Abstract
Introduction:
The overuse of blood tests burdens the healthcare system and can detrimentally impact patient care. Risks of frequent blood sampling include infection and clinician-induced anemia, which can negatively impact patients and their families. Pediatric cancer patients are particularly vulnerable as they are immunocompromised with a small blood volume. Four blood tests had become a daily practice. Therefore, we aimed to reduce the number of blood tests taken per bed day within the inpatient pediatric cancer unit by 15% within 8 months.
Methods:
This quality improvement project combined several strategies to reduce test frequency and empower clinicians on the rationale for blood test ordering. Recommendations were developed collaboratively presented in a summary table. Targeted behavior-change methodology built engagement and momentum for the change. All clinicians were challenged to STOP and THINK about why a test is necessary for each patient. The primary outcome measure was the frequency of the tests taken per bed day. Frequency was compared between pre- and postimplementation plus follow-up periods across 2019–2021.
Results:
26,941 blood tests were captured in 1,558 admissions. The intervention led to an overall blood test reduction of 37% over 8 months. Liver Function Tests were the standout, with a 52% decrease in test frequency.
Conclusions:
A strategy incorporating education and culture change, combined with clear guidance on testing frequency, significantly reduced the ordering frequency of blood tests without increased patient harm.
Keywords: pediatric, oncology, blood test, quality improvement
INTRODUCTION
Blood tests have become a fundamental aspect of clinical decision-making within healthcare. They are incorporated universally across specialties, including diagnosis, health monitoring, response to treatment, and symptom management.1 Although they play a crucial role in clinical decision-making, they need to be ordered and used in a considered and appropriate way. The overuse of these tests burdens the healthcare system. It can detrimentally impact patient care, with evidence suggesting an ongoing increase in the number of requested blood tests without considering each test’s rationale.1–3 Tamburrano et al identified a consistent rise in blood tests by approximately 5% per annum over the past ten years in the United States and Europe.4 Indeed, health economists estimate that a fifth of spending in healthcare is ineffective and even wasteful.5
Within Australia, driven by the rising demands for hospital services, blood tests, and budgetary constraints, the National Coalition of Public Pathology (NCOPP) was endorsed under the Department of Health and Ageing to undertake a review on encouraging “quality pathology ordering.” The NCOPP described a quality order as the right test at the right time on the right patient for the right condition.6 The report identified cultural behaviors as a major barrier to successfully reducing blood tests. The report aligns with the strategic direction of both local and international campaigns, such as the Sensible Test Ordering Project7 and the Choosing Wisely Initiative.8
Within our cancer center, routine daily blood tests had become standard of care for all inpatients. However, tests were being ordered and performed often without careful consideration of the specific clinical situation. Central venous catheters (CVC) made the collection of blood tests easy to perform. Although blood tests are valuable, overordering has wide-ranging implications. For example, frequent blood sampling can result in clinician-induced anemia,9 exacerbated in pediatric cancer patients by their small blood volume and underlying impaired bone marrow function, and increase the need for blood transfusions.
Additionally, associated underlying disease and treatment-related immunosuppression, alongside frequent accessing of CVCs, has long been shown to increase the risk of catheter-related bloodstream infections.10 Patients may also feel increased psychological anxiety, anticipating that results will impact their treatment course. Also, the financial expenditure, clinician and nursing time, and consumables present additional burdens on the health system.
Treatment-driven protocols provide a basis for blood test ordering in the pediatric cancer setting; however, there is no international consensus for the frequency of blood tests. Furthermore, the evidence guiding retesting intervals across adult and pediatric specialties is also scarce.
This quality improvement (QI) project focused on challenging ingrained and historical behaviors regarding blood test ordering and collection frequency within the inpatient unit. The child’s individual needs guided the safe reduction of blood test frequency.
AIM
We aimed to reduce the number of blood tests taken per bed day within the inpatient pediatric cancer unit by 15% within 8 months.
METHOD
Context
The project took place in the inpatient cancer unit of an Australian quaternary level pediatric hospital. The cancer service covers over 40% of the state’s pediatric cancer diagnoses and includes inpatient and outpatient wards and outreach services across the state.
The cancer service consists of approximately 175 full-time equivalent staff, including senior oncologists, fellows, junior medical officers (JMOs), registered nurses (RNs), and allied health professionals. Approximately 60 bedside RNs and 8 JMOs covered the 2 units during the intervention period. Patients and families are actively involved in the decision-making process throughout the treatment course. Funding for the blood tests in Australian public hospitals is government-funded. We undertook this project during an international pandemic.
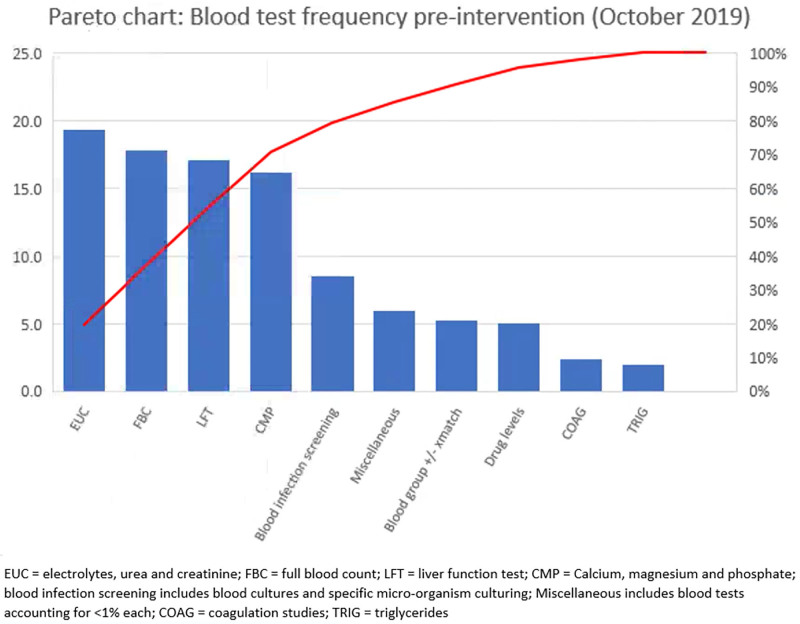
Four blood tests, Full Blood Count (FBC), Electrolytes, Urea, Creatinine (EUC), Liver Function Tests (LFT), and Calcium Magnesium Phosphate, accounted for approximately 70% of all blood orders within the unit (Fig. 1). For this article, the term blood tests will refer to these blood order sets. JMOs are primarily responsible for blood test ordering, and RNs can also order tests. Blood tests are routinely taken via CVC between 02:00 and 06:00 when senior medical cover is minimal. No comprehensive written patient management guidelines were available to support the decision-making process for test frequency. These contextual factors assisted in identifying change ideas and implementation strategies to support the cultural transition brought on by this QI project.
Fig. 1.
Pareto Chart: Blood test frequency preintervention.
Intervention
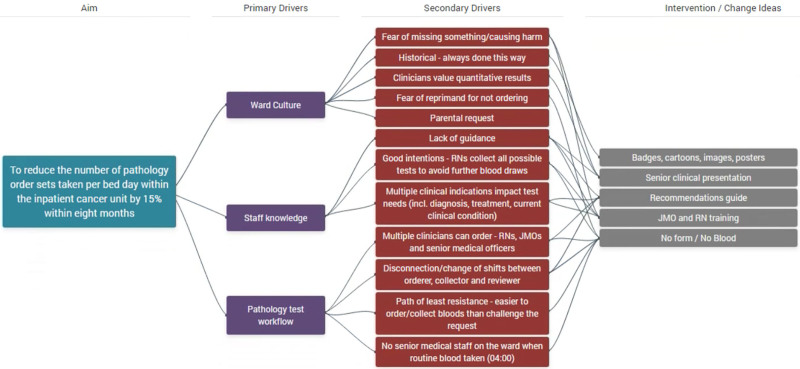
We developed a strategy to address blood tests, including local multidisciplinary expert agreement and the best available evidence. Through systematic analysis, clinician involvement, and patient and family feedback, we identified that ward culture, staff knowledge, and blood test workflow were primary drivers for the current state of practice. The change strategy consisted of multiple elements that addressed different aspects of the process and staff specialties summarized in a Driver Diagram (Fig. 2). We held collaborative, multidisciplinary meetings to review the best available evidence and discuss the expected clinical course for various patients; for example, the anticipated myelosuppression from chemotherapy and subsequent recovery and the complications and trajectory of febrile neutropenia admissions.
Fig. 2.
Driver diagram.
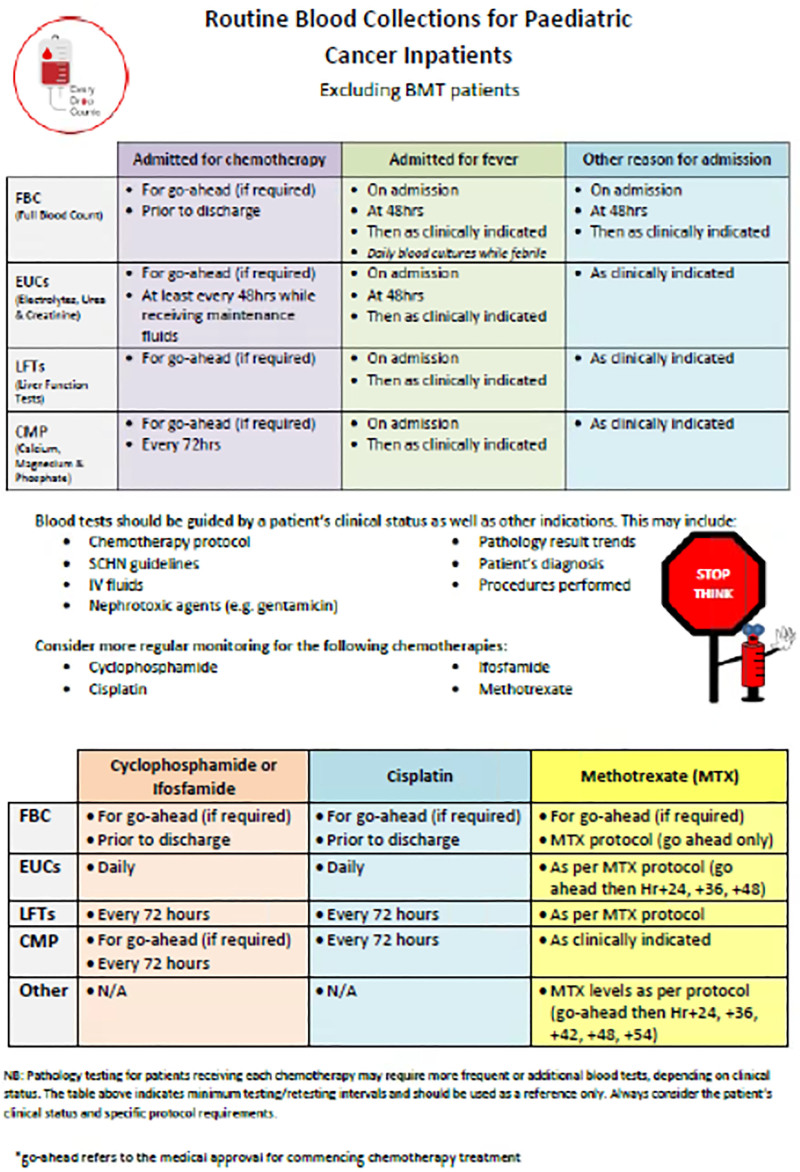
From this, written guidelines represented in a recommendations table (Fig. 3) identified the standard frequency of the blood tests related to common reasons for admission. We developed the guidelines through an iterative process with senior clinician involvement, encompassing opportunities for feedback to refine the table and gain a consensus opinion of the clinical appropriateness for each test. Importantly, this also engaged senior clinicians to embrace the change through a codesign approach.
Fig. 3.
Recommendation table for routine blood collections.
Targeted education sessions were delivered separately to JMOs and RNs outlining the project, providing the rationale for blood tests, and educating how the interventions will improve patient care. We challenged all clinicians to STOP and THINK about ordering tests and RNs not to order routine blood tests unless clinically appropriate with the slogan “No Form/No Blood.” A culture change campaign, including a logo, posters, badges, cartoons, lanyards, and other material, was distributed to frontline staff to support the adoption and sustainability of the project. Branding with the slogan “Every Drop Counts” aligned this project with other blood management initiatives within the unit. This compounding of QI projects fed into the larger hemovigilance approach and aligned with a national healthcare priority.
Study of the Interventions
We analyzed 4 blood tests (including 39 discrete parameters). The blood tests and discrete parameters are listed in Table (Supplemental Digital Content 1, http://links.lww.com/PQ9/A370). The change strategy’s impact was assessed by comparing blood test frequency during a preimplementation phase (June 2019–February 2020) and postimplementation phase (March–October 2020). Additionally, we gathered data during a follow-up phase (November 2020–October 2021) to evaluate sustainability. We gathered preimplementation data retrospectively, and postimplementation data, including the follow-up period, prospectively at month’s end.
Patients who received a transplant or cellular therapy within the last 12 months were excluded due to their clinical complexity.
We conducted baseline and follow-up staff surveys with an anonymous questionnaire (Material, Supplemental Digital Content 1, http://links.lww.com/PQ9/A371) to identify culture change and knowledge acquisition. This survey assessed staff knowledge, ward culture, and the changing environment.
Measures
The primary process measure was the total number of FBCs, EUCs, LFTs, and CMPs per bed day per month pre- and postimplementation. We used bed days to account for ward activity. We analyzed each of the 4 blood tests separately to compare the baseline ordering frequency to postimplementation frequency. This difference represented the reduction in test frequency. Outcome measures included cost savings, blood volume saved, and equivalent blood transfusions.
Balance measures included any unintentional harm resulting from the absence of blood monitoring. We tracked balance measures through the hospital’s incident management system and regular engagement with clinicians to mitigate the risk of harm.
Analysis
We used process control charts to track the outcome measure monthly. We calculated the baseline mean of blood tests per bed day per month, with upper and lower control limits (±3 standard deviations). Standard rules for identifying special cause variation were applied using 8 consecutive points below the centerline. Additionally, Poisson regression analysis examined the difference in blood test ordering per period. A P value of ≤0.05 was considered significant. The hospital’s analysis unit provided monthly blood tests, bed days, and demographics.
We used patient demographic comparators to validate the uniformity of the patient population pre- and postintervention. We used the consolidated framework for implementation research with rapid-cycle evaluation.11
We extracted additional activities, diagnoses, and demographics to determine if there was variation in service delivery:
Average length of stay per admission, number of distinct admissions, and number of new diagnoses admissions for febrile neutropenia represented markers of activity.
We calculated savings per 1,000 bed days for pathology costs, consumable costs, and blood volume.
We calculated pathology costs at rollout using Medicare Benefits Schedule Category 6, August 1, 2019. Service fees were not included.
For benefits analysis, we calculated:
consumables on a cost per item based on standard blood collection equipment;
blood volume using a standard 5 mL discard and 3 mL per order set;
the equivalent volume of blood transfusions saved based on 240 mL per transfusion.
This study follows the SQUIRE V.2.0 publication guidelines for reporting.12
Ethical Considerations
The project team gained ethical approval via the hospital’s quality governance structure (activity number 6,391 approved November 1, 2019). Ethical approval included a review of patient electronic medical records and surveys of staff and consumers.
RESULTS
During the pre- and postimplementation phase, we included 26,941 blood tests during 1,558 admissions. Table 1 represents the absolute number of order sets. Table 2 compares patient demographics pre- and postintervention. During the study period, no significant changes occurred in chemotherapy protocols or local guidelines.
Table 1.
Blood Test Comparison Pre- and Postimplementation
| Preimplementation Tests(June 2019 to Feb 2020) | Postimplementation tests(March to Oct 2020) | Reduction Mean/bedday |
P * | |||
|---|---|---|---|---|---|---|
| Absolute tests | Mean/bedday | Absolute tests | Mean/bedday | |||
| FBC | 4,020 | 0.95 | 2,780 | 0.65 | 32% | p < 0.0001 |
| EUC | 4,654 | 1.10 | 3,643 | 0.85 | 23% | p < 0.0001 |
| LFT | 3,822 | 0.91 | 1,853 | 0.43 | 52% | p < 0.0001 |
| CMP | 3,801 | 0.90 | 2,368 | 0.55 | 37% | p < 0.0001 |
*Poisson regression based on mean blood tests/bedday.
CMP, calcium magnesium phosphate; EUC, electrolytes, urea, creatinine.; FBC, full blood count; LFT, Liver Function Tests.
Table 2.
Activity Comparison Pre- and Postimplementation
| Preimplementation | Postimplementation | |
|---|---|---|
| Time period | June 2019 to Feb 2020 | March to Oct 2020 |
| Total inpatient beddays | 4,260 | 4,305 |
| Average LOS per encounter | 3.53 days | 3.97 days |
| Number distinct encounters | 757 | 675 |
| New diagnosis (denovo + relapse) | 121 | 133 |
| Denovo disease | 98 | 107 |
| Relapse diagnosis | 23 | 24 |
| Fever immunosuppressed patient | 74 | 86 |
LOS, length of stay.
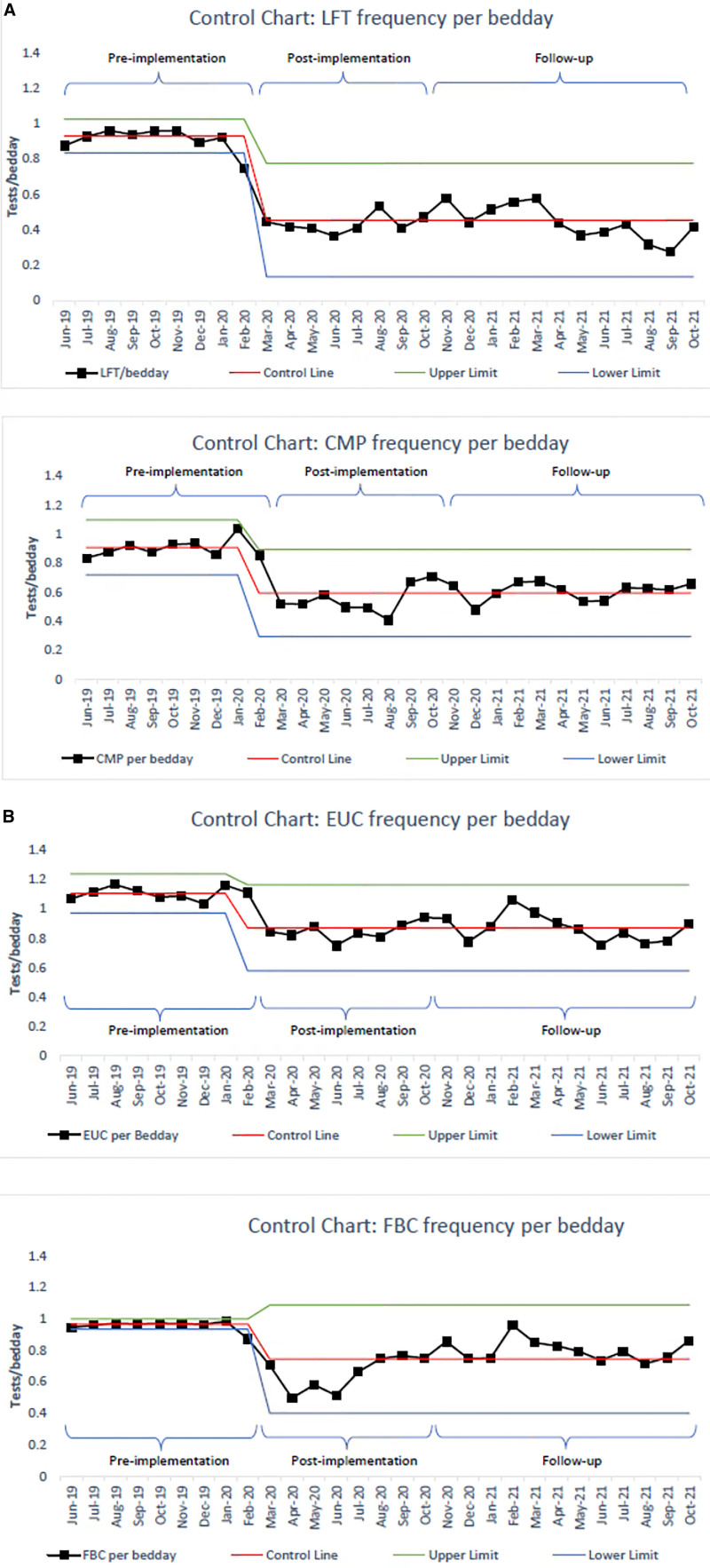
Over the 8-month postimplementation phase, there was a reduction in all 4 blood tests, ranging from 23% to 52% reduction (Table 1 and Fig. 4A, B). LFTs displayed the most significant reduction. All 4 blood tests demonstrate 8 consecutive points below the centerline. CMPs and LFTs demonstrated a sustained reduction throughout the follow-up phase. Both EUCs and FBCs demonstrated a trend back to the centerline, with both touching the preimplementation mean at 1 point. However, all 4 blood tests demonstrate 8 consecutive points below the centerline in the follow-up phase (Fig. 4B).
Fig. 4.
A, Control chart: LFT and CMP per bed day. B, Control Chart: FBC and EUC per bed day. CMP, calcium magnesium phosphate; EUC, electrolytes, urea, creatinine.; FBC, full blood count; LFT, Liver Function Tests.
Over the period between March and October 2020, the calculated savings ranged from $13,000 to $17,000 each month based on pathology fees alone. Additionally, $1,200–$1,500 per month in consumables was saved. This finding equates to a calculated savings of over $130,000 (AUD) over the 8 months. The total blood volume saved was calculated as 48 L over 8 months, equivalent to 200 units of packed red blood cells.
Analysis of balancing measures identified 1 episode of misadventure. This event resulted from a lack of LFT monitoring during regular paracetamol administration of a patient with underlying hepatotoxicity. There were no serious sequelae, with LFTs returning to baseline following paracetamol cessation. Although the incident did not directly align with the project recommendations, it is unlikely to have occurred based on previous practice.
Key clinicians involved in ordering blood tests received a questionnaire. At baseline, 76% felt blood tests were taken too frequently. Over 80% of RNs and JMOs self-reported they had changed practice due to the interventions at follow-up. The 3 main contributors to practice change were self-reported: support and empowerment not to order blood tests, written recommendation guidelines, and the nursing directed imperative “No form/No blood.”
DISCUSSION
Empowerment and education of medical and nursing teams created a behavior change that has resulted in an overall reduction in blood test ordering of 37% in pediatric cancer patients. This reduction has been sustained over the follow-up period and out-performed our aim of a 15% reduction. Moreover, each blood test demonstrated a statistically significant decrease in test ordering frequency, ranging from 23% to 52% reduction. This result had significant clinical and financial benefits without causing increased harm.
Clinician empowerment was self-reported by RNs and JMOs as the most significant influence in changing practice. We also identified written guidelines and the imperative “No form/No blood” as having a significant impact.
FBCs and EUCs demonstrated a trend toward the centerline following the initial reduction in blood test ordering. This finding may be due to an initial “over compliance” with not ordering tests. Although fewer tests are needed, blood test results still provide vital information for clinicians, and achieving the right balance is necessary. Therefore, FBCs and EUCs are likely to be required more frequently than CMPs and LFTs for the clinical management of pediatric oncology patients. Additionally, the points at which EUCs and FBCs touch the centerline may be explained by the cancer unit undergoing a significant staffing change in January 2021. New senior oncologists joined the team, and JMOs changed in January 2021. This change required training to reframe the new staff with the current ordering culture within the unit.
This project is unique in pediatric cancer, yet initiatives attempting to reduce the burden of blood testing on health services are not novel. Change ideas to limit blood requests have focused on improving knowledge, changing systems, and financial implications.2–4,6,13 Wertheim et al demonstrated a reduction in blood ordering in an adult general medical service.2 Similar to our project, change ideas included education and the development of written guidelines. However, the change strategy by Wertheim et al also included alterations in electronic ordering and written progress notes. A report undertaken by the NCOPP reviewed strategies to prevent the overuse of blood tests. The report concluded that numerous interventions demonstrate short-term results; however, strategies that resulted in sustainable success were challenging to quantify.6 They identified interventions that made simple changes focused around behavior science, with active support from senior clinicians and engagement with junior clinicians, were most likely to result in long-term success.
Routine blood testing has been a long-entrenched practice within our unit. As a result, behavior change within the clinical setting can be challenging, with contextual factors and clinician engagement identified as potential barriers. These barriers are not uncommon with QI and addressing these is vital for integrating change successfully.14 Rapport et al discuss enabling factors that support the implementation process, drawing on five foundational concepts: diffusion, dissemination, implementation, adoption, and sustainability.15
We feel several elements contributed to the success of this project. Intuitively, the initial focus was on diffusion and dissemination. An important enabler for our projects’ success was the unit’s understanding that change needed to occur. Initial discussions with senior clinicians identified they felt less frequent testing was appropriate; however, they were unsure how to implement this safely. The baseline surveys reflected this opinion, with over 3-quarters of respondents indicating that blood tests were taken too frequently. The multifaceted strategy, focusing on education, support, and guidance, allowed clinicians to rationalize the need to order a test. By providing recommendations rather than a directive for order frequency, we engaged clinicians in a decision-making process that empowered them to question the rationale and learn in the process. Additionally, the awareness campaign created a discussion that aligned senior and junior medical and nursing perspectives.
Comparing baseline and follow-up perspectives on test frequency shows a significant shift in clinician thinking. This shift away from daily testing demonstrates clinicians were questioning why a specific blood test is appropriate for a particular patient on a specific day. This result has been shown clinically in the differences between test frequencies following implementation. In addition, comparing the number of individual blood tests per bed day showed varying results (Fig. 4). This variability implies clinicians are considering which test is appropriate for the patient. In essence, this thought process is the core of value-based blood testing.6
Reducing blood tests taken from pediatric cancer patients has significant clinical benefits. Blood withdrawal through a CVC has an inherent risk of infection16 confounded by a vulnerable, immunocompromised patient population. Additionally, blood volume is taken from the patient with each blood withdrawal. Therefore, the pediatric patient population is at higher risk of clinician-induced anemia with a smaller blood volume than adults. Furthermore pediatric cancer treatments rely heavily on aggressive, intensively timed myelosuppressive treatment regimens.17 Over the 8 months, the calculated blood volume saved was 48 L and would be expected to lessen the extent of clinician-induced anemia and the requirement for blood transfusions.
Considering the national interest in the quality use of blood tests and the ever-growing cost of health care, strategies to promote safe, patient-centered approaches to reduce blood tests in a financially beneficial way are vital. This project has the potential for direct scalability with other pediatric cancer services. Individual units will need to consider the contextual factors that may support or create barriers to integrating these recommendations into their service. There is transferability with other adult and pediatric subspecialties. Individual blood tests need to be relevant, considering the most significant impact on the clinical area.
Of note, we demonstrated significant results during the project despite the international pandemic (COVID-19).
LIMITATIONS
This project has several limitations. First, as a single-center QI project, results may require further evaluation to determine generalizability. Second, we have demonstrated a significant reduction sustained for a short period (20 months); however, long-term sustainability is vital for the project’s success. Finally, the investigators intend to continue to monitor blood test frequency with ongoing engagement with senior clinicians.
The project did not directly measure a reduction in CVC infection rates or clinician-induced hypovolemia; this is a potential future direction.
CONCLUSION
Here, we report a demonstrable and sustained reduction in blood test ordering. We have found that a multifaceted strategy incorporating education and a culture change approach can significantly reduce the ordering frequency of blood tests for hospitalized pediatric oncology patients. We have integrated a patient-centered decision-making framework for clinically appropriate blood testing by challenging ingrained behaviors across the medical and nursing cancer service.
We demonstrated meaningful clinical benefits for the patient and substantial financial savings. To our knowledge, this is the first initiative to develop clinician-led guidelines providing recommendations on blood test frequency for pediatric oncology patients. Our findings benefit pediatric oncology and can be transferable to wider pediatric and adult subspecialties.
Supplementary Material
Footnotes
Published online June 14, 2022
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
The authors listed above certify that they have read and approved the article, that the requirements for authorship have been met, and each author believes that the article represents honest work.
REFERENCES
- 1.Freedman DB. Towards better test utilization—strategies to improve physician ordering and their impact on patient outcomes. EJIFCC. 2015;26:15–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim BM, Aguirre AJ, Bhattacharyya RP, et al. An educational and administrative intervention to promote rational laboratory test ordering on an academic general medicine service. Am J Med. 2017;130:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinitsky L, Brierley J. Reducing the number of unnecessary liver function tests requested on the Paediatric Intensive Care Unit. BMJ Qual Improv Rep. 2017;6:u214071.w5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamburrano A, Vallone D, Carrozza C, et al. Evaluation and cost estimation of laboratory test overuse in 43 commonly ordered parameters through a Computerized Clinical Decision Support System (CCDSS) in a large university hospital. PLoS One. 2020;15:e0237159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limb M. A fifth of healthcare spending is wasted, says OECD report. BMJ. 2017;356:j215. [DOI] [PubMed] [Google Scholar]
- 6.National Coalition of Public Pathology (NCOPP). Encouraging Quality Pathology Ordering in Australia’s Public Hospitals. Final Report. Canberra: NCOPP; 2012. [Google Scholar]
- 7.Emergency Care Institute New South Wales. Sensible Test Ordering Project (STOP) Brief. 2013.
- 8.Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med. 2014;89:990–995. [DOI] [PubMed] [Google Scholar]
- 9.Dhanani JA, Barnett AG, Lipman J, et al. Strategies to reduce inappropriate laboratory blood test orders in intensive care are effective and safe: a before-and-after quality improvement study. Anaesth Intensive Care. 2018;46:313–320. [DOI] [PubMed] [Google Scholar]
- 10.van den Bosch CH, van der Bruggen JT, Frakking FNJ, et al. Incidence, severity and outcome of central line related complications in pediatric oncology patients; A single center study. J Pediatr Surg. 2019;54:1894–1900. [DOI] [PubMed] [Google Scholar]
- 11.Keith RE, Crosson JC, O’Malley AS, et al. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: a rapid-cycle evaluation approach to improving implementation. Implement Sci. 2017;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25:986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baricchi R, Zini M, Nibali MG, et al. Using pathology-specific laboratory profiles in clinical pathology to reduce inappropriate test requesting: two completed audit cycles. BMC Health Serv Res. 2012;12:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braithwaite J, Marks D, Taylor N. Harnessing implementation science to improve care quality and patient safety: a systematic review of targeted literature. Int J Qual Health Care. 2014;26:321–329. [DOI] [PubMed] [Google Scholar]
- 15.Rapport F, Clay-Williams R, Churruca K, et al. The struggle of translating science into action: foundational concepts of implementation science. J Eval Clin Pract. 2018;24:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell T, O’Grady NP. Prevention of central line-associated bloodstream infections. Infect Dis Clin North Am. 2017;31:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roubinian N, Carson JL. Red blood cell transfusion strategies in adult and pediatric patients with malignancy. Hematol Oncol Clin North Am. 2016;30:529–540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.