Abstract
PURPOSE
High allelic ratio (HAR) FLT3/ITD (AR > 0.4) mutations confer poor prognosis in pediatric acute myeloid leukemia (AML). COG AAML1031 studied the feasibility and efficacy of adding sorafenib, a multikinase tyrosine kinase inhibitor to standard chemotherapy and as single-agent maintenance therapy in this population.
MATERIALS AND METHODS
Patients were treated in three cohorts. The initial safety phase defined the maximum tolerated dose of sorafenib starting in induction 2. Cohorts 2 and 3 added sorafenib in induction and as single-agent maintenance. Clinical outcome analysis was limited to n = 72 patients in cohorts 2/3 and compared with n = 76 HAR FLT3/ITD+ AML patients who received identical chemotherapy without sorafenib. Sorafenib pharmacokinetics and plasma inhibitory activity were measured in a subset of patients.
RESULTS
The maximum tolerated dose of sorafenib was 200 mg/m2 once daily; dose-limiting toxicities included rash (n = 2; 1 grade 3 and 1 grade 2), grade 2 hand-foot syndrome, and grade 3 fever. Pharmacokinetics/plasma inhibitory activity data demonstrated that measured plasma concentrations were sufficient to inhibit phosphorylated FLT3. Although outcomes were superior with sorafenib in cohorts 2 and 3, patients treated with sorafenib also underwent hematopoietic stem-cell transplant more frequently than the comparator population. Multivariable analysis that accounted for both hematopoietic stem-cell transplant and favorable co-occurring mutations confirmed sorafenib's benefit. Specifically, risk of an event was approximately two-fold higher in HAR FLT3/ITD+ patients who did not receive sorafenib (event-free survival from study entry: hazard ratio [HR] 2.37, 95% CI, 1.45 to 3.88, P < .001, disease-free survival from complete remission: HR 2.28, 95% CI, 1.08 to 4.82, P = .032, relapse risk from complete remission: HR 3.03, 95% CI 1.31 to 7.04, P = .010).
CONCLUSION
Sorafenib can be safely added to conventional AML chemotherapy and may improve outcomes in pediatric HAR FLT3/ITD+ AML.
INTRODUCTION
Fms-like tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase, and mutations in FLT3 occur in 10%-15% of pediatric de novo acute myeloid leukemia (AML) patients.1,2 Children with high allelic ratio (HAR; AR > 0.4) FLT3 internal tandem duplication (ITD) mutant AML have inferior outcomes with survival of approximately 25%-30% historically; hematopoietic stem-cell transplant (HSCT) has improved outcomes to 50%-65%.1,3-6 Co-occurrence of an NPM1 mutation, seen in 20%-30% of FLT3/ITD+ AML, confers more favorable outcome, with event-free survival (EFS) of approximately 60%.7
CONTEXT
Key Objective
Pediatric high allelic ratio (HAR) FLT3/ITD+ acute myeloid leukemia (AML) is a high-risk disease subset. Children's Oncology Group protocol AAML1031 tested sorafenib, a tyrosine kinase inhibitor (TKI), in the treatment of children with this AML subtype. Patients who consented to treatment received sorafenib in combination with conventional chemotherapy; a subset of patients were also eligible for sorafenib maintenance.
Knowledge Generated
Sorafenib was safe and tolerable and significantly improved event-free survival and disease-free survival while lowering relapse risk in children with HAR FLT3/ITD+ AML. Multivariable analysis that accounted for stem-cell transplant and favorable co-occurring mutations confirmed sorafenib's benefit. The utility of maintenance treatment warrants further investigation, given limited patient exposure.
Relevance
Treatment of pediatric HAR FLT3/ITD+ AML should entail consideration of TKIs. Contemporary pediatric AML trials are studying the feasibility and efficacy of second-generation TKIs in combination with chemotherapy and as a postconsolidation maintenance approach.
FLT3/ITD alterations constitutively activate FLT3, and tyrosine kinase inhibitors (TKIs) are approved for adults with FLT3-mutated AML.8,9 Type I inhibitors, including gilteritinib, inhibit both FLT3/ITD mutations and tyrosine kinase domain–activating mutations. By contrast, type II inhibitors, such as sorafenib, are largely inactive against the latter.10 Sorafenib targets KIT, PDGFR, VEGF, RET, and RAF pathway signaling along with FLT3. Studies of sorafenib in adults with FLT3-mutant AML demonstrate safety despite targeting multiple pathways but impact on outcome is variable.11-16 After two early pediatric studies demonstrated feasibility of administering sorafenib in pediatric AML,17,18 COG AAML1031 broadened this experience by adding sorafenib to chemotherapy for patients with HAR FLT3/ITD+ AML and as single-agent maintenance. We hypothesized that sorafenib could be added safely and would improve remission induction and survival outcomes.
MATERIALS AND METHODS
Patients and Treatment
Details of the primary AAML1031 randomization are published.19 At enrollment, patients were randomly assigned to either arm A (standard chemotherapy) or arm B (standard chemotherapy with bortezomib) and underwent centralized FLT3/ITD mutation testing. Dexrazoxane use as a cardioprotectant was per treating physician discretion. Patients with an FLT3/ITD AR > 0.4 were eligible for enrollment on arm C. If consented, patients initially randomly assigned to arm A continued standard chemotherapy with sorafenib, whereas arm B patients discontinued bortezomib when signing arm C consent. After arms A/B closed,19 patients were enrolled on arm D (same as arm A) until FLT3/ITD results returned; if positive, they were eligible for arm C. AAML1031 was approved by the National Cancer Institute's Central Institutional Review Board (IRB) and local IRBs (n = 184). Patients and families provided informed consent and assent as appropriate. The trial was conducted in accordance with the Declaration of Helsinki and was registered at ClinicalTrials.gov (identifier: NCT01371981). The clinical Protocol (online only) included three aims for patients with HAR FLT3/ITD+ AML: (1) feasibility of sorafenib administration, (2) assessment of antileukemic activity of sorafenib, and (3) analysis of pharmacokinetics (PK) and plasma inhibitory activity (PIA) in subjects receiving sorafenib. Analytic plans/power analyses are provided in the protocol.
Treatment Cohorts
The initial safety phase (cohort 1 [C1], n = 12, Data Supplement, online only) defined the maximum tolerated dose of sorafenib when administered in induction 2 and subsequent courses. Targeted toxicities were compared against predetermined rates that would mandate treatment arm closure. During the safety phase, sorafenib was initiated at 200 mg/m2 once daily; given lack of protocol defined dose-limiting toxicities that would warrant treatment de-escalation, the recommended dosing of sorafenib for subsequent cohorts remained 200 mg/m2. Following completion of C1, the study was amended (cohort 2 [C2], Data Supplement) to start sorafenib on day 11 of induction 1 and to administer concomitantly with chemotherapy in subsequent cycles. This design maximized sorafenib exposure while allowing for delayed drug start during induction 1, given centralized FLT3 testing. Moreover, by starting sorafenib after chemotherapy in induction 1, risk for overlapping toxicities of two investigational drugs (sorafenib and bortezomib) was lessened. A year of sorafenib maintenance, administered after HSCT or completion of chemotherapy (if no HSCT donor was identified) was added for patients enrolled in C2 given preliminary evidence for benefit of maintenance therapy.20-23 Maintenance dosing was 100 mg/m2/daily with potential intrapatient escalation to a maximum of 150 mg/m2 twice daily. After interim analyses suggested potential cardiac risk with this dosing schedule, the study was subsequently amended (cohort 3 [C3], Data Supplement) to start sorafenib after chemotherapy completion each cycle. Patients eligible for arm C but diagnosed during periods of arm C closure were eligible to transition to arm C after induction when the arm reopened but were not included in efficacy analysis. Targeted toxicities of all arm C cohorts were described in evaluable patients. To minimize potential confounding influence of bortezomib, toxicities of n = 53 FLT3/ITD+ patients enrolled on arm C after initial treatment assignment to arm A were compared with those of 34 arm A patients with HAR FLT/ITD+ AML who either declined arm C participation or were treated on arm A while arm C was closed.
Sorafenib-Exposed Versus Sorafenib-Unexposed Patients
Long-term clinical outcome analysis was limited to C2/C3, given lack of induction 1 sorafenib exposure in C1. Patients in C2/C3 who did not receive drug during induction I were also excluded. Outcome measures for patients with FLT3/ITD+ AML enrolled on arm C2 and C3 were compared with children with FLT3/ITD+ AML (AR > 0.4) who received similar treatment without sorafenib. Specifically, this unexposed group included patients who enrolled on AAML1031 but remained on their initial treatment arm (arm A: n = 19, arm B: n = 23) because of declination of arm C enrollment (n = 14) or closure of arm C during their time on protocol therapy (n = 28). Since AAML1031 observed equivalent outcomes between arms A and B,19 patients with HAR FLT3/ITD+ AML from both arms were included in the response comparison. In addition, n = 34 HAR FLT3/ITD+ patients on AAML0531 arm A (standard chemotherapy without gemtuzumab ozogamicin) were also defined as the unexposed cohort.24 Ultimately, a total of 72 patients from AAML1031 arm C were included in the sorafenib-exposed analyses and compared with n = 76 patients on AAML1031/AAML0531 who did not receive sorafenib (sorafenib-unexposed, Data Supplement).
Statistical Analyses
Data were current as of June 30, 2021. The significance of observed difference in proportions was tested using the chi-squared test and Fisher's exact test when data were sparse. The Kruskal-Wallis test was used to determine the significance between differences in medians of groups. The Kaplan-Meier method was used to calculate overall survival (OS), EFS, and disease-free survival (DFS).25 Nonparametric maximum likelihood estimation was used to estimate the cumulative incidence of relapse risk (RR).26 OS was defined as time from study entry until death. EFS was defined as time from study entry until either death, refractory disease, or relapse of any type, whichever occurred first. DFS was defined as time from end of induction 1 for patients in complete remission (CR) until relapse or death. RR was defined as time from the end of induction 1 for patients in CR to relapse, where deaths without a relapse were considered competing events. The statistical significance of predictor variables was tested with the log-rank statistic for OS, EFS, and DFS, and with Gray's statistic for RR.26 Three-year estimates were summarized with their corresponding log-log 95% CIs. Cox proportional hazards models were used to estimate hazard ratios (HRs) for univariable and multivariable analyses of OS, EFS, and DFS.27 Competing risk regression models were used to estimate the subgroup HR for univariable and multivariable analyses of RR. Receipt of HSCT on protocol therapy was analyzed as a time-varying covariate (TVC) to control for HSCT effect. To minimize impact of TKI exposure after removal from protocol therapy, sorafenib-unexposed patients were censored at date of elective withdrawal from protocol therapy. All P values were two-sided.
PK and Pharmacodynamic Analysis
Sorafenib PK and PIA were measured in a subset of patients who consented to this optional study and provided evaluable samples at prescribed time points. A noncompartmental PK analysis characterized the concentration × time profile and trough concentrations at steady state for sorafenib and the N-oxide metabolite.28 Pharmacodynamic testing was conducted by determining PIA using previously described techniques.29
RESULTS
Study Distribution
A total of 1,645 de novo AML patients enrolled on AAML1031; 1,609 were study-eligible. Of the 1,609 enrolled, n = 136 (8.5%) had HAR FLT3/ITD+ AML (AR > 0.4) and were eligible for arm C enrollment, of which 92 patients (68%) consented. An additional n = 42 HAR FLT3/ITD+ patients enrolled on arm A/B patients did not participate in arm C (Fig 1B). Of 24 HAR FLT3/ITD+ patients randomly assigned to bortezomib before arm C enrollment, only 19 received bortezomib in close proximity to sorafenib.
FIG 1.
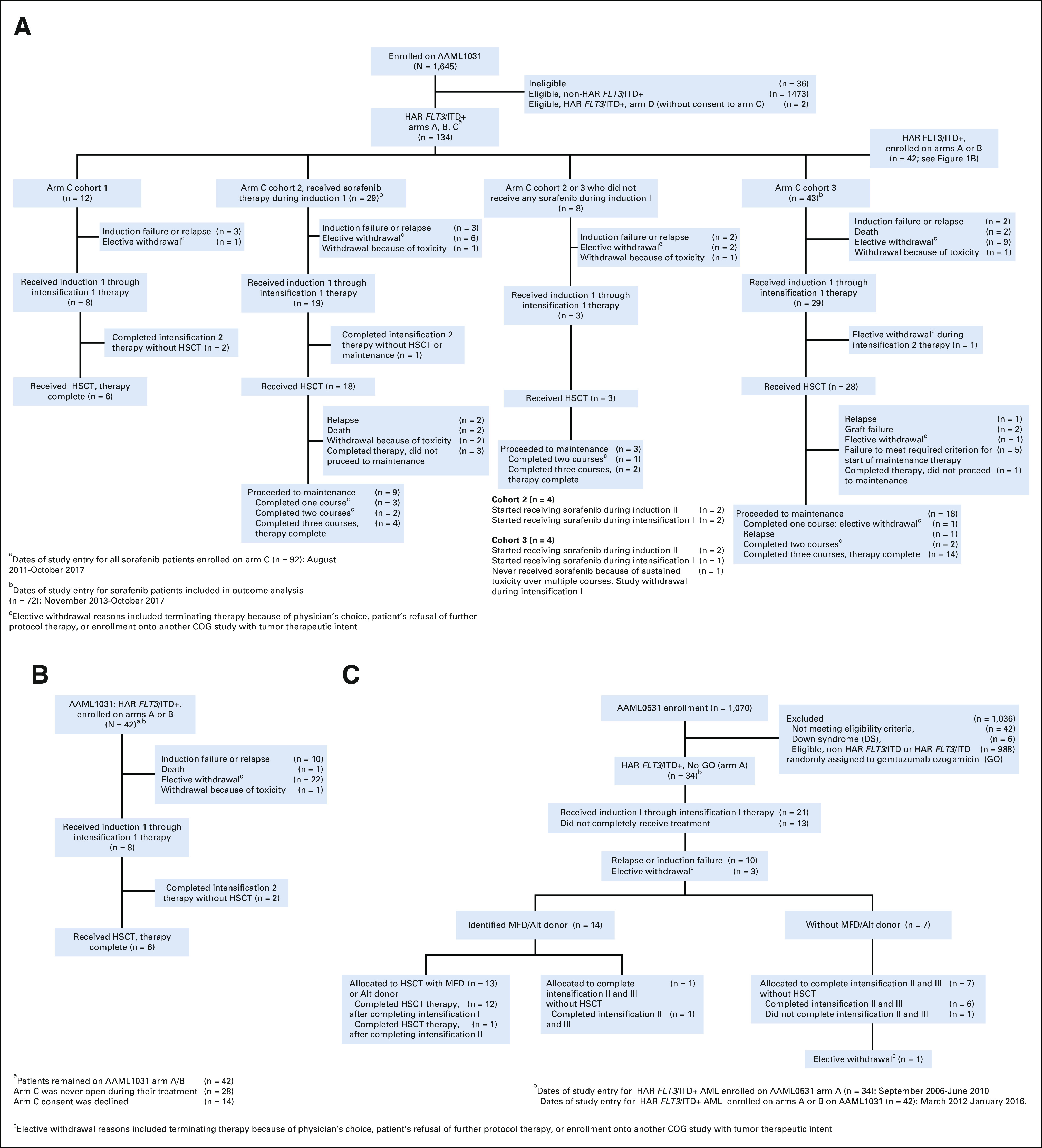
Distribution of HAR FLT3/ITD+ AML patients included in analysis. (A) CONSORT diagram of AAML1031 overall and by Arm C cohort, (B) Flow diagram of AAML1031 Arms A/B (sorafenib-unexposed), and (C) Flow diagram for AAML0531 (sorafenib-unexposed). Alt, alternative donor (donor availability defined for intermediate- and high-risk patients only); AML, acute myeloid leukemia; HAR, high allelic ratio; HSCT, hematopoietic stem-cell transplant; MFD, matched family donor.
Arm C C1 Analysis (safety phase)
The maximum tolerated dose of sorafenib in C1 was 200 mg/m2 once daily. Dose-limiting toxicities observed in C1 included rash (grade 2 [n = 1] and grade 3 [n = 1]), grade 2 hand-foot syndrome (n = 1), and grade 3 fever (n = 1). Rates of targeted toxicities for C1 were similar to that of arm C patients in later cohorts (Data Supplement).
Targeted Toxicity for FLT3/ITD+ Patients on Arm A Versus C
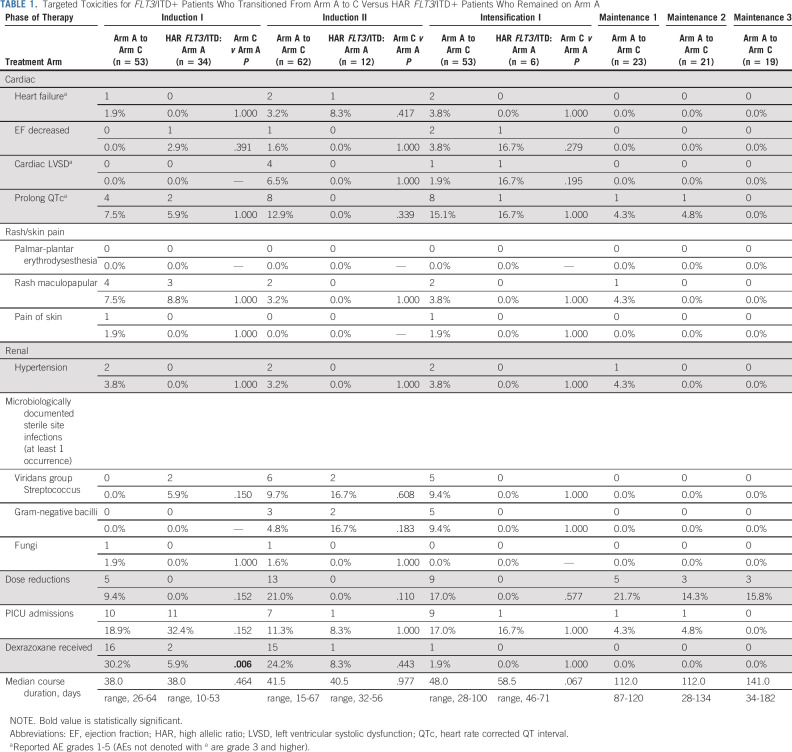
FLT3/ITD+ patients initially enrolled on arm A before arm C enrollment (n = 53) were compared with n = 34 FLT3/ITD+ patients who remained on arm A (standard therapy). Targeted toxicities were similar, both overall (Table 1) and across cohorts and treatment phases for arm C patients who initially were treated on arm A (Data Supplement). Moreover, rates of chemotherapy dose reduction and intensive care unit admission were similar (Table 1). Interestingly, patients on arm C were more likely to receive dexrazoxane as a cardioprotectant with anthracycline therapy during induction I (P = .006, Table 1). No significant unanticipated toxicities were identified in the sorafenib cohort.
TABLE 1.
Targeted Toxicities for FLT3/ITD+ Patients Who Transitioned From Arm A to C Versus HAR FLT3/ITD+ Patients Who Remained on Arm A
Interim cardiac toxicity analyses identified a preliminary signal of increased cardiac toxicity in 7/33 (22%) C2 patients as defined by grade 3 ejection fraction (EF) decline (n = 3), grade 2 EF decline (n = 2), grade 3 left-ventricular systolic dysfunction (n = 1), grade 2 cardiac other (shortening fraction decline, n = 1), and grade 1 cardiac other (shortening fraction decline, n = 1). Two patients met criteria for permanent discontinuation of sorafenib and two tolerated restart. The remaining five discontinued protocol therapy before rechallenge was possible. This toxicity concern prompted amendment of the chemotherapy schedule to start sorafenib after completion of standard chemotherapy in a given cycle (cohort 3, C3, Data Supplement). Ultimately, the cardiac toxicity observed in arm C was comparable to that of arm A (Table 1). Differences in median EF were also comparable between arms C and A and similar across arm C cohorts (Table 1, Data Supplement).
Feasibility of Sorafenib Maintenance
Sorafenib maintenance was restricted to 80 patients in C2/C3; 30/80 (38%) received at least one cycle (4 months of therapy) and 20/80 (25%) completed all maintenance treatment. Approximately 62% of patients did not receive any maintenance treatment; 45/80 (56%) went off protocol therapy prior to being eligible for maintenance (Fig 1) and the remaining 5/80 (6%) failed to meet maintenance eligibility criteria. Maintenance toxicity rates were similar to that of earlier treatment cycles (Table 1, Data Supplement).
Arm C Clinical Characteristics and Induction Response by Cohort
To identify potential confounders that could have clinical impact, clinical covariates were compared between sorafenib cohorts. No statistically significant differences were observed with the exception of patients who received sorafenib during induction 1 (eg, C2 or C3) had decreased burden of disease if found to be minimal residual disease–positive (Data Supplement).
Clinical Characteristics and Treatment Response for Sorafenib-Exposed Versus -Unexposed Cohorts
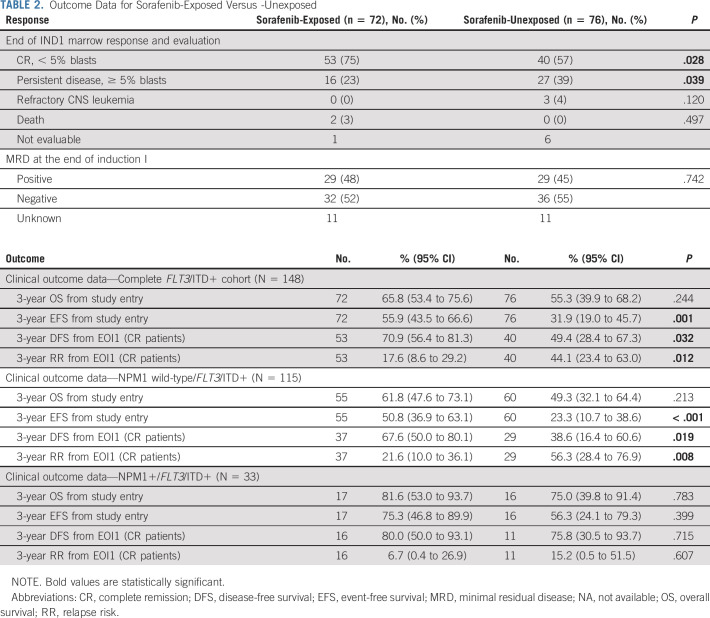
Clinical characteristics were similar for the sorafenib-exposed versus sorafenib-unexposed cohorts with the exception that children of Hispanic ethnicity were more common in the unexposed cohort and HSCT occurred more frequently in those treated with sorafenib. Notably, prevalence of co-occurring NPM1 mutation was similar for the two cohorts (Data Supplement). Patients who received sorafenib were more likely to achieve morphologic CR at the end of induction 1 and were less likely to have persistent disease. However, rates of minimal residual disease were not significantly different (Table 2) for the two groups.
TABLE 2.
Outcome Data for Sorafenib-Exposed Versus -Unexposed
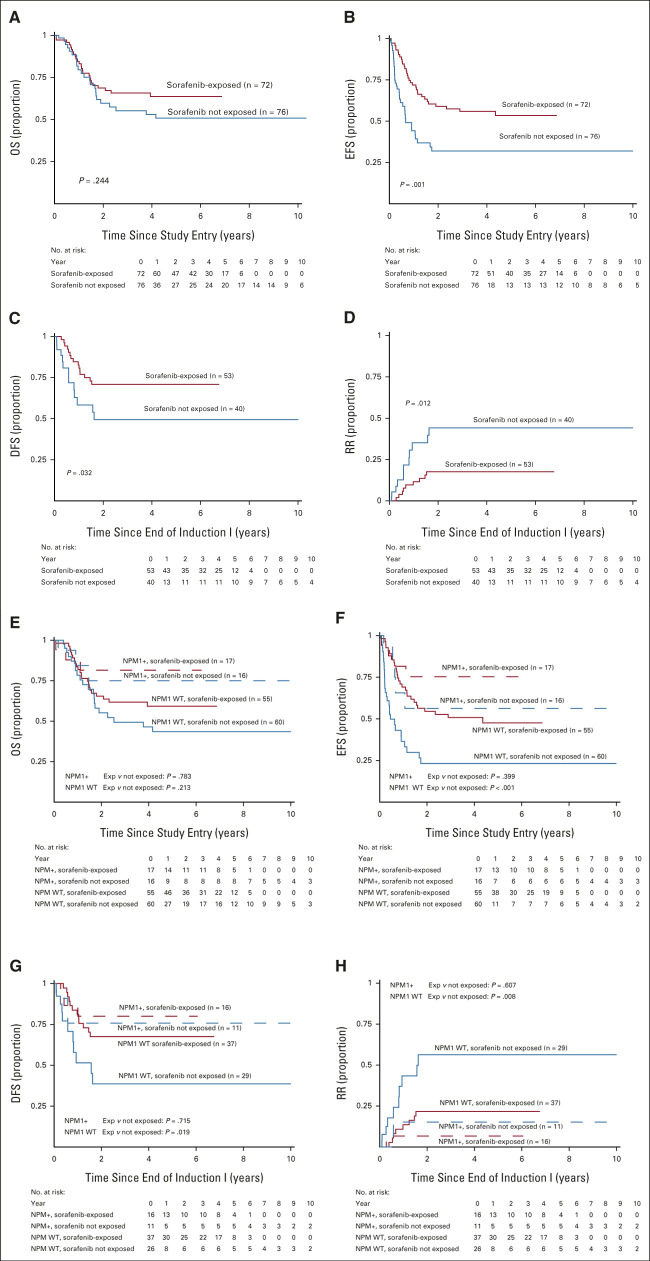
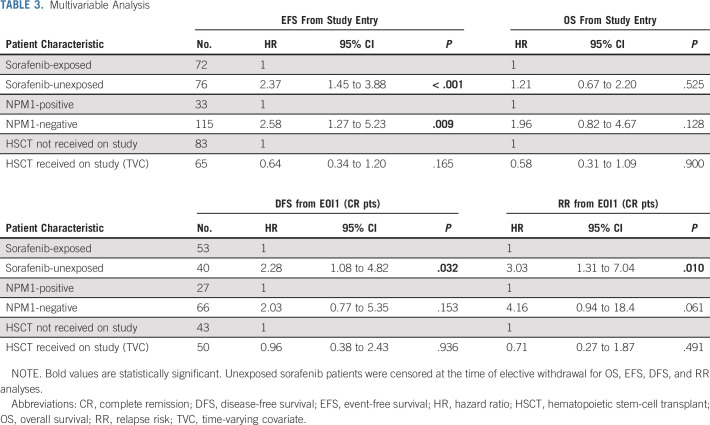
The median (range) of follow-up time for patients alive at last contact was 5.3 (0.3-13.1) years. There were 32 EFS events among patients exposed to sorafenib (n = 72) and 35 events among patients who were unexposed (n = 76). Comparison of long-term outcomes suggested that sorafenib exposure was associated with improved EFS from study entry as well as DFS and RR from CR but not OS (Table 2, Figs 2A-2D). Secondary analyses, in which sorafenib-exposed versus -unexposed were both censored at the date of last contact, demonstrated similar findings (Data Supplement) as did censoring of both groups at the time of elective withdrawal (Data Supplement). Subanalysis by NPM1 status demonstrated that FLT3/ITD+ NPM1+ patients treated with sorafenib did not show a statistically significant improvement in outcome with sorafenib (Table 2, Figs 2E-2H). Although outcomes appeared overall superior for those children with FLT3/ITD+ AML who were treated with sorafenib, they also underwent HSCT more frequently than the comparator population (64% v 25%, P < .001). In multivariable analysis including NPM1 status and HSCT as a TVC, there was significantly worse EFS, DFS, and RR in sorafenib-unexposed patients (EFS from study entry: HR 2.37, 95% CI, 1.45 to 3.88, P < .001, DFS from CR: HR 2.28, 95% CI, 1.08 to 4.82, P = .032, RR from CR: HR 3.03, 95% CI, 1.31 to 7.04, P = .010, Table 3).
FIG 2.
Outcomes for sorafenib-exposed versus -unexposed patients: (A-D) Overall and (E-H) by NPM1 status. (A) OS from study entry, (B) EFS from study entry, (C) DFS from CR, (D) RR from CR, (E) OS from study entry, (F) EFS from study entry by NPM1 status, (G) DFS from CR by NPM1 status, and (H) RR from CR by NPM1 status. CR, complete remission; DFS, disease-free survival; EFS, event-free survival; OS, overall survival; RR, relapse risk; WT, wild-type.
TABLE 3.
Multivariable Analysis
Correlative Studies: PK and PIA Analysis
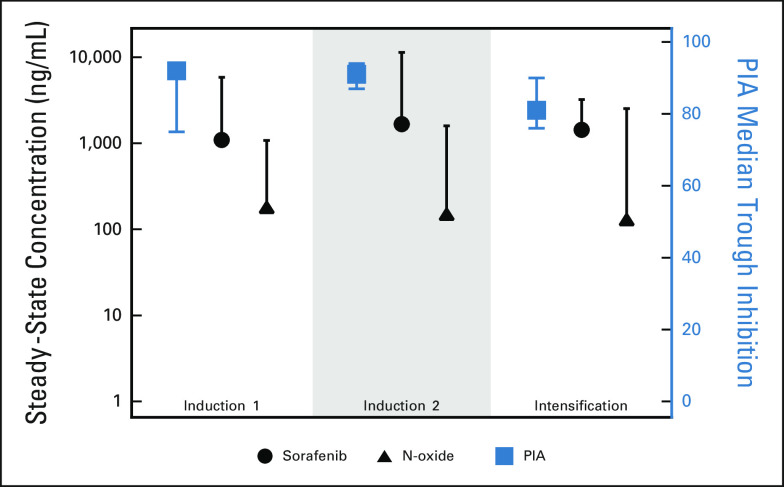
Optional PK and PIA data that were obtained during the first 3 courses of chemotherapy demonstrated that the steady-state concentrations of sorafenib and N-oxide metabolite were similar across treatment cycles and that measured plasma concentrations were sufficient to inhibit phosphorylated FLT3 (Fig 3). With PIA assay, the median trough FLT3 inhibition was 92%, 91%, and 81%, respectively, for the first 3 courses of therapy, suggesting sorafenib, at the dosing prescribed, was able to, in a subset of patients, target FLT3 and inhibit its function. There were no significant differences in clinical characteristics or outcome for arm C patients who contributed to PK/PD data (n = 52) versus not (n = 40; Data Supplement).
FIG 3.
PK/PD effects of sorafenib. The median and upper range steady-state concentration of sorafenib and the N-oxide metabolite in induction I (n = 23), induction 2 (n = 33), and intensification I (n = 28) is noted in black. PIA median trough inhibition and associated 95% CI are noted in red. Data are contributed by a total of n = 52 patients enrolled at 10 different institutions. PIA, plasma inhibitory activity; PK, pharmacokinetics.
DISCUSSION
These data demonstrate that sorafenib dosing of 200 mg/m2/day was tolerable in conjunction with conventional chemotherapy, significantly improved EFS, RR, and DFS, and provided potent FLT3 inhibition. Importantly, HSCT use and NPM1 status did not explain the clinical benefit seen. Although sorafenib did not improve OS, this may reflect use of TKI therapy after withdrawal from protocol therapy or at time of relapse. Our findings build on previously published pediatric studies of sorafenib that demonstrated tolerability and on-target effects.17,18 Studies of TKI efficacy in younger adults with AML previously demonstrated benefit of midostaurin in FLT3-mutant AML30 and sorafenib in younger adults with AML, regardless of FLT3 mutation status.14,15 However, a more recent study of sorafenib in adults with HAR FLT3/ITD+ AML shows less clear benefit.16 Although our results are compelling, we recognize that the higher-than-anticipated rates of attrition and intermittent periods of study closure are limitations in our study. Despite this, the improved EFS and DFS and reduced RR observed with treatment would support its use.
Importantly, first-generation FLT3 inhibitors have off-target effects that may increase systemic toxicity by targeting multiple signaling pathways. Despite this risk, the sorafenib/chemotherapy toxicity profile observed was overall comparable to that of standard therapy, although an early cardiac toxicity signal in C2 prompted change in dosing schedule for induction 2 and beyond. Interestingly, a majority of patients experiencing cardiac toxicity had preceding exposure to bortezomib (6/7; 86%), which was associated with higher rates of overall study toxicity.19 Despite this early concern for cardiac dysfunction, rates of cardiac toxicity were ultimately comparable for FLT3/ITD+ patients treated with and without sorafenib. Interestingly, more patients enrolled on arm C received dexrazoxane compared with arms A and B, a difference that may reflect practice change after the cardiac toxicity concern was raised as well as evolving data regarding various mechanisms of TKI cardiotoxicity.31-34 Additional data regarding long-term cardiac function after completion of sorafenib treatment are being sought.
To our knowledge, our study is also the first prospective trial of sorafenib maintenance for FLT3/ITD+ AML in children, an effective intervention in adult FLT3/ITD+ AML.35-37 As the majority of children (62%) did not receive any maintenance therapy, a greater understanding of barriers that preclude maintenance treatment are needed. As early relapse was seen in a subset of patients after HSCT before sorafenib start, earlier initiation of TKI therapy after HSCT (eg, before day 40) may be warranted. Moreover, as a subset of patients failed to meet criteria for drug start within the window of time allowed after HSCT or were removed from protocol therapy after HSCT before eligible, a greater understanding of barrier to maintenance treatment is needed. Use of a second-generation TKI with less off-target effects, such as gilteritinib, may enable initiation of maintenance treatment at an earlier stage of hematopoietic recovery and ensure greater compliance. Discontinuous dosing of sorafenib during maintenance treatment may also facilitate greater compliance, albeit with a potential risk of resistance mutation development.
The efficacy analyses presented have the well-established limitation of historical controls and incomplete TKI exposure data after study withdrawal. To control for differential rates of HSCT between sorafenib-exposed/-unexposed patients, HSCT was treated as a TVC. Although this analytic approach appropriately adjusts for the differential HSCT exposure, it does not address differences resulting from changes in HSCT conditioning or supportive care. We also electively censored sorafenib-unexposed patients at the time of elective withdrawal to minimize the impact of unobserved TKI exposure after study withdrawal. We performed secondary analyses using different censoring approaches (Data Supplement) that suggest outcomes remained superior in the sorafenib-exposed cohort. We recognize that although sorafenib improved EFS, DFS, and RR, it had less definitive impact on OS, suggesting that those who did not receive sorafenib might benefit from FLT3 inhibition at the time of recurrence. Moreover, the role of sorafenib in the more favorable NPM1+/FLT3/ITD+ AML is less clear and warrants further study in a larger subset of patients.
Despite these limitations, these data are the largest analysis of sorafenib efficacy in pediatric FLT3/ITD+ AML. The presently open COG phase III study, AAML1831, builds on our sorafenib experience by testing gilteritinib in both FLT3/ITD+ AML and children with clinically relevant FLT3-activating mutations. For treatment of pediatric FLT3/ITD+ AML outside of a study context, these data provide compelling support for sorafenib combined with conventional chemotherapy.
ACKNOWLEDGMENT
The AAML1031 study team would like to acknowledge the patients and families who participated in the AAML1031 clinical trial.
Jessica A. Pollard
Consulting or Advisory Role: Syndax, Kura Oncology
Patrick Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Servier, Kite, a Gilead company, Amgen, Kura Oncology, Takeda
Brian Fisher
Consulting or Advisory Role: Astellas Pharma
Research Funding: Pfizer (Inst), Merck (Inst)
John Levine
Consulting or Advisory Role: Talaris, Bluebird Bio, Mesoblast, Therakos, SymBio Pharmaceuticals, X4 Pharmaceuticals, Equillium, Jazz Pharmaceuticals, OncoImmune, Omeros
Research Funding: Incyte (Inst), Kamada (Inst), Biogen (Inst), Mesoblast (Inst), MaaT Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: GVHD biomarkers patent licensed to VIracor
Lisa Eidenschink Brodersen
Employment: Hematologics Inc
Leadership: Hematologics Inc
Michael R. Loken
Employment: Hematologics Inc
Leadership: Hematologics Inc
Stock and Other Ownership Interests: Hematologics Inc
Consulting or Advisory Role: Newlink Genetics
Andrew Wood
Patents, Royalties, Other Intellectual Property: PAT055863-US-PCT. Inventor assigned to Genomics Institute of the Novartis Research Foundation involving combination inhibition of ALK, PAT055863-US-PCT. Inventor assigned to Genomics Institute of the Novartis Research Foundation involving combination inhibition of ALK
E. Anders Kolb
Travel, Accommodations, Expenses: Roche/Genentech
Lillian Sung
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Alan Gamis
Consulting or Advisory Role: Novartis
Richard Aplenc
Expert Testimony: Vorys
No other potential conflicts of interest were reported.
See accompanying article on page 2058
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented as an oral abstract at the 61st Annual Meeting of American Society of Hematology, Orlando, FL, December 7, 2019 (Blood 134s:292, 2019).
SUPPORT
Supported by Chair's Grant of the Children's Oncology Group - U10 CA98543-08 and Statistics and Data Center Grant No CA U10CA180899, NCTN Operations Center Grant U10CA180886, St Baldrick's Foundation, National Cancer Institute Grant No RO1CA114563 (S.M.), and St Baldrick's Career Development Award (J.A.P.).
CLINICAL TRIAL INFORMATION
NCT01371981 (COG AAML1031)
AUTHOR CONTRIBUTIONS
Conception and design: Jessica A. Pollard, Todd A. Alonzo, Robert Gerbing, Patrick Brown, Elizabeth Fox, Brian Fisher, Lisa Eidenschink Brodersen, Michael R. Loken, Susana Raimondi, Lillian Sung, Alan Gamis, Soheil Meshinchi, Richard Aplenc
Administrative support: All authors
Provision of study materials or patients: All authors
Collection and assembly of data: Jessica A. Pollard, Todd A. Alonzo, Robert Gerbing, Patrick Brown, Elizabeth Fox, Brian Fisher, Betsy Hirsch, Samir Kahwash, Lisa Eidenschink Brodersen, Michael R. Loken, Susana Raimondi, Lillian Sung, E. Anders Kolb, Soheil Meshinchi, Richard Aplenc
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sorafenib in Combination With Standard Chemotherapy for Children With High Allelic Ratio FLT3/ITD+ Acute Myeloid Leukemia: A Report From the Children's Oncology Group Protocol AAML1031
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jessica A. Pollard
Consulting or Advisory Role: Syndax, Kura Oncology
Patrick Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Servier, Kite, a Gilead company, Amgen, Kura Oncology, Takeda
Brian Fisher
Consulting or Advisory Role: Astellas Pharma
Research Funding: Pfizer (Inst), Merck (Inst)
John Levine
Consulting or Advisory Role: Talaris, Bluebird Bio, Mesoblast, Therakos, SymBio Pharmaceuticals, X4 Pharmaceuticals, Equillium, Jazz Pharmaceuticals, OncoImmune, Omeros
Research Funding: Incyte (Inst), Kamada (Inst), Biogen (Inst), Mesoblast (Inst), MaaT Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: GVHD biomarkers patent licensed to VIracor
Lisa Eidenschink Brodersen
Employment: Hematologics Inc
Leadership: Hematologics Inc
Michael R. Loken
Employment: Hematologics Inc
Leadership: Hematologics Inc
Stock and Other Ownership Interests: Hematologics Inc
Consulting or Advisory Role: Newlink Genetics
Andrew Wood
Patents, Royalties, Other Intellectual Property: PAT055863-US-PCT. Inventor assigned to Genomics Institute of the Novartis Research Foundation involving combination inhibition of ALK, PAT055863-US-PCT. Inventor assigned to Genomics Institute of the Novartis Research Foundation involving combination inhibition of ALK
E. Anders Kolb
Travel, Accommodations, Expenses: Roche/Genentech
Lillian Sung
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Alan Gamis
Consulting or Advisory Role: Novartis
Richard Aplenc
Expert Testimony: Vorys
No other potential conflicts of interest were reported.
REFERENCES
- 1.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML Blood 1083654–36612006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolouri H, Farrar JE, Triche T, Jr, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions Nat Med 24103–1122018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia Blood 9789–942001 [DOI] [PubMed] [Google Scholar]
- 4.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials Blood 981752–17592001 [DOI] [PubMed] [Google Scholar]
- 5.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis Blood 994326–43352002 [DOI] [PubMed] [Google Scholar]
- 6. Schlenk RF, Krauter J, Frohling S, et al. Postremission therapy with an allogeneic transplantation from an HLA-matched family donor seems to overcome the negative prognostic impact of FLT3-ITD in younger patients with acute myeloid leukemia exhibiting a normal karyotype. ASH Annual Meeting Abstracts. 2005;106:2353. [Google Scholar]
- 7. Tarlock K, Alonzo TA, Gerbing RB, et al. Distinct co-occurring mutational profiles in acute myeloid leukemia confers prognostic significance in children and young adults with FLT3/ITD mutations. Blood. 2018;132:443. [Google Scholar]
- 8.Meshinchi S, Appelbaum FR.Structural and functional alterations of FLT3 in acute myeloid leukemia Clin Cancer Res 154263–42692009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravandi F, Alattar ML, Grunwald MR, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation Blood 1214655–46622013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennedy VE, Smith CC. FLT3 mutations in acute myeloid leukemia: Key concepts and emerging controversies. Front Oncol. 2020;10:612880. doi: 10.3389/fonc.2020.612880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia J Clin Oncol 281856–18622010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravandi F, Arana Yi C, Cortes JE, et al. Final report of phase II study of sorafenib, cytarabine and idarubicin for initial therapy in younger patients with acute myeloid leukemia Leukemia 281543–15452014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serve H, Krug U, Wagner R, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: Results from a randomized, placebo-controlled trial J Clin Oncol 313110–31182013 [DOI] [PubMed] [Google Scholar]
- 14.Rollig C, Serve H, Huttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): A multicentre, phase 2, randomised controlled trial Lancet Oncol 161691–16992015 [DOI] [PubMed] [Google Scholar]
- 15. Rollig C, Serve H, Hüttmann A, et al. The addition of sorafenib to standard AML treatment results in a substantial reduction in relapse risk and improved survival. Updated results from long-term follow-up of the randomized-controlled soraml trial. Blood. 2017;130:721. [Google Scholar]
- 16.Wei AH, Kennedy GA, Morris KL, et al. Results of a phase 2, randomized, double-blind study of sorafenib versus placebo in combination with intensive chemotherapy in previously untreated patients with FLT3-ITD acute myeloid leukemia (ALLG AMLM16) Blood 13636–382020. 32430502 [Google Scholar]
- 17.Inaba H, Rubnitz JE, Coustan-Smith E, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia J Clin Oncol 293293–33002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubnitz JE, Lacayo NJ, Inaba H, et al. Clofarabine can replace anthracyclines and etoposide in remission induction therapy for childhood acute myeloid leukemia: The AML08 multicenter, randomized phase III trial J Clin Oncol 372072–20812019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aplenc R, Meshinchi S, Sung L, et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: A report from the Children's Oncology Group Haematologica 1051879–18862020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winkler J, Rech D, Kallert S, et al. Sorafenib induces sustained molecular remission in FLT3-ITD positive AML with relapse after second allogeneic stem cell transplantation without exacerbation of acute GVHD: A case report Leuk Res 34e270–e2722010 [DOI] [PubMed] [Google Scholar]
- 21.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: Sustained regression before and after allogeneic stem cell transplantation Blood 1136567–65712009 [DOI] [PubMed] [Google Scholar]
- 22. Metzelder S, Finck A, Fey M, et al. Sorafenib monotherapy is effective in relapsed and RefractoryFlt3-ITD positive acute myeloid leukemia, particularly after allogenic stem cell transplantation. ASH Annual Meeting Abstracts. 2010;116:3314. [Google Scholar]
- 23.Tarlock K, Chang B, Cooper T, et al. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia Pediatr Blood Cancer 621048–10542015 [DOI] [PubMed] [Google Scholar]
- 24.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children's Oncology Group trial AAML0531 J Clin Oncol 323021–30322014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan E, Meier P.Nonparametric estimation from incomplete observation J Am Stat Assoc 53457–4811958 [Google Scholar]
- 26.Fine JP, Gray RJ.A proportional hazards model for the subdistribution of a competing risk J Am Stat Assoc 94496–5091999 [Google Scholar]
- 27.Cox D.Regression models and life-tables J R Stat Soc Series B Stat Methodol 34187–2201972 [Google Scholar]
- 28.Lathia C, Lettieri J, Cihon F, et al. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics Cancer Chemother Pharmacol 57685–6922006 [DOI] [PubMed] [Google Scholar]
- 29.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): A pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors Blood 1083477–34832006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation N Engl J Med 377454–4642017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duran JM, Makarewich CA, Trappanese D, et al. Sorafenib cardiotoxicity increases mortality after myocardial infarction Circ Res 1141700–17122014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. 2018;2:13. doi: 10.1038/s41698-018-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MH, Kerkela R, Force T.Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics Circulation 11884–952008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu C, Shemisa K. Sorafenib-associated heart failure complicated by cardiogenic shock after treatment of advanced stage hepatocellular carcinoma: A clinical case discussion. Case Rep Cardiol. 2017;2017:7065759. doi: 10.1155/2017/7065759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN) J Clin Oncol 382993–30022020 [DOI] [PubMed] [Google Scholar]
- 36.Burchert A.Maintenance therapy for FLT3-ITD-mutated acute myeloid leukemia Haematologica 106664–6702021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunner AM, Li S, Fathi AT, et al. Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission Br J Haematol 175496–5042016 [DOI] [PMC free article] [PubMed] [Google Scholar]