Abstract
Measles elimination hinges on vaccination coverage remaining above 95% to retain sufficient community protection. Recent declines in routine measles vaccinations due to the COVID-19 pandemic coupled with prior models indicating the country was close to the 92% herd immunity benchmark are a cause for concern. We evaluated population-level measles susceptibility in the US, including sensitivity analyses accounting for pandemic-related impacts on immunization. We estimated the number of children aged 0–18 currently susceptible to measles and modeled susceptibility proportions in decreased vaccination scenarios. Participants were respondents to the NIS-Teen survey between 2008 and 2017 that also had provider-verified vaccination documentation. The exposure of interest was vaccination with a measles-containing vaccine (MCV), and the age at which they were vaccinated for all doses given. Using age at vaccination, we estimated age-based probabilities of vaccination and modeled population levels of MCV immunization and immunity vs. susceptibility. Currently, 9,145,026 children (13.1%) are estimated to be susceptible to measles. With pandemic level vaccination rates, 15,165,221 children (21.7%) will be susceptible to measles if no attempt at catch-up is made, or 9,454,436 children (13.5%) if catch-up vaccinations mitigate the decline by 2–3%. Models based on increased vaccine hesitancy also show increased susceptibility at national levels, with a 10% increase in hesitancy nationally resulting in 14,925,481 children (21.37%) susceptible to measles, irrespective of pandemic vaccination levels. Current levels of measles immunity remain below herd immunity thresholds. If pandemic-era reductions in childhood immunization are not rectified, population-level immunity to measles is likely to decline further.
Keywords: Measles, MMR vaccine, Vaccine uptake, COVID-19, Vaccine hesitancy, NIS-Teen
1. Introduction
Measles vaccine coverage in the US is high: 90.8% of children received at least one measles, mumps, and rubella (MMR) vaccine by 24 months old [1]. Due to high vaccine coverage [2], [3], in 2000, the World Health Organization (WHO) declared measles eliminated from the United States (US) [4].
Despite high MMR vaccine coverage, international travel in areas of endemic measles still poses a risk of outbreaks in the US. The US Centers for Disease Control and Prevention (CDC) reported in 2019 the US experienced its highest number of measles cases since 1992: 1,282, with 1,249 cases documented from January-September 2019 [5]. 86% of cases were associated with tight-knit communities with low vaccine acceptance, illustrating that while national coverage remains high, sustained transmission can remain in communities with low vaccination coverage due to vaccine hesitance [6], [7]. The effects of vaccine hesitancy are compounded by additional barriers to vaccination, including poverty and lack of health insurance [8], [9]. The WHO specifies measles elimination as the absence of endemic transmission for 12 or more months in a region with a verified surveillance system. Although the outbreak in 2019 was large, the US kept its measles elimination status when the outbreak ended just short of 12 months of transmission [7].
Due to the COVID-19 pandemic, routine childhood vaccination coverage in the US has been negatively impacted. Although these declines and recovery vary by age, estimates range from 10 to 35% decreases, including measles-containing vaccines (MCV) [10], [11], [12], [13], [14]. The highly contagious nature of measles, coupled with the additive nature of each birth cohort’s unimmunized children adding to the susceptible pool without intervention, and similar pandemic-era delays in MCV campaigns globally, means that larger clusters of measles-susceptible children could reignite endemic transmission in the US [15].
A 2016 modeling study estimated 1 in 8 US children under the age of 18 are potentially susceptible to measles. This study considers vaccination coverage, which is consistently high nationally, and addresses issues surrounding vaccination such as waning immunity, vaccine efficacy, immunization timing, and loss of immunity due to chemotherapies. While only 1 out of 10 children in the US go unvaccinated, likely 1 out of 8 children are susceptible to measles. This study also could not account for the impact of COVID-19 pandemic-related drops in vaccination on measles susceptibility in the US [15]. To address this gap, we updated our prior modeling study, accounting for drops and potential recovery of childhood measles vaccination in the US during the COVID-19 pandemic.
2. Materials and methods
All analyses used public NIS-Teen datasets from 2008 to 2017. NIS-Teen is a nationally representative survey of parents of adolescents aged 13–17 living in the United States. In addition to routine adolescent vaccinations, NIS-Teen also collects data on measles containing vaccines (MCV) coverage. The CDC carries this out using household and cell phone surveys for the general public and mailed surveys for medical providers to verify patient vaccinations [16]. As part of the provider verification process, age at receipt of each MCV dose is assessed [17]. The CDC also completes a similar NIS-Child survey, but given our interest in evaluating national susceptibility up to 18 years of age, the NIS-Teen survey was selected as the datasets for the baseline model and sensitivity analyses.
Provider-verified data were used to compute age- and dose-specific probabilities of receipt of MCV. All analyses were conducted using SAS version 9.4 (Cary, NC), using PROC SURVEYFREQ and weighting for complex survey analysis [17], [18]. Only children with provider-verified immunization data were included in this analysis.
Estimates of measles-containing vaccine coverage were generated by taking the probabilities of the first and second dose administrations at each individual age, from 0 to 17 years. These age- and dose-specific data were aggregated and birth cohorts representing the years that surveyed children were born (1990–2005) were gathered from the National Center for Health Statistics [19]. The smallest birth cohort size (1997; n = 3,880,894) was used for all years to calculate the most conservative, lowest whole number of un- and under-vaccinated children [20].
Aggregated data was used to generate conditional probabilities for each age and both MCV doses. First-dose probabilities were incorporated into the model directly, while second-dose probabilities were summed across the remaining ages after the first dose. For example, a child aged 5 years old receiving their first dose could only receive a second dose at age 5 or older. These summed second dose probabilities were then added to the model as the probability of a second dose, given the first was received. Measles, mumps, and rubella vaccine (MMR), the most commonly given MCV, has an estimated effectiveness of 93% (single dose) and 97% (two doses) against measles [21]; these values were incorporated into the model to account for susceptibility among vaccinated children.
We assumed mothers were immune to measles, and passive protection from maternally transferred anti-measles antibodies lasted approximately 6 months after birth [22], [23]. Because this model increments age by years, we assumed that half of all children under 1 year of age were immune to measles.
Some cancer treatments have been shown to decrease antibody titers below those that would confer immunity. Current literature indicates this can occur in approximately 25% of the children undergoing therapies [24], [25]. We used American Cancer Society estimates that 1 in 285 children under 19 years old will contract cancer [26], prorated this estimate across the ages under study, and assumed that 50% of children with cancer would undergo therapies, and 25% of those children would lose functional immunity.
Three sensitivity analyses were performed to simulate 1) sustained current pandemic conditions, 2) conditions 5 years post-pandemic, and 3) conditions surrounding increased vaccine hesitancy possibly arising after the implementation of the COVID-19 vaccination program. The baseline model and sensitivity analyses were plotted as age (0 to 17 years) against the percentage of the population considered immune.
Well-child visit rates have dropped substantially due to COVID-19 beginning in spring 2020 [10], [11], [12], [13], [14], with related decreases in administration of MMR vaccines to half of the documented rates in the same month of the prior year in some pediatric clinics [10], [11], including MMR vaccinations [12]. To model this decrease conservatively, we reduced the probabilities of receiving either the first or second dose at each age, based on the baseline model, proportionally, assuming that parents of younger children would be more hesitant towards COVID-19 exposure than those of older children [27]. For ages 0–5, 6–10, and 11–17, we estimated 10%, 7%, and 5% decreases in vaccination, respectively.
To model impacts over the next five years, we assumed that those children born within that five-year window would not have decreased vaccination rates; we thus returned the ages 0–5 vaccination probabilities to their values in the baseline model as the “normal” schedule. Ages 6–10 remained at 7% decrease and ages 11–17 remained at 5% decreased to simulate only a partial attempt at a catch-up campaign.
We assumed current vaccine hesitancy for MCVs to be accounted for by the baseline model, in a category that indicates how many children missed doses, for any reason. We assumed that 30% of the population would express hesitancy related to COVID-19 vaccines [27]. To account for possible increased hesitancy towards MCVs concurrent with increased hesitancy related to COVID-19 vaccines, we modeled proportional decreases in select age groups. We again assumed that parents of younger children are more vaccine hesitant than those with older children, decreasing vaccination probabilities by age (10%, 7%, 5%, and 3% decreases for ages 0–2, 3–6, 7–13, and 14–17 years, respectively) [28], [29].
3. Results
3.1. Baseline model results
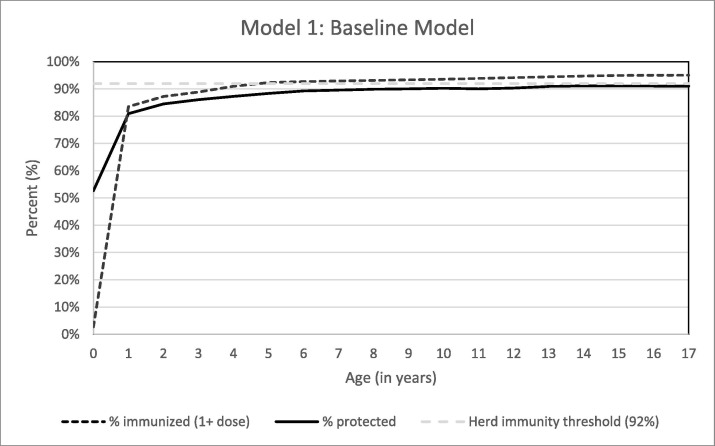
Among birth cohorts 2000–2017 for ages 0–17, we estimated approximately 9,145,026 children (13.1%) are susceptible to measles, with 60,711,066 (86.9%) of children immune to measles. Age-specific immunity increases as age groups increase, from 52.6% of children less than one year old, to 91.0% of children aged 17 years (Fig. 1 ).
Fig. 1.
Percent of Children Immunized and Protected from Measles.
3.2. Sensitivity analysis results
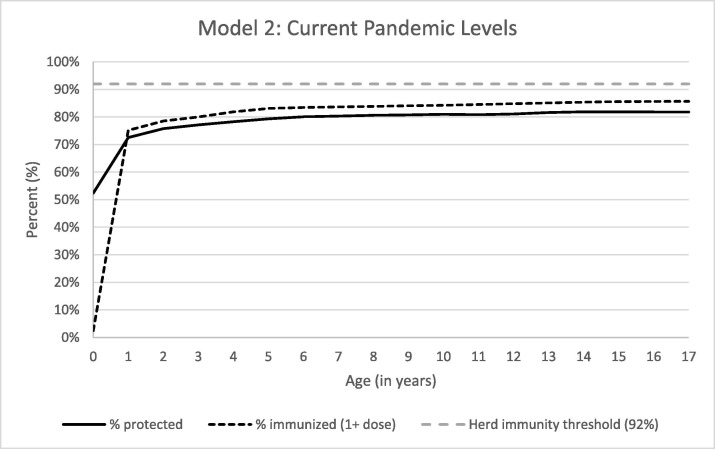
With assumptions simulating no sufficient recovery in MCV uptake, we estimated that the number of susceptible children would rise to 15,165,221 (21.7%) children, with declines in all age-specific immunity levels compared to the baseline model. Age-specific immunity was also decreased from age 1 year onwards compared to the baseline. Percent immunity for any age group did not reach 80% until 6-year-olds, the latest age recommended for second MMR dose, and only reaching a high of 81.9% by 15 years of age, where in the baseline model at least 80% immunity occurs by 1-year of age (Fig. 2 ).
Fig. 2.
Percent of Children Immunized and Protected from Measles Based on Current Vaccination Rates.
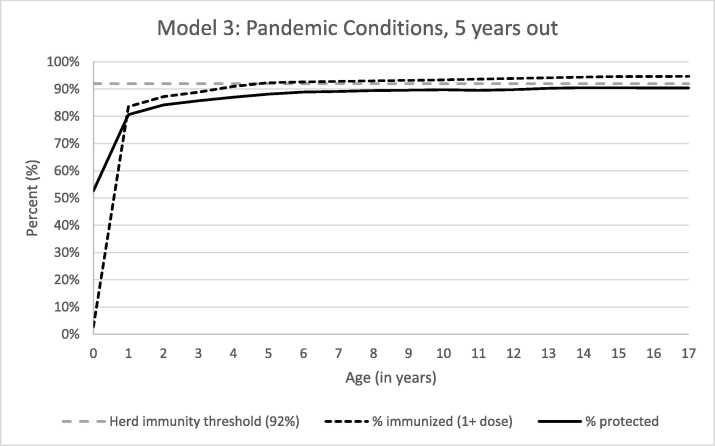
In the model assuming at least partial recovery in MCV uptake, we estimated 9,45,436 children (13.5%) would be susceptible to measles 5 years after pandemic end. Age-specific immunity for ages 6 to 17-years old was lower than in the baseline model. Immunity for ages 13 to 17-years old exceeded 90%, though the highest age-specific immunity (90.5% for ages 14 and 15) did not meet the herd immunity threshold (Fig. 3 ).
Fig. 3.
Percent of Children Immunized and Protected from Measles, 5 Years Post-Pandemic.
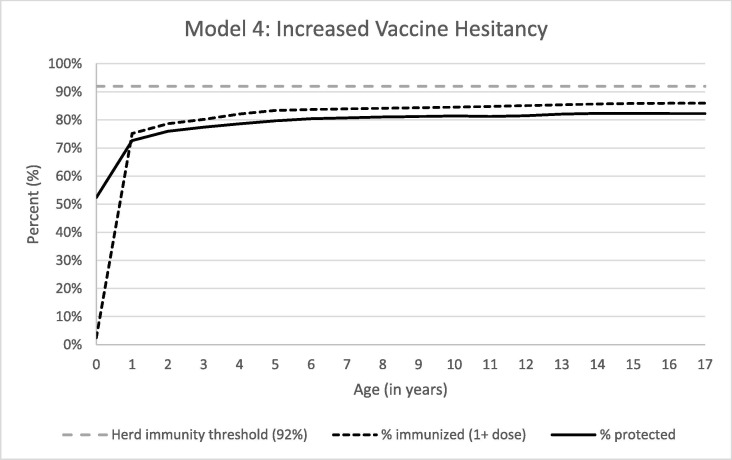
With the baseline model and assumptions for increased vaccine hesitancy based around COVID-19 vaccine hesitancy spillover, we estimated that 14,925,481 (21.4%) children would be susceptible. Age-specific immunity again did not exceed 90% and did not surpass 80% until age 6 (Fig. 4 ).
Fig. 4.
Percent of Children Immunized and Protected from Measles with Increased Vaccine Hesitancy.
4. Discussion
This model updated measles susceptibility estimates in the US, considering several COVID-19 pandemic and post-pandemic effects on vaccine coverage [15]. Children in birth cohorts from 2000 to 2017 failed to meet the herd immunity threshold for measles immunity of 92% [30]. This coverage estimate is based on a wide variety of characteristics, including maternal antibodies and cancer prevalence. Due to the previous successes of US vaccination programs, outbreaks in recent years have largely been confined to communities with recent international travel and low vaccine coverage. Given the vaccination gaps illustrated in the 2019 US Measles outbreak, however, we can ill-afford complacency with regard to vaccination rates.
We also assessed age-specific immunity to measles; no individual age group was estimated to meet the 92% benchmark. These are national-level estimates, which can mask clusters of unprotected children. As seen in the 2019 outbreak, pockets of unvaccinated children remain a source of measles transmission [7]. Given the decline of the national immunity levels, it is likely these communities have grown in size. In the surrounding areas, individuals may have a higher vaccination coverage, but may also have a higher exposure rate. This increases the risk not only of outbreaks in these unvaccinated communities, but in the wider area surrounding them as well.
While the percentage of children who have received one dose of MCV remains above 92%, numbers of functionally immune do not. This illustrates the multipronged problem of immunization coverage vs. functional immunity, including factors such as vaccine effectiveness, number of doses, loss of immunity, and other factors not covered by this model. Thus, when vaccination coverage dips slightly, it can result in gaps in immunity leading to outbreaks.
We conducted sensitivity analyses based on reports of decreased well-child and immunization visits across the United States during the COVID-19 pandemic. Estimates of the approximate decrease in childhood vaccinations vary, with estimates in reduction of vaccine coverage ranging from approximately 25% to 50% [10], [11], [12], [13], [14]. For current (2021) pandemic-level vaccination rates, 21.7% of all children aged 0–17 years would be susceptible to measles. Results from these analyses re-emphasize the importance of high vaccine coverage, and the reality that even a modest decline could result in the re-emergence of measles as an endemic virus.
Small clusters of unvaccinated children have been at high risk for measles each time an internationally-sourced outbreak occurs [7]. These outbreaks occurred despite maintenance of population immunity above the threshold range of 92%-95%. Further reductions in population immunity increase the possibility of larger, longer measles outbreaks.
COVID-19 pandemic mitigation measures are likely responsible for low measles transmission in the US in 2020–21 [6]. However, it is imperative to focus efforts on recovering from pandemic-related drops in vaccination coverage. Interruptions in childhood immunization schedules globally mean an increased risk of measles outbreaks globally [31]. Increases in international travel and increasing numbers of pockets of susceptible children in the US may lead to more and larger measles outbreaks as travel and distancing restrictions are lifted [32].
One sensitivity analysis shows a potential future in which decreased vaccination rates are not addressed, and no attempt for a “catch up” campaign is made. Such a scenario is unlikely but illustrates a “floor” from which these catch-up rates can be compared. If vaccination rates decrease across age groups even by 10%, the percent of children functionally immune against measles declines to 78.3% (compared to 86.9% immune in the baseline model). In addition to this population-level decrease, age-specific immunity also suffers, with no age group reaching greater than 82% immunity. Measles attack rates are estimated to be 90% among unvaccinated people living in close contact, meaning the virus has a very high critical vaccination threshold required to interrupt transmission in potential outbreaks [33].
A second sensitivity analysis estimates measles immunity in post-pandemic years with a policy that ignores vaccination gaps that began during the pandemic, and instead focuses solely on ensuring that children born during and after the pandemic do not fall behind on the immunization schedule. This analysis reveals that while age-specific immunity for those children (aged 0 to 5 years) returned to rates seen in the baseline model, age-specific rates for those children 6 and older remain decreased. This decrease results in 86.5% immunity at the population level, with only adolescents 13–17 reaching over 90% immunity. This model also fails to achieve herd immunity threshold in all age groups and overall. This method of attempting to close the gap is not sufficient. By failing to address the gaps among those children during the pandemic, clusters of school aged children will remain unprotected and at risk for propagating outbreaks in the community. This, coupled with the pandemic-related disruption for in-person schooling could also cause school tracking and enforcement of vaccination requirements to become increasingly difficult, illustrating the need for continued vigilance and monitoring to ensure appropriate catch-up vaccination. This highlights that strict enforcement of vaccine requirements by schools and encouragement from pediatricians is vital to conducting meaningful vaccine catch-up efforts.
A final sensitivity analysis investigated increased vaccine hesitancy and a subsequent decline in numbers of children vaccinated with MCV. According to Mayo Clinic, approximately 22% of eligible people in the US have not had any COVID-19 vaccinations, with 77% partially vaccinated and only 66% considered fully vaccinated [27]. Another Gallup poll shows approximately 11% of US adults think that vaccines are more dangerous than helpful, with most of those answers coming from adults with lower education levels, older than 50, and parents with children under the age of 18 [34]. With this model, we illustrate the deadliness of vaccine hesitancy, including possibilities of spillover hesitancy related to the current COVID-19 pandemic and concerns about the development of the COVID-19 vaccine.
Hesitancy towards COVID-19 vaccines does not exist in a vacuum, and this model explores the potential effect of the COVID-19 pandemic on hesitancy towards MCVs. Increased vaccine hesitancy that results in 10% of each age group missing MCVs results in coverage similar to the first sensitivity analysis with COVID-19 disruptions alone. Increased awareness and positive perceptions resulting from established public health programs could possibly result in rebound vaccination. Hesitancy towards the COVID-19 vaccines could also be increased due to extenuating circumstances such as Emergency Use Authorization or novel technologies unfamiliar to laypeople. Without current evidence of these occurrences, however, we felt that to model using the worst-case scenario is more useful for establishing a baseline for vaccine recovery efforts.
This study has some limitations. First, the aggregated data used to record the age at first and subsequent doses for MCVs was based on survey responses via the CDC’s annual NIS-Teen Survey. This dataset contains deidentified data aggregated without respect to geographical area, resulting in variations across the country in terms of vaccination rates and age-specific vaccination probabilities. Estimates of population level immunity do not directly correlate with sustained transmission of measles in the US, but they can serve as sentinels for potential sustained transmission. Additionally this model used only those survey responses for which provider-verification of immunizations could be obtained, possibly excluding those children who are not regularly followed by a pediatrician.
Maternal antibodies transferred via the placenta can provide a transient period of immunity for infants. However, the exact age at which this immunity wanes below protective levels is unclear and may occur as early as 6 months or as late as 12 months. Given this variability, we chose to model the effects of maternal antibodies as a percentage of children under one year of age that were functionally immune.
Diagnoses of cancer and subsequent immunity loss from suppressive therapies were based on percentages given by the American Cancer Society and were not based in actual incidence for each study year. This could result in more/fewer children with cancer, and as a result, more/fewer children having undergone chemotherapies that cause their vaccine conferred immunity to dip below protective levels. Though the numbers for those treated with chemotherapies and those that subsequently lost immunity were based on assumptions, given how small a proportion children with cancer make up, estimates of immunity for the population would largely be unaltered unless those numbers fluctuated a great deal.
Finally, though lost immunity due to cancer and immunosuppressive therapies was added into the model to give a better representation of functional immunity at a population level, there is evidence to suggest that immunity conferred by the MMR vaccine, can wane over time, potentially leaving more adults vulnerable [35]. More research is needed to assess how waning immunity functions within the scope of outbreak prevention and population immunity for those that age out of pediatric vaccination schedules.
5. Conclusions
Highly infectious diseases like measles act as the proverbial canary in the coal mine, identifying weaknesses in public health infrastructure. Where childhood vaccination rates dip, measles will often be the first pathogen to re-emerge. Our analyses illustrate a reduction in measles immunity below herd immunity thresholds, consistent across several different policy conditions. The COVID-19 pandemic provides a chance to strengthen our vaccination policies, practices, and condemnation of misinformation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
National Immunization Survey-Teen datasets for 2008 – 2017 are online and available from the Centers for Disease Control and Prevention in Atlanta, Georgia (https://www.cdc.gov/nchs/nis/data_files_teen.htm & https://www.cdc.gov/vaccines/imz-managers/nis/datasets-teen.html)
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the National Center for Health Statistics or the Centers for Disease Control and Prevention.
References
- 1.FastStats. 2021, August 3. Available from: https://www.cdc.gov/nchs/fastats/immunize.htm.
- 2.Carlson A., Riethman M., Gastañaduy P., Lee A., Leung J., Holshue M., et al. Notes from the Field: community outbreak of measles — Clark County, Washington, 2018–2019. MMWR Morb Mortal Wkly Rep. 2019;68(19):446–447. doi: 10.15585/mmwr.mm6819a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald R., Ruppert P.S., Souto M., Johns D.E., McKay K., Bessette N., et al. Notes from the Field: measles outbreaks from imported cases in Orthodox Jewish Communities — New York and New Jersey, 2018–2019. MMWR Morb Mortal Wkly Rep. 2019;68(19):444–445. doi: 10.15585/mmwr.mm6819a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summary and Conclusions: Measles Elimination Meeting, 16–17 March 2000. J Infect Dis 2004;189(Supplement_1):S43–7. [DOI] [PubMed]
- 5.Measles Elimination in the U.S. | CDC. 2020, November 5. Available from: https://www.cdc.gov/measles/elimination.html.
- 6.CDC. 2020, December 2. Measles cases and outbreaks. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/measles/cases-outbreaks.html.
- 7.Patel M., Lee A.D., Clemmons N.S., Redd S.B., Poser S., Blog D., et al. National update on measles cases and outbreaks — United States, January 1–October 1, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(40):893–896. doi: 10.15585/mmwr.mm6840e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W., Elam-Evans L.D., Hill H.A., Yankey D. Employment and socioeconomic factors associated with children’s up-to-date vaccination status. Clin Pediatr. 2017;56(4):348–356. doi: 10.1177/0009922816660540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill H.A., Elam-Evans L.D., Yankey D., Singleton J.A., Kang Y. Vaccination coverage among children aged 19–35 months — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(40):1123–1128. doi: 10.15585/mmwr.mm6740a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques-Carroll LA, et al. Decline in child vaccination coverage during the COVID-19 pandemic—Michigan Care improvement registry, May 2016–May 2020. 2, n.d. [DOI] [PubMed]
- 11.Hoffman J. Vaccine rates drop dangerously as parents avoid doctor’s visits. The New York Times. 2020, April 23. Available from: https://www.nytimes.com/2020/04/23/health/coronavirus-measles-vaccines.html.
- 12.Santoli J.M., Lindley M.C., DeSilva M.B., Kharbanda E.O., Daley M.F., Galloway L., et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):591–593. doi: 10.15585/mmwr.mm6919e2. [DOI] [PubMed] [Google Scholar]
- 13.Patel Murthy B., Zell E., Kirtland K., Jones-Jack N., Harris L., Sprague C., et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations — 10 U.S. Jurisdictions, March–September 2020. MMWR Morb Mortal Wkly Rep. 2021;70(23):840–845. doi: 10.15585/mmwr.mm7023a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSilva M.B., Haapala J., Vazquez-Benitez G., Daley M.F., Nordin J.D., Klein N.P., et al. Association of the COVID-19 pandemic with routine childhood vaccination rates and proportion up to date with vaccinations across 8 US Health Systems in the vaccine safety datalink. JAMA Pediatrics. 2022;176(1):68–77. doi: 10.1001/jamapediatrics.2021.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bednarczyk R.A., Orenstein W.A., Omer S.B. Estimating the number of measles-susceptible children and adolescents in the United States using data from the national immunization survey-teen (NIS-Teen) Am J Epidemiol. 2016;184(2):148–156. doi: 10.1093/aje/kwv320. [DOI] [PubMed] [Google Scholar]
- 16.Vaccination Coverage | NIS Child | 2011 Adding Cell Phone Use | CDC. 2019, March 14. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/nis/child/dual-frame-sampling.html.
- 17.NIS | About the National Immunization Surveys | Vaccines | CDC. (2021, January 25). Available from: https://www.cdc.gov/vaccines/imz-managers/nis/about.html.
- 18.NIS | NIS-Teen Data and Documentation for 2015 to Present | National Immunization Surveys | Vaccines | CDC. 2021, January 25. Available from: https://www.cdc.gov/vaccines/imz-managers/nis/datasets-teen.html.
- 19.NIS - Datasets for the National Immunization Survey—Teen. 2019, March 2. Available from: https://www.cdc.gov/nchs/nis/data_files_teen.htm.
- 20.NCHS Data Visualization Gallery—Natality Trends in the United States. 2020, January 9. Available from: <https://www.cdc.gov/nchs/data-visualization/natality-trends/index.htm>.
- 21.Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps, 2013. n.d. Retrieved May 26, 2022, from https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm#Tab1.
- 22.Gans H., Yasukawa L., Rinki M., DeHovitz R., Forghani B., Beeler J., et al. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis. 2001;184(7):817–826. doi: 10.1086/323346. [DOI] [PubMed] [Google Scholar]
- 23.Leuridan E., Hens N., Hutse V., Ieven M., Aerts M., Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: Longitudinal study. BMJ (Clinical Research Ed) 2010;340(may18 2):c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
- 24.Bochennek K., Allwinn R., Langer R., Becker M., Keppler O.T., Klingebiel T., et al. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine. 2014;32(27):3357–3361. doi: 10.1016/j.vaccine.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 25.Zignol M., Peracchi M., Tridello G., Pillon M., Fregonese F., D’Elia R., et al. Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy. Cancer. 2004;101(3):635–641. doi: 10.1002/cncr.20384. [DOI] [PubMed] [Google Scholar]
- 26.Cancer Facts & Figures 2014. n.d. Retrieved February 14, 2021, from https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2014.html.
- 27.U.S. COVID-19 vaccine tracker: See your state’s progress. n.d. Mayo Clinic. Retrieved May 26, 2022, from https://www.mayoclinic.org/coronavirus-covid-19/vaccine-tracker.
- 28.Funk C. n.d. Parents of young children are more ‘vaccine hesitant.’ Pew Research Center. Retrieved November 18, 2020, from https://www.pewresearch.org/fact-tank/2017/02/06/parents-of-young-children-are-more-vaccine-hesitant/.
- 29.He K., Mack W.J., Neely M., Lewis L., Anand V. Parental perspectives on immunizations: impact of the COVID-19 pandemic on childhood vaccine hesitancy. J Community Health. 2022;47(1):39–52. doi: 10.1007/s10900-021-01017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plotkin SA, Orenstein W, Offit PA, Edwards KM. 2017. Vaccines E-book. Elsevier. Available from: http://ebookcentral.proquest.com/lib/emory/detail.action?docID=5508004.
- 31.Durrheim D.N., Andrus J.K., Tabassum S., Bashour H., Githanga D., Pfaff G. A dangerous measles future looms beyond the COVID-19 pandemic. Nat Med. 2021;27(3):360–361. doi: 10.1038/s41591-021-01237-5. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar S., Zlojutro A., Khan K., Gardner L. Measles resurgence in the USA: How international travel compounds vaccine resistance. Lancet Infect Dis. 2019;19(7):684–686. doi: 10.1016/S1473-3099(19)30231-2. [DOI] [PubMed] [Google Scholar]
- 33.Pinkbook: Measles | CDC. 2021, April 6. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/meas.html.
- 34.Survey: Anti-Vaccine Arguments Are Starting to Gain Traction. n.d. US News & World Report. Retrieved January 27, 2021, from https://www.usnews.com/news/healthiest-communities/articles/2020-01-14/survey-fewer-people-now-support-vaccinating-their-kids-than-in-2001.
- 35.Seagle E.E., Bednarczyk R.A., Hill T., Fiebelkorn A.P., Hickman C.J., Icenogle J.P., et al. Measles, mumps, and rubella antibody patterns of persistence and rate of decline following the second dose of the MMR vaccine. Vaccine. 2018;36(6):818–826. doi: 10.1016/j.vaccine.2017.12.075. [DOI] [PubMed] [Google Scholar]