272 | 322 | −50 | 0.85 (0.75-0.95) |
| Kidney (C64) | 182 | 178 | 4 | 1.02 (0.88-1.18) | 711 | 820 | −109 | 0.87 (0.80-0.93) | 612 | 661 | −49 | 0.93 (0.85-1.00) | 1505 | 1658 | −153 | 0.91 (0.86-0.95) |
| Urinary tract (C66.9-C68) | 98 | 132 | −34 | 0.75 (0.60-0.91) | 1528 | 1588 | −60 | 0.96 (0.91-1.01) | 2642 | 2768 | −126 | 0.95 (0.92-0.99) | 4268 | 4488 | −220 | 0.95 (0.92-0.98) |
| Meninges, brain, spinal cord (C70-C72) | 290 | 435 | −145 | 0.67 (0.59-0.75) | 526 | 610 | −84 | 0.86 (0.79-0.94) | 253 | 354 | −101 | 0.71 (0.63-0.81) | 1069 | 1399 | −330 | 0.76 (0.72-0.81) |
| Thyroid (C73.9) | 350 | 443 | −93 | 0.79 (0.71-0.88) | 392 | 496 | −104 | 0.79 (0.71-0.87) | 174 | 226 | −52 | 0.77 (0.66-0.89) | 916 | 1165 | −249 | 0.79 (0.74-0.84) |
| Lymphoid | 419 | 537 | −118 | 0.78 (0.71-0.86) | 834 | 1069 | −235 | 0.78 (0.73-0.84) | 1004 | 1149 | −145 | 0.87 (0.82-0.93) | 2257 | 2755 | −498 | 0.82 (0.79-0.85) |
| Myeloid | 116 | 155 | −39 | 0.75 (0.62-0.90) | 289 | 360 | −71 | 0.80 (0.71-0.90) | 430 | 540 | −110 | 0.80 (0.72-0.88) | 835 | 1055 | −220 | 0.79 (0.74-0.85) |
| Other tumor sitesa | 121 | 143 | −22 | 0.84 (0.70-1.01) | 332 | 378 | −46 | 0.88 (0.79-0.98) | 372 | 445 | −73 | 0.84 (0.75-0.93) | 825 | 966 | −141 | 0.85 (0.80-0.91) |
| Unknown primary siteb | 491 | 529 | −38 | 0.93 (0.85-1.01) | 1614 | 1709 | −95 | 0.94 (0.90-0.99) | 1632 | 1838 | −206 | 0.89 (0.85-0.93) | 3737 | 4076 | −339 | 0.92 (0.89-0.95) |
| All sites | 8307 | 9516 | −1209 | 0.87 (0.85-0.89) | 30 250 | 35 036 | −4786 | 0.86 (0.85-0.87) | 40 034 | 47 149 | −7115 | 0.85 (0.84-0.86) | 78 591 | 91 700 | −13 109 | 0.86 (0.85-0.86) |
| All but nonmelanoma of skin | 6630 | 7659 | −1029 | 0.87 (0.84-0.89) | 24 501 | 27 911 | −3410 | 0.88 (0.87-0.89) | 25 976 | 29 232 | −3256 | 0.89 (0.88-0.90) | 57 107 | 64 802 | −7695 | 0.88 (0.87-0.89) |
O: observed incident cancers in the CPR, E: expected incident cancers in the CPR, Dif: estimated number of undetected cancers, difference between O and E. The sum of the estimated undetected cancers from all tumor sites may not be equal to the total in the table. SIR (95% CI): standardized incidence ratio (95% confidence interval). 95% CIs including 1 are not statistically significant (colorless), SIRs >1 indicate significant overdiagnosis (yellow) and <1, significant underdiagnosis (red).
Other tumor sites include small intestine, placenta, penis, eye and adnexa, endocrine glands without thyroid.
Unknown primary site includes all unspecific tumour sites in specimens from the CPR.
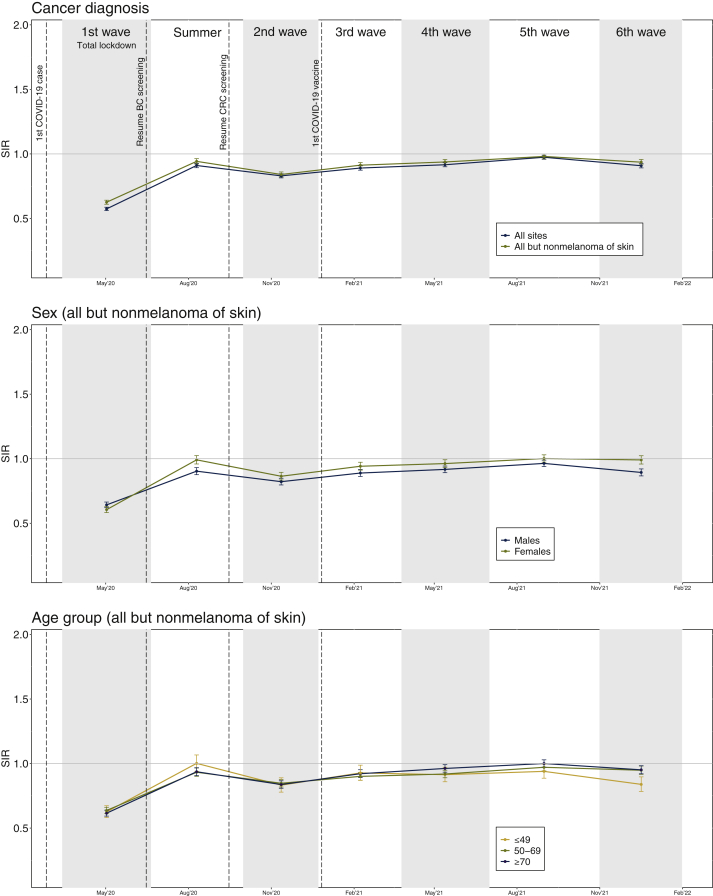
Figure 1.
Global comparison of cancer incidence in the Catalan Pathology Registry between pre-pandemic (2019) and pandemic (March 2020 to January 2022) periods by sex, age groups, and pandemic wave. SIR (figure dots). Whiskers represent 95% confidence intervals (95% CI) for estimated SIR. 95% CIs including value 1 (horizontal line) are not statistically significant. SIR significant values over or below 1 indicate over- and underdiagnosis of cancer, respectively.
BC, breast cancer; CRC, colorectal cancer; SIR, standardized incidence ratio.
Overall, cancer diagnoses dropped significantly in all but the fifth pandemic wave (Figure 1), with a gradual return to pre-pandemic levels after spring 2020 (first wave −37%, summer 2020 −6%, second wave −16%, third wave −9%, fourth wave −6%, fifth wave −2%, and sixth wave −6%). In men, observed diagnoses were significantly lower than expected cases in all waves and in summer 2020 (first −36%, summer 2020 −9%, second −18%, third −11%, fourth −8%, fifth −4%, sixth −11%). In contrast, data for women indicated underdiagnosis only in the first four waves (first −39%; second −13%, third −6%, fourth −4%) and a stabilization approaching pre-pandemic diagnostic levels in summer 2020 and in the fifth and sixth waves (summer 2020 −1%, fifth 0%, sixth −1%; Supplementary Tables S3-S5, available at https://doi.org/10.1016/j.esmoop.2022.100486). Figure 2 shows specific cancer diagnosis for each tumor site during the entire pandemic.
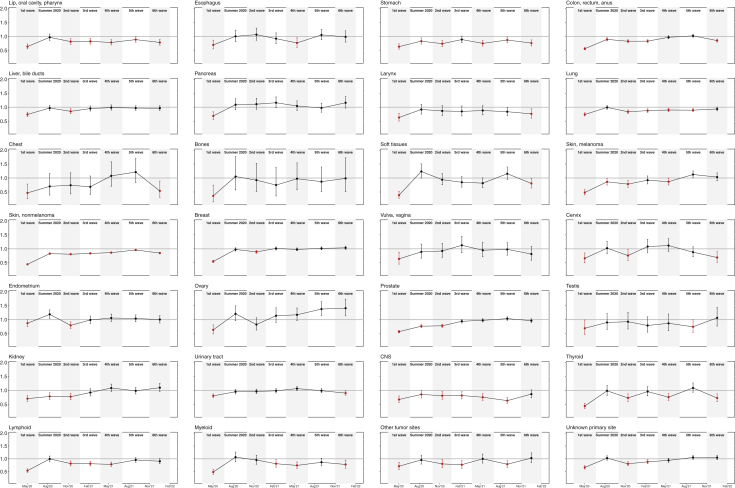
Figure 2.
Comparison of cancer incidence in the Catalan Pathology Registry between pre-pandemic (2019) and pandemic (March 2020 to January 2022) periods by tumor site and pandemic wave.Liver, bile duct: liver, gallbladder, extrahepatic bile ducts; chest: thymus, heart, mediastinum, pleura; soft tissues: soft tissues, peritoneum, retroperitoneum; urinary tract: urethra, bladder, ureter, renal pelvis; CNS: meninges, brain, spinal cord.
SIR (figure dots). Whiskers represent 95% confidence intervals (95% CI) for estimated SIR. 95% CIs including value 1 (horizontal line) are not statistically significant. SIR significant values over or below 1 (red color) indicate over- and underdiagnosis of cancer, respectively.
SIR, standardized incidence ratio.
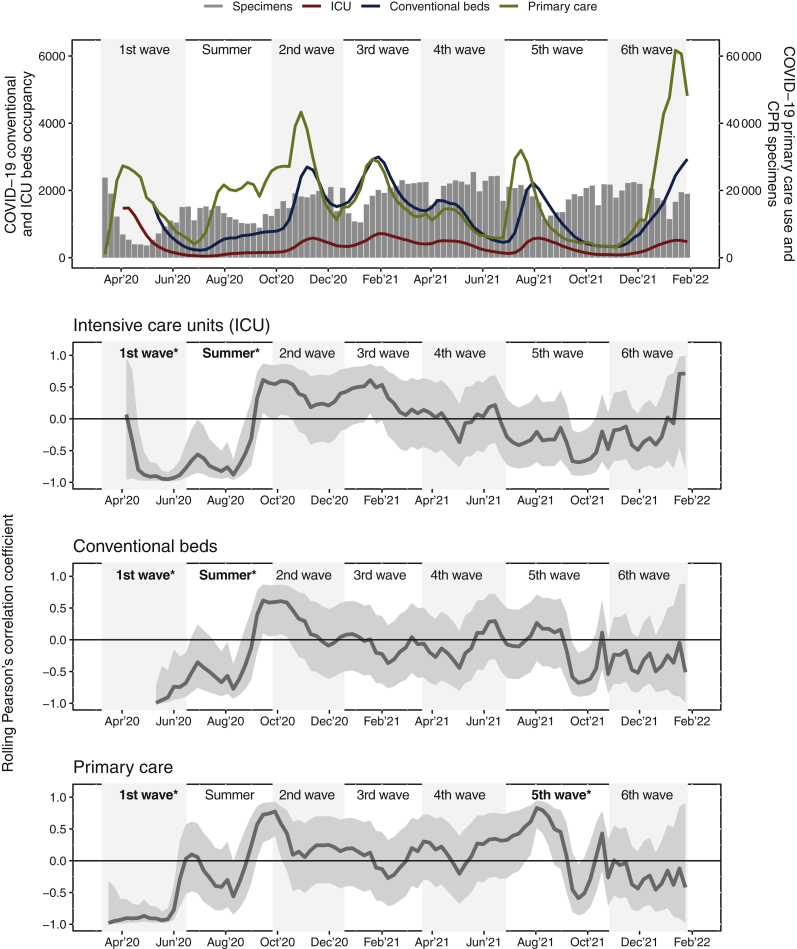
In the first wave, cancer diagnosis was inversely and significantly correlated with high COVID-19 caseload in primary care (R: −0.91, 95% CI −0.97 to −0.75), conventional hospital wards (R: −0.91, 95% CI −0.99 to −0.48), and ICUs (R: −0.91, 95% CI −0.98 to −0.70). In the summer of 2020, this pattern persisted only for conventional wards and ICUs (R: −0.56, 95% CI −0.83 to −0.09; R: −0.68, 95% CI −0.88 to −0.28, respectively). In the rest of the pandemic, no association was detected between cancer diagnosis and health care workload due to COVID-19. In the fifth wave, a positive association was found only in primary care (R: 0.48, 95% CI 0.02-0.77) (Figure 3).
Figure 3.
Catalan COVID-19 health care caseload due to COVID-19 and its rolling correlation with incident cancers from the Catalan Pathology Registry (CPR) during the pandemic. Conventional and ICU beds occupancy and primary care caseload due to COVID-19 are shown as a weekly average. CPR specimens are per week. Shaded bands represent the rolling correlation and 95% confidence interval. Shaded bands containing 0 (horizontal line) are statistically not significant. ∗Significant Pearson’s correlation coefficients (R): RICU 1st wave −0.91 (−0.98 to −0.70), RConventional beds 1st wave −0.91 (−0.99 to −0.48), RPrimary care 1st wave −0.91 (−0.97 to −0.75), RICU Summer 2020 −0.68 (−0.88 to −0.28), RConventional beds Summer 2020: −0.56 (−0.83 to −0.09), RPrimary care 5th wave: 0.48 (0.02-0.77). COVID-19 pandemic waves: (i) 1st wave, 14 March-20 June 2020; (ii) summer 2020, 21 June-30 September 2020; (iii) 2nd wave, 1 October-23 December 2020; (iv) 3rd wave, 24 December 2020-25 March 2021; (v) 4th wave, 26 March-1 July 2021, (vi) 5th wave, 2 July-31 October 2021, and (vii) 6th wave, 1 November 2021-31 January 2022.
ICU, intensive care unit.
Cancer diagnoses dropped similarly and significantly among the 0-49, 50-69, and >70 years age groups during the pandemic (pandemic: −13%, −12%, −11%; 2020: −19%, −20%, −21%; 2021: −10%, −7%, −4%, respectively) (Figure 1, Table 1; Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2022.100486). A significant level of underdiagnosis was maintained throughout the first four waves in all three age groups (first −37%, −36%, −38%; second −17%, −15%, −16%; third −7%, −10%, −8%; fourth −9%, −8%, −4%, respectively). In the fifth wave, cancer detection stabilized at pre-pandemic levels in all age groups except 0-49 years. (5th −6%, −3%, 0%), whereas it dropped significantly again in the sixth one (sixth −16%, −5%, −5%) (Supplementary Tables S6-S12, available at https://doi.org/10.1016/j.esmoop.2022.100486). Pediatric cancers (0-14 years) presented significant overall cancer underdiagnosis throughout the pandemic (−35%), attributable specifically to hematological malignancies, retinoblastoma, and hepatic and bone tumors (Supplementary Table S13, available at https://doi.org/10.1016/j.esmoop.2022.100486).
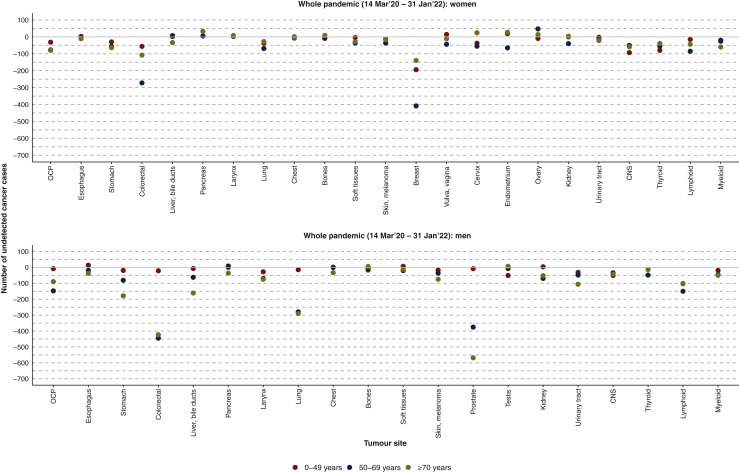
As of February 2022, after two pandemic years, the relative decrease and estimated number of undetected cancers in the CPR was, according to tumor site, colorectal cancer (−15%, −1300), followed by cancers of the prostate (−14%, −950), breast (−8%, −750), lung (−12%, −700), lymphoid malignancies (−18%, −500), head and neck (−19%, −430), stomach (−22%, −430), nervous system (−24%, −330), liver (−8%, −250), thyroid (−21%, −250), myeloid malignancies (−21%, −220), urinary tract (−5%, −220), melanoma (−12%, −190), larynx (−18%, −160), and kidney (−9%, −150). Unknown primary site, other tumor sites, and chest tumors (different from lung) have likewise not returned to pre-pandemic diagnostic levels (missing cancers: −500). In contrast, cancers of the pancreas, esophagus, bone, vulva, vagina, and endometrium have recovered (SIRs 95% CI crosses the value 1). Only ovarian cancer has shown a significant increase in diagnosis during the pandemic (+9%, 50) (Table 1). Undetected cases according to sex, age, and tumor site are shown in Figure 4.
Figure 4.
Estimated undetected cancer cases in the Catalan Pathology Registry during the COVID-19 pandemic by sex, age, and tumour site.OCP: lip, oral cavity, pharynx; colorectal: colon, rectum, anus; liver, bile duct: liver, gallbladder, extrahepatic bile ducts; chest: thymus, heart, mediastinum, pleura; soft tissues: soft tissues, peritoneum, retroperitoneum; urinary tract: urethra, bladder, ureter, renal pelvis; CNS: meninges, brain, spinal cord.
CNS, central nervous system.
The only cancer consistently underdiagnosed throughout the entire pandemic was nonmelanoma skin cancer (Figure 1; Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100486). Diagnosis of breast and colorectal cancers fell sharply during the first wave (breast −45%, −650; colorectal −44%, −600), especially in the 50-69-year age group (breast −52%, −350; colorectal −51%, −280) (Figure 2; Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2022.100486). Overall, breast cancer diagnoses returned to pre-pandemic levels in the summer of 2020 and then from the third wave onwards (summer 2020 −2%, −30; third +1%, 15; fourth −2%, −30; fifth +2%, 25; sixth +4%, 40). In contrast, colorectal cancer reached pre-pandemic diagnostic levels from the fourth wave (summer 2020 −10%, −120; second −17%, −200; third −17%, − 215; fourth −3%, −40; fifth +2%, 40) but declined significantly again in the sixth wave (sixth −15%, −170). Lung cancer was also significantly underdiagnosed throughout the whole pandemic except in the summer of 2020 (−1%, −5) and in the sixth wave (−6%, −50). Prostate cancer, however, after a very significant decline in the first wave (−43%, −460), gradually recovered to baseline levels from the third wave on (summer 2020 −24%, −200; second −22%, −220; third −6%, −50; fourth −3%, −30; fifth +3%, 40; sixth −4%, −40) (Figure 2; Supplementary Tables S6-S12, available at https://doi.org/10.1016/j.esmoop.2022.100486).
Certain tumors with a worse prognosis, such as those of the head and neck (−19%), stomach (−22%), larynx (−18%), nervous system (−24%), and hematological malignancies (lymphoid: −18%, myeloid: −21%) also presented a significant drop in diagnosis in both sexes throughout the pandemic (Table 1, Figures 2 and 4).
Discussion
According to the CPR, Catalonia saw a substantial reduction in incident cancers over two pandemic years compared with the preceding year. Our data show an estimated 7700 undetected cancers, or 13 000 if nonmelanoma skin cancer is included. Excluding nonmelanoma skin cancer, an estimated 12% of expected cancer cases were left undetected during the pandemic.65 Although the decline in cancer diagnosis was concentrated in 2020 with a trend towards normality in 2021, diagnostic levels of incident cancers have not yet fully recovered. Data up to 31 January 2022 show that cancers with a generally good prognosis (skin, testis) have not yet returned to pre-pandemic diagnostic levels, nor have cancers with a relatively good prognosis if diagnosed early (colorectal, prostate, breast, larynx, thyroid, cervix, urinary system) or those with a poor prognosis (head and neck, stomach, liver, nervous system, lung, and hematological). The only cancers that did not present suboptimal diagnosis levels in the pandemic were those of the esophagus, pancreas, bone, vulva, vagina, endometrium, and ovary; the last showed even higher rates of diagnosis. In fact, the pandemic had less impact on women than on men, mainly due to the early recovery of diagnoses of breast and gynecological tumors and the lower incidence of colorectal and tobacco-related cancers.65 Although published studies over this large timeframe are limited, our results show an impact of the pandemic on cancer diagnosis similar to that of other European countries during the first wave,4, 5, 6, 7, 8,11,12 but perhaps with signs of a slower recovery afterward.7
The decrease in detected incidence in the CPR has been uneven during the pandemic. The most important drop was observed in the first wave, in line with previous reports from Catalan primary care38, 39 and elsewhere in Europe, North America, and Hong Kong.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The strong inverse correlation between COVID-19 workload in health care settings and cancer diagnosis reflects the sudden, overwhelming pressure on the Catalan health care system as COVID-19 cases began to skyrocket in the spring of 2020. The decline in detected cases during this wave is probably largely attributable to the temporary suspension of organized cancer screening programs and non-essential surgery, the substitution of face-to-face visits with telemedicine, and patients’ fear of going to health care facilities. After the first wave lay bare the fragility of the health system, Catalan policymakers reinforced community identification of COVID-19 cases and contact tracing, redefined priority circuits for high suspicion of cancer in primary care, expanded ICU capacity, and reactivated delayed cancer surgeries and cancer screening.66 It was reassuring to note that by the summer of 2020, cancer diagnosis in Catalonia had almost recovered to baseline levels for most malignancies.
In the second wave (October to December 2020), incident cancers fell again, but to a lesser magnitude. This smoother decline suggests that health interventions implemented in the summer of 2020 were successful in mitigating the new pandemic shock. Even milder was the drop in cancer diagnoses in the third wave (January to March 2021), which is partly attributable to the launch of the vaccination campaign against COVID-19 in the high-risk population on 27 December 2020.50 In early 2021, diagnosis of breast and prostate cancers reached pre-pandemic levels in all age groups. The resumption of population-based breast cancer screening in June 2020 (before the pandemic, 30% of invasive breast cancers were detected by screening; unpublished data), together with the lower impact on referrals and first active treatments in both cancers, could partly explain the rebound that has continued to date.11,12,30,43,44,67
In the fourth wave (March to July 2021), cancer underdiagnosis continued to abate. For the first time in the pandemic, colorectal cancer diagnosis normalized to pre-pandemic levels, and these levels persisted until the sixth wave, when data once again indicated suboptimal detection. Invitations to the fecal immunochemical test (FIT) based colorectal cancer screening program resumed in September 2020, but ∼43% of the target population had not yet received their invitation by December 2020. Given that before the pandemic ∼11% of invasive colorectal cancers in Catalonia were detected by screening (unpublished data), the lower rates of participation, adherence to colonoscopy, and cancer detection due to the increased FIT positivity threshold during the pandemic68 could explain why colorectal cancer diagnoses did not return to baseline levels until 6 months after reopening the screening program. The progressive increase in endoscopies and elective surgeries as the pandemic progressed probably helped the rebound in diagnosis of this cancer in all age groups, not only in the target age group of the colorectal cancer screening.9,31,43,44
During the fifth wave (July to October 2021), overall cancer diagnoses reached the 2019 baseline levels for the first time since the start of the pandemic. This wave was characterized by community transmission of the COVID-19 delta variant, which primarily affected adolescents and young adults, in whom COVID-19 vaccination coverage was still relatively low.50,69 The good vaccination rates in adults—among the highest in Europe3—and the concentration of cases of the delta variant among young people,50 who are at lower risk of developing both cancer and severe COVID-19 disease, helped to preserve the health system’s capacity to detect cancers. The sixth wave (November 2021 to January 2022) was marked by the fast-spreading omicron variant,48 a new but discreet drop in overall cancer diagnoses, and, for the first time in the pandemic, a return to pre-pandemic levels of lung cancer diagnosis. The lower impact of this wave can be largely attributed to previously administered booster doses among the high-risk population and the elderly,50,70 the concentration of transmission in low-risk children and young adults,50 and the presumed lower severity of this variant.71
Cancer incidence in children under the age of 15 years is low, reaching at most about 200 incident cases yearly in Catalonia before the pandemic.72,73 Since the pandemic began, we have estimated a loss of ∼90 pediatric cancers (52% corresponding to leukemia’s, lymphomas, and tumors of the nervous system). Worldwide, substantial disruptions in pediatric cancer diagnosis and management were described in the first pandemic wave, particularly in low- and middle-income countries.74 Whereas a study conducted in the UK reported a significant reduction in cancer diagnoses during the first wave,75 however, two studies from Canada and Germany76,77 did not find a decrease in the first pandemic year. These heterogeneous published data, combined with the insufficient statistical power of our pediatric series which did not allow us to analyze incident cases according to pandemic waves for all tumor types, mean that our estimates must be interpreted with great caution.
Clinically diagnosed cancers and those that may not require histological verification, especially hematological cancers and tumors of the prostate and nervous system, could have generated a possible underestimation of the impact of the COVID-19 pandemic in Catalonia. In addition to pediatric, bone, and testicular cancers, there was insufficient statistical power for some age groups, which may not have been adequately evaluated. Due to the lack of clinical data, it was not possible for us to assess stage at diagnosis. Due to the long study period and the fact that two-thirds of undetected cancers during the pandemic occurred in the last 9 months of 2020, staging would have contributed to a better interpretation of the cancer diagnosis rebound in 2021. In particular, for cancers with a relatively good prognosis, staging might have helped characterize cancers with delayed diagnosis versus cancers that debuted in 2021. Short- and long-term clinical consequences, such as stage at diagnosis and changes in survival rates, will need to be carefully evaluated later through population-based cancer registries. Other possible limitations of our study include its focus on patients diagnosed in the public health system, without taking into account the increased use of private insurance in low educational groups under 50 years of age, although its impact would be quite limited due to the low cancer risk in this age group. Moreover, we could not account for the loss of population that occurred during the pandemic due to the fall in internal and external immigration. The impact of this attrition on our results would be limited, however, since the calculation of the expected cancers was based on the Catalan population pyramids updated in January 2022, which took into account both excess mortality and the migratory balance.60
To our knowledge, this is the first Spanish study to evaluate the impact of the whole pandemic on cancer diagnosis on a large scale and with minimal selection bias. Pending cancer incidence data from Spanish population-based cancer registries,78 the early data collection in the CPR could inform the development of national cancer control plans. In summary, at 2 years since the start of the COVID-19 pandemic, there may still be 7700 individuals with undetected cancers in Catalonia. Colorectal (n = 1300), prostate (n = 950), and breast (n = 750) cancers alone could account for 40% of these cases. Public health interventions to address decreases in cancer diagnoses will be critical to minimize the potentially increased incidence of advanced cancers because of delayed detection.
Acknowledgements
We thank the Catalan Agency for Health Quality and Assessment for the health care and COVID-19 epidemiological data, the pathologists who have continued to diagnose despite the pandemic, and to Meggan Harris, for her support with the English edition.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Islam N., Shkolnikov V.M., Acosta R., et al. Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries. BMJ. 2021;373:n1137. doi: 10.1136/bmj.n1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchie H., Mathieu E., Rodés-Guirao L., et al. Coronavirus Pandemic (COVID-19) https://ourworldindata.org/coronavirus Published online at OurWorldInData.org. 2020. Available at.
- 4.Dinmohamed A.G., Visser O., Verhoeven R.H.A., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock H.M., Tambuyzer T., Verdoodt F., et al. Decline and incomplete recovery in cancer diagnoses during the COVID-19 pandemic in Belgium: a year-long, population-level analysis. ESMO Open. 2021;6(4):100197. doi: 10.1016/j.esmoop.2021.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skovlund C.W., Friis S., Dehlendorff C., Nilbert M.C., Mørch L.S. Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta Oncol. 2021;60(1):20–23. doi: 10.1080/0284186X.2020.1858235. [DOI] [PubMed] [Google Scholar]
- 7.Skovlund CW Friis S., Christensen J., Nilbert M.C., Mørch L.S. Drop in cancer diagnosis during the COVID-19 pandemic in Denmark: assessment of impact during 2020. Acta Oncol. 2022;61(5):658–661. doi: 10.1080/0284186X.2021.2024879. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton A.C., Donnelly D.W., Loughrey M.B., et al. Inequalities in the decline and recovery of pathological cancer diagnoses during the first six months of the COVID-19 pandemic: a population-based study. Br J Cancer. 2021;125(6):798–805. doi: 10.1038/s41416-021-01472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutter M.D., Brookes M., Lee T.J., Rogers P., Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2021;70(3):537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 10.Andrew T.W., Alrawi M., Lovat P. Reduction in skin cancer diagnoses in the UK during the COVID-19 pandemic. Clin Exp Dermatol. 2020;46(1):145–146. doi: 10.1111/ced.14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gathani T., Clayton G., MacInnes E., Horgan K. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer. 2021;124:710–712. doi: 10.1038/s41416-020-01182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gathani T., Reeves G., Dodwell D., et al. Impact of the COVID-19 pandemic on breast cancer referrals and diagnoses in 2020 and 2021: a population-based study in England. Br J Surgery. 2022;109(2):e29–e30. doi: 10.1093/bjs/znab426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earnshaw C.H., Hunter H.J.A., McMullen E., Griffiths C.E.M., Warren R.B. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183(4):792–794. doi: 10.1111/bjd.19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacob L., Loosen S.H., Kalder M., Luedde T., Roderburg C., Kostev K. Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany. Cancers. 2021;13:408. doi: 10.3390/cancers13030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob L., Kalder M., Kostev K. Decrease in the number of patients diagnosed with cancer during the COVID-19 pandemic in Germany. J Cancer Res Clin Oncol. 2022 doi: 10.1007/s00432-022-03922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maluchnik M., Podwojcic K., Wieckowska B. Decreasing access to cancer diagnosis and treatment during the COVID-19 pandemic in Poland. Acta Oncol. 2021;60(1):28–31. doi: 10.1080/0284186X.2020.1837392. [DOI] [PubMed] [Google Scholar]
- 17.Aparicio A., Layese R., Hemery F., et al. Effect of lockdown on digestive system cancer care amongst older patients during the first wave of COVID-19: The CADIGCOVAGE multicentre cohort study. Dig Liver Dis. 2022;54(1):10–18. doi: 10.1016/j.dld.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsibulak I., Reiser E., Bogner G., et al. Decrease in gynecological cancer diagnoses during the COVID-19 pandemic: an Austrian perspective. Int J Gynecol Cancer. 2020;30(11):1667–1671. doi: 10.1136/ijgc-2020-001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vincentiis L., Carr R.A., Mariani M.P., Ferrara G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018-2019: an audit study from cellular pathology. J Clin Pathol. 2021;74(3):187–189. doi: 10.1136/jclinpath-2020-206833. [DOI] [PubMed] [Google Scholar]
- 20.Zadnik V., Mihor A., Tomsic S., et al. Impact of COVID-19 on cancer diagnosis and management in Slovenia - preliminary results. Radiol Oncol. 2020;54(3):329–334. doi: 10.2478/raon-2020-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langabeer S.E. Reduction in molecular diagnostics of myeloproliferative neoplasms during the COVID-19 pandemic. Ir J Med Sci. 2021;190(1):27–28. doi: 10.1007/s11845-020-02303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turkington R.C., Lavery A., Donnelly D., Cairnduff V., McManus D.T., Coleman H.G. The impact of the COVID-19 pandemic on Barrett’s esophagus and esophagogastric cancer. Gastroenterology. 2021;160:2169–2171. doi: 10.1053/j.gastro.2021.01.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman H.W., Chen Z., Niles J., et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maganty A., Yu M., Anyaeche V.I., et al. Referral pattern for urologic malignancies before and during the COVID-19 pandemic. Urol Oncol. 2021;39:268–276. doi: 10.1016/j.urolonc.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litchman G.H., Rigel D.S. The immediate impact of COVID-19 on US dermatology practices. J Am Acad Dermatol. 2020;83(2):685–686. doi: 10.1016/j.jaad.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vardhanabhuti V., Ng K.S. Differential impact of COVID-19 on cancer diagnostic services based on body regions: a public facility-based study in Hong Kong. Int J Radiat Oncol Biol Phys. 2021;111(2):331–336. doi: 10.1016/j.ijrobp.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EsKander A., Li Q., Yu J., et al. Incident cancer detection during the COVID-19 pandemic. J Natl Compr Canc Netw. 2022 doi: 10.6004/jnccn.2021.7114. [DOI] [PubMed] [Google Scholar]
- 28.Slotman E., Schreuder K., Nijsten T.E.C., et al. The impact of the COVID-19 pandemic on keratinocyte carcinoma in the Netherlands: trends in diagnoses and magnitude of diagnostic delays. J Eur Acad Dermatol Venereol. 2022;36(5):680–687. doi: 10.1111/jdv.17976. [DOI] [PubMed] [Google Scholar]
- 29.van Not O.J., van Breeschoten J., van den Eertwegh A.J.M., et al. The unfavorable effects of COVID-19 on Dutch advanced melanoma care. Int J Cancer. 2022;150(5):816–824. doi: 10.1002/ijc.33833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nossiter J., Morris M., Parry M.G., et al. Impact of the Covid-19 pandemic on the diagnosis and treatment of men with prostate cancer. BJU Int. 2022 doi: 10.1111/bju.15699. [DOI] [PubMed] [Google Scholar]
- 31.Morris E.J.A., Goldacre R., Spata E., et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6:199–208. doi: 10.1016/S2468-1253(21)00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigliar E., Cepurnaite R., Iaccarino A., et al. Cytopathology practice during the COVID-19 postlockdown: an Italian experience. Cancer Cytopathol. 2021;129(7):548–554. doi: 10.1002/cncy.22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manso L., de Velasco G., Paz-Ares L. Impact of the COVID-19 outbreak on cancer patient flow and management: experience from a large university hospital in Spain. ESMO Open. 2020;4(suppl 2) doi: 10.1136/esmoopen-2020-000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogado J., Obispo B., Gullón P., Lara M.A. Impact of the COVID-19 pandemic in cancer diagnosis in the first and second waves in one of the most affected cancer areas in the city of Madrid (Spain) Int J Cancer. 2021;148:1794–1795. doi: 10.1002/ijc.33462. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Medina S., Gil S., Jimenez B., et al. Significant decrease in annual cancer diagnoses in Spain during the COVID-19 pandemic: a real-data study. Cancers. 2021;13:3215. doi: 10.3390/cancers13133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suárez J., Mata E., Guerra A., et al. Impact of the COVID-19 pandemic during Spain's state of emergency on the diagnosis of colorectal cancer. J Surg Oncol. 2021;123:32–36. doi: 10.1002/jso.26263. [DOI] [PubMed] [Google Scholar]
- 37.Amador M., Matias-Guiu X., Sancho-Pardo G., et al. Impact of the COVID-19 pandemic on the care of cancer patients in Spain. ESMO Open. 2021;6(3):100157. doi: 10.1016/j.esmoop.2021.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coma E., Guiriguet C., Mora N., et al. Impact of the COVID-19 pandemic and related control measures on cancer diagnosis in Catalonia: a time-series analysis of primary care electronic health records covering about five million people. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pifarré i Arolas H., Vidal-Alaball J., Gil J., López F., Nicodemo C., Saez M. Missing diagnoses during the COVID-19 pandemic: a year in review. Int J Environ Res Public Health. 2021;18:5335. doi: 10.3390/ijerph18105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanvisens A., Puigdemont M., Rubió-Casadevall J., et al. Differences in the impact of COVID-19 on pathology laboratories and cancer diagnosis in Girona. Int J Environ Res Public Health. 2021;18:13269. doi: 10.3390/ijerph182413269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch X., Torres M., Moreno P., López-Soto A. Delays in cancer diagnostic testing at a quick referral unit in Spain during COVID-19. Diagnostics (Basel) 2021;11(11):2096. doi: 10.3390/diagnostics11112096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayo M., Potugari B., Bzeih R., et al. Cancer screening during the COVID-19 pandemic: a systematic review and meta-analysis. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1109–1117. doi: 10.1016/j.mayocpiqo.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.COVIDSurg Collaborative∗ Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;22:1507–1517. doi: 10.1016/S1470-2045(21)00493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Joode K., Dumoulin D.W., Engelen V., et al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients' perspective. Eur J Cancer. 2020;136:132–139. doi: 10.1016/j.ejca.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sessa C., Cortes J., Conte P., et al. The impact of COVID-19 on cancer care and oncology clinical research: an experts' perspective. ESMO Open. 2022;7(1):100339. doi: 10.1016/j.esmoop.2021.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spencer K., Jones C.M., Girdler R., et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. 2021;22:309–320. doi: 10.1016/S1470-2045(20)30743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onesti C.E., Tagliamento M., Curigliano G., et al. Expected medium- and long-term impact of the COVID-19 outbreak in oncology. JCO Glob Oncol. 2021;7:162–172. doi: 10.1200/GO.20.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinho-Gomes A.C., Allen A., Rae M., Ryder J. Omicron: we must protect the health and wellbeing of the public health workforce. BMJ. 2021;375:n3123. doi: 10.1136/bmj.n3123. [DOI] [PubMed] [Google Scholar]
- 49.Editorial COVID-19 in Spain: a predictable storm? Lancet Public Health. 2020;5(11):E568. doi: 10.1016/S2468-2667(20)30239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.COVID data. Epidemiological and healthcare situation in Catalonia. Catalan Agency for Health Quality and Assessment (AQuAS). Generalitat de Catalunya https://dadescovid.cat/critics . Available at.
- 51.Sanz X., Pareja L., Rius A., et al. Definition of a SNOMED CT pathology subset and microglossary, based on 1.17 million biological samples from the Catalan Pathology Registry. J Biomedical Informatics. 2018;78:167–176. doi: 10.1016/j.jbi.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Sanz X., Pareja L., Rius A., et al. How cancer registries can detect neoplasms in pathology laboratories that code with SNOMED CT terminology? An actual, simple and flexible solution. Int J Med Inform. 2020;141:104167. doi: 10.1016/j.ijmedinf.2020.104167. [DOI] [PubMed] [Google Scholar]
- 53.Martos C., Crocetti E., Visser O., et al. 1.1, EUR 29089 EN, Publications Office of the European Union: Luxembourg; Cancer registries-version: 2018. A proposal on cancer data quality checks: one common procedure for European. [Google Scholar]
- 54.International Classification of Diseases for Oncology, 2nd revision (ICDO-3.2). International Association for Cancer Registries http://www.iacr.com.fr/index.php?option=com_content&view=category&layout=blog&id=100&Itemid=577 . Available at.
- 55.Ribes J., Gálvez J., Melià A., Clèries R., Messeguer X., Bosch F.X. Automatization of a hospital-based tumor registry. Gac Sanit. 2005;19(3):221–228. doi: 10.1157/13075955. [DOI] [PubMed] [Google Scholar]
- 56.Engle R. Dynamic conditional correlation: a simple class of multivariate generalized autoregressive conditional heteroskedasticity models. J Bus Econ Stat. 2002;20(3):339–350. [Google Scholar]
- 57.Rothman K.J., Greenland S., Lash T. Lippincott Williams & Wilkins; Philadelphia: 2008. Modern Epidemiology. [Google Scholar]
- 58.Achilleos S., Quattrocchi A., Gabel J., et al. Excess all-cause mortality and COVID-19-related mortality: a temporal analysis in 22 countries, from January until August 2020. Int J Epidemiol. 2022;51(1):35–53. doi: 10.1093/ije/dyab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vila-Corcoles A., Satue-Gracia E., Vila-Rovira A., et al. COVID19-related and all-cause mortality risk among middle-aged and older adults across the first epidemic wave of SARS-COV-2 infection: a population-based cohort study in Southern Catalonia, Spain, March-June 2020. BMC Public Health. 2021;21(1):1795. doi: 10.1186/s12889-021-11879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Statistical Institute of Catalonia https://www.idescat.cat/pub/?id=ep&n=9124 . Available at.
- 61.Breslow NE, Day NE (eds). Statistical methods in cancer research Vol II. The Design and Analysis of Cohort Studies. IARC Scientific Publications Nº 82. International Agency for Research on Cancer: Lyon, 1987. [PubMed]
- 62.Steliarova-Foucher E., Colombet M., Ries L.A.G., et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18(6):719–731. doi: 10.1016/S1470-2045(17)30186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudis B. Intro to the viridis color palette https://cran.microsoft.com/snapshot/2015-09-17/web/packages/viridis/vignettes/intro-to-viridis.html . Available at.
- 64.R Core Team . R Foundation for Statistical Computing, Vienna, Austria; 2021. R: A language and environment for statistical computing.https://www.R-project.org/ Available at. [Google Scholar]
- 65.Clèries R., Ameijide A., Marcos-Gragera R., et al. Predicting the cancer burden in Catalonia between 2015 and 2025: the challenge of cancer management in the elderly. Clin Transl Oncol. 2018;20(5):647–657. doi: 10.1007/s12094-017-1764-5. [DOI] [PubMed] [Google Scholar]
- 66.Report on the extension and modification of the special measures in the field of Public Health in force for the containment of the epidemic outbreak of the COVID-19 pandemic in Catalonia. Salut/Agència de Salut Pública de Catalunya. Barcelona. Available at https://salutweb.gencat.cat/web/.content/_departament/decicions-i-actuacions-rellevancia-juridica/Resolucions-administratives-judicials-amb-especial-rellevancia-publica/informes-covid19/informe-slt-2147.pdf. Accessed July 9, 2021.
- 67.Toss A., Isca C., Venturelli M., et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6(2):100055. doi: 10.1016/j.esmoop.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vives N., Binefa G., Vidal C., et al. Short-term impact of the COVID-19 pandemic on a population-based screening program for colorectal cancer in Catalonia (Spain) Prev Med. 2022;155:106929. doi: 10.1016/j.ypmed.2021.106929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singanayagam A., Hakki S., Dunning J., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2021;21(12):e363. doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nealon J., Cowlinga B.J. Omicron severity: milder but not mild. Lancet. 2022;399(10323):412–413. doi: 10.1016/S0140-6736(22)00056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Larrañaga N., Sanchez M.J., Ardanaz E., et al. Incidence patterns and trends of non-central nervous system solid tumours in children and adolescents. a collaborative study of the Spanish population based cancer registries. J Cancer. 2016;7(3):335–343. doi: 10.7150/jca.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marcos-Gragera R., Galceran J., Martos C., et al. Incidence and survival time trends for Spanish children and adolescents with leukaemia from 1983 to 2007. Clin Transl Oncol. 2017;19(3):301–316. doi: 10.1007/s12094-016-1531-z. [DOI] [PubMed] [Google Scholar]
- 74.Graetz D., Agulnik A., Ranadive R., et al. Global effect of the COVID-19 pandemic on paediatric cancer care: a cross-sectional study. Lancet Child Adolesc Health. 2021;5(5):332–340. doi: 10.1016/S2352-4642(21)00031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saatci D., Oke J., Harnden A., Hippisley-Cox J. Childhood, teenage and young adult cancer diagnosis during the first wave of the COVID-19 pandemic: a population-based observational cohort study in England. Arch Dis Child. 2022 doi: 10.1136/archdischild-2021-322644. [DOI] [PubMed] [Google Scholar]
- 76.Pelland-Marcotte M.C., Xie L., Barber R., et al. Incidence of childhood cancer in Canada during the COVID-19 pandemic. CMAJ. 2021;193(47):E1798–E1806. doi: 10.1503/cmaj.210659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erdmann F., Wellbrock M., Trübenbach C., et al. Impact of the COVID-19 pandemic on incidence, time of diagnosis and delivery of healthcare among paediatric oncology patients in Germany in 2020: evidence from the German childhood cancer registry and a qualitative survey. Lancet Reg Health Eur. 2021;9:100188. doi: 10.1016/j.lanepe.2021.100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soerjomataram I., Bardot A., Aitken J., et al. Impact of the COVID-19 pandemic on population-based cancer registry. Int J Cancer. 2022;150(2):273–278. doi: 10.1002/ijc.33792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.