Abstract
Although coronavirus disease 2019 (COVID-19) vaccination is known to carry a slight risk of myocarditis and pericarditis, it remains unclear whether it has any impact on coronary artery disease. Here we present a case without particular thrombotic diathesis with a diagnosis of ST segment elevation acute myocardial infarction (STEMI) 19 h after a third dose of a COVID-19 mRNA vaccine. A primary percutaneous coronary intervention procedure for occluded right coronary artery with thrombus aspiration alone was successful in this patient. However, the relationship between STEMI and COVID-19 mRNA vaccination is uncertain, and additional studies to validate thrombogenetic effects of COVID-19 mRNA vaccines are needed. This case was helpful in distinguishing STEMI from myocarditis and pericarditis, which are recognized rare cardiac side effects of COVID-19 vaccination. It is important not to hesitate to perform coronary angiography procedures to rule out the possibility of STEMI occurrence, as in this case.
Keywords: COVID-19, Vaccine, Acute myocardial infarction
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is still developing. The number of COVID-19 cases now exceeds 5 five million in Japan, with around 25,000 fatalities (about 0.5 % mortality) as of March 8, 2022 [1]. To combat the pandemic, the development of vaccines against COVID-19 was accelerated; of these, the efficacy of mRNA formulations was demonstrated and has been focused on in many countries [2], [3]. Moreover, booster shots of COVID-19 mRNA vaccines have been demonstrated to reduce the rate of COVID-19 infection and severe illness [4].
Side effects of the same kind of COVID-19 mRNA vaccines used in Japan may occur in >90 % of cases, as a recent manuscript pointed out in a Slovakian population [5]. These side effects were mostly mild, of short duration, and did not require medical attention. However, rare severe side effects have also been reported, including cardiac adverse events, most often acute myocarditis and pericarditis [6]. In contrast, it appears that only 5 cases with acute myocardial infarction (AMI) have been reported [7] and most of these case reports referred to the relationship between vaccination and AMI occurrence as being uncertain [8], [9], [10]. Nonetheless, it is important to acknowledge that mechanisms responsible for thrombotic events in COVID-19 are still not fully understood [11]. Hence, here we present a case diagnosed with ST segment elevation AMI (STEMI) following a third dose of COVID-19 mRNA vaccine. It must be noted that the occurrence of this case does not imply any suggestion that vaccination with COVID-19 mRNA formulations should be avoided.
2. Case report
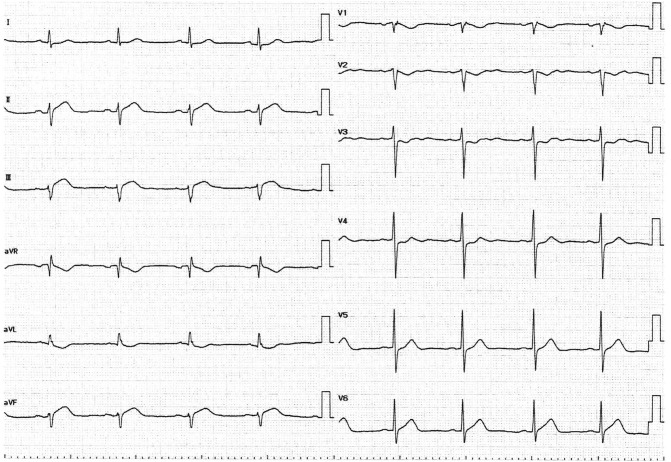
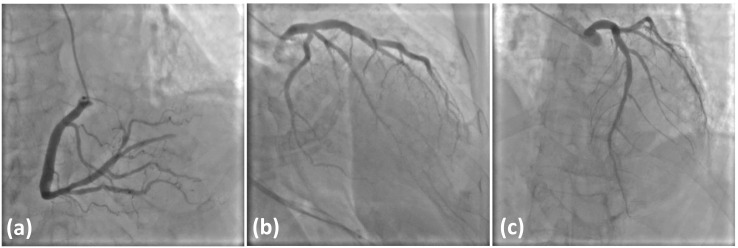
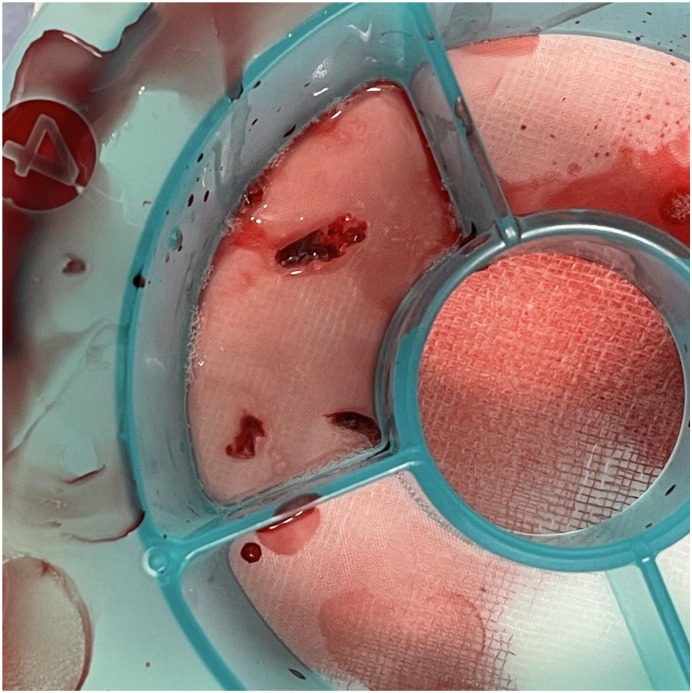
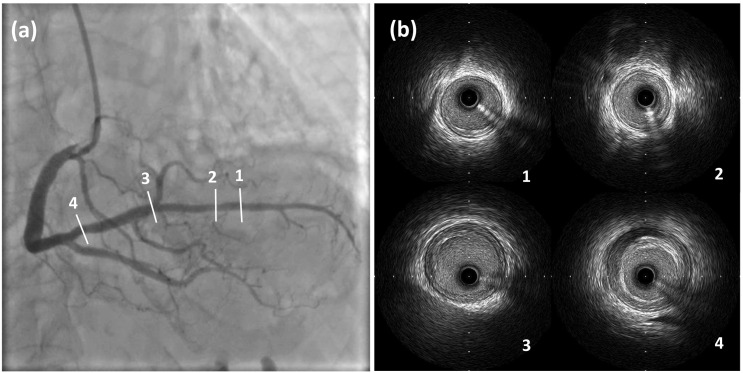
A 76-year-old woman visited the emergency department 3 h after complaining of chest pain. She had hypertension, dyslipidemia, and bronchial asthma which was under treatment with oral and inhaled medicines. She was allergic to Japanese cedar, cypress, penicillin, and non-steroid anti-inflammatory drugs. She had received the first and second dose of the Pfizer-BioNTech vaccine without any allergic reactions, and had then received a third dose of the same vaccine 19 h before the onset of cardiac symptoms. Her electrocardiogram on arrival showed sinus rhythm with ST segment elevation in the II, III, and aVF leads (Fig. 1 ) and cardiac ultrasound examination showed infero-posterior hypokinesia of left ventricular wall motion. Blood myocardial enzymes were not elevated on arrival (Table 1 ). Following diagnosis of STEMI based on symptomology and the above findings, emergency coronary angiography (CAG) was performed. This revealed that the distal part of the right coronary artery was totally occluded by thrombus (Fig. 2(a)) without residual stenotic or occluded lesion in left coronary artery (Fig. 2(b) (c)). Primary percutaneous coronary intervention (PCI) was performed on this lesion as the STEMI culprit. After guiding catheter engagement and guidewire crossing, thrombus aspiration through the catheter was attempted to bypass the lesion, and several thrombi were aspirated (Fig. 3 ). Recanalization with Thrombolysis in Myocardial Infarction (TIMI) 3 flow was obtained. (Fig. 4(a)) Final intravascular ultrasound imaging revealed no plaque rupture, dissection, residual thrombi, or atherosclerotic plaques in the right coronary artery (Fig. 4(b)). PCI was therefore finished successfully without additional ballooning or stent implantation.
Fig. 1.
Electrocardiogram on arrival.
Table 1.
Blood sample on arrival.
| Leukocytes | 13,800/μl |
| Neutrophils | 89.0 % |
| Lymphocytes | 10.0 % |
| Eosinophils | 1.0 % |
| Erythrocytes | 5.11 × 104/μl |
| Hemoglobin | 14.7 g/dl |
| Hematocrit | 47.0 % |
| Platelets | 49.8 × 104/μl |
| APTT | 26 s |
| PT | 10.1 s |
| INR | 0.85 |
| D dimer | 1.1 μg/ml |
| BNP | 34.5 pg/ml |
| LDL-C | 107 mg/dl |
| HDL-C | 61 mg/dl |
| TG | 147 mg/dl |
| CK | 144 U/l |
| CK-MB | 11 U/l |
| AST | 36 U/l |
| ALT | 55 U/l |
| LDH | 293 U/l |
| Alb | 4.1 g/dl |
| Creatinine | 0.71 mg/dl |
| BUN | 17 mg/dl |
| eGFR | 60.2 |
| Uric acid | 5.3 mg/dl |
| Na | 144 mEq/l |
| K | 3.3 mEq/l |
| Glucose | 131 mg/dl |
| Troponin I | 0.03 ng/ml |
| HbA1c | 5.7 % |
Fig. 2.
Initial coronary angiography.
(a) (b) Left coronary angiography.
(c) Right coronary angiography.
Fig. 3.
Aspirated thrombi from the right coronary artery.
Fig. 4.
(a) Final coronary angiography post-percutaneous coronary intervention procedure.
(b) Final intravascular ultrasound imaging.
The peak CK value was elevated to 1671 IU/L, but no cardiac complications or other thrombo-embolic events occurred. During hospitalization, no atrial fibrillation was documented, and other examinations such as magnetic resonance imaging of the head, as well as whole-body computed tomography with contrast-enhancement, showed no thrombo-embolic findings. No abnormal findings regarding screenable thrombotic diathesis were seen in the blood values (Table 2 ). The patient was discharged from hospital after eight days.
Table 2.
Blood sample regarding thrombotic diathesis during hospitalization.
| Thrombin-antithrombin III complex | 2.2 ng/ml |
| α2 antiplasmin inhibitor | 0.8 μg/ml |
| Coagulation factor V | 72.0 % |
| Protein C Antigen | 118.0 % |
| Protein C Activity | 149.0 % |
| Protein S Antigen | 112.0 % |
| Free Protein S | 89.0 % |
| Thrombomodulin | 14.6 U/ml |
| Anti-nuclear antibody | ×40 |
| Rheumatoid factor | 6 IU/ml |
| Lupus Anticoagulant | 1.1 |
| Anti β2-glycoprotein I antibody | 1.2 U/ml |
| Anti cardiolipin antibody IgG | 34.5 pg/ml |
3. Discussion
A case with STEMI occurring within 24 h of the third dose of a COVID-19 mRNA vaccine is described in this report. The patient exhibited total occlusive lesions implicating thrombosis, and successful PCI was achieved using thrombus aspiration alone without any balloon or stenting. In this way, the patient benefited from avoiding dual antiplatelet therapy after PCI. Previous reviews in the literature, and also different case reports, have recorded some AMI occurrences following COVID-19 mRNA vaccination [7], [12]. However, AMI occurred in all those cases following the first or second dose of COVID-19 vaccine. In contrast, STEMI occurred in the patient reported here following the third dose of COVID-19 mRNA vaccine, within 24 h. No similar cases appear to have been reported in PubMed at the time of writing.
Around the same time as we treated the patient presented here, a similar case with STEMI occurrence following the third dose of COVID-19 mRNA vaccination after 17 h was encountered. However, this patient's CAG showed severe stenotic lesions composed of atherosclerotic plaques in the middle part of the left anterior descending artery without any suspicion of thrombosis. This case was therefore considered seminally different from the present report because there was a question of whether a relationship existed between STEMI occurrence and COVID-19 mRNA vaccination.
There have been several previous reports about relationships between COVID-19 vaccination and AMI onset. One concerns the occurrence of thrombotic events with thrombocytopenia mediated by platelet factor-4 antibody 5–10 days after COVID-19 vaccination [13]. However, the clinical features of the case in this report including no thrombocytopenia and rapid onset after vaccination do not match this pathological condition. As another possible cause, rare acute hypersensitivity with autoimmune responses against excipients in the COVID-19 vaccine, or the general stress of vaccination known as Kounis syndrome [14], may have been responsible. Indeed, our patient did exhibit sensitivity to various allergens, and the possibility of allergy against components of the Pfizer-BioNTech vaccine cannot be excluded. Nonetheless, this is unlikely because she had already received the same vaccine twice previously.
Formally, there are no data directly demonstrating a positive correlation between COVID-19 mRNA vaccination and AMI occurrence, unlike the situation with myocarditis or pericarditis [6]. Showkathali et al. reported that there were no significant differences in the incidence of coronary thrombo-embolic events regardless of vaccination status [15]. Case et al. also reported that the incidence of coronary artery disease including acute coronary syndrome did not differ before and after COVID-19 vaccination [16]. However, those studies were retrospective observational studies, and relationships between COVID-19 vaccination and AMI occurrence cannot be completely excluded from their results. In fact, Showkathali et al. also reported that the burden of thrombosis in patients suffering coronary thrombo-embolic events is significantly higher and TIMI flow is significantly lower in vaccinated than non-vaccinated patients. They pointed out that COVID-19 vaccination could potentially lead to a high thrombotic state with immune responses as well as high prothrombotic states as a feature of COVID-19 disease itself [15]. These findings suggest that COVID-19 vaccination may contribute to thrombogenesis in the coronary artery, and there may be a positive relationship between STEMI and COVID-19 vaccination.
However, although myocarditis and pericarditis are generally cited as the serious cardiac side effects after COVID-19 vaccination [6], the possibility of AMI occurrence as in this case should be kept in mind. The symptoms of myocarditis and pericarditis are similar to STEMI, including chest pain and ST segment elevation in the electrocardiogram, and CAG procedures are needed for differential diagnosis. As presented above, a relationship between STEMI occurrence and COVID-19 vaccination is conceivable, so there should be no hesitation to undertake CAG procedures. It is important to note that thrombogenesis due to COVID-19 disease or vaccination has not yet been reported; therefore, additional case reports and observational studies are essential for validating any relationship between them and the occurrence of thrombo-embolic events.
4. Conclusion
STEMI occurred within 24 h of a third dose of COVID-19 mRNA vaccine in the case described here. It is important to suspect not only myocarditis and pericarditis but also AMI on the appearance of chest discomfort after COVID-19 mRNA vaccination, and CAG procedures may be helpful for differential diagnosis. Whether there is any relationship between STEMI occurrence and COVID-19 mRNA vaccination is uncertain, pending further studies and case reports.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- 1.Situation report, Ministry of Health, Labour and Welfare n.d. https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou_00006.html (accessed March 8, 2022).
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/nejmoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riad A., Hocková B., Kantorová L., Slávik R., Spurná L., Stebel A. 2021. Side effects of mRNA-based COVID-19 vaccine: nationwide phase IV study among healthcare workers in Slovakia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai F.T.T., Li X., Peng K., Huang L., Ip P., Tong X., et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine. Ann Intern Med. 2022:1–10. doi: 10.7326/m21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Çınar T., Hayıroçlu M.I., Çiçek V., Selçuk M., Yavuz S., Orhan A.L. Review of the current literature regarding cardiac adverse events following COVID-19 vaccination. Rev Assoc Med Bras. 2021;67:1751–1758. doi: 10.1590/1806-9282.20210940. [DOI] [PubMed] [Google Scholar]
- 8.Sung J.G., Sobieszczyk P.S., Bhatt D.L. Acute myocardial infarction within 24 hours after COVID-19 vaccination. Am J Cardiol. 2021;156:129–131. doi: 10.1016/j.amjcard.2021.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajstra M., Jaroszewicz J., Gąsior M. Acute coronary tree thrombosis after vaccination for COVID-19. JACC Cardiovasc Interv. 2021;14:e103–e104. doi: 10.1016/j.jcin.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iqbal S., Adnan G., Farhad A., Ahmed I., Rahman M.N. Acute myocardial infarction after coronavirus vaccine: a rare adverse effect. Cureus. 2022;14:10–14. doi: 10.7759/cureus.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFadyen J.D., Stevens H., Peter K. The emerging threat of (Micro)Thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127:571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aye Y.N., Mai A.S., Zhang A., Lim O.Z.H., Lin N., Ng C.H., et al. Acute myocardial infarction and myocarditis following COVID-19 vaccination. QJM An Int J Med. 2021:1–5. doi: 10.1093/qjmed/hcab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/nejmoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kounis N.G., Koniari I., Kouni S., Mplani V., Velissaris D., Plotas P., et al. Rare acute hypersensitivity myocardial infarction (Kounis syndrome) and hypersensitivity myocarditis following COVID-19 vaccination. QJM An Int J Med. 2022:1–2. doi: 10.1093/qjmed/hcac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Showkathali R., Yalamanchi R., Narra L., Vinayagamoorthy N., Gunasekaran S., Nayak R., et al. Coronary thrombo-embolic events after Covid-19 vaccination- a single Centre study. Indian Heart J. 2022:3–6. doi: 10.1016/j.ihj.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Case B.C., Rosenfeld B., Shea C., Rappaport H., Zhang C., Medranda G.A., et al. Implications of COVID-19 vaccination on hospital encounters and outcomes. Am J Cardiol. 2022 doi: 10.1016/j.amjcard.2022.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]