Abstract
Importance:
Chimeric Antigen Receptor (CAR)-T cell therapy is a novel therapy for patients with relapsed or refractory hematological malignancies. Most CAR-T cell therapy recipients will experience clinical features of the immune effector cell-associated neurotoxicity syndrome (ICANS), a potentially life-threatening condition.
Objective:
To describe the clinical, biological, and radiological findings associated with ICANS among adults with hematological malignancies treated with CAR-T cell therapy and the acute or long-term outcomes of ICANS.
Evidence Review:
A literature search of Ovid Medline, EMBASE, PubMed, Scopus, Web of Science Core Collection, Cochrane Library, and Google Scholar was conducted from each database’s inception through February 1, 2022, using search terms reflecting CAR-T and ICANS. We included studies that enrolled adults (age 18 years or older) who received CAR-T cell therapy as management for hematological malignancies and reported on the clinical presentation, predictors, or the acute or long-term outcomes of ICANS. Two reviewers independently extracted data following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis reporting guidelines. Quality was assessed using Joanna Briggs Institute critical appraisal tool for cohort studies.
Findings:
Of 2928 studies screened, 23 observational studies (10 prospective, 11 retrospective, one mixed-design, and one cross-sectional) met the eligibility criteria and included 1666 participants in total. The most common hematological malignancies were diffuse large B-cell lymphoma, acute lymphocytic leukemia, non-Hodgkin lymphoma, and chronic lymphocytic leukemia. ICANS onset was most often associated with the presence and severity of cytokine release syndrome, c-reactive protein (CRP), and ferritin levels. Aphasia was the most common ICANS-related symptom reported, although the neurological manifestations of ICANS were highly variable. Neuroimaging studies (MRI or CT) were often normal in cases of ICANS; however, electroencephalograms often showed generalized background slowing, abnormal rhythmic and periodic discharge patterns. The pooled mean (± Standard Deviation) onset of ICANS was 6.4 ± 3.2 days, with a pooled mean duration of 8.3 ±10.5 days. Two (9%) of the 23 studies reported 5 ICANS related deaths among 233 participants. A subset of patients experienced persistent neurocognitive complaints ≥ 1-year post-CAR-T cell therapy.
Conclusion and Relevance:
The clinical presentation, onset, severity, long-term sequelae, and grading system of ICANS are variable. Future studies should consider using a consensus grading/reporting scale that would permit cross-trial comparisons of the safety profile of various CAR-T products and enable interventions to be developed to mitigate or manage these neurotoxicities.
Funding:
SJG is supported by National Cancer Institute Grant No. (5-K12-CA120780-13; PI: William Kim) and National Institute on Aging Grant No, (1 R03 AG074030-01; PI: Shakira Grant). SG is supported by the UAB Walter B. Frommeyer Fellowship in Investigative Medicine.
Registration:
PROSPERO CRD42020207864
Introduction
Chimeric Antigen Receptor (CAR)-T cell therapy represents a novel immunotherapeutic strategy that has changed the treatment landscape for those with heavily pre-treated acute lymphoblastic leukemia,1,2 large B cell lymphoma,3,4 mantle cell lymphoma,5 and most recently multiple myeloma.6
Immune effector cell-associated neurotoxicity syndrome (ICANS), a clinical syndrome characterized by a constellation of neuropsychiatric symptoms, is one major toxicity associated with CAR-T cell therapy that can affect approximately 20–70% of CAR-T cell therapy recipients.7 While the ICANS course is often mild in severity and limited in duration,8–10 some patients will develop severe, life-threatening ICANS,9,11,12 or have long-term neuropsychiatric effects persisting one year or more following treatment.13,14 Despite these observations, identifying those at greatest risk for severe ICANS and the subset of patients likely to experience long-term neuropsychiatric effects remain poorly understood.
Our systematic review summarizes the biological, clinical, radiological, and electroencephalography (EEG) features of ICANS and outcomes in adults with hematologic malignancies treated with CAR-T cell therapy.
Methods
This systematic review was conducted according to a published protocol (CRD42020207864) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis and Synthesis Without Meta-analysis in Systematic Review reporting guidelines (Supplementary Table 1).15,16
Search Strategy
A medical librarian (AG) searched the following databases: Cochrane Library, Google Scholar, Ovid Medline, Ovid EMBASE, PubMed, Scopus, and Web of Science Core Collection from the inception of each database to February 1, 2022 (Supplementary Table 2) using controlled vocabulary and keywords with synonyms that reflected CAR-T therapy and ICANS concepts. Reference lists and citations associated with the included studies were also reviewed.
Study Selection
Studies included were observational or interventional, enrolling at least five adult CAR-T cell recipients with hematological malignancies and reporting on ICANS as adjudicated by the primary papers. Studies reporting clinical, radiographic, EEG findings, biological correlates, or outcomes of ICANS were also included.
Selected citations from the initial search were imported into the Endnote X9™ database. After removing duplicates, the remaining articles were uploaded into Covidence™. All studies were selected and reviewed by two reviewers (S.J.G. and S.G.). A third reviewer (D.M) was consulted when consensus could not be reached to resolve discrepancies.
Data Extraction
Data were extracted by (S.J.G and S.G) using a pre-piloted excel document (Supplementary Table 3). Extracted data included study-level and individual participant characteristics, ICANS symptoms and signs as adjudicated by the primary studies, radiographic and EEG findings, biologic correlates, and outcomes of patients with ICANS. Each study’s quality was evaluated using the Joanna Briggs Institute (JBI) Critical Appraisal Tool.17
Definition of Outcomes
The primary outcome of interest was ICANS as adjudicated by the primary studies and guided by the American Society of Transplantation and Cellular Therapy’s (ASTCT) definition of ICANS as a pathologic process involving the central nervous system, developing after immune therapy and results in the activation or engagement of endogenous or infused T cells or other immune effector cells. Data on the clinical features of ICANS were abstracted for each eligible study and included aphasia, altered level of consciousness, impairment of neurocognitive skills, motor weakness, seizures, and cerebral edema anxiety, delirium, dizziness, dyscalculia, headache, myoclonus, seizure, and tremor.8,10,18
Statistical Analysis
While our original intent was to perform a quantitative meta-synthesis of the results, considerable heterogeneity in the study design, patient characteristics, and clinical outcomes precluded meta-analysis. Instead, we chose to summarize the results descriptively, presenting pooled data when appropriate. Specifically, we computed pooled mean and pooled standard deviations using weighted averages across individual studies. For studies reporting medians instead of means, we used the Quantile Estimation method as outlined by McGrath et al. to estimate the mean and standard deviation.19
Results
Our initial literature search yielded 2928 potential studies after removing duplicate publications. Following the further exclusion of studies without ICANS data, and those published in abstract form alone, (Supplementary Table 4), 140 full-text articles were reviewed, yielding 23 studies for the systematic review (Figure 1).
Figure 1.
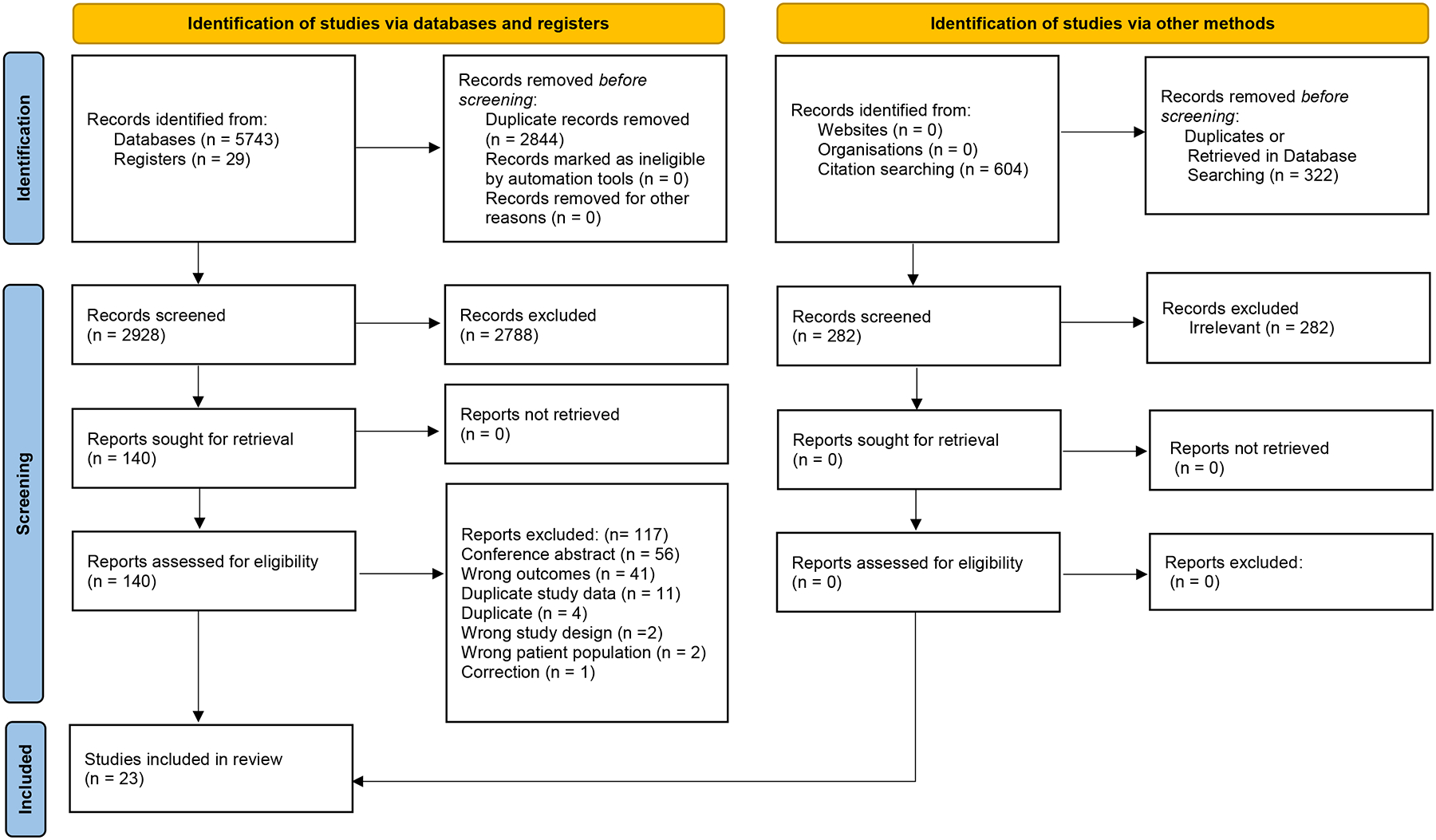
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart depicts the identification, screening, and inclusion criteria yielding 19 studies for systemic review. Adapted from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Study Characteristics
Table 1 summarizes the study characteristics. All studies were observational (10 prospective cohort,9,10,20–27 11 retrospective cohort,8,13,28–36 1 mixed prospective and retrospective cohort,12 and 1 cross-sectional).14 Most studies (17/23) were published between 2020–2021,13,14,21–27,29–34,36 with the remainder published between 2017–2019.8–10,12,20,28 One study evaluated post-marketing adverse events (n=804), therefore did not report on the unique number of patients;33 the 18 remaining studies enrolled 1,666 participants.8–10,12–14,20–32,34–36 Most studies (19/23) only enrolled adult participants (aged 18–85 years).8,9,12–14,21–27,30,31,35,36 Four studies included mixed cohorts of pediatric and young adults (age range 2–30.5 years).20,23,28,34 The pooled mean across 18 (78%) of 23 studies was 53.5 years ± Standard Deviation 10.5.8,9,12–14,20–28,30,31,35,36 CD19 was the most common CAR-T target evaluated across 23 studies.8–10,13,14,21–25,28–34 One study evaluated a CD22 CAR-T product,20 and for one study the CAR-T cell target was not reported.12 The most prevalent hematological malignancies were diffuse large B-Cell lymphoma (DLBCL) (8 studies),20–22,25,30,31,33,34 acute lymphocytic leukemia (ALL) (7 studies),10,13,14,20,26–29,34–36 non-Hodgkin’s lymphoma (NHL) (4 studies),13,14,29,32 and chronic lymphocytic leukemia (CLL) (4 studies).8,13,14,29 Nine (39%) of 23 studies reported the CAR-T product for study participants.21,22,24,28,30–34 In three (13.0%) of 23 studies, reported on the use of axicabatagene ciloleucel,22,24,31 two (8.7%) reported use of tisagenlecleucel,28,32 and four (17.4%) included patients who received either both axicabatagene ciloleucel or tisagenlecleuel.21,30,33,34
Table 1.
Characteristics of the 19 Studies Included in the Systematic Review
| CHARACTERISTICS | NO. OF STUDIES (N=19) | REFERENCES |
|---|---|---|
| Year Published | ||
| 2017–2019 | 6 | Gust 2017,9 Gofshteyn 201828, Santomasso 201810, Shalabi 201820, Karschnia 20198, Rubin 201912 |
| 2020–2022 | 17 | Belin 202021, Cordeiro 202013, Gajra 202033, Maziara 202032, Ruark 202014, Rubin 202024, Sokolov 202029, Strati 202022, Wudhikarn 202030, Gust 202123, Holtzman 202131, Brown 202134, Maillet 202125, Yuen 202135, Greenbaum 202126, Beuchat 202236, Wudhikarn 202127 |
| Sample Size | ||
| < 50 | 7 | Shalabi 201820, Karschnia 20198, Ruark 202014, Sokolov 202029, Holtzman 202131, Brown 202134, Yuen 202135 |
| 50–99 | 8 | Gofshteyn 201828, Santomasso 201810, Belin 202021, Cordeiro 202013, Wudhikarn 202030, Maillet 202125, Beuchat 202236, Wudhikarn 202127 |
| 100–199 | 6 | Gust 2017,9 Rubin 201912, Maziara 202032, Strati 202022, Gust 202123, Greenbaum 202126 |
| ≥200 | 2 | Rubin 202024, Gajra 202033 |
| Age | ||
| Pediatric and young adult cohort (mean, SD)* | 4 | Gofshteyn 2018 (11.5y, 4.3)28, Shalabi 2018 (17.9y, 6.9)20, Gust 2021 (cEEG-14.3y, 7.5; rEEG- 10.7y, 8.2)23, Brown 202134 |
| Adult cohort (mean, SD)* | 15 | Gust 2017,9 Santomasso 201810, Karschnia 2019 (56y, 16.3)8, Rubin 2019 (54.6y, 12.3)12, Belin 2020 (51.7y, 12.8)21, Cordeiro 2020 (51.7y, 11.5)13, Gajra 202033, Maziara 202032, Ruark 2020 (51.3y, 12.3)14, Rubin 2020 (60y,12.5)24, Sokolov 202029, Strati 2020 (54.3y, 14.4)22, Wudhikarn 2020 (55.7y, 15.5)30, Holtzman 2021(53.7y, 12.2)31, Maillet 2021(58y,14)25, Yuen 202135, Greenbaum 202126, Beuchat 2022 (60y, 11)36, Wudhikarn 202127 |
| Study Design | ||
| prospective cohort | 8 | Gust 2017,9 Santomasso 201810,Shalabi 201820, Belin 202021, Rubin 202024, Strati 202022, Gust 202123, Maillet 202125, Greenbaum 202126, Wudhikarn 202127 |
| retrospective cohort | 9 | Gofshteyn 201828, Karschnia 20198, Cordeiro 202013, Gajra 202033, Maziara 202032, Sokolov 202029, Wudhikarn 202030, Holtzman 202131, Brown 202134, Yuen 202135, Beuchat 202236 |
| mixed prospective and retrospective cohort | 1 | Rubin 201912 |
| cross-sectional | 1 | Ruark 202014 |
| Cancer Type | ||
| Diffuse large B-Cell lymphoma (DLBCL) | 11 | Shalabi 201820, Belin 202021, Gajra 202033, Strati 202022, Wudhikarn 202030, Holtzman 202131, Brown 202134, Maillet 202125, Yuen 202135, Greenbaum 202126, Wudhikarn 202127 |
| Acute lymphocytic leukemia (ALL) | 8 | Gofshteyn 201828, Santomasso 201810,Shalabi 201820, Cordeiro 202013, Ruark 202014, Sokolov 202029, Brown 202134, Beuchat 202236 |
| Non-Hodgkins lymphoma (NHL) | 4 | Cordeiro 202013, Maziara 202032, Ruark 202014, Sokolov 202029 |
| Chronic lymphocytic leukemia (CLL) | 4 | Karschnia 20198, Cordeiro 202013, Ruark 202014, Sokolov 202029 |
| Lymphoma (not classified) | 4 | Gust 2017,9 Rubin 201912, Rubin 202024, Beuchat 202236 |
| Leukemia (not classified) | 2 | Rubin 201912, Rubin 202024 |
| Follicular Lymphoma | 2 | Belin 202021, Sokolov 202029 |
| Hepatocellular Carcinoma | 1 | Karschnia 20198 |
| Multiple Myeloma | 1 | Beuchat 202236 |
| Sarcoma | 1 | Rubin 201912 |
| CAR-T Cell Target | ||
| CD19 | 17 | Gust 2017,9 Gofshteyn 201828, Santomasso 201810, Karschnia 20198, Belin 202021, Cordeiro 202013, Gajra 202033, Maziara 202032, Ruark 202014, Rubin 202024, Sokolov 202029, Strati 202022, Wudhikarn 202030, Gust 202123, Holtzman 202131, Brown 202134, Maillet 202125, Yuen 202135, Greenbaum 202126, Beuchat 202236, Wudhikarn 202127 |
| CD22 | 1 | Shalabi 201820 |
| BCMA | 1 | Yuen 202135 |
| Not indicated | 1 | Rubin 201912 |
| CAR-T Product | ||
| Axicabtagene ciloleucel | 3 | Rubin 202024, Strati 202022, Holtzman 202131 |
| Tisagenlecleucel | 2 | Gofshteyn 201828, Maziara 202032 |
| Axicabtagene ciloleucel and Tisagenlecleucel | 4 | Gajra 202033, Belin 202021, Wudhikarn 202030, Brown 202134 |
Risk-of-Bias Assessment
The quality rating for each study was independently assessed using the JBI checklist for cohort studies. The overall quality rating of all studies but one was considered good. The Quality Rating Scheme and results are summarized in Supplementary Table 5.
Studies evaluating the incidence of ICANS
Ninteen (83%) of 23 studies reported the incidence of ICANS which ranged from 37.5–77%.9,10,12,14,20–22,24–28,30–36 The remaining studies included cohorts of participants with ICANS only,8,29 or data was not included to allow estimation of ICANS incidence.13,23 ICANS was most often graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) in 17 (74%) of 23 studies.8–10,12,14,21,22,24–27,29,31,32,34,36 Seventeen (74%) of 23 studies reported on the number of grade 3–4 ICANS events.8–10,12,14,21,22,24–27,29,31,32,34–36 Two studies reported five grade 5 (death directly related to ICANS) events.9,12 Eleven (48%) of 23 studies reported the time to onset of ICANS following infusion of the CAR-T product. Across these studies the pooled mean onset of ICANS was 6.4 days ± 3.2.8,10,21,22,24,26,27,30,31,34,35 Seven (30%) of 23 studies also reported on the duration of ICANS symptoms. Across these studies the pooled mean duration of ICANS was 8.3 days ± 10.5.8,9,21,22,25,27,34
Studies evaluating risk factors for ICANS
Thirteen (57%) of 23 studies8–10,12,21,22,24,26–28,30,33,34 reported factors associated with ICANS development (Figure 2). Six (50%) of 12 studies reported that the presence and severity of cytokine release syndrome (CRS) predicted the onset of ICANS.10,24,27,28,30,33 In two other studies, earlier time to onset of CRS (median range of 1–2 days)12,24 after CAR T-cell infusion was also associated with ICANS development. Other identified factors included elevated C-reactive protein levels in six (50%) of 12 studies,8,12,21,22,24,27 and elevated serum ferritin levels in six (50%) of 12 studies.8,21,22,24,27,34 Older adult patient age was also associated with ICANS onset in three (27%) of 11 studies.10,24,33 One study by Greenbaum et al.26 found the Endothelial Activation and Stress Index (EASIX) score (lactate dehydrogenase [LDH; U/L] × creatinine [mg/dL]/platelets [PLTs; 109 cells/L]) in combination with serum ferritin and c-reactive protein levels to be predictive for ICANS development.
FIGURE 2.
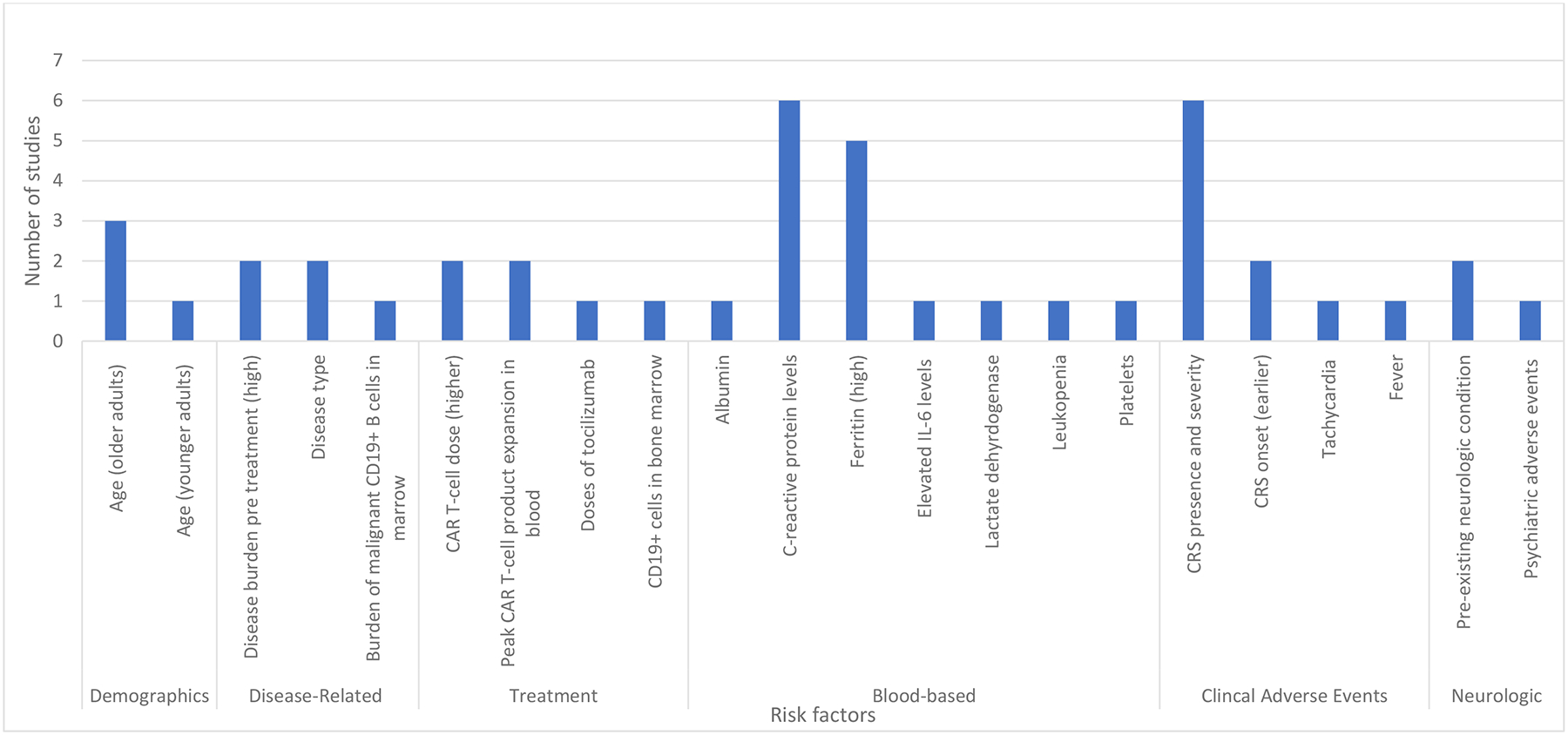
Risk Factors Associated with ICANS Onset Across 13 studies
Demographic, disease-related, blood-based, and clinical risk factors associated with ICANS onset across 11 studies. The most frequently noted risk factors were C-reactive protein levels, presence and severity of cytokine release syndrome, and high ferritin levels. Abbreviations: cytokine release syndrome (CRS), interleukin (IL)
Studies evaluating the clinical presentation of ICANS
Seventeen (74%) of 23 studies reported on the clinical presentation of ICANS (Figure 3).8–10,12,20–22,25–31,33–36 Aphasia was the most common symptom of ICANS reported in 15 (88%) of 17 studies reporting on clinical features of ICANS.8–10,12,20–22,25,27–31,33,34 Generalized or absence seizures were reported as a clinical feature of ICANS in 13 (87%) of the 17 studies..8,10,12,20,21,25,27–31,33,34,36 Additional symptoms of ICANS included encephalopathy8,10,12,21,28,33 in six (35%) of 17 studies and headache9,10,12,21,27,30,33,35 in eight (47%) of 17 studies. Additionally, 10 (59%) of 17 studies reported on disorders of consciousness8–10,12,20,21,25,27,31,34 and nine studies reported confusional states8,21,27,30,31 or disorientation.10,20,21,35 Only three studies reported stroke as a clinical manifestation of ICANS.12,31,33 Overall, 19 stroke cases were reported, among 254 participants enrolled in two studies.12,31 Of these, three were ischemic.12,31 For the remaining 16 cases identified in a retrospective database analysis of the Food and Drug Administration’s adverse events reporting systems, frequencies on the type of stroke were not reported.33
FIGURE 3.
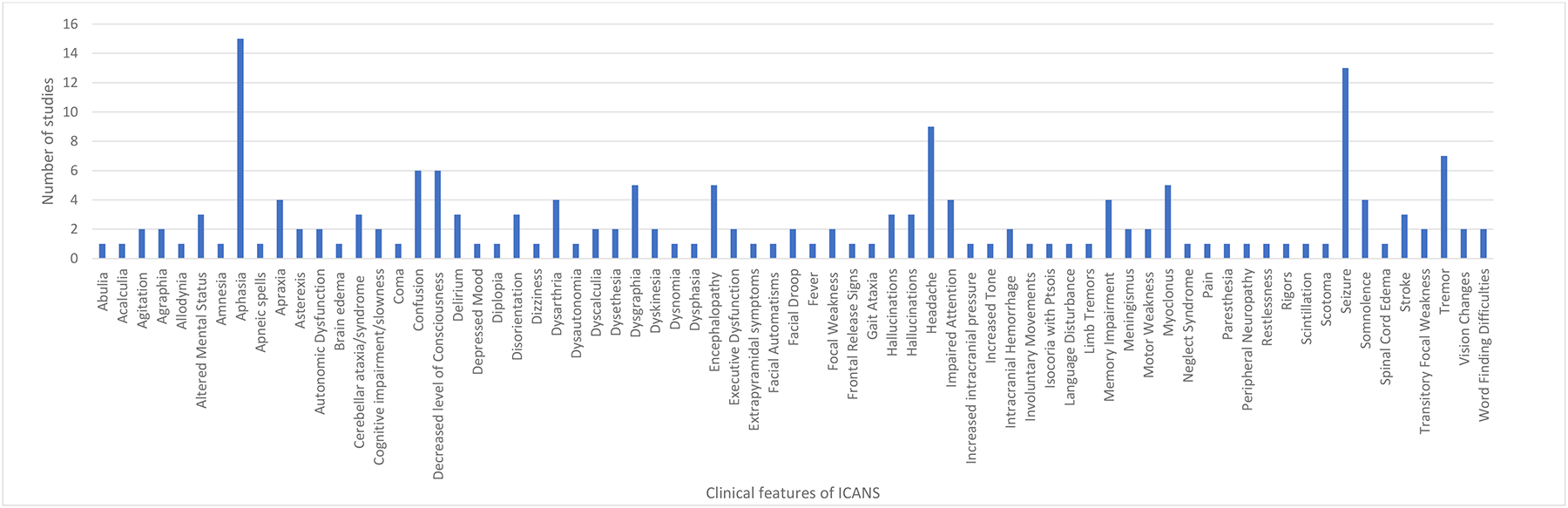
Clinical Features of ICANS Across 17 studies
Clinical features for ICANS noted across 14 studies were highly variable but most often included aphasia, seizure, headache, hallucinations, and encephalopathy.
Studies reporting on the role of neuroimaging and EEG in the diagnostic evaluation of ICANS
Fourteen (61%) of 23 studies reported on the role of neuroimaging and EEG to evaluate ICANS (Figure 4).8–10,12,21–23,27–29,31,34,35 All 14 studies used magnetic resonance imaging (MRI),8–10,12,21–23,28,29,31,34 and 12 (86%) of 14 studies used EEG.8–10,12,21–23,29,31,34–36 Additional neuroimaging included computerized tomography (CT),8,12,21,28,35 CT angiography,12 and Positron Emission Tomography-Computed Tomography (PET-CT).12 Eleven (78%) of 14 studies reported MRI or CT of the brain as either normal or unchanged from pre-CAR T-cell therapy infusion.8–10,12,21–23,29,31,34,36 Among these 14 studies, 9 (64%) of 14 reported abnormal MRI findings involving the pons,8–10,23,31 thalamus,9,10,22,23 white matter,22,23,31,34 corpus callosum,8,10 leptomeninges,9,22 and temporal lobe.12,22 Ten (71%) of 14 reported the abnormalities on MRI of the brain as T2/FLAIR hyperintensities.8–10,22,23,29,31,34–36 Six (43%) of 14 studies reported MRI or CT brain findings such as stroke,12,22,31 intracranial hemorrhage,8,9,12,31,34, and diffuse cerebral edema.9,12 Across 8 (80%) of 12 studies, findings on EEG included generalized background slowing, abnormal rhythmic and periodic discharge patterns.8–10,12,21–23,29,31,34–36 Five (42%) of 12 studies reported seizure-related activity on EEG.10,12,22,23,29
FIGURE 4.
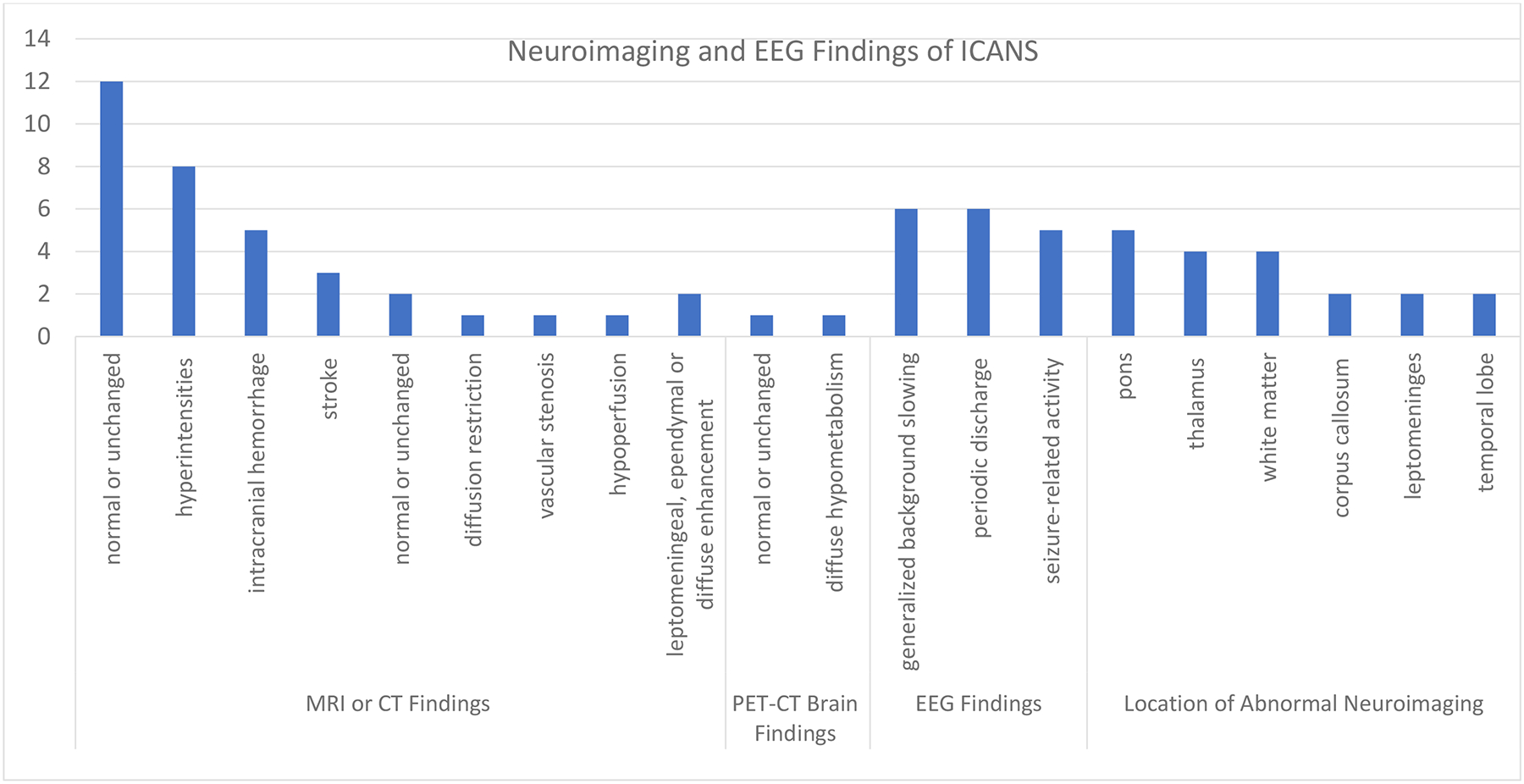
Neuroimaging and EEG Finding of ICANS Onset Across 14 studies
Neuroimaging findings with MRI and CT across 11 studies were most often normal, but when abnormalities were noted, they were cited most frequently in the pons. EEG findings across 11 studies most often included generalized slowing, periodic discharges, and epileptic activity. Abbreviations: magnetic resonance imaging (MRI), electroencephalography (EEG).
Studies evaluating biological correlates of ICANS
Only one study evaluated cerebrospinal fluid (CSF) markers and their association with ICANS development.9 Six (26%) of 23 studies reported correlative analyses of blood-based markers (Figure 5).9,10,20,28,31,34 The most commonly identified blood-based markers associated with ICANS development were Interleukin 6,9,10,20,34 Interleukin 2,10,20,28 Interleukin 15,10,20,28 and Interferon-gamma (IFN-gamma).9,10,34 Additional markers reported included Granulocyte-macrophage colony-stimulating factor (GM-CSF),10,20 Interleukin 10,10,20 Monocyte Chemoattractant Protein-1 (MCP-1),9,10 and Tumor Necrosis Factor-alpha (TNF-alpha).9,20
FIGURE 5.
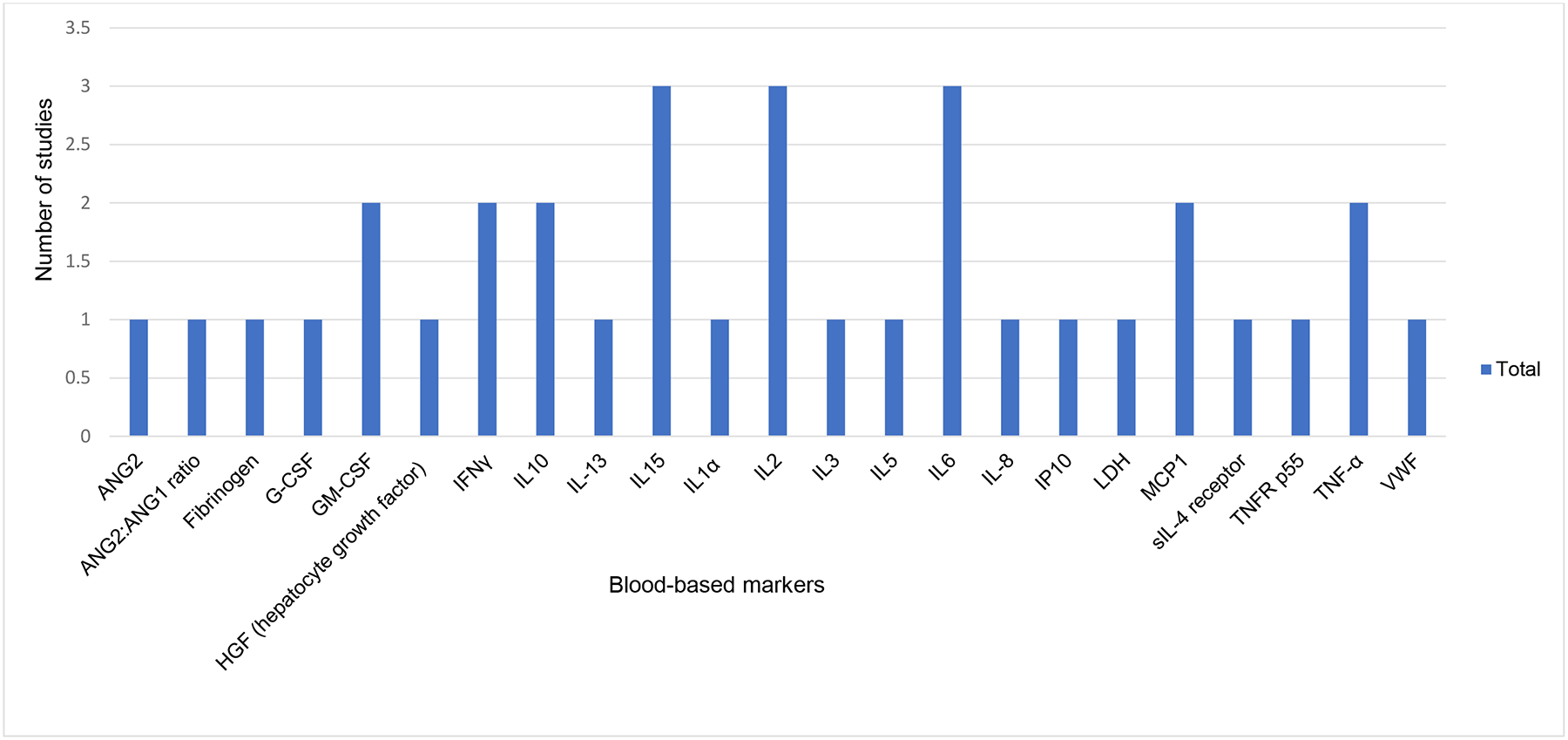
Blood-based Biologic Markers associated with ICANS Across 6 studies
Various blood-based markers were associated ICANS across 6 studies. IL15, IL2, IL6 were not most frequently cited markers. Abbreviations: angiopoietin (ANG), granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulated factor (GM-CSF), hepatocyte growth factor (HGF), interferon gamma (INFγ), interleukin (IL), monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-alpha (TNF-alpha), von Willebrand factor (VWF).
Studies reporting on the management of ICANS
Nine (39%) of 23 studies reported on the management of ICANS, which often included supportive care, steroids (dexamethasone or methylprednisone) either as monotherapy or in combination with the anti-IL-6 receptor monoclonal antibody, tocilizumab.8–10,21,22,27,31,34,36 Of these studies, only one reported using the anti-IL-6 receptor monoclonal antibody, siltuximab, and in the same study, the IL-1 receptor antagonist anakinra was used in the management of ICANS.36 Among the studies reporting tocilizumab use, most patients had a preceding diagnosis of CRS. In four (44%) of the nine studies reporting ICANS management, prophylactic anti-seizure medication was reported. The most commonly used anti-seizure medication was levetiracetam.
Studies evaluating long-term neurological outcomes following CAR-T cell therapy
Two related studies reported on the neurological outcomes of patients greater than one year after receiving CAR-T cell therapy.13,14 In the first study, a retrospective chart review, nine (10%) of 86 patients developed 11 new neurologic findings, including three cerebrovascular accidents and one case of transient ischemic attack, Alzheimer’s dementia, and peripheral neuropathy.13 Eight of 86 (9%) patients developed mood disorders such as depression and anxiety, which required interventions.13 In the second study, patient-reported measures were used to identify 15 (38%) of 40 patients with at least one cognitive difficulty (difficulties with concentration, memory, word-finding, and problem-solving).14 Two (9 %) of 23 studies reported neurological outcomes for patients 1 to 12 months after treatment with CAR-T cell therapy.8,25 One of these studies reported the following neurological complaints: confusion, myoclonus, and extremity weakness affecting two patients.8 However, a second study showed no exacerbation or development of new neurological symptoms in 56 patients 6–12 months after receiving CAR-T cell therapy.25
Studies reporting death due to ICANS
Two (9%) of 23 studies enrolling 233 participants reported a total of five deaths due to ICANS.9,12 In one study, death occurred five days following CAR T-cell infusion in a 21-year-old male with relapsed B-cell acute lymphoblastic leukemia.12 The ICANS events resulting in death in this patient were acute obtundation, which progressed to coma and brain death. In a study of 133 patients, 4 (3%) died due to ICANS.9 In one patient, death was due to multifocal brainstem hemorrhage and edema associated with disseminated intravascular coagulation. In the same study, two patients died from acute cerebral edema and one from cortical laminar necrosis, which resulted in a persistent minimally conscious state until death four months after CAR-T cell infusion.9
Discussion
This systematic review included 23 studies. Data presented summarized the clinical presentation, risk factors, neuroimaging and EEG findings, biologic correlates, management, and clinical outcomes of ICANS in adults who received CAR-T cell therapy for hematological malignancies. Our findings indicate that the incidence and clinical presentation of ICANS are highly variable.
ICANS onset was associated with the presence and severity of CRS, older patient age at the time of CAR-T infusion, and elevation in inflammatory markers (c-reactive protein and ferritin). Aphasia, seizures, headache, encephalopathy, and other disorders leading to a decreased level of consciousness, were the most common clinical features of ICANS. ICANS developed early, usually within the first week following the CAR-T infusion, most often resolving within 5–6 days of symptom onset. Notably, the grading of ICANS varied across all studies. In 2019 the American Society for Transplantation and Cellular Therapy proposed a new grading system to harmonize existing scoring systems.18 The use of this consensus grading scale would permit cross-trial comparisons of the safety profile of various CAR-T products and enable interventions to be developed to mitigate or manage these neurotoxicities.
Across studies that evaluated neuroimaging in cases with clinically confirmed ICANS, neuroimaging was most often normal. However, neuroimaging could be considered when excluding other conditions which may mimic the neurological symptoms of ICANS. EEG was most often abnormal when assessed in those with clinically confirmed ICANS. Despite its utility, EEG testing may be unavailable in some clinical settings. Several pro-inflammatory markers have been associated with ICANS development, including Interleukin (IL) 2, IL-6 and IL-15, interferon-gamma (IFN-gamma), and tumor necrosis factor-alpha (TNF-alpha), but the routine use of these markers in clinical practice is limited. Alternatives include evaluating the trends and peak levels of c-reactive protein and ferritin, which can be used to predict the risk of ICANS development.
Overall, only nine of the 23 included studies reported on the management of ICANS.8–10,21,22,27,31,34,36 Treatment most often involved supportive care alone in mild cases. However, most required glucocorticoid treatment and anti-seizure prophylaxis. Among the studies reporting coadministration of tocilizumab and steroids for ICANS, these patients also had concomitant CRS. Based on prior studies, using tocilizumab to manage ICANS in the absence of concurrent CRS is not recommended due to its poor blood-brain barrier penetration and concerns around worsening ICANS related to tocilizumab’s ability to cause elevations in CSF IL-6 levels.9,37–39
Few studies reported in our systematic review evaluated long-term neurologic outcomes following CAR-T cell therapies. However, two reports from a single institution suggest that neuropsychiatric difficulties could persist beyond one year following CAR-T therapy. In one of these reports of long-term neurological complications among CAR-T cell therapy recipients, patients also reported clinically meaningful depression and anxiety symptoms.13 In the other published report, over 37% of patients reported at least one cognitive difficulty, predominantly difficulties with memory.14 These findings suggest a need for further research to characterize long-term patient-centered outcomes, which would then lend to developing interventions to prevent or mitigate the impact of ICANS. Finally, five fatal ICANS events were reported across two studies. These fatal outcomes support the need for early identification and management of those with symptoms of ICANS to prevent rapid clinical deterioration and death. Furthermore, there is a need to develop risk prediction tools capable of identifying the subset of patients at risk for severe ICANS development and targeted interventions to mitigate these neurologic complications.
Strengths and Limitations
Our study has limitations. First, to maintain our focus on hematological malignancies in the adult population, we excluded studies that reported ICANS in those with solid tumors or studies conducted exclusively in pediatric populations. Second, because of the heterogeneity of studies, a meta-analysis could not be performed. Thirdly, some relevant literature may not have been retrieved due to changes in the terminology used to define ICANS, previously known as CAR-T cell-related neurotoxicity or encephalopathy and cytokine release encephalopathy syndrome (CRES). We attempted to address this by conducting citation chasing and citation chaining of the included studies. Finally, all included studies were observational, which introduces the risk of confounding. We attempted to address confounding effects by assessing and reporting the risk of bias.
Despite these limitations, our systematic review has the following strengths. First, to our knowledge, this is the first systematic review to synthesize the literature on the clinical, biological, and radiological findings associated with ICANS among adults with hematological malignancies treated with CAR-T cell therapy. Second, this review reports on the long-term neurological complications of CAR-T cell therapy, which has seldom been the focus of previously published reports. Third, our study enables the identification of gaps in the diagnosis, classification, and long-term follow-up of CAR-T cell therapy recipients. Finally, our findings highlight a need for future studies designed to predict more accurately those at greatest risk for developing high-grade ICANS and long-term neurologic complications of CAR-T cell therapy.
Emerging data
The 2021 annual meetings of the American Society of Clinical Oncology and the American Society of Hematology showcased research abstracts examining the clinical manifestations, predictors, biomarkers, and management of ICANS (Table 2). These studies demonstrated similar incidences and time to onset and resolution of ICANS as those identified in our systematic review. The emergence of serum neurofilament light chain levels as a possible biomarker of ICANS onset and severity is especially promising. In the future, this biomarker could be potentially applied to the clinical setting, thus aiding in the early identification of those at risk for ICANS development. Additionally, the EASIX score has emerged as a possible predictor of ICANS onset and severity in those receiving CAR-T cell therapy. The EASIX score consists of easily and routinely measured labs, allowing easier adoption into clinical practice. Two highlighted abstracts focused on the role of the Interleukin-1 antagonist anakinra in the prevention or management of ICANS, with results suggesting lower and less severe rates of ICANS in those treated with anakinra.40,41 This preliminary data also suggests a dose-dependent response to anakinra among those with ICANS.41
Table 2.
Summary of select abstracts presented at the 2021 Annual Meetings of the American Society of Clinical Oncology and the American Society of Hematology
| First Author | Title | Study Aim | Study design | Population | Sample Size | CAR-T target and product | Outcome(s) |
|---|---|---|---|---|---|---|---|
| Clinical manifestations of Immune effector cell-associated neurotoxicity syndrome (ICANS) | |||||||
| Einslee et al.42 | Incidence, mitigation, and management of neurologic adverse events in patients with multiple myeloma (MM) treated with ciltacabtagene autoleucel (cilta-cel) in CARTITUDE-2 | To describe the clinical features and management of ICANS | Phase II open label | Relapsed multiple myeloma | n=20 | BCMA: Cilta-cel (JNJ-68284528) | ICANS developed in n=4 (20%); median time to onset of symptoms: 8 days (range: 7–11), median duration of ICANS: 2 days (range: 1–2). Management of ICANS included: levetiracetam and steroids; all 3 patients with ICANS had concurrent cytokine release syndrome (CRS) and all recovered. One patient developed isolated facial paralysis on Day 29 post infusion, that resolved 51 days post onset of symptoms and with dexamethasone therapy. |
| Biomarkers and predictors of ICANS development | |||||||
| Gauthier et al.43 | CD19 CAR T-cell product type independently impacts CRS and ICANS severity in patients with aggressive NHL | To assess the impact of 3 CD19 CAR T-cell products (axicabtagene ciloleucel, tisagenlecleucel, and JCAR014) on CRS and ICANS severity | retrospective | Relapsed, refractory Non-Hodgkin’s lymphoma | n=136 | CD19: Axicabtagene ciloleucel, Tisagenlecleucel BCMA: JCAR014 |
Factors associated with ICANS severity CAR T-cell product type (Tisagenlecleucel vs axicabatagene ciloleucel OR = 0.14, p <.001; JCAR014 vs axicabatagene ciloleucel, OR = 0.31, p = 0.009) Pre-lymphodepletion LDH (OR, 3.96 per log10 increase; p = 0.04) Age (OR per 10-year increase, 1.32; p =.06) |
| Butt et al.44 | Pre-Infusion Neurofilament Light Chain (NfL) Levels Predict the Development of Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) - a Multicenter Retrospective Study | To evaluate pre-infusion levels of plasma neurofilament light chain (NfL), a marker of neurodegeneration, as a predictive biomarker of ICANS | retrospective | not reported | not reported | not reported | n=30 (36%) with ASTCT Gr 1–4 ICANS, higher NfL were found in those who developed ICANS compared to those who did not ([87.6 v 29.4 pg/ml], p = 0.00004); NfL correlated with ICANS development (r = 0.74, p < 0.0001) |
| Korell et al.45 | Easix Predicts Severe Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neuro-Toxicity Syndrome (ICANS) in Patients Receiving CD19-Directed Chimeric Antigen Receptor T (CAR-T) Cell Therapy | To evaluate and validate the Endothelial Activation and Stress Index (EASIX)) as a predictor for CRS and ICANS in patients receiving CD19-directed CAR-T cells | retrospective | Relapsed, refractory large B-cell lymphoma Acute lymphoblastic leukemia Mantle cell lymphoma Chronic lymphocytic leukemia Follicular lymphoma |
n=107 (training cohort) n=93 (validation cohort) |
CD19: Axicabtagene ciloleucel, Tisagenlecleucel | n=24 patients (22%) developed ICANS grades 1–4. Grade ≥ 3 ICANS occurred in 11 patients (11%; median onset 8 (4–17) days). EASIX values (pre-lymphodepletion, d0, d3, d7) were significantly higher in patients who developed either grade 3–4 CRS, ICANS, or both; EASIX predicted grade 3–4 CRS and ICANS before lymphodepleting therapy (-pre), on day 0 and on day 3 in both cohorts |
| Management of ICANS | |||||||
| Gazeau et al.41 | Safety and Efficacy of Two Anakinra Dose Regimens for Refractory CRS or ICANS after CAR T-Cell Therapy | To describe the safety and efficacy of two anakinra dose regimens to treat refractory CRS and/or ICANS after CAR T-cell therapy | retrospective | Multiple myeloma Mantle cell lymphoma Transformed follicular lymphoma Diffuse large B-cell lymphoma Steroid-refractory ICANS |
n=26 | not reported | Intervention: Anakinra 100–200mg/day subcutaneously (SC) in 13 patients (pts) or 8mg/kg/day SC or intravenously (IV) in 13 patients. Treatment with anakinra led to improved ICANS/CRS rates in 73% of patients with higher response in those who received the higher dose of anakinra |
| Frigault et al.40 | A Phase II Trial of Anakinra for the Prevention of CAR-T Cell Mediated Neurotoxic | To evaluate whether or not anakinra could be administered prophylactically to prevent severe CRS and neurologic events (NE) in patients receiving axicabtagene ciloleucel | Phase II, open-labelled | Relapsed/refractory large cell lymphoma | N=6 | CD19: Axicabtagene ciloleucel | Intervention: Anakinra 200 mg SC starting 4 hours prior to axicabatagene ciloleucel infusion and daily thereafter for a total of 7 days. 2 of 6 patients experienced grade 3 ICANS |
Conclusion
Our findings highlight the variability in the biological, clinical, radiological, and EEG findings of ICANS following CAR-T cell therapy and the need for further work to characterize the long-term neurologic outcomes of these patients. As CAR-T cell therapies continue to gain popularity in clinical practice, there is a need to bridge the evidence gaps. Future research studies should focus on early identification and risk prediction modeling for ICANS development. The use of consensus grading scales of ICANS in future studies would provide cross-trial comparisons critical for developing interventions to reduce and manage neurotoxicities related to CAR-T therapies. Additional studies are needed to characterize the long-term neurologic complications following CAR T-cell therapy fully. Studies ought to leverage real-world populations when examining the effects of CAR-T cell therapies.
| Suggested future research directions for ICANS related studies: |
|---|
|
|
|
|
|
Supplementary Material
Key Points.
ICANS typically develops within the first 7–10 days following CAR-T cell infusion and often develops in the setting of cytokine release syndrome
Clinical features of ICANS are highly variable but commonly include aphasia, seizures, headache, encephalopathy, and altered level of consciousness
Most symptoms of ICANS resolve within 21 days of onset and can be managed with supportive care, steroids, and anti-seizure prophylaxis; tocilizumab (a monoclonal antibody to interleukin-6 receptor) should be reserved for those who have concurrent cytokine release syndrome
We systematically demonstrate the association between ICANS and the following: presence and severity of cytokine release syndrome (CRS), c-reactive protein (CRP), and ferritin levels, and the diagnostic utility of magnetic resonance imaging (MRI), computerized tomography (CT) and electroencephalogram (EEG)
Acknowledgements
Funding/Support:
Dr. Grant is supported by a 5-K12-CA120780-13 grant from the National Cancer Institute (PI: William Kim) and a National Institute on Aging Grant (1 R03 AG074030-01; PI: Shakira Grant). Dr. Giri is supported by the University of Alabama at Birmingham Walter B. Frommeyer Fellowship in Investigative Medicine.
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosure: The authors have no conflicts of interest to disclose
References
- 1.Maude SL, Frey N, Shaw PA, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. New England Journal of Medicine. 2014-10-16 2014;371(16):1507–1517. doi: 10.1056/nejmoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New England Journal of Medicine. 2018-02-01 2018;378(5):439–448. doi: 10.1056/nejmoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine. 2017-12-28 2017;377(26):2531–2544. doi: 10.1056/nejmoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine. 2019-01-03 2019;380(1):45–56. doi: 10.1056/nejmoa1804980 [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. New England Journal of Medicine. 2020-04-02 2020;382(14):1331–1342. doi: 10.1056/nejmoa1914347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munshi NC, Anderson LD, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. New England Journal of Medicine. 2021-02-25 2021;384(8):705–716. doi: 10.1056/nejmoa2024850 [DOI] [PubMed] [Google Scholar]
- 7.Garcia Borrega J, Gödel P, Rüger MA, et al. In the Eye of the Storm: Immune-mediated Toxicities Associated With CAR-T Cell Therapy. Hemasphere. Apr 2019;3(2):e191. doi: 10.1097/hs9.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019-05-16 2019;133(20):2212–2221. doi: 10.1182/blood-2018-12-893396 [DOI] [PubMed] [Google Scholar]
- 9.Gust J, Hay KA, Hanafi L-A, et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discovery. 2017-12-01 2017;7(12):1404–1419. doi: 10.1158/2159-8290.cd-17-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santomasso BD, Park JH, Salloum D, et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discovery. 2018-08-01 2018;8(8):958–971. doi: 10.1158/2159-8290.cd-17-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torre M, Solomon IH, Sutherland CL, et al. Neuropathology of a Case With Fatal CAR T-Cell-Associated Cerebral Edema. J Neuropathol Exp Neurol. Oct 1 2018;77(10):877–882. doi: 10.1093/jnen/nly064 [DOI] [PubMed] [Google Scholar]
- 12.Rubin DB, Danish HH, Ali AB, et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain. May 1 2019;142(5):1334–1348. doi: 10.1093/brain/awz053 [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transplant. Jan 2020;26(1):26–33. doi: 10.1016/j.bbmt.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruark J, Mullane E, Cleary N, et al. Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors after Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant. Jan 2020;26(1):34–43. doi: 10.1016/j.bbmt.2019.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. bmj. 2020;368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moola SMZ, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M,, Qureshi RMP, Lisy K, Mu P-F. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. Available from https://synthesismanual.jbi.global. 2020. [Google Scholar]
- 18.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. Apr 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mcgrath S, Zhao X, Steele R, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Statistical Methods in Medical Research. 2020-09-01 2020;29(9):2520–2537. doi: 10.1177/0962280219889080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalabi H, Wolters PL, Martin S, et al. Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy. Journal of Immunotherapy. 2018-09-01 2018;41(7):350–358. doi: 10.1097/cji.0000000000000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belin C, Devic P, Ayrignac X, et al. Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Scientific Reports. 2020-12-01 2020;10(1)doi: 10.1038/s41598-020-76055-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strati P, Nastoupil LJ, Westin J, et al. Clinical and radiologic correlates of neurotoxicity after axicabtagene ciloleucel in large B-cell lymphoma. Blood Advances. 2020-08-25 2020;4(16):3943–3951. doi: 10.1182/bloodadvances.2020002228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gust J, Annesley CE, Gardner RA, Bozarth X. EEG Correlates of Delirium in Children and Young Adults With CD19-Directed CAR T Cell Treatment-Related Neurotoxicity. J Clin Neurophysiol. Mar 1 2021;38(2):135–142. doi: 10.1097/WNP.0000000000000669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin DB, Al Jarrah A, Li K, et al. Clinical Predictors of Neurotoxicity After Chimeric Antigen Receptor T-Cell Therapy. JAMA Neurol. Aug 10 2020;doi: 10.1001/jamaneurol.2020.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maillet D, Belin C, Moroni C, et al. Evaluation of mid-term (6–12 months) neurotoxicity in B-cell lymphoma patients treated with CAR T cells: a prospective cohort study. Neuro-Oncology. 2021;doi: 10.1093/neuonc/noab077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenbaum U, Strati P, Saliba RM, et al. CRP and ferritin in addition to the EASIX score predict CAR-T-related toxicity. Blood Adv. Jul 27 2021;5(14):2799–2806. doi: 10.1182/bloodadvances.2021004575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wudhikarn K, Bansal R, Khurana A, et al. Age defining immune effector cell associated neurotoxicity syndromes in aggressive large B cell lymphoma patients treated with axicabtagene ciloleucel. Am J Hematol. Nov 1 2021;96(11):E427–e430. doi: 10.1002/ajh.26330 [DOI] [PubMed] [Google Scholar]
- 28.Gofshteyn JS, Shaw PA, Teachey DT, et al. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Annals of Neurology. 2018-10-01 2018;84(4):537–546. doi: 10.1002/ana.25315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokolov E, Karschnia P, Benjamin R, et al. Language dysfunction-associated EEG findings in patients with CAR-T related neurotoxicity. BMJ Neurology Open. 2020-06-01 2020;2(1):e000054. doi: 10.1136/bmjno-2020-000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wudhikarn K, Pennisi M, Garcia-Recio M, et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Advances. 2020-07-14 2020;4(13):3024–3033. doi: 10.1182/bloodadvances.2020001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtzman NG, Xie H, Bentzen S, et al. Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes. Neuro Oncol. Jan 30 2021;23(1):112–121. doi: 10.1093/neuonc/noaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maziarz RT, Schuster SJ, Romanov VV, et al. Grading of neurological toxicity in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv. Apr 14 2020;4(7):1440–1447. doi: 10.1182/bloodadvances.2019001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gajra A, Zettler ME, Phillips EG Jr., et al. Neurological adverse events following CAR T-cell therapy: a real-world analysis. Immunotherapy. Oct 2020;12(14):1077–1082. doi: 10.2217/imt-2020-0161 [DOI] [PubMed] [Google Scholar]
- 34.Brown BD, Tambaro FP, Kohorst M, et al. Immune Effector Cell Associated Neurotoxicity (ICANS) in Pediatric and Young Adult Patients Following Chimeric Antigen Receptor (CAR) T-Cell Therapy: Can We Optimize Early Diagnosis? Front Oncol. 2021;11:634445–634445. doi: 10.3389/fonc.2021.634445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuen C, Rezania K, Kelly T, Bishop MR. Clinical predictors of chimeric antigen receptor T-cell therapy neurotoxicity: a single-center study. Immunotherapy. Oct 2021;13(15):1261–1269. doi: 10.2217/imt-2021-0084 [DOI] [PubMed] [Google Scholar]
- 36.Beuchat I, Danish HH, Rubin DB, et al. EEG findings in CAR T-cell-associated neurotoxicity: Clinical and radiological correlations. Neuro Oncol. Feb 1 2022;24(2):313–325. doi: 10.1093/neuonc/noab174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Teachey DT, Pequignot E, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. Jul 2016;434:1–8. doi: 10.1016/j.jim.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gust J, Finney OC, Li D, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. Jul 2019;86(1):42–54. doi: 10.1002/ana.25502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locke FL, Neelapu SS, Bartlett NL, et al. Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (axi-cel; KTE-C19) Treatment for Patients with Refractory,Aggressive Non-Hodgkin Lymphoma (NHL). Blood. 2017;130(Supplement 1):1547–1547. doi: 10.1182/blood.V130.Suppl_1.1547.1547 [DOI] [Google Scholar]
- 40.Frigault MJ, Gallagher KME, Wehrli M, et al. A Phase II Trial of Anakinra for the Prevention of CAR-T Cell Mediated Neurotoxicity. Blood. 2021;138(Supplement 1):2814–2814. doi: 10.1182/blood-2021-146927 [DOI] [Google Scholar]
- 41.Gazeau N, Barba P, Iacoboni G, et al. Safety and Efficacy of Two Anakinra Dose Regimens for Refractory CRS or Icans after CAR T-Cell Therapy. Blood. 2021;138(Supplement 1):2816–2816. doi: 10.1182/blood-2021-147454 [DOI] [Google Scholar]
- 42.Einsele H, Parekh SS, Madduri D, et al. Incidence, mitigation, and management of neurologic adverse events in patients with multiple myeloma (MM) treated with ciltacabtagene autoleucel (cilta-cel) in CARTITUDE-2. Journal of Clinical Oncology. 2021;39(15_suppl):8028–8028. doi: 10.1200/JCO.2021.39.15_suppl.8028 [DOI] [Google Scholar]
- 43.Gauthier J, Cearley A, Perkins P, et al. CD19 CAR T-cell product type independently impacts CRS and ICANS severity in patients with aggressive NHL. Journal of Clinical Oncology. 2021;39(15_suppl):7532–7532. doi: 10.1200/JCO.2021.39.15_suppl.7532 [DOI] [Google Scholar]
- 44.Butt OH, Zhou AY, Caimi PF, et al. Pre-Infusion Neurofilament Light Chain (NfL) Levels Predict the Development of Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) - a Multicenter Retrospective Study. Blood. 2021;138(Supplement 1):2841–2841. doi: 10.1182/blood-2021-149674 [DOI] [Google Scholar]
- 45.Korell F, Penack O, Schmitt M, et al. Easix Predicts Severe Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neuro-Toxicity Syndrome (ICANS) in Patients Receiving CD19-Directed Chimeric Antigen Receptor T (CAR-T) Cell Therapy. Blood. 2021;138(Supplement 1):3861–3861. doi: 10.1182/blood-2021-152875 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


