Abstract
Background:
The human microbiome plays a critical role in human health and disease. The diversity and composition of the human microbiome varies across human body sites. A dysbiotic gut microbiome is associated with carcinogenesis, therapeutic drug response, and side effects of cancer treatments.
Objectives:
This paper aims to demonstrate the role of the gut microbiome in oncology care and nursing implications for clinical cancer care.
Methods:
A review of the literature was conducted to determine influencing factors and roles of the gut microbiome in oncology care. The roles of the gut microbiome included treatment-induced dysbiosis of the gut microbiome, treatment-related symptoms such as gastrointestinal and psychoneurological symptoms, and human microbiome-associated interventions, including prebiotics, probiotics, and fecal microbiome transplant.
Practice Implications:
By understanding the definition of the human microbiome and its influencing factors, oncology nurses in clinical practice could educate, screen, and monitor cancer patients who have a higher risk of gut microbiome dysbiosis. Knowledge of the gut microbiome and its impact on cancer outcomes can help oncology nurses interpret associations between the gut microbiome and treatment-related toxicities and symptoms. Oncology nurses can guide patients to build a healthy gut microbiome across the trajectory of cancer treatment and survivorship.
Introduction
The human microbiome is defined as a collection of the microorganisms (e.g., bacteria, archaea, eukaryotes, and viruses) and their genomes harbored in or on the human body (Marchesi & Ravel, 2015). The diversity and composition of the human microbiome varies across different body sites. For example, the gastrointestinal tract is primarily dominated by anaerobic microbes, critically associated with food digestion and metabolism, and maintaining homeostasis of the immune system. A healthy vagina is comparatively acidic with the dominance of Lactobacillus species to prevent yeast infections, sexually transmitted infections, and urinary tract infections. Additionally, the skin has the most variable and least stable microbiome owing to constant exposure to various conditions, including humidity, salinity, and temperature (Kennedy & Chang, 2020).
The human microbiome is a complex subject. Table 1 provides key definitions for terms used throughout this paper. Compared to other body sites, the microbiome in the gut has been studied extensively among cancer populations. The human gut hosts 500 to 1,000 microbial species on average (Knight & Buhler, 2015). A dysbiotic gut microbiome (i.e., loss of keystone taxa, loss of diversity, shifts in metabolic capacity, or increase of pathogens) is associated with carcinogenesis and interference with therapeutic drug metabolism, such as chemotherapy (Roy & Trinchieri, 2017). Recently, dysbiotic gut microbiome is identified as a promising biomarker of toxicities associated with cancer treatment (J. Bai et al., 2020; Touchefeu et al., 2014). Specifically, a disturbed gut microbiome potentially contributes to frequent gastrointestinal symptoms (Touchefeu et al., 2014) and psychoneurological symptoms (J. Bai et al., 2020; Song & Bai, 2020).
Table 1.
Concepts for the Human Microbiome
| Concept | Description/Definition |
|---|---|
| Alpha diversity | Diversity within an individual site or sample diversity |
| Archaea | A domain of single-celled organisms which lack cell nuclei |
| Beta diversity | Diversity between separate samples |
| Eukaryotes | Organisms whose cells have a nucleus enclosed within a nuclear envelope |
| Fecal microbiome transplant | An investigational treatment in which a fecal preparation from a carefully screened, healthy stool donor is transplanted into the colon of the patient via multiple routes, such as colonoscopy, naso-enteric tube, and capsules |
| Genome | All genetic material of an organism |
| Gut microbial dysbiosis | A disrupted profile of the gut microbiome, including loss of keystone taxa, loss of diversity, shifts in metabolic capacity, or increase of pathogens |
| Gut microbiome | Microbes and their microbial genomes in the gastrointestinal tract |
| Microbiome | The collection of microbes and their microbial genomes at a given site (e.g., vagina or gastrointestinal tract) |
| Gut-brain axis | The bidirectional network involving multiple biological systems that allows communication between gut bacteria and the brain |
| Microbiota | The microbial taxa associated with humans. The human body is colonized by a vast number of microbes, collectively called the human microbiota |
| Prebiotics | Non-digestible food ingredients that can benefit the host’s health via changing the composition and function of the gut microbiome |
| Probiotics | Living bacteria that can benefit the host’s health via changing the gut microbiome |
| Psychoneurological symptoms (PNS) | A cluster of co-occurring symptoms with potentially common biological or pathophysiological mechanisms, including pain, fatigue, anxiety, depression, sleep disturbance, and cognitive dysfunction |
| Taxa | A population of phylogenetically related organisms |
Note: Based on references of Marchesi & Ravel, 2015; Roy & Trinchieri, 2017; Bai et al., 2020; Touchefeu et al., 2014
Influencing Factors of the Gut Microbiome
Various factors can affect the diversity and composition of the gut microbiome (Figure 1). Over 20% of the microbiota variability is shaped by environmental factors such as the use of antibiotics, living environment, and anthropometric measurements, while family factors such as genetics only explain 2% of taxa variance (Rothschild et al., 2018). The priority effects (i.e., the order and timing of gut microbiota arrival) and microbial transmission (e.g., infant delivery and feeding modalities) can determine the microbial development in early life (Sprockett, Fukami, & Relman, 2018). Both the Human Microbiome Project and the American Gut have identified a series of individual factors that change the gut microbiome, including sociodemographic characteristics (e.g., sex, age, and race), health behaviors (e.g., diet and physical activity) (Singh et al., 2017), and chronic conditions such as inflammatory bowel disease. Additionally, the gut microbiome has been explored in various cancers across the continuum of diagnosis, treatment, and survivorship. Cancer treatments such as chemotherapy disrupt the gut microbiome, resulting in gastrointestinal and psychoneurological toxicities and symptoms including gut barrier failure, inflammation, and gut-brain axis (J. Bai et al., 2020; Touchefeu et al., 2014).
Figure 1.
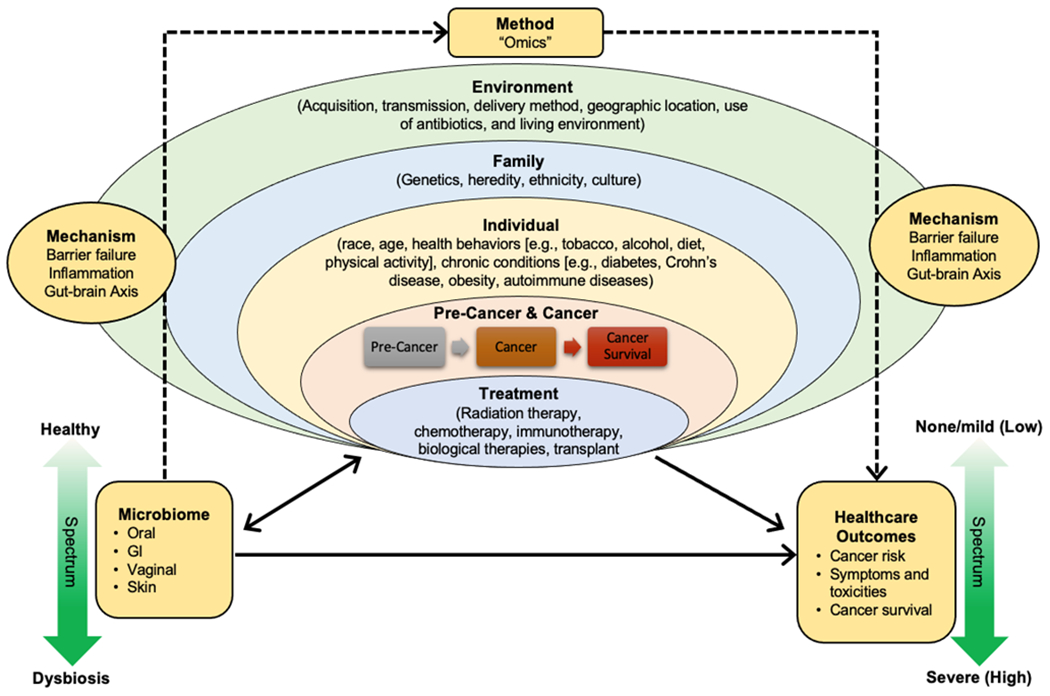
Influencing Factors of the Gut Microbiome in Cancer
Gut Microbiome in Oncology Nursing Care
Treatment-induced gut microbial dysbiosis.
Cancer treatments, particularly chemotherapy and radiation therapy (RT), can influence the diversity and composition of the gut microbiome (Table 2). Adult patients’ gut microbiome was significantly disrupted across chemotherapy, with decreases in the abundance of healthy gut microbiotas, including Firmicutes, Actinobacteria, Bacteroides, Bifidobacteria, Clostridium cluster IV and XIVa, and increases in pathological microbes such as Proteobacteria and Enterobacteriaceae (Montassier et al., 2014). Similarly, a marked reduction in the number of anaerobic bacteria (e.g., Bifidobacteria, Clostridium cluster XIVa, Faecalibacterium, Lactobacillus, and Streptococci) and an increase in Enterococci were found in children with cancer receiving chemotherapy (Rajagopala et al., 2016; van Vliet et al., 2009). RT can also influence the gut microbiome diversity and composition. The reduced richness of the gut microbiome community, as well as a decreased abundance of Firmicutes and increased abundance of Fusobacteria, were observed in gynecologic cancer patients treated with RT. Moreover, the overall gut microbiome pattern can be remodeled after the completion of RT. Studies in pelvic cancer patients demonstrate that RT could lead to an increased abundance of Clostridium_XIVa, Proteobacteria, and Gammaproteobacteria and a decreased abundance of Faecalibacterium, Lachnospiracea, Oscillibacter, Roseburia, and Streptococcus (Wang et al., 2019). Among pediatric cancer patients, the reduced microbiome diversity was observed throughout RT; for instance, decreased relative abundance of Firmicutes and increased relative abundance of Proteobacteria, Streptococcus, Bacteroides, Dorea, Subdoligranulum, and Escherichia-Shigella were reported after RT completion (Sahly, Moustafa, Zaghloul, & Salem, 2019). Understanding how gut microbiome dysbiosis at the time of the treatment may influence cancer treatment toxicities and symptoms requires further investigation.
Table 2.
Taxa Associated with Cancer and Cancer Treatment-related Symptoms
| Outcomes or Toxicities | Key Taxa |
|---|---|
| Patients with cancer (vs healthy controls) |
|
| High gastrointestinal symptoms (vs low gastrointestinal symptoms) |
|
| High psychoneurological symptoms (vs low psychoneurological symptoms) |
|
| Low psychoneurological symptoms (vs high psychoneurological symptoms) |
|
Gastrointestinal symptoms.
Gastrointestinal symptoms such as diarrhea, constipation, and oral mucositis, frequently occur in cancer patients owing to cytotoxic treatments. Patients with significant gastrointestinal symptoms may have a decreased diversity of healthy microbial communities, such as a lower abundance of Actinobacteria, Lactobacillus, Faecalibacterium, Roseburia, and Bifidobacterium, and an increase in pathologically relevant microbiome species, such as Escherichia coli, Enterobacter, and Staphylococcus (Montassier et al., 2015). Among cancer patients who developed gastrointestinal symptoms during RT, a modified bacterial profile with a higher abundance of Phascolarctobacterium, Lachnospiraceae, Erysipelotrichaceae, Clostridium XI and XVIII, and Fecalitalea was reported (Mitra et al., 2020). Relationships between the gut microbiome and gastrointestinal symptoms are still unknown in childhood cancer patients. The pathogenesis of gut microbiome for cancer treatment-related gastrointestinal symptoms may be associated with the following pathways: inflammatory cytokines; intestinal permeability; bacteria translocation; changes in the epithelial surface microbiota pattern, intestinal protection from noxious stimuli, epithelial repair mechanisms; and the release of immune cells and molecules (van Vliet, Harmsen, de Bont, & Tissing, 2010).
Psychoneurological symptoms (PNS).
PNS, including pain, fatigue, anxiety, depression, sleep disturbance, and cognitive impairment, are prevalent among cancer populations undergoing cancer treatments. Recent studies have demonstrated associations between the gut microbiome and PNS (J. Bai et al., 2020; González-Mercado et al., 2020). Higher PNS was associated with decreased microbial diversity, a lower abundance of Firmicutes, Ruminiclostridium, Phascolarctobacterium, Subdoligranulum, but a higher abundance of Bacteroidetes, Anaerofustis, Tyzzerella, Intestinimonas, and Family XIII AD3011. Patients with lower PNS had a higher abundance of Lactococcus, Phascolarctobacterium, Acidaminococcaceae, and Desulfovibrio than those with a higher PNS (J. Bai et al., 2020). In rectal cancer patients treated with chemoradiation therapy, an enriched abundance of Bacteroides, Blautia1, Ruminococcaceae, Oscillibacter, and Lactobacillus was observed among patients with no symptoms. In contrast, lower alpha diversity and higher abundance of Blautia2 were reported among patients with two or more PNS (González-Mercado et al., 2020). Associations between the gut microbiome and PNS in children undergoing chemotherapy and RT are limited and require further studies. The gut microbiome may modulate the occurrence and severity of PNS via endocrine, immune, and neural pathways through the microbiome-gut-brain axis (Song & Bai, 2020), a bi-directional network to connect the communications between the gut and the brain.
Human microbiome-related interventions.
Several interventions, including probiotics, prebiotics, or fecal microbiome transplant (FMT), have been studied and found to prevent or treat dysbiotic gut microbiome in various chronic conditions, including cancer (Figure 2). Probiotics, defined as living bacteria that can benefit the host’s health via changing the gut microbiome, can positively affect cancer patients by reducing their experience of PNS. A 12-week probiotics treatment (i.e., Lacidofil) in colorectal cancer patients resulted in a significant reduction in patients suffering from irritable bowel symptoms and improved colorectal cancer-related anxiety, depression, and quality of life (QOL) (Lee et al., 2014). Prebiotics are defined as non-digestible food ingredients that can benefit the hosts’ health by changing the composition and function of the gut microbiome. The effects of prebiotics, such as enteral formula containing fermentable dietary fibers (i.e., fructooligosaccharides), have been examined in the gut microbiome dysbiosis in childhood cancer patients (Zheng et al., 2006). In contrast, FMT is rarely examined in cancer populations. Further studies are needed to understand the mechanism by which prebiotics, probiotics, or FMT mediate protection against cancer therapy symptoms and to help define specific interventions to diminish the adverse effects of cancer treatments.
Figure 2.
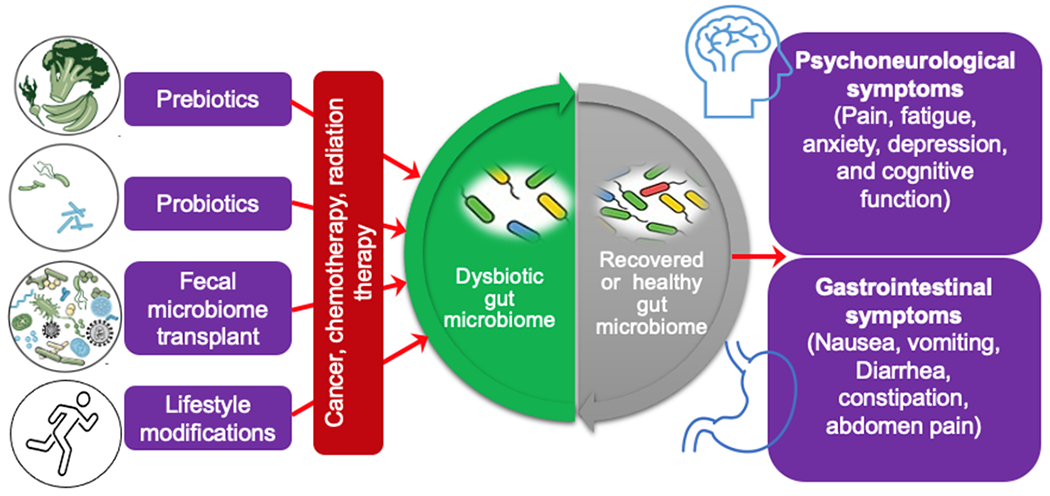
Target the gut microbiome for therapeutic management of cancer treatment-related symptoms. Based on the current knowledge of the bacterial microbiome in cancer treatment-related symptoms, prebiotics, probiotics, fecal microbiome transplantation, and lifestyle changes are suggested approaches for the early prevention and management of cancer treatment-related symptoms via adjusting the gut microbiome.
Nursing Implications
Understanding the role of the gut microbiome in oncology care helps provide nurses with the tools to monitor risks for dysbiotic gut microbiome, screen cancer treatment-related gastrointestinal symptoms and PNS, and prompts nurses to educate patients on interventions that can improve gut health. First, oncology nurses need to understand the potential risk factors for gut microbiome dysbiosis. Many cancer treatments increase the risk of gut microbiome dysbiosis. Second, oncology nurses can use the gut microbiome to screen and monitor cancer treatment-related gastrointestinal and PNS symptoms that may affect cancer patients’ QOL. Third, oncology nurses can guide patients to maintain a healthy gut microbiome. While personalized interventions, such as FMT, are still under development, nurses can educate patients on how to maintain a healthy gut microbiome by using a healthy diet and lifestyle, prebiotics, and probiotics.
Conclusion
Cancer treatment can lead to a dysbiotic gut microbiome, specifically associated with gastrointestinal symptoms and PNS, leading to a decrease in a patient’s QOL. By understanding the role of the gut microbiome, oncology nurses can screen patients with a dysbiotic gut microbiome, examine associations between the gut microbiome and treatment toxicities and symptoms, and guide patients to adjust their diet or to consider use of probiotics to build a healthy gut microbiome.
Acknowledgement:
Support by grants from the National Institute of Health/National Institute of Nursing Research (1K99NR017897 and 4R00NR017897, PI: Jinbing Bai), NCI R25CA203650 (PI: Melinda Irwin). We thanked Mrs. Rebecca Meador for editing this paper.
References
- Bai J, Bruner DW, Fedirko V, Beitler JJ, Zhou C, Gu J, … Xiao C (2020). Gut microbiome associated with the psychoneurological symptom cluster in patients with head and neck cancers. Cancers, 12(9), 2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Bruner DW, Fedirko V, Beitler JJ, Zhou C, Gu J, … Xiao C (2020). Gut Microbiome Associated with the Psychoneurological Symptom Cluster in Patients with Head and Neck Cancers. Cancers (Basel), 12(9). doi: 10.3390/cancers12092531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mercado VJ, Henderson WA, Sarkar A, Lim J, Saligan LN, Berk L, … Melkus GD (2020). Changes in Gut Microbiome Associated With Co-Occurring Symptoms Development During Chemo-Radiation for Rectal Cancer: A Proof of Concept Study. Biol Res Nurs, 1099800420942830. doi: 10.1177/1099800420942830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy Megan S., & Chang Eugene B. (2020). Chapter One - The microbiome: Composition and locations. In Kasselman Lora J. (Ed.), Progress in Molecular Biology and Translational Science (Vol. 176, pp. 1–42): Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight Rob, & Buhler Brendan. (2015). Follow your gut : the enormous impact of tiny microbes (First TED Books hardcover edition. ed.).
- Lee Jee-Yon, Chu Sang-Hui, Jeon Justin Y., Lee Mi-Kyung, Park Ji-Hye, Lee Duk-Chul, … Kim Nam-Kyu. (2014). Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis, 46(12), 1126–1132. doi: 10.1016/j.dld.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Marchesi Julian R., & Ravel Jacques. (2015). The vocabulary of microbiome research: a proposal. Microbiome, 3(1), 31. doi: 10.1186/s40168-015-0094-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra Aparna, Biegert Greyson Willis Grossman, Delgado Andrea Y, Karpinets Tatiana V, Solley Travis N, Mezzari Melissa P, … Lin Lilie. (2020). Microbial Diversity and Composition Is Associated with Patient-Reported Toxicity during Chemoradiation Therapy for Cervical Cancer. International Journal of Radiation Oncology* Biology* Physics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montassier E, Batard E, Massart S, Gastinne T, Carton T, Caillon J, … de La Cochetiere MF (2014). 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb Ecol, 67(3), 690–699. doi: 10.1007/s00248-013-0355-4 [DOI] [PubMed] [Google Scholar]
- Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley des Varannes S, Massart S, … Knights D (2015). Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther, 42(5), 515–528. doi: 10.1111/apt.13302 [DOI] [PubMed] [Google Scholar]
- Rajagopala Seesandra V., Yooseph Shibu, Harkins Derek M., Moncera Kelvin J., Zabokrtsky Keri B., Torralba Manolito G., … Nelson Karen E. (2016). Gastrointestinal microbial populations can distinguish pediatric and adolescent Acute Lymphoblastic Leukemia (ALL) at the time of disease diagnosis. BMC Genomics, 17(1), 1–10. doi: 10.1186/s12864-016-2965-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild Daphna, Weissbrod Omer, Barkan Elad, Kurilshikov Alexander, Korem Tal, Zeevi David, … Segal Eran. (2018). Environment dominates over host genetics in shaping human gut microbiota. Nature, 555, 210. doi: 10.1038/nature25973 [DOI] [PubMed] [Google Scholar]
- Roy S, & Trinchieri G (2017). Microbiota: a key orchestrator of cancer therapy. Nature Reviews: Cancer, 17(5), 271–285. doi: 10.1038/nrc.2017.13 [DOI] [PubMed] [Google Scholar]
- Sahly Nourhan, Moustafa Ahmed, Zaghloul Mohamed, & Salem Tamer Z. (2019). Effect of radiotherapy on the gut microbiome in pediatric cancer patients: a pilot study. PeerJ, 7, e7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, … Liao W (2017). Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 15(1), 73. doi: 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BC, & Bai J (2020). Microbiome-gut-brain axis in cancer treatment-related psychoneurological toxicities and symptoms: a systematic review. Support Care Cancer. doi: 10.1007/s00520-020-05739-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprockett D, Fukami T, & Relman DA (2018). Role of priority effects in the early-life assembly of the gut microbiota. Nat Rev Gastroenterol Hepatol, 15(4), 197–205. doi: 10.1038/nrgastro.2017.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, … de La Cochetiere MF (2014). Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Alimentary Pharmacology & Therapeutics, 40(5), 409–421. doi: 10.1111/apt.12878 [DOI] [PubMed] [Google Scholar]
- van Vliet MJ, Harmsen HJM, de Bont ESJM, & Tissing WJE (2010). The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog, 6(5), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet MJ, Tissing WJ, Dun CA, Meessen NE, Kamps WA, de Bont ES, & Harmsen HJ (2009). Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis, 49(2), 262–270. doi: 10.1086/599346 [DOI] [PubMed] [Google Scholar]
- Wang Zhongqiu, Wang Qingxin, Wang Xia, Zhu Li, Chen Jie, Zhang Bailin, … Zhiyong Yuan. (2019). Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. Journal of cellular and molecular medicine, 23(5), 3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Steenhout P, Kuiran D, Qihong W, Weiping W, Hager C, … Clemens RA (2006). Nutritional support of pediatric patients with cancer consuming an enteral formula with fructooligosaccharides. Nutrition Research, 26(4), 154–162. [Google Scholar]


