SUMMARY
Central nervous system (CNS) metastases constitute a challenge for the design of ALK fusion-positive lung cancer trials. The ASCEND-7 study of ceritinib demonstrates the feasibility of broadening CNS eligibility criteria to include symptomatic brain and leptomeningeal disease and highlights design features that contemporary trials will need to incorporate.
In this issue of Clinical Cancer Research, Chow and colleagues report the results of the phase 2 ASCEND-7 trial of ceritinib in patients with ALK fusion-positive lung cancers (1). The trial was unique in that it only treated patients with central nervous system (CNS) metastases. The inclusion of patients with symptomatic brain metastases and leptomeningeal disease was particularly laudable, recognizing that many trials exclude these populations. By doing so, the investigators teach us what should be an intuitive lesson: drugs that are predicted to work well in the CNS should be studied in those patients that need them the most.
Broadening CNS inclusion criteria is critical as brain metastases constitute a frequent problem in advanced ALK fusion-positive lung cancers (2). Whereas a series of trials using other later generation ALK inhibitors showed evidence of intracranial activity (1, 3–7) (Figure 1), many required stricter CNS entry criteria. ASCEND-7 enrolled patients with at least one progressive intracranial lesion or leptomeningeal disease (LMD), and permitted symptomatic as well as untreated disease. Patients were divided into four arms depending on whether they had received prior ALK inhibitor therapy and/or prior radiation therapy; a fifth arm was designated for LMD (1).
FIGURE 1. Intracranial and overall response rates to ALK tyrosine kinase inhibitor therapy in ALK-positive lung cancers.
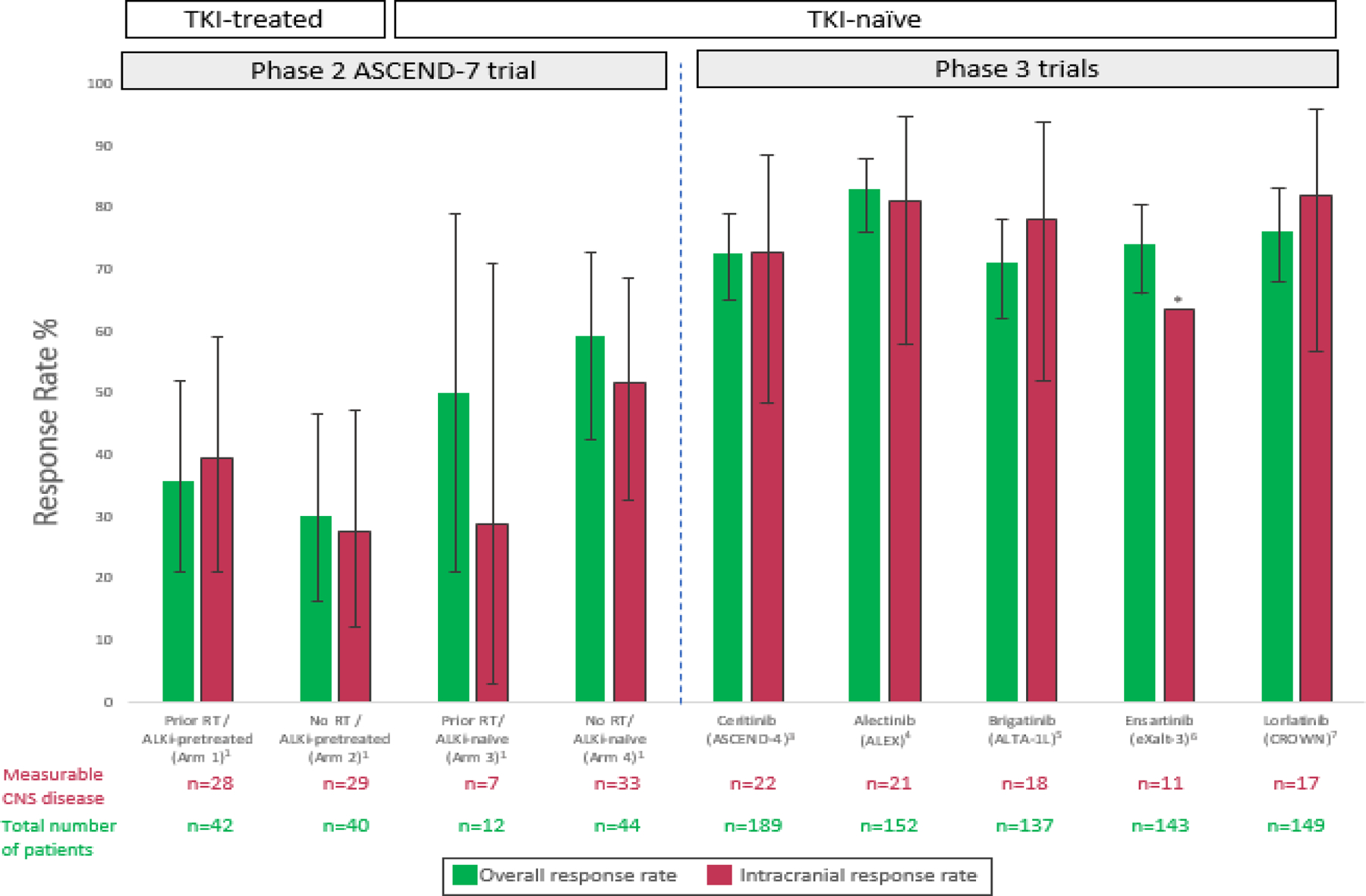
The intracranial and overall response rates to ceritinib and select other second and third-generation ALK tyrosine kinase inhibitors are shown. Notably the cohort of patients enrolled in the ASCEND-4 study of ceritinib included some with neurologically stable CNS disease, but eligibility was more lenient in ASCEND-7, which was designed as a dedicated CNS study. Error bars indicate 95% confidence intervals (CI) where available. The * symbol indicates that information on the 95% CI was not available.
The CNS-centric ASCEND-7 design represents a model for targeted therapy studies in oncogene-driven cancers. At the same time, it highlights challenges that accompany the treatment of a population with a wider range of pre-trial fitness levels and characteristics. For example, 37% of screened patients did not ultimately meet eligibility criteria. Those who were eligible included patients with a World Health Organization performance status of 2 and a substantial intracranial tumor burden (up to an intracranial target sum of ~16 cm in patients on the trial) (1). Increasing the granularity with which studies capture the pre-treatment burden of CNS disease should be considered; interrogation of circulating tumor cells (CTCs) or DNA in the cerebrospinal fluid (CSF) may offer additional information when safe and feasible (8).
The ASCEND-7 investigators demonstrated benefit with ceritinib even in this sicker population. While whole-body overall and intracranial response rates ranged from approximately 30% to 60% in the non-LMD trial arms, the rate of progressive disease as a best overall response was low (~6–17%). In patients with LMD, the intracranial response rate was 13%, the intracranial disease control rate exceeded 60%, and survival exceeded 7 months. Furthermore, because patterns of disease progression on ceritinib were carefully characterized, the study uncovered that CNS disease remained controlled in a proportion of patients (~8–21%) despite extracranial progression (1). The inclusion of additional established and exploratory metrics (e.g. RANO criteria, volumetric assessment, CTC or variant allele frequency changes) may benefit efficacy assessments in future CNS trials (8). The authors also suggest that more intensive radiologic monitoring is warranted, particularly for patients with LMD (1).
While cross-trial comparisons come with important caveats, it is not surprising that the ASCEND-7 ceritinib report card differs from that of other ALK kinase inhibitors such as alectinib, brigatinib, ensartinib, and lorlatinib (1, 3–7) (Figure 1). Beyond expanded eligibility criteria, other factors were potentially at play. Whereas ceritinib is estimated to have a potency 20x that of crizotinib, it is a substrate for the p-glycoprotein (P-GP/ABCB1) and BCRP/ABCG2 efflux pumps that limit CNS penetration (1, 2, 9). Among three patients in ASCEND-7 for whom CSF ceritinib concentrations were available, the CSF to blood ratio ranged from just 13–35% (1); conclusions from this small number of patients must be interpreted cautiously. Nonetheless, it is notable that the CSF to serum ratios of lorlatinib, for example, have been reported as being closer to 75%, suggesting a potential mechanism of decreased efficacy with ceritinib (10).
The challenges of achieving optimal brain and CSF penetrance were magnified in the trial by difficulties maintaining consistent drug levels with a ceritinib starting dose of 750 mg in the fasted state daily. Most patients (79%) experienced a grade 3/4 adverse event, and 80% had at least one dose interruption or dose level reduction. High rates of inconsistent drug exposure challenge adequate ALK wild type and mutant target coverage, especially for a drug requiring 4 weeks to achieve steady state (1). A dose of 450 mg of ceritinib taken with food has shown comparable systemic efficacy to the 750 mg fasted dose and additional investigation is required to show the intracranial activity of such a dose, especially in patients with LMD (1, 2).
Molecular heterogeneity is another potential confounder. ASCEND-7 accrued patients based on fluorescence in situ hybridization testing that has been shown to yield false positive results in selected cases. Furthermore, 5’ partners and fusion breakpoints could not be assessed and an exploratory analysis of upstream gene or variant diversity as a modifier of efficacy was not possible. In ALK kinase inhibitor pre-treated patients, the spectrum of on-target or off-target resistance alterations was not available. With more cases, CSF circulating tumor DNA collection could have potentially allowed for the characterization of intracranial versus extracranial resistance both to ceritinib and pre-trial kinase inhibitor therapy. Experience with other small molecule inhibitors, including for classical sensitizing EGFR alterations, suggests that resistance patterns in the CNS may differ. Such differences may be due to suboptimal drug exposure leading to decreased selective pressure for the emergence of resistance alterations as well as to differences in the co-alteration profile associated with tropism to the brain (2, 8).
While much remains to be done to optimize outcomes for patients with oncogene-driven CNS disease, Chow and colleagues’ work represents a critical contribution to the field (1). ASCEND-7 adds to historical evidence that stakeholders are capable of executing patient-centric study designs that are more broadly inclusive on a CNS disease front. Prospective clinical trials should not dissuade patients with LMD from enrolling; such patients can and should participate in these protocols as the need in this community is indisputably high.
Finally, ceritinib’s place in the landscape of ALK fusion-positive lung cancers will need to be defined, especially as next-generation targeted therapy strategies emerge. These include “fourth-generation” single agents (such as TPX-0131, NVL-655), which have been rationally designed to abrogate complex ALK kinase domain mediated resistance and provide meaningful CNS sanctuary site coverage. Combinatorial strategies that target putative bypass resistance also continue to be explored. These drug development programs can rise to the challenge of trial inclusivity, including for CNS disease, by following simple advice: take a page from the ceritinib playbook.
ACKNOWLEDGMENTS:
Y.R. Murciano-Goroff acknowledges receipt of training through an institutional K30 grant from the NIH (CTSA UL1TR00457). She has received funding from a Kristina M. Day Young Investigator Award from Conquer Cancer, the ASCO Foundation, funded by Dr. Charles M. Baum and Carol A. Baum. This work is supported in part by the NIH/NCI Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
CONFLICTS OF INTEREST:
Y.R. Murciano-Goroff reports travel, accommodation, and expenses from AstraZeneca and honoraria from Virology Education. She acknowledges research funding to the institution from Loxo Oncology at Eli Lilly, Elucida Oncology, Taiho Oncology, and Hengrui Pharmaceutical. She acknowledges royalties from Wolters Kluwer. G. Harada has no conflicts of interest to report. A. Drilon acknowledges: HONORARIA/ADVISORY BOARDS: Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, ArcherDX, Monopteros, Novartis, EMD Serono, Medendi, Repare RX, Nuvalent, Merus, Chugai Pharmaceutical, Remedica Ltd, mBrace, AXIS, EPG Health, Harborside Nexus, Liberum, RV More, Ology, Amgen, TouchIME, Janssen, Entos, Treeline Bio, Prelude, Applied Pharmaceutical Science, Inc. ASSOCIATED RESEARCH PAID TO INSTITUTION: Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar. ROYALTIES: Wolters Kluwer. OTHER (Food/Beverage): Merck, Puma, Merus, Boehringer Ingelheim CME HONORARIA: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, AXIS, EPG Health, JNCC/Harborside.
Abbreviations:
- CNS
Central nervous system
- LMD
leptomeningeal disease
- CTCs
circulating tumor cells
- CSF
cerebrospinal fluid
REFERENCES
- 1.Chow LQM, Barlesi F, Bertino EM, van den Bent MJ, Wakelee HA, Wen PY, et al. ASCEND-7: Efficacy and safety of ceritinib treatment in patients with ALK-positive non-small cell lung cancer metastatic to the brain and/or leptomeninges. Clin Cancer Res 2022;online ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Remon J, Besse B. Brain metastases in oncogene-addicted non-small cell lung cancer patients: incidence and treatment. Front Oncol 2018;8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soria J-C, Tan DSW, Chiari R, Wu Y-L, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 4.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017;377:829–838. 2018 [DOI] [PubMed] [Google Scholar]
- 5.Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 2018;379:2027–2039. [DOI] [PubMed] [Google Scholar]
- 6.Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol 2021;7:1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 2020;383:2018–2029. [DOI] [PubMed] [Google Scholar]
- 8.Boire A, Brandsma D, Brastianos PK, Le Rhun E, Ahluwalia M, Junck L, et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol 2019;21:571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kort A, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Brain accumulation of the EML4-ALK inhibitor ceritinib is restricted by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance protein (BCRP/ABCG2). Pharmacol Res 2015;102:200–7. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel-Vinay S, et al. , Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]


