Abstract
Non-infectious pulmonary toxicity (NPT) is a significant complication of allogeneic hematopoietic cell transplantation (alloHCT) and includes idiopathic pneumonia syndrome (IPS), diffuse alveolar hemorrhage (DAH), and cryptogenic organizing pneumonia (COP) with an overall incidence ranging 1–15% in different case series and variable mortality rates. A registry study of the epidemiology and outcomes of NPT after alloHCT has not been conducted. The primary objective was to assess the incidence of and risk factors for IPS, DAH, and COP; the secondary objective was to assess overall survival (OS) in patients developing NPT. This retrospective study included adult patients who underwent alloHCT between 2008 and 2017 and reported to the Center for International Blood and Marrow Transplant Research (CIBMTR®). Multivariable Cox proportional hazards regression models were developed to identify the risk factors for development of NPT and for OS, by including pre-transplant clinical variables and time-dependent variables of neutrophil and platelet recovery, and acute GVHD post-transplant. This study included 21,574 adult patients, with a median age of 55 years. Per the HCT-Comorbidity Index (HCT-CI), 24% and 15% patients had moderate and severe pulmonary comorbidity, respectively. The cumulative incidence of NPT at 1-year was 8.1% (95% confidence interval [95CI], 7.7–8.5%). Individually, 1-year cumulative incidence of IPS, DAH, and COP was 4.9% (95CI, 4.7–5.2%), 2.1% (95CI, 1.9–2.3%), and 0.7% (95CI, 0.6–0.8%), respectively. Multivariable analysis showed severe pulmonary comorbidity, grade II-IV acute GVHD, mismatched unrelated donor and cord blood transplant, and HCT-CI score ≥1 significantly increased the risk of NPT. In contrast, alloHCT performed in ≥2014, non-TBI and TBI-based non-myeloablative conditioning and platelet recovery were associated with a decreased risk. In a landmark analysis at day+100 post-transplant, the risk of DAH was significantly lower in patients who had platelet recovery by day+100. Multivariable analysis for OS demonstrated that NPT significantly increased the mortality risk (HR 4.2, p<0.0001).
Keywords: non-infectious pulmonary toxicity, allogeneic hematopoietic cell transplantation, diffuse alveolar hemorrhage, idiopathic pneumonia syndrome, cryptogenic organizing pneumonia
INTRODUCTION
Allogeneic hematopoietic cell transplantation (alloHCT) is a potentially curative treatment for a variety of malignant and non-malignant diseases. However, alloHCT outcomes are limited by significant complications including pulmonary toxicity, which are a major driver of non-relapse mortality (NRM)1 However, limited data primarily from single-center retrospective studies have been reported on the prevention and management of non-infectious pulmonary toxicity (NPT), which is associated with a high mortality rate after alloHCT2. Despite advances in supportive care, pulmonary complications still develop in 30–60% of alloHCT recipients resulting in NRM as high as 50% in those patients3,4. Previously reported risk factors include pre-existing pulmonary toxicity of chemotherapies, total body irradiation (TBI) in alloHCT conditioning, graft-versus-host disease (GVHD), and comorbidities such as restrictive lung disease and smoking history. Smaller studies have suggested increased risk with TBI-based myeloablative (MAC) conditioning for developing NPT5.
NPT early on after alloHCT has traditionally included the following entities: idiopathic pneumonia syndrome (IPS), diffuse alveolar hemorrhage (DAH), and cryptogenic organizing pneumonia (COP)6. IPS is defined by widespread alveolar injury with signs and symptoms of pneumonia in the absence of active lower respiratory tract infection2. Clinical symptoms can be insidious and non-specific, and diagnosis may require high index of suspicion after excluding infectious and alternative pathologies2,6,7. IPS can occur as early as 2–6 weeks post-transplant with an incidence of 2–15%2,4,8,9. Treatment is empirical, with high-dose systemic corticosteroids as the established standard10. Tumor necrosis factor (TNF)-α inhibitor, etanercept has been shown to benefit IPS patients in a few clinical trials; nonetheless, high mortality rate has been demonstrated, even in responders2,4,8,11,12. DAH presents with progressively bloodier returns from bronchoalveolar lavage and evidence of widespread alveolar injury and can cause rapidly progressive acute respiratory failure13,14. DAH has been shown to develop in the first 4 weeks post-transplant with an incidence of 2–14% and is associated with mortality rates ranging from 64%–100%15–17. COP is marked by a restrictive pattern on pulmonary function test (PFT) with pathology showing patchy granulation tissue invading alveolar ducts with interstitial inflammation18. The incidence of COP is 1–10%, usually between 2 and 15 months after alloHCT, but responses to corticosteroids are generally favorable2,17,19,20.
As the data demonstrate, there is significant heterogeneity in the published literature on the incidence and variability in outcomes of early NPT. Historically, this has stemmed from lack of consensus on evaluation, treatment, and response, evolution of cancer treatment and transplant regimens, including supportive care over time, lack of prospective clinical trials data, and the limitations of retrospective, single-center studies, reflecting the associated geographical biases in approaching and managing NPT. We conducted a retrospective registry study to better understand the epidemiology of NPT developing early on after alloHCT, to confirm the risk factors for NPT, and to examine the impact of NPT on transplant outcomes.2
METHODS
Data Sources
The CIBMTR® (Center for International Blood and Marrow Transplant Research®) is a nonprofit research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin (MCW). More than 330 medical centers worldwide submit clinical data to the CIBMTR® on HCT and other cellular therapies; currently, the CIBMTR’s Research Database includes long-term clinical data for more than 585,000 patients. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits and patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and onsite audits of participating centers ensure data quality. Studies conducted by the CIBMTR® are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
Patients
This analysis included adult patients (aged 18 years and older) undergoing a first alloHCT as reported to the CIBMTR® between the years 2008 and 2017. Patients with any disease indication, graft source, and donor source were included. Transplant recipients with less than 100 days of follow-up were excluded.
Objectives, Endpoints and Definitions
The primary objectives were to evaluate the incidence of and the risk factors for development of IPS, DAH, COP, and the composite NPT. Other transplant complications involving lungs, including peri-engraftment respiratory distress syndrome (PERDS), pulmonary veno-occlusive disease, and late-onset post-transplant pulmonary entities of interstitial lung diseases (ILD) and bronchiolitis obliterans syndrome (BOS) were not included in this analysis. The secondary objective was to evaluate the impact of NPT on overall survival (OS) after alloHCT. IPS, DAH, and COP were diagnosed and reported by the contributing transplant centers based on the clinical, histopathologic, and imaging data obtained. OS was defined as time from alloHCT to death from any cause, with surviving patients censored at last follow-up.
Statistical Analysis
NPT as a composite endpoint was reached if the patient developed any one of the three toxicities: IPS, DAH, or COP. Death from any cause was a competing risk. Multivariable proportional cause-specific hazards model and Cox models with forward stepwise selection and significance level 0.01 were developed to identify the risk factors for NPT and OS. Covariates included transplant indication (underlying disease), Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) score (minus the pulmonary component), presence and severity of pulmonary comorbidity (moderate or severe, as defined in the HCT-CI), graft source, donor type, conditioning intensity and use of TBI, neutrophil recovery (defined as achievement of absolute neutrophil count >500/μL for 3 consecutive days), platelet recovery (defined as platelet count >20,000/μL for 3 consecutive days, without transfusion in 7 previous days), grade II-IV acute GVHD, and year of transplant. Conditioning intensities were defined by the CIBMTR consensus criteria21. Pre-transplant pulmonary comorbidity severity was defined per HCT-CI as moderate (FEV1 and/or DLCO 66–80%, dyspnea on slight activity) or severe (FEV1 and/or DLCO ≤65%, dyspnea at rest, requiring supplemental oxygen)22. Neutrophil and platelet recovery as well as acute GVHD were added as time-dependent covariates in the regression model. The assumption of proportional hazards for each factor was tested by examining time-varying effects. Potential interactions between the main effect and significant covariates were also tested. In addition, landmark analyses at day +100 were conducted to examine the impact of NPT on overall survival after alloHCT. For the landmark survival analysis, covariates included transplant disease indication, age, HCT-CI score (minus the pulmonary component), Karnofsky Performance Score (KPS), smoking history, pulmonary comorbidity severity, prior autoHCT, year of transplant, and donor source. Adjusted OS and cumulative incidence of NPT, IPS, DAH, and COP were calculated based on the final regression model. A center effect was tested using the score test of homogeneity. When there was a significant center effect, the marginal regression model was fitted to account for it. All analyses were conducted using SAS® v9.4 (Cary, NC).
RESULTS
Patients, Disease, and Transplant Characteristics
A total of 21,574 adult alloHCT recipients were included in the study (Table 1). Median age at alloHCT was 55 years (range, 18–88 years), 59% were male, and 74% were Caucasian. Fifty-nine percent of patients had a KPS ≥90%. Based on the HCT-CI score, 24% and 15% patients had moderate and severe pulmonary impairment, respectively. Prior to transplant, 3% patients had a history of mechanical ventilation, 5% had a history of invasive fungal infection, and 40% had a smoking history. Most patients had a matched sibling or matched unrelated donor (64%). Peripheral blood stem cell graft was the most often used graft source (71%). MAC, reduced intensity (RIC), and non-myeloablative (NMA) conditioning regimens were used in 49%, 34%, and 17% of patients, respectively. Twenty percent patients received a TBI-based MAC and 19% had TBI-based NMA regimen. The median follow-up of survivors was 49 months (range, 3–131 months).
Table 1.
Baseline characteristics of patients receiving first allogeneic transplant between 2008 and 2017
| Characteristic | N (%) |
|---|---|
| Number of patients | 21574 |
| Number of centers | 255 |
| Age, median (range) | 55 (18–88) |
| Male | 12672 (59) |
| Race | |
| Caucasian | 15993 (74) |
| Ethnicity | |
| Not Hispanic or Latino | 17962 (83) |
| KPS 90–100 | 12833 (59) |
| HCT-CI (minus pulmonary comorbidity) | |
| 0 | 6018 (28) |
| 1 | 3001 (14) |
| 2 | 2884 (13) |
| 3 | 3712 (17) |
| 4 | 2262 (10) |
| 5+ | 3231 (15) |
| Pulmonary comorbidity (based on HCT-CI) | |
| Moderate | 5163 (24) |
| Severe | 3242 (15) |
| History of mechanical ventilation | 646 (3) |
| History of smoking cigarettes | 8541 (40) |
| Disease indication | |
| AML | 8003 (37) |
| MDS/MPN | 6044 (28) |
| ALL | 2362 (11) |
| Lymphoma | 2081 (10) |
| Non-malignant disease | 1107 (5) |
| CML | 722 (3) |
| CLL | 696 (3) |
| MM/PCD | 300 (1) |
| Other malignant disease | 259 (1) |
| Refined-Disease Risk Index groups | |
| Low | 2061 (10) |
| Intermediate | 10846 (50) |
| High | 5955 (28) |
| Very high | 745 (3) |
| Prior autologous transplant | 1277 (6) |
| Donor type | |
| Matched unrelated | 8270 (38) |
| HLA-identical sibling | 5576 (26) |
| Cord blood | 2911 (13) |
| Haploidentical | 1978 (9) |
| Mismatched unrelated | 1891 (9) |
| Other | 900 (5) |
| Graft source | |
| Peripheral blood | 15335 (71) |
| Bone marrow | 3328 (15) |
| Umbilical cord blood | 2911 (13) |
| Conditioning regimen intensity | |
| MAC | 10665 (49) |
| RIC | 7242 (34) |
| NMA | 3667 (17) |
| TBI and intensity | |
| Myeloablative TBI | 4285 (20) |
| Non-myeloablative TBI | 4109 (19) |
| GVHD prophylaxis | |
| TAC + MMF or MTX or other | 13256 (61) |
| CSA + MMF or MTX | 3837 (18) |
| ptCy | 2246 (10) |
| Ex-vivo T-cell depletion or CD34 selection | 694 (3) |
| ATG/Alemtuzumab | |
| ATG alone | 5885 (27) |
| Alemtuzumab alone | 656 (3) |
| Year of transplant | |
| 2008–2013 | 10888 (51) |
| 2014–2017 | 10686 (49) |
| Follow-up of survivors, median (range) | 49 (3–131) |
Abbreviations: alloHCT = allogeneic hematopoietic cell transplantation; KPS = Karnofsky Performance Score, HCT-CI = Hematopoietic Cell Transplantation-Comorbidity Index; AML = acute myeloid leukemia; ALL = acute lymphoblastic leukemia; CML = chronic myelogenous leukemia; MDS = myelodysplastic syndrome; MPN = myeloproliferative neoplasm; NHL = non-Hodgkin’s lymphoma; CLL = chronic lymphocytic leukemia; PCD = plasma cell disorders; MAC = myeloablative conditioning; RIC = reduced intensity conditioning; NMA = non-myeloablative conditioning; TBI = total body irradiation; Cy = cyclophosphamide; Flu = fludarabine; Bu = busulfan; Mel = melphalan; TAC = tacrolimus; MTX = methotrexate; MMF = mycophenolate mofetil; ptCy = post-transplant cyclophosphamide; ATG = anti-thymocyte globulin
Other donors included any matched related (not siblings), 1-locus mis-matched related donors, and cases with related donors who do not have HLA-matching information.
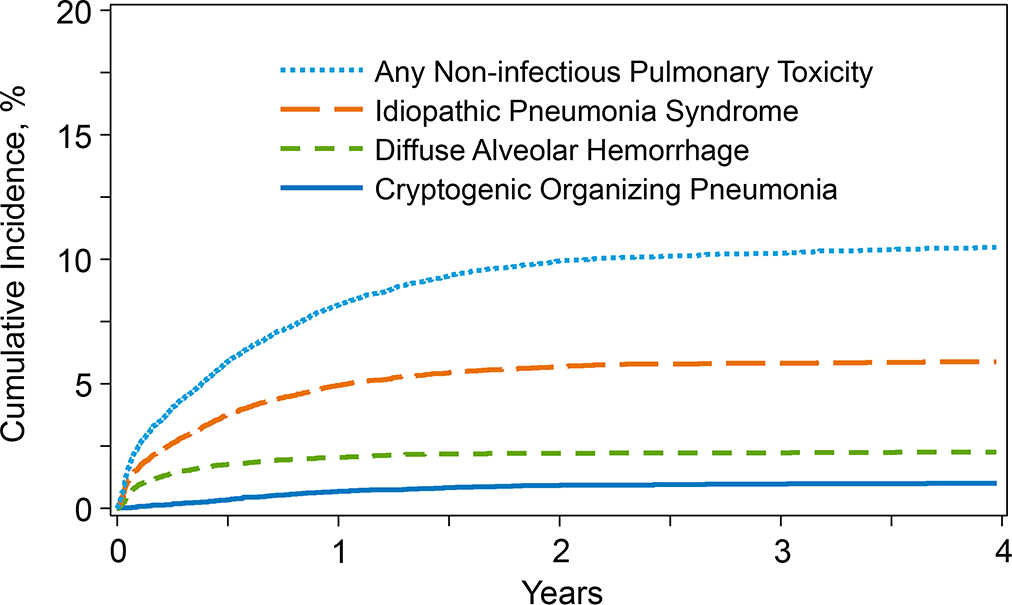
Incidence of NPT
The cumulative incidence of NPT as a composite endpoint at 1-year post-transplant was 8.1% (95% Confidence Interval [95%CI], 7.7–8.5%). The cumulative incidence of IPS, DAH, and COP was 4.9% (95% CI, 4.7–5.2%), 2.1% (95% CI, 1.9–2.3%), and 0.7% (95% CI, 0.6–0.8%), respectively (Table 2 and Figure 1). Individually, the median time to onset of IPS, DAH, and COP was 3.7, 1.7, and 8.2 months, respectively. Amongst patients who were reported to have NPT (n=1802) in the database, 39% (n=708) received an interventional diagnostic procedure and 29% (n=518) had no invasive testing/diagnostic intervention performed. The data on the remaining 32% (n=576) were not reported by the respective centers. Of the cases who had testing done (n=708; 39%): 596 (84%) had bronchoalveolar lavage (BAL) or other diagnostic testing performed, 67 (9%) underwent transbronchial biopsy, 44 (6%) had open/thoracoscopic (video-assisted) lung biopsy completed. Of those with IPS in the study cohort, 168 (13.2%) had diagnostic testing performed, 514 (40.3%) did not have diagnostic testing, testing was not reported for 593 (46.5%) cases (Supplemental Table 1). Of the study patients labelled with DAH, 82.4% (n=402) had diagnostic testing completed, whereas 13.7% (n=67) had no diagnostic testing performed and 3.9% (n=19) had testing not reported. Of the study patients diagnosed with COP, 168 (82.8%) patients underwent testing, and 35 (17.2%) did not have diagnostic testing performed.
Table 2.
Cumulative Incidence of non-infectious pulmonary toxicity (NPT) after allogeneic transplantation
| Outcomes | N | Probability (95% Confidence Interval) |
|---|---|---|
| Non-infectious pulmonary toxicity (IPS, DAH or COP) | 21508 | |
| 3 months | 4.0 (3.8–4.3)% | |
| 6 months | 5.9 (5.6–6.2)% | |
| 1-year | 8.1 (7.7–8.5)% | |
| 3-year | 10.3 (9.8–10.7)% | |
| Idiopathic pneumonia syndrome (IPS) | 21526 | |
| 3 months | 2.6 (2.4–2.8)% | |
| 6 months | 3.8 (3.5–4)% | |
| 1-year | 4.9 (4.7–5.2)% | |
| 3-year | 5.8 (5.5–6.2)% | |
| Diffuse alveolar hemorrhage (DAH) | 21552 | |
| 3 months | 1.4 (1.3–1.6)% | |
| 6 months | 1.8 (1.6–2)% | |
| 1-year | 2.1 (1.9–2.3)% | |
| 3-year | 2.3 (2.1–2.5)% | |
| Cryptogenic organizing pneumonia (COP) | 21553 | |
| 3 months | 0.2 (0.1–0.2)% | |
| 6 months | 0.3 (0.3–0.4)% | |
| 1-year | 0.7 (0.6–0.8)% | |
| 3-year | 1.0 (0.8–1.1)% |
NPT = non-infectious pulmonary toxicity; alloHCT = allogeneic hematopoietic cell transplantation
Figure 1. Cumulative Incidence of Non-infectious Pulmonary Toxicity after allogeneic transplant.
Risk Factors for NPT
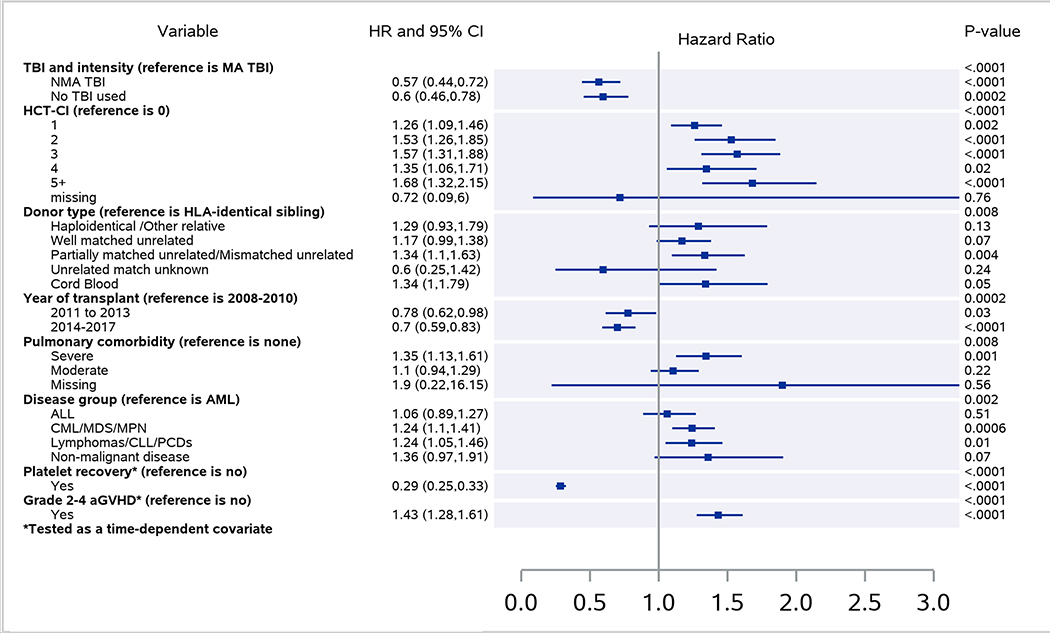
Multivariable analysis showed that severe pulmonary comorbidity based on pre-transplant pulmonary function testing (PFT) (HR 1.35, p=0.0009), grade II-IV acute GVHD (HR 1.43, p<0.0001), mismatched unrelated donor (HR 1.33, p=0.005), cord blood transplant (HR 1.34, p=0.046) and HCT-CI ≥1 (vs. 0; HR 1.26–1.56, p<0.0001) significantly increased the risk of NPT (Figure 2). In contrast, year of HCT ≥2014 (HR 0.70, p<0.0001), conditioning without TBI (HR 0.60, p=0.0002) and TBI-based NMA conditioning (HR 0.57, p<0.0001) (vs. TBI-based MAC), and platelet recovery (HR 0.29, p<0.001) were associated with a significantly lower risk of NPT (Table 3). We also analyzed the impact of in vivo T cell depletion (anti-thymocyte globulin [ATG] and alemtuzumab) in the conditioning and use of granulocyte-colony stimulating factor (G-CSF) post-transplant but did not find any significant association with NPT in the multivariable models.
Figure 2. Multivariable analysis Forest plot of risk factors for NPT after alloHCT.
NPT = non-infectious pulmonary toxicity; alloHCT = allogeneic hematopoietic cell transplantation
Table 3.
Multivariable analysis of non-infectious pulmonary toxicity (NPT) after allogeneic transplantation
| Variable | N | HR | 95% CI | P-value |
|---|---|---|---|---|
| 1. Non-infectious Pulmonary Toxicity | ||||
| TBI and Conditioning Intensity | ||||
| MA TBI | 4216 | 1 | <.0001 | |
| NMA TBI | 4010 | 0.566 | 0.442–0.725 | <.0001 |
| No TBI | 12937 | 0.596 | 0.455–0.78 | 0.0002 |
| HCT-CI (minus pulmonary) | ||||
| 0 | 8514 | 1 | <.0001 | |
| 1 | 5193 | 1.262 | 1.09–1.461 | 0.0019 |
| 2 | 2287 | 1.527 | 1.261–1.849 | <.0001 |
| 3 | 2300 | 1.568 | 1.307–1.882 | <.0001 |
| ≥4 | 2385 | 1.484 | 1.231–1.788 | <.0001 |
| Donor type | ||||
| HLA-identical sibling | 5492 | 1 | 0.0089 | |
| Haploidentical /Other relative | 2612 | 1.288 | 0.929–1.786 | 0.1288 |
| Well matched unrelated | 8161 | 1.169 | 0.988–1.383 | 0.0694 |
| Mismatched unrelated | 1867 | 1.33 | 1.092–1.621 | 0.0046 |
| Cord Blood | 2845 | 1.342 | 1.005–1.792 | 0.0465 |
| Year of transplant | ||||
| 2008 to 2010 | 6318 | 1 | 0.0003 | |
| 2011 to 2013 | 4413 | 0.777 | 0.615–0.982 | 0.0346 |
| ≥2014 | 10432 | 0.702 | 0.592–0.833 | <.0001 |
| Pulmonary comorbidity | ||||
| None | 12443 | 1 | 0.0071 | |
| Severe | 3171 | 1.352 | 1.132–1.615 | 0.0009 |
| Moderate | 5081 | 1.105 | 0.944–1.294 | 0.2127 |
| Disease group | ||||
| AML | 7889 | 1 | 0.0021 | |
| ALL | 2469 | 1.061 | 0.887–1.27 | 0.5145 |
| CML/MDS/MPN | 6646 | 1.245 | 1.099–1.41 | 0.0006 |
| Lymphomas/CLL/PCDs | 3073 | 1.239 | 1.049–1.462 | 0.0114 |
| Non-malignant disease | 1086 | 1.36 | 0.971–1.903 | 0.0734 |
| Platelet recovery | ||||
| No | 2325 | 1 | <.0001 | |
| Yes | 18838 | 0.286 | 0.252–0.325 | <.0001 |
| Grade II-IV acute GVHD | ||||
| No | 12480 | 1 | <.0001 | |
| Yes | 8683 | 1.434 | 1.276–1.611 | <.0001 |
| 2. Idiopathic pneumonia syndrome | ||||
| HCT-CI (minus pulmonary) | ||||
| 0 | 8508 | 1 | <.0001 | |
| 1 | 5188 | 1.26 | 1.075–1.477 | 0.0044 |
| 2 | 2281 | 1.437 | 1.173–1.761 | 0.0005 |
| 3 | 2301 | 1.595 | 1.308–1.944 | <.0001 |
| ≥4 | 2381 | 1.489 | 1.211–1.83 | 0.0002 |
| TBI and Conditioning Intensity, Acute GVHD | ||||
| MA TBI+ no acute GVHD | 2264 | 1 | 0.0018 | |
| NMA TBI+ no acute GVHD | 2460 | 0.478 | 0.3788–0.6031 | <.0001 |
| No TBI+ no acute GVHD | 7740 | 0.5149 | 0.4335–0.6117 | <.0001 |
| MA TBI+ acute GVHD | 1947 | 1.0066 | 0.7878–1.2862 | 0.9579 |
| NMA TBI+ acute GVHD | 1547 | 0.7469 | 0.5494–1.0153 | 0.0625 |
| No TBI+ acute GVHD | 5186 | 0.8785 | 0.7157–1.0784 | 0.2156 |
| Year of transplant | ||||
| 2008 to 2010 | 6315 | 1 | 0.0081 | |
| 2011 to 2013 | 4411 | 0.848 | 0.716–1.004 | 0.0561 |
| ≥2014 | 10418 | 0.801 | 0.695–0.923 | 0.0022 |
| Platelet recovery | ||||
| No | 2311 | 1 | <.0001 | |
| Yes | 18833 | 0.338 | 0.281–0.407 | <.0001 |
| 3. Diffuse alveolar hemorrhage | ||||
| Disease group | ||||
| AML | 7892 | 1 | 0.0076 | |
| ALL | 2468 | 0.992 | 0.724–1.36 | 0.9622 |
| CML/MDS/MPN | 6655 | 1.517 | 1.197–1.922 | 0.0006 |
| Lymphomas/CLL/PCDs | 3074 | 1.292 | 0.959–1.741 | 0.0915 |
| Non-malignant disease | 1088 | 1.373 | 0.867–2.174 | 0.1769 |
| Pulmonary comorbidity | ||||
| None | 12449 | 1 | 0.0003 | |
| Severe | 3172 | 1.665 | 1.3–2.132 | <.0001 |
| Moderate | 5085 | 1.373 | 1.093–1.725 | 0.0064 |
| TBI and Conditioning Intensity | ||||
| MA TBI | 4220 | 1 | <.0001 | |
| NMA TBI | 4012 | 0.598 | 0.449–0.797 | 0.0004 |
| No TBI | 12945 | 0.542 | 0.426–0.69 | <.0001 |
| GVHD prophylaxis | ||||
| Ex-vivo T-cell depletion/CD34+ selection | 669 | 1 | <.0001 | |
| PtCy +/− other(s) | 2198 | 1.031 | 0.535–1.987 | 0.9269 |
| CNI + MMF | 5936 | 1.559 | 0.867–2.8 | 0.1377 |
| CNI + MTX | 9668 | 0.828 | 0.457–1.501 | 0.535 |
| CNI + other | 2109 | 1.049 | 0.548–2.008 | 0.8858 |
| Year of transplant | ||||
| 2008 to 2010 | 6319 | 1 | <.0001 | |
| 2011 to 2013 | 4419 | 0.644 | 0.497–0.833 | 0.0008 |
| ≥2014 | 10439 | 0.582 | 0.462–0.733 | <.0001 |
| Grade II-IV Acute GVHD | ||||
| No | 12490 | 1 | 0.0006 | |
| Yes | 8687 | 1.486 | 1.185–1.864 | 0.0006 |
| Platelet recovery | ||||
| No | 2327 | 1 | <.0001 | |
| Yes | 18850 | 0.15 | 0.116–0.194 | <.0001 |
| 4. Cryptogenic organizing pneumonia | ||||
| TBI and Conditioning Intensity | ||||
| MA TBI | 4218 | 1 | 0.007 | |
| NMA TBI | 4013 | 0.753 | 0.423–1.338 | 0.333 |
| No TBI | 12947 | 0.471 | 0.29–0.765 | 0.002 |
| Grade II-IV Acute GVHD | ||||
| No | 12490 | 1 | 0.001 | |
| Yes | 8688 | 1.815 | 1.26–2.613 | 0.001 |
| Platelet recovery | ||||
| No | 2327 | 1 | ||
| Yes | 18851 | 0.502 | 0.329–0.765 | 0.001 |
Abbreviations: NPT = non-infectious pulmonary toxicity; alloHCT = allogeneic hematopoietic cell transplantation; HLA = human leukocyte antigen; haplo-related = haploidentical related donor; HCT-CI = Hematopoietic Cell Transplantation-Comorbidity Index; KPS = Karnofsky Performance Score; AML = acute myeloid leukemia; ALL = acute lymphoid leukemia; CML = chronic myeloid leukemia; MDS = myelodysplastic syndrome; MPN = myeloproliferative neoplasm; CLL = chronic lymphocytic leukemia; PCD = plasma cell disorders; PB = peripheral blood stem cell graft, BM = bone marrow graft; ptCy = posttransplant cyclophosphamide; CNI = calcineurin inhibitor; MMF= mycophenolate mofetil; MTX = methotrexate.
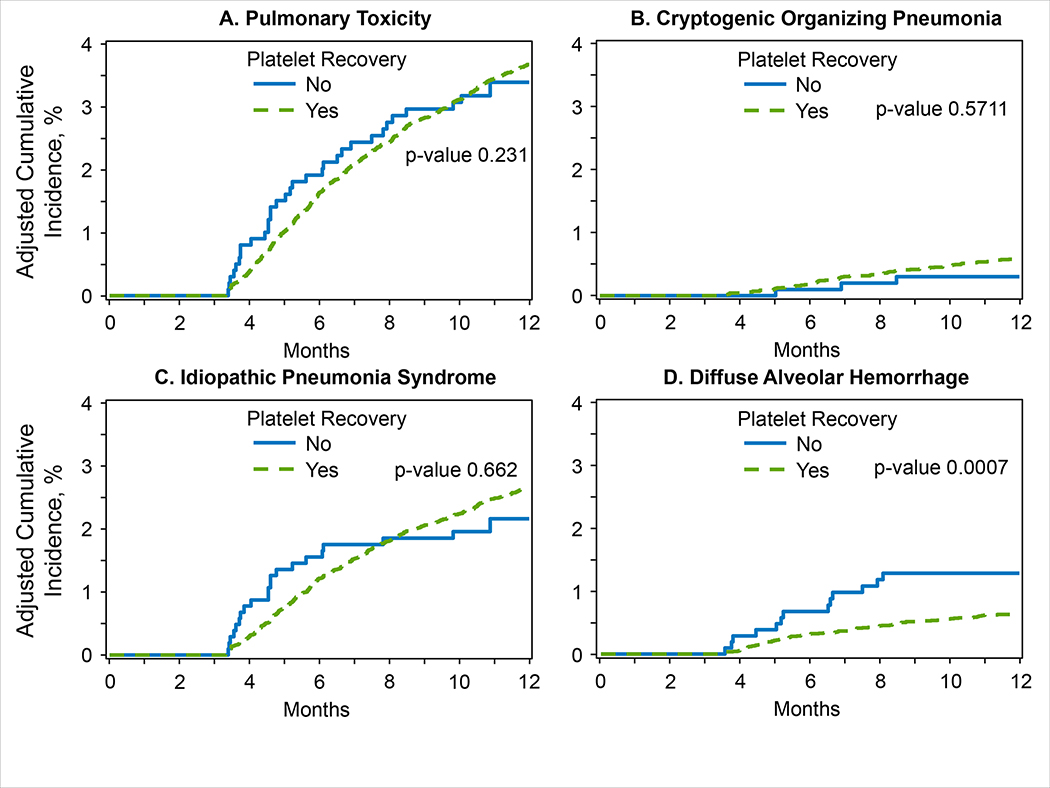
Risk factors for IPS included HCT-CI ≥1 (vs. 0; HR 1.26–1.59, p0.0002), while transplantation in ≥2014 (HR 0.80, p=0.002), and platelet recovery (HR 0.34, p<0.0001) decreased the risk of IPS (Table 3). The analysis showed a significant interaction between TBI-conditioning intensity and time-dependent variable of grade II-IV acute GVHD (p=0.002): patients receiving TBI-based NMA and non-TBI-based regimens had a significantly lower risk of IPS, if they did not develop grade II-IV acute GVHD (HR 0.48 and 0.52, p<0.0001, respectively), but not if they had acute GVHD (HR 0.75, p=0.06 and 0.88, p=0.21, respectively), when compared with TBI-based MAC recipients (regardless of acute GVHD onset). For DAH, significant risk factors included severe pulmonary dysfunction (HR 1.66, p<0.0001), underlying disease of CML, MDS, or MPN (HR 1.52, p=0.0006), and grade II-IV acute GVHD (HR 1.49, p=0.0006); in contrast, alloHCT performed in ≥2014 (HR 0.58, p<0.0001), TBI-based NMA and non-TBI-based conditioning (vs. TBI-based MAC; HR 0.60, p=0.0004 and HR 0.54, p<0.0001), and platelet recovery (HR 0.15, p<0.0001) were associated with significantly decreased risk of DAH (Table 3). Finally, grade II-IV acute GVHD (HR 1.81, p=0.001) significantly increased the risk of COP, while platelet recovery (HR 0.50, p=0.001) and non-TBI-based conditioning (HR 0.47, p=0.002) were associated with significantly decreased risk (Table 3). In addition, a landmark analysis was conducted at day +100 after alloHCT to evaluate the impact of platelet recovery on NPT risk (Figure 3A–D). The risk of DAH was significantly lower in patients who had achieved platelet recovery by day +100 (HR 0.37, p=0.0007) (Figure 3D). The risk of IPS and COP was not significantly affected by platelet recovery by day +100 (Figure 3B–C).
Figure 3. A-D. Cumulative incidence of NPT after alloHCT by platelet recovery.
Survival Outcomes
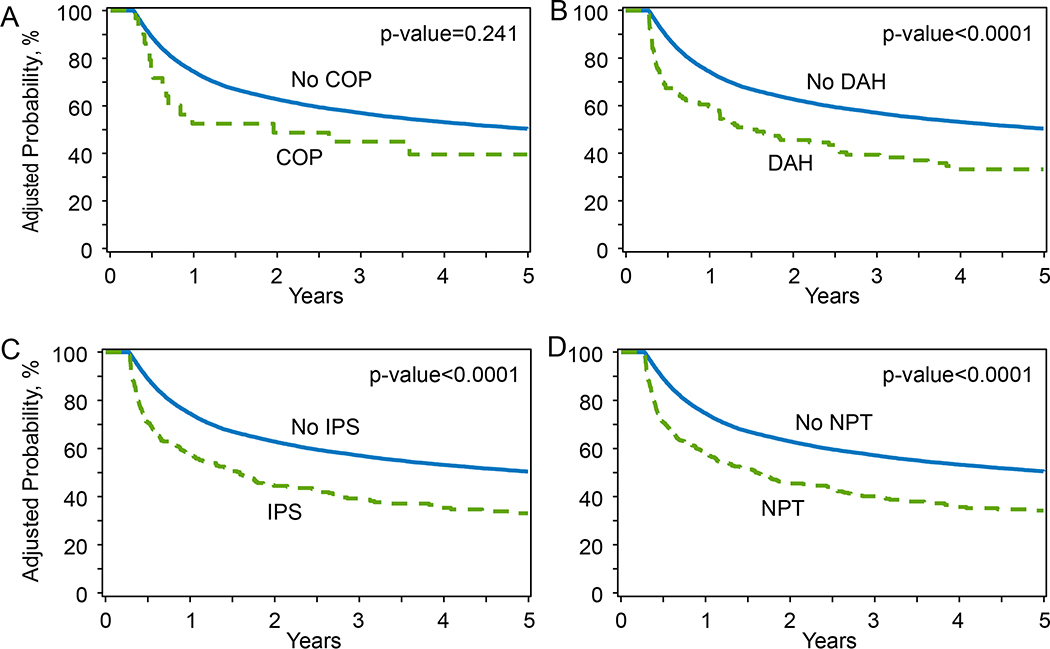
Three-year OS probability for the study cohort was 48.9% (95%CI 48.1–49.6%). Multivariable analysis for OS demonstrated that development of NPT significantly increased the risk of overall mortality (HR 4.19, p<0.0001) (Table 4). for the multivariable models for IPS, DAH, and COP individually showed increased mortality risk with HRs of 4.16, 5.6, and 1.93 (p<0.0001 for all), respectively. The effect of other variables on the probability of overall survival after alloHCT is shown in Table 4 and Supplemental Table 2. Importantly, smoking history (HR 1.11, p<0.0001), HCT-CI ≥1 (HR 1.10–1.45, p<0.01) and severe pulmonary comorbidity (HR 1.27, <0.0001) significantly increased mortality risk. We also conducted a day+100 landmark analysis to evaluate the impact of NPT on OS after alloHCT (Supplemental Table 3, Figures 4A–D) and showed a significant decrease in OS in patients developing NPT. Patients who were alive and developed NPT by day+100 had a 1-year OS of 58.4% (95%CI, 54–63%), compared to 74.7% (95%CI, 74–75.3%) in patients who survived until day+100 without developing NPT (P<0.0001). Similarly, 3-year OS was significantly improved in patients surviving without NPT by day+100 (57% vs. 39%, p<0.0001).
Table 4.
Multivariable analysis for overall survival examining the effect of non-infectious pulmonary toxicity (NPT) after allogeneic transplantation
| Variable | N | HR | 95% CI | P-value |
|---|---|---|---|---|
| Non-infectious Pulmonary Toxicity | ||||
| No | 19641 | 1 | <.0001 | |
| Yes | 1828 | 4.187 | 3.78II-IV.635 | <.0001 |
| Disease group | ||||
| AML | 8001 | 1 | <.0001 | |
| ALL | 2505 | 0.912 | 0.855–0.974 | 0.0059 |
| CML/MDS/MPN | 6745 | 0.93 | 0.887–0.976 | 0.0033 |
| Lymphomas/CLL/PCDs | 3121 | 0.782 | 0.729–0.84 | <.0001 |
| Non-malignant diseases | 1097 | 0.56 | 0.471–0.666 | <.0001 |
| Age Group | ||||
| 18–39 | 5062 | 1 | <.0001 | |
| 40–64 | 12036 | 1.343 | 1.274–1.417 | <.0001 |
| ≥65 | 4371 | 1.638 | 1.524–1.761 | <.0001 |
| Race | ||||
| Caucasian | 15914 | 1 | 0.0011 | |
| African-American | 1643 | 1.19 | 1.081–1.311 | 0.0004 |
| Asian/Pacific Islander | 1336 | 0.979 | 0.851–1.127 | 0.769 |
| Hispanic | 1800 | 1.054 | 0.971–1.145 | 0.2099 |
| KPS | ||||
| >90% | 3849 | 1 | 0.0001 | |
| ≤90% | 17221 | 1.187 | 1.096–1.286 | <.0001 |
| History of smoking | ||||
| No | 12170 | 1 | <.0001 | |
| Yes | 8499 | 1.108 | 1.063–1.153 | <.0001 |
| HCT-CI (minus pulmonary) | ||||
| 0 | 8636 | 1 | <.0001 | |
| 1 | 5264 | 1.105 | 1.047–1.167 | 0.0003 |
| 2 | 2311 | 1.214 | 1.129–1.305 | <.0001 |
| 3 | 2332 | 1.225 | 1.135–1.322 | <.0001 |
| ≥4 | 2429 | 1.45 | 1.351–1.556 | <.0001 |
| Pulmonary comorbidity | ||||
| None | 12624 | 1 | <.0001 | |
| Severe | 3220 | 1.27 | 1.194–1.351 | <.0001 |
| Moderate | 5144 | 1.031 | 0.975–1.09 | 0.2806 |
| Prior autologous transplant | ||||
| No | 20202 | 1 | <.0001 | |
| Yes | 1267 | 1.226 | 1.136–1.323 | <.0001 |
| GVHD prophylaxis | ||||
| Ex-vivo T-cell depletion / CD34 selection | 687 | 1 | <.0001 | |
| PtCy +/− other(s) | 2235 | 0.989 | 0.782–1.251 | 0.9277 |
| CNI+MMF | 5999 | 1.148 | 0.932–1.415 | 0.1933 |
| CNI+MTX | 9762 | 0.974 | 0.777–1.22 | 0.8164 |
| CNI+/−other | 2118 | 1.017 | 0.803–1.29 | 0.8863 |
| Year of transplant | ||||
| 2008 to 2010 | 6379 | 1 | <.0001 | |
| 2011 to 2013 | 4471 | 0.824 | 0.772–0.879 | <.0001 |
| ≥2014 | 10619 | 0.739 | 0.693–0.787 | <.0001 |
| Donor type, Graft source | ||||
| HLA-identical sibling BM | 636 | 1 | <.0001 | |
| HLA-identical sibling PB | 4925 | 1.284 | 1.089–1.513 | 0.0029 |
| Haplo-related BM | 846 | 1.407 | 1.167–1.697 | 0.0003 |
| Haplo-related PB | 1814 | 1.543 | 1.281–1.86 | <.0001 |
| Matched unrelated BM | 1436 | 1.341 | 1.151–1.563 | 0.0002 |
| Matched unrelated PB | 6826 | 1.339 | 1.147–1.564 | 0.0002 |
| Mismatched unrelated BM | 366 | 1.778 | 1.458–2.169 | <.0001 |
| Mismatched unrelated PB | 1521 | 1.644 | 1.373–1.967 | <.0001 |
| Cord Blood | 2904 | 1.711 | 1.376–2.127 | <.0001 |
Abbreviations: OS = overall survival; NPT = non-infectious pulmonary toxicity; alloHCT = allogeneic hematopoietic cell transplantation; HLA = human leukocyte antigen; haplo-related = haploidentical related donor; HCT-CI = Hematopoietic Cell Transplantation-Comorbidity Index; KPS = Karnofsky Performance Score; AML = acute myeloid leukemia; ALL = acute lymphoid leukemia; CML = chronic myeloid leukemia; MDS = myelodysplastic syndrome; MPN = myeloproliferative neoplasm; CLL = chronic lymphocytic leukemia; PCD = plasma cell disorders; PB = peripheral blood stem cell graft, BM = bone marrow graft; ptCy = posttransplant cyclophosphamide; CNI = calcineurin inhibitor; MMF= mycophenolate mofetil; MTX = methotrexate.
Figure 4. A-D. Day +100 landmark analysis for OS in patients with and without IPS, DAH, COP, and any NPT.
OS = overall survival; IPS = idiopathic pneumonia syndrome; DAH = diffuse alveolar hemorrhage; COP = cryptogenic organizing pneumonia; NPT = non-infectious pulmonary toxicity
DISCUSSION
This retrospective analysis of 21,574 patients in a registry-based study examined the incidence of and the risk factors for NPT as well as its effect on survival after allogeneic transplant. We identified a 1-year cumulative incidence of IPS, DAH, and COP of 4.9%, 2.1%, and 0.7%, respectively. The analysis demonstrated that TBI-based MAC and HCT-CI score ≥1 increased the risk for IPS, while severe pulmonary comorbidity, myelodysplastic and myeloproliferative disorders (CML, MDS, MPN) were significant risk factors for DAH; TBI-based MAC and acute GVHD were predictive for both DAH and COP. Severe but not moderate pulmonary comorbidity on pre-transplant PFT, and mismatched unrelated donor and cord blood transplants predicted for significantly higher risk of NPT. In contrast, transplants performed in more recent years, non-TBI and TBI-based NMA regimens, and platelet recovery were associated with a lower risk of NPT. The occurrence of acute GVHD abrogated the favorable effect of non-TBI and NMA conditioning regimens on IPS risk. The more frequent use of RIC/NMA regimens and less frequent use of myeloablative TBI in the last decade, and improved supportive care likely explain the decreased risk of NPT between 2014 and 2017 (vs. 2008–2010).
NPT after alloHCT represent a varied and multifaceted set of problem with significant contribution to morbidity and mortality23. Many prior reports included single-institution retrospective datasets with smaller sample sizes with significant heterogeneity, thereby limiting our understanding of the epidemiology of and outcomes associated with NPT. Prior studies have shown the incidence of IPS ranges from 2–15%, DAH from 2–14%, and COP from 1–10%4,15,19,24. Previous reports have identified risk factors for IPS as MAC, particularly with TBI, age over 40 years, and severe acute GVHD5,25. Older age, MAC especially with TBI, and acute GVHD have been previously shown to increase the risk of DAH26,27. In addition, further work suggested delayed or failed neutrophil engraftment as a risk factor for DAH, with delayed platelet engraftment a risk factor after cord blood transplants28. COP, in contrast, has been shown to be associated with HLA disparity, female donor-to-male recipient, use of PBSC graft, and acute and chronic GVHD18 29.
The results also revealed that IPS and DAH are likely to develop earlier in the post-transplant course than COP with a median time to onset of IPS and DAH of 112 and 50 days, respectively, whereas COP occurred at a median of 246 days post-transplant. This likely reflects the differences in the underlying pathophysiology of each of these disorders. In IPS, for example, data from murine models suggest that conditioning agents trigger lung epithelial injury followed by excessive activation of pulmonary macrophages and alloreactive T lymphocytes.2 The association of IPS with higher comorbidity index and TBI-based MAC suggests that pre-existing pulmonary comorbidity (such as chemotherapy-associated interstitial pneumonitis or smoking-related emphysema) coupled with subsequent conditioning regimen-related toxicity drive the development of IPS. In contrast, the pathogenesis of DAH is thought to be prompted by alveolar epithelial injury leading to inflammation followed by dysregulated cytokine release resulting in capillary endothelial injury30. Our study has demonstrated a strong association of DAH with platelet recovery as the onset of DAH correlated with non-engraftment of platelets with a median time of 1.7 months. Post-transplant thrombocytopenia can be associated with thrombotic microangiopathy, which also has been reported as a risk factor for DAH28. Furthermore, transfusion-related acute lung injury (TRALI) or transfusion-associated circulatory overload (TACO), a byproduct of supportive care treatment, can damage the capillary endothelium and worsen pre-existing alveolar injury31. Based on the increased risk of DAH and COP in patients developing acute GVHD, and decreased risk in recipients of non-TBI-based conditioning regimens, the data suggest a role for alloreactivity in conjunction with regimen-related toxicity in the development of NPT32.
The study has several limitations, including its retrospective nature, the observational database of the registry notwithstanding. One caveat of this analysis is the inherent possibility of misdiagnosis or delayed diagnosis of NPT. We acknowledge the possibility that NPT cases may have not been correctly diagnosed, thereby affecting the incidence estimates. Intrinsically, these entities can have overlapping clinical features with infectious and other non-infectious processes (such as cardiac or renal) and may necessitate lung biopsy to confirm the diagnosis, which may not be feasible. Underutilization of invasive diagnostic procedures may be due to obvious safety concerns in already compromised transplant patients. Patchy distribution of disease may also limit the yield of bronchoscopic biopsies as compared to surgical lung biopsies33. Our data show that diagnosis of NPT was largely based on clinical context, and only a small percentage of patients had invasive diagnostic procedure performed and in a significant proportion, data were not reported. Inherent with real-world practice, diagnostic pathways for NPT are often not universally established and/or implemented across transplant centers. The study is also limited by the fact that the registry does not capture the details of NPT diagnosis, such as its severity (e.g., proportion of patients requiring mechanical ventilation after developing NPT), treatments, and subsequent response. Another limitation is the inability to ascertain the effect of infections, including respiratory, on the development of NPT. Pre-transplant therapies such as bleomycin, checkpoint inhibitors, thoracic irradiation, that can increase predisposition to pulmonary complications, could not be included as a variable in the analysis; we presume, nonetheless, that those patients had residual effects of therapy-induced interstitial pneumonitis on pre-transplant PFTs, which were captured in the analysis.
However, the study results do represent the real-world evidence on posttransplant NPT, diagnosed based on clinical grounds, with imaging, laboratory, and histopathologic support. A prospective study with an algorithmic approach to diagnosing and managing NPT mandated in the protocol will minimize this limitation. More frequent utilization of invasive diagnostic testing such as transbronchial or thoracoscopic lung biopsy will increase the probability of making an accurate diagnosis. As the clinical presentation of various NPTs can overlap making the diagnosis difficult, the use of a composite NPT endpoint incorporating all three entities circumvented the potential limitation of capturing a misclassified NPT (e.g., IPS in lieu of COP) in the analysis. A large, diverse, and contemporaneous cohort of alloHCT patients with mature follow up data is a major strength of this analysis. The study population included a variety of malignant and non-malignant diseases receiving different conditioning and GVHD prophylaxis regimens, donor types and graft sources, adding to the generalizability of the study results. This analysis focused on NPT occurring early in the post-transplant period, which encompassed the bulk of IPS, DAH, and COP burden, and was shown to have onset even after the first year of alloHCT. It is important to note that we excluded BOS in this analysis given its typical presentation later in the course after alloHCT and its association with chronic GVHD4,34. PERDS, which manifests with fever, diffuse infiltrates on imaging, hypoxemia, and an erythematous rash in the absence of infection, occurs in patients with engraftment syndrome (diffuse systemic capillary leak disorder), may be considered to represent a subset of IPS, and is not individually captured in the CIBMTR® database4,35,36.
In conclusion, this registry-based analysis of alloHCT patients highlights several risk factors for the development of early NPT including severe pulmonary dysfunction, TBI-based MAC, and acute GVHD. Identification of baseline pre-transplant variables can help elucidate the risk of development of NPT and guide selection of conditioning, graft source, and GVHD prophylaxis. We confirm that post-transplant NPT is associated with a several-fold higher mortality risk. It is, therefore, prudent to consider the risk of NPT in patients with severe pulmonary dysfunction based on pretransplant PFT: counseling patients to stop smoking early and tailoring the conditioning to non-TBI-based MAC regimen would be important considerations. It remains to be seen if recent changes in treatments against hematologic malignancies (targeted and immunotherapy approaches), novel transplant conditioning and GVHD platforms (post-transplant cyclophosphamide for GVHD prophylaxis and ruxolitinib for severe acute GVHD) have an effect on the risk of NPT and outcomes in alloHCT patients developing NPT. Future research should focus on understanding the mechanisms contributing to NPT development and identifying biomarkers to predict and risk stratify these patients and to develop effective novel therapies. Advances in novel therapeutic approaches for NPT that will improve survival represent an area of significant unmet clinical need.
Supplementary Material
HIGHLIGHTS.
Non-infectious pulmonary toxicity (NPT), a significant complication of allogeneic transplant, has a 1-year cumulative incidence of 8.1% (95% CI, 7.7–8.5%).
Severe pulmonary comorbidity, mismatched unrelated donor and cord blood transplant, HCT-CI score ≥1 and grade II-IV acute GVHD significantly increase the risk of NPT, whereas non-TBI and TBI-based non-myeloablative conditioning and platelet recovery are associated with a decreased risk.
NPT is associated with increased risk of overall mortality.
ACKNOWLEDGMENT (Other members of the Working Committee):
Catherine Lee, Robert Peter Gale, Peiman Hematti, Zachariah DeFilipp, Marjolein van der Poel, Shatha Farhan, Miguel Angel Diaz, Sherif Badawy, Jane Liesveld, Amy K. Keating, Jan Cerny, Jean-Yves Cahn, David A Rizzieri, Siddhartha Ganguly, Hisham Abdel-Azim, Mahmoud Aljurf, Sunita Nathan, Kasiani Myers, Muhammad Bilal Abid, Jean Yared, Attaphol Pawarode
FINANCIAL DISCLOSURE:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon; Karius; Karyopharm Therapeutics; Kiadis Pharma; Kite Pharma Inc; Kite, a Gilead Company; Kyowa Kirin International plc; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Medac GmbH; Medexus; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OncoImmune, Inc.; Oncopeptides, Inc.; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc; Pfizer, Inc.; Pharmacyclics, LLC; Priothera; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Tscan; Vertex; Vor Biopharma; Xenikos BV.
DATA USE STATEMENT:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Footnotes
CONFLICTS OF INTEREST:
N.S.M served as a consultant for Incyte. A.S. is the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of SCD sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287) and by Novartis (NCT04443907). The industry sponsors provide funding for the clinical trial, which includes salary support paid to A.S.’s institution. A.S. also is a paid consultant for Spotlight Therapeutics and Medexus Inc. A.S. has received research funding from CRISPR Therapeutics; H.M. reports Consultation and research funding: CRISPR Therapeutics; T.N. reports institution received research support from Novartis (clinical trial support) and Karyopharm (drug supply for clinical trial) but no direct support; H.M.L. reports Advisory board membership with Jazz Pharmaceuticals; M.S. reports Advisory board membership with Jazz Pharmaceuticals; S.C. reports payment or honoraria from GSK and Sanofi, and research support for clinical trial to the institution from Amgen, Janssen, BMS, Syndax and Sanofi.
The study results were presented as a virtual poster at 2020 ASH Annual Meeting and Exposition in Dec 2020.
REFERENCES
- 1.Socie G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. May 1 2003;101(9):3373–85. doi: 10.1182/blood-2002-07-2231 [DOI] [PubMed] [Google Scholar]
- 2.Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. May 1 2011;183(9):1262–79. doi: 10.1164/rccm.2007-413ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. Sep 2001;28(5):425–34. doi: 10.1038/sj.bmt.1703142 [DOI] [PubMed] [Google Scholar]
- 4.Yanik G, Kitko C. Management of noninfectious lung injury following hematopoietic cell transplantation. Curr Opin Oncol. Mar 2013;25(2):187–94. doi: 10.1097/CCO.0b013e32835dc8a5 [DOI] [PubMed] [Google Scholar]
- 5.Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. Oct 15 2003;102(8):2777–85. doi: 10.1182/blood-2003-05-1597 [DOI] [PubMed] [Google Scholar]
- 6.Patriarca F, Poletti V, Costabel U, et al. Clinical presentation, outcome and risk factors of late-onset non-infectious pulmonary complications after allogeneic stem cell transplantation. Current stem cell research & therapy. May 2009;4(2):161–7. [DOI] [PubMed] [Google Scholar]
- 7.Zhu KE, Hu JY, Zhang T, Chen J, Zhong J, Lu YH. Incidence, risks, and outcome of idiopathic pneumonia syndrome early after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. Dec 2008;81(6):461–6. doi: 10.1111/j.1600-0609.2008.01149.x [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi H, Takahashi Y, Watanabe N, et al. Incidence, clinical features, and risk factors of idiopathic pneumonia syndrome following hematopoietic stem cell transplantation in children. Pediatr Blood Cancer. May 2012;58(5):780–4. doi: 10.1002/pbc.23298 [DOI] [PubMed] [Google Scholar]
- 9.Yanik GA, Ho VT, Levine JE, et al. The impact of soluble tumor necrosis factor receptor etanercept on the treatment of idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Blood. Oct 15 2008;112(8):3073–81. doi: 10.1182/blood-2008-03-143412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo S, Yu J, Jenkins IC, et al. Diagnostic and Prognostic Plasma Biomarkers for Idiopathic Pneumonia Syndrome after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. Apr 2018;24(4):678–686. doi: 10.1016/j.bbmt.2017.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, Double-Blind, Placebo-Controlled Trial of Soluble Tumor Necrosis Factor Receptor: Enbrel (Etanercept) for the Treatment of Idiopathic Pneumonia Syndrome after Allogeneic Stem Cell Transplantation: Blood and Marrow Transplant Clinical Trials Network Protocol. Biology of Blood and Marrow Transplantation. 2014/06/01/ 2014;20(6):858–864. doi: 10.1016/j.bbmt.2014.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenger DS, Triplette M, Crothers K, et al. Incidence, Risk Factors, and Outcomes of Idiopathic Pneumonia Syndrome after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. Feb 2020;26(2):413–420. doi: 10.1016/j.bbmt.2019.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Lassence A, Fleury-Feith J, Escudier E, Beaune J, Bernaudin JF, Cordonnier C. Alveolar hemorrhage. Diagnostic criteria and results in 194 immunocompromised hosts. Am J Respir Crit Care Med. Jan 1995;151(1):157–63. doi: 10.1164/ajrccm.151.1.7812547 [DOI] [PubMed] [Google Scholar]
- 14.Agustí C, Ramirez J, Picado C, et al. Diffuse alveolar hemorrhage in allogeneic bone marrow transplantation. A postmortem study. Am J Respir Crit Care Med. Apr 1995;151(4):1006–10. doi: 10.1164/ajrccm/151.4.1006 [DOI] [PubMed] [Google Scholar]
- 15.Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. Nov 15 2002;166(10):1364–8. doi: 10.1164/rccm.200208-792OC [DOI] [PubMed] [Google Scholar]
- 16.Robbins RA, Linder J, Stahl MG, et al. Diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Am J Med. Nov 1989;87(5):511–8. doi: 10.1016/s0002-9343(89)80606-0 [DOI] [PubMed] [Google Scholar]
- 17.Haider S, Durairajan N, Soubani AO. Noninfectious pulmonary complications of haematopoietic stem cell transplantation. European respiratory review : an official journal of the European Respiratory Society. Jun 30 2020;29(156)doi: 10.1183/16000617.0119-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. Jul 2007;13(7):749–59. doi: 10.1016/j.bbmt.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Soubani AO, Pandya CM. The spectrum of noninfectious pulmonary complications following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. 2010;3(3):143–57. doi: 10.1016/s1658-3876(10)50025-6 [DOI] [PubMed] [Google Scholar]
- 20.Nakasone H, Onizuka M, Suzuki N, et al. Pre-transplant risk factors for cryptogenic organizing pneumonia/bronchiolitis obliterans organizing pneumonia after hematopoietic cell transplantation. Bone Marrow Transplant. Oct 2013;48(10):1317–23. doi: 10.1038/bmt.2013.116 [DOI] [PubMed] [Google Scholar]
- 21.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. Dec 2009;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieruszewski PM, Herasevich S, Gajic O, Yadav H. Respiratory failure in the hematopoietic stem cell transplant recipient. World J Crit Care Med. Oct 16 2018;7(5):62–72. doi: 10.5492/wjccm.v7.i5.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keates-Baleeiro J, Moore P, Koyama T, Manes B, Calder C, Frangoul H. Incidence and outcome of idiopathic pneumonia syndrome in pediatric stem cell transplant recipients. Bone Marrow Transplant. Aug 2006;38(4):285–9. doi: 10.1038/sj.bmt.1705436 [DOI] [PubMed] [Google Scholar]
- 25.Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys. Nov 1 2005;63(3):876–84. doi: 10.1016/j.ijrobp.2005.02.032 [DOI] [PubMed] [Google Scholar]
- 26.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant. Oct 2006;12(10):1038–46. doi: 10.1016/j.bbmt.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 27.Kharbanda S, Panoskaltsis-Mortari A, Haddad IY, et al. Inflammatory cytokines and the development of pulmonary complications after allogeneic hematopoietic cell transplantation in patients with inherited metabolic storage disorders. Biol Blood Marrow Transplant. Apr 2006;12(4):430–7. doi: 10.1016/j.bbmt.2005.12.026 [DOI] [PubMed] [Google Scholar]
- 28.Keklik F, Alrawi EB, Cao Q, et al. Diffuse alveolar hemorrhage is most often fatal and is affected by graft source, conditioning regimen toxicity, and engraftment kinetics. Haematologica. Dec 2018;103(12):2109–2115. doi: 10.3324/haematol.2018.189134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freudenberger TD, Madtes DK, Curtis JR, Cummings P, Storer BE, Hackman RC. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood. Nov 15 2003;102(10):3822–8. doi: 10.1182/blood-2002-06-1813 [DOI] [PubMed] [Google Scholar]
- 30.Fan K, McArthur J, Morrison RR, Ghafoor S. Diffuse Alveolar Hemorrhage After Pediatric Hematopoietic Stem Cell Transplantation. Front Oncol. 2020;10:1757. doi: 10.3389/fonc.2020.01757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. Apr 25 2019;133(17):1840–1853. doi: 10.1182/blood-2018-10-860809 [DOI] [PubMed] [Google Scholar]
- 32.Bergeron A, Cheng GS. Bronchiolitis Obliterans Syndrome and Other Late Pulmonary Complications After Allogeneic Hematopoietic Stem Cell Transplantation. Clin Chest Med. Dec 2017;38(4):607–621. doi: 10.1016/j.ccm.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 33.Yao HM, Zuo W, Wang XL, Zhang W. Findings on cryptogenic organizing pneumonia: a case report and literature review. J Int Med Res. Apr 2020;48(4):300060520920068. doi: 10.1177/0300060520920068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. Dec 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 35.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. May 2001;27(9):893–8. doi: 10.1038/sj.bmt.1703015 [DOI] [PubMed] [Google Scholar]
- 36.Cornell RF, Hari P, Drobyski WR. Engraftment Syndrome after Autologous Stem Cell Transplantation: An Update Unifying the Definition and Management Approach. Biol Blood Marrow Transplant. Dec 2015;21(12):2061–2068. doi: 10.1016/j.bbmt.2015.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.