Abstract
Animals have evolved mechanisms, such as cell competition, to remove dangerous or nonfunctional cells from a tissue. Tumor Necrosis Factor signaling can eliminate clonal malignancies from Drosophila imaginal epithelia, but why this pathway is activated in tumor cells, but not normal tissue, is unknown. We show that the ligand driving elimination is present in basolateral circulation, but remains latent because it is spatially segregated from its apically-localized receptor. Polarity defects associated with malignant transformation cause receptor mislocalization, allowing ligand binding and subsequent apoptotic signaling. This process occurs irrespective of the neighboring cells’ genotype and is thus distinct from cell competition. Related phenomena at epithelial wound sites are required for efficient repair. This mechanism of polarized compartmentalization of ligand and receptor can generally monitor epithelial integrity to promote tissue homeostasis.
One sentence summary:
Transformed cells and wounds activate a polarity-enforced latent signaling system to trigger death or repair respectively.
Epithelial architecture is the fundamental organizing principle of animal tissues. Polarized epithelial sheets provide a contiguous barrier that allows an organ to function in a milieu distinct from the external environment. To maintain the barrier, epithelia must detect threats to their integrity and resolve them. Integrity can be compromised by both physical damage and the production of structurally-defective cells. The latter is a frequent feature of oncogenic transformation, and it is important to eliminate such cells before a tumor can form. Deleterious cells can be removed by cell competition, a broadly utilized mechanism in which ‘winner’ cells of one genotype often induce apoptosis in neighboring ‘loser’ cells (1, 2). In Drosophila imaginal discs, cells mutant for the conserved apicobasal polarity regulators scribble (scrib) or discs-large (dlg) form malignant, ‘neoplastic’ tumors that kill the animal (3, 4). However, prior to tumor growth, small clones of these polarity-deficient cells are efficiently eliminated, allowing a healthy organ to develop. The mechanisms involved have been described as cell competition during which the Drosophila TNF ligand Eiger (Egr) binds the TNF receptor Grindelwald (TNFR, Grnd) in mutant cells, activating the JNK Basket (Bsk), which induces apoptosis (reviewed in (5–7). How polarity loss is coupled to TNF pathway activation to remove oncogenic clones is not known.
We investigated TNF-TNFR interactions during polarity-deficient cell elimination by co-culturing imaginal discs ex vivo alongside Egr-Venus (EgrV)-expressing fat bodies (a major endocrine organ) (Fig. 1A)(8). EgrV secreted into media associated strongly with clones of dlg-depleted cells (Fig. 1B–C, E, Fig. S1A–B, K–L). Increased EgrV binding is specific for polarity-deficient elimination: it is seen in scrib mutant clones but not loser cells outcompeted by Myc-overexpressing neighbors (Fig. S1G–J). Egr binding, like cell elimination, depends on Grnd (Fig. 1D–F, Fig. 2I, Fig. S1C, Fig S3C–E). We used patched-GAL4 (ptc-GAL4) to conditionally deplete dlg, generating a consistent stripe of apoptotic cells that accumulate EgrV (Fig. 1G–L, Fig. S1D–E, Fig. S3A–B)(9, 10). As in clones, Grnd depletion blocked elimination and led to overgrowth (Fig. 1K, Fig. S1F). Mechanical wounding also activates JNK signaling (11), and wound sites bind secreted Egr in a Grnd-dependent manner (Fig. S2A–I). Increased Egr-Grnd binding is thus associated with malignant cell elimination and physical wounding, which both disrupt epithelial integrity.
Fig. 1. Heightened binding of Egr to polarity-deficient cells.
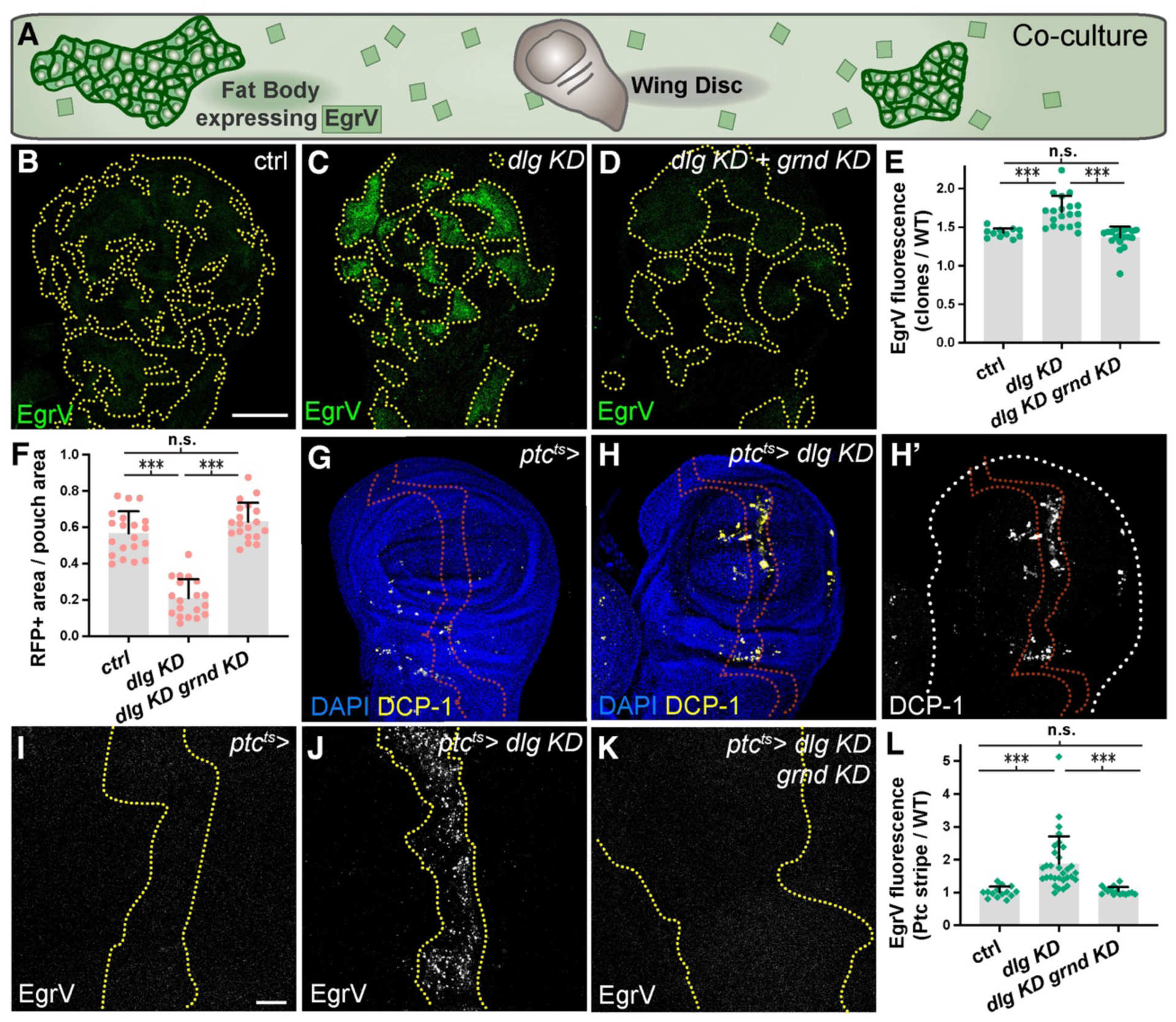
(A): Diagram of EgrV-expressing fat bodies co-cultured ex vivo with imaginal discs.
(B-F): Binding of EgrV and elimination of dlg-depleted clones (C) are dependent on cell-autonomous Grnd (D, control in B, dotted lines demarcate clones). Quantitated in E, F.
(G-H): Depletion of dlg with ptc-Gal4 (dotted lines indicate stripe of expression) enhances cell death (DCP-1) (H, control in G, quantitation in Fig. 2D)
(I-L): EgrV binding, absent in control (I), is elevated in the dlg-depleted stripe (J), and this requires Grnd (K). Quantitated in L.
Scale bars: 100 μm in B, 10 μm in I. See Materials and Methods and Table S3 for information on statistical tests.
Fig. 2. Egr required for cell elimination derives from circulation.
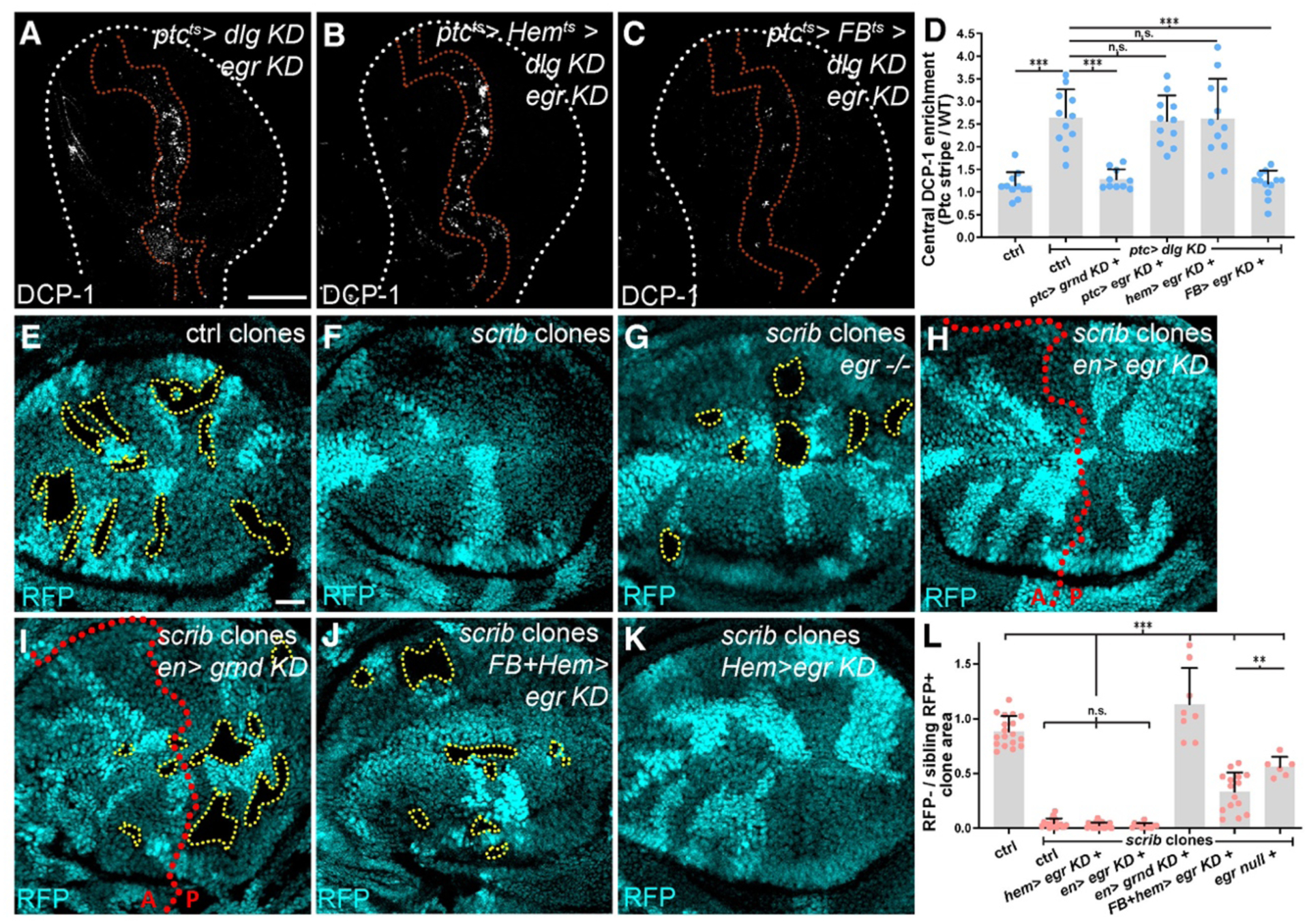
(A-D): dlg cell elimination in the stripe (DCP-1, red dotted lines) is not prevented by co-depletion of autonomous egr (A), nor by egr co-depletion in hemocytes (B). Depletion of egr from fat body and stripe prevents dlg cell apoptosis (C). Quantitation of apoptosis shows requirement for autonomous Grnd and fat body-produced Egr (D).
(E-L): Although WT mitotic clones survive (E), scrib clones are eliminated (F). scrib clones survive in entirely egr-mutant animal (G), but are eliminated in a field of egr-depleted cells (H). As with autonomous depletion of grnd (I), scrib clone elimination is blocked when egr is depleted in fat body and hemocytes (J), but not in hemocytes alone (K). Quantitation in L. Dotted lines mark clone boundaries or posterior (P) compartment gene depletion.
Scale bars: 100 μm in A, 25 μm in E.
Since JNK activation in both cases above is associated with Egr binding, we investigated the underlying mechanism. Data argue against elevated Grnd levels (Fig. S5A–R), changes in Grnd N-glycosylation (8)(Fig. S5S), altered endocytic dynamics (12)(Fig. S4A–L), or elevated Egr levels (Fig. S6A–Q) as mediators of dlg-depleted cell apoptosis. In functional experiments, neither autocrine nor paracrine epithelial Egr was required (Fig. 2A, D–F, H, L, Fig. S3F, J). Because polarity-deficient clones survive in an animal completely devoid of Egr (Fig. 2G, L)(10, 12, 13), the Egr required for elimination must come from another source.
Both fat body and hemocytes (innate immune cells) produce Egr (14–17). We co-depleted egr and dlg simultaneously from both the disc stripe and these tissues. Hemocytes did not associate with Dlg-deficient cells, and co-depletion of hemocyte Egr had no impact on elimination (Fig. 2B, D, Fig. S3G, Fig. S6R, S). Co-depletion of fat body Egr prevented apoptosis in the stripe: Dlg-deficient cells persisted and overgrew (Fig. 2C–D, Fig. S3H–J). scrib mutant disc clones persisted upon Egr depletion in fat body and hemocytes (Fig. 2J, L), but not when Egr was depleted in hemocytes alone (Fig. 2K–L). Wound healing was also perturbed by depletion of fat body Egr (Fig. S2J–K). Together, these data indicate that circulating Egr is essential for full activation of Grnd and JNK signaling in response to epithelial interruptions.
The above results prompt consideration of ligand and receptor localization in this signaling axis. Fat body-produced Egr is secreted into hemolymph (circulatory fluid), which bathes the disc basolateral surface (Fig. 3A)(15). However, steady-state Grnd is apically polarized (Fig. 3B, C)(7). Co-culture experiments revealed that EgrV binds only basally, suggesting limited access of circulating Egr to Grnd (Fig. 3C, Fig S8D–E). Dextran assays demonstrated that discs do not display transepithelial permeability, but luminal access can be induced by wounding (Fig. 3D–E). We tested whether fat body-produced EgrV could bind to an extracellular nanobody targeted to either polarized epithelial surface in vivo (Fig. S8A)(18). EgrV bound robustly to basal nanobodies (Fig. 3F) but only slightly to apical nanobodies, and basal signal of these cells was higher (Fig. 3G). After mechanical wounding of the latter discs, EgrV bound apically instead (Fig. 3H). Enhanced EgrV binding was also seen when Dlg-depleted cells mispolarize apical nanobodies (Fig. S8B–C). Thus, TNF ligand and its receptor are normally segregated by the epithelial barrier. However, inducing transepithelial permeability was not sufficient to initiate cell elimination (Fig. S7A–J).
Fig. 3. Egr binds basolaterally to polarity-deficient cells.
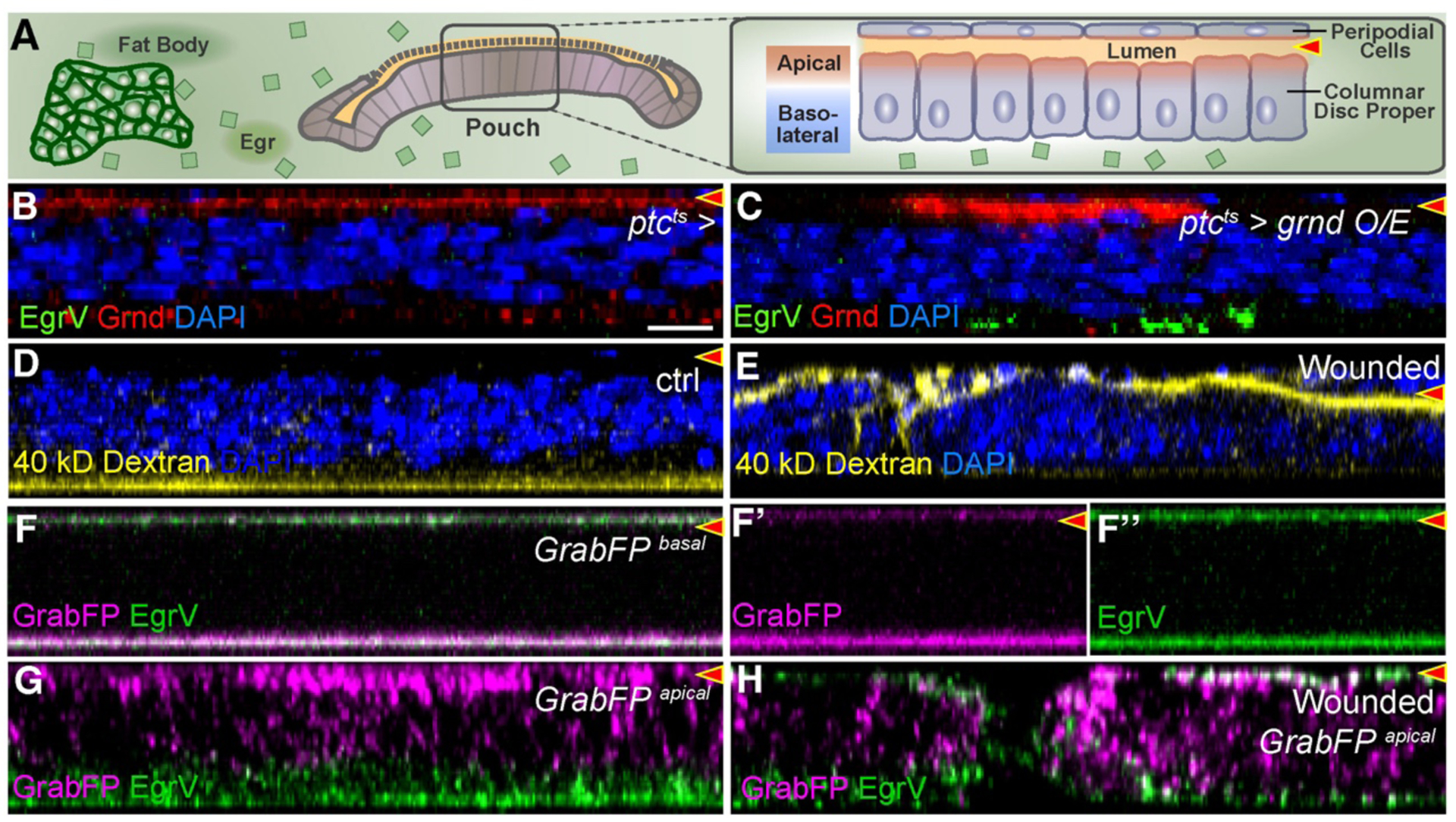
X-Z cross-sections show disc proper below and peripodium above. Lumen is indicated by red arrowhead.
(A): Diagram showing relationship of disc epithelial barrier to hemolymph. Egr secreted by fat body bathes the basolateral surface but is excluded from apical surface and lumen.
(B-C): Grnd is apically localized (B), even when overexpressed (C), but bound EgrV is exclusively basolateral.
(D-E): Dextran in media is excluded from lumen of intact discs (D) but can enter wounded discs (E).
(F): Basolateral GrabFP binds strongly at basal surface to EgrV produced by fat bodies. Signal at top is peripodial basal surface. F’ and F” show left half of F.
(G-H): Apical GrabFP binds EgrV only at the basolateral surface (G), but wounding enables strong apical binding of EgrV as well (H).
Scale bar: 10 μm in B
We therefore examined Grnd localization during polarity-deficient cell elimination and found it mispolarized basolaterally (Fig. 4A–B, Fig. 3C, Fig. S8F–G). This is not due to cell death or basal extrusion (Fig. S5P–R). When these discs are co-cultured, bound EgrV is predominantly basal (Fig. 4A–B). Inhibiting JNK rescued apoptosis but not basal Grnd localization, and again EgrV bound basally (Fig. 4C, Fig. S8H). In wounded cells also, EgrV bound predominantly basally, although tissue damage prevented rigorous analysis of Grnd localization (Fig. 4D, Fig. S8I). These data suggest that receptor mispolarization allows access to basally circulating ligand, triggering JNK activation, and adaptive homeostatic responses including cell elimination and wound-healing.
Fig. 4. Mispolarization of Grnd permits Egr binding and cell elimination.
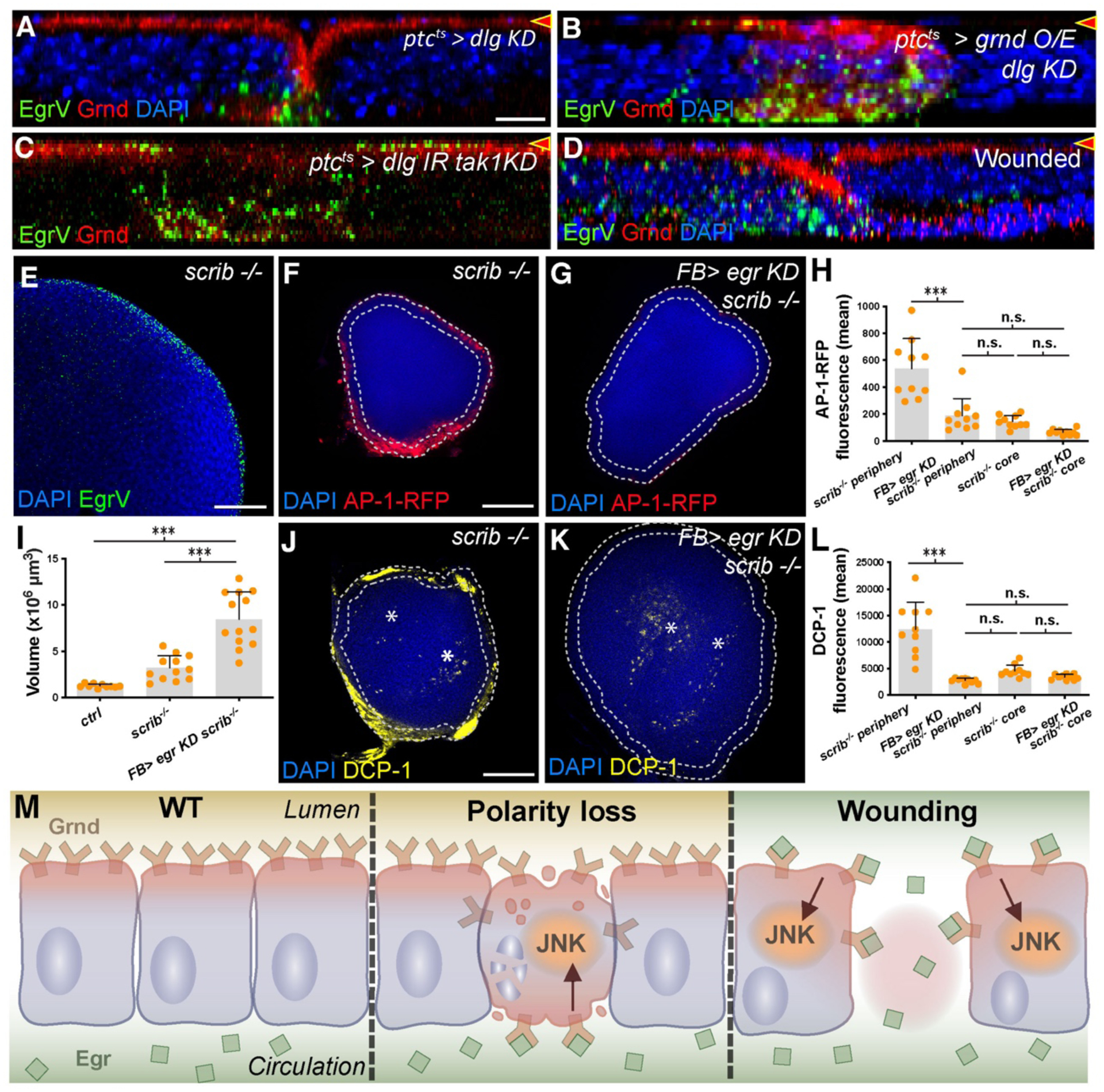
(A-D): X-Z sections as in Fig. 3. dlg-depleted cells mislocalize Grnd basolaterally (A), evident especially when Grnd is overexpressed (B). EgrV binds basally to dlg-depleted cells. Co-depletion of dlg and tak1 (C) blocks cell elimination but polarity defects remain. Mispolarized Grnd binds EgrV at basal surface. Wounded discs (D) show altered Grnd localization and basal EgrV binding.
(E-L): X-Y sections. Wing discs containing only scrib mutant cells bind Egr preferentially at periphery (E). JNK signaling (F) is elevated in periphery compared to core and is dependent on circulating Egr (G, quantitated in H). scrib discs grow larger when circulating Egr is depleted (I), and peripheral apoptosis (J, DCP-1) is reduced (K, asterisks indicate DCP-1+ cells in core; quantitated in L).
(M): Model for role of polarized segregation of TNF ligand and receptor in epithelial homeostasis.
Scale bar: 10 μm in A, 50 μm in E, 100 μm in F, and J.
Elimination of polarity-deficient cells has long been described as a form of cell competition, albeit regulated by pathways distinct from Minute or Myc competition (1, 2, 5–7). A defining feature of cell competition is that elimination of ‘loser’ cells requires neighboring ‘winners’ of a different genotype. Yet circulating Egr can access basolaterally-mislocalized Grnd in any polarity-deficient cell, regardless of its neighbor. We therefore reconsidered the requirement for WT cells in death of scrib-class mutant cells.
We asked whether polarity-deficient discs containing no WT cells showed the same dependence on circulating Egr as polarity-deficient cells with WT neighbors. scrib discs bound EgrV specifically at their hemolymph-contacting periphery, paralleling the activation of JNK reporters (Fig. 4E–F, Fig. S9A–B)(19). Apoptosis in scrib discs is also enriched peripherally, compared to the ‘core’ which lacks EgrV binding (Fig. 4J). Peripheral apoptosis and JNK activation were normalized when circulating Egr was depleted, and scrib discs were larger, consistent with a hypothesis that multilayered tissue architecture allows some scrib cells to evade hemolymph Egr and overproliferate to form tumors (Fig. 4F–L)(19). scrib disc periphery mitotic rates were elevated in fat body Egr-depleted animals, likely due to relief of JNK-mediated cell cycle stalling (Fig. S9C–E) (20). Thus, the same mechanisms that eliminate small clones of polarity-deficient cells also kill polarity-deficient cells and limit their growth in a non-competitive situation. These results challenge the paradigm that elimination of scrib cells is due to classical cell competition, and suggest that the mechanism we describe is a distinct pathway coupling epithelial organization to tissue homeostasis.
All epithelia need to monitor their integrity and respond when breaches are detected. Since most tumors arise in epithelial tissues, preventing the growth of malignant clones within them must also be a priority. Here we show a mechanism for tumor elimination that arises from an intrinsic property of the epithelial barrier –its ability to compartmentalize a luminal environment segregated from the external milieu. Drosophila TNF circulates systemically and bathes basal organ surfaces, but is latent due to TNFR’s strict apical localization. However, when neoplastic cells arise, their altered polarity induces basal localization of TNFR, where it binds ligand and triggers apoptotic signaling. A similar axis promotes wound healing: if the epithelium is physically ruptured, ligand can meet receptor and contribute to a pro-healing JNK signaling program. Thus, a common molecular mechanism inherent to epithelial geometry -a mechanism which recognizes polarity changes as a Damage-Associated Molecular Pattern (DAMP)(21) -- underlies both homeostatic programs (Fig. 4M). The function of the TNF/TNFR system described here as an in vivo sensor of epithelial integrity raises the possibility that ligand-receptor segregation (22) may be a theme general to epithelial maintenance.
Supplementary Material
Acknowledgements:
We thank S. Yoo and C. Liu for help with wounding experiments. This work was funded by NIH grants GM090150 and GM130388 to DB and Independent Research Fund Denmark fellowship 0131-00010B to SUG. GdV, SUG and DB designed the research and wrote the manuscript; GdV and SUG conducted experiments and analyzed data. Authors declare no competing interests. All data are available in the main text or the supplementary materials. Materials are available upon request.
Footnotes
REFERENCES
- 1.Johnston LA, Cold Spring Harb. Perspect. Med 4 (2014), doi: 10.1101/cshperspect.a014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madan E, Gogna R, Moreno E, Curr. Opin. Cell Biol 55 (2018), pp. 150–157. [DOI] [PubMed] [Google Scholar]
- 3.Bilder D, Genes Dev. 18, 1909–1925 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Stephens R et al. , J Mol Biol. 430, 3585–3612 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Morata G, Calleja M, Semin. Cancer Biol 63 (2020), pp. 19–26. [DOI] [PubMed] [Google Scholar]
- 6.Nagata R, Igaki T, Dev. Growth Differ 60 (2018), pp. 522–530. [DOI] [PubMed] [Google Scholar]
- 7.Andersen DS et al. , Nature. 522, 482–486 (2015). [DOI] [PubMed] [Google Scholar]
- 8.de Vreede G et al. , Dev. Cell 45, 595–605.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang C-C et al. , Proc. Natl. Acad. Sci. U. S. A 112, 1785–1790 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordero JB et al. , Dev. Cell 18, 999–1011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repiso A, Bergantiños C, Corominas M, Serras F, Dev. Growth Differ 53 (2011), pp. 177–185. [DOI] [PubMed] [Google Scholar]
- 12.Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T, Dev. Cell 16, 458–465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G, Proc. Natl. Acad. Sci. U. S. A 109, 484–489 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parisi F, Stefanatos RK, Strathdee K, Yu Y, Vidal M, Cell Rep. 6, 855–867 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Agrawal N et al. , Cell Metab. 23, 675–684 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Muzzopappa M, Murcia L, Milan M, Proc. Natl. Acad. Sci. U. S. A 114, E7291–E7300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogarty CE et al. , Curr. Biol 26, 575–584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmansa S, Alborelli I, Bieli D, Caussinus E, Affolter M, Elife. 6 (2017), doi: 10.7554/eLife.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji T et al. , Dis. Model. Mech 12, dmm040147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosolo A et al. , Elife. 8 (2019), doi: 10.7554/eLife.41036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen GY, Nuñez G, Nat. Rev. Immunol 10 (2010), pp. 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermeer PD et al. , Nature. 422, 322–326 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Igaki T et al. , EMBO J. 21, 3009–3018 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodra A, de la Cova C, Gerhold AR, Johnston LA, G3 Genes, Genomes, Genet 10, 4707–4712 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee N, Bohmann D, PLoS One. 7 (2012), doi: 10.1371/journal.pone.0034063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betschinger J, Mechtler K, Knoblich JA, Nature. 422, 326–330 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Zecca M, Struhl G, Development. 134, 3011–3020 (2007). [DOI] [PubMed] [Google Scholar]
- 28.de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA, Cell. 117, 107–116 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Zeitler J, Hsu CP, Dionne H, Bilder D, J. Cell Biol 167, 1137–1146 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurucz E et al. , Proc. Natl. Acad. Sci. U. S. A 100, 2622–2627 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindelin J et al. , Nat Methods. 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Vreede G, Schoenfeld JS, Windler SL, Morrison HA, Bilder D, Development. 141, 2796–2802 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballesteros-Arias L, Saavedra V, Morata G, Oncogene. 33, 4377–4384 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Tepass U, Tanentzapf G, Annu. Rev. Genet 35 (2001), pp. 747–784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


