Abstract
Isatuximab is an approved anti‐CD38 monoclonal antibody with multiple antitumor modes of action. An exposure‐response (E‐R) analysis using data from patients with relapsed/refractory multiple myeloma (RRMM) enrolled in a phase Ib clinical study who received isatuximab at doses from 5 to 20 mg/kg weekly for 1 cycle (4 weeks) followed by every 2 weeks thereafter (qw/q2w) in combination with pomalidomide/dexamethasone (n = 44) was first used to determine the optimal dose/schedule for the phase III ICARIA‐MM study. It was complemented by an E‐R analysis from a second phase Ib study of patients who received isatuximab at doses from 3 to 10 mg/kg q2w or 10 or 20 mg/kg qw/q2w in combination with lenalidomide/dexamethasone (n = 52). Plasma trough concentration at week 4 (CT4W) was the best predictor for response, and the benefit of the initial 4‐weekly administration was confirmed. Although the predicted overall response rate (ORR) was higher at 20 mg/kg vs. 10 mg/kg, the 95% confidence intervals were overlapping. Considering the high probability of success to reach the targeted ORR of greater than or equal to 60%, 10 mg/kg qw/q2w was selected. Results of the E‐R analysis from the lenalidomide/dexamethasone study and published disease modeling using data from both phase Ib clinical studies reinforced 10 mg/kg qw/q2w as the optimal dose/schedule for the phase III ICARIA‐MM study. E‐R analysis showed that higher CT4W was associated with higher ORR. Developed models supported the phase III isatuximab dosing regimen selection/confirmation of 10 mg/kg qw/q2w for use in combination with pomalidomide/dexamethasone in patients with RRMM.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
There is no cure for multiple myeloma despite advances in drug discovery. Isatuximab is an anti‐CD38 monoclonal antibody that has multiple biological mechanisms with which to kill tumor cells. Mouse xenograft studies have demonstrated enhanced antitumor activity with a combination of isatuximab and pomalidomide/dexamethasone (Pd) compared with the activity of either drug alone.
WHAT QUESTION DID THIS STUDY ADDRESS?
What is the relationship between isatuximab exposure and efficacy outcomes? Do the data support the isatuximab dosing regimen selection/confirmation when administered in combination in patients with relapsed/refractory multiple myeloma (RRMM)?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The combination of isatuximab 10 mg/kg weekly/every 2 weeks and Pd has clinical benefit in patients with RRMM.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
The model‐based drug development approaches can be applied successfully to select and justify a dosing regimen.
INTRODUCTION
Multiple myeloma (MM) is a malignant plasma cell disease characterized by clonal proliferation of plasma cells in the bone marrow and excessive production of a monoclonal immunoglobulin ([Ig] usually of the IgG or IgA type or light chain [paraprotein, M‐protein, or M‐component]). 1 MM remains an incurable disease despite the availability of new classes of major drugs, including immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), and monoclonal antibodies, which have improved survival. 2
Isatuximab is an immunoglobulin G1 (IgG1) monoclonal antibody that binds to a specific epitope of CD38, 3 whereby it kills tumor cells via multiple mechanisms, including antibody‐dependent cellular‐mediated cytotoxicity, antibody‐dependent cellular phagocytosis, complement‐dependent cytotoxicity, direct induction of apoptosis (pro‐apoptosis) without crosslinking, and inhibition of CD38 ectoenzymatic activity. 4
Isatuximab was first evaluated as a single agent for the treatment of relapsed/refractory multiple myeloma (RRMM) in a phase I/II trial (NCT01084252). In the phase I dose escalation/expansion study, isatuximab was administered at doses from 0.0001 to 20 mg/kg every 2 weeks (q2w) and 10 and 20 mg/kg weekly (qw), and it was well‐tolerated in heavily pretreated patients with RRMM, with the greatest efficacy at doses greater than or equal to 10 mg/kg. The phase II study initially investigating isatuximab at 3 and 10 mg/kg q2w or 10 mg/kg q2w/every 4 weeks (q4w; q2w for cycle 1 followed by q4w thereafter; 1 cycle = 4 weeks) was amended based on pharmacokinetic (PK) analyses of phase I data, adding a fourth treatment arm at 20 mg/kg qw/q2w (qw for cycle 1 followed by q2w thereafter). The selection of the dose (20 mg/kg qw/q2w) and dosing schedule of isatuximab as a single agent in RRMM was supported by combining exposure‐response (E‐R) analysis and disease modeling of tumor burden (i.e., PK/pharmacodynamics for serum M‐protein). 5 Weekly administrations together with a high dose at the first cycle allow for an optimized response, with the efficacious isatuximab concentration being more rapidly reached. 5 , 6
Isatuximab was further evaluated in two phase Ib combination studies with IMiDs, including lenalidomide and pomalidomide. Patients with RRMM received isatuximab intravenously (i.v.) administered either in two dose schedules (3, 5, or 10 mg/kg q2w or 10 or 20 mg/kg qw/q2w in combination with lenalidomide/dexamethasone) or at 5, 10, or 20 mg/kg qw/q2w with pomalidomide/dexamethasone (Pd; NCT01749969 and NCT02283775, respectively). The efficacy and safety data from these studies combined with E‐R analyses and disease modeling supported the initiation of a phase III study of isatuximab 10 mg/kg qw/q2w in combination with Pd (ICARIA‐MM). ICARIA‐MM trial results demonstrated that isatuximab combined with Pd led to statistically significant improvements compared with Pd alone in patients with RRMM. Finally, E‐R analyses were performed to confirm the clinical benefit and support the approval of isatuximab 10 mg/kg qw/q2w in combination with Pd.
On the basis of this pivotal study, isatuximab (Sarclisa) is approved in a number of countries in combination with Pd for the treatment of adult patients with RRMM who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor. 7 , 8
METHODS
Study designs and covariates
To determine the optimal dose/schedule for the phase III ICARIA‐MM trial, the E‐R analyses were first conducted in 44 of 45 patients in the phase Ib combination study of isatuximab with Pd. 9 To strengthen the choice of the dose, we performed additional E‐R analyses with 52 of 57 evaluable patients with RRMM from a phase Ib combination study of isatuximab with another IMiD (lenalidomide/dexamethasone [Rd]). 10 These investigations complemented the disease modeling approach conducted on the same datasets during drug development. 6 Isatuximab i.v. was administered at doses from 3 to 20 mg/kg q2w or qw/q2w (NCT01749969 and NCT02283775, respectively). Then, E‐R analyses were conducted for dose confirmation using data from the phase III, randomized, open‐label, multicenter, international ICARIA‐MM study, which compared the combination of isatuximab 10 mg/kg qw/q2w with Pd (Isa‐Pd, n = 148/153) vs. Pd (n = 149/154) in patients with RRMM (NCT02990338). 11
Covariates, such as demographics, disease characteristics, and other baseline characteristics, were tested in univariate and multivariate analyses (see Table S1). Exposure‐efficacy and exposure‐safety analyses were performed using SAS version 9.4 and R version 3.4.3.
Isatuximab PK exposure
For the exposure‐efficacy/safety analysis, the following PK end points were considered: plasma trough concentration (C trough) at week 4 (CT4W); C trough at week 1 (CT1W) and cumulative area under the plasma concentration‐time curve (AUC) over 1 week (AUC1W) or C trough at week 2 (CT2W) or 2 weeks (AUC2W) tested in the phase III and phase I, respectively. Other PK end points included cumulative AUC over 4 weeks (AUC4W); maximum plasma concentration (C max) calculated on the first administration of cycle 2 (C maxD1C2); C max calculated on the first administration of cycle 1 (C maxD1C1); and maximal value of C max on the first administration of cycle 1 to the first administration of cycle 2 (MaxC max). Additional details are included in the Methods S1.
Optimal dose selection
Overall response rate
Because isatuximab appeared to be well‐tolerated in patients with RRMM from the two phase Ib studies, the E‐R analyses focused on efficacy. For the overall response rate (ORR), patients achieving partial response or better were considered as responders, and patients with any other response were defined as nonresponders. The influence of exposure metrics on ORR was first explored graphically using boxplots and mosaic plots by PK parameter quartiles. A logistic regression model was then developed to assess the existence and functional form (linear, log‐linear, or maximum effect [E max]) of the relationship between isatuximab PK parameters and probability of ORR. The Akaike information criterion (AIC) and/or AUC criteria of those models as well as the p value of the PK estimates were used to select the best PK and its link function. Once the best isatuximab PK predictor and its link function were identified, univariate analyses were conducted to assess the effect of each covariate adjusted on this PK effect. Variables with possible correlation with ORR (p < 0.10 in the univariate analysis) were included in the E‐R model as potential covariates, with stepwise inclusion and deletion of covariates (multivariate analysis with a significance level of 0.10 for variable entry and 0.05 for removal at each step).
Dose confirmation
Exposure‐efficacy (ORR and progression‐free survival [PFS]) analysis (n = 148 Isa‐Pd; n = 149 Pd) was conducted with data from the phase III ICARIA‐MM study alone, whereas exposure‐safety (selected adverse events [AEs]) used pooled data from phase I and phase III studies (n = 192 isatuximab/pomalidomide arm; n = 149 pomalidomide control arm).
Overall response rate and progression‐free survival
The influence of exposure metrics on ORR was first explored graphically using boxplots and mosaic plots by PK parameter quartiles and Kaplan–Meier (K‐M) plot of PFS in the control arm and test arm by PK parameter quartiles.
A survival parametric Weibull distribution model (PFS) or logistic regression model (ORR) was then developed to assess the existence and functional form (linear, log‐linear, or E max) of the relationship between isatuximab PK parameters and PFS or probability of ORR. As a first step, demographic or baseline characteristics potentially influential of the efficacy end points (ORR or PFS) in the absence of isatuximab administration were screened based on the control arm population (Pd) only. This step was done to screen the prognostic factors prior testing the impact of isatuximab exposure. The second step was to select the best PK exposure parameter and the best link function based on the whole PK/pharmacodynamic population after adjustment on the prognostic factors identified in the first step. The AIC and/or area under the receiver operating characteristic curve (AUROC) criteria of those models as well as the p values of the PK estimates were used to select the best PK and it link function.
Demographic or baseline characteristics potentially influential of the efficacy end points (ORR or PFS) in the presence of isatuximab administration were screened (p value ≤0.1) for the final model based on the whole population and adjusted on the best PK parameter and it link function selected in step two.
Finally, the multivariate analyses performed, including the best isatuximab PK parameter and in case of more than two influential covariates, a stepwise inclusion and deletion of those covariates was used with significance level of 0.10 for variable entry and of 0.05 for removal at each step.
In addition, the interaction between the previously selected covariates and the PK parameter were tested to assess the homogeneity of the PK parameter effect over levels of the covariate(s). Goodness of fit was assessed by the Hosmer‐Lemeshow test.
Details on model evaluation can be found in Methods S1.
Safety
The influence of PK exposure on safety end points was first explored graphically, and then the incidence rates of AEs were summarized according to quartiles of the exposure metrics and corresponding 95% confidence intervals (CIs). Subgroup analyses by Ig MM type were also explored. Univariate and multivariate logistic analyses using logistic regressions were to be performed based on the findings from these exploratory analyses.
RESULTS
Optimal combination dose selection
Efficacy data were evaluable from 52 of 57 and 44 of 45 patients with PK in the Isa‐Rd and Isa‐Pd studies, respectively. The characteristics of the patients in the E‐R dataset were representative of the entire population (Table S2). Of the patients included in the efficacy analysis of ORR, 29 (55.8%) and 28 (63.6%) experienced a partial response (PR) or better in the Isa‐Rd and Isa‐Pd studies, respectively, with no clear evidence of a dose–response relationship between isatuximab doses of 10 and 20 mg/kg (Table S3, Table S4). 9 , 10
Therefore, combined E‐R analyses and disease modeling 6 were performed to guide optimal dose/schedule selection for isatuximab in combination. The exploratory analyses showed that higher exposure was observed in responders compared with nonresponders. As previously reported with monotherapy data, 5 univariate logistic regression analyses identified CT4W as the best predictor for response (i.e., lowest p value of 0.055), with an increase in ORR as log CT4W increased (Figure 1, Figure 2). In contrast to Isa‐Rd, log CT4W was marginally statistically non‐significant at the 0.05 level, but was kept in the Isa‐Pd model because CT4W was the best predictor of response in the ER analyses for monotherapy, 12 where a large range of doses were tested (i.e., 1–20 mg/kg in 170 patients). This result may be due to the small sample size of the Isa‐Pd study and to an imbalance in number of patients by dose group, with the majority of patients (68%) treated at the dose of 10 mg/kg (n = 30) qw/q2w.
FIGURE 1.
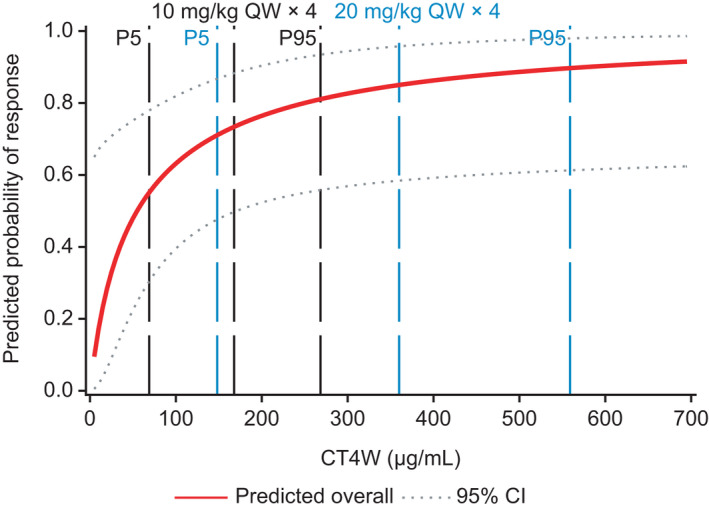
Final log‐linear model for predicting the probability of responding to treatment with isatuximab plus pomalidomide/dexamethasone includes log plasma trough concentration at week 4 (CT4W) and β2‐microglobulin. For a given CT4W value, patients with low β2‐microglobulin values had a higher probability to respond. Solid line represents median. CI, confidence interval; P, percentile; QW, weekly
FIGURE 2.
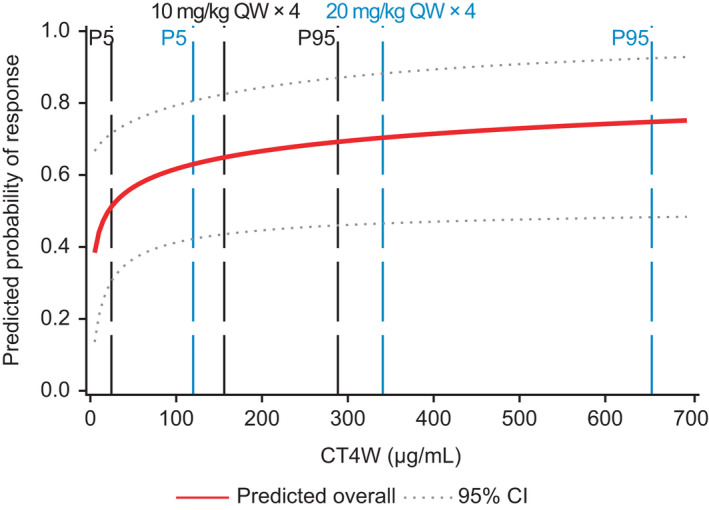
Final log‐linear model for predicting the probability of responding to treatment with isatuximab plus lenalidomide/dexamethasone includes log plasma trough concentration at week 4 (CT4W), β2‐microglobulin, and the number of prior lines. Solid line represents median. CI, confidence interval; P, percentile; QW, weekly
For Isa‐Rd, both log MaxC max and log CT4W were found statistically significantly correlated with ORR (p < 0.05). Log CT4W was selected using AIC and AUROC: the two largest AUROC values were obtained for log MaxC max and log CT4W. The AIC was in favor of log CT4W, with a value of 65.1 for log CT4W and 68.5 for log MaxC max.
In addition to log CT4W, baseline β2‐microglobulin (and number of prior lines of therapy for Isa‐Rd only) was a significant predictor of ORR. For a given CT4W, the model predicted a higher ORR when β2‐microglobulin was <3.5 mg/L and when the number of prior lines of treatment was less than or equal to 5.
Model‐predicted ORRs were close to the observed ORRs in the studies, indicating a good quality of fit for the model. In the Isa‐Pd study, the predicted ORR increased from 12% at 3 mg/kg q2w to 25%, 47%, and 65% at 5, 10, and 20 mg/kg q2w, respectively, indicative of dose response using the same schedule of administration (Figure 3). In addition, the predicted ORR increased to 33%, 50%, 67%, and 78% at 3, 5, 10, and 20 mg/kg qw/q2w, respectively, confirming findings from the monotherapy setting that the initial 4‐weekly administration is beneficial. For both combinations, the predicted probability of response was slightly higher at 20 mg/kg qw/q2w compared with 10 mg/kg qw/q2w, but the 95% CIs were largely overlapping (Figure 4). Clinical trial simulations showed that the probability of success was already high with isatuximab 10 mg/kg qw/q2w to reach the targeted ORR of 60% for the Pd combination (83%) and for the targeted ORR of 50% for the Rd combination in more pretreated patients (96%; see Table S5). Together with the disease modeling performed by Koiwai et al., 6 the 10 mg/kg qw/q2w dose was proposed as the optimal dose/schedule for further combination studies.
FIGURE 3.
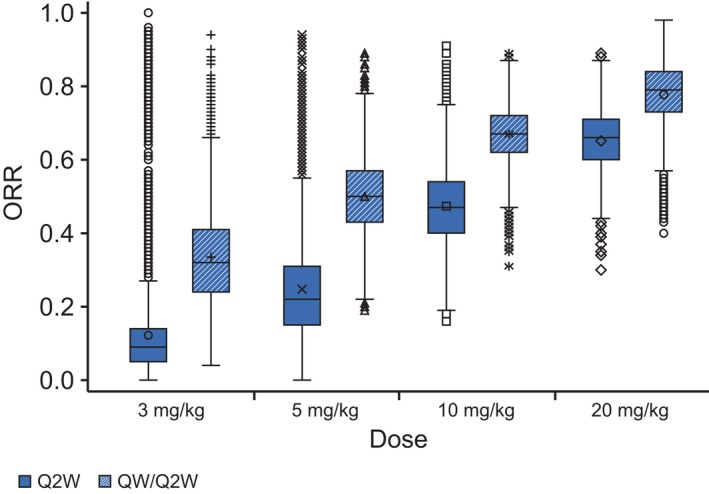
Mean predicted overall response rate (ORR) increases with increasing isatuximab dose. Results for 5000 trials based on simulated plasma trough concentration at week 4 (CT4W; using the final model) from 42 resampled patients treated with isatuximab plus pomalidomide/dexamethasone, with 100 patients each. Boxplots based on the model with log CT4W and β2‐microglobulin (<3.5, ≥3.5). Symbols represent individual values for each treatment group. Q2W, every 2 weeks; QW, weekly
FIGURE 4.
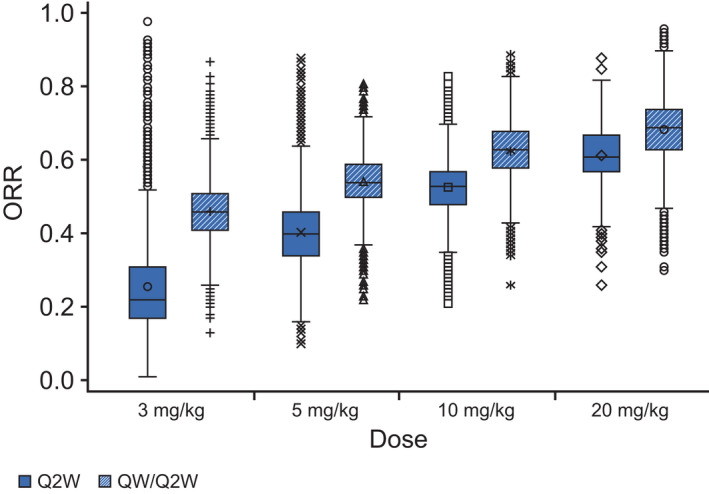
Mean predicted overall response rate (ORR) increases with increasing isatuximab dose. Results for 5000 trials based on simulated plasma trough concentration at week 4 (CT4W; using the final model) from 52 resampled patients treated with isatuximab plus lenalidomide/dexamethasone, with 100 patients each. Boxplots based on the model with log CT4W, β2‐microglobulin (<3.5, ≥3.5) and number of prior lines (≤5, >5). Symbols represent individual values for each treatment group. Q2W, every 2 weeks; QW, weekly
Dose confirmation
Demographics, disease characteristics, and other baseline characteristics of patients in the ICARIA‐MM study were evaluated for correlation between efficacy or safety and PK exposure parameters. The baseline demographic and disease characteristics of patients per quartile of CT4W are presented in Table 1. In the first exposure quartile (Q1), patients tended to have lower baseline albumin (i.e., <35 g/L) and higher baseline β2‐microglobulin (i.e., ≥3.5 mg/L), bone marrow plasma cells (i.e., ≥50%), and lactate dehydrogenase (i.e., >upper limit of normal [ULN]).
TABLE 1.
Patient characteristics for all quartiles in the phase III ICARIA‐MM study
| Pd | Isa‐Pd | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Baseline eGFR, ml/min/1.73 m2, mean (SD) | 72.0 (24.6) | 71.1 (28.5) | 69.7 (23.8) | 75.4 (30.7) | 70.3 (23.6) |
| Baseline lymphocytes, giga/L, mean (SD) | 1.2 (0.8) | 1.3 (0.7) | 1.3 (0.7) | 1.1 (0.5) | 1.2 (0.5) |
| Number of prior lines, mean (SD) | 3.3 (1.4) | 3.3 (1.3) | 3.8 (2.0) | 3.8 (1.9) | 3.3 (1.8) |
| Baseline plasma cells in bone marrow, %, mean (SD) | 33.1 (27.6) | 49.7 (27.9) | 35.4 (31.8) | 27.6 (24.3) | 18.7 (19.4) |
| Time since diagnosis to randomization, years, mean (SD) | 5.29 (3.71) | 4.59 (3.06) | 5.31 (3.01) | 6.44 (3.90) | 4.61 (2.72) |
| Baseline albumin <35 vs. ≥35, % | |||||
| <35 | 30.9 | 45.9 | 43.2 | 32.4 | 13.5 |
| ≥35 | 69.1 | 54.1 | 56.8 | 67.6 | 86.5 |
| Baseline β2‐microglobulin <3.5 vs. ≥3.5, % | |||||
| <3.5 | 44.5 | 37.8 | 41.7 | 65.7 | 62.2 |
| ≥3.5 | 55.5 | 62.2 | 58.3 | 34.3 | 37.8 |
| Baseline chromosomal aberration risk estimated, % | |||||
| High risk | 22.8 | 24.3 | 16.2 | 8.1 | 8.1 |
| Standard risk | 51.0 | 59.5 | 62.2 | 73.0 | 81.1 |
| Unknown or missing | 26.2 | 16.2 | 21.6 | 18.9 | 10.8 |
| MM subtype at initial diagnosis: IgG vs. non‐IgG, % | |||||
| IgG | 65.1 | 83.8 | 64.9 | 67.6 | 48.6 |
| Non‐IgG | 34.9 | 16.2 | 35.1 | 32.4 | 51.4 |
| Derived IgG type at study entry, % | |||||
| IgG | 75.8 | 97.3 | 75.7 | 78.4 | 62.2 |
| Non‐IgG | 24.2 | 2.7 | 24.3 | 21.6 | 37.8 |
| Baseline serum LDH group 1, % | |||||
| ≤ULN | 67.8 | 59.5 | 54.1 | 91.9 | 73.0 |
| >ULN | 32.2 | 40.5 | 45.9 | 8.1 | 27.0 |
| Baseline eGFR <60, 60 to <90, and ≥90, % | |||||
| Normal (≥90 ml/min/1.73 m2) | 19.1 | 12.1 | 14.7 | 23.5 | 25.0 |
| Mild impairment (<90 and ≥60 ml/min/1.73 m2) | 47.5 | 51.5 | 44.1 | 38.2 | 36.1 |
| Moderate or severe impairment (<60 ml/min/1.73 m2) | 33.3 | 36.4 | 41.2 | 38.2 | 38.9 |
| Baseline plasma cells in bone marrow <50 vs. ≥50, % | |||||
| <50 | 68.8 | 48.6 | 64.9 | 77.8 | 89.2 |
| ≥50 | 31.3 | 51.4 | 35.1 | 22.2 | 10.8 |
| R‐ISS stage at study entry, % | |||||
| Stage I | 20.8 | 16.2 | 16.2 | 40.5 | 32.4 |
| Stage II | 65.1 | 62.2 | 78.4 | 54.1 | 59.5 |
| Stage III | 14.1 | 21.6 | 5.4 | 5.4 | 8.1 |
| Plasmacytomas in MRI/CT at baseline flag, % | |||||
| N | 93.3 | 81.1 | 94.6 | 91.9 | 94.6 |
| Y | 6.7 | 18.9 | 5.4 | 8.1 | 5.4 |
| Baseline measurable paraprotein, % | |||||
| Non‐measurable | 2.7 | 5.4 | 8.1 | ||
| Serum M‐protein | 69.8 | 64.9 | 73.0 | 70.3 | 64.9 |
| Serum M‐protein and urine M‐protein | 18.1 | 18.9 | 18.9 | 16.2 | 13.5 |
| Urine M‐protein | 9.4 | 16.2 | 8.1 | 8.1 | 13.5 |
| Baseline ECOG status, % | |||||
| 0 | 45.6 | 27.0 | 32.4 | 35.1 | 48.6 |
| 1 | 45.0 | 56.8 | 59.5 | 56.8 | 48.6 |
| 2 | 9.4 | 16.2 | 8.1 | 8.1 | 2.7 |
| Baseline hepatic status | |||||
| Normal (total bilirubin ≤ULN and AST ≤ULN) | 85.1% | 78.4% | 75.7% | 94.6% | 94.6% |
| Mild impairment (total bilirubin ≤ULN and AST > ULN, or ULN < total bilirubin ≤1.5 ULN and AST any) | 14.2% | 21.6% | 24.3% | 5.4% | 5.4% |
| Moderate impairment (1.5 ULN < total bilirubin ≤3 ULN and AST any) | 0.7% | ||||
Note: The quartiles for plasma trough concentration at week 4 are Q1 (<86.0 μg/ml), Q2 (86.0 to <142.4 μg/ml), Q3 (142.4 to <187.5 μg/ml), and Q4 (187.5–357.1 μg/ml).
Abbreviations: AST, aspartate aminotransferase; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate; IgG, immunoglobulin G; Isa‐Pd, isatuximab plus pomalidomide/dexamethasone; LDH, lactate dehydrogenase; MM, multiple myeloma; MRI, magnetic resonance imaging; Pd, pomalidomide/dexamethasone; Q, quartile; R‐ISS, Revised International Staging System; SD, standard deviation; ULN, upper limit of normal.
There were more patients with IgG MM type, plasmacytomas, Revised International Staging System (R‐ISS) stage III, and high‐risk chromosomal aberrations in Q1 than in the other quartiles of CT4W. Of note, among the 148 patients in the Isa‐Pd arm of ICARIA‐MM, 102 patients completed their 4‐weekly administration within 4 weeks (cycle 1), with most of the patients being in the highest quartiles of exposure: patients with four isatuximab weekly administrations at cycle 1 accounted for 21.6%, 70.3%, 89.2%, and 94.6% of patients in Q1, Q2, Q3, and Q4 of CT4W, respectively.
Overall response rate and progression‐free survival
Efficacy data were evaluable from 148 and 149 patients with PK exposure parameters in the Isa‐Pd and Pd arms, respectively. Parameter estimates are shown in Table S6 and Table S7. PK exposure summaries for different dosing regimens are shown in Table S8. Of the patients included in the efficacy analysis of ORR, 92 (62.2%) and 54 (36.2%), respectively, experienced a PR or better. Demographic or baseline characteristics potentially influential of ORR were screened based on the Pd arm. After logistic regression with stepwise selection of covariates, baseline bone marrow plasma cells by category (<50% vs. ≥50%) was the only covariate that remained significant (p < 0.05). After adjusting on the baseline bone marrow plasma cells, CT4W was found to be the best predictor (p < 0.0001) of ORR, with the probability of ORR increasing with a linear increase of CT4W (linear form of logit function CT4W link; Figure 5), with the predicted responders below and above the median CT4W being 52% and 72%, respectively (Table S9). This model was chosen over the model with log link function because linear CT4W provides slightly better AIC and AUROC values compared with those from the model with log CT4W (CT4W: 372.19 [AIC] and 0.6944 [AUROC]; log CT4W: 372.31 [AIC] and 0.6944 [AUROC]), although the two models were very close. Besides CT4W, the final model included time since diagnosis (TSD), Revised International Staging System (R‐ISS) stage, and an interaction between TSD and R‐ISS stage (see Appendix S1 for final model equation). When TSD was included in the stepwise selection, it was selected for the final model with R‐ISS; those covariates increased the AUROC for the model with only CT4W from 0.66 to 0.74. In addition, the interaction between R‐ISS and TSD was significant and improved slightly the AUROC to 0.75.
FIGURE 5.
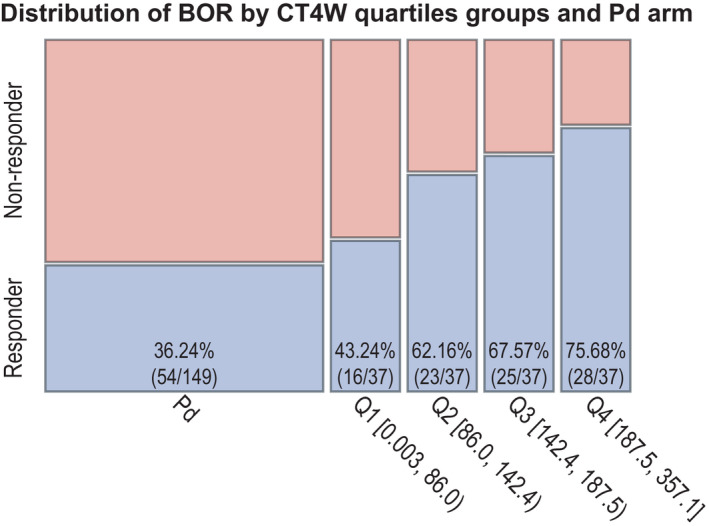
The proportion of responders to isatuximab increases with increased plasma trough concentration at week 4 (CT4W) in the phase 3 ICARIA‐MM study. BOR, best overall response; Pd, pomalidomide/dexamethasone; Q, quartile
Overall response rate increased as TSD increased, and ORR decreased with higher R‐ISS stage, with a longer TSD slope for stage III. The model‐predicted ORRs showed that there is good agreement between the model‐predicted probability of the ORR rate in the median subgroups of exposure in patients with R‐ISS stages I, II, and III when compared with the observed rate of response and 95% CI, indicating a good quality of fit for the model. When CT4W was above or equal to the median, the model‐predicted ORRs were 80%, 72%, and 41% in patients with R‐ISS stages I, II, and III, respectively (Figure S1). In addition, based on simulations, the probability to respond to the treatment would have been higher if the patients would have completed the 4‐weekly administrations, especially in the lowest quartile of exposure, with a predicted responder probability of 35% in the Pd arm, 45% in low‐exposure Q1, 48% in low‐exposure Q1 assuming all patients completed the 4‐weekly administrations, and 50% for patients who completed the 4‐weekly administrations (Figure S2).
For the PFS analysis, from the K‐M analysis, median (95% CI) PFS was 11.5 (8.94–13.9) and 7.03 (4.57–8.38) months in the 148 and 149 patients of the Isa‐Pd and Pd arms, respectively. In addition, PFS improved with increasing isatuximab CT4W in the Isa‐Pd arm, with a better PFS in the highest quartile of exposure (Q4). To account for the potential effect of confounding variables, model‐based analyses were conducted. Among the different PK exposure parameters tested, CT4W was found to be linearly associated with improved PFS. Multivariate analysis identified three factors in addition to isatuximab exposure (plasmacytomas, albumin, and R‐ISS at baseline; see Appendix S1 for final model equation). For all different quartile groups, there was generally good agreement between model‐predicted PFS and the corresponding K‐M curves (the latter lying within the 95% model prediction interval).
Different case–control analyses were performed adjusting for risk factors (those identified in the PFS model) using nearest‐neighbor matching based on Mahalanobis distance. Grouping the lowest quartiles (Q1 and Q2) and the highest quartiles (Q3 and Q4) gave a better insight of treatment benefit. Patients with isatuximab in the low‐ and high‐exposure groups (i.e., ≤ or >median CT4W) showed an increase in median PFS of 14.5 and 15.8 weeks compared with matched active controls, respectively. This is translated into a hazard ratio (HR) of 0.773 (log‐rank p = 0.0785) for the lower‐exposure CT4W group (≤median CT4W) and an HR of 0.534 (log‐rank p = 0.0655) for the higher‐exposure CT4W group (>median CT4W; Figure 6). These results illustrate that the patients with CT4W less than or equal to the median benefit from the isatuximab treatment, including those in the lower quartile of exposure.
FIGURE 6.
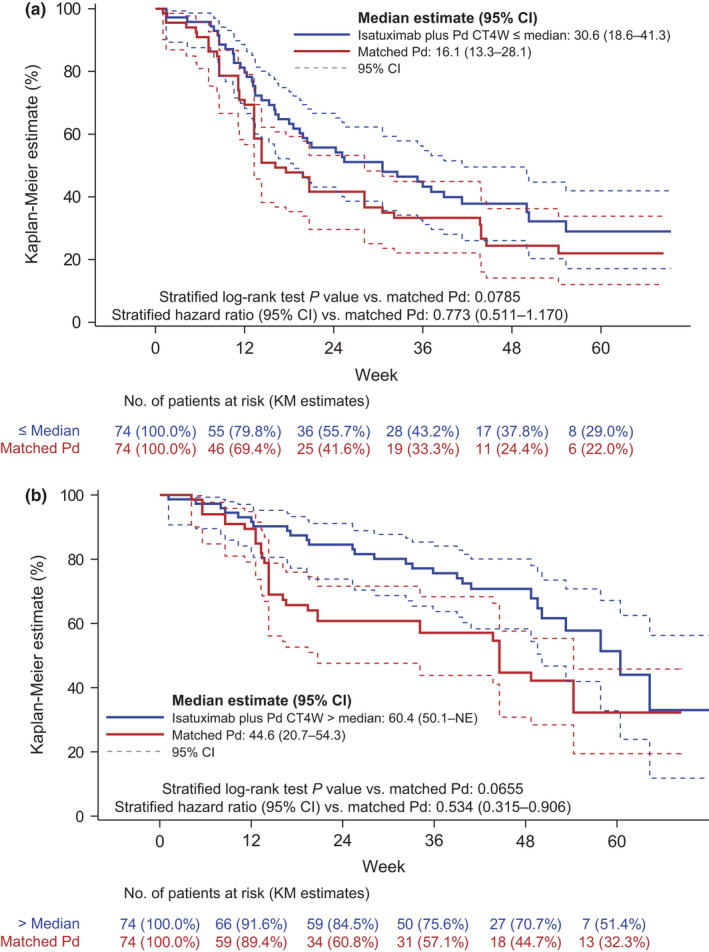
Isatuximab‐treated patients exhibit increased median progression‐free survival compared with matched patients with (a) low (below median plasma trough concentration at week 4 [CT4W]) and (b) high (above median CT4W) exposure in the phase III ICARIA‐MM study. Using Kaplan–Meier (KM) estimates, all isatuximab exposure quartiles had a positive treatment effect (hazard ratio >1) compared with their matching pomalidomide/dexamethasone (Pd) controls. The lowest quartiles (Q1 and Q2) and highest quartiles (Q3 and Q4) were grouped to provide better insight of treatment benefit. CI, confidence interval; NE, not evaluable
The model‐predicted distribution of PFS is presented in some populations of interest, essentially defined by each class of the qualitative covariates in the final model (R‐ISS stage and with or without plasmacytomas). Those curves were obtained using the theoretical distribution defined by the final model in fixing, for each population of patients considered, the CT4W value to its typical value (median) in the same population and albumin level set at 36.9 g/L (median value). Therefore, the predicted distribution of PFS in patients by isatuximab exposure quartile indicated that patients with R‐ISS stage III and plasmacytomas had shorter PFS whereas patients with higher isatuximab exposure had longer PFS.
As there is no interaction between the covariates and CT4W in the PFS Weibull parametric model adjusted on covariates, the HR associated with CT4W or the relative PFS rate depends only on the exposure metric CT4W values, and it is given by the exponent of its value, its coefficient, and the shape parameters estimates. The HR was estimated at the CT4W median, 5th and 95th percentiles of each CT4W quartile (median or quartile) subgroup vs. Pd for different populations (completers, simulations, and all populations). Based on this, patients would also benefit to complete the 4‐weekly administrations at cycle 1, as shown by a decreased HR for the lower quartiles of exposure (≤median CT4W), which is more evident in the lowest quartile (Q1) for the patients who received the 4‐weekly administrations, or by using the simulations compared with the whole population (HR = 0.56 [completers], 0.60 [simulations], and 0.68 [all populations]).
Safety
There was no apparent relationship between an increase of isatuximab C max after the first administration (C max,C1D1) and infusion reactions, nor between an increase in C max,C1D1 or the MaxC max and an increase in the incidence of the examined AEs of interest, including infusion reactions, thrombocytopenia, neutropenia, lymphopenia, respiratory events, cardiac arrhythmia, cardiac and nervous system disorders, and infections, based on data from ICARIA‐MM alone and pooled with the phase Ib study, except for those events related to treatment effect (anemia and infections; Figure S3).
Nevertheless, when comparing with the Pd control arm of ICARIA‐MM, there was a trend for higher neutropenia and cardiac arrhythmias and disorders in the Isa‐Pd arm, but with no difference between the quartiles of isatuximab exposure for neutropenia and a trend toward fewer cardiac events in the highest‐exposure quartile. Overall, comparable results were observed for the other PK metrics (AUC and C trough) tested at any timepoint over cycle 1 and for the subgroup analysis (IgG and non‐IgG MM–type patients).
DISCUSSION
Isatuximab appeared to be well‐tolerated in patients with RRMM from the two phase Ib studies, and no clear dose response was observed. Therefore, combined E‐R analysis with disease modeling was undertaken to guide optimal dose/schedule selection. 6 As previously reported for isatuximab monotherapy, CT4W was the best predictor of ORR, with an increase in ORR as CT4W increased. 13 Isatuximab CT4W was selected as the measure of exposure for the characterization of E‐R relationships of both efficacy end points (i.e., ORR and PFS). As the developed population PK model described a time‐dependent clearance that may be due to the potential effect of changes in disease status on PK, using the early exposure measurement of CT4W could avoid such a confounding effect in the characterization of causal E‐R relationships. 13
Baseline β2‐microglobulin (and the number of prior lines of therapy for Isa‐Rd only) was a significant predictor of ORR, with a higher ORR in patients with lower β2‐microglobulin at baseline (<3.5 mg/L). β2‐microglobulin is one of the two factors used for MM staging (<3.5 mg/L for stage I and ≥3.5 mg/L for stages II and III). Therefore, it is not unexpected that patients with higher β2‐microglobulin level have a lower predicted response rate. Interestingly, β2‐microglobulin was also found to be an influential covariate for PKs, 13 with faster linear clearance (lower exposure) in patients with higher β2‐microglobulin levels. Isa‐Pd and Isa‐Rd models differ by the presence of an additional covariate (i.e., the number of lines of prior therapy for Isa‐Rd). The populations were slightly different, with more heavily pretreated patients receiving Isa‐Rd (median number of prior lines 5.0 vs. 3.0). Furthermore, the population in the phase III study was comparable in terms of number of prior lines of therapy to the phase I study of the Isa‐Pd combination, and E‐R analyses confirmed the finding of the phase I study, where β2‐microglobulin (as part of R‐ISS), but not the number of lines, was found to be influential on ORR.
Clinical trial simulations showed that the predicted ORR at 10 mg/kg qw/q2w was higher compared with 10 mg/kg q2w, indicating consistency with monotherapy findings that 4‐weekly administrations would be beneficial. The probability of success to reach a targeted ORR greater than or equal to 60% was high with 10 mg/kg qw/q2w, and there was no clear clinical benefit of increasing the dose from 10 to 20 mg/kg qw/q2w. The results were consistent with the disease modeling performed by Koiwai et al. to characterize the relationship between serum M‐protein kinetics and PK profile in the subset of patients evaluable for serum M‐protein disease assessment. 6 Data also demonstrated a clear serum M‐protein decrease at weeks 8 to 12 at doses greater than or equal to 10 mg/kg. There was minimal added value to increase the dose from 10 to 20 mg/kg qw/q2w, with less than a 1.3‐fold difference between these two dose regimens in terms of serum M‐protein reduction at week 8 or 12 of treatment with both Rd and Pd combinations.
Therefore, this analysis from the phase I trial of isatuximab plus Pd, complemented by the analysis from the phase I isatuximab plus Rd trial, supported the rationale for the selection of the 10 mg/kg qw/q2w isatuximab regimen for use in combination with Pd in patients with RRMM, 4 , 9 , 10 , 14 , 15 and in the phase III ICARIA‐MM study. 11 , 16
E‐R analyses and clinical data from the phase III ICARIA‐MM study confirmed the efficacy and safety of the 10 mg/kg qw/q2w dose/schedule selected. 17 The E‐R analysis was based on ORR and PFS. For ORR, the probability to respond to isatuximab treatment was found to increase with a linear increase of CT4W.
In the selection models, based on AUROC/AIC criteria and p value, log transformed CT4W was the best predictor of response. In the confirmation models, CT4W and log transformed CT4W were the best predictors of response based on p values, AIC, and AUROC values. The two models (log or linear) were very close, with linear CT4W providing a slightly better AIC and AUROC value compared with those from the model with log CT4W. For this reason, linear CT4W was kept in the model.
The difference in the link exposure between the selection and confirmation model may be related to the broader range of exposure tested in the phase I study (doses from 5 to 20 mg/kg qw/q2w) whereas only one dose was tested in the phase III (10 mg/kg qw/q2w). The monotherapy data were indeed better fitted using an Emax model corresponding to the largest range of exposure (doses from 1 to 20 mg/kg qw, q2w, or qw/q2w).
This is also consistent with the final model using len/dex phase I data (doses from 3 to 20 mg/kg q2w or qw/q2w), as the relationship between ORR and CT4W were better fitted by a log linear model than by E max or linear models since this model gave the lowest AIC value (65.1 for log CT4W, 67.2 for E max, and 72.1 for CT4W).
The final model also included TSD, R‐ISS stage, and an interaction between TSD and R‐ISS. Bone marrow plasma cells was found to be influential on ORR based on the Pd arm. The presence of bone marrow plasma cells was found to be one of the few significant covariates when introduced in the base model but was not retained in the final model. Often, these were patients with missing bone marrow plasma cell counts. ORR increased as TSD increased, whereas ORR decreased with higher R‐ISS stage, with a longer TSD slope for stage III. The inclusion of R‐ISS in the model is consistent with the previously developed models for ORR with phase I combination data where β2‐microglobulin was selected as an influential covariate. ORR increases as TSD increases, and decreases with higher R‐ISS stage, with a steeper TSD slope in stage III. Regarding TSD, the effect might be counterintuitive because patients with a shorter TSD are usually earlier‐line patients, which often means less prior treatment and better ORR. However, in the overall population, there were more patients with TSD less than or equal to 5 years in R‐ISS stages II and III than in stage I (59.7% and 66.7% vs. 50%), indicative of the presence of aggressive disease in some patients with a short TSD. The correlation between R‐ISS and TSD, which was not found to be statistically significant, explains the direction of TSD effect on ORR. Therefore, this phenomenon might be attributed to the presence of aggressive disease in some patients with a short TSD and possibly a selection phenomenon of indolent disease where patients in phase III trials receiving late lines (i.e., patients with MM diagnosis) respond well to a new drug.
When considering the 37 patients in the lowest quartile of the E‐R curve, most are IgG MM‐type patients. In the population PK analysis, IgG MM‐type patients have a higher linear clearance at steady state, with a 44% lower isatuximab C trough at 4 weeks compared with those who had non‐IgG MM type. IgG MM type was not the only reason for the lower exposure in Q1, as only eight (21.6%) patients received their 4‐weekly isatuximab administrations and 27 (73.0%) patients encountered isatuximab dose delay and/or dose omissions due to occurrence of certain AEs during cycle 1, including 11 due to occurrence of neutropenia or febrile neutropenia at cycle 1. Consistently, most (21/27) also had pomalidomide dose reductions/omissions at cycle 1. This higher incidence of isatuximab and pomalidomide dose modifications is likely linked to the more aggressive MM characteristics of these patients in this quartile, leading to more bone marrow function impairment. They had lower baseline albumin, higher baseline β2‐microglobulin, more bone marrow plasma cells, and higher lactate dehydrogenase. There were also more patients with plasmacytomas, R‐ISS stage III, and high‐risk chromosomal aberrations, which are reflective of higher disease burden or poor prognosis characteristics in MM. Therefore, patients in the low part of the slope in the E‐R curve were identified as patients with isatuximab dose omission leading to lower exposure but also having poorer prognostic factors, which may have increased risk for developing AEs and resulting in isatuximab dose omissions and pomalidomide dose reduction/delay. This suggests that the E‐R analyses for patients with low isatuximab exposure are confounded by their baseline disease characteristics and by the high incidence of dose modifications for both isatuximab and pomalidomide.
The E‐R analysis also revealed that median PFS increased as CT4W increased. After controlling for the potential confounding factors identified in a model‐based analysis, patients with isatuximab in the low and high quartiles showed an increase in median PFS of 14.5 and 15.8 weeks, respectively, compared with active controls. In addition, the estimation of the HR vs. Pd showed that patients with less than or equal to median CT4W benefit from the isatuximab treatment, including those in the lower quartile of exposure. However, the risk of disease progression would decrease if the patients completed the 4‐weekly administrations, confirming that patients who completed the 4‐weekly administrations would have a higher benefit from the treatment.
The interaction of IgG MM type with isatuximab CT4W was not found to be significant for either ORR or PFS, indicating that the response would be similar in the two populations for a similar level of CT4W exposure. Overall, these results were consistent with the disease model developed using data from 256 evaluable serum M‐protein patients of the ICARIA‐MM phase III study. 18
Taken together, the findings from phase I analyses support the rationale for the selection of isatuximab 10 mg/kg qw/q2w for the phase III ICARIA‐MM study of patients with RRMM. Model‐based drug development was successfully applied to support this dosing regimen. E‐R and clinical data from the ICARIA‐MM study confirmed the safety and efficacy of the selected isatuximab regimen. Overall, these clinical pharmacology data support the proposed regimen of 10 mg/kg q.w. for 4 weeks followed by q2w when given in combination with Pd in patients with RRMM. 11 , 19 , 20
CONFLICT OF INTEREST
F.R. is a contractor for Sanofi on behalf of IviData Life Sciences. K.K., N.G.‐D., B.S., H.‐T.T., C.B., J.B.F., L.N., H.v.d.V., C.V.‐F., and D.S. are employed by Sanofi and may hold stock and/or stock options in the company.
AUTHOR CONTRIBUTIONS
F.R., K.K., N.G.‐D., B.S., H.‐T.T., C.B., J.B.F., L.N., H.v.d.V., C.V.‐F., and D.S. wrote the manuscript. F.R., N.G.‐D., B.S., C.V.‐F., and D.S. designed the research. F.R., N.G.‐D., and B.S. performed the research. F.R., N.G.‐D., B.S., H.‐T.T., C.V.‐F., and D.S. analyzed the data. F.R., N.G.‐D., B.S., H.‐T.T., C.V.‐F., and D.S. contributed new reagents/analytical tools.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors wish to thank the patients participating in this study and their families, as well as the global network of investigators, research nurses, study coordinators, and operations staff. Medical writing support was provided by Erin Burns‐Tidmore, PhD, of Elevate Medical Affairs, contracted by Sanofi for publication support services.
Rachedi F, Koiwai K, Gaudel‐Dedieu N, et al. Exposure‐response analyses for selection/confirmation of optimal isatuximab dosing regimen in combination with pomalidomide/dexamethasone treatment in patients with multiple myeloma. CPT Pharmacometrics Syst Pharmacol. 2022;11:766‐777. doi: 10.1002/psp4.12789
Funding information
These studies were sponsored by Sanofi.
REFERENCES
- 1. Kumar S, Paiva B, Anderson KC, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328‐e346. [DOI] [PubMed] [Google Scholar]
- 2. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang H, Acharya C, An G, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal‐associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30:399‐408. [DOI] [PubMed] [Google Scholar]
- 4. Martin T, Strickland S, Glenn M, et al. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J. 2019;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thai H‐T, Liu L, Koiwai K, et al. Exposure‐response analysis and disease modeling for selection of optimal dosing regimen of isatuximab as single agent in patients with multiple myeloma. 2019. 2019 European Hematology Association Congress, June 14, 2019.
- 6. Koiwai K, el‐Cheikh R, Thai HT, et al. PK/PD modeling analysis for dosing regimen selection of isatuximab as single agent and in combination therapy in patients with multiple myeloma. CPT Pharmacometrics Syst Pharmacol. 2021;10:928‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2021;14:127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Medicines Agency (EMA). Medicines. Sarclisa . 2021. Accessed May 24, 2021. https://www.ema.europa.eu/en/medicines/human/summaries‐opinion/sarclisa‐0
- 9. Martin T, Baz R, Benson DM, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129:3294‐3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mikhael J, Richardson P, Usmani SZ, et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood. 2019;134:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low‐dose dexamethasone versus pomalidomide and low‐dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA‐MM): a randomised, multicentre, open‐label, phase 3 study. Lancet. 2019;394:2096‐2107. [DOI] [PubMed] [Google Scholar]
- 12. Thai HT, Liu L, Semiond D, et al. Model‐based drug development to support isatuximab dosing regimen selection in phase II multiple myeloma patients. Page 25; 2016; Lisboa, Portugal.
- 13. Fau JB, el‐Cheikh R, Brillac C, et al. Drug‐disease interaction and time‐dependent population pharmacokinetics of isatuximab in relapsed/refractory multiple myeloma patients. CPT Pharmacometrics Syst Pharmacol. 2020;9:649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin TG, Hsu K, Strickland SA, Glenn MJ, Mikhael J, Charpentier E. A phase I trial of SAR650984, a CD38 monoclonal antibody, in relapsed or refractory multiple myeloma. J Clin Oncol. 2014;32:8532. [Google Scholar]
- 15. Richter JR, Martin TG, Vij R, et al. Updated data from a phase II dose finding trial of single agent isatuximab (SAR650984, anti‐CD38 mAb) in relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2016;34:8005. [Google Scholar]
- 16. Richardson PG, Attal M, Campana F, et al. Isatuximab plus pomalidomide/dexamethasone versus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma: ICARIA phase III study design. Future Oncol. 2018;14:1035‐1047. [DOI] [PubMed] [Google Scholar]
- 17. Richardson PG, Oriol A, Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:781‐794. [DOI] [PubMed] [Google Scholar]
- 18. Thai HT, Gaudel‐Dedieu N, Cerou M, et al. Model‐based approach to evaluate isatuximab monthly dosing regimen in relapsed/refractory multiple myeloma patients. Abstract 1372. (American Association for Cancer Research Annual Meeting, Philadelphia, PA). 2021. Accessed June 16, 2021. https://ashpublications.org/blood/article/136/Supplement%201/44/473815/Model‐Based‐Approach‐to‐Evaluate‐Isatuximab?msclkid=49b60fdeaddb11ecb6bb6d6e25a4395e
- 19. Schjesvold FH, Richardson PG, Facon T, et al. Isatuximab plus pomalidomide and dexamethasone in elderly patients with relapsed/refractory multiple myeloma: ICARIA‐MM subgroup analysis. Haematologica. 2021;106:1182‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dimopoulos MA, Leleu X, Moreau P, et al. Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: ICARIA‐MM subgroup analysis. Leukemia. 2021;35:562‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


