ABSTRACT
Background
Bovine milk oligosaccharides (BMOs) have several demonstrated and hypothesized benefits including roles in cognitive development and antipathogenic activities, making them promising ingredients for infant formulas and nutraceutical applications. BMO extraction from bovine milk is challenged by low concentrations relative to nonbioactive simple sugars like lactose. BMO abundances are known to vary with a cow's lactation stage, breed, and parity, but these characteristics are difficult to modify in existing dairy herds. In contrast, diet modification is an accessible target, and is already known to influence milk yield, lipid content, protein levels, and monosaccharide compositions.
Objectives
To determine the impact of a low starch high fiber versus a high starch low fiber diet on overall BMO profiles and individual BMO abundances in Holstein dairy cattle.
Methods
Milk samples were collected from 59 midlactation Holsteins in a crossover study featuring dietary modification with either a low starch high fiber or high starch low fiber feed. BMO profiles were evaluated by nano-LC quadrupole time-of-flight tandem MS, and differences in BMO abundances between diets were evaluated using linear mixed effects modeling.
Results
A total of 19 BMOs were identified across the sample set, including 4 large fucosylated compounds. Seven BMOs were found to have significantly more positive percent changes in yield-adjusted abundance from the pre-experiment baseline period for milk samples collected during feeding with the low starch high fiber diet compared with the high starch low fiber diet.
Conclusions
Consuming the low starch high fiber diet promoted greater overall BMO production than the high starch low fiber diet in a population of midlactation Holsteins. Additionally, this study afforded the opportunity to investigate the impact of other factors potentially influencing BMO abundances, furthering understanding of how dairy herd management practices can positively impact milk composition and support the potential use of BMOs as functional ingredients.
Keywords: bovine milk oligosaccharides, diet, fiber, liquid chromatography-mass spectrometry, prebiotics
Consuming a low starch high fiber diet promoted greater overall bovine milk oligosaccharide production than a low fiber high starch diet in a population of midlactation Holstein dairy cattle.
Introduction
Bovine milk oligosaccharides (BMOs) are a class of carbohydrates found in cow milk composed of between 3 and 11 monosaccharide subunits connected by glycosidic linkages. The core of a BMO structure is either a lactose [galactose(β1-4)glucose] or lactosamine [galactose(β1-4)N-acetylglucosamine] reducing end. These core structures may then be expanded through the addition of further galactose (Gal), N-acetylglucosamine (GlcNAc), or N-acetylgalactosamine (GalNAc) units and decorated with α2-3- or α2-6-linked N-acetylneuraminic acid (Neu5Ac) or N-glycolylneuraminic acid (Neu5Gc) or, less commonly, α1-2- or α1-3-linked fucose (Fuc) (1). BMOs may be classified as either acidic or neutral based on the presence or absence of sialic acid (Neu5Ac or Neu5Gc) in their structures. Neutral BMOs can be further designated as either neutral fucosylated or neutral unfucosylated based on whether or not they contain fucose monomers. BMOs discussed herein are referred to by their monosaccharide composition as the number of hexose_N-acetylhexosamine_fucose_N-acetylneuraminic acid_N-glycolylneuraminic acid (Hex_HexNAc_Fuc_Neu5Ac_Neu5Gc), followed by an isomer designation when applicable. Following this nomenclature, acidic BMOs can be identified by the presence of a nonzero number in either the fourth or fifth position of the 5-digit numerical code, whereas neutral fucosylated BMOs can be distinguished by the presence of a nonzero number as the third digit of the compositional code, as shown in Supplemental Table 1 and Supplemental Figure 1.
BMOs have numerous demonstrated health and development benefits which are particularly relevant for human infants. BMOs exhibit antiadhesive and antipathogenic activity against major enteric pathogens including Campylobacter jejuni (2) and enterotoxigenic Escherichia coli (ETEC) (3). The 2 most abundant acidic BMOs, 3′-sialyllactose (3′-SL) and 6′-sialyllactose (6′-SL) have also been shown to exhibit antipathogenic effects against enteropathogenic E. coli (EPEC) (4), S-fimbriated E. coli (5), Salmonella entericaser. Fyris (4), and Pseudomonas aeruginosa (6). In addition, BMOs have demonstrated improved gut barrier function in vitro (7), as well as decreased gut permeability, increased lean body mass, and healthy organ growth in animal models of infant undernutrition (8, 9). Sialylated milk oligosaccharides including 3′-SL and 6′-SL have also been linked with increased sialylation of cerebellum gangliosides, upregulated genes for myelination and ganglioside synthesis in the hippocampus, and improved learning outcomes in animal models (10–12).
Due to their structural similarities with human milk oligosaccharides, BMOs are also hypothesized to have prebiotic activity. Recent in vitro studies featuring BMOs support this hypothesis. Isolated BMOs or sialyllactose have been shown to promote the in vitro growth of the beneficial infant gut microbes Parabacteroides distasonis, Bifidobacterium breve, and B. longum ssp. longum (13) as well as the probiotic B. animalis ssp. lactis (14). In addition, BMOs have been shown to promote the colonization of B. longum ssp. infantis when coadministered in mouse models (15).
Despite the clear benefits, isolating BMOs for use in products like infant formulas and nutraceuticals is challenging due to their low concentrations both in milk and dairy processing streams like whey permeate. Unlike human milk oligosaccharides which are present in concentrations of ∼12–16 g/L in colostrum and 5–11 g/L in milk (16–19), BMOs are only found at ∼1 g/L in bovine colostrum and fall to 80–100 mg/L in mature milk (20, 21). Increasing the concentrations of BMOs in milk would facilitate their isolation. In addition, modifying BMO profiles to be more similar to human milk oligosaccharides with greater abundances of larger and more fucosylated structures would improve the bioactivity of the resultant BMO isolate.
BMO abundances have been previously shown to vary with lactation time point (20, 21), cow breed (22–26), and parity (20, 23). However, these factors are difficult to modify in existing dairy herds. Cow diet has been well documented to influence the yield (27–30), lipid profiles (28, 29, 31–36), nitrogen content (27, 29, 32–34, 36), and monosaccharide composition (37) of cow milk. Dietary supplementation with chitooligosaccharides in sows has also been previously linked with increased abundances of some pig milk oligosaccharides (38). Although a connection between diet and milk oligosaccharides has not yet been shown in ruminants, cow diet is an easily modified factor that has the potential to favorably impact BMO profiles and concentrations.
The impact of the ratio of dietary fiber to starch on BMO content is of particular interest because the balance of these components in feed influences both the ruminal buffering capacity and the digesta passage rate in the cow. These factors, in turn, affect the balance between the breakdown of feed components in the rumen and the absorption of breakdown products in the rumen and small intestine. Although the biochemical pathways for BMO synthesis and the precursors involved have not yet been fully elucidated, the absorption of more energetically favorable building blocks, as influenced by the composition of digestion breakdown products absorbed in the small intestine, may favor BMO production. In this study, BMO profiles were evaluated in a herd of Holstein dairy cattle across a 3-period crossover study design with the objectives of identifying significant variations in BMO profiles and abundances based on dietary fiber to starch ratio, cow parity, and lactation time point.
Methods
Study design
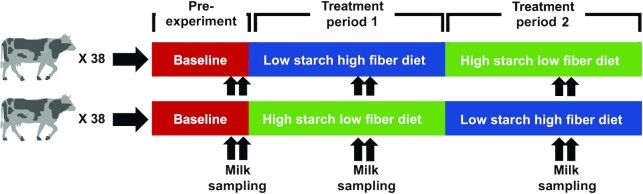
Milk samples were collected from 76 midlactation Holstein dairy cattle in a crossover study design that included sampling during a 4-wk pre-experimental baseline period and 2 subsequent 70-d treatment periods during which cows were fed either a low starch high fiber diet [LSHF; 37% neutral detergent fiber (NDF), 13% starch] or a high starch low fiber diet (HSLF; 29% NDF, 27% starch). At the end of each period, cows were assigned to the opposite diet, as shown in Figure 1, such that each cow acted as its own control. There was an 11-d transition period between each period. Milk samples were collected across the 3 dietary periods, with a sample collected from each cow during 2 consecutive morning milkings in the final week of the pre-experimental baseline period and week 5 of each experimental period (39).
FIGURE 1.
Crossover design of this study featuring a baseline period followed by 2 70-d treatment periods, with milk samples collected for oligosaccharide profiling on 2 consecutive days in the final week of the baseline period and during the fifth week of each dietary period.
Both treatment diets and the baseline diet were composed of a combination of beet pulp, alfalfa silage, corn silage, canola meal, high moisture corn, corn distillers’ grains, roasted soybeans, and soy hulls, mixed at different proportions such that the diets differed in fiber and starch levels but were balanced for protein availability and other key nutrients (Supplemental Table 2). The baseline diet fed in the pre-experiment period was formulated to have starch and fiber contents halfway between those of the LSHF and HSLF diets. Cows were assigned to the 2 groups in a balanced manner based on evaluation of their parity, dry matter intake, milk production, and body weight during the pre-experimental baseline period. Cows were housed in indoor tie-stalls throughout the duration of the study. Feed was provided ad libitum once a day, with feed amounts adjusted daily to allow a maximum of 10% refusals individually, determined based on the refusals measured 2 d prior. Cows were milked 3 times per day (04:00, 10:30, and 18:00). All milk for BMO analysis was collected during the first morning milking after teats were stripped (3 streams of milk), treated, and disinfected with Gladiator Barrier (BouMatic) and towel dried. Raw milk was collected for BMO analysis on 2 consecutive days after 5 wk of consumption of each experimental diet in portions of ∼48 mL each. Aliquots were stored at −10°C immediately after collection and shipped on dry ice to the USDA Agricultural Research Service (ARS) Western Human Nutrition Research Center. Here, the samples were thawed, portioned into smaller 2 mL aliquots, and stored at –20°C until later analysis.
All feeding and milk collection portions of this study were conducted at the USDA-ARS Dairy Forage Research Center Dairy Farm (Prairie du Sac, WI) under protocols approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (Protocol #A005945).
Sample subset selection
From the full set of 456 available milk samples, 338 (from 59 cows) were selected for BMO analysis. A total of 38 from 9 cows were excluded because the cows received antibiotic treatment during the corresponding or prior study period. Four samples were removed because ≥1 sample was missing in a given period or collected outside of the morning milking. Four samples were removed because they were collected after 300 d of lactation and had particularly low milk yields. A total of 54 samples from 9 cows were removed due to technical issues related to accurately estimating their feed intake. In addition, a total of 18 samples from 5 cows were removed because they were outliers for a given period for either lactose concentration or BMO abundances, as evaluated by the SE with a cut-off of 3 (39).
Oligosaccharide extraction and multiplexing
Oligosaccharides were extracted, labeled, and analyzed from milk samples as described previously (40, 41) with some modifications. Samples were skimmed to remove lipids, and for each cow, skimmed milk samples collected on consecutive days within the same period were pooled to minimize the influence of day-to-day variations in milk composition. Pooled samples then underwent ethanol precipitation to remove proteins, followed by C18 microplate solid phase extraction (SPE) to remove peptides, and graphitized carbon microplate SPE to remove lactose and salts. A 4% acetonitrile/0.1% trifluoroacetic acid solution was used for solid phase equilibration and sample washing during graphitized carbon microplate SPE to maximize lactose removal while minimizing BMO loss.
Extracted oligosaccharides from samples were then isobarically labeled with aminoxy tandem mass tags (TMTs) with reporter ions of 127,128, 129,130, or 131 Da, and a previously characterized BMO mixture (42) labeled with the aminoxy TMT 126 Da reporter ion was used as an internal standard. Labeled samples were multiplexed such that each set of 6 aminoxy TMTs contained the labeled internal standard and 5 unique samples, each labeled with a different 1 of the 5 remaining TMTs. Multiplexed samples underwent an additional SPE clean-up employing Oasis Hydrophilic-Lipophilic Balance cartridges to remove excess labeling reagents prior to LC-MS analysis.
LC-MS/MS analysis
Glycoprofiling of oligosaccharides in the collected samples was conducted using nano-LC chip quadrupole time-of-flight MS (nano-LC-chip-Q-ToF MS) using our previously published LC-MS method (41) with slight modifications. Briefly, samples were dissolved in 3% acetonitrile, passed through 0.2 μm polyethersulfone filters, and loaded onto the nano-LC chip with a 40 nL enrichment column and a 75 μm × 43 mm analytical column, packed with 5 μm particles of 250 Å pore size. Flow rates were operated at 4 μL/min (enrichment column) and 0.3 μL/min (analytical column). Mobile phase solvents were 3% acetonitrile/0.1% formic acid (A), and 89.9% acetonitrile/0.1% formic acid (B). After equilibrating both the analytical and enrichment columns with 100% A, a 65-min gradient was used for chromatographic separation. The gradient was ramped from 4 to 20.6% B from 0 to 23 min, 20.6 to 50% B from 23 to 30 min, 50 to 100% B from 30 to 35 min, held at 100% B from 35 to 50 min, then lowered from 100 to 0% B from 50 to 50.1 min.
Mass spectra were collected in positive mode over a scan range of 400 to 2500 m/z at a rate of 2 spectra/s for MS scans and 100 to 2500 m/z at a rate of 1 spectra/s for MS/MS. The drying gas was held at 350°C with a flow of 5 L/min. An in-house library of BMO masses assembled from the literature (1, 23, 43–45) was entered in the acquisition software as a list for targeted fragmentation. The 5 most abundant precursors in each MS scan matching the targeted list were fragmented, with a quadrupole isolation window of ∼4 m/z. A minimum precursor threshold of 5000 ion counts/spectrum was set to ensure substantial reporter ion abundance in the MS/MS scans. Capillary voltage was varied from 1900 to 1975 V as needed to maintain a stable spray. In-run mass calibration was performed with infused calibrant ions of m/z 922.009798 and 1221.990637.
BMOs were identified using a customized bioinformatics library of BMO compounds assembled from prior publications (1, 23, 43–45) and their identities were confirmed by the examination of MS/MS spectra using Agilent MassHunter B.07.00 (Agilent Technologies). For relative quantification, raw data was exported in .mzData format with MassHunter and then imported into SimGlycan Enterprise Edition 5.61 (PREMIER Biosoft) (46). BMOs with confirmed identities were added to a custom library on the SimGlycan server, which was used by the software to identify those BMOs in the data files through matching retention time and precursor mass using the “High Throughput Search and Score” feature. Precursor ion and reporter ion m/z tolerances were set to 10 ppm and 0.025 Da, respectively. For each BMO, the reporter ion abundances from all the MS/MS spectra were summed, and the ratios of these sums were calculated. The reporter ion abundances for each sample were normalized to the signal for the TMT 126-labeled BMO internal standard to give the BMO relative abundances (Supplemental Table 3).
Statistical analysis
Glycoprofiling relative abundances were log transformed to improve normality as evaluated by the Shapiro–Wilks test prior to comparative statistical analysis, with the exception of the results for the BMOs with compositions 2_1_0_0_0 isomer 2 and 4_4_1_0_0, which were transformed via a cube root, and 3_6_1_0_0 and 5_4_1_0_0, which did not require a transformation to achieve a normal distribution (Shapiro–Wilks test, P >0.05). Relative abundances were also multiplied by the average morning milk weight (lbs) for the corresponding period to give yield-adjusted relative abundances.
Yield-adjusted relative abundance results were log transformed to improve normality as evaluated by the Shapiro–Wilks test prior to comparative statistical analysis, with the exception of BMO with composition 3_6_1_0_0, which did not require a transformation to achieve a normal distribution (Shapiro–Wilks test, P >0.05). Transformed relative abundances and yield-adjusted relative abundances were evaluated with 2-sided Student's t-tests to compare the 2 postdiet arms and 1-factor ANOVA with posthoc evaluation using Tukey's test to compare the 3 diet time points. Linear mixed effects modeling was used to determine the significance (α = 0.05) of the effects of diet, cow ID, treatment period, dietary sequence, parity, milk yield, and lactation time point on the oligosaccharide profiles. In addition, the percent change in transformed relative abundances and percent change in transformed yield-adjusted relative abundances were calculated as:
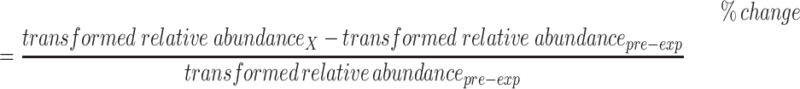 |
(1) |
where X is a dietary treatment period. Percent change in relative abundances from the pre-experimental baseline period were evaluated similarly using 2-sided Student's t-test, 1-factor ANOVA with posthoc evaluation using Tukey's test, and linear mixed effects modeling.
Calculation of Pearson's correlations was conducted on transformed data, with all Pearson correlation figures and their significances generated using the R package corrplot (47). Principal component analysis was conducted on untransformed data. All statistical analyses were conducted using R version 4.0.2.
Results
Identification of BMOs and their abundance in milk
Milk samples from all of the cows in the study showed a high degree of similarity in BMO composition. The abundance of 19 major BMOs was measured in all samples, including 5 acidic structures and 4 neutral fucosylated compounds. Identified BMOs ranged in size from degrees of polymerization of 3 to 10.
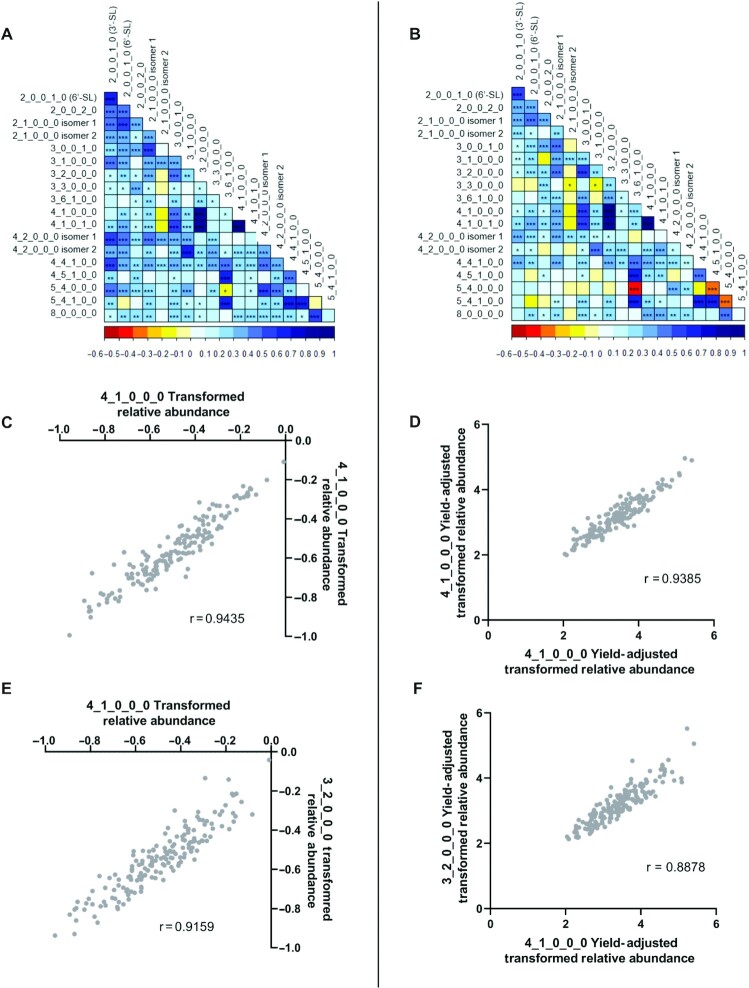
Correlations in abundance between BMOs were identified for transformed data, both without and with adjustment for milk yield. In both cases, the strongest correlations were observed between the BMOs 4_1_0_0_0 and 4_1_0_1_0 (Figure 2C and D) and between 4_1_0_0_0 and 3_2_0_0_0 (Figure 2E and F). Significant positive correlations were also observed among the 4 identified fucosylated BMOs, as well as between the 2 sialyllactose isomers (Figure 2A and B).
FIGURE 2.
Pearson's correlations among oligosaccharide pairs for nonyield corrected (left) and yield-corrected (right) relative abundance (signal intensity) data organized as heat maps for all oligosaccharide pairs (A & B), as well as individual plots for the strongest correlations between 4_1_0_0_0 and 4_1_0_1_0 (C, r = 0.9435 & D, r = 0.9385), and between 4_1_0_0_0 and 3_2_0_0_0 (E, r = 0.9159 & F, r = 0.8878). BMOs are described by their monosaccharide compositions as the number of hexose_N-acetylhexosamine_fucose_N-acetylneuraminic acid_N-glycolyneuraminic acid (Hex_HexNAc_Fuc_Neu5Ac_Neu5Gc), followed by the isomer number, as appropriate. *0.01 < P ≤0.05, **0.001 < P ≤0.01, ***P <0.001. BMO, bovine milk oligosaccharide.
Sources of variation in BMO profiles
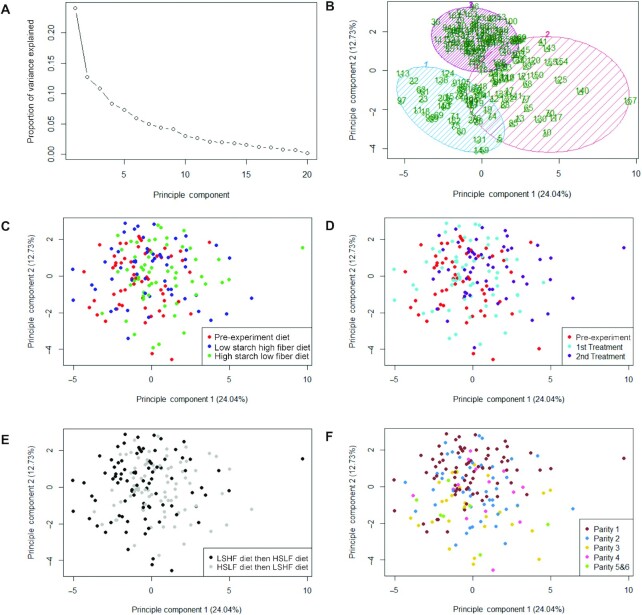
A wide spread of BMO abundances was observed both within and across treatment groups. Principal component analysis was conducted to evaluate which, if any, of the main recorded study variables contributed to the observed variation. Although some clustering was present (Figure 3B), very little separation based on cow diet, dietary treatment period, or diet sequence was observed (Figure 3C–E). Some separation did occur based on parity, particularly between parity 1 and parities 5 and 6 along the second principal component (Figure 3F). Thus, the largest source of variance in BMO profiles remains as 1 or more unrecorded factors.
FIGURE 3.
(A) Scree plot, (B) cluster plot, and principal component analysis of BMO relative abundance data organized by (C) diet, (D) study period, (E) diet sequence, and (F) parity. BMO, bovine milk oligosaccharide.
Diet effects on BMO profiles
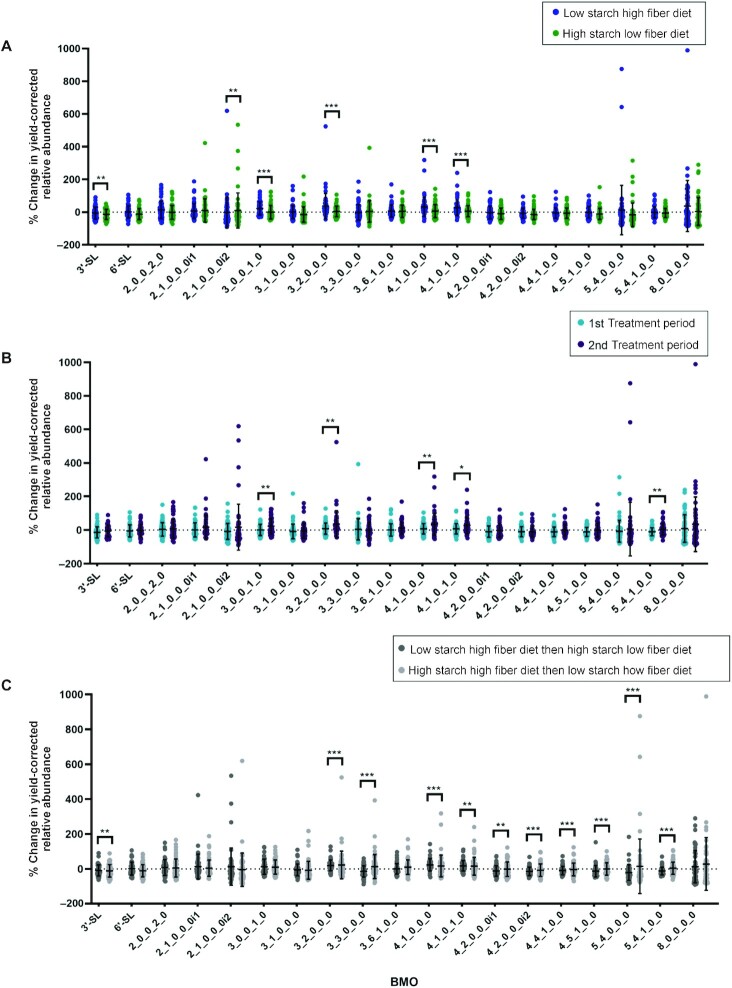
The percent change in the transformed relative abundance from the pre-experiment baseline diet differed significantly (P <0.001) between the LSHF and HSLF dietary treatments for 4 BMOs (with compositions 3_0_0_1_0, 3_2_0_0_0, 4_1_0_0_0, and 4_1_0_1_0) based on initial t-test comparisons (Figure 1A). For all 4 of these oligosaccharides the abundance was significantly higher (P <0.05) in samples from cows fed the LSHF diet compared with both the HSLF diet and the pre-experimental diet (Supplemental Figure 2).
The dietary treatment period significantly influenced (P <0.05) the percent change in relative abundance from the pre-experiment baseline period for 8 BMOs (Figure 4B). Similarly, t-test comparisons showed that the percent change in the transformed relative abundances from the pre-experimental baseline period significantly differed (P <0.05) for 7 BMOs based on the sequence of dietary treatments (Figure 4C). All 4 of the BMOs for which the percent change in their transformed relative abundances from the pre-experiment baseline period differed significantly by diet also differed significantly by treatment period and dietary treatment sequence. An interaction between diet and period was observed for 3_1_0_0_0, 3_2_0_0_0, 4_1_0_0_0, and 4_1_0_1_0 with more negative percent changes in transformed relative abundances (corresponding with smaller percent changes from the pre-experiment baseline period in the untransformed abundance data) for both the second dietary treatment period and the LSHF diet for all 4 BMOs, as shown in Supplemental Figure 3.
FIGURE 4.
BMO percent change in relative abundance (percent change in signal intensity) data organized by (A) diet, (B) study period, and (C) diet sequence with BMOs described by monosaccharide composition as the number of hexose_N-acetylhexosamine_fucose_N-acetylneuraminic acid_N-glycolylneuraminic acid (Hex_HexNAc_Fuc_Neu5Ac_Neu5Gc), followed by the isomer number, as appropriate. Statistics are from parametric analyses of transformed data and graphs present untransformed data. *0.01 < P ≤0.05, **0.001 < P ≤0.01, ***P <0.001. BMO, bovine milk oligosaccharide.
Linear mixed effects modeling was conducted to determine whether the effects of diet on the percent change in the transformed relative abundances from the pre-experiment baseline diet remained significant after adjustment for other study parameters including treatment period, diet sequence, parity, days in milk, cow ID, and milk yield. The influence of diet was not significant (P >0.05) for nearly all linear mixed effects models constructed for 3_0_0_1_0 and 4_1_0_1_0; however, the effect of diet remained significant (P <0.05) across all models for 3_2_0_0_0 and 4_1_0_0_0. In addition, the effect of diet emerged as significant for 3_1_0_0_0 in all linear mixed effect models including cow ID as a variable. In summary, the LSHF diet increased the abundance of 3_2_0_0_0, 4_1_0_0_0, and possibly also 3_1_0_0_0. No BMOs were decreased in abundance on the LSHF diet relative to the HSLF diet.
Parity affects BMO profiles
Significant differences (P <0.05) were observed between milk samples from cows of different parities for 6 BMOs, with the most significant differences in BMO abundances being observed between parities 1 and 3 (Figure 5). 6′-SL, 4_2_0_0_0 isomer 1, and 5_4_0_0_0 increase with increasing parity whereas 3_6_1_0_0, 4_5_1_0_0, and 5_4_1_0_0 decrease with increasing parity.
FIGURE 5.
BMO relative abundance data (signal intensity) organized by parity with BMOs described by monosaccharide composition as the number of hexose_N-acetylhexosamine_fucose_N-acetylneuraminic acid_N-glycolylneuraminic acid (Hex_HexNAc_Fuc_Neu5Ac_Neu5Gc), followed by the isomer number, as appropriate. Statistics are from parametric analyses of transformed data and graphs present untransformed data. Parities that share a letter are not significantly different (α = 0.05). Arrows indicate the direction of average relative abundance changes across the first 3 parties. BMO, bovine milk oligosaccharide.
Differences in BMO abundances are not merely due to changes in yield
BMO abundances were also adjusted for milk yield by multiplying BMO abundance by the average milk weight collected during the morning milkings on the days of each sample collection during the corresponding study period.
Similar to the nonyield-adjusted data, the yield-adjusted relative abundances of 3_0_0_1_0, 3_2_0_0_0, 4_1_0_0_0, and 4_1_0_1_0 were all highest with the LSHF diet (Supplemental Figure 4A). Adjusting for yield, however, also revealed differences in BMO abundances between dietary treatments for the additional BMOs 3′-SL and 2_1_0_0_0 isomer 2, which had significantly lower abundances (P <0.05) with the HSLF diet compared to the pre-experiment baseline diet (Supplemental Figure 4A).
As with the nonyield-adjusted data, t-test comparisons showed that the percent change in the transformed yield-adjusted relative abundances from the pre-experimental baseline period significantly differed (P <0.05) based on study period and/or the sequence of dietary treatments for most of the same BMOs that showed significant differences between diets (Figure 6B and C).
FIGURE 6.
BMO percent change in yield-adjusted relative abundance (percent change in yield-adjusted signal intensity) data organized by (A) diet, (B) study period, and (C) diet sequence with BMOs described by monosaccharide composition as the number of hexose_N-acetylhexosamine_fucose_N-acetylneuraminic acid_N-glycolylneuraminic acid (Hex_HexNAc_Fuc_Neu5Ac_Neu5Gc), followed by the isomer number, as appropriate. Statistics are from parametric analyses of transformed data and graphs present untransformed data. *0.01 < P ≤0.05, **0.001 < P ≤0.01, ***P <0.001. BMO, bovine milk oligosaccharide.
Linear mixed effects modeling was conducted to determine whether the effects of diet on the percent change in the transformed yield-adjusted relative abundances from the pre-experiment baseline diet remained significant when other study parameters including treatment period, diet sequence, parity, days in milk, and cow ID were also accounted for. The influence of diet became nonsignificant (P >0.05) for all linear mixed effects models constructed for 3′-SL and 2_1_0_0_0 isomer 2; however, the effect of diet remained significant (P <0.05) across all models for 3_0_0_1_0, 3_2_0_0_0, 4_1_0_0_0, and 4_1_0_1_0. In addition, the effect of diet emerged as significant for 3_1_0_0_0, 4_2_0_0_0 isomer 2, and 5_4_0_0_0 in all linear mixed effect models including cow ID as a variable. In summary, when the influence of differences in milk yield are accounted for, no BMOs decreased in abundance with the LSHF diet compared with the HSLF diet. In addition, the LSHF diet increased the abundance of 3_0_0_1_0, 3_2_0_0_0, 4_1_0_0_0, and 4_1_0_1_0 and may have also increased the abundance of 3_1_0_0_0, 4_2_0_0_0 isomer 2, and 5_4_0_0_0.
Discussion
Diet
Two acidic BMOs (3_0_0_1_0 and 4_1_0_1_0) and 2 neutral unfucosylated BMOs (3_2_0_0_0 and 4_1_0_0_0) exhibited significantly more positive percent changes of the transformed abundances from the pre-experiment baseline diet for the LSHF diet compared with the HSLF diet, based on initial t-test comparisons (P <0.001).
Interestingly, the relative abundances of 3_2_0_0_0 and 4_1_0_0_0 were found to correlate with each other in the present study (Figure 2), as well as in a recent analysis of milk from 634 Danish Jerseys and Holstein-Friesians (23). Given these correlations across differences in both breed and feeding, the changes in abundance of these compounds with diet in the present study suggest that dietary fiber levels may impact a key enzyme or reaction involved in the synthesis of both of these oligosaccharides. Further, the fact that BMO abundances only increase with the LSHF diet suggests that substrate increases the shared synthesis of these correlated BMOs.
Two previous studies have also investigated the influence of diet on BMO profiles. Vicaretti et al. compared milk samples from cows that were either exclusively grass fed or consumed a feed composed of alfalfa and corn silage, earlage, and grain (48). No significant differences in BMO profiles were observed between cows in the 2 dietary groups; however, only 6 cows were included per dietary group, likely causing the study to be too underpowered to observe any meaningful differences between diets, if such a difference existed. In addition, differences in breed composition and farm between the 2 dietary groups may have had a confounding influence on the data.
Liu et al. compared BMO profiles between 32 Holstein-Friesian dairy cows with diets supplemented with either almond hulls or citrus peels to a base total mixed ration of corn grain, canola meal, and alfalfa cubes (49). As a result of BMO measurements only being taken at 1 time point during the study, the identified BMOs were found to have greater intercow variation within dietary treatment groups than intergroup variation, preventing any conclusions from being made about the influence of the diets on BMO production.
Although the present study also showed minimal effects of diet on nonyield-adjusted BMO profiles, our results are more meaningful and conclusive as a result of the greater study power and the use of a crossover study design, which accounted for both the inherent cow-to-cow variation through the inclusion of pre-experimental baseline BMO profiling as well as many potential confounding factors that may have impacted the results of prior studies. The design of the present study is also advantageous in the inclusion of cows from a single breed, all located on the same farm, and all without access to an alternative feed source (i.e., pasture) outside of the study diets. In addition, in the present study, cows in the 2 groups were balanced by parity and pre-experimental average milk yields.
Beyond the influence of diet, this study also affords the opportunity to investigate the impact of other BMO-influencing factors in a large set of milk samples from midlactation dairy cattle.
Parity
Similar to the differences in BMO abundances between cows of different parities observed in previous studies (20, 23), primiparous cows were found to have significantly lower abundances of 6′-SL, 4_2_0_0_0 isomer 1, and 5_4_0_0_0 in their milk compared with cows in either their second or third parity (Figure 5). Unlike prior studies however, cows in the present study were also shown to have significantly higher abundances of the large neutral fucosylated BMOs 3_6_1_0_0, 4_5_1_0_0, and 5_4_1_0_0 in the first parity compared with those in the third parity (Figure 5), a direct contrast to the previously observed trend. This pattern of some BMOs increasing in abundance with increasing parity while other BMOs decrease, suggests that trade-offs may occur in BMO synthesis pathways as the mammary gland is remodeled with each lactation cycle through epigenetics (50, 51). The higher abundances of larger fucosylated oligosaccharides, which have greater demonstrated bioactivities (52), in earlier parities may also be evidence of the corresponding fucosylation genes being naturally activated prior to the first lactation and silenced during subsequent lactations.
Lactation time point
Nearly all previous BMO studies with samples collected at >1 time point have focused on detecting BMO in early lactation, with samples collected only through to the end of the second week postpartum (20, 21, 48, 53). However, most milk used for commercial purposes is collected outside of this timeframe, and very little is known about if and how BMO profiles change over time in mature cow milk. McJarrow and van Amelsfort-Schoonbeek followed the concentrations of 5 BMOs in bulk milk samples across a milking season in grass-fed New Zealand Jersey and Friesian dairy cattle and noted a seasonal variation (26); however, no parallel study has been conducted with nongrass-fed cows or cows from other breeds or regions to determine whether similar patterns in BMO variations occur.
The number of days in milk at the time of milk sample collection significantly influenced BMO abundances for several BMOs, including 2_1_0_0_0 isomer 1, 3_0_0_1_0, 3_2_0_0_0, 3_6_1_0_0, 4_1_0_0_0, 4_1_0_1_0, 4_4_1_0_0, and 5_4_1_0_0. Although the correlation coefficients for the abundances of these oligosaccharides over time are not particularly strong – likely due in part to the wide degree of natural variation in BMO abundances between cows – general increasing trends can be observed for these 6 BMOs across lactation (Figure 7). This trend disappears when looking at the yield-adjusted relative abundance data, suggesting that the apparent increase in abundances for these BMOs in later lactation may be due, at least in part, to a concentrating effect caused by similar levels of total BMO production despite decreasing total milk volumes. Although this concentrating effect has been previously hypothesized (54), this is the first report, to our knowledge, of yield-adjusted BMO concentrations across the lactation cycle.
FIGURE 7.
Increasing trends of BMO relative abundance (signal intensity) across lactation for (A) 3_0_0_1_0, (B) 3_2_0_0_0, (C) 3_6_1_0_0, (D) 4_1_0_0_0, (E) 4_4_1_0_0, and (F) 5_4_1_0_0. BMOs are described by their monosaccharide composition as the number of hexose_N-acetylhexosamine_fucose_N-acetylneuraminic acid_N-glycolylneuraminic acid (Hex_HexNAc_Fuc_Neu5Ac_Neu5Gc). BMO, bovine milk oligosaccharide.
Unmeasured factors
The principal component analyses suggest that the largest source of variance in BMO abundance is due to 1 or more factors that were not measured in the study. Prior work demonstrating differences in BMO concentration between breeds (23, 55) suggests that genetics is an important determinant of BMO production and is therefore likely to be ≥1 source of variance. Future studies involving both breed and feeding will be needed to increase BMO production.
Correlations in BMO abundances
Significant correlations in abundance between several BMOs were identified both without and with adjustment for milk yield (Figure 2), providing insight into their co-occurrences in milk from milk consumption and milk synthesis perspectives, respectively. The strongest correlations were observed between the BMOs 4_1_0_0_0 and 4_1_0_1_0 (nonyield-adjusted r = 0.94, yield-adjusted r = 0.94) and between 4_1_0_0_0 and 3_2_0_0_0 (nonyield-adjusted r = 0.92, yield-adjusted r = 0.89), suggesting that these 3 BMOs may share a common core structure or key glycosyltransferase enzyme. Significant positive correlations were also observed among the 4 identified fucosylated BMOs, which may indicate a shared fucosyltransferase enzyme utilized in their synthesis. In addition, the negative correlation of 5_4_0_0_0 with 5_4_1_0_0 may suggest that 5_4_0_0_0 is a precursor structure for its larger, fucosylated BMO, causing its abundance to decrease as it is used to create its fucosylated counterpart. Overall, the correlations among BMO abundances provide tantalizing clues regarding BMO synthesis that should be investigated in future studies.
Yield-adjusted BMO abundances
Although most previous BMO studies have focused more on a consumer perspective and, therefore, have not accounted for milk yield in their analyses, adjusting for milk yield is important for understanding whether milk oligosaccharide production is truly increasing, decreasing, or remaining unchanged from a biological and mechanistic perspective on milk production.
Analysis of the transformed yield-adjusted percent change in relative abundance from the pre-experiment baseline diet in the present data with linear mixed effects modeling including cow ID as a variable revealed 7 BMOs that differed significantly by diet, with all 7 BMOs featuring a more positive percent change from the pre-experiment baseline diet for the LSHF diet compared with the HSLF diet.
The observed significant changes in transformed yield-adjusted relative abundances from the baseline period for 7 out of the 19 measured BMOs suggests that there is indeed a relation between cows’ dietary fiber and starch intake levels and their production of milk oligosaccharides. Among these 7 BMOs are 2 acidic (3_0_0_1_0 and 4_1_0_1_0) and 5 neutral unfucosylated compounds (3_1_0_0_0, 3_2_0_0_0, 4_1_0_0_0, 4_2_0_0_0 isomer 2, and 5_4_0_0_0). The significant impact of dietary fiber levels on the yield-adjusted abundances of these BMOs but not the 4 identified neutral fucosylated BMOs may indicate that these fucosylated compounds do not share the same core structures as the 7 impacted unfucosylated BMOs or that the availability of fucose or the occurrence of the fucosylation reaction is a limiting factor in the synthesis of these fucosylated BMOs under the conditions of the present study.
Additional investigations into the biological mechanisms of milk oligosaccharide synthesis and the absorption of carbohydrates and potential carbohydrate precursors from the digestive track in cows are needed to better understand the observed relation between bovine dietary fiber intake and yield-adjusted BMO abundances. The inclusion of more detailed analysis of the dietary fiber consumed by the cows (i.e., monosaccharide compositions and linkage analysis) as well as linkage analysis of the produced BMOs in future studies will aid in the further investigation of the observed link between the cow dietary fiber to starch intake ratio and BMO production.
Conclusions
In this study we implemented a 3-period crossover design paired with high-throughput nano-LC-chip-Q-ToF MS analysis to evaluate the impact of dietary fiber and starch ratios on BMO abundances. A total of 19 BMOs were identified across 338 samples from 59 cows, including 7 BMOs with a more positive percent change in yield-adjusted abundance from the pre-experimental baseline period with a LSHF diet compared with a HSLF diet. In addition, significant differences were observed for 6 BMOs based on parity, including 3 for which abundances were greater in primiparous cows compared with their secundiparous or triparous herd mates. Parity had a mixed effect on BMO abundances with some increasing and others decreasing with increasing parity, the LSHF diet only increased BMOs, suggesting the utility of this diet regardless of other cow-specific factors.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to extend special thanks to Randall Robinson for his advisement during the tandem mass tagging and mass spectrometry optimization processes. We would like to thank Shan Betzold and Kurt Pickar for animal care, and Amélie Fischer, Alicia Pelletier, and Diane Amundson for data and sample collection.
The authors’ responsibilities were as follows—KFK, JWF, NKF, and DB: designed the research; SDD and ZW: conducted the research; SDD and DGL: analyzed the data; SDD wrote the manuscript; and all authors: read and approved the final manuscript.
Notes
This work was supported by USDA Agricultural Research Service (USDA-ARS) [Non-assistance cooperative agreement (NACA) #58-8040-8-013], project CA-D-FST-2187-H, USDA-ARS Project #5090-31000-025-00D, USDA-ARS Project # 2032-51530-026-000-D, and by the USDA-ARS Grand Challenge Synergy project “Dairy Agriculture for People and Planet”.
Author disclosures: JWF is an Editor on Current Developments of Nutrition and played no role in the journal's evaluation of the manuscript. DGL, KFK, JWF, and NKF are employees of the USDA. The USDA is an Equal Opportunity Employer. DB is a cofounder of Evolve Biosystems, a company focused on diet-based manipulation of the gut microbiota. Evolve Biosystems played no role in the design, execution, interpretation, or publication of this work. SDD and ZW report no conflicts of interest.
Supplemental Figures 1–4 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ARS, Agricultural Research Service; BMO, bovine milk oligosaccharide; Fuc, fucose; Gal, galactose; Hex, hexose; HexNAc, N-acetylhexosamine; HSLF, high starch low fiber; LSHF, low starch high fiber; NDF, neutral detergent fiber; Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; Q-ToF, quadrupole time-of-flight; SPE, solid phase extraction; TMT, tandem mass tag; 3′-SL, 3′-sialyllactose; 6′-SL, 6′-sialyllactose.
Contributor Information
Sierra D Durham, Email: sddurham@ucdavis.edu, Department of Food Science and Technology, University of California, Davis, CA, USA.
Danielle G Lemay, Agricultural Research Service, USDA, Western Human Nutrition Research Center, Davis, CA, USA.
Zhe Wei, Department of Food Science and Technology, University of California, Davis, CA, USA.
Kenneth F Kalscheur, Agricultural Research Service, USDA, US Dairy Forage Research Center, Madison, WI, USA.
John W Finley, Agricultural Research Service, USDA, Office of National Programs, Beltsville, MD, USA.
Naomi K Fukagawa, Agricultural Research Service, USDA, Beltsville Human Nutrition Research Center, Beltsville, MD, USA.
Daniela Barile, Department of Food Science and Technology, University of California, Davis, CA, USA; Foods for Health Institute, University of California, Davis, CA, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB, Barile D. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology. 2013;23(6):664–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lane JA, Mariño K, Naughton J, Kavanaugh D, Clyne M, Carrington SD, Hickey RM. Anti-infective bovine colostrum oligosaccharides: Campylobacterjejuni as a case study. Int J Food Microbiol. 2012;157(2):182–8. [DOI] [PubMed] [Google Scholar]
- 3. Martín-Sosa S, Martín J-J, Hueso P. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichiacoli human strains. J Nutr. 2002;132(10):3067–72. [DOI] [PubMed] [Google Scholar]
- 4. Coppa GV, Zampini L, Galeazzi T, Facinelli B, Ferrante L, Capretti R, Orazio G. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichiacoli, Vibriocholerae, and Salmonellafyris. Pediatr Res. 2006;59(3):377–82. [DOI] [PubMed] [Google Scholar]
- 5. Parkkinen J, Rogers GN, Korhonen T, Dahr W, Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichiacoli. Infect Immun. 1986;54(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marotta M, Ryan JT, Hickey RM. The predominant milk oligosaccharide 6′-sialyllactose reduces the internalisation of Pseudomonasaeruginosa in human pneumocytes. J Funct Foods. 2014;6:367–73. [Google Scholar]
- 7. Perdijk O, van Baarlen P, Fernandez-Gutierrez MM, van den Brink E, Schuren FHJ, Brugman S, Savelkoul HFJ, Kleerebezem M, van Neerven RJJ. Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production in vitro. Front Immunol. 2019;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boudry G, Hamilton MK, Chichlowski M, Wickramasinghe S, Barile D, Kalanetra KM, Mills DA, Raybould HE. Bovine milk oligosaccharides decrease gut permeability and improve inflammation and microbial dysbiosis in diet-induced obese mice. J Dairy Sci. 2017;100(4):2471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charbonneau MR, O'Donnell D, Blanton LV, Totten SM, Davis JCC, Barratt MJ, Cheng J, Guruge J, Talcott M, Bain JRet al. . Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell. 2016;164(5):859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Obelitz-Ryom K, Bering SB, Overgaard SH, Eskildsen SF, Ringgaard S, Olesen JL, Skovgaard K, Pankratova S, Wang B, Brunse Aet al. . Bovine milk oligosaccharides with sialyllactose improves cognition in preterm pigs. Nutrients. 2019;11(6):1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oliveros E, Vázquez E, Barranco A, Ramírez M, Gruart A, Delgado-García J, Buck R, Rueda R, Martín M. Sialic acid and sialylated oligosaccharide supplementation during lactation improves learning and memory in rats. Nutrients. 2018;10(10):1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobi SK, Yatsunenko T, Li D, Dasgupta S, Yu RK, Berg BM, Chichlowski M, Odle J. Dietary isomers of sialyllactose increase ganglioside sialic acid concentrations in the corpus callosum and cerebellum and modulate the colonic microbiota of formula-fed piglets. J Nutr. 2016;146(2):200–8. [DOI] [PubMed] [Google Scholar]
- 13. Jakobsen LMA, Sundekilde UK, Andersen HJ, Nielsen DS, Bertram HC. Lactose and bovine milk oligosaccharides synergistically stimulate B. longum subsp. longum growth in a simplified model of the infant gut microbiome. J Proteome Res. 2019;18(8):3086–98. [DOI] [PubMed] [Google Scholar]
- 14. Marsaux B, Van den Abbeele P, Ghyselinck J, Prioult G, Marzorati M, Bogićević B. Synbiotic effect of Bifidobacteriumlactis CNCM I-3446 and bovine milk-derived oligosaccharides on infant gut microbiota. Nutrients. 2020;12(8):2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jena PK, Sheng L, Nagar N, Wu C, Barile D, Mills DA, Wan Y-JY. Synbiotics Bifidobacterium infantis and milk oligosaccharides are effective in reversing cancer-prone nonalcoholic steatohepatitis using Western diet-fed FXR knockout mouse models. J Nutr Biochem. 2018;57:246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin S, De Castro CA, Sprenger N, Binia A, Affolter M, Garcia-Rodenas CL, Beauport L, Tolsa J-F, Fumeaux CJF. Human milk oligosaccharides in the milk of mothers delivering term versus preterm infants. Nutrients. 2019;11(6):1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samuel TM, Binia A, de Castro CA, Thakkar SK, Billeaud C, Agosti M, Al-Jashi I, Costeira MJ, Marchini G, Martínez-Costa Cet al. . Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci Rep. 2019;9(1):11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertino E, Fabris C, Coppa GV. Preterm milk oligosaccharides during the first month of lactation. Pediatrics. 2011;128(6):e1520–e1531. [DOI] [PubMed] [Google Scholar]
- 19. Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104(9):1261–71. [DOI] [PubMed] [Google Scholar]
- 20. Fischer-Tlustos AJ, Hertogs K, van Niekerk JK, Nagorske M, Haines DM, Steele MA. Oligosaccharide concentrations in colostrum, transition milk, and mature milk of primi- and multiparous Holstein cows during the first week of lactation. J Dairy Sci. 2020:103(4):3683–95. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura T, Kawase H, Kimura K, Watanabe Y, Ohtani M, Arai I, Urashima T. Concentrations of sialyloligosaccharides in bovine colostrum and milk during the prepartum and early lactation. J Dairy Sci. 2003;86(4):1315–20. [DOI] [PubMed] [Google Scholar]
- 22. Sunds AV, Bunyatratchata A, Robinson R, Glantz M, Paulsson M, Leskauskaite D, Pihlanto A, Inglingstad R, Devold TG, Vegarudet al. . Comparison of bovine milk oligosaccharides in native North European cattle breeds. Int Dairy J. 2021;114:104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson RC, Poulsen NA, Colet E, Duchene C, Larsen LB, Barile D. Profiling of aminoxyTMT-labeled bovine milk oligosaccharides reveals substantial variation in oligosaccharide abundance between dairy cattle breeds. Sci Rep. 2019;9(1):5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sischo WM, Short DM, Geissler M, Bunyatratchata A, Barile D. Comparative composition, diversity, and abundance of oligosaccharides in early lactation milk from commercial dairy and beef cows. J Dairy Sci. 2017;100(5):3883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sundekilde U, Downey E, O'Mahony J, O'Shea C-A, Ryan C, Kelly A, Bertram H. The effect of gestational and lactational age on the human milk metabolome. Nutrients. 2016;8(5):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McJarrow P, van Amelsfort-Schoonbeek J. Bovine sialyl oligosaccharides: seasonal variations in their concentrations in milk, and a comparison of the colostrums of Jersey and Friesian cows. Int Dairy J. 2004;14(7):571–9. [Google Scholar]
- 27. Sánchez-Duarte JI, Kalscheur KF, Casper DP, García AD. Performance of dairy cows fed diets formulated at 2 starch concentrations with either canola meal or soybean meal as the protein supplement. J Dairy Sci. 2019;102(9):7970–9. [DOI] [PubMed] [Google Scholar]
- 28. Ranathunga SD, Abdelqader MM, Kalscheur KF. Nutrient digestion by dairy cows fed diets replacing starch with non-forage fiber. In: Oltjen JW, Kebreab E, Lapierre H (eds.), Energy and Protein Metabolism and Nutrition in Sustainable Animal Production. Wageningen, NL: Wageningen Academic Publishers; 2013, pp. 61–63. [Google Scholar]
- 29. Cant JP, DePeters EJ, Baldwin RL. Effect of dietary fat and postruminal casein administration on milk composition of lactating dairy cows. J Dairy Sci. 1991;74(1):211–19. [Google Scholar]
- 30. Thomson DJ, Beever DE, Haines MJ, Cammell SB, Evans RT, Dhanoa MS, Austin AR. Yield and composition of milk from Friesian cows grazing either perennial ryegrass or white clover in early lactation. J Dairy Res. 1985;52(1):17–31. [Google Scholar]
- 31. Xue F, Sun F, Jiang L, Hua D, Wang Y, Nan X, Zhao Y, Xiong B. Effects of partial replacment of dietary forage using kelp powder (Thalluslaminariae) on ruminal fermentation and lactation performances of dairy cows. Animals. 2019;9(10):852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miron J, Zuckerman E, Adin G, Solomon R, Shoshani E, Nikbachat M, Yosef E, Zenou A, Weinberg ZG, Chen Yet al. . Comparison of two forage sorghum varieties with corn and the effect of feeding their silages on eating behavior and lactation performance of dairy cows. Anim Feed Sci Technol. 2007;139(1–2):23–39. [Google Scholar]
- 33. Carroll SM, DePeters EJ, Taylor SJ, Rosenberg M, Perez-Monti H, Capps VA. Milk composition of Holstein, Jersey, and Brown Swiss cows in response to increasing levels of dietary fat. Anim Feed Sci Tech. 2006;131:451–73. [Google Scholar]
- 34. Miron J, Yosef E, Maltz E, Halachmi I. Soybean hulls as a replacement of forage neutral detergent fiber in total mixed rations of lactating cows. Anim Feed Sci Technol. 2003;106(1–4):20–28. [Google Scholar]
- 35. Jahreis G, Fritsche J, Steinhart H. Conjugated linoleic acid in milk fat: high variation depending on production system. Nutr Res. 1997;17(9):1479–84. [Google Scholar]
- 36. Spain JN, Alvarado MD, Polan CE, Miller CN, McGilliard ML. Effect of protein source and energy on milk composition in midlactation dairy cows. J Dairy Sci. 1990;73(2):445–52. [Google Scholar]
- 37. Asakuma S, Ueda Y, Akiyama F, Uemura Y, Miyaji M, Nakamura M, Murai M, Urashima T. Effect of grazing on the concentrations of total sialic acid and hexose in bovine milk. J Dairy Sci. 2010;93(10):4850–4. [DOI] [PubMed] [Google Scholar]
- 38. Cheng LK, Wang LX, Xu QS, Huang LJ, Zhou DS, Li Z, Li SG, Du YG, Yin H. Chitooligosaccharide supplementation improves the reproductive performance and milk composition of sows. Livestock Science. 2015;174:74–81. [Google Scholar]
- 39. Aguinis H, Gottfredson RK, Joo H. Best-practice recommendations for defining, identifying, and handling outliers. Organizational Research Methods. 2013;16(2):270–301. [Google Scholar]
- 40. Robinson RC, Colet E, Tian T, Poulsen NA, Barile D. An improved method for the purification of milk oligosaccharides by graphitised carbon-solid phase extraction. Int Dairy J. 2018;80:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robinson RC, Poulsen NA, Barile D. Multiplexed bovine milk oligosaccharide analysis with aminoxy tandem mass tags. PLoS One. 2018;13(4):e0196513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mehra R, Barile D, Marotta M, Lebrilla CB, Chu C, German JB. Novel high-molecular weight fucosylated milk oligosaccharides identified in dairy streams. PLoS One. 2014;9(5):e96040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barile D, Marotta M, Chu C, Mehra R, Grimm R, Lebrilla CB, German JB. Neutral and acidic oligosaccharides in Holstein-Friesian colostrum during the first 3 days of lactation measured by high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. J Dairy Sci. 2010;93(9):3940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tao N, DePeters EJ, German JB, Grimm R, Lebrilla CB. Variations in bovine milk oligosaccharides during early and middle lactation stages analyzed by high-performance liquid chromatography-chip/mass spectrometry. J Dairy Sci. 2009;92(7):2991–3001. [DOI] [PubMed] [Google Scholar]
- 45. Tao N, DePeters EJ, Freeman S, German JB, Grimm R, Lebrilla CB. Bovine milk glycome. J Dairy Sci. 2008;91(10):3768–78. [DOI] [PubMed] [Google Scholar]
- 46. Meitei NS, Apte A, Snovida SI, Rogers JC, Saba J. Automating mass spectrometry-based quantitative glycomics using aminoxy tandem mass tag reagents with Simglycan. J Proteomics. 2015;127:211–22. [DOI] [PubMed] [Google Scholar]
- 47. Wei T, Simko V, Levy M, Xie Y, Jin Y, Zemla J, Freidank M, Cai J, Protivinsky T. Corrplot: visualization of a correlation matrix. R package version 0.90. 2021. [Google Scholar]
- 48. Vicaretti SD, Mohtarudin NA, Garner AM, Zandberg WF. Capillary electrophoresis analysis of bovine milk oligosaccharides permits an assessment of the influence of diet and the discovery of nine abundant sulfated analogues. J Agric Food Chem. 2018;66(32):8574–83. [DOI] [PubMed] [Google Scholar]
- 49. Liu Z, Moate P, Cocks B, Rochfort S. Simple liquid chromatography-mass spectrometry method for quantification of major free oligosaccharides in bovine milk. J Agric Food Chem. 2014;62(47):11568–74. [DOI] [PubMed] [Google Scholar]
- 50. Lemay DG, Pollard KS, Martin WF, Freeman-Zadrowski C, Hernandez J, Korf I, German JB, Rijnkels M. From genes to milk: genomic organization and epigenetic regulation of the mammary transcriptome. PLoS One. 2013;8(9):e75030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rijnkels M, Freeman-Zadrowski C, Hernandez J, Potluri V, Wang L, Li W, Lemay DG. Epigenetic modifications unlock the milk protein gene loci during mouse mammary gland development and differentiation. PLoS One. 2013;8(1):e53270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weinborn V, Li Y, Shah IM, Yu H, Dallas DC, German JB, Mills DA, Chen X, Barile D. Production of functional mimics of human milk oligosaccharides by enzymatic glycosylation of bovine milk oligosaccharides. Int Dairy J. 2020;102:104583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fong B, Ma K, McJarrow P. Quantification of bovine milk oligosaccharides using liquid chromatography-selected reaction monitoring-mass spectrometry. J Agric Food Chem. 2011;59(18):9788–95. [DOI] [PubMed] [Google Scholar]
- 54. Martin M-J, Martin-Sosa S, Garcia-Pardo L-A, Hueso P. Distribution of bovine milk sialoglycoconjgates during lactation. J Dairy Sci. 2001;84(5):995–1000. [DOI] [PubMed] [Google Scholar]
- 55. Poulsen NA, Robinson RC, Barile D, Larson LB, Buitenhuis B. A genome-wide association study reveals specific transferases as candidate loci for bovine milk oligosaccharide synthesis. BMC Genomics. 2019;20(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.