Abstract
Objective
This systematic review aimed at estimating the prevalence of post-acute COVID-19 symptoms in view of published literature that studied prolonged clinical manifestations after recovery from acute COVID-19 infection.
Methods
Relevant databases were searched for extraction of articles. For data synthesis, based on the distribution of quantitative variables, they were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Qualitative variables were presented as frequency (n) and percentages (%).
Results
Twenty-one articles qualified for the final analysis. The most common persistent clinical manifestations were fatigue (54.11%), dyspnea (24.38%), alopecia (23.21%), hyperhidrosis (23.6%), insomnia (25.98%), anxiety (17.29%), and arthralgia (16.35%). In addition to these symptoms, new-onset hypertension, diabetes, neuropsychiatric disorders, and bladder incontinence were also reported.
Conclusion
Clinical features of post-acute COVID-19 infection can manifest even after 60 days of initial infection. Multidisciplinary care along with regular follow-up must be provided to such patients.
Keywords: COVID-19, Long COVID, SARS-CoV-2
Highlights
-
•
Clinical features of post-acute COVID-19 infection can manifest even after 60 days of initial infection.
-
•
Prolonged symptoms and signs are being reported in observational studies and case reports every day. Although such symptoms are usually experienced in survivors of critical illness, the post-acute effects of COVID-19 are equally being reported in patients with mild severity of disease who do not require hospitalization.
-
•
Necessary future research includes stratification of these post-acute effects with gender, age, and comorbid conditions in acute, subacute, and chronic phases of the disease.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) presented as clustered cases of atypical pneumonia in the city of Wuhan in the Hubei province of China. In March 2020, coronavirus disease 2019 (COVID-19) was declared as a pandemic by World Health Organization (WHO) and since then approximately 148 million people have been infected with the virus [1]. There is a well-established pool of scientific knowledge about the acute effects of COVID-19 and unprecedented efforts of the scientific community have now shifted towards the long-lasting sequelae of the disease, effects of which are yet to be seen [[2], [3], [4], [5]].
The term “long COVID” was used in social media to indicate persistence of symptoms after weeks or months of recovery from SARS-COV-2 infection. It is also called “post-acute COVID-19 syndrome” due to its remitting and relapsing nature. There can be persistence of one or more symptom or appearance of new symptoms. As most patients with post-acute COVID-19 syndrome are PCR negative, it indicates that there is microbiological recovery. However, there is a time lag between microbiological recovery and clinical recovery. There are several barriers in diagnosing post-acute COVID-19 because the time for clinical recovery varies with severity of illness; while associated complications make it difficult to define the cut-off time for the diagnosis.
Prolonged symptoms and signs are being reported in observational studies and case reports every day [6]. Although such symptoms are usually experienced in survivors of critical illness, the post-acute effects of COVID-19 are equally being reported in patients with mild severity of disease who do not require hospitalization [7]. Therefore, this systematic review was conducted to estimate the prevalence of persisting COVID-19 signs and symptoms after recovery.
2. Methods
2.1. Search strategy
A protocol for the selection of articles and carrying out the systematic review of the literature was made after a consensus among the authors and subject experts, but it was not deposited in a registry. Data was collected after protocol approval from the ethical review board of Foundation University Medical College (ID#FFH/ADC/021/21).
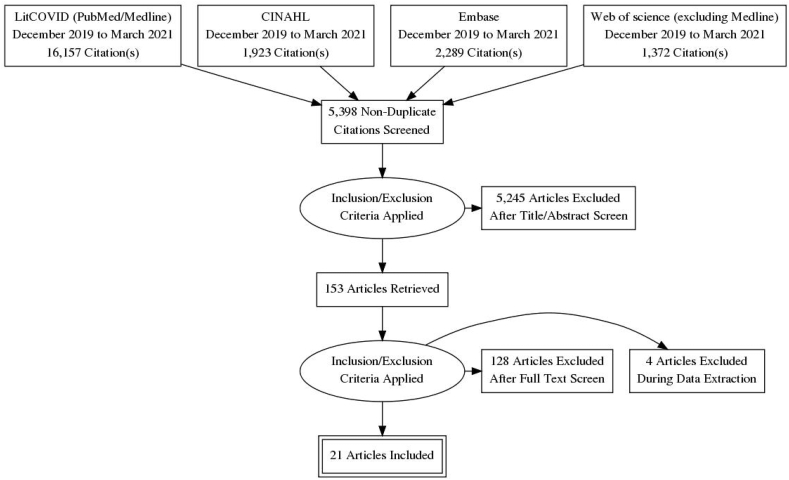
The search terms used in the search strategy were as follows: (((“long-haul” coronavirus disease OR post-acute COVID-19 OR “convalescent” COVID-19) OR prolonged coronavirus infection OR coronavirus disease [Mesh]) OR “severe acute respiratory syndrome coronavirus 2” chronic disease [Supplementary Concept]) OR recurrent OR lingering OR complications of “COVID-19” [Mesh] OR “betacoronavirus” [Mesh])) AND 2019/12 [PDAT]: 2030 [PDAT]))). The systematic review followed the Preferred Reporting Items for Systematic Reviewers and Meta-analysis (PRISMA) guidelines and the PRISMA flowchart is demonstrated in Fig. 1 [9].
Fig. 1.
PRISMA flow chart.
2.2. Selection criteria
The main databases used for study selection were PubMed and Medline through LitCOVID (accessed on 17th April 2021) [8], the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (accessed on 12th April 2021), Embase, and Web of Science (accessed on 12th April 2021). Articles published before 1st May 2021 were included in the search. We included randomized clinical trials, observational, cross-sectional, and cohort studies which were in the English language, and peer-reviewed published articles that reported signs and symptoms after at least two weeks from the recovery of acute COVID-19 in adults. Investigations on children (<18 years) were excluded. Only studies with more than 50 participants were included. Post-acute COVID-19 syndrome was defined as symptomatology after two weeks of recovery from COVID-19.
2.3. Data extraction
Pre-prints, case reports, editorials, and data notes were excluded. After the initial search and removal of duplicates, all the search was imported on EndNote version 20 (Clarivate Analytics™). All the screening and inclusion of the articles were conducted by two independent reviewers (TA, SMJZ) blinded to each other's decisions. Once the initial screening was finished, all the included studies were referenced in Mendeley.
The two reviewers (TA, SMJZ) reviewed full texts for final inclusion. Where there was a dispute, a third reviewer (MA) resolved it between them. The descriptive variables extracted were country, setting, follow-up time, sample size, mean age and percentage of gender, outcomes, symptoms, and signs, and names used for post-acute COVID-19 syndrome. No automation tool was used.
2.4. Data synthesis
For statistical analysis, Statistical Package for the Social Sciences (SPSS) version 26 (IBM Corp. Armonk, NY, USA) was used, and based on the distribution of quantitative variables, they were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Qualitative variables were presented as frequency (n) and percentages (%).
2.5. Risk of bias assessment
All included articles were assessed using the Newcastle-Ottawa Scale (NOS) [10]. Scales are provided resources 1 and 2.
3. Results
A total of 21,741 titles and abstracts were screened for this review. Of these, 153 full texts were reviewed and according to the review protocol, 56 were excluded because of inappropriate sample size, 47 presented acute COVID-19 symptoms, 23 were case series, and 6 excluded as data notes. A total of 21 studies were included for final analysis and review and their general characteristics are shown in Table 2. Many studies assessed a specific long-term symptom after COVID-19 recovery and the PRISMA flowchart for study selection is presented in Fig. 1. A total of 10 studies were from Europe and three from the United States. Others were from Mexico, Saudi Arabia, China, Australia, and Bangladesh. All studies included were on either previously hospitalized or non-hospitalized patients, and most of them had mild, moderate, and severe states of COVID-19 patients. Total follow-up time was more than one month in the majority of the studies and the number of the patient cohort was 54,730 participants with a median age of 54 years. Except for two studies, there was no stratification among gender differences between post-COVID-19 symptoms.
Table 2.
General article characteristics and study population. Standard deviation (SD), interquartile range (IQR).
| # | Author [ref] | Country | Setting | Follow-up (number of days) | Study participants | Sample size (n) | Age; mean ± SD/median (IQR) | Males; % | Outcome variables |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Carfì et al. [14] | Italy | Single-centered | 60 | Patients meeting the following criteria (no fever for 3 consecutive days, improvement in symptoms, and 2 negative test results for SARS-CoV-2 virus 24 h apart) | 143 | 56.5 ± 14.6 | 62.9% | Quality of life assessment after acute COVID-19, length of hospital stay, Number of persistent symptoms. Fever, fatigue, red eyes, chest pain, cough, anosmia, dysgeusia, myalgia, diarrhea |
| 2 | Mandal et al. [16] | London, United Kingdom | Multi-centric (3 hospitals) | 45 | Patients with abnormal blood tests or imaging at discharge. | 384 | 59.9 ± 16.1 | 62% | Symptom persistence including breathlessness, cough, fatigue, and, poor sleep quality. Laboratory parameters including TLC, platlet count, Lymphocyte count, D dimers, LFTs, and CRP levels |
| 3 | Chopra et al. [21] | United States | Multi-centric (38 hospitals) | 60 | ICU/Hospitalized COVID-19 patients discharged between 16 March and July 1, 2020 at 38 hospitals. |
488 | 62 (50–72) years | 51.8% | Mortality and rehospitalization, Primary care follow-up, New/worsened symptoms, Return to normal activity, Emotional impact, Financial loss/impact |
| 4 | El Sayed et al. [17] | Saudi Arabia | Single centered | 14 | Patients of COVID-19 after 2 consecutive negative PCR tests attending pulmonology clinic for follow-up | 200 | 36.58 ± 9.85 | 57% | Assessment of fatigue and anhedonia using validated scales. |
| 5 | Mahmud et al. [6] | Dhaka, Bangladesh | Single centered | 30 | Discharged COVID-19 patients | 355 | 39.8 ± 13.4 | 58.3% | The frequency and interval of a spectrum of post COVID-19 symptoms were assessed. These include post viral fatigue, persistent cough, insomnia, Circadian rhythm sleep disorders, headache, vertigo, Post-exertional dyspnea, rash, pneumonia, restless leg syndrome, chest pain, Adjustment disorder, Nasal blockage, Excessive sweating, Disturbance of memory, New-onset diabetes or hypertension, myalgias, and Precipitation of gout |
| 6 | Carvalho-Schneider et al. [28] | France | Single centered | 60 | Post COVID-19 patients with or without clinical signs of pneumonia but without a need for oxygen therapy (mild/moderate disease) | 150 | 49 ± 15 years | 44% | Persisting symptoms at Day 30 and 60 which included Fever, dyspnea, chest pain, abnormal auscultation, flu-like symptoms, digestive disorders, weight loss, anosmia, palpitations, arthralgia, cutaneous rashes |
| 7 | Marwa et al. [36] | Egypt | Single centered | 14 | Patients recovered from COVID-19 | 287 | 32.3 ± 8.5 | 35.8% | Fatigue, anxiety, joint pain, continuous headache, chest pain, dementia, depression, dyspnea, blurred vision, tinnitus, intermittent fever, obsessive compulsive disorder |
| 8 | Galván-Tejada et al. [32] | Mexico | Multi centeric | 14 | Cases: Patients who had a laboratory-confirmed diagnosis of SARS-CoV-2, and in whom at least fourteen days have passed since the appearance of symptoms. Controls: Patients with no laboratory or clinically proven COVID-19 infection |
141 cases and 78 controls. (Total 218) | Means of 39.14 years for females and 39.01 for males respectively | 49% | Fever, myalgia, rhinorrhea or coryza, asthenia, cough, cephalgia, red eyes, odynophagia, nausea, vomit or diarrhea, anosmia or dysgeusia, stomach pain or discomfort, dyspnea, chills |
| 9 | Moreno-Pérez et al. [18] | Spain | Single centric | 98 | Hospitalized Patients who had laboratory proven SARS-COV-2 | 277 | 56.0 (42.0–67.5) | 52.7% | Post- COVID syndrome. These include pneumonia, fatigue, anosmia, dyspnea, persistent cough, headache fever, diarrhea, neurological symptoms, and laboratory features |
| 10 | Halpin et al. [22] | United Kingdom | Single centered | 30–60 | Hospitalized Patients who had laboratory proven SARS-COV-2 and were discharged from hospital | 100 | For ward patients: 70.5 (20–93) For ICU patients: 58.5 (34–84) | 54% | Fatigue, Breathlessness, Neuropsychological symptoms, Speech and swallowing problems, weight loss/gain, bowel/bladder incontinence, Perceived health, quality of life, and Vocation change since COVID‐19 illness. |
| 11 | Huang et al. [7] | China | Single centered | 186 | patients with laboratory confirmed COVID-19 who were discharged between Jan 7, and May 29, 2020 | 1733 | 57·0 (47·0–65·0) | 52% | Fatigues, sleeping problems, hairloss, anosmia, palpitations, joint pain, decreased appetite, taste disorder, chest pain, myalgias, rashes, swallowing difficulty, Low grade fever, eGFR, and quality of life |
| 12 | Xiong et al. [27] | China | Single centered | 90 | All COVID-19 survivors who were diagnosed with COVID-19 according to WHO interim guidance and were discharged from the hospital by March 1, 2020 | 538 | 52.0 (41.0–62.0) years | 45.5% | Fatigue, swelling, myalgias, arthralgia, chills, limb edema, dizziness, chest pain, post activity polypnea, cough sputum, throat pain, palpitations, discontinuous flushing, new onset hypertension, depression, anxiety, and alopecia |
| 13 | Tenforde et al. [35] | United States | Single centered | 14–21 | adults aged ≥18 years who had a first positive RT-PCR test for SARS-CoV-2, and reported persistence COVID-19 symptoms | 270 | 26% patients aged between 18 and 34 years, 32% aged between 35 and 49 years, and 47% aged ≥50 years | 48.14% | Risk Factors for Delayed Return to Usual Health Among COVID-19 patients were evaluated. The outcome variables included age, comorbids, ethinicity, gender |
| 14 | Taquet et al. [24] | United States | Multicentric, electronic records | 14–90 days | Discharged COVID-19 patients with no previous psychiatric illness | 44,779 | 49.3 (19.2)< | 45.1% | New onset psychiatric illness disorders psychotic, insomnia, mood disorders (depressive episodes) anxiety disorders (PTSD, panic disorder, adjustment disorder and generalized anxiety disorder). |
| 15 | Townsend et al. [15] | Ireland | Single centered, outpatient clinic | 56–84 | Mild, moderate Symptomatic patients and Hospitalized patients |
128 | 49.5 ± 15 years | 46.1% | Persistent fatigue |
| 16 | Garrigues et al. [37] | France | Single centered | 110 | Discharged COVID-19 patients who were Hospitalized in ward or ICU | 120 | 63.2 (15.7) years | 62.5% | Cough, chest pain, fatigue, dyspnea, ageusia, anosmia, hair loss, attention disorder, memory loss, sleep disorder |
| 17 | Horvath et al. [38] | Australia | Multicentric, computed records | 83 | Discharged COVID-19 patients with mild to moderate disease intensity | 102 | 45 (17–87) years | 40% | Smell reduction, taste change, cough, fever, headaches, worsening nasal blockage, runny nose, fatigue, sore throat. |
| 18 | Arnold et al. [39] | United Kingdom | Single centered | 28 | patients (≥18years of age) admitted with COVID-1 | 110 | 60 (46–73) years | 56% | Fever, cough arthralgia, myalgias, chest pain, anosmia, diarrhea, abdominal pain, headache, insomnia, deranged blood tests, spirometry and chest C ray |
| 19 | Osikomaiya et al. [40] | Nigeria | Multi- centered | 14 | Discharged COVID-19 patients who were Hospitalized in ward or ICU | 274 | 41.8 ± 11.8 years | 66.1% | Fever, fatigue, weight loss, malaise, cough, dyspnea, chest pain, anosmia, loss of appetite, dizziness, palpitations, insomnia vertigo, dysgeusia |
| 20 | Leth et al. [41] | Denmark | Single centered | 84 | Hospitalized COVID-19 that were discharged after negative PCR | 71 patients | 58 (48–73) | 43% | Difficulty in concentration, paresthesia's, headache, anosmia, taste impairment, cough dyspnea, expectoration, sore throat, |
| 21 | Sudre et al. [23] | United Kingdom | Multi-center | 90 | Mobile health app users with PCR positive COVID-19 patients/negative matched controls | 4182 | 44 (28, 56) | 28.5% | Number of symptoms, duration of symptoms, quality of life, |
The general quality of the studies was assessed using the Newcastle-Ottawa scale for observational studies (Table 1). There were 35 post-acute symptoms and signs presented in the study cohort of these articles. They are stratified in Table 3. The most common manifestations were fatigue (54.11%), dyspnea (24.38%), alopecia (23.21%), hyperhidrosis (23.6%), insomnia (25.98%), anxiety (17.29%), and arthralgia (16.35%). Thirteen studies reported fatigue and anosmia, 15 dyspnea, 12 chest pain, and 11 non-productive coughs, and 5 studies showed more than one symptom. Apart from constitutional symptoms of COVID-19, personality and sleep disorders, bladder and bowel incontinence, new-onset hypertension, and diabetes were also seen in the included studies.
Table 1.
Newcastle-Ottawa Scale assessment of pooled studies, * = one score 0 = no score.
| Studies | Selection |
Comparability | Outcomes |
Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohorts | Selection of non-exposed cohorts | Ascertainment of exposure | Outcome not present at the start of the study | Assessment of outcomes | Length of follow-up | Adequacy of follow-up | |||
| Cardi et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Mandal et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Chopra et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| El Sayed et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Mahmud et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Carvalho-Schnieider et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Marva et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Galván-Tejada et al. | * | * | * | * | ** | * | * | * | ********* |
| Moreno-Pérez et al. | * | 0 | * | * | 0 | * | * | 0 | ***** |
| Halpin et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Huang et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Xiong et al. | * | 0 | * | * | 0 | * | * | 0 | ***** |
| Tenford et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Taquet et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Townsend et al. | * | 0 | * | * | 0 | * | 0 | 0 | **** |
| Garrigues et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Horvath et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Arnold et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Osikomaiya et al. | * | 0 | * | * | 0 | * | * | * | ****** |
| Leth et al. | * | 0 | * | * | 0 | * | * | 0 | ***** |
| Sudra et al. | * | 0 | * | * | 0 | * | * | 0 | ***** |
Table 3.
Post-acute COVID-19 signs and symptoms after recovery (pooled prevalence, %).
| Clinical characteristics of post-acute COVID-19 | Studies (n) | Number of patients with symptoms (n) | Total number of patients (n) | Pooled Prevalence; % |
|---|---|---|---|---|
| Fatigue | 13 | 2412 | 4457 | 54.11% |
| Hyperhidrosis | 1 | 127 | 538 | 23.6% |
| Migraine-like Headache | 8 | 221 | 3006 | 0.03% |
| Vertigo | 2 | 11 | 629 | 1.74% |
| Alopecia/Telogen effluvium | 3 | 537 | 2313 | 23.21% |
| Dyspnea | 15 | 790 | 3242 | 24.38% |
| Anosmia | 13 | 497 | 3924 | 12.66% |
| Dry eyes | 1 | 21 | 146 | 14.38% |
| Blurred vision | 3 | 70 | 838 | 8.35% |
| Dysgeusia/Ageusia | 7 | 293 | 3009 | 9.73% |
| Arthralgia | 5 | 198 | 1211 | 16.35% |
| Myalgias | 9 | 204 | 3527 | 5.78% |
| Adjustment disorder | 1 | 2 | 355 | 0.56% |
| Anxiety | 3 | 160 | 925 | 17.29% |
| Dementia | 1 | 82 | 287 | 28.57% |
| Dizziness | 3 | 123 | 2467 | 4.98% |
| Depression | 2 | 117 | 825 | 14.18% |
| Cough | 11 | 434 | 2527 | 17.17% |
| Expectoration | 1 | 16 | 538 | 2.97% |
| Insomnia | 6 | 725 | 2790 | 25.98% |
| Obsessive compulsive disorder | 1 | 14 | 287 | 4.87% |
| New-onset hypertension | 3 | 10 | 1180 | 0.84% |
| New-onset diabetes | 2 | 3 | 642 | 0.46% |
| Palpitations | 5 | 252 | 2952 | 8.53% |
| Discontinuous flushing | 1 | 26 | 538 | 4.83% |
| Restless leg syndrome | 1 | 2 | 355 | 0.56% |
| Pedal edema | 1 | 14 | 538 | 2.6% |
| Memory disturbances | 4 | 69 | 849 | 8.12% |
| Rash/Cutaneous signs | 4 | 87 | 2417 | 3.59% |
| Chest pain | 12 | 459 | 4422 | 10.37% |
| Sore throat/odynophagia | 6 | 150 | 2886 | 5.19% |
| Bowel/bladder incontinence | 1 | 8 | 100 | 8% |
| Tinnitus | 1 | 48 | 287 | 16.72% |
| Weight loss | 3 | 42 | 504 | 8.33% |
| Diarrhea/Vomiting/gastrointestinal issues | 7 | 236 | 2911 | 8.1% |
| More than one symptom | 5 | 456 | 1533 | 29.74% |
4. Discussion
The most important pathophysiological mechanism in acute COVID-19 is direct viral toxicity leading to endothelial damage and microvascular injury [11]. This can cause immune system dysregulation and hyperinflammatory states, hypercoagulability, and downregulation of the angiotensin-converting enzyme 2 (ACE2) pathway [11]. In contrast to this, the post-acute effects of COVID-19 are an overlap of phylogenetic similarities with SARS-COV-1 and Middle-Eastern respiratory syndrome (MERS) viruses [12]. However, SARS-COV-2 has a higher affinity for ACE2 compared with SARS-COV-1, and this mechanism may be the contributing factor in the widespread transmission of SARS-COV-2. Furthermore, potential mechanisms behind post-acute symptoms and signs in COVID-19 recovered patients seem multifactorial: the pathophysiologic changes caused by the virus, inflammatory and immune-mediated cell damage, and sequelae of recovery from critical illness [13].
This systematic review demonstrated that 68% of patients have at least one post-acute symptom after recovery from COVID-19. A total of 21 studies were included in this review which fulfilled our inclusion criteria and overall, 35 signs and symptoms of post-acute COVID-19 were identified in this cohort of patients (Table 3). The most common symptoms were fatigue, dyspnea, hyperhidrosis, dementia, depression, alopecia, and cough. The majority of presenting symptoms or signs were similar to the acute presentation of COVID-19. However, a possibility remains for other effects to be identified later in this pandemic. In the following discussion, we will elaborate on the most common symptoms and signs of post-acute COVID-19 to understand each disease in more detail.
Overall, the most common symptom among all the included patients was the feeling of tiredness or fatigue (54.11%) [6,7,[14], [15], [16], [17], [18]]. It was present after three months’ follow-up in critical COVID-19 patients admitted to intensive care units (ICUs) [15]. This phenomenon has been established in survivors of critical illness (post-ICU syndrome), even after years of recovery, where half the patients report symptoms of chronic fatigue syndrome, including incapacitating fatigue, generalized body pain, neurocognitive disturbances, insomnia, and increased sympathetic drive [19]. Viruses like Epstein-Barr virus, cytomegalovirus, and herpes virus have been implicated in causing chronic fatigue syndrome and this review adds SARS-COV-2 as the causative agent of chronic fatigue [20].
Neuropsychiatric symptoms are also reported in some studies, including headache, insomnia, anxiety, depression, bladder and bowel incontinence, ageusia, migraine, and dementia [6,16,[21], [22], [23], [24]]. Similar to chronic fatigue syndrome, the etiology, and pathophysiology of neuropsychiatric symptoms in COVID-19 are multifactorial and unclear. In a cohort of 355 patients in Bangladesh, and 143 patients in Italy, a cumulative 63% of the patients were screened positive in at least one of the domains evaluated for neuropsychiatric sequelae (depression, anxiety, insomnia, obsessive-compulsive disorders, etc.) [6,14]. Clinical depression and anxiety were reported in approximately 17% of patients following COVID-19 [6]. Memory loss in the form of dementia and ageusia is also reported in a few studies, including cognitive impairment with or without fluctuations [25]. All these symptoms could be related to the social stigma of contracting a potentially fatal illness, some effects of sedatives in critical COVID-19 patients with delirium, and hypercoagulability leading to cerebrovascular disease. In addition, post-recovery sleep disturbances can also precipitate psychiatric disorders [26]. Mental health assessment and mental health attention models are very important in the post-acute COVID-19 stage, as they can contribute to a better quality of life in this cohort. Telogen effluvium and alopecia are also reported in three studies, which is defined as temporary hair loss due to excessive shedding of Telogen hair after COVID-19. Although self-limiting, this condition can cause emotional distress in many patients [27].
Dyspnea (24.38%) and cough (17.17%) were the most prominent pulmonary symptoms in this review [28]. Several studies have demonstrated persistent high resolution computed tomography (HRCT) lung abnormalities after 60 days from the initial presentation [29]. In addition, previous studies have exhibited lung dysfunction in more than 50% of the patients compared to our study cohort [7,30]. A decreased diffusion capacity due to loss of lung volume is the most commonly reported pathophysiologic impairment in post-acute effects of COVID-19, which is directly related to the severity of acute illness [31,32]. This observation is consistent with SARS and MERS and seems to be the contributing factor in long-term pulmonary sequelae of COVID-19. There is the viral-dependent invasion of endothelial-epithelial barrier causing infiltration of monocytes and macrophages, leading to extravasation of protein-rich exudate filling the alveolar space. This is similar to acute respiratory distress syndrome (ARDS) [33]. There are reports of pulmonary vascular micro and macrothrombosis in 20% of the patients with critical COVID-19 pneumonia and the severity of the endothelial injury and widespread microangiopathy seen on lung histopathology is greater than that seen in ARDS from other viruses [34,35].
Several other constitutional symptoms are demonstrated in this review [[36], [37], [38], [39], [40], [41]]. The most important of them are weight loss, new-onset diabetes and hypertension, expectoration, blurred vision, and dry eyes. Chest pain is reported in up to 10% of COVID-19 survivors at 60 days follow up, while ongoing palpitations were reported in 8.53% at 6-months follow up. Apart from acute coronary syndrome (ACS) and myocarditis, an increased incidence of takotsubo cardiomyopathy is being reported in this pandemic compared with the pre-pandemic period (7.8% vs. 1.5%, respectively) [42]. Mechanisms contributing to cardiovascular sequelae in post-acute COVID-19 seem to be downregulation of ACE2 and renin-angiotensin-aldosterone system (RAAS), cytokine storm-related deterioration of myocardial integrity, pericarditis, and arrhythmias [43].
A recent meta-analysis identified studies assessing the long-term effects of COVID-19. They included 15 studies and estimated that 80% of the infected patients with SARS-COV-2 developed one or more long-term symptoms [44]. One other living systematic review included 39 studies and showed weakness (41%), general malaise (33%), and fatigue (31%) as the most commonly occurring symptoms [45]. Similarly, our estimated that 68% of the patients developed one or more symptoms after COVID-19 recovery with fatigue, dyspnea, and dementia as the most common symptoms.
This systematic review had several limitations. One is the small number of studies with underpowered sample size, creating a potential bias and variation in defined outcomes leading to the heterogeneity of the results. Many studies used a self-reporting method which can produce an interobserver bias and almost all studies enrolled COVID-19 patients in mild, moderate, and severe disease category with variable follow-up times references. This can produce heterogeneous results. There was a predefined assessment of symptoms in every study assessed, which can lead to unreported outcomes. Although high viral load is implicated in the long-term sequelae of COVID-19, there is no definition of the effect of late effects of COVID-19 and its associated symptoms. A critical illness survivor can have prolonged symptoms while a patient with mild disease can recover early from the same problem. Hence, there is a need for prospective studies to determine if the post-acute COVID-19 effects are a continuation of SARS-COV-2 or complications of premorbid conditions.
4.1. Future directions
The provision of post-hospital discharge care of COVID-19 patients is an evolving field and may differ across institutions. The current mainstay of treatment involves the use of dexamethasone and antivirals along with early rehabilitation interventions during the post-hospitalization stage, with management largely dictated by the severity of the disease. Therefore, clinicians who are meticulously reporting and managing those afflicted with the syndrome have a crucial role in the future towards creating the appropriate protocols and management plans that will significantly improve patient outcomes. Moreover, studies and active research are required to optimise the management of post-hospital discharge care of COVID-19 patients.
5. Conclusion
The multiorgan sequelae of SARS-COV-2 infection beyond the acute infection are increasingly being recognized with an increasing clinical experience and pool of data becoming available rapidly on COVID-19. This updated systematic review of 21 studies and 54,730 patients is the largest cohort of patients with post-acute effects of COVID-19 evaluated to date. It demonstrated that post-acute effects of COVID-19 can persist even at six months and from the clinical point of view, medical professionals should look for the symptoms and signs in patients recovered from COVID-19. Necessary future research includes stratification of these post-acute effects with gender, age, and comorbid conditions in acute, subacute, and chronic phases of the disease. This will lead to a better understanding of the delayed sequelae of COVID-19. Through this review, it is clear that acute care of COVID-19 does not conclude at hospital discharge, and interdisciplinary care is needed for comprehensive care of these patients at homes and outpatient clinics. Hence, healthcare systems must establish dedicated COVID-19 clinics, where specialists from various disciplines can provide unanimous care.
Ethical approval
NA.
Sources of funding
NA.
Author contribution
TA, JM; concept, literature search, first draft, final draft, methodology, analysis; AKA, SMJZ; literature review, study selection, first draft; RI; first and final draft; KK; first draft, supervision, methodology; FK, FK, LA; literature search, first draft; MA, RA; literature search, first draft; MA, SH, ASR, TK; first draft, analysis, literature search; AUW, TT, RA, IA, MA; first and final draft, methodology; UI; supervision, concept; final draft, analysis.
Please state any conflicts of interest
NA.
Registration of research studies
-
1.
Name of the registry: NA
-
2.
Unique Identifying number or registration ID: NA
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): NA
Guarantor
Dr. Jahanzeb Malik and Dr. Talal Almas.
Consent
Written and informed consent was obtained and is available to the editor in chief upon request.
Provenance and peer-review
Not commissioned, externally peer-reviewed.
Funding
Authors did not receive any specific funding for this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103995.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Roser Max, Ritchie Hannah, Ortiz-Ospina Esteban, Joe Hasell. Coronavirus pandemic (COVID-19) 2020. https://ourworldindata.org/coronavirus Published online at OurWorldInData.org. Retrieved from. [Online Resource]
- 2.Malik J., Malik A., Javaid M., Zahid T., Ishaq U., Shoaib M. Thyroid function analysis in COVID-19: a retrospective study from a single center. PLoS One. 2021 Mar 30;16(3) doi: 10.1371/journal.pone.0249421. PMID: 33784355; PMCID: PMC8009384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik J., Javaid M., Majedi O., Ishaq U., Zahid T. Paying in blood: a case of thrombocytopenia in covid-19. Cureus. 2020 Aug 16;12(8) doi: 10.7759/cureus.9791. PMID: 32953307; PMCID: PMC7491688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik J., Javed N., Naeem H., Sattar R.A., Ikram U. COVID-19 associated pneumonia and pleural effusion masquerading as heart failure in rheumatic heart disease. Eur J Case Rep Intern Med. 2020 Jul 10;7(8) doi: 10.12890/2020_001842. PMID: 32789145; PMCID: PMC7417058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shafi A.M.A., Shaikh S.A., Shirke M.M., Iddawela S., Harky A. Cardiac manifestations in COVID-19 patients-A systematic review. J. Card. Surg. 2020 Aug;35(8):1988–2008. doi: 10.1111/jocs.14808. Epub 2020 Jul 11. PMID: 32652713; PMCID: PMC7404674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmud R., Rahman M.M., Rassel M.A., Monayem F.B., Sayeed S.K.J.B., Islam M.S., et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. PLoS One. 2021 Apr 8;16(4) doi: 10.1371/journal.pone.0249644. PMID: 33831043; PMCID: PMC8031743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 Jan 16;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. Epub 2021 Jan 8. PMID: 33428867; PMCID: PMC7833295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q., Allot A., Lu Z. LitCovid: an open database of COVID-19 literature. Nucleic Acids Res. 2021 Jan 8;49(D1):D1534–D1540. doi: 10.1093/nar/gkaa952. PMID: 33166392; PMCID: PMC7778958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021:88. doi: 10.1016/j.ijsu.2021.105906. 105906. [DOI] [PubMed] [Google Scholar]
- 10.Ma L.L., Wang Y.Y., Yang Z.H., Huang D., Weng H., Zeng X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020 Feb 29;7(1):7. doi: 10.1186/s40779-020-00238-8. PMID: 32111253; PMCID: PMC7049186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020 Jul;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. Epub 2020 Jul 10. PMID: 32651579. [DOI] [PubMed] [Google Scholar]
- 12.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021 Mar;19(3):141–154. doi: 10.1038/s41579-020-00459-7. Epub 2020 Oct 6. PMID: 33024307; PMCID: PMC7537588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020 May;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. Epub 2020 Mar 30. PMID: 32225175; PMCID: PMC7328981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020 Aug 11;324(6):603–605. doi: 10.1001/jama.2020.12603. PMID: 32644129; PMCID: PMC7349096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend L., Dowds J., O'Brien K., Sheill G., Dyer A.H., O'Kelly B., et al. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021 Jan;8 doi: 10.1513/AnnalsATS.202009-1175OC. Epub ahead of print. PMID: 33413026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., et al. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2020 Nov;10 doi: 10.1136/thoraxjnl-2020-215818. thoraxjnl-2020-215818. Epub ahead of print. PMID: 33172844; PMCID: PMC7661378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Sayed S., Shokry D., Gomaa S.M. Post-COVID-19 fatigue and anhedonia: a cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol Rep. 2021 Mar;41(1):50–55. doi: 10.1002/npr2.12154. Epub 2020Dec17. PMID: 33332756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno-Pérez O., Merino E., Leon-Ramirez J.M., Andres M., Ramos J.M., Arenas-Jiménez J., et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J. Infect. 2021 Mar;82(3):378–383. doi: 10.1016/j.jinf.2021.01.004. Epub 2021 Jan 12. PMID: 33450302; PMCID: PMC7802523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011 Mar 24;11:37. doi: 10.1186/1471-2377-11-37. PMID: 21435231; PMCID: PMC3071317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wostyn P. COVID-19 and chronic fatigue syndrome: is the worst yet to come? Med. Hypotheses. 2021 Jan;146 doi: 10.1016/j.mehy.2020.110469. Epub 2021 Jan 2. PMID: 33401106; PMCID: PMC7836544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chopra V., Flanders S.A., O'Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. 2021 Apr;174(4):576–578. doi: 10.7326/M20-5661. Epub 2020 Nov 11. PMID: 33175566; PMCID: PMC7707210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J. Med. Virol. 2021 Feb;93(2):1013–1022. doi: 10.1002/jmv.26368. Epub 2020 Aug 17. PMID: 32729939. [DOI] [PubMed] [Google Scholar]
- 23.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., et al. Attributes and predictors of long COVID. Nat. Med. 2021 Apr;27(4):626–631. doi: 10.1038/s41591-021-01292-y. Epub 2021 Mar 10. PMID: 33692530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatr. 2021 May;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. Epub 2021 Apr 6. PMID: 33836148; PMCID: PMC8023694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020 Jul;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. Epub 2020 May 18. PMID: 32437679; PMCID: PMC7234781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacaro V., Chiabudini M., Buonanno C., De Bartolo P., Riemann D., Mancini F., et al. Insomnia in the Italian population during covid-19 outbreak: a snapshot on one major risk factor for depression and anxiety. Front. Psychiatr. 2020 Dec 15;11 doi: 10.3389/fpsyt.2020.579107. PMID: 33384625; PMCID: PMC7769843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Q., Xu M., Li J., Liu Y., Zhang J., Xu Y., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin. Microbiol. Infect. 2021 Jan;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. Epub 2020 Sep 23. PMID: 32979574; PMCID: PMC7510771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021 Feb;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. Epub 2020 Oct 5. PMID: 33031948; PMCID: PMC7534895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balbi M., Conti C., Imeri G., Caroli A., Surace A., Corsi A., et al. Post-discharge chest CT findings and pulmonary function tests in severe COVID-19 patients. Eur. J. Radiol. 2021 May;138 doi: 10.1016/j.ejrad.2021.109676. Epub 2021 Mar 20. PMID: 33798931; PMCID: PMC7980523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y.M., Shang Y.M., Song W.B., Li Q.Q., Xie H., Xu Q.F., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020 Aug;25 doi: 10.1016/j.eclinm.2020.100463. Epub 2020 Jul 15. PMID: 32838236; PMCID: PMC7361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020 Jun 29;21(1):163. doi: 10.1186/s12931-020-01429-6. PMID: 32600344; PMCID: PMC7323373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galván-Tejada C.E., Herrera-García C.F., Godina-González S., Villagrana-Bañuelos K.E., Amaro J.D.L., Herrera-García K., et al. Persistence of COVID-19 symptoms after recovery in Mexican population. Int. J. Environ. Res. Publ. Health. 2020 Dec 14;17(24):9367. doi: 10.3390/ijerph17249367. PMID: 33327641; PMCID: PMC7765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huppert L.A., Matthay M.A., Ware L.B. Pathogenesis of acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2019 Feb;40(1):31–39. doi: 10.1055/s-0039-1683996. Epub 2019 May 6. PMID: 31060086; PMCID: PMC7060969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020 Jun;18(6):1421–1424. doi: 10.1111/jth.14830. Epub 2020 May 6. PMID: 32271988; PMCID: PMC7262324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, march-june 2020. MMWR Morb. Mortal. Wkly. Rep. 2020 Jul 31;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. PMID: 32730238; PMCID: PMC7392393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamal M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021 Mar;75(3) doi: 10.1111/ijcp.13746. Epub 2020 Nov 3. PMID: 32991035; PMCID: PMC7536922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020 Dec;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. Epub 2020 Aug 25. PMID: 32853602; PMCID: PMC7445491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horvath L., Lim J.W.J., Taylor J.W., Saief T., Stuart R., Rimmer J., et al. Smell and taste loss in COVID-19 patients: assessment outcomes in a Victorian population. Acta Otolaryngol. 2021 Mar;141(3):299–302. doi: 10.1080/00016489.2020.1855366. Epub 2020 Dec 12. PMID: 33307905. [DOI] [PubMed] [Google Scholar]
- 39.Arnold D.T., Hamilton F.W., Milne A., Morley A.J., Viner J., Attwood M., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020 Dec 3;76(4):399–401. doi: 10.1136/thoraxjnl-2020-216086. Epub ahead of print. PMID: 33273026; PMCID: PMC7716340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osikomaiya B., Erinoso O., Wright K.O., Odusola A.O., Thomas B., Adeyemi O., et al. 'Long COVID': persistent COVID-19 symptoms in survivors managed in Lagos State, Nigeria. BMC Infect. Dis. 2021 Mar 25;21(1):304. doi: 10.1186/s12879-020-05716-x. PMID: 33765941; PMCID: PMC7993075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leth S., Gunst J.D., Mathiasen V., Hansen K., Søgaard O., Østergaard L., et al. Persistent symptoms in patients recovering from COVID-19 in Denmark. Open Forum Infect. Dis. 2021 Jan 29;8(4) doi: 10.1093/ofid/ofab042. PMID: 33875970; PMCID: PMC7928683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jabri A., Kalra A., Kumar A., Alameh A., Adroja S., Bashir H., et al. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw. Open. 2020 Jul 1;3(7) doi: 10.1001/jamanetworkopen.2020.14780. PMID: 32644140; PMCID: PMC7348683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020 Jul 7;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. Epub 2020 Apr 15. PMID: 32293910. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv [Preprint] 2021 Jan;30 doi: 10.1101/2021.01.27.21250617. 2021.01.27.21250617, Update in: Sci Rep. 2021 Aug 9;11(1):16144. PMID: 33532785; PMCID: PMC7852236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michelen M., Manoharan L., Elkheir N., Cheng V., Dagens A., Hastie C., et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021 Sep;6(9) doi: 10.1136/bmjgh-2021-005427. PMID: 34580069; PMCID: PMC8478580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.