Abstract
Aims
The delayed administration of epinephrine has been proven to worsen the neurological outcomes of patients with out-of-hospital cardiac arrest (OHCA) and shockable rhythm or asystole. We aimed to investigate whether the delayed administration of epinephrine might also worsen the neurological outcomes of patients with witnessed OHCA and initial pulseless electrical activity (PEA).
Methods and results
The JAAM-OHCA Registry is a multicentre registry including OHCA patients between 2014 and 2017. Patients with emergency medical services (EMS)-treated OHCA and initial PEA rhythm were included. The primary exposure was the time from the EMS call to the administration of epinephrine. The secondary exposure was the time to epinephrine dichotomized as early (≤15 min) or delayed (>15 min). The primary outcome was the achievement of a favourable neurological outcome, defined as Cerebral Performance Categories Scale 1–2 at 30 days after OHCA. Out of 34 754 patients with OHCA, 3050 patients were included in the present study. After adjusting for potential confounders, the delayed administration of the epinephrine was associated with a lower likelihood of achieving a favourable neurological outcome [adjusted odds ratio (OR) 0.96; 95% confidence interval (CI) 0.93–0.99; P = 0.016]. The percentage of patients who achieved a favourable neurological outcome in the delayed epinephrine group was lower than that in the early epinephrine group (1.3% vs. 4.7%; adjusted OR 0.33; 95% CI 0.15–0.72; P = 0.005). A restricted cubic spline analysis demonstrated that delayed epinephrine administration could decrease the likelihood of achieving a favourable neurological outcome; this was significant within the first 10 min.
Conclusions
The delayed administration of epinephrine was associated with worse neurological outcomes in patients with witnessed OHCA patients with initial PEA.
Keywords: Resuscitation, Out-of-hospital cardiac arrest, Epinephrine, Pulseless electrical activity
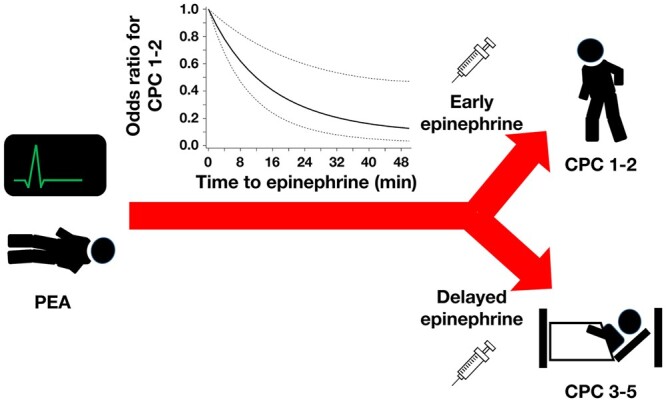
Graphical Abstract
Graphical Abstract.
Introduction
According to the Heart Disease and Stroke Statistics 2020 Update from the American Heart Association, the weighted national estimate of emergency department visits with a principal diagnosis of cardiac arrest was 56.8 per 100 000 population or 183 629 people out of the total population of the USA.1 Although the outcomes of witnessed out-of-hospital cardiac arrest (OHCA) have improved with the revision of The International Liaison Committee on Resuscitation (ILCOR) guidelines,2 in the Cardiac Arrest Registry to Enhance Survival (CARES), in 2018, the rate of survival to hospital discharge was only 10.4% and the rate of survival with a good functional status was 8.2%. As for patients who initially presented a shockable rhythm, the number of survivors with a favourable neurological outcome increased as the use of public access defibrillators increased.3 However, there has been no significant improvement in the neurological outcomes of OHCA in patients who initially presented a non-shockable rhythm.2,4
Pulseless electrical activity (PEA) has been increasing over the past decades with a corresponding decrease in the shockable rhythm.5 The analysis of the Swedish Registry of Cardiopulmonary Resuscitation demonstrated that the survival of patients with PEA increased from 1% to 5%, while the survival rate of patients in asystole increased modestly from 0.6% to 1.3%.6 These studies indicate that PEA and asystole should be considered separate entities, and it would be worthwhile investigating treatment strategies to improve not only survival but also the neurological outcomes of patients who initially present PEA.
Epinephrine has been reported to increase 30-day survival in comparison to placebo,7 and it is recommended as first-line drug for the resuscitation of patients with PEA.8 A subgroup analysis of a randomized trial of epinephrine administration during OHCA showed that the return of spontaneous circulation (ROSC) rate was three-fold higher in the epinephrine group in the subgroup of patients who initially presented a non-shockable rhythm, while there was no differences in the ROSC rate in the subgroup of patients who initially presented a shockable rhythm.9 These findings might indicate the usefulness of epinephrine in OHCA who initially presented a non-shockable rhythm.
Several observational studies have shown that the early administration of epinephrine is associated with better neurological outcomes in patients with OHCA10–12; however, these studies included patients with both shockable and non-shockable rhythms. Hansen et al.13 showed that the delay in the administration of epinephrine was associated with reduced odds of achieving a favourable neurological outcome in patients who initially presented a non-shockable rhythm; however, the benefit of epinephrine was limited to patients with asystole. It has not been determined whether the early administration of epinephrine could improve the neurological outcomes in OHCA of non-traumatic origin with initial PEA.
The aim of the present study was to determine whether the time to the administration of epinephrine could affect the neurological outcomes in patients with witnessed OHCA patients with initial PEA.
Methods
JAAM-OHCA registry
The Japanese Association for Acute Medicine–Out-of-Hospital Cardiac Arrest (JAAM-OHCA) Registry is a prospective, multicentre registry of patients with OHCA who are transported to critical care medical centres or hospitals with an emergency care department.14 Prehospital data were obtained from the All Japan Utstein Registry of the Fire and Disaster Management Agency as previously reported.14 In-hospital data were collected via an Internet-based system by physicians or medical staff at each institution. The JAAM-OHCA Registry committee integrated the prehospital and in-hospital data.
Patient selection
The present study employed this registry from 2014 to 2017. Patients with witnessed non-traumatic OHCA with initial PEA who received epinephrine were included in this analysis.
Primary and secondary exposures
A previous study showed an association between the prognosis of OHCA patients and the time from emergency medical services (EMS) agency arrival on the scene to the administration of epinephrine13; however, the time from EMS call to EMS arrival on-the scene might differ according to the distance between the nearest EMS station and the place patients collapsed. Considering this fact, the primary exposure in this study was the time (in minutes) from the EMS call to the first administration of epinephrine. A previously mentioned study dichotomized time from EMS arrival on-the scene to the administration of epinephrine into the early (<10 min) and delayed (≥10 min) and showed that the delayed group had worse outcomes in comparison to the early group. Given that mean time from EMS call to EMS arrival on the scene was 4–5 min, we divided eligible patients into the early (≤15 min) and delayed (>15 min) administration groups as the secondary exposure. Based on the Guidelines for cardiopulmonary resuscitation (CPR), the dose of epinephrine was 1 mg.15 Paramedics who have completed training are allowed to administer epinephrine on the ambulance in Japan.16 Epinephrine could be administered by physicians, nurses, or paramedics.
Outcomes
The primary outcome was the achievement of a favourable neurological outcome, defined as a Cerebral Performance Categories Scale of 1–2 at 30 days after OHCA. Cerebral Performance Categories was assessed in each participating hospital. The secondary outcome was 30-day survival after OHCA.
Statistical analysis
Patient characteristics were compared using the Pearson’s χ2 test for categorical variables, and Student’s t-test or the Wilcoxon rank sum test for continuous variables where applicable, and are presented as the mean ± standard deviation or median with interquartile range.
We conducted several statistical analyses to examine the relationship between the timing of the administration of epinephrine and the achievement of a favourable neurological outcomes. First, we evaluated the timing of the administration of epinephrine as a continuous variable by a multivariable logistic regression model adjusted for age, sex, aetiology of OHCA (cardiac/non-cardiac), doctor car or helicopter transportation, presence of an eyewitnesses, intubation, time from EMS call to CPR, time from EMS call to the arrival of EMS on the scene. The multivariable logistic regression model included the targeted temperature management (TTM), extracorporeal membrane oxygenation (ECMO), and intra-aortic balloon pumping (IABP) in addition to the covariates listed above. An analysis of outcomes by using a combination of multiple imputation and a multivariate analysis was also conducted to assess the effects of missing values on outcomes. For all missing baseline data, multiple imputation was performed (n = 10) by predictive mean matching for continuous variables and a logistic regression model for binary variables. The odds ratios (ORs) for outcomes were estimated by a multivariate logistic regression model that included the same baseline covariates as above. Estimates from 10 iterations were combined with the use of Rubin’s rule. Odds ratios were presented with 95% confidence intervals (CIs) and P-values. As a sensitivity analysis, we divided eligible patients into early (<29 min, median) and delayed (≥29 min) administration groups, and applied the same analysis as described above. Second, we evaluated the timing of epinephrine administration as a categorical variable, and applied the multivariable logistic regression analysis described above and a multiple imputation analysis.
The potential non-linear associations between the OR for a favourable neurological outcome and the timing of epinephrine administration were examined using restricted cubic splines adjusted for age, sex, and aetiology of OHCA. All tests were two-tailed, and P-values of <0.05 were considered to indicate statistical significance. All analyses were performed using the SAS statistical package (version 9.4, SAS Institute, Cary, NC, USA). The analysis code and the data derived in this research will be shared by the corresponding author upon reasonable request.
Ethics approval
This study protocol was organized to ensure compliance with the Declaration of Helsinki and the Guidelines for the Epidemiological Research published by the Japanese Ministry of Health, Labour and Welfare. The original study protocol was approved by the Institutional Review Board (IRB) at Kyoto University as the corresponding institution, as well as each participating hospital.
Consent to participate/consent for publication
To give patients or their family members the opportunity to refuse to be included in this registry, the special committee and each participating institution showed a document regarding opt-out consent on the website and/or the board of the emergency department, and the requirement for informed consent was waived.
Results
Patient characteristics
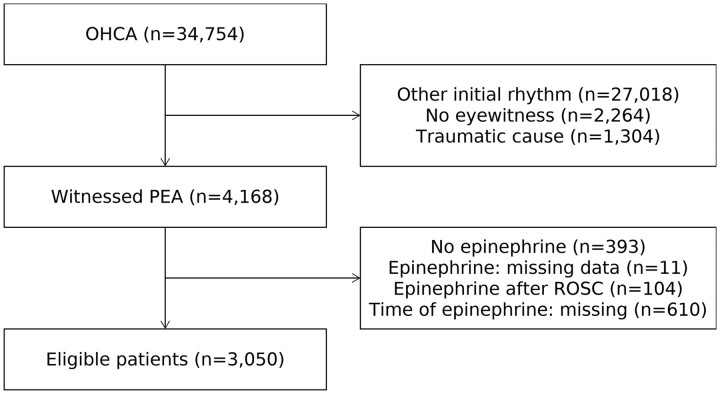
From January 2014 to December 2016, 34 754 consecutive patients with OHCA were screened and 4168 patients with witnessed non-traumatic PEA were identified (Figure 1). Out of these, 393 patients without epinephrine administration, 11 patients whose records were missing information about epinephrine administration, 104 patients who received epinephrine after an ROSC, and 610 patients with missing information about the timing of epinephrine administration were excluded. The remaining 3050 patients were included in the present analysis.
Figure 1.
Patient selection. PEA, pulseless electrical activity.
The patient characteristics are shown in Table 1. The mean age was 73.7 years, 1836 (60.2%) patients were male. The time from the EMS call to CPR [8 (2–11) min vs. 4 (1–7) min; P < 0.001], time from the EMS call to arrival on the scene [8 (7–10) min vs. 7 (6–8) min; P < 0.001], and the time from the EMS call to epinephrine administration [30 (23–37) min vs. 14 (13–15) min; P < 0.001] were longer in the delayed group in comparison to the early group. The frequency of cardiac arrest due to cardiac causes was lower in the early group; however, the difference did not reach statistical significance (58.0% vs. 63.9%; P = 0.092). The frequency of bystander CPR (36.1% vs. 50.7%; P < 0.001) and the use of doctor car or doctor helicopter (10.9% vs. 25.4%; P < 0.001) was lower in the delayed group.
Table 1.
Patient characteristics
| Variables | ≤15 min (n = 213) | >15 min (n = 2837) | P-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 75.0 ± 13.0 | 73.6 ± 14.8 | 0.14 |
| Male | 123 (57.8) | 1713 (60.4) | 0.45 |
| Cause of cardiac arrest | |||
| Cardiac cause | 136 (63.9) | 1644 (58.0) | 0.092 |
| Non-cardiac cause | 77 (36.2) | 1193 (42.1) | 0.092 |
| Cerebral vascular disease | 9 (4.2) | 151 (5.3) | 0.49 |
| Lung disease | 9 (4.2) | 232 (8.2) | 0.039 |
| Malignancy | 6 (2.8) | 85 (3.0) | 0.88 |
| Other | 53 (24.9) | 725 (25.6) | 0.83 |
| Intervention | |||
| Bystander CPR | 108 (50.7) | 1025 (36.1) | <0.001 |
| Intubation | 211 (99.1) | 2808 (99.0) | 0.10 |
| Doctor car or doctor helicopter | 54 (25.4) | 308 (10.9) | <0.001 |
| Time course | |||
| Time from call to CPR, min | 4 (1–7) | 8 (2–11) | <0.001 |
| Time from call to EMS arrival on the scene, min | 7 (6–8) | 8 (7–10) | <0.001 |
| Time from call to epinephrine, min | 14 (13–15) | 30 (23–37) | <0.001 |
Data are shown as n (%) or the means ± standard deviation otherwise specified.
CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; GCS, Glasgow coma scale; ROSC, return of spontaneous circulation; SMD, standardized mean difference.
Clinical outcomes
Each additional minute of time from witnessed OHCA to the administration of epinephrine was associated with a 5% decrease in the odds of a favourable neurological outcome in the univariate analysis (unadjusted OR 0.95; 95% CI 0.92–0.98; P = 0.002), a 4% decrease in the multivariate analysis (adjusted OR 0.96; 95% CI 0.93–0.99; P = 0.016), and a 4% decrease in the combination of multiple imputation and a multivariate analysis (adjusted OR 0.96; 95% CI 0.93–0.99; P = 0.010) (Table 2). A shorter time from witness to the administration of epinephrine was associated with better 30-day survival in univariate analysis (unadjusted OR 0.96; 95% CI 0.95–0.98; P < 0.001), multivariate analysis (adjusted OR 0.96; 95% CI 0.95–0.98; P < 0.001), and multiple imputation analysis (adjusted OR 0.96; 95% CI 0.95–0.98; P < 0.001) (Table 2). Missing patterns of patient characteristics were shown in Supplementary material online, Table S1.
Table 2.
The univariate, multivariate, and multiple imputation analyses when the time to epinephrine was analysed as a continuous variable
| Time to epinephrine as continuous | Time to epinephrine as categorical | ||||
|---|---|---|---|---|---|
| Variable | Adjusted OR (95% CI) | P-value | ≤15 min (n = 213) | >15 min (n = 2837) | P-value |
| Univariate analysis | |||||
| CPC 1–2 | 0.95 (0.92–0.98) | 0.002 | 1 (reference) | 0.28 (0.14–0.56) | <0.001 |
| Alive | 0.96 (0.95–0.98) | <0.001 | 1 (reference) | 0.56 (0.33–0.95) | 0.032 |
| Multivariate analysis | |||||
| CPC 1–2 | 0.96 (0.93–0.99) | 0.016 | 1 (reference) | 0.33 (0.15–0.72) | 0.005 |
| Alive | 0.96 (0.95–0.98) | <0.001 | 1 (reference) | 0.60 (0.35–1.05) | 0.073 |
| Multiple imputation | |||||
| CPC 1–2 | 0.96 (0.93–0.99) | 0.010 | 1 (reference) | 0.58 (0.40–0.85) | 0.006 |
| Alive | 0.96 (0.95–0.98) | <0.001 | 1 (reference) | 0.78 (0.59–1.03) | 0.083 |
CI, confidence interval; OR, odds ratio.
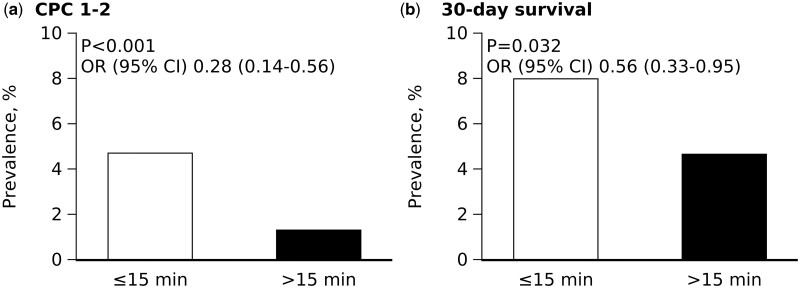
When the time to epinephrine was analysed as a categorical variable, the delayed epinephrine group had worse neurological outcomes in comparison to the early group in the univariate analysis (1.3% vs. 4.7%; OR 0.28; 95% CI 0.14–0.56; P < 0.001) (Figure 2), the multivariate analysis (adjusted OR 0.33; 95% CI 0.15–0.72; P = 0.005), and the multiple imputation analysis (adjusted OR 0.58; 95% CI 0.40–0.85; P = 0.006) (Table 2). It was still significantly associated with a worse neurological outcome (adjusted OR 0.28; 95% CI 0.13–0.61; P = 0.001), even in the multivariable analysis, which included TTM, ECMO, and IABP (Supplementary material online, Table S2). When the time to epinephrine was dichotomized based on the median value (29 min), the delayed epinephrine group consistently had worse neurological outcomes in the univariate analysis (OR 0.41; 95% CI 0.22–0.77; P = 0.006) and multivariate analysis (OR 0.46; 95% CI 0.24–0.88; P = 0.020) (Supplementary material online, Table S3).
Figure 2.
Favourable neurological outcome and 30-day survival Cerebral Performance Categories 1–2 at 30 days (A) and 30-day survival (B) in the early and delayed epinephrine groups. CI, confidence interval; OR, odds ratio; PS, propensity score.
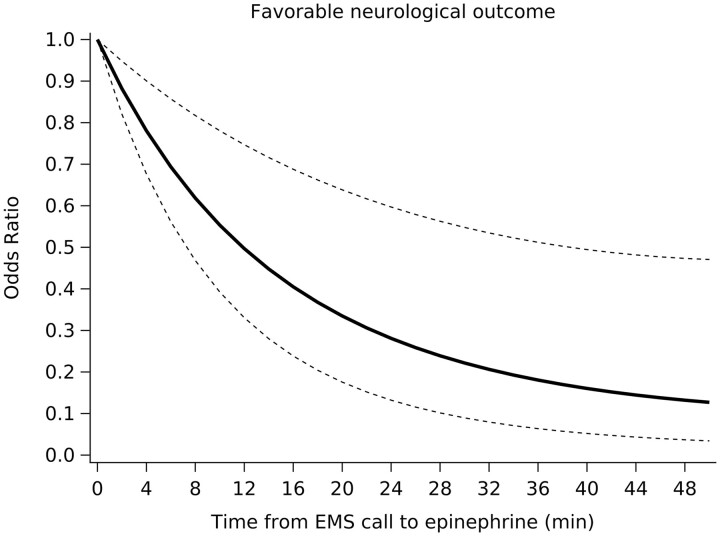
A non-linear relationship was observed between the odds of a favourable neurological outcome and the time to the administration of epinephrine, with the odds of a favourable neurological outcome rapidly decreasing within 10 min (Figure 3).
Figure 3.
Odds of favourable neurological outcome according to the time from the emergency medical services call to epinephrine administration. The solid line indicates the adjusted odds ratio; dotted line, 95% confidence interval; a reference when the adjusted odds ratio for the time from witness to epinephrine administration is 0 min.
Discussion
The major finding of the present study was that each minute of delay to the administration of epinephrine was associated with a 6% reduction in the likelihood of achieving favourable neurological outcome in patients with witnessed OHCA with initial PEA. This association remained significant even after adjustment for other important factors, including aetiology of OHCA (cardiac/non-cardiac), doctor car or helicopter transportation, presence of an eyewitnesses, intubation, time from EMS call to CPR, time from EMS call to the arrival of EMS on the scene, TTM, ECMO, and IABP. The restricted cubic spline analysis demonstrated that the odds of a favourable neurological outcome rapidly decreased within 10 min.
The use of epinephrine is recommended in the ILCOR International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations (CoSTR)17; however, its effectiveness for patients with OHCA has long been discussed. A randomized controlled trial showed that epinephrine use was associated with higher rates of short-term survival but not with survival to hospital discharge.9 Another randomized controlled trial demonstrated that the survival rate at hospital discharge of patients who received epinephrine showed no significant improvement.18 However, the statistical analyses of these trials were underpowered, which limited their ability to detect significant differences. A recent randomized controlled trial and two meta-analyses showed that—in comparison to placebo—the use of epinephrine improved the rate of survival to hospital discharge in OHCA patients.7,19,20 However, epinephrine did not improve the neurological outcomes and the time to epinephrine administration was not taken into account in these trials.
Previous studies showed that epinephrine was useful, especially when patients initially presented shockable rhythm. Ewy et al.21 showed that in patients with OHCA with shockable rhythm, the rate of survival to hospital discharge was greater in those treated with epinephrine. The early administration of epinephrine (≤10 min) was also associated with a favourable neurological outcome in adult bystander-witnessed OHCA10–12; however, it was only observed in the subset of patients with shockable rhythm.11 These studies indicated that epinephrine had beneficial effects for patients who initially presented a shockable rhythm but was underpowered in patients with non-shockable rhythms, including PEA.
Hansen et al.13 showed that the delay in the administration of epinephrine was associated with reduced odds of achieving a favourable neurological outcome in patients who initially presented a non-shockable rhythm; however, the benefit of epinephrine was limited to patients with asystole. In this study, 60% of the patients were not witnessed; thus, the exact time from collapse to the administration of epinephrine could not be inferred and PEA might transition to asystole in some cases. In this study, Hansen et al. did not include the postresuscitation hospital care into the analysis, which could impact patient outcomes. The present study showed that delayed administration of epinephrine was still significantly associated with a worse neurological outcome even in the multivariable analysis which included TTM, ECMO, and IABP (Supplementary material online, Table S2). It further supported the usefulness of early administration of epinephrine in PEA. Taken together, early administration (within 10–15 min) of epinephrine in non-shockable rhythm is as useful as shockable rhythms in improving survival and neurological outcomes.
The analysis of the Get With The Guidelines-Resuscitation database showed that the earlier administration of epinephrine was associated with a higher neurologically intact survival rate in adult and paediatric patients with in-hospital cardiac arrest who initially presented a non-shockable rhythm.22–24 Combined with the present analysis, earlier administration of epinephrine can be beneficial for patients with OHCA and in-hospital cardiac arrest who initially present both asystole and PEA.
In the present study, we focused on witnessed PEA and showed that the earlier administration of epinephrine was associated with better neurological outcomes in patients with initial PEA. The findings support the usefulness of the earlier administration of epinephrine in this group of patients. We observed a rapid decrease of the odds of achieving favourable neurological outcome within 10 min after cardiac arrest. This is concordant with the report of the analysis of the Get With The Guidelines-Resuscitation database, which showed that within 10 min, there was a stepwise decrease in survival with an increasing interval of time to epinephrine.22 These findings support the concept that it is crucial to administer epinephrine as soon as possible to patients with initial PEA.
An experimental study demonstrated that epinephrine, through its alpha-1 agonist action caused platelet activation, which promoted thrombosis25 and had adverse effects on the cerebral microvascular blood flow, such as increasing the severity of cerebral ischaemia during CPR.26 The delayed administration of epinephrine prolonged CPR, which might result in the accumulation of a higher dose of epinephrine, possibly hindering cerebral microvascular blood flow. This may be one of the reasons why the delayed administration of epinephrine was harmful.
Our study showed that the delayed administration of epinephrine decreased the percentage of patients who achieved a favourable neurological outcome by 4%. However, given that the incidence of OHCA was 183 629 out of the total population of the USA27 or 127 018 out of the total population of Japan, and 20% of the OHCA patients were in PEA (approximately 70 000 people out of the total population of the USA),14 even small increases in the percentage of patients who achieve a favourable neurological outcome could have a significant clinical impact.
Study limitations
The present study was associated with several limitations. First, the quality of CPR was not assessed in this study. A retrospective study demonstrated that the adherence to the advanced cardiovascular life support protocol throughout an event was correlated with an increased rate of ROSC in the setting of cardiac arrest.28 Second, there may have been difficulties in obtaining vascular access in the delayed epinephrine administration group, which might have affected the results because repeated attempts could lead to the interruption of CPR. Third, comorbidities were not recorded in our database, which could have affected the results. Fourth, the present study was not a prospective randomized trial and unmeasured factors might have influenced the outcomes. However, we performed several analyses and obtained the same results. Despite these limitations, we analysed a large national database that included more than 30 000 OHCA patients, which supports the generalizability and conclusion drawn in the present study.
Conclusions
The delayed administration of epinephrine was associated with worse neurological outcomes in patients with witnessed non-traumatic OHCA with initial PEA.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Supplementary Material
Acknowledgements
This study was conducted on behalf of all the members and institutions of the JAAM-OHCA Registry. The participating institutions of the JAAM-OHCA Registry are listed at the following URL (http://www.jaamohca-web.com/list/). This registry was constructed based on the design, concept, and system of the CRITICAL Study in Osaka as well as ‘The establishment of data registry system for evaluating emergency medical decision regarding cardiovascular diseases (J-ACUTE)’. We also thank Ms. Narumi Funayama and Mr. Hiroki Chiba for their support of the JAAM-OHCA Registry.
Funding
This registry was supported by research funding from the JAAM and a scientific research grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (16K09034 and 15H05006) and the Ministry of Health, Labour, and Welfare of Japan (25112601).
Conflict of interest: K.H. received honoraria from Daiichi Sankyo, Nippon Boehringer Ingelheim, Pfizer, and Bristol-Myers Squibb, Bayer. H.T. received honoraria from Otsuka, Takeda Pharmaceutical, Mitsubishi-Tanabe Pharma, Daiichi Sankyo, Nippon Boehringer Ingelheim, Bayer, Pfizer, Novartis Pharma, Ono Pharmaceutical, MSD, Teijin Pharma, and Bristol-Myers Squibb and Astellas Pharma; and received research funding from Nippon Boehringer Ingelheim, Mitsubishi-Tanabe Pharma, Japan Tobacco, Daiichi Sankyo, IQVIA Services Japan, Takeda Pharmaceutical, Bayer Yakuhin, Sanofi, Acterion Pharmaceuticals Japan, and MSD. The other authors declare no conflicts of interest in association with the present study.
Contributor Information
Nobuyuki Enzan, Department of Cardiovascular Medicine, Faculty of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Ken ichi Hiasa, Department of Cardiovascular Medicine, Faculty of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Kenzo Ichimura, School of Medicine, Pulmonary, Allergy and Critical Care Medicine, Stanford University, 300 Pasteur Drive, Grand Bld Rm S126B, Stanford, CA 94305 USA.
Masaaki Nishihara, Emergency and Critical Care Center, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Takeshi Iyonaga, Emergency and Critical Care Center, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Yuji Shono, Emergency and Critical Care Center, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Takeshi Tohyama, Center for Clinical and Translational Research, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Kouta Funakoshi, Center for Clinical and Translational Research, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Takanari Kitazono, Emergency and Critical Care Center, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Hiroyuki Tsutsui, Department of Cardiovascular Medicine, Faculty of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
References
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Kaneko H, Hara M, Mizutani K, Yoshiyama M, Yokoi K, Kabata D, Shintani A, Kitamura T.. Improving outcomes of witnessed out-of-hospital cardiac arrest after implementation of International Liaison Committee on Resuscitation 2010 Consensus: a nationwide prospective observational population-based study. J Am Heart Assoc 2017;6:e004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kitamura T, Kiyohara K, Sakai T, Matsuyama T, Hatakeyama T, Shimamoto T, Izawa J, Fujii T, Nishiyama C, Kawamura T, Iwami T.. Public-access defibrillation and out-of-hospital cardiac arrest in Japan. N Engl J Med 2016;375:1649–1659. [DOI] [PubMed] [Google Scholar]
- 4. Andrew E, Nehme Z, Lijovic M, Bernard S, Smith K.. Outcomes following out-of-hospital cardiac arrest with an initial cardiac rhythm of asystole or pulseless electrical activity in Victoria, Australia. Resuscitation 2014;85:1633–1639. [DOI] [PubMed] [Google Scholar]
- 5. Mehta C, Brady W.. Pulseless electrical activity in cardiac arrest: electrocardiographic presentations and management considerations based on the electrocardiogram. Am J Emerg Med 2012;30:236–239. [DOI] [PubMed] [Google Scholar]
- 6. Bergstrom M, Schmidbauer S, Herlitz J, Rawshani A, Friberg H.. Pulseless electrical activity is associated with improved survival in out-of-hospital cardiac arrest with initial non-shockable rhythm. Resuscitation 2018;133:147–152. [DOI] [PubMed] [Google Scholar]
- 7. Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, Regan S, Long J, Slowther A, Pocock H, Black JJM, Moore F, Fothergill RT, Rees N, O'Shea L, Docherty M, Gunson I, Han K, Charlton K, Finn J, Petrou S, Stallard N, Gates S, Lall R; PARAMEDIC2 Collaborators . A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med 2018;379:711–721. [DOI] [PubMed] [Google Scholar]
- 8. Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, Neumar RW, O'Neil BJ, Paxton JH, Silvers SM, White RD, Yannopoulos D, Donnino MW.. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S444–S464. [DOI] [PubMed] [Google Scholar]
- 9. Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L.. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA 2009;302:2222–2229. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka H, Takyu H, Sagisaka R, Ueta H, Shirakawa T, Kinoshi T, Takahashi H, Nakagawa T, Shimazaki S, Ong Eng Hock M.. Favorable neurological outcomes by early epinephrine administration within 19 minutes after EMS call for out-of-hospital cardiac arrest patients. Am J Emerg Med 2016;34:2284–2290. [DOI] [PubMed] [Google Scholar]
- 11. Hayashi Y, Iwami T, Kitamura T, Nishiuchi T, Kajino K, Sakai T, Nishiyama C, Nitta M, Hiraide A, Kai T.. Impact of early intravenous epinephrine administration on outcomes following out-of-hospital cardiac arrest. Circ J 2012;76:1639–1645. [DOI] [PubMed] [Google Scholar]
- 12. Nakahara S, Tomio J, Nishida M, Morimura N, Ichikawa M, Sakamoto T.. Association between timing of epinephrine administration and intact neurologic survival following out-of-hospital cardiac arrest in Japan: a population-based prospective observational study. Acad Emerg Med 2012;19:782–792. [DOI] [PubMed] [Google Scholar]
- 13. Hansen M, Schmicker RH, Newgard CD, Grunau B, Scheuermeyer F, Cheskes S, Vithalani V, Alnaji F, Rea T, Idris AH, Herren H, Hutchison J, Austin M, Egan D, Daya M; Resuscitation Outcomes Consortium Investigators . Time to epinephrine administration and survival from nonshockable out-of-hospital cardiac arrest among children and adults. Circulation 2018;137:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitamura T, Iwami T, Atsumi T, Endo T, Kanna T, Kuroda Y, Sakurai A, Tasaki O, Tahara Y, Tsuruta R, Tomio J, Nakata K, Nachi S, Hase M, Hayakawa M, Hiruma T, Hiasa K, Muguruma T, Yano T, Shimazu T, Morimura N; special committee that aims to improve survival after out-of-hospital cardiac arrest (OHCA) by providing evidence-based therapeutic strategy and emergency medical system from the Japanese Association for Acute Medicine (JAAM) . Medicine (JAAM) sctatisaoohcaObpebtsaemsftJAfA. The profile of Japanese Association for Acute Medicine—out-of-hospital cardiac arrest registry in 2014-2015. Acute Med Surg 2018;5:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panchal AR, Bartos JA, Cabanas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, O'Neil BJ, Peberdy MA, Rittenberger JC, Rodriguez AJ, Sawyer KN, Berg KM; Adult Basic and Advanced Life Support Writing Group . Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020;142:S366–S468. [DOI] [PubMed] [Google Scholar]
- 16. Naito H, Yumoto T, Yorifuji T, Tahara Y, Yonemoto N, Nonogi H, Nagao K, Ikeda T, Sato N, Tsutsui H.. Improved outcomes for out-of-hospital cardiac arrest patients treated by emergency life-saving technicians compared with basic emergency medical technicians: a JCS-ReSS study report. Resuscitation 2020;153:251–257. [DOI] [PubMed] [Google Scholar]
- 17. Callaway CW, Soar J, Aibiki M, Böttiger BW, Brooks SC, Deakin CD, Donnino MW, Drajer S, Kloeck W, Morley PT, Morrison LJ, Neumar RW, Nicholson TC, Nolan JP, Okada K, O'Neil BJ, Paiva EF, Parr MJ, Wang TL, Witt J; Advanced Life Support Chapter Collaborators . Part 4: advanced life support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2015;132:S84–S145. [DOI] [PubMed] [Google Scholar]
- 18. Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL.. Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation 2011;82:1138–1143. [DOI] [PubMed] [Google Scholar]
- 19. Aves T, Chopra A, Patel M, Lin S.. Epinephrine for out-of-hospital cardiac arrest: an updated systematic review and meta-analysis. Crit Care Med 2020;48:225–229. [DOI] [PubMed] [Google Scholar]
- 20. Vargas M, Buonanno P, Iacovazzo C, Servillo G.. Epinephrine for out of hospital cardiac arrest: a systematic review and meta-analysis of randomized controlled trials. Resuscitation 2019;145:151–157. [DOI] [PubMed] [Google Scholar]
- 21. Ewy GA, Bobrow BJ, Chikani V, Sanders AB, Otto CW, Spaite DW, Kern KB.. The time dependent association of adrenaline administration and survival from out-of-hospital cardiac arrest. Resuscitation 2015;96:180–185. [DOI] [PubMed] [Google Scholar]
- 22. Donnino MW, Salciccioli JD, Howell MD, Cocchi MN, Giberson B, Berg K, Gautam S, Callaway C; for the American Heart Association's Get With The Guidelines-Resuscitation Investigators . Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ 2014;348:g3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andersen LW, Berg KM, Saindon BZ, Massaro JM, Raymond TT, Berg RA, Nadkarni VM, Donnino MW; American Heart Association Get With the Guidelines–Resuscitation Investigators . Time to epinephrine and survival after pediatric in-hospital cardiac arrest. JAMA 2015;314:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khera R, Chan PS, Donnino M, Girotra S; American Heart Association’s Get With The Guidelines-Resuscitation Investigators . Hospital variation in time to epinephrine for nonshockable in-hospital cardiac arrest. Circulation 2016;134:2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larsson PT, Wallen NH, Egberg N, Hjemdahl P.. Alpha-adrenoceptor blockade by phentolamine inhibits adrenaline-induced platelet activation in vivo without affecting resting measurements. Clin Sci (Lond) 1992;82:369–376. [DOI] [PubMed] [Google Scholar]
- 26. Ristagno G, Tang W, Huang L, Fymat A, Chang YT, Sun S, Castillo C, Weil MH.. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med 2009;37:1408–1415. [DOI] [PubMed] [Google Scholar]
- 27. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 28. McEvoy MD, Field LC, Moore HE, Smalley JC, Nietert PJ, Scarbrough SH.. The effect of adherence to ACLS protocols on survival of event in the setting of in-hospital cardiac arrest. Resuscitation 2014;85:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.