Abstract
Clostridium difficile is a Gram-positive bacillus with fecal-oral transmission and is currently one of the most common nosocomial infections worldwide, which was renamed Clostridioides difficile in 2016. Clostridioides difficile infection (CDI) is a prevalent infection in cirrhosis and negatively affects prognosis. This study aimed to provide a concise review with clinical practice implications. The prevalence of CDI in cirrhotic patients increases, while the associated mortality decreases. Multiple groups of risk factors increase the likelihood of CDI in patients with cirrhosis, such as antibiotic use, the severity of cirrhosis, some comorbidities, and demographic aspects. Treatment in the general population is currently described in the latest guidelines. In patients with cirrhosis, rifaximin and lactulose have been shown to reduce CDI risk due to their modulatory effects on the intestinal flora, although conflicting results exist. Fecal microbiota transplantation (FMT) as a treatment for the second or subsequent CDI recurrences has demonstrated a good safety and efficacy in cirrhosis and CDI. Future validation in more prospective studies is needed. Screening of asymptomatic patients appears to be discouraged for the prevention currently, with strict hand hygiene and cleaning of the ward and medical equipment surfaces being the cornerstone of minimizing transmission.
1. Introduction
Clostridium difficile (C. difficile) is a Gram-positive, anaerobic, spore-producing bacillus widespread in the human intestine and the natural environment [1]. In 2016, it was officially renamed Clostridioides difficile [2]. Generally, its spores are transmitted by the fecal-oral route and colonize and proliferate in the large intestine [3]. C. difficile can release two major protein exotoxins (TcdA and TcdB) that induce colitis in susceptible individuals. However, not all colonized populations are symptomatic [3]. Symptoms evolve from colonization to infection, and colonization by toxigenic strains is an independent risk factor for Clostridioides difficile infection (CDI) [4]. C. difficile was first isolated in the stool of a newborn in 1935 [5], and until the 1970s, this group was perceived to be symbiotically related to humans [3, 5]. Following the introduction of antibiotics, the incidence of CDI has escalated and now constitutes one of the most common nosocomial infection pathogens [3]. As per a 2015 United States (US) survey, CDI is the most common healthcare-associated infection in the US, standing at approximately 15% [6]. In a recent extensive systematic review [7], the overall incidence of CDI in European countries varied from the lowest in Spain (2.33 per 10,000 patient days) to the highest in Poland (7.88 per 10,000 patient days). The incidence of CDI overall was 53.5 cases per 100,000 adults in 2019 in a recent epidemiological survey in Hong Kong, China [8]. In an analysis of health system data conducted in the US, CDI hospital management required nearly 2.4 million days of hospital stays in the ten years from 2005 to 2015, which imposed a substantial financial burden on the country [9]. Of note is that community-based CDI is incrementally on the rise, further exacerbating the disease burden associated with CDI [10]. Antibiotic use [11], old age [12], gastric acid inhibitors [13], and hospitalization [14] are the critical risk factors for the development of such infections.
Cirrhosis is the end stage of chronic liver disease and is responsible for a heavy burden of illness and death worldwide. In 2017, cirrhosis caused more than one million deaths [15, 16], making it the eleventh leading cause of death [15]. Data from 2019 showed that cirrhosis contributed to 560.4 age-standardized disability-adjusted life years (DALYs) per 100,000 population (one DALY represents one life-year of full health lost) [17]. Infection is a significant comorbidity in patients with cirrhosis, increasing mortality risk [18–20]. Since risk factors for the development of CDI are also frequently reported in patients with cirrhosis [21], CDI is also a prevalent type of infection in cirrhosis and hurts the prognosis of patients. Cirrhosis with CDI have a worse prognosis and more extended hospital stays than those without CDI [22]. Meanwhile, the incidence of CDI is double that of noncirrhotic patients, and there are more CDI-related complications compared with patients without cirrhosis [23]. Recurrent CDI (R-CDI) disease burden in cirrhotic patients is even more challenging [22]. Fecal microbiota transplantation (FMT) is the recommended treatment for R-CDI [24]. Still, its implementation in patients with cirrhosis is questionable [25, 26] as it raises the possibility of additional adverse events in decompensated cirrhosis [25]. Other relevant therapies such as rifaximin [27] and lactulose [28] have also shown evidence in reducing CDI. Rational understanding of the impact of CDI in cirrhosis and treatment options to improve outcomes and lower the burden of disease on patients is therefore highly regarded.
Given the magnitude of the disease burden posed by CDI in cirrhosis and the controversial nature of some of the issues, therefore, this study aims to provide clinicians with a synthesis of the latest status on the epidemiology, risk factors, prognosis, and therapeutic aspects of CDI in patients with cirrhosis and briefly characterize the impact of cirrhosis in CDI hospitalization. As the pathogenesis in cirrhosis, clinical presentation, and diagnosis of CDI have been well described [28, 29], these sections will not be discussed in this review.
2. Method
The electronic databases PubMed and Embase were retrieved manually to obtain relevant literature. The reference lists in the primary included literature were also checked internally to search for matches. Only publications in the English language were included. There was no restriction on the year of publication for the documents. We excluded studies that primarily involved patients receiving liver transplants, as the profile of CDI in this specific population is somewhat different from that of the general cirrhotic population. Studies that included populations younger than 18 years were excluded. Index terms included “cirrhosis,” “Clostridium difficile,” “Clostridioides difficile,” “Clostridium difficile infection,” “chronic liver disease,” and “infection.” A critical evaluation was carried out for all studies included in this paper.
3. Epidemiology
Several studies have reported the prevalence of CDI in patients with cirrhosis using large nationwide databases. In a study conducted in an extensive commercial database in the US, the prevalence of CDI in cirrhosis was 134.93 per 100,000 of 133,400 patients diagnosed with cirrhosis between 2018–2021 [30]. Nationwide Readmissions Database (NRD) in the US revealed that the prevalence of CDI in patients with cirrhosis was 2.8% from 2011 to 2014, with higher inpatient mortality compared with cellulitis and urinary tract infections (UTI) (17.6% vs. 7.6%, 11.8%), respectively, and the presence of sepsis and organ failure was also most common in CDI [31]. Another study using the National Inpatient Sample (NIS) [32], which investigated trends in CDI hospitalizations for end-stage liver disease (ESLD) from 2005 to 2014, found that the prevalence of CDI among inpatients with decompensated cirrhosis increased approximately twofold from 1.3% in 2005 to 2.7% in 2014, with an annual rate of increase of 7.8%. However, mortality in patients with in-hospital ESLD including CDI decreased notably from 15.4% in 2005 to 11.1% in 2015, a decrease that improved diagnostic and therapeutic approaches can explain. Similar results were observed in several other studies that used NIS in patients with advanced cirrhosis to describe CDI prevalence and mortality [23, 33, 34].
Some local data also provide epidemiological figures for CDI in cirrhosis. In a study from a tertiary hospital in Romania [35], CDI occurred in 7.3% of 231 patients with cirrhosis coexisting with hepatic encephalopathy (HE) (mainly stage 2 or 3) between 2012 and 2014, with an overall CDI incidence of 57.2 cases per 10,000 patient days. In a small prospective study conducted in Romania in 2015, among 200 Child-Pugh B and C patients hospitalized for decompensation, 9% developed CDI during their hospitalization [36]. Another prospective study, also conducted in a Romanian tertiary hospital, included 122 patients with cirrhosis and spontaneous bacterial peritonitis (SBP) who also received norfloxacin as secondary prophylaxis from 2018 to 2019, in which 18.8% of the population presented with CDI (median follow-up of 7 months) [37]. In a further over six years study, CDI incidence was 11.8% in 388 cirrhotic patients, and notably, 30.8% of the cirrhotic patients received the antibiotic rifaximin to prevent HE [38]. In a study of patients with variceal bleeding, also conducted in Romania, the incidence of CDI was 6.8% between 2017 and 2019 [39]. Finally, in another hospital in China, the Infectious Diseases Department reported 26 cases of CDI in 526 cirrhotic inpatients over six months in 2015 (4.9%) [4].
The incidence of R-CDI in patients with cirrhosis was studied in a cohort study conducted at Indiana University Hospital from 2012 to 2016, with an 11.9% incidence of R-CDI among those hospitalized with CDI in patients with cirrhosis [21].
The prevalence of cirrhosis among 366,283 inpatients with CDI between 2011 and 2014 was 3.4%, according to the survey conducted in the NRD [40]. Of these cirrhotic patients, 63.1% had decompensated cirrhosis. Another two studies using NIS yielded a 3.97% and 4.18% prevalence of cirrhosis in 2012–2015 and 2016-2017, respectively [41, 42]. A further study implemented in a US health system diagnosed cirrhosis in 9.13% of 526 CDI inpatients from 2014 to 2017 [43]. However, an additional 2011 study based on long-term care facilities (LTCFs) showed only 326 (0.72%) cirrhotic patients out of 45,500 CDI admissions [44]. This is presumably explained by the fact that the number of CDI admissions rather than the specific number of people was considered (some patients had readmissions), and the database only included individuals ≥65 years (median age 82 years), which resulted in a significantly higher prevalence of CDI.
In summary, these nationwide population studies in the US demonstrate an overall increasing trend in CDI prevalence in patients with cirrhosis, in contrast to decreasing associated mortality. CDI incidence in local hospitals reported in the literature is even higher. The incidence of R-CDI in cirrhosis is not uncommon. Cirrhosis accounts for approximately 3-4% of CDI hospitalizations in nationwide studies. However, data from other parts of the world are still lacking (Table 1).
Table 1.
Epidemiological profile related to CDI and cirrhosis. Abbreviations: CDI, Clostridioides difficile infection; US, United States; SNOMED–CT, systematized nomenclature of medicine clinical terms; NRD, nationwide readmissions database; NIS, national inpatient sample; ICD, international classification of diseases; CLD, chronic liver disease; HE, hepatic encephalopathy; EIA, enzyme immunoassay; SBP, spontaneous bacterial peritonitis; GDH, glutamate dehydrogenase; PCR, polymerase chain reaction; LTCFs, long-term care facilities.
| Reference | Country | Study type | Study duration | Patient cohort | Database | CDI diagnostic methods | Epidemiology |
|---|---|---|---|---|---|---|---|
| CDI in cirrhosis | |||||||
| [30] | US | Retrospective | 2018–2021 | 133,400 patients with cirrhosis | Explorys | SNOMED-CT | Prevalence: 134.93 per 100,000 |
| [31] | US | Retrospective | 2011–2014 | 1,798,830 patients with cirrhosis | NRD | ICD-9 | Prevalence: 2.8% |
| [32] | US | Retrospective | 2005–2014 | 590,980 patients with decompensated cirrhosis | NIS | ICD-9 | Prevalence: from 1.3% in 2005 to 2.7% in 2014; in-hospital mortality: from 15.4% in 2005 to 11.1% in 2014 |
| [33] | US | Retrospective | 1998–2014 | 3, 049, 696 patients with advanced cirrhosis | NIS | ICD-9 | Prevalence: from 0.8% in 1998 to 2.6% in 2014; in-hospital mortality: from 20.7% in 1998 to 11.3% in 2014 |
| [34] | US | Retrospective | 1998–2007 | 742, 391 patients with cirrhosis | NIS | ICD-9 | Prevalence: from 0.7% in 1998 to 1.6% in 2007; in-hospital mortality: from 13.4% in 1998 to 12.3% in 2007 |
| [23] | US | Retrospective | 2009 | 114,108 patients with CLD | NIS | ICD-9 | Incidence: 189.4/10,000 |
| [35] | Romania | Retrospective | 2012–2014 | 231 patients with cirrhosis and HE | Tertiary hospital | EIA | Incidence: 7.3% |
| [36] | Romania | Prospective | 2015 | 200 patients with decompensated cirrhosis | Tertiary hospital | EIA | Incidence: 9% |
| [37] | Romania | Prospective | 2018–2019 | 122 patients with cirrhosis and SBP receiving secondary prophylaxis with norfloxacin | Tertiary hospital | EIA | Incidence: 18.8% |
| [38] | Spain | Retrospective | 2009–2014 | 367 patients with cirrhosis and 30.8% received rifaximin | Tertiary hospital | Rapid detection test | Incidence: 11.8% |
| [39] | Romania | Retrospective | 2017–2019 | 367 patients with cirrhosis and variceal bleeding | Tertiary hospital | EIA | Incidence: 6.8% |
| [4] | China | Retrospective | 2015 | 526 patients with cirrhosis | Tertiary hospital | EIA | Incidence: 4.9% |
|
| |||||||
| R-CDI in cirrhosis | |||||||
| [21] | US | Retrospective | 2012–2016 | 257 patients with cirrhosis and CDI | Tertiary hospital | EIA | Incidence: 11.9% |
|
| |||||||
| Cirrhosis in CDI | |||||||
| [40] | US | Retrospective | 2011–2014 | 366,283 CDI inpatients | NRD | ICD-9 | Prevalence: 3.4% |
| [41] | US | Retrospective | 2012–2015 | 1,327,595 CDI inpatients | NIS | ICD-9 | Prevalence: 3.97% |
| [42] | US | Retrospective | 2016–2017 | 196,945 CDI inpatients | NIS | ICD-9 | Prevalence: 4.18% |
| [43] | US | Retrospective | 2014–2017 | 526 CDI inpatients | Tertiary hospital | GDH and PCR | Prevalence: 9.13% |
| [44] | US | Retrospective | 2011 | 45,500 CDI inpatients | LTCFs | ICD-9 | Prevalence: 0.72% |
4. Risk Factors
The risk factors for the development and progression of cirrhosis have been well established. Age >65 years, multiple hospitalizations, inpatient stays >20 days, hypoproteinemia, Clostridioides difficile colonization (CDC), HE, antibiotic, and proton pump inhibitors (PPIs) use were found to be associated with the development of CDI in a study conducted to identify risk factors for CDI in patients with cirrhosis [45]. Furthermore, many studies have also reported risk factors for CDI development [22, 23, 30, 32, 35, 37, 39, 46–49] although heterogeneity exists between studies. The risk factors concluded from these studies are largely in line with the previous research and can be broadly classified into several categories, namely medications (PPIs, antibiotics, etc.), severity and etiology of cirrhosis (Child-Pugh grade, Charlson index, etc.), presence of complications (HE, hypoproteinemia/malnutrition, infections, hepatorenal syndrome, ascites, etc.), hospitalizations (multiple hospitalizations, extended hospital stays, etc.), demographic characteristics of the patients (advanced age, female, ethnicity), and CDC. Several issues require further clarification in this regard. Firstly, studies have shown females to be more prone to CDI [50]. This was confirmed in a couple of studies on cirrhosis patients [22, 30, 32, 38, 47]. Experimental and human studies have demonstrated differences in the gut microbiome concerning gender, and such effects are mediated by sex hormone levels [51, 52]. However, studies on the sex differences in C. difficile abundance have not yet emerged. Secondly, etiological variants in cirrhosis may also be a risk factor for CDI. Nonalcoholic fatty liver disease (NAFLD) is associated with an increased risk of CDI [53] although no studies have shown that this etiology increases CDI risk in cirrhosis. A few studies suggest that alcoholic etiology is a risk factor for CDI [37, 47]. Lastly, medication use such as rifaximin and PPIs shows conflicting results in this context. Several studies have shown rifaximin to be protective and therapeutic (as mentioned later) [27, 54–56]. PPIs are risk factors for CDI in many studies, but PPIs were not shown to cause CDI in an evidence-based review, although there may be an increased risk of infectious diarrhea [57]. In other words, no substantial evidence is available for a causative relationship for PPIs on CDI in the general population and the cirrhotic population although an increased risk is identified. The impact of these agents on CDI in cirrhosis needs to be further supported in high-quality studies. A study showed risk factors for R-CDI in cirrhosis, including Charlson Comorbidity Index and lactulose use, which is aligned with the risk factors for CDI [21] (Figure 1).
Figure 1.
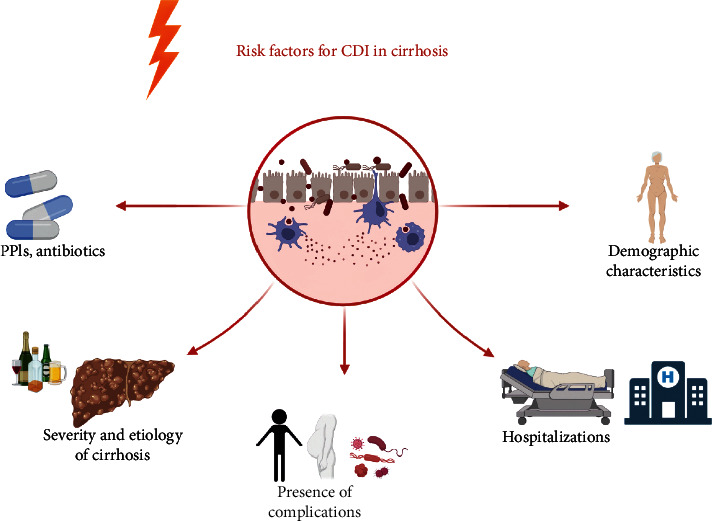
Risk factors for CDI development in patients with cirrhosis. The risk factors for the development of CDI in patients with cirrhosis have been described in different studies. In general, they can be divided into several categories: namely medications (PPIs, antibiotics, etc.), severity and etiology of cirrhosis (Child-Pugh grade, Charlson index, alcoholic etiology, etc.), presence of complications (HE, hypoproteinemia/malnutrition, infections, hepatorenal syndrome, ascites, etc.), hospitalizations (multiple hospitalizations, extended hospital stays, ICU admissions), demographic characteristics (advanced age, female, ethnicity) and CDC. Abbreviations: CDI, Clostridioides difficile infection; PPIs, proton pump inhibitors; HE, hepatic encephalopathy; ICU, intensive care unit; CDC, Clostridioides difficile colonization.
Overall, the risk factors for the development of CDI in cirrhosis fall into several broad categories, that is, certain established drug exposures, progression of cirrhosis and specific etiology, presence of complications, hospitalization, patient demographic characteristics, and CDC, of which several still warrant further exploration. Being aware of the predisposing factors for the occurrence of CDI in patients with cirrhosis has positive implications for timely insight and subsequent prevention and treatment by clinicians.
5. Prognosis
Increased mortality and comorbidity are associated with infection in cirrhosis. Given the dramatic advances in healthcare management, in-hospital mortality in cirrhotic patients has declined [58], and mortality in CDI patients has also been dropping annually. However, its associated mortality and burden of complications remain significantly overwhelming compared with other populations, increasing mortality by about 50% in patients with cirrhosis and CDI versus those without CDI [33]. Therefore, understanding and predicting the prognosis of this population is essential to mitigate the risk of undesirable outcomes. Extensive publications have reported increased mortality of CDI in patients with cirrhosis [22, 23, 31, 33, 34, 36, 39, 44, 47, 48, 59]. However, one study has not established the impact of CDI development on mortality in patients with cirrhosis and SBP receiving norfloxacin as secondary prophylaxis [37]. Norfloxacin has been shown in in vitro studies as a quinolone to down-regulate inflammation, which may be a protective effect [60, 61]. Yet another study indicated that CDI was associated with increased 30-day mortality but not with increased overall mortality [48]. Alongside increased mortality, CDI could potentially carry an additional risk of complications, including sepsis [31], organ failure [31], portal vein thrombosis [62], and readmission [21]. Caution should be taken, as readmission is associated with increased severity of cirrhosis and mortality [21, 40]. Studies on the outcomes of CDI in cirrhosis are summarized in Table 2.
Table 2.
Studies reporting the effect size of the outcomes of CDI in cirrhosis. Abbreviations: CDI, Clostridioides difficile infection; US, United States; NIS, national inpatient sample; 95%CI, 95% confidence interval; OR, odds ratio; aOR, adjusted odds ratio; NA, not available; HE, hepatic encephalopathy; SBP, spontaneous bacterial peritonitis; LTCFs, long-term care facilities.
| Reference | Study period | Country | Database | Outcome metrics | Effect size (95%CI) | Adjustment factors |
|---|---|---|---|---|---|---|
| [22] | 2015 | US | NIS | Mortality | aOR: 1.55 (1.29–1.85) | Hospital location, teaching status, insurance status, complications of cirrhosis and infections |
| [23] | 2009 | US | NIS | Mortality | aOR: 2.29 (1.90–2.76) | Demographic (age in decade-long intervals, gender, race) and socioeconomic characteristics (primary payer and income level) |
| [31] | 2011–2014 | US | NRD | Mortality; sepsis; any organ failure; 2+ organ failures; 30-day readmission | OR:2.00 (1.91–2.28); 3.99 (3.86–4.12); 3.00 (2.90–3.11); 3.25 (3.12–3.39); 1.01 (0.95–1.06), respectively | NA |
| [33] | 1998–2014 | US | NIS | Mortality | aOR: 1.47 (1.40–1.56) | Age >65, gender, HE, SBP, variceal bleed, presence of ascites, and Elixhauser comorbidity index |
| [44] | 2011 | US | LTCFs | Mortality | aOR: 1.27 (1.24–1.30) | NA |
The impact of cirrhosis on inpatients with CDI has been addressed in several studies. In a retrospective study using the NIS database between 2012 and 2015, the presence of cirrhosis in CDI admissions was associated with increased mortality, with an adjusted hazard ratio (aOR) of 1.65 and a 95% confidence interval (CI) of 1.53–1.77 [41]. Another study revealed similar results using the NRD between 2011 and 2014 [40]. A further study conducted in 526 CDI admissions found a significantly higher mortality among the cirrhotic population than the noncirrhotic group (39.6% vs. 14.6%, p=0.001) [43]. An association was also established with the presence of cirrhosis and 30-day readmission for CDI [63]. Nonetheless, a study using data from the NIS during 2016-2017 found that cirrhosis was not associated with increased all-cause mortality (aOR 1.31, 95% CI 0.89–1.93) [42]; this may represent a change that has evolved in more recent years.
CDI is an independent predictor of mortality in patients with cirrhosis [32, 33, 64]. Predicting mortality in patients with cirrhosis and CDI for targeted intervention is thus crucial. The model for end-stage liver disease (MELD) was identified as the only predictor of 30-day mortality in one study [59]. A second study suggested that hypoalbuminemia (albumin <3 g/dL) and intensive care unit (ICU) admission were independent predictors of short-term mortality [48]. A consideration of the discrepancy may arise from differences in the measured outcomes in the two studies. The outcomes in the study that yielded MELD as the sole predictor were 30-day mortality, 30-day colectomy, any requirement for ICU admission, and R-CDI within 90 days, whereas in the latter, the primary outcomes were 30-day mortality and overall mortality. ICU admission was adopted as an outcome instead of a prognostic indicator in the first study. The further point is that the latter excluded the MELD score in the multivariate analysis. Therefore, the inclusion of MELD in the multivariate analysis allowed for consideration of the severity of cirrhosis, detracting from the prognostic value of hypoalbuminemia [59]. To conclude, in a broad sense, both suggest that the severity of cirrhosis is a predictor of death among the CDI population in cirrhosis.
6. Treatment
6.1. General Considerations
Recently, two American guidelines have described the treatment options for CDI in the general population [65, 66]. For the initial episode of nonsevere CDI, oral vancomycin 125 mg 4 times daily for ten days or oral fidaxomicin 200 mg twice daily for ten days is recommended [65]. In contrast, fidaxomicin is superior to vancomycin for the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) guidelines [66]. Oral metronidazole 500 mg 3 times daily for ten days may be an alternative selection if the above two first-line agents are not available or in a low-risk CDI population. However, initial therapy for severe CDI remains with two first-line drugs at the same dose and for the same duration. As initial treatment for fulminant CDI, oral vancomycin 500 mg 4 times daily in combination with parenteral metronidazole 500 mg every 8 hours is recommended. An additional vancomycin enema of 500 mg can be administered every 6 hours if ileus is present. Sufficient capacity must also be available for resuscitation [65]. For the first recurrence of CDI, a tapering/pulsed dose of vancomycin is recommended (if the standard regimen was used for the initial episode). If the initial treatment is given with metronidazole or vancomycin, fidaxomicin is recommended [65]. In the IDSA and SHEA guidelines, fidaxomicin is preferred to vancomycin, and bezlotoxumab (a human monoclonal antibody against C. difficile toxin B) 10 mg/kg given intravenously is also recommended as adjunctive therapy to antibiotic therapy [66]. The notable difference in the two guidelines for the second or subsequent recurrence of CDI is that the American College of Gastroenterology (ACG) guidelines recommend FMT for this population [65]. In contrast, the IDSA and SHEA guidelines suggest that FMT be performed after at least two recurrences treated with antibiotics [66] (Table 3).
Table 3.
Recommendations of the latest two American guidelines on the therapeutic aspects of CDI. Abbreviations: ACG, American college of gastroenterology; IDSA, infectious diseases society of America; SHEA, society for healthcare epidemiology of America; CDI, Clostridioides difficile infection; NA, not available; FMT, fecal microbiota transplantation; SOC, standard of care.
| Clinical definition | ACG guideline [65] | IDSA and SHEA guideline [66] | ||
|---|---|---|---|---|
| Recommendations | Strength of recommendation, quality of evidence | Recommendations | Strength of recommendation, quality of evidence | |
| Initial episode of nonsevere CDI | Oral vancomycin 125 mg 4 times daily for ten days; oral fidaxomicin 200 mg twice daily for ten days; oral metronidazole 500 mg 3 times daily for ten days in low-risk patients | Strong recommendation, low quality of evidence; strong recommendation, moderate quality of evidence; strong recommendation, moderate quality of evidence, respectively | Preferred: Fidaxomicin 200 mg given twice daily for ten days; Alternative: Vancomycin 125 mg given four times daily by mouth for ten days; if above agents are unavailable: Metronidazole, 500 mg 3 times daily by mouth for 10–14 days | Conditional recommendation, moderate certainty of evidence |
| Initial episode of severe CDI | Vancomycin 125 mg 4 times a day for ten days; fidaxomicin 200 mg twice daily for ten days | Strong recommendation, low quality of evidence; conditional recommendation, very low quality of evidence, respectively | Preferred: Fidaxomicin 200 mg given twice daily for ten days; Alternative: Vancomycin 125 mg given four times daily by mouth for ten days | Conditional recommendation, moderate certainty of evidence |
| Fulminant CDI | Adequate volume resuscitation and 500 mg of oral vancomycin every 6 hours daily for the first 48–72 hours; combination therapy with parenteral metronidazole 500 mg every 8 hours; addition of vancomycin enemas 500 mg every 6 hours if ileus; FMT for severe and fulminant CDI refractory to antibiotic therapy | Strong recommendation, very low quality of evidence; conditional recommendation, very low quality of evidence; conditional recommendation, very low quality of evidence; strong recommendation, low quality of evidence, respectively | Vancomycin 500 mg 4 times daily by mouth or by nasogastric tube and intravenously administered metronidazole 500 mg every 8 hours; rectal instillation of vancomycin if ileus | NA |
| First CDI recurrence | Tapering/pulsed dose vancomycin for a first recurrence after an initial course of fidaxomicin, vancomycin, or metronidazole; fidaxomicin for a first recurrence after an initial course of vancomycin or metronidazole | Strong recommendation, very low quality of evidence; conditional recommendation, moderate quality of evidence, respectively | Preferred: Fidaxomicin 200 mg given twice daily for ten days or twice daily for five days followed by once every other day for 20 days; Alternative: Vancomycin by mouth in a tapered and pulsed regimen or 125 mg given four times daily for ten days; Adjunctive treatment: Bezlotoxumab 10 mg/kg given intravenously once during the administration of SOC antibiotics | Conditional recommendation, low certainty evidencea |
| Second or subsequent CDI recurrence | FMT delivered through colonoscopy or capsules; by enema, if other methods are unavailable; repeat FMT for a recurrence of CDI within eight weeks of an initial FMT; suppressive oral vancomycin for not candidates for FMT, relapsed after FMT, or require ongoing or frequent courses of antibiotics | Strong recommendation, moderate quality of evidence; conditional recommendation, low quality of evidence; conditional recommendation, very low quality of evidence; conditional recommendation, very low quality of evidence, respectively | Fidaxomicin 200 mg given twice daily for ten days or twice daily for five days followed by once every other day for 20 days; vancomycin by mouth in a tapered and pulsed regimen or 125 mg 4 times daily for ten days followed by rifaximin 400 mg 3 times daily for 20 days; FMT after antibiotic treatments for at least two recurrences; Adjunctive treatment: Bezlotoxumab 10 mg/kg given intravenously once during the administration of SOC antibiotics | Conditional recommendation, very low certainty of evidenceb |
afidaxomicin rather than vancomycin. bbezlotoxumab as a co-intervention along with SOC antibiotics rather than SOC antibiotics alone.
In addition, the latest guideline from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) [67] is also available. In the ESCMID guideline, the standard of care (SOC) for initial CDI is fidaxomicin 200 mg twice daily for ten days or vancomycin 125 mg 4 times daily for ten days, and for those at high risk of recurrence, fidaxomicin or SOC plus bezlotoxumab is considered. If the preferred option is not available, metronidazole 500 mg 3 times daily for ten days is recommended. For the first recurrence, SOC plus bezlotoxumab or fidaxomicin is recommended, while FMT or SOC plus bezlotoxumab is recommended for a second recurrence, and if it fails, vancomycin is used by tapering and pulsed. The guideline refers to severe CDI as a separate clinical type, regardless of the number of previous episodes.
6.2. Rifaximin
Rifaximin, a derivative of rifamycin, is poorly absorbed in the gut and is currently prescribed as a therapeutic agent for recurrent HE and exerts its antibacterial activity by inhibiting RNA synthesis in bacteria [68, 69]. Rifaximin is a therapeutic for CDI [70–72]. In the latest guideline [66], rifaximin 400 mg 3 times daily for 20 days as continuation therapy to vancomycin can be offered as a treatment for the second or subsequent recurrences of CDI. There are some conflicting effects of rifaximin on CDI in cirrhosis. A few studies suggest that rifaximin is a risk factor for developing CDI in patients with cirrhosis [47, 49]. A few reasons may explain in these studies that rifaximin may have increased CDI risk in patients with cirrhosis. First, in the survey by Bajaj et al. [47], the risk of nosocomial infection was increased in a regression model including rifaximin use, but the model was not robust enough, and rifaximin was used as a surrogate for HE as a variable in this study, and HE is a known risk factor for the development of CDI. Secondly, in another Spanish study including 46 patients with cirrhosis and CDI, 34.1% were rifampin-resistant strains, and 84.6% were in patients who had previously received rifaximin [38]. This is in line with the study also conducted in Spain that reported an increased risk of CDI due to rifaximin, which also reported a high incidence of rifaximin-resistant strains [49]. Rifaximin-resistant strains were significantly more often female, had a higher incidence of HE and portal hypertension, and were more frequently treated with rifaximin or rifamycin [38], which may contribute to the increased incidence of CDI. Apart from these local data, rifaximin was shown to reduce CDI development while treating HE [27, 59, 73], and rifaximin also showed no increase in rifaximin-resistant strains during the treatment of HE in a systematic review and meta-analysis [74]. Thus, alongside HE treatment, rifaximin has shown a more positive effect on CDI in cirrhosis, and yet further prospective studies are needed.
6.3. Lactulose
Lactulose is a nondigestible oligosaccharide frequently combined with rifaximin as prevention for HE [75]. It promotes the growth of indigenous host microorganisms as a prebiotic and enhances colonization resistance to CDI [76, 77]. Similarly, lactulose can be used as a substitute for HE or the severity of cirrhosis and is, therefore, a risk factor for developing CDI [47] and R-CDI [21] in some studies. A case-control study revealed a significantly lower incidence of CDI with the combination of lactulose and rifaximin compared with lactulose alone (12.5% vs. 27.9%, p=0.02). A nested controlled study confirmed the positive effect of lactulose on CDI [78], including 112 patients with decompensated cirrhosis and incident CDI and 928 matched controls, and lactulose significantly reduced the incidence of CDI after excluding patients who received rifaximin (aOR 0.52, 95% CI 0.31–0.89, p=0.02). Controversy remains regarding the use of lactulose in patients with cirrhosis to reduce CDI risk concurrently, and prospective studies are awaited to elucidate the issue further.
6.4. Fecal Microbiota Transplantation
FMT has demonstrated superior efficacy in recurrent CDI as solid evidence of the role of microbiota in the diseases [79]. Since FMT was first recommended in guidelines in 2013 as a treatment for the third recurrence of CDI [80], it has been officially endorsed for its role in the treatment of recurrent CDI and the latest guidelines [65, 66], and as mentioned above, FMT is recommended as the treatment for second or further recurrences of CDI. However, the administration of FMT in patients with cirrhosis and CDI has not been much specified. In an FMT Working Group review in 2011 [25], decompensated cirrhosis and other forms of severe immunodeficiency were regarded as conditions that would lead to increased risk of adverse events with FMT and were not recommended for implementation. Recently, however, the use of FMT has appeared to gain more clarity regarding its safety and efficacy in patients with cirrhosis and even decompensated cirrhosis. The trial of FMT outcomes in recurrent HE demonstrated a favorable effect on hospitalization, cognitive improvement, and dysbiosis in patients with cirrhosis [81]. Similar findings were obtained for long-term FMT with a high safety profile [82]. Based on these encouraging results, positive effects were noted in patients with cirrhosis and recurrent CDI. A retrospective study included 63 patients with cirrhosis (median MELD, 14.5; 24 patients with decompensated cirrhosis) undergoing FMT in multiple centers from 2012–2018, yielding a final FMT success of 85.7%, with adverse events (AEs) and serious adverse events (SAEs) occurring in 21 and 5 patients, respectively [26]. The AEs that may be linked to FMT consisted of abdominal pain/cramping and diarrhea. The occurrence of SAEs was rare, and the five cases included hospitalization associated with a Crohn's disease flare, fecal urgency, dehydration due to acute kidney injury, and cirrhotic decompensation possibly involved with FMT. Efficacy and safety of FMT in patients with cirrhosis and CDI were demonstrated, notwithstanding adverse events. Although FMT has shown a positive effect on CDI in the cirrhotic population, more well-designed studies are warranted for closer validation, and meticulous follow-up is still essential to systematically monitor the emergence of complications in clinical practice [83].
6.5. Summary
CDI treatment in the cirrhotic population currently has general considerations and some specific possible alternatives. Recently, two American guidelines, a European guideline, and a Taiwanese guideline have recommended CDI treatment, and there are discrepancies between these guidelines. A potential therapeutic effect on the reduced incidence of CDI has been shown in several studies by the two agents for HE prevention, rifaximin and lactulose. However, controversial results remain, and more large sample studies are needed to demonstrate the issue in the future. FMT has shown promising safety and efficacy in patients with cirrhosis and CDI.
7. Prevention
Prevention of the development of CDI in patients with cirrhosis necessitates several interventions. The first is the introduction of potentially appropriate screening strategies, and the second is minimizing identified and controllable risk factors. Finally, emphasis should be placed on hand hygiene and the decontamination of medical equipment and wards.
Saab and colleagues present two strategies for screening and treating CDI [84]. The first strategy involved screening all cirrhotic patients and treating those who were positive instead of treating only individuals with symptomatic CDI without screening. A Markov model was developed to compare the respective healthcare costs and patient outcomes between the proposed strategies. Screening for CDI in all populations showed a 3.54-fold reduction in associated medical costs and lower mortality among patients with symptomatic CDI. This study demonstrated that screening and treating asymptomatic patients were cost-effective and prevented more complications than not screening. However, this contradicted the available clinical guidelines [65]. The guidelines recommended only testing for C. difficile in the diarrheal stools and discouraged treatment of C. difficile carriers. Additional concerns from other authors have prompted discussions on CDI screening [36, 85]. Zacharioudakis et al. provided a systematic review and meta-analysis of the prevalence of toxicogenic CDC and the risks of infection in hospitalized populations, and the prevalence of colonization in the asymptomatic people was found to be 8.1%, with a significantly higher risk of developing CDI (21.8% vs. 3.4%) [86]. However, only 154 patients (1.8%) were screened for CDI in this analysis, including 8725 inpatients [85]. As most patients screened would not progress to CDI, it might seem impractical to screen asymptomatic populations. Furthermore, asymptomatic patients are a source of C. difficile transmission in the general population. In cirrhosis, the risk of transmission should be increased due to impaired immunity and complications. Given the widespread availability of disinfection measures today, e.g., hand washing, however, the potential for transmission between these asymptomatic patients would be limited [85]. A separate study found that CDI developed after antibiotic therapy in 200 patients with cirrhosis and identified multiple antibiotic therapies as the only independent risk factor. Therefore, Pop et al. indicated that screening for CDI in the asymptomatic population should only be implemented if the cirrhotic population is at high risk for CDI [36].
Several studies have demonstrated that screening asymptomatic hospitalized populations can reduce the incidence of nosocomial CDI and may be recommended for clinical practice [87–91]. Nevertheless, the models in these studies were established in the general hospitalizations with no further evidence of generalization in the distinct subpopulation of cirrhosis. More research is needed to support CDI screening in an asymptomatic patient with cirrhosis. Testing of symptomatic patients in the cirrhotic population should currently be mandatory.
Infection control-based approaches (antibiotic stewardship, improved hygiene concepts to reduce transmission within the ward) remain the cornerstone of the prevention of hospitalized CDI patients. Strict disinfection routines, including cleaning stethoscopes and other medical equipment, and thorough sterilization of wards to eliminate possible residual spores on surfaces, are fundamental to prevent transmission [85]. These basic precautions are even further emphasized in patients with cirrhosis. The prevention and treatment of hypoalbuminemia are of clinical relevance in preventing infections [92], notably CDI in patients with cirrhosis [93], and serum levels of the effective albumin may be more significant [94]. Antibiotics and PPIs administered for complications are frequently used in patients with cirrhosis; the judicious use of broad-spectrum antibiotics and PPIs is probably supportive in preventing CDI.
8. Conclusion
Currently known as one of the most common nosocomial infections, CDI is as well a common type of infection in the cirrhotic population. Nationwide databases have shown that the prevalence of CDI in cirrhosis has been on the rise in recent years, while the associated mortality has been falling. Large databases indicate that cirrhosis comprises approximately 3-4% of CDI admissions, with local hospital data varying considerably. CDI imposes a heavy economic burden on cirrhosis, carrying higher mortality and the development of complications. The severity of cirrhosis may be a predictor of death from CDI. Numerous factors may contribute to the susceptibility of individuals with cirrhosis to CDI, such as antibiotics and PPIs and severity and complications of cirrhosis and hospitalization. Appropriate prevention and treatment are crucial to reduce the disease burden of CDI in the cirrhotic population. The treatment of CDI is specified in the latest guidelines. In the setting of cirrhosis, agents such as rifaximin and lactulose used to prevent recurrent HE have shown controversial results. FMT as a preferred option for the treatment of second or subsequent recurrent CDI has also demonstrated a promising safety and efficacy profile in cirrhosis and CDI, but careful follow-up is still necessary. Screening of asymptomatic populations currently appears not to be recommended, but thorough disinfection of wards and medical equipment remains the cornerstone of preventing CDI transmission.
Data Availability
All data are presented in the article, and no additional data are available.
Conflicts of Interest
The authors declare no conflicts of interest in this study.
Authors' Contributions
Y.L. proposed the idea for the article, carried out the literature search, wrote the manuscript, and prepared the illustrations and tables. M.C. revised the manuscript as the corresponding author and provided comments. All authors read and approved the final version of the manuscript.
References
- 1.Smits W. K., Lyras D., Lacy D. B., Wilcox M. H., Kuijper E. J. Clostridium difficile infection. Nature Reviews Disease Primers . 2016;2(1) doi: 10.1038/nrdp.2016.20.16020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawson P. A., Citron D. M., Tyrrell K. L., Finegold S. M. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe . 2016;40:95–99. doi: 10.1016/j.anaerobe.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Leffler D. A., Lamont J. T. Clostridium difficile infection. New England Journal of Medicine . 2015;372(16):1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y., Gu H., Lv T., et al. Longitudinal investigation of carriage rates and genotypes of toxigenic Clostridium difficilein hepatic cirrhosis patients. Epidemiology and Infection . 2019;147:p. e166. doi: 10.1017/s0950268819000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czepiel J., Dróżdż M., Pituch H., et al. Clostridium difficile infection: review. European Journal of Clinical Microbiology & Infectious Diseases . 2019;38(7):1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magill S. S., O’Leary E., Janelle S. J., et al. Changes in prevalence of health care-associated infections in U.S. Hospitals. New England Journal of Medicine . 2018;379(18):1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finn E., Andersson F. L., Madin-Warburton M. Burden of Clostridioides difficile infection (CDI)—a systematic review of the epidemiology of primary and recurrent CDI. BMC Infectious Diseases . 2021;21(1):p. 456. doi: 10.1186/s12879-021-06147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo C. L. T., Kwong T. N. Y., Mak J. W. Y., et al. Trends in incidence and clinical outcomes of Clostridioides difficile infection, Hong Kong. Emerging Infectious Diseases . 2021;27(12):3036–3044. doi: 10.3201/eid2712.203769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S., Palazuelos-Munoz S., Balsells E. M., Nair H., Chit A., Kyaw M. H. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infectious Diseases . 2016;16(1):p. 447. doi: 10.1186/s12879-016-1786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna S., Pardi D. S., Aronson S. L., et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. American Journal of Gastroenterology . 2012;107(1):89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullish B. H., Williams H. R. Clostridium difficile infection and antibiotic-associated diarrhoea. Clinical Medicine . 2018;18(3):237–241. doi: 10.7861/clinmedicine.18-3-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asempa T., Nicolau D. Clostridium difficile infection in the elderly: an update on management. Clinical Interventions in Aging . 2017;12:1799–1809. doi: 10.2147/cia.S149089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok C. S., Arthur A. K., Anibueze C. I., Singh S., Cavallazzi R., Loke Y. K. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. American Journal of Gastroenterology . 2012;107(7):1011–1019. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 14.Lang V., Gunka K., Ortlepp J. R., Zimmermann O., Groß U. Risk factors of patients with diarrhea for having Clostridioides (Clostridium) difficile infection. Frontiers in Microbiology . 2022;13 doi: 10.3389/fmicb.2022.840846.840846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asrani S. K., Devarbhavi H., Eaton J., Kamath P. S. Burden of liver diseases in the world. Journal of Hepatology . 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Sepanlou S. G., Safiri S., Bisignano C., et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet Gastroenterology & Hepatology . 2020;5(3):245–266. doi: 10.1016/s2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jepsen P., Younossi Z. M. The global burden of cirrhosis: a review of disability-adjusted life-years lost and unmet needs. Journal of Hepatology . 2021;75(Suppl 1):S3–S13. doi: 10.1016/j.jhep.2020.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj J. S., Kamath P. S., Reddy K. R. The evolving challenge of infections in cirrhosis. New England Journal of Medicine . 2021;384(24):2317–2330. doi: 10.1056/NEJMra2021808. [DOI] [PubMed] [Google Scholar]
- 19.Van der Merwe S., Chokshi S., Bernsmeier C., Albillos A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. Journal of Hepatology . 2021;75(Suppl 1):S82–s100. doi: 10.1016/j.jhep.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Trebicka J., Fernandez J., Papp M., et al. Predict identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. Journal of Hepatology . 2021;74(5):1097–1108. doi: 10.1016/j.jhep.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Phatharacharukul P., Purpura R. D., Gandhi D., et al. Incidence and risk factors of recurrent Clostridioides difficile infection in patients with cirrhosis. Clinical and Translational Gastroenterology . 2020;11(7) doi: 10.14309/ctg.0000000000000189.e00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj J. S., Ananthakrishnan A. N., Hafeezullah M., et al. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: a national and tertiary center perspective. American Journal of Gastroenterology . 2010;105(1):106–113. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 23.Dotson K. M., Aitken S. L., Sofjan A. K., Shah D. N., Aparasu R. R., Garey K. W. Outcomes associated with Clostridium difficile infection in patients with chronic liver disease. Epidemiology and Infection . 2018;146(9):1101–1105. doi: 10.1017/s0950268818001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shane A. L., Mody R. K., Crump J. A., et al. 2017 infectious diseases society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clinical Infectious Diseases . 2017;65(12):1963–1973. doi: 10.1093/cid/cix959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakken J. S., Borody T., Brandt L. J., et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clinical Gastroenterology and Hepatology . 2011;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y.-W., Alhaffar D., Saha S., et al. Fecal microbiota transplantation is safe and effective in patients with Clostridioides difficile infection and cirrhosis. Clinical Gastroenterology and Hepatology . 2021;19(8):1627–1634. doi: 10.1016/j.cgh.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feuerstadt P., Hong S. J., Brandt L. J. Chronic rifaximin use in cirrhotic patients is associated with decreased rate of C. difficile infection. Digestive Diseases and Sciences . 2020;65(2):632–638. doi: 10.1007/s10620-019-05804-2. [DOI] [PubMed] [Google Scholar]
- 28.Trifan A., Stoica O., Stanciu C., et al. Clostridium difficile infection in patients with liver disease: a review. European Journal of Clinical Microbiology & Infectious Diseases . 2015;34(12):2313–2324. doi: 10.1007/s10096-015-2501-z. [DOI] [PubMed] [Google Scholar]
- 29.Musa S., Moran C., Rahman T. Clostridium difficile infection and liver disease. Journal of Gastrointestinal and Liver Diseases: JGLD . 2010;19(3):303–310. [PubMed] [Google Scholar]
- 30.Sahra S., Abureesh M., Amarnath S., et al. Clostridioides difficile infection in liver cirrhosis patients: a population-based study in United States. World Journal of Hepatology . 2021;13(8):926–938. doi: 10.4254/wjh.v13.i8.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atteberry P., Biederman B., Jesudian A., et al. Mortality, sepsis, and organ failure in hospitalized patients with cirrhosis vary by type of infection. Journal of Gastroenterology and Hepatology . 2021;36(12):3363–3370. doi: 10.1111/jgh.15633. [DOI] [PubMed] [Google Scholar]
- 32.Kim D., Yoo E. R., Li A. A., Tighe S. P., Cholankeril G., Ahmed A. Trends in hospitalizations for Clostridioides difficile infection in end-stage liver disease, 2005-2014. Digestive Diseases and Sciences . 2021;66(1):296–307. doi: 10.1007/s10620-020-06162-0. [DOI] [PubMed] [Google Scholar]
- 33.Rosenblatt R., Mehta A., Cohen‐Mekelburg S., et al. The rise of Clostridioides difficile infections and fall of associated mortality in hospitalized advanced cirrhotics. Liver International . 2019;39(7):1263–1270. doi: 10.1111/liv.14077. [DOI] [PubMed] [Google Scholar]
- 34.Singal A. K., Salameh H., Kamath P. S. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalised patients in the United States. Alimentary Pharmacology & Therapeutics . 2014;40(1):105–112. doi: 10.1111/apt.12797. [DOI] [PubMed] [Google Scholar]
- 35.Stoica O. C., Stanciu C., Cojocariu C., et al. Clostridium difficile infection in hospitalized cirrhotic patients with hepatic encephalopathy. Journal of Gastrointestinal and Liver Diseases: JGLD . 2015;24(4):423–428. doi: 10.15403/jgld.2014.1121.244.csd. [DOI] [PubMed] [Google Scholar]
- 36.Pop A., Procopet B., Stefanescu H., Cavasi A., Tantau M., Andreica V. Clostridium difficile screening in cirrhosis: one for all, or some for one? Digestive Diseases and Sciences . 2015;60(12):3825–3826. doi: 10.1007/s10620-015-3913-4. [DOI] [PubMed] [Google Scholar]
- 37.Girleanu I., Trifan A., Huiban L., et al. The risk of Clostridioides difficile infection in cirrhotic patients receiving norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis-a real life cohort. Medicina . 2021;57(9):p. 964. doi: 10.3390/medicina57090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reigadas E., Alcalá L., Gómez J., et al. Breakthrough Clostridium difficile infection in cirrhotic patients receiving rifaximin. Clinical Infectious Diseases . 2018;66(7):1086–1091. doi: 10.1093/cid/cix918. [DOI] [PubMed] [Google Scholar]
- 39.Voicu M. N., Popescu F., Florescu D. N., et al. Clostridioides difficile infection among cirrhotic patients with variceal bleeding. Antibiotics . 2021;10(6):p. 731. doi: 10.3390/antibiotics10060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruger A. J., Durkin C., Mumtaz K., Hinton A., Krishna S. G. Early readmission predicts increased mortality in cirrhosis patients after Clostridium difficile infection. Journal of Clinical Gastroenterology . 2019;53(8):e322–e327. doi: 10.1097/mcg.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 41.Abdalla A. O., Pisipati S., Elnaggar M., Rishi M., Doshi R., Gullapalli N. Outcomes of Clostridioides difficile infection in patients with liver cirrhosis: a nationwide study. Gastroenterology Research . 2020;13(2):53–57. doi: 10.14740/gr1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iriana S., Sharma S., McDonough S., Zarate E. R., Adler D. G. Outcomes among inpatients with cirrhosis and Clostridioides difficile infection in the modern era: results from an analysis of the national inpatient sample. Annals of Gastroenterology . 2021;34(5):721–727. doi: 10.20524/aog.2021.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantri N., Patel H., Badipatla K. R., et al. Clostridioides difficile infection and liver cirrhosis - a retrospective, cohort study. Clinical and Experimental Gastroenterology . 2021;14:229–235. doi: 10.2147/ceg.S308862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziakas P. D., Joyce N., Zacharioudakis I. M., et al. Prevalence and impact of Clostridium difficile infection in elderly residents of long-term care facilities, 2011: a nationwide study. Medicine . 2016;95(31) doi: 10.1097/md.0000000000004187.e4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan D., Huang Y.-D., Chen Y.-B., et al. Risk factors for Clostridium difficile infection in cirrhotic patients. Hepatobiliary and Pancreatic Diseases International . 2019;18(3):237–241. doi: 10.1016/j.hbpd.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Lee D. U., Fan G. H., Ahern R. R., Karagozian R. The effect of malnutrition on the infectious outcomes of hospitalized patients with cirrhosis: analysis of the 2011-2017 hospital data. European Journal of Gastroenterology and Hepatology . 2021;32(2):269–278. doi: 10.1097/meg.0000000000001991. [DOI] [PubMed] [Google Scholar]
- 47.Bajaj J. S., O’Leary J. G., Tandon P., et al. Nosocomial infections are frequent and negatively impact outcomes in hospitalized patients with cirrhosis. American Journal of Gastroenterology . 2019;114(7):1091–1100. doi: 10.14309/ajg.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith E. Z., Northup P. G., Argo C. K. Predictors of mortality in cirrhosis inpatients with Clostridium difficile infection. Journal of Clinical Gastroenterology . 2018;52(8):747–751. doi: 10.1097/mcg.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 49.Bouza E., Alcalá L., Marín M., et al. An outbreak of Clostridium difficile PCR ribotype 027 in Spain: risk factors for recurrence and a novel treatment strategy. European Journal of Clinical Microbiology & Infectious Diseases . 2017;36(10):1777–1786. doi: 10.1007/s10096-017-2991-y. [DOI] [PubMed] [Google Scholar]
- 50.Natarajan M., Rogers M. A., Bundy J., et al. Gender differences in non-toxigenic Clostridium difficile colonization and risk of subsequent C. difficile infection. Clinical Research in Infectious Diseases . 2015;2(2):p. 1017. [PMC free article] [PubMed] [Google Scholar]
- 51.Markle J G M, Frank D. N., Mortin-Toth S., et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science . 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 52.Mueller S., Saunier K., Hanisch C., et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Applied and Environmental Microbiology . 2006;72(2):1027–1033. doi: 10.1128/aem.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papić N., Jelovčić F., Karlović M., Marić L. S., Vince A. Nonalcoholic fatty liver disease as a risk factor for Clostridioides difficile infection. European Journal of Clinical Microbiology & Infectious Diseases . 2020;39(3):569–574. doi: 10.1007/s10096-019-03759-w. [DOI] [PubMed] [Google Scholar]
- 54.Mullen K. D., Sanyal A. J., Bass N. M., et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clinical Gastroenterology and Hepatology . 2014;12(8):1390.e2–1397.e2. doi: 10.1016/j.cgh.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 55.Scarpignato C., Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy . 2005;51(1):36–66. doi: 10.1159/000081990. [DOI] [PubMed] [Google Scholar]
- 56.Kang S. H., Lee Y. B., Lee J.-H., et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Alimentary Pharmacology & Therapeutics . 2017;46(9):845–855. doi: 10.1111/apt.14275. [DOI] [PubMed] [Google Scholar]
- 57.Johnson D. A., Katz P. O., Armstrong D., et al. The safety of appropriate use of over-the-counter proton pump inhibitors: an evidence-based review and delphi consensus. Drugs . 2017;77(5):547–561. doi: 10.1007/s40265-017-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanwal F., Tansel A., Kramer J. R., Feng H., Asch S. M., El-Serag H. B. Trends in 30-day and 1-year mortality among patients hospitalized with cirrhosis from 2004 to 2013. American Journal of Gastroenterology . 2017;112(8):1287–1297. doi: 10.1038/ajg.2017.175. [DOI] [PubMed] [Google Scholar]
- 59.Hong S. J., Feuerstadt P., Brandt L. J. MELD is the only predictor of short-term mortality in cirrhotic patients with C. difficile infection. Digestive and Liver Disease . 2019;51(2):275–280. doi: 10.1016/j.dld.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 60.Gómez-Hurtado I., Zapater P., Bellot P., et al. Interleukin-10-mediated heme oxygenase 1-induced underlying mechanism in inflammatory down-regulation by norfloxacin in cirrhosis. Hepatology . 2011;53(3):935–944. doi: 10.1002/hep.24102. [DOI] [PubMed] [Google Scholar]
- 61.Juanola O., Gómez-Hurtado I., Zapater P., et al. Selective intestinal decontamination with norfloxacin enhances a regulatory T cell-mediated inflammatory control mechanism in cirrhosis. Liver International . 2016;36(12):1811–1820. doi: 10.1111/liv.13172. [DOI] [PubMed] [Google Scholar]
- 62.Nadinskaia M. Y., Kodzoeva K. B., Ulyanova K. A., et al. Risk factors associated with portal vein thrombosis in liver cirrhosis: a case-control study. Terapevticheskii Arkhiv . 2019;91(2):73–81. doi: 10.26442/00403660.2019.02.000153. [DOI] [PubMed] [Google Scholar]
- 63.Sharma S., Weissman S., Walradt T., et al. Readmission, healthcare consumption, and mortality in Clostridioides difficile infection hospitalizations: a nationwide cohort study. International Journal of Colorectal Disease . 2021;36(12):2629–2635. doi: 10.1007/s00384-021-04001-w. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Tsao G., Surawicz C. M. Clostridium difficile infection: yet another predictor of poor outcome in cirrhosis. American Journal of Gastroenterology . 2010;105(1):114–116. doi: 10.1038/ajg.2009.604. [DOI] [PubMed] [Google Scholar]
- 65.Kelly C. R., Fischer M., Allegretti J. R., et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. American Journal of Gastroenterology . 2021;116(6):1124–1147. doi: 10.14309/ajg.0000000000001278. [DOI] [PubMed] [Google Scholar]
- 66.Johnson S., Lavergne V., Skinner A. M., et al. Clinical practice guideline by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clinical Infectious Diseases . 2021;73(5):e1029–e1044. doi: 10.1093/cid/ciab549. [DOI] [PubMed] [Google Scholar]
- 67.van Prehn J., Reigadas E., Vogelzang E. H., et al. European society of clinical microbiology and infectious diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clinical Microbiology and Infection . 2021;27(Suppl 2):S1–S21. doi: 10.1016/j.cmi.2021.09.038. [DOI] [PubMed] [Google Scholar]
- 68.Gerard L., Garey K. W., DuPont H. L. Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Review of Anti-infective Therapy . 2005;3(2):201–211. doi: 10.1586/14787210.3.2.201. [DOI] [PubMed] [Google Scholar]
- 69.Debbia E. A., Maioli E., Roveta S., Marchese A. Effects of rifaximin on bacterial virulence mechanisms at supra- and sub-inhibitory concentrations. Journal of Chemotherapy . 2008;20(2):186–194. doi: 10.1179/joc.2008.20.2.186. [DOI] [PubMed] [Google Scholar]
- 70.Basu P P, Dinani A., Rayapudi K., et al. Rifaximin therapy for metronidazole-unresponsive Clostridium difficile infection: a prospective pilot trial. Therapeutic Advances in Gastroenterology . 2010;3(4):221–225. doi: 10.1177/1756283x10372985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mattila E., Arkkila P., Mattila P. S., Tarkka E., Tissari P., Anttila V. J. Rifaximin in the treatment of recurrent Clostridium difficile infection. Alimentary Pharmacology & Therapeutics . 2013;37(1):122–128. doi: 10.1111/apt.12111. [DOI] [PubMed] [Google Scholar]
- 72.Johnson S., Schriever C., Galang M., Kelly C. P., Gerding D. N. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clinical Infectious Diseases . 2007;44(6):846–848. doi: 10.1086/511870. [DOI] [PubMed] [Google Scholar]
- 73.Neff G. W., Jones M., Jonas M., et al. Lack of Clostridium difficile infection in patients treated with rifaximin for hepatic encephalopathy. Journal of Clinical Gastroenterology . 2013;47(2):188–192. doi: 10.1097/MCG.0b013e318276be13. [DOI] [PubMed] [Google Scholar]
- 74.Kimer N., Krag A., Møller S., Bendtsen F., Gluud L. L. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Alimentary Pharmacology & Therapeutics . 2014;40(2):123–132. doi: 10.1111/apt.12803. [DOI] [PubMed] [Google Scholar]
- 75.Bloom P. P., Tapper E. B., Young V. B., Lok A. S. Microbiome therapeutics for hepatic encephalopathy. Journal of Hepatology . 2021;75(6):1452–1464. doi: 10.1016/j.jhep.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kondepudi K. K., Ambalam P., Nilsson I., Wadström T., Ljungh A. Prebiotic-non-digestible oligosaccharides preference of probiotic bifidobacteria and antimicrobial activity against Clostridium difficile. Anaerobe . 2012;18(5):489–497. doi: 10.1016/j.anaerobe.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Ito Y., Moriwaki H., Muto Y., Kato N., Watanabe K., Ueno K. Effect of lactulose on short-chain fatty acids and lactate production and on the growth of faecal flora, with special reference to Clostridium difficile. Journal of Medical Microbiology . 1997;46(1):80–84. doi: 10.1099/00222615-46-1-80. [DOI] [PubMed] [Google Scholar]
- 78.Agarwalla A., Weber A., Davey S., et al. Lactulose is associated with decreased risk of Clostridium difficile infection in decompensated cirrhosis. Clinical Gastroenterology and Hepatology . 2017;15(6):953–954. doi: 10.1016/j.cgh.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Zhang F., Cui B., Cui B., et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein & Cell . 2018;9(5):462–473. doi: 10.1007/s13238-018-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Surawicz C. M., Brandt L. J., Binion D. G., et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. American Journal of Gastroenterology . 2013;108(4):478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 81.Bajaj J. S., Kassam Z., Fagan A., et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology . 2017;66(6):1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bajaj J. S., Fagan A., Gavis E. A., Kassam Z., Sikaroodi M., Gillevet P. M. Long-term outcomes of fecal microbiota transplantation in patients with cirrhosis. Gastroenterology . 2019;156(6):1921.e3–1923.e3. doi: 10.1053/j.gastro.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olmedo M., Reigadas E., Valerio M., et al. Is it reasonable to perform fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with liver cirrhosis? Revista Espan˜ola de Quimioterapia . 2019;32(2):205–207. [PMC free article] [PubMed] [Google Scholar]
- 84.Saab S., Alper T., Sernas E., Pruthi P., Alper M. A., Sundaram V. Hospitalized patients with cirrhosis should Be screened for Clostridium difficile colitis. Digestive Diseases and Sciences . 2015;60(10):3124–3129. doi: 10.1007/s10620-015-3707-8. [DOI] [PubMed] [Google Scholar]
- 85.Vindigni S. M., Surawicz C. M. Cirrhosis and C. difficile: a deadly duo? Digestive Diseases and Sciences . 2015;60(10):2865–2867. doi: 10.1007/s10620-015-3777-7. [DOI] [PubMed] [Google Scholar]
- 86.Zacharioudakis I. M., Zervou F. N., Pliakos E. E., Ziakas P. D., Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. American Journal of Gastroenterology . 2015;110(3):381–390. doi: 10.1038/ajg.2015.22. [DOI] [PubMed] [Google Scholar]
- 87.Baron S. W., Ostrowsky B. E., Nori P., et al. Screening of Clostridioides difficile carriers in an urban academic medical center: understanding implications of disease. Infection Control & Hospital Epidemiology . 2020;41(2):1–5. doi: 10.1017/ice.2019.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maghdoori S., Moghadas S. M. Assessing the effect of patient screening and isolation on curtailing Clostridium difficile infection in hospital settings. BMC Infectious Diseases . 2017;17(1):p. 384. doi: 10.1186/s12879-017-2494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartsch S. M., Curry S. R., Harrison L. H., Lee B. Y. The potential economic value of screening hospital admissions for Clostridium difficile. European Journal of Clinical Microbiology & Infectious Diseases . 2012;31(11):3163–3171. doi: 10.1007/s10096-012-1681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lanzas C., Dubberke E. R. Effectiveness of screening hospital admissions to detect asymptomatic carriers of Clostridium difficile: a modeling evaluation. Infection Control & Hospital Epidemiology . 2014;35(8):1043–1050. doi: 10.1086/677162. [DOI] [PubMed] [Google Scholar]
- 91.Meltzer E., Smollan G., Huppert A., et al. Universal screening for Clostridioides difficile in a tertiary hospital: risk factors for carriage and clinical disease. Clinical Microbiology and Infection . 2019;25(9):1127–1132. doi: 10.1016/j.cmi.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 92.Fernández J., Angeli P., Trebicka J., et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clinical Gastroenterology and Hepatology . 2020;18(4):963.e14–973.e14. doi: 10.1016/j.cgh.2019.07.055. [DOI] [PubMed] [Google Scholar]
- 93.Wiedermann C. J. Hypoalbuminemia as surrogate and culprit of infections. International Journal of Molecular Sciences . 2021;22(9):p. 4496. doi: 10.3390/ijms22094496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baldassarre M., Naldi M., Zaccherini G., et al. Determination of effective albumin in patients with decompensated cirrhosis: clinical and prognostic implications. Hepatology . 2021;74(4):2058–2073. doi: 10.1002/hep.31798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the article, and no additional data are available.


