Abstract
Objectives
Previously considered solely an opportunistic pathogen, Clostridium innocuum (CI) was recently reported in Taiwan to be an emerging cause of antibiotic-associated diarrhea and clinically indistinguishable from Clostridioides difficile (CD) infection. We previously identified CI culture supernatant being cross-reactive with commercial CD toxin enzyme immunoassays. We aimed to identify and characterize the cross-reacting protein and determine whether it functioned as a human toxin.
Methods
We performed western blots using CI culture supernatants and CD anti-toxin antibodies and identified interacting bands. We identified protein(s) using tandem mass spectrometry and evaluated them by cytotoxicity assays.
Results
CI, but not CD, was isolated from stool of 12 children and adults with diarrhea. Culture supernatant from 6/12 CI isolates, and an ATCC reference strain, tested positive for CD toxins (total 7/13 isolates) by commercial EIA. Using two of these isolates, we identified two ~40 kDa hypothetical proteins, CI_01447 and CI_01448, and confirmed cross-reactivity with CD anti-toxin antibodies by enzyme immunoassay and western blot. Whole-genome sequencing confirmed all 13 isolates contained both genes, which were highly conserved. We observed no cytopathic or cytotoxic effects to HeLa cells when treated with these proteins. We identified amino acid sequence similarity to the NlpC/P60 family of proteins.
Conclusions
Our findings do not suggest CI proteins CI_01448 and CI_01447, which cross-react with antibodies against CD toxins A and B, are toxic to HeLa cells. Further studies are needed to determine the function of these cross-reacting proteins and the potential virulence factors that could be responsible for CI diarrheal disease.
Keywords: Clostridium innocuum, antibiotic-associated diarrhea, Clostridioides difficile, toxin A, toxin B, NlpC/P60
INTRODUCTION
Antibiotic-associated diarrhea (AAD) occurs in 5–35% [1, 2] of patients who receive antibiotics. Clostridioides difficile is the most frequently identified causal pathogen of AAD and is responsible for 25% of all AAD cases [3]. Diagnosing C. difficile infection (CDI) is a challenge because of frequent use of highly sensitive nucleic acid amplification testing (NAAT) that does not differentiate colonization and infection [4]. Thus, it is possible that other infectious and non-infectious etiologies can be overlooked in a patient with a positive NAAT related to C. difficile colonization but in whom C. difficile is not the actual cause of their diarrheal illness.
Clostridium innocuum may be one such overlooked etiology in patients with a CDI-like illness. In 2018, a retrospective study from Taiwan [5] reported that stools from 5% of patients being evaluated for CDI were culture-positive for C. innocuum but culture-negative for C. difficile. The C. innocuum isolates obtained from these patients were multidrug resistant (including to vancomycin), cytotoxic to a human cell line, and pathogenic in a murine intestinal model of infection. Together, these data suggested that C. innocuum was an emerging diarrheal pathogen. These findings were unexpected given that C. innocuum has been characterized as clinically ”innocuous” because it was avirulent in both a murine intraperitoneal infection model and after intramuscular injection in guinea pigs [6], and until recently C. innocuum was very rarely reported as an opportunistic pathogen in patients who were immunocompromised or had comorbidities [7, 8].
Prior to the important publication by Chia, et al., our research laboratory made some initial unpublished observations about C. innocuum. We occasionally isolate C. innocuum from stool collected from children who present with diarrhea. These children were initially thought to have CDI because their stool tested positive by toxin B gene PCR, but we were unable to culture C. difficile from some of these stools.[9] This typically occurs in samples with high tcdB PCR cycle threshold values (i.e., very low stool inoculum of toxigenic C. difficile), as other groups have reported [10, 11], suggesting that these children had C. difficile colonization rather than CDI. In these cases, when using media thought to be selective for C. difficile, we occasionally noted single colony morphology that was equivocal for C. difficile, appearing smaller and more white in color than typically observed. To rule out toxigenic C. difficile, we used commercial enzyme immunoassays (EIA) to identify C. difficile-specific toxins A and/or B in culture supernatant of these isolates. Although these EIAs were used on bacterial culture supernatants rather than stool as the manufacturer intended, these isolate supernatants unexpectedly tested positive for toxin A/B. This pattern suggested that though these isolates were not C. difficile, they secreted a protein that cross reacts with antibodies against toxins A and/or B. These isolates were subsequently identified as C. innocuum.
The isolation of C. innocuum, but not C. difficile, from stools of patients with diarrhea and positive toxin B gene PCR results were perplexing. For example, it was unclear if C. innocuum was the source of the positive stool toxin B gene PCR (e.g., the possibility of C. innocuum having acquired the gene for toxin B). If not, it was also unclear if C. innocuum was a causal agent for the diarrheal illnesses in these children; this remains an unanswered question and outside of the scope of the present study. As a first step in resolving the observed EIA cross-reactivity, the present study sought to characterize the observed interaction with the C. difficile-specific EIA that suggested that C. innocuum secretes a protein that may share structural similarities with toxins A and/or B and cross reacts with C. difficile antitoxin antibodies. This cross-reactive protein heretofore is referred to as EIA-reactive factor (ErF). We hypothesized that ErF may be a human toxin and contribute to C. innocuum-associated diarrheal illness. The objectives of this study were to identify ErF and determine whether it functioned as human toxin that could potentially cause a diarrheal illness.
MATERIALS and METHODS
Clostridium innocuum strain selection and enzyme immunoassay (EIA) cross-reactivity
The isolates used in this study were derived from three sources. First, we compiled a convenience sample of C. innocuum isolates that were unexpectedly identified in stool cultures of children who had been included in various studies of CDI. Eleven isolates in total were identified from various clinical epidemiological studies of CDI in children; all children had been diagnosed with CDI by a stool toxin B gene PCR assay performed in a clinical microbiology laboratory [12–14]. However, limited clinical data are available for these patients. Two additional C. innocuum strains, were included: ATCC reference strain 14501 [6, 15] and LC-LUMC-CI-001 (LC-CI). ATCC reference strain 14501 was derived from a patient described in the original clinical report of C. innocuum in 1962.[6] This isolate was derived from a human pyogenic infection, although it is not known which of the eight infections described in the report serves as the source of the reference strain. LC-LUMC-CI-001 (referred to as LC-CI in the present paper) is a strain isolated from stool of an adult thought to have C. innocuum-associated diarrhea [16]. This patient first had CDI, followed by a recurrent CDI-like illness but whose stool was negative for C. difficile by PCR and culture, and positive for C. innocuum by culture, at the time of diarrhea recurrence. Thus, of the strains used in this study, the clinical data available for the adult patient with the LC-CI strain most strongly supports C. innocuum being the cause of the diarrheal illness.
Stool was cultured for C. difficile using pre-reduced taurocholate-cycloserine-cefoxitin-fructose agar (TCCFA) in an anaerobic chamber and incubated at 37°C for 48 hours. Colonies with morphology that were equivocal for C. difficile first underwent toxigenic stool culture using a commercial lateral flow EIA for glutamate dehydrogenase (GDH) and toxin A/B (C. difficile QUIK CHEK Complete; Techlab, Blacksburg, VA). This EIA was designed and intended to be used on clinical stool specimens, but we deviated from manufacturer recommendations for use and instead performed EIA on cultured isolates, as previously suggested, using the following methods [10]. Single colonies from TCCFA were first sub-cultured on blood agar for 24 hours, and a single colony was then incubated in tryptic soy broth (TSB) for 24 h or until an optical density (OD600) of 0.6 was reached. Broth cultures were centrifuged for 10 min at 4,100 × g and filtered with 0.45 um filter. Filtered supernatant was processed by EIA and interpreted following manufacture’s protocols. All isolates included were GDH-negative by EIA (i.e., ruling out C. difficile), and toxin A/B EIA results were confirmed using an additional commercial lateral flow EIA from another manufacturer (Immunocard® Toxins A&B; Meridian Bioscience, Cincinnati, OH) that was also used on bacterial isolates, which again is a deviation from its intended use. Isolates underwent matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) using the Bruker biotyper; all 13 isolates were confirmed as C. innocuum with an identification score of 1.80–2.06. Using growth conditions described below, genomic DNA was extraction from single colony cultured overnight using QIAamp BiOstic Bacteremia DNA Kit (Qiagen, Hilden, Germany). Whole genome sequencing was then performed using the Illumina (San Diego, CA) platform for library preparation (Nextera) and sequencing (MiSeq). Our bioinformatics analyses have been described previously.[17] The 13 isolate sequences included in this study have been deposited in DDBJ/ENA/GenBank under the accession numbers listed in the Supplemental Table S1.
Bacterial strains, plasmids, media, and culture conditions.
All bacterial strains and plasmids used for these experiments are listed in Table 1. All C. innocuum and C. difficile cultures were grown in 50 ml falcon tubes at 37°C in anaerobic chamber (5% CO2, 5% H2, bal N2) for 24–48 h in TSB.
Table 1.
Strains and plasmids used in this study.
| Strain/plasmid | Relevant genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| C. innocuum | ||
| LC-LUMC-CI-001 | Clinical isolate | [16] |
| ATCC 14501 | Reference isolate | Smith and King (ATCC® 14501™) [6, 15] |
| C. difficile | RT106/DH/ST-42 | [32] |
| E. coli | ||
| BL21 (DE3) (Magic) | [19] | |
| Plasmids | ||
| pMCSG53 | ampicillin resistance a N-terminal 6xHis-tag with a tobacco etch virus (TEV) protease cleavage site | [18] |
| pMCSG53_CI_01447 | N-terminal 6xHis-tagged CI_01448 cloned into pMCSG53; ampR | This study |
| pMCSG53_CI_01448 | N-terminal 6xHis-tagged CI_01447 cloned into pMCSG53; ampR | This study |
Plasmid Construction and transformation into Escherichia coli
Genes encoding proteins of interest, CI_01447 and CI_01448, were cloned into plasmid pMCSG53 [18] using Gibson Assembly Cloning Kit (New England Biolabs, Ipswich, MA) per the manufacturer’s instructions. The plasmid pMCSG53 contains an N-terminal 6×His tag followed by a TEV protease cleavage site, encoding ampicillin resistance, and genes for rare codons [18]. Primers for cloning and sequencing are indicated in Table 2. The plasmid was transformed into E. coli BL21 (DE3) (Magic) cells [19].
Table 2.
Primers used for cloning and sequencing in this study
| Oligonucleotide | Sequence |
|---|---|
| pMCSG-01447 F | AACCTGTACTTCCAATCCAATGACGGATGGCACGGAAGT |
| pMCSG-01447 R | GATCCGTTATCCACTTCCAATGTTACTTGTTCACAC |
| pMCSG-01448-F | CTGTACTTCCAATCCAATGCCAAGTTTGATAAAAATGGA |
| pMCSG-01448-R | GATCCGTTATCCACTTCCAATGTTACGGACGGC |
| 01448 FL-For | CTGTACTTCCAATCCAATGCCAAGTTTGATAAAAATGGA |
| 01448 FL-Rev | GATCCGTTATCCACTTCCAATGTTACGGACGGC |
| 01447 FL-For | CTGTACTTCCAATCCAATGACGGATGG |
| 01447 FL-Rev | GATCCGTTATCCACTTCCAATTTATTTGTTTACGCG |
| T7 | TAATACGACTCACTATAGGG |
| T7 Rev | TAGTTATTGCTCAGCGGTGG |
Bacterial pellet protein extraction
A 200 ml overnight culture of C. innocuum was centrifuged for 10 min at 3,000 × g. Bacterial cell pellets were combined and resuspended in 5 ml of lysis buffer (0.5 % SDS, 50 mM AmBic, 50 mM NaCl, and 0.1% HALT Protease Inhibitor). Cells were sonicated on ice with three 30-s bursts at 4 W, followed by centrifugation for 10 min at 3,000 × g to remove cell debris and unbroken cells.
Trichloroacetic acid-acetone precipitation on bacterial cell supernatants
Bacterial cell supernatants from an overnight were filtered using a 0.45 μm filter and combined with 100% trichloroacetic acid (TCA) in a 1:4 ratio. Precipitation occurred overnight at 4°C with shaking. The following day, precipitations were centrifuged for 30 min at 4,100 × g. Supernatants were then discarded, and pellets were resuspended in 1 ml pre-chilled acetone and incubated 4°C for 15 min with shaking. This was repeated twice, after which pellets were dried at room temperature in a laboratory fume hood for 10 min. Pellets were resuspended in 1 ml PBS and centrifuged 30 min at 4,100 × g. Supernatants were decanted, and pellets were resuspended in a final volume of 1 ml PBS.
Recombinant protein expression and purification from E. coli
E. coli cells transformed with pMCSG53_CI_01447 or pMCSG53_CI_01448 were used to inoculate overnight starter cultures grown in LB supplemented with 120 μg/ml ampicillin and 50 μg/ml kanamycin at 37°C and 200 rpm. The next day, 3 liters of Dubelco Terrific Broth medium supplemented with 200 μg/ml ampicillin and 50 μg/ml kanamycin were inoculated at 1:100 dilution with the overnight culture and incubated at 37°C and rotation 220 rpm. Protein expression was induced at OD600=1.8–2 by addition of 0.6 mM IPTG and the culture was further incubated at 25°C and 200 rpm for 16 hours [20]. The cells were harvested by centrifugation at 6000 rpm for 10 min, re-suspended (1g of cells: 5 ml of lysis buffer) in lysis buffer (50 mM Tris pH 8.3, 0.5 M NaCl, 10% glycerol, 0.1% IGEPAL CA-630) and frozen at −30°C until purification. Frozen pellets were thawed and sonicated at 50% amplitude, in 5 s × 10 s cycle for 40 min in an ice bath. The lysate was clarified by centrifugation at 36,000 × g for 40 min at 4°C, the supernatant was collected, and the protein was purified as previously described [21] with some modifications described below for CI_01447 and CI_01448.
CI_01447 was purified by Ni-IMAC followed by size exclusion gel chromatography (SEC) using an ÅKTAxpress system (GE Healthcare). The supernatant was loaded onto a His-Trap FF (Ni-NTA) column in loading buffer (10 mM Tris-HCl pH 8.3, 500 mM NaCl, 1 mM Tris (2-carboxyethyl) phosphine (TCEP), and 5% glycerol). The column was washed with 10 column volumes (cv) of loading buffer and 10 (cv) of washing buffer (10 mM Tris-HCl pH 8.3, 1M NaCl, 25 mM imidazole, 5% glycerol) Protein was eluted with elution buffer (10 mM Tris pH 8.3, 500 mM NaCl, 1 M imidazole, 0.1 mM TCEP), loaded onto a Superdex 200 26/600 column and separated in loading buffer. The protein was collected and analyzed by SDS-PAGE. The 6xHis-tag was cleaved by recombinant TEV protease in ratio 1:20 (protease:protein) overnight at room temperature. The cleaved protein was separated from recombinant TEV protease, un-cleaved protein and 6xHistag peptide by Ni-NTA-affinity chromatography using loading buffer followed by loading buffer with 25 mM imidazole. The cleaved protein was collected in the flow through in loading buffer, analyzed by SDS-PAGE for purity and 6xHis tag cleavage, concentrated to 10.2 mg/ml and stored in 10 mM Tris-HCl pH 8.3, 500 mM NaCl, 0.1 mM TCEP at −80°C.
CI_01448 was purified by Ni-IMAC followed by SEC using an ÅKTAxpress system (GE Healthcare). The supernatant was loaded onto a His-Trap FF(Ni-NTA) column in loading buffer (10 mM Tris-HCl pH 7.5, 500 mM NaCl, 1 mM TCE), and 5% glycerol). The column was washed with 10 column volumes (cv) of loading buffer and 10 (cv) of washing buffer (10 mM Tris-HCl pH 7.5, 1M NaCl, 25 mM imidazole, 5% glycerol). The protein was eluted with elution buffer (10 mM Tris pH 7.5, 500 mM NaCl, 1 M imidazole, 0.1 mM TCEP), loaded onto a Superdex 200 26/600 column and separated in loading buffer. The protein was collected and analyzed by SDS-PAGE. The 6xHis-tag was cleaved by recombinant TEV protease at 1:20 protease:protein overnight at room temperature. The cleaved protein was separated from recombinant TEV protease, un-cleaved protein and 6xHis-tag peptide by Ni-NTA-affinity chromatograph using loaded buffer followed by loaded buffer supplemented with 25 mM imidazole. The cleaned protein was collected in the flow through in loading buffer, analyzed by SDS-PAGE for purity and 6xHis tag cleavage, concentrated to 6.6 mg/ml and stored in 10 mM Tris-HCl pH 7.5, 500 mM NaCl, 0.1 mM TCEP at −80°C.
Immunoblot analysis
Protein concentrations were determined using a modified Lowry assay (Thermo Scientific, Waltham, MA) with bovine serum albumin as a standard. Equal amounts of protein (40 μg) per sample were combined with SDS-loading buffer (2X Laemmli buffer) with 5% 2-Mercaptoethanol, and run on pre-casted 7.5% acrylamide gels (Bio-Rad, Hercules, CA) at 100V for 90 min. For immunoblots performed with recombinant protein a total of 50 ng was used of CI_01447 and CI_01448 was used. For C. difficile TcdA and TcdB, 1 ng was used. Proteins transferred onto polyvinylidene difluoride (PVDF) membranes using a Power Blotter–Semi-dry Transfer System (Thermo Fisher Scientific, Waltham, MA). Membranes were incubated in blocking buffer (5% Skim milk, 0.1% Tween 20 in PBS) for 2 h at room temperature or overnight at 4°C with gentle shaking. Blots were probed with a 1: 5,000 dilution of chicken Clostridium difficile anti-toxin A, conjugated with HRP (ACdTA-HRP), or with chicken Clostridium difficile anti-toxin B, conjugated with HRP (AcdTB-HRP) (Exalpha Biologicals, Inc., Shirley, MA). Blots were subsequently developed using Immun-Star WesternC chemiluminescence reagents (Bio-Rad, Hercules, CA) and imaged using Syn G: BOX Chemi XX6 (Syngene International Limited, India).
Tandem mass spectrometry (LC-MS/MS) preparation and analyses
Samples were run on an SDS-PAGE gel and gel bands were then excised. Excised gel material was then washed in 100 mM Ammonium Bicarbonate (AmBic)/Acetonitrile (ACN) and reduced with 10 mM dithiothreitol at 50°C for 30 minutes. Cysteines were alkylated with100 mM iodoacetamide in the dark for 30 minutes at room temperature. Gel band was washed in 100 mM AmBic/ACN prior to adding 600 ng trypsin for overnight incubation at 37 °C. After incubation, the supernatant was collected, and the gel was washed for 10 min with gentle shaking in 50% ACN/5% formic acid (FA) at room temperature. The wash supernatant was collected and saved in peptide solution. The wash step was repeated twice more with 80% ACN/5% FA, and 100% ACN/5% FA. All wash supernatants were collected in peptide solution and then lyophilized using speedvac drying. After lyophilization, peptides were reconstituted with 5% ACN/0.1% FA in water.
Peptides were analyzed by LC-MS/MS using a Dionex UltiMate 3000 Rapid Separation nanoLC coupled to an Orbitrap Elite Mass Spectrometer (Thermo Fisher Scientific Inc, Waltham, MA). Samples were loaded onto the trap column, which was 150 μm × 3 cm in-house packed with 3 μm ReproSil-Pur® beads. The analytical column was a 75 μm × 10.5 cm PicoChip column packed with 3 μm ReproSil-Pur® beads (New Objective, Inc. Woburn, MA). The flow rate was kept at 300nL/min. Solvent A was 0.1% FA in water and Solvent B was 0.1% FA in ACN. The peptide was separated on a 120-min analytical gradient from 5% ACN/0.1% FA to 40% ACN/0.1% FA. MS1 scans were acquired from 400–2000m/z at 60,000 resolving power and automatic gain control (AGC) set to 1×106. The 15 most abundant precursor ions in each MS1 scan were selected for fragmentation by collision-induced dissociation at 35% normalized collision energy in the ion trap; previously selected ions were dynamically excluded from re-selection for 60 seconds.
Proteins were identified from the MS raw files using Mascot search engine (Matrix Science, London, UK; version 2.5.1). MS/MS spectra were searched against the C. innocuum database. All searches included carbamidomethyl cysteine as a fixed modification and oxidized Met, deamidated Asn and Gln, acetylated N-term as variable modifications. Three missed tryptic cleavages were allowed. The MS1 precursor mass tolerance was set to 10 ppm and the MS2 tolerance was set to 0.6 Da. The search result was visualized by Scaffold (version 4.9.0. Proteome Software, Inc., Portland, OR). A 1% false discovery rate cutoff was applied at the peptide level. Only proteins with a minimum of two peptides above the cutoff were considered for further study.
Sequence alignment
Protein sequences of CI_01447 and CI_01448 were acquired from our previously published whole genome sequence of LC-CI [16]. Using default parameters, a multispecies NCBI BLASTp (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins) was performed for CI_01447 and CI_01448. Top proteins alignments were then selected, and the multiple sequence alignment was generated using the identified locus of various organisms on Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) [22]. MUSCLE [23, 24] was used to perform multiple sequence alignments for CI_01447 and CI_01448 in our cohort of C. innocuum isolates. For sequence alignment of CI_01447 and CI_01448 to full-length glycosylating toxin TcdB from C. difficile (WP_009902069.1), Blastp alignment was performed using default parameters.
Cytotoxicity assays
HeLa cells provided by ATCC were seeded at 20,000 cells per well into 96-well polystyrene tissue culture plates (Corning, Corning, NY) in 200 μl Eagle’s minimum essential medium with phenol red, 10% fetal bovine serum, and 1% Penicillin-Streptomycin-Glutamine. HeLa cells were allowed to grow for 22 h, after which cells were rinsed with PBS, and sterile RPMI medium 1640 (Gibco Inc, Billings, MT) was added to each well. C. innocuum bacterial cultures were grown as described and cytotoxicity assay were performed as described with modifications [5]. For recombinant protein cytotoxicity assays, CI_01447 or CI_01448 at a final concentration of 100 μg/ml were added to each well. A PBS negative control was added to some wells to measure background lactate dehydrogenase (LDH) release, and 1% Triton X-100 in PBS was used as a positive control to measure total cell lysis. The plate was incubated at 37 °C under 5% CO2 for 24 h. Cytotoxicity was quantified by measuring LDH release using the Invitrogen™ CyQUANT™ LDH Cytotoxicity Assay (Thermo fisher Scientific, Waltham, MA) following the manufacturers protocol. After 24 h, supernatant was removed from the top of each well for measurement of LDH. Cytotoxicity was calculated as a percentage of total cell lysis (wells with Triton X-100 in PBS) as follows: % cytotoxicity = [(Sample OD490 – Sample OD680) – (PBS OD490 – PBS OD680)] / [(Triton X-100 OD490 – Triton X-100 OD680) – (PBS OD490 – PBS OD680)] × 100. Cytotoxicity assays were performed with at least two biological replicates, each with three technical replicates.
Cell rounding assays
HeLa cells were grown as described and seeded at 100,000 cells per well in 24-well polystyrene tissue culture plates (Corning, Corning, NY). After 22 h, medium was changed and a total of 100 ug/ml of CI_01448, CI_01447, 4 ug/ml of TcdA, or PBS were added to each well, and cells were incubated for 24 h. Representative images were acquired using FLoid Cell Imaging Station (Life Technologies Invitrogen, Carlsbad, CA) at 20X magnification. Experiments were performed with at least two biological replicates, each with three technical replicates.
Statistical analyses
All experiments were carried out in at least duplicate. Wilcoxon rank sum test was used to compare continuous data between two groups, and Kruskal-Wallis test followed by Dunn’s multiple comparisons test was used to compare continuous data among multiple groups. Two-sided p values < 0.05 were considered statistically significant. All statistical analyses were performed using the Prism9 software v9.0.2 (Graph Pad, La Jolla, CA).
RESULTS
C. innocuum cross-reactivity with C. difficile EIA
In total, 13 isolates (12 stool culture isolates and the ATCC 14501 reference strain) were identified as C. innocuum by MALDI-TOF and whole genome sequencing. Of the 13 C. innocuum isolates, culture supernatant from six (46%) were positive for toxin A/B by EIA, including both the C. innocuum reference strain and LC-CI isolate. Toxin EIA was repeated with a second commercial EIA from another manufacturer, and results were concordant except for one sample that was toxin A/B EIA-positive on the second EIA. All bacterial isolates were negative for the C. difficile toxin B gene by PCR. Thus, these results indicated that 7/13 (54%) C. innocuum isolates secreted or released detectable concentrations of the EIA cross-reactive factor (ErF).
Identification of ErF
To identify ErF, we selected two EIA-positive C. innocuum strains as candidates for additional experiments to identify ErF (Table 1), the ATCC 14501 reference strain [6, 15], and LC-CI isolated from an adult thought to have C. innocuum-associated diarrhea [16]. We first confirmed C. innocuum protein interaction with C. difficile anti-toxin A or anti-toxin B antibodies by performing western blots (Fig. 1) on total cell extracts from bacterial pellets and concentrated supernatants. In immunoblots probed with either anti-toxin A (Fig. 1A) or anti-toxin B (Fig. 1B), we identified an ~40 kDa protein band present in the supernatant but not the pellet of LC-CI and ATCC 14501.
Figure 1. Proteins present in C. innocuum bacterial supernatants can be identified by C. difficile-specific toxin antibodies.
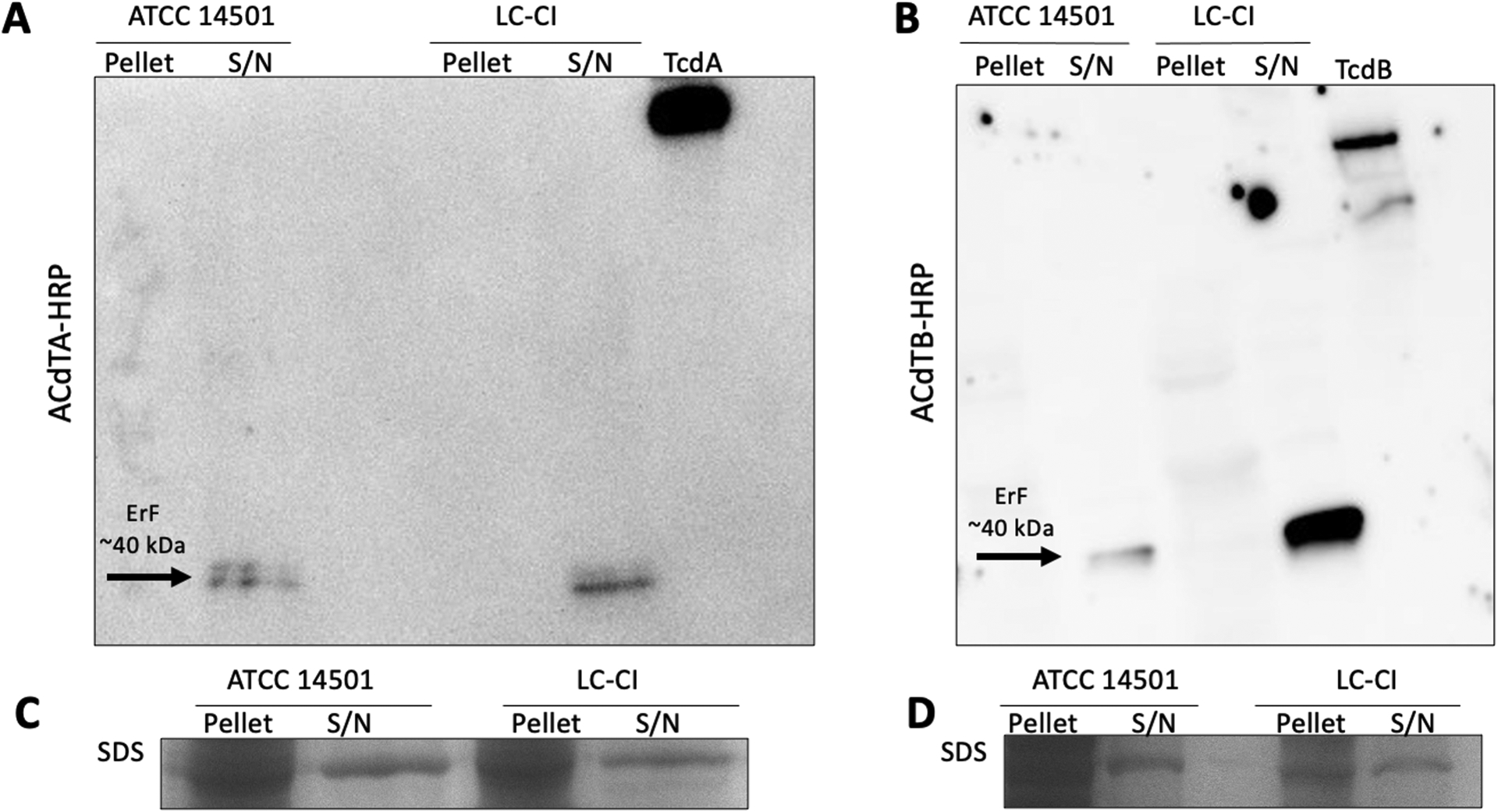
Representative images of immunoblots from bacterial pellets and supernatants (S/N) from C. innocuum LC-CI and ATCC 14501 probed with (A) C. difficile anti-toxin A antibody (ACdTA-HRP), and (B) C. difficile anti-toxin B antibody (ACdTB-HRP). (C-D) Coomassie stained SDS-gel were used as loading controls. All experiments were performed in at least biological duplicate; purified C. difficile TcdA (308 kDa) and TcdB (270 kDa) were used as positive controls. Arrows identify protein bands from C. innocuum that interact with C. difficile anti-toxin A and B antibodies at ~40 kDa. A total of 40 μg of protein from pellets and supernatants was loaded, and 1 ng of TcdA or TcdB was loaded.
We then extracted the corresponding band identified by western blot from a Coomassie stained SDS-PAGE gel and performed LC-MS/MS on the extracted band. Two distinct C. innocuum hypothetical proteins, CI_01447 (43 kDa) and CI_01448 (44 kDa), were identified from the supernatants of LC-CI and ATCC 14501(Fig. S1). Using our modified EIA protocol, we observed GHD−/Toxin+ EIA for recombinantly purified proteins. Using purified protein, we confirmed by western blot that CI_01447 and CI_01448 each interacted with anti-toxin A (Fig. 2A) and anti-toxin B (Fig. 2B) antibodies. Thus, these experiments confirmed the identity of ErF as both CI_01447 and CI_01448.
Figure 2. Western blots of purified CI_01447 and CI_01448 probed with anti-toxin A and B antibodies.
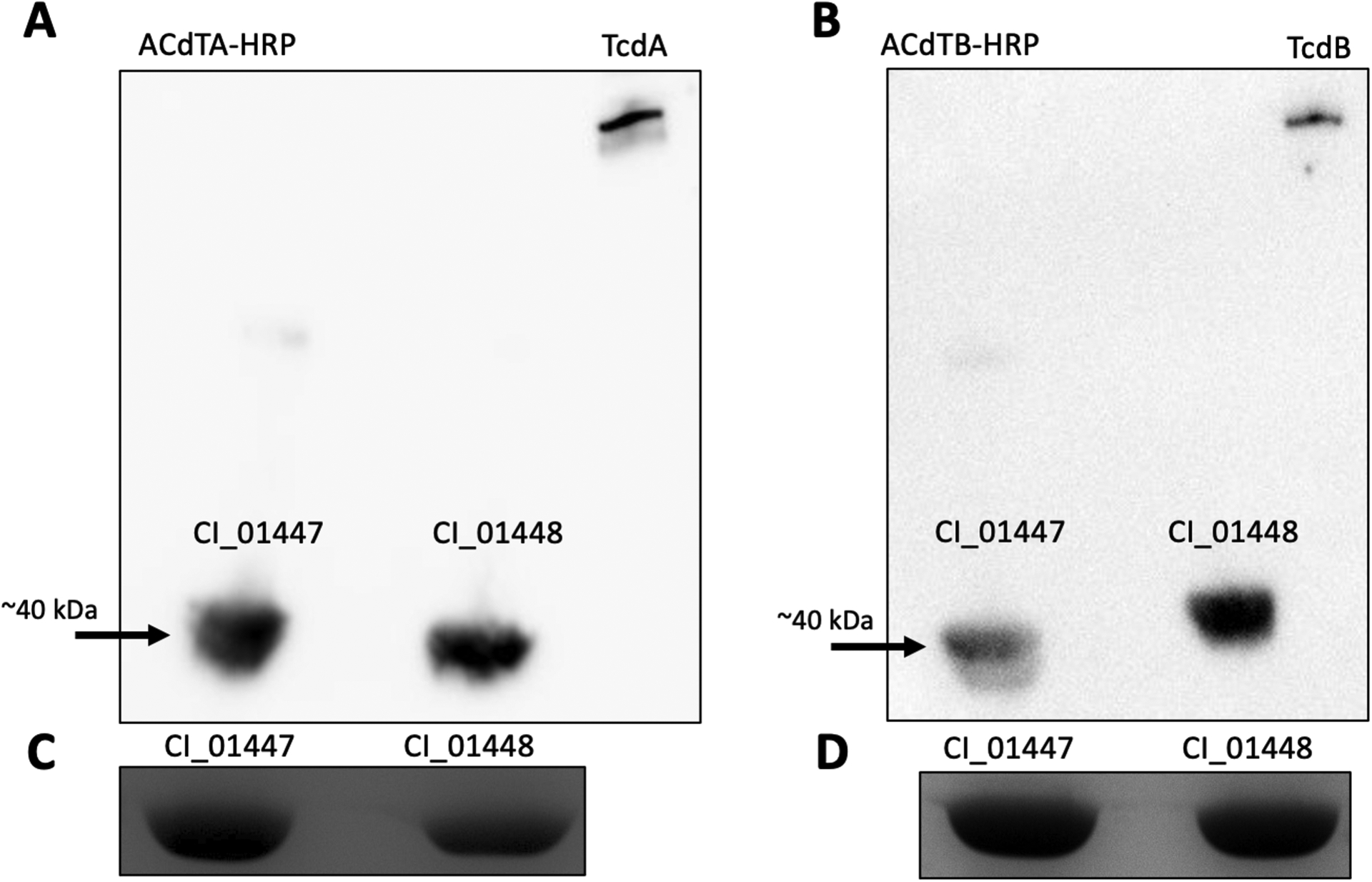
Representative immunoblots performed with purified CI_01447 and CI_01448 probed with (A) C. difficile anti-toxin A antibody (ACdTA-HRP), and (B) C. difficile anti-toxin B antibody (ACdTB-HRP). (C-D) Coomassie stained SDS-gel were used as loading controls. All experiments were performed in at least biological duplicate; purified C. difficile TcdA (308 kDa) and TcdB (270 kDa) were used as positive controls. Arrows identify protein bands interacting with C. difficile anti-toxin A and B antibodies at ~40 kDa. A total of 50 ng of recombinant C. innocuum proteins and 1 ng of TcdA or TcdB was loaded.
C. innocuum LC-CI, and proteins CI_01447 and CI_01447 are not cytotoxic to HeLa cells
With the suspicion that C. innocuum may cause a CDI-like gastrointestinal illness, and our observation that C. innocuum supernatant cross-reacted with C. difficile antitoxin antibodies in two commercial EIAs, we hypothesized that ErF may be a human toxin similar to C. difficile toxin A and/or B. We next asked if C. innocuum LC-CI was cytotoxic to human cells, and if CI_01447 and CI_01448 proteins acted similarly to C. difficile toxins A and B. Using LDH and cell rounding assays, we evaluated the cytotoxicity of LC-CI and recombinant proteins CI_01447 and CI_01448 to HeLa cells (Fig.3–4). HeLa cells exposed to C. innocuum LC-CI did not release LDH (Fig. 3A) or demonstrate cell rounding (Fig. 4A) indicative of cell intoxication as seen with C. difficile RT106/DH/ST-42. We observed similar results when HeLa cells were treated with recombinant CI_01447 or CI_01448 (Fig 3B, 4B). These data demonstrate that neither LC-CI nor CI_01447 or CI_01448 were cytotoxic to HeLa cells.
Figure 3. C. innocuum LC-CI and CI_01447 or CI_01447 cytotoxicity towards HeLa cells.
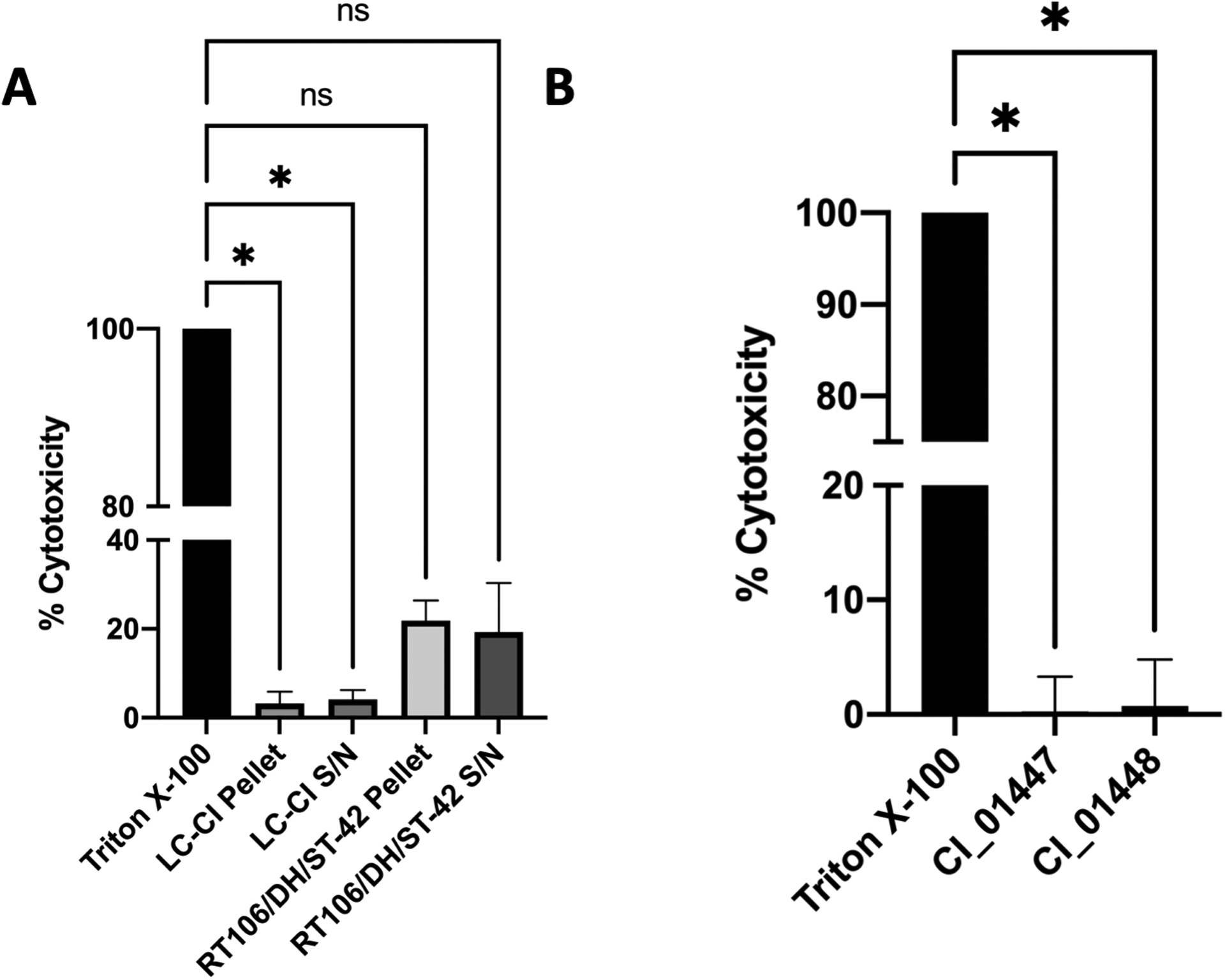
Lactate dehydrogenase release determined from HeLa cells incubated with (A) bacterial pellets or supernatant (S/N) from C. innocuum LC-CI or C. difficile RT106/DH/ST-42, or (B) recombinant protein CI_01447 and CI_01448. Percent cytotoxicity is relative to Triton X-100 positive control. Experiments were completed in at least duplicate with technical duplicates. Error bars indicate standard deviation. ns, not significant; *, p-value < 0.05.
Fig 4. Visual assessment of cell rounding with C. innocuum supernatants and purified recombinant CI_01447 or CI_01448 protein.
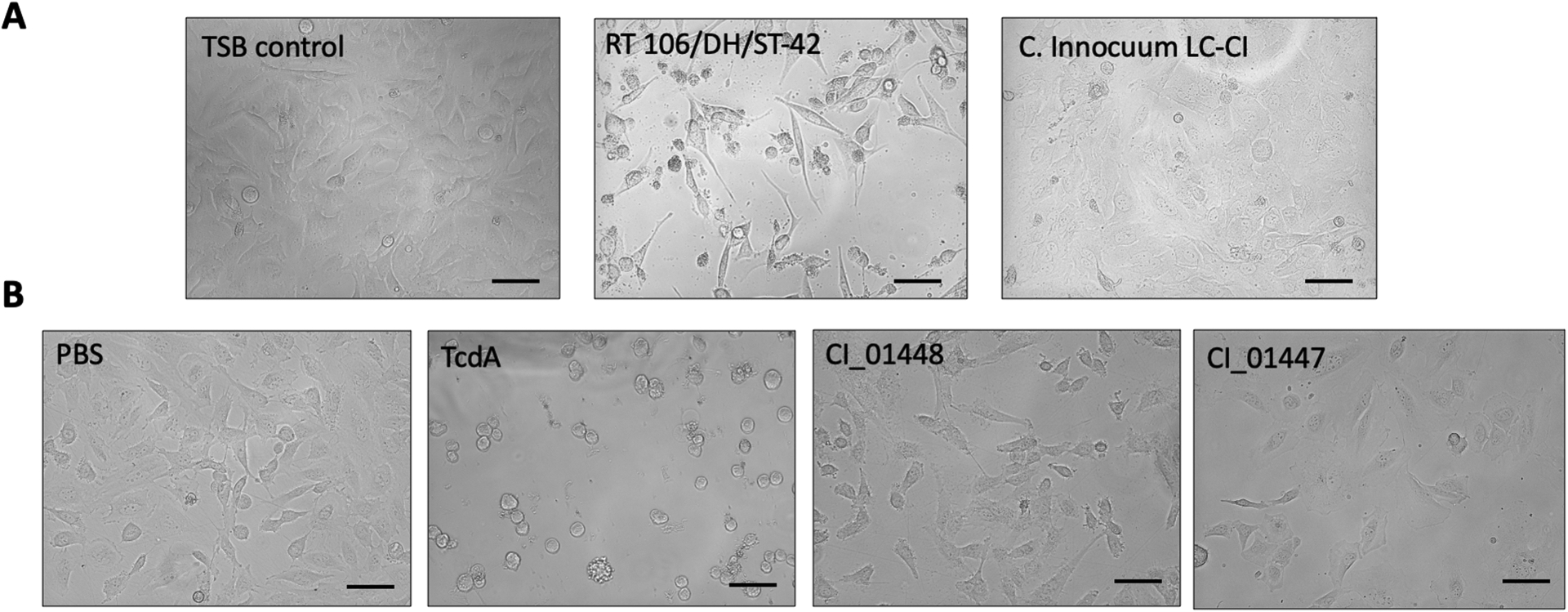
Representative images of HeLa cells treated with (A) supernatant (S/N) from C. innocuum LC-CI, C. difficile RT106/DH/ST-42 or (B) recombinant protein CI_01447 and CI_01448. C. difficile supernatant or TcdA was used as a positive control; PBS or TSB medium was used as a negative control. Each experiment was performed at least twice consisting of technical duplicates. Scale bars 100 μm.
CI_01447 and CI_01448 are similar to NlpC/P60 family of proteins
Using the amino acid sequences of CI_01447 and CI_01448, we generated sequence alignments of CI_01447 and CI-01448 to C. difficile TcdB to evaluate sequence similarity. We found CI_01447 and CI_01448 had limited sequence similarity (30% sequence identity) to TcdB (Fig. S2A–B). Furthermore, our proteins aligned to the combined repetitive oligopeptide (CROP) region of TcdB (Fig. S2C), which is the region responsible for C. difficile toxin binding to epithelial cells and is not responsible for cytotoxic activity [25–27].
Based on these results, we searched for proteins similar to CI_01447 (Fig. S3A) and CI_01448 (Fig. S3B) in other organisms. Of the 100 amino acid sequences, we identified 58 hits homologous to CI_01447 and 60 hits homologous to CI_01448 that belonged to the NlpC/P60 family of peptidases known to affect bacterial cell wall dynamics [28]. Homologs were identified in multiple Gram-positive bacteria, including C. innocuum, Erysipelotrichaceae bacterium, Longicatena, Amedibacterium intestinale, Amedibacillus dolichus, Absiella sp., Faecalitalea sp., Holdemanella sp, Faecalicoccus sp.,and Clostridium sp. Query coverage was between 150–450 amino acids in length with 30–100% percent sequence identity.
The Expasy PrositeScan [29] tool confirmed CI_01447 and CI_01448 each contained a NlpC/P60 domain and identified the possible residues responsible. For CI_01447, the suggested domain was from residues 273–397, with the possible catalytic residues identified as a triad of a cysteine (C) and two histidine (H) (C310, H371, H359). Similarly, NlpC/P60 domain for CI_01448 was identified as residues 295–414, with the catalytic triad of C325, H372, and H384. These alignments and analyses strongly suggested that CI_01447 and CI_01448 are NlpC/P60 proteins.
We next compared amino acid sequence alignments for CI_01447 and CI_01448 across the 13 C. innocuum isolates included in this study, all of which underwent whole genome sequencing (Fig S4). CI_01447 and CI_01448 were highly conserved across the C. innocuum isolates with percent identity scores of 99.8%−100% for CI_01447 (Fig S4A) and 99.3%−100% for CI_01448 (Fig S4B). The purposed catalytic residues of the C-H-H triad remained conserved in all the isolates. Further supporting that these proteins may have a more essential function and not associated with a specific genotype.
DISCUSSION
In this study, we aimed to identify the C. innocuum protein that cross reacts with C. difficile anti-toxin A and B antibodies. We conducted this study following the clinical observations by Chia and colleagues suggesting that C. innocuum was an emerging pathogen causing a CDI-like and potentially severe gastrointestinal illness [5]. We hypothesized that the proteins secreted by C. innocuum may be human toxins with the propensity to cause diarrhea. We identified two novel and previously undescribed proteins secreted by C. innocuum, annotated as CI_01447 and CI_01448. However, we failed to identify evidence of cytotoxicity to HeLa cells. Thus, the present study does not support that these cross-reacting proteins are toxins and/or responsible for human disease.
Prior to the 2018 publication [5], C. innocuum had been primarily characterized as an intestinal commensal and rare opportunistic pathogen in immunocompromised and/or medically complex patients [7]. Beyond these clinical studies, C. innocuum has been poorly described in the literature and no other studies have investigated C. innocuum as a potential gastrointestinal pathogen. A limitation of our study is that we are unable to definitively determine if, and to what extent, C. innocuum contributed to patient illness. It is possible that alternate infectious or non-infectious diarrheal etiologies were present, and C. innocuum was merely colonizing the gastrointestinal tract. Additional studies are needed to validate Chia and colleagues’ initial observations and better estimate the pathogenicity of C. innocuum in the general population. Moreover, if pathogenicity is confirmed in the general population, additional studies are needed to identify and characterize C. innocuum virulence factors.
Despite the interactions of CI_01447 and CI_01448 with the C. difficile EIAs and toxin A and B antibodies, initially suggesting similarity to C. difficile TcdA and TcdB, we found that CI_01447 and CI_01448 were not cytotoxic to HeLa cells. Amino acid sequence alignment between CI_01447 and CI_01448 and C. difficile TcdB demonstrated low sequence similarity that was limited to the binding region (CROP) of TcdB, a domain not responsible for cytotoxicity activity [25–27]. Our experiments confirmed that CI_01447 and CI_01448 were not toxins as indicated through LDH release or cell rounding.
To further elucidate the possible function of CI_01447 and CI_01448, amino acid sequence alignments were performed and revealed high sequence similarity, up to 100% identity, to several NlpC/P60 proteins across several genera of Gram-positive bacteria. CI_01447 and CI_01448 were found ubiquitously in our small cohort of C. innocuum patient isolates irrespective of clinical status, including those isolates without C. difficile EIA cross-reactivity, suggesting either differential expression/secretion of the proteins or limitations of the assay. These data suggest that CI_01447 and CI_01448 may have an alternative role for the as part of the NlpC/P60 family of proteins. The NlpC/P60 family of proteins have been shown to be essential to bacterial proliferation as they play various roles in peptidoglycan remodeling [28, 30, 31]. Additional analyses of CI_01447 and CI_01448 are required to determine its activity.
It is important to note that the two commercial EIAs used in this study were performed using bacterial isolates, not stool samples. Therefore, the assays were not used according to manufacturer’s recommendations. We have additionally performed these EIAs on patient stools that were culture-positive for C. innocuum but both PCR- and culture-negative for C. difficile (unpublished data). We have not identified false positive EIA test results using these stool samples. Therefore, the data presented here should not be misinterpreted as failure of these test to adequate assess C. difficile infection. Further, because the specific antibodies used in these EIAs are proprietary, we were unable to determine the specific binding domains responsible for the observed cross-reactivity. Notably, not all isolate supernatant was positive by EIA despite CI_01447 and CI_01448 being present in and highly conserved among all 13 study isolates. It is unclear if this is related to EIA sensitivity for these particular cross-reactive proteins or differences in protein expression. Because of the lack of cytotoxicity of these proteins, this finding was not explored further.
In summary, we aimed to identify and assess the toxigenicity of proteins that are secreted by the potentially emerging pathogen C. innocuum and cross-react with EIAs designed to specifically recognize C. difficile toxins A and/or B. We have identified two cross-reactive proteins in C. innocuum, CI_01447 and CI_01448. These proteins lacked cytotoxicity to HeLa cells. They additionally shared sequence similarity with the NlpC/P60 protein family containing catalytic cysteine residues. Further investigation is needed to confirm and evaluate CI_01447 and CI_01448 as NlpC/P60 proteins, as well as confirming pathogenicity of C. innocuum as a gastrointestinal pathogen.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant R21 AI144549 to L.K.K. and T32 916225 to K.E.C.). The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Proteomics services were performed by the Northwestern Proteomics Core Facility, generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center, instrumentation award (S10OD025194) from NIH Office of Director, and the National Resource for Translational and Developmental Proteomics supported by P41 GM108569. Recombinant protein was provided by the Center for Structural Genomics of Infectious Diseases (NIAID contract no. HHSN272201700060C to K.J.F.S).
References
- [1].McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol 3 (2008) 563–78. [DOI] [PubMed] [Google Scholar]
- [2].McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis 16 (1998) 292–307. [DOI] [PubMed] [Google Scholar]
- [3].Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis 18 Suppl 4 (1994) S265–72. [DOI] [PubMed] [Google Scholar]
- [4].Kociolek LK. Strategies for Optimizing the Diagnostic Predictive Value of Clostridium difficile Molecular Diagnostics. J Clin Microbiol 55 (2017) 1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chia JH, Wu TS, Wu TL, Chen CL, Chuang CH, Su LH, et al. Clostridium innocuum is a vancomycin-resistant pathogen that may cause antibiotic-associated diarrhoea. Clinical Microbiology and Infection 24 (2018) 1195–9. [DOI] [PubMed] [Google Scholar]
- [6].Smith LD, King E. Clostridium innocuum, sp. n., a sporeforming anaerobe isolated from human infections. J Bacteriol 83 (1962) 938–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cherny KE, Muscat EB, Reyna ME, Kociolek LK. Clostridium innocuum: Microbiological and clinical characteristics of a potential emerging pathogen. Anaerobe 71 (2021) 102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chia JH, Feng Y, Su LH, Wu TL, Chen CL, Liang YH, et al. Clostridium innocuum is a significant vancomycin-resistant pathogen for extraintestinal clostridial infection. Clin Microbiol Infect 23 (2017) 560–6. [DOI] [PubMed] [Google Scholar]
- [9].Cherny KE. Characterization of structure and function of a Clostridium innocuum hypothetical protein that is cross-reactive with C. difficile anti-toxin antibodies. 12th International Conference on the Molecular Biology and Pathogenesis of the Clostridia (Clostpath 2021) Virtual; 2021. [Google Scholar]
- [10].Burnham CA, Carroll KC. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev 26 (2013) 604–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Montecalvo MA, Sisay E, McKenna D, Wang G, Visintainer P, Wormser GP. Use of a Perianal Swab Compared With a Stool Sample to Detect Symptomatic Clostridium difficile Infection. Infect Control Hosp Epidemiol 38 (2017) 658–62. [DOI] [PubMed] [Google Scholar]
- [12].Kociolek LK, Patel SJ, Shulman ST, Gerding D. Molecular epidemiology of Clostridium difficile infections in children: A retrospective cohort study. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America 36 (2015) 445–51. [DOI] [PubMed] [Google Scholar]
- [13].Balaji A, Ozer EA, Kociolek LK. Clostridioides difficile Whole-Genome Sequencing Reveals Limited Within-Host Genetic Diversity in a Pediatric Cohort. Journal of clinical microbiology 57 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Balaji A, Espinosa R, Balmert LC, Todd K, Steed K, Kociolek LK. Performance of toxin enzyme immunoassays and PCR cycle threshold for differentiating Clostridium difficile infection from colonization in children with diarrhea. ID Week. San Francisco, CA; 2018. [Google Scholar]
- [15].Cherny KE, Ozer EA, Kochan TJ, Kociolek LK. Complete Genome Sequence of Clostridium innocuum Strain ATCC 14501. Microbiology Resource Announcements 9 (2020) e00452–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cherny KE, Ozer EA, Kochan TJ, Johnson S, Kociolek LK, Rasko D. Complete Genome Sequence of Clostridium innocuum Strain LC-LUMC-CI-001, Isolated from a Patient with Recurrent Antibiotic-Associated Diarrhea. Microbiology Resource Announcements 9 (2020) e00365–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kociolek LK, Gerding DN, Espinosa RO, Patel SJ, Shulman ST, Ozer EA. Clostridium difficile Whole Genome Sequencing Reveals Limited Transmission Among Symptomatic Children: A Single-Center Analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 67 (2018) 229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eschenfeldt WH, Makowska-Grzyska M, Stols L, Donnelly MI, Jedrzejczak R, Joachimiak A. New LIC vectors for production of proteins from genes containing rare codons. J Struct Funct Genomics 14 (2013) 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kwon K, Peterson SN. High-throughput cloning for biophysical applications. Methods Mol Biol 1140 (2014) 61–74. [DOI] [PubMed] [Google Scholar]
- [20].Millard CS, Stols L, Quartey P, Kim Y, Dementieva I, Donnelly MI. A less laborious approach to the high-throughput production of recombinant proteins in Escherichia coli using 2-liter plastic bottles. Protein Expr Purif 29 (2003) 311–20. [DOI] [PubMed] [Google Scholar]
- [21].Shuvalova L. Parallel protein purification. Methods Mol Biol 1140 (2014) 137–43. [DOI] [PubMed] [Google Scholar]
- [22].Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic acids research 47 (2019) W636–W41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5 (2004) 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research 32 (2004) 1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gupta P, Zhang Z, Sugiman-Marangos SN, Tam J, Raman S, Julien JP, et al. Functional defects in Clostridium difficile TcdB toxin uptake identify CSPG4 receptor-binding determinants. J Biol Chem 292 (2017) 17290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Orrell KE, Mansfield MJ, Doxey AC, Melnyk RA. The C. difficile toxin B membrane translocation machinery is an evolutionarily conserved protein delivery apparatus. Nature Communications 11 (2020) 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen P, Lam KH, Liu Z, Mindlin FA, Chen B, Gutierrez CB, et al. Structure of the full-length Clostridium difficile toxin B. Nat Struct Mol Biol 26 (2019) 712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Anantharaman V, Aravind L. Evolutionary history, structural features and biochemical diversity of the NlpC/P60 superfamily of enzymes. Genome Biol 4 (2003) R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Castro E, Sigrist CJA, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, et al. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Research 34 (2006) W362–W5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hess J, Gentschev I, Szalay G, Ladel C, Bubert A, Goebel W, et al. Listeria monocytogenes p60 supports host cell invasion by and in vivo survival of attenuated Salmonella typhimurium. Infect Immun 63 (1995) 2047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ishikawa S, Hara Y, Ohnishi R, Sekiguchi J. Regulation of a new cell wall hydrolase gene, cwlF, which affects cell separation in Bacillus subtilis. J Bacteriol 180 (1998) 2549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ozer EA, Hauser AR, Gerding DN, Espinosa RO, Hecht DW, Kociolek LK. Complete Genome Sequence of Clostridioids difficile Epidemic Strain DH/NAP11/106/ST-42, Isolated from Stool from a Pediatric Patient with Diarrhea. Genome Announcements 5 (2017) e00923–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


