Abstract
Introduction
We analyzed the exclusion of pregnant and breastfeeding individuals and those capable of pregnancy in COVID-19 vaccine and clinical treatment trials.
Methods
Inclusion and exclusion criteria were extracted from all listed COVID-19 vaccine and treatment clinical trials from May 1, 2020, to October 31, 2020, using the U.S. National Library of Medicine database. We report rates of rates of exclusion for pregnant and lactating individuals and requirements for contraception for pregnancy-capable participants in COVID-19 vaccine and treatment clinical trials. The analysis included the association between clinical trial exclusion and vaccine and treatment type, study location, sponsor, and phase.
Results
Pregnant and lactating individuals were explicitly excluded from most COVID-19 vaccine and treatment clinical trials. Of the 90 vaccine trials, 88 (97.8%) excluded pregnant individuals, 73 (81.1%) excluded lactating individuals, and 56 (62.2%) required contraception use. Of the 495 treatment trials, 350 (70.7%) excluded pregnant individuals, 269 (54.3%) excluded lactating individuals, and 91 (18.4%) required contraception use. Although vaccine type was not associated with pregnancy exclusion, it was associated with lactation exclusion (p = .01) and contraception requirement (p < .001). Treatment type was associated with pregnancy exclusion, lactation exclusion, and contraception requirement (all p < .001).
Conclusions
COVID-19 vaccination and treatment clinical trials mirrored historical trends restricting participation owing to pregnancy, lactation, and contraception nonuse, despite known safety profiles. People of childbearing potential should be considered for and afforded the same opportunity as males to make informed decisions on study participation, particularly in the setting of a global pandemic.
The World Health Organization declared the SARS-CoV-2 outbreak a global pandemic on March 11, 2020. Failure to contain the virus caused rapid spread to almost 76 million individuals worldwide by December 22, 2020, and initiated a surge of clinical trials to find safe and effective prevention and treatment (WHO Coronavirus Disease [COVID-19] Dashboard, 2020). This process is especially important for those at an increased risk for contracting COVID-19 and those with predispositions to more severe illness, such as immunocompromised individuals, those with preexisting heart or lung disease, the elderly, and pregnant people (People with Certain Medical Conditions, 2022). COVID-19 during pregnancy requires special attention; current data suggest an increased risk of adverse outcomes for both the birthing person and infant, including preterm birth, stillbirth, and increased risk of pregnancy complications (Ellington et al., 2020; Yang et al., 2020).
Pregnant and lactating individuals have been historically excluded from clinical trials, even in the absence of scientific evidence of harmful side effects to the pregnant person or teratogenicity in the developing fetus secondary to the intervention. Reasons for exclusion include the labeling of pregnant people as a vulnerable population, the restrictive interpretations of language of federal regulations, the physiologic complexity of pregnancy warranting additional institutional review board requirements, the potential for increased financial burden, and the fear of legal liability (Blehar et al., 2013). Altogether, representation of reproductive-aged women in clinical trials has been impeded. The exclusion of this population limits our understanding of interventions used in the prevention and treatment of pregnant and lactating individuals as well as the outcomes of pregnancy-specific conditions for women and their offspring (Blehar et al., 2013). In accordance with regulations set by the U.S. Department of Department of Health and Human Services, pregnant and lactating humans should be granted the autonomy to consent for participation in a clinical trial when the anticipated benefits outweigh the risks and safety data in pregnant and lactating animals and nonpregnant people has been established (Subpart B—Additional Protections for Pregnant Women, Human Fetuses and Neonates Involved in Research, 2001).
Excluding pregnant and lactating people in prevention and treatment clinical trials leads to inequities in health care during pregnancy. Without evidence-based data, these individuals are forced to make medical decisions affecting both themselves and their fetus while lacking the clear recommendations that most of the population has. Thus, the lack of clinical data regarding the safety and efficacy of these interventions during pregnancy prevents pregnant and lactating people from deriving the same benefits from clinical research that the general population does.
This study's purpose was to quantify the exclusion of pregnant, lactating, and pregnancy-capable individuals from COVID-19 clinical trials based on pregnancy, lactation, and nonuse of contraception. We investigated the potential impacts of geographic location and sponsor for each clinical trial, hypothesizing higher exclusion rates in trials occurring in the United States and those sponsored by public entities. Additionally, we anticipated lower rates of exclusion in advanced phases of clinical trials.
Methods
We conducted a comprehensive search of all COVID-19 vaccination and treatment trials from May 1, 2020, through October 31, 2020, using the U.S. National Library of Medicine database (Clinicaltrials.gov). For vaccines, we refined our search by entering “COVID-19” under “condition” and “vaccine” under “other terms.” We categorized each registered clinical trial into the following vaccine subtypes: nucleic acid (RNA, DNA), inactivated (protein/peptide subunit, inactivated SARS-CoV-2 antigen/virion, inactivated plasma), live-attenuated (adenovirus vector, MF59 adjuvant, VSV), and vaccines repurposed from their original use for the treatment of COVID-19 (BCG, MMR, mycobacterium). For other treatments, our search was modified by entering the name of each treatment in “other terms.” Although there are a multitude of treatment clinical trials being conducted, we narrowed our search based on the National Institutes of Health COVID-19 Treatment Guidelines at the time of our data extraction (Coronavirus Disease 2019 [COVID-19] Treatment Guidelines, 2020). The treatments were classified into antiviral therapies (remdesivir, hydroxychloroquine with or without azithromycin, HIV protease inhibitors) or immune-based therapies, which were subsequently categorized into blood-derived products (mesenchymal stem cells, convalescent plasma) and immunomodulators (monoclonal antibodies, corticosteroids, interferons).
For each clinical trial, we extracted data regarding the sponsor (pharmaceutical company vs. nonpharmaceutical organization), location (United States vs. international), and study phase. We further examined the inclusion and exclusion criteria for each study to determine whether pregnant and breastfeeding people were included and whether the study had a contraception requirement for participants.
Trials in which pregnancy-capable individuals would be ineligible for participation, such as studies for the elderly or those exclusively studying males, were excluded a priori.
Exact χ2 tests were used to assess the association of vaccine type, initiating party, location, and phase with pregnancy exclusion, lactation exclusion, and contraception requirement status. Trials with locations in both U.S. and international settings were removed from analysis, because our goal was to compare U.S. studies with those conducted in other countries. Similar associations were assessed for treatment trials via χ2 tests.
Institutional review board approval was not indicated for the initiation of this study because the data extracted from Clinicaltrials.gov are available publicly.
Results
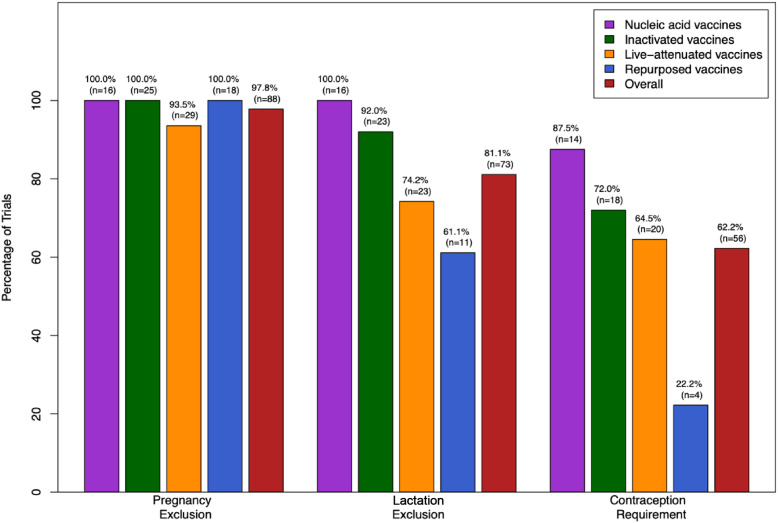
A review of COVID-19 vaccine candidates revealed a notable pattern of exclusion. Of the 90 vaccine clinical trials, the overwhelming majority excluded pregnant and lactating people from participation (Figure 1 ). Furthermore, 62.2% of these trials required the use of a reliable form of contraception for pregnancy-capable individuals while participating in the study (Figure 1).
Figure 1.
Exclusion of pregnant and lactating individuals, and the contraception use requirements for women of childbearing potential, in COVID-19 vaccine clinical trials by vaccine type.
When differentiating between vaccine subtypes, pregnant individuals were excluded from 100.0% of nucleic acid, inactivated, and repurposed vaccine trials and 93.5% of live-attenuated vaccine trials (Figure 1). There was no association between pregnancy exclusion status and vaccine type. Lactating people were excluded from 100.0% of nucleic acid vaccine clinical trials, 92.0% of inactivated vaccine clinical trials, 74.2% of live-attenuated vaccine clinical trials, and 61.1% of repurposed vaccine clinical trials (Figure 1). Vaccine type was significantly associated with rates of lactation exclusion (p = .01). Specifically, nucleic acid vaccine trials were more likely to exclude breastfeeding individuals compared with live-attenuated vaccine trials (p = .03) and repurposed vaccine trials (p = .01). Additionally, inactivated vaccine trials were more likely to exclude breastfeeding individuals compared with repurposed vaccine trials (p = .04). Contraception use was required in 87.5% of nucleic acid vaccine trials, 72.0% of inactivated vaccine trials, 64.5% of live-attenuated vaccine trials, and 22.2% of repurposed vaccine trials (Figure 1). Vaccine type demonstrated an association with the presence of contraception requirements for study participation in reproductive-aged individuals (p < .001). Specifically, trials studying nucleic acid, inactivated, or live-attenuated vaccines were more likely to require contraception than trials studying repurposed vaccines (p < .001, p = .003, and p = .01, respectively).
Table 1 reports vaccine exclusion patterns based on the sponsor type, geographical location, and trial phase. There was no association between the exclusion of pregnant and lactating individuals and the sponsor. However, pharmaceutical company-initiated trials were significantly more likely to require contraception use for people of childbearing potential. Neither the location nor the phase of the vaccine clinical trial were significantly associated with the exclusion of pregnant or lactating individuals or contraception requirements.
Table 1.
Clinical Trials for COVID-19 Vaccine Options: Pregnancy, Lactation, and Contraception Requirement Criteria by Initiating Party, Location, and Phase (n = 90)
| Pregnancy Exclusion |
Lactation Exclusion |
Contraception Requirement |
||||
|---|---|---|---|---|---|---|
| n (%) | p Value | n (%) | p Value | n (%) | p Value | |
| Sponsor | ||||||
| Nonpharmaceutical | 42 (100.0) | .50 | 32 (76.2) | .29 | 21 (50.0) | .03 |
| Pharmaceutical | 46 (95.8) | 41 (85.4) | 35 (72.9) | |||
| Location∗ | ||||||
| U.S. | 11 (100.0) | .99 | 9 (81.8) | .99 | 8 (72.7) | .53 |
| International | 70 (98.6) | 60 (84.5) | 44 (62.0) | |||
| Phase† | ||||||
| 1 | 27 (100.0) | .79 | 24 (88.9) | .36 | 21 (77.8) | .16 |
| 1/2 | 22 (95.7) | 18 (78.3) | 15 (65.2) | |||
| 2 | 5 (100.0) | 5 (100.0) | 3 (60.0) | |||
| 2/3 | 2 (100.0) | 2 (100.0) | 1 (50.0) | |||
| 3 | 30 (96.8) | 23 (74.2) | 16 (51.6) | |||
| 4 | 2 (100.0) | 1 (50.0) | 0 (0.0) | |||
Trials that included both U.S. and international locations were excluded for this analysis (n = 8).
The p value represents overall χ2 test.
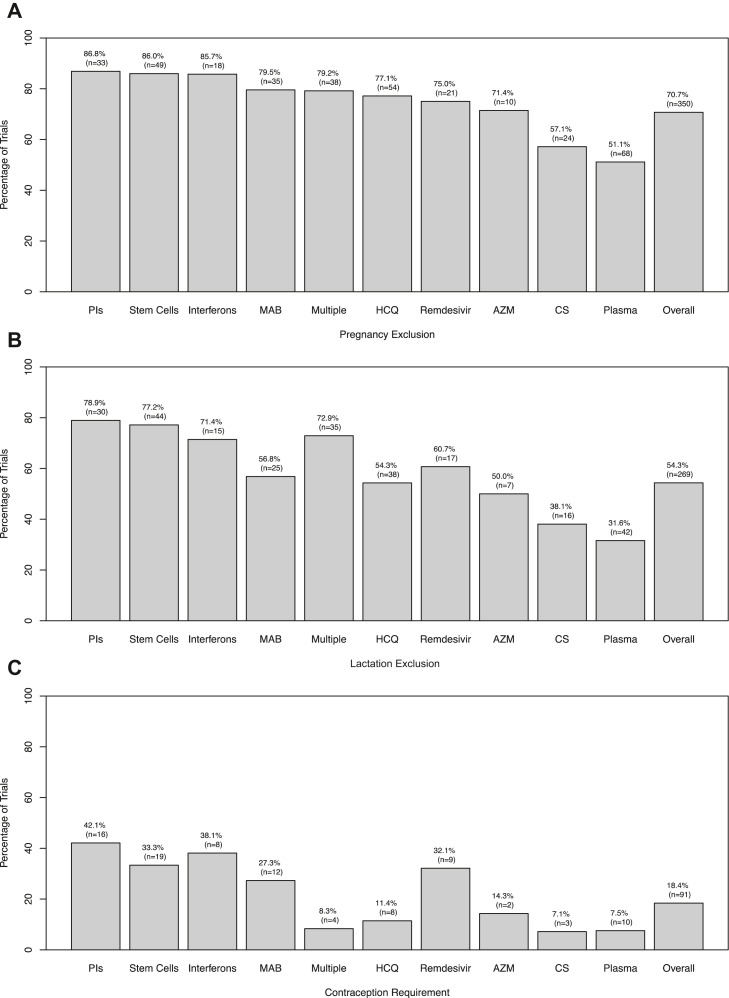
A review of 495 COVID-19 therapeutic treatment trials revealed patterns of exclusion. Overall, 70.7% of trials excluded pregnant individuals and 54.3% excluded lactating individuals; 18.4% of trials had additional regulations requiring contraceptive use for reproductive-aged participants with childbearing potential (Figure 2 ).
Figure 2.
Percent of COVID-19 clinical trials with pregnancy exclusion (A), lactation exclusion (B), and contraception use requirements (C) by treatment type. Abbreviations: AZM, azithromycin; CS, corticosteroids; HCQ, hydroxychloroquine; MAB, monoclonal antibodies; PIs, protease inhibitors.
Pregnancy and lactation exclusions and the presence of contraception requirements were associated with the type of treatment (p < .001 for all three associations). Trials for corticosteroids and plasma were least likely to exclude pregnant (Figure 2A) and breastfeeding (Figure 2B) individuals. Similarly, the lowest percentages of contraception requirements were observed in corticosteroid and plasma-based trials, although studies of hydroxychloroquine, azithromycin, and multiple treatment regimens had contraception requirements in less than 15% of all trials (Figure 2C). Studies for both remdesivir and interferons excluded pregnant individuals in 75.0% and 85.7% of trials, respectively, and breastfeeding individuals in 60.7% and 71.4% of trials, respectively (Figure 2).
There was no significant association between pregnancy and breastfeeding exclusion in COVID-19 treatment trials and the sponsor initiating the study (pharmaceutical vs. nonpharmaceutical). However, pharmaceutical-sponsored trials were more likely to require contraception use (Table 2 ). The location of the trial (U.S. or international) had no observed association with pregnancy and breastfeeding exclusion status or requirements for contraception. Finally, there was an association between treatment clinical trial phase, pregnancy and lactation exclusion status, and contraception requirement (Table 2).
Table 2.
Clinical Trials for COVID-19 Treatment Options: Pregnancy, Lactation, and Contraception Requirement Criteria by Sponsor, Location, and Phase (n = 495)
| Pregnancy Exclusion |
Lactation Exclusion |
Contraception Requirement |
||||
|---|---|---|---|---|---|---|
| n (%) | p Value | n (%) | p Value | n (%) | p Value | |
| Sponsor | ||||||
| Nonpharmaceutical | 299 (69.4) | .09 | 228 (52.9) | .09 | 66 (15.3) | <.001 |
| Pharmaceutical | 51 (79.7) | 41 (64.1) | 25 (39.1) | |||
| Location∗ | ||||||
| U.S. | 98 (68.1) | .29 | 87 (60.4) | .12 | 30 (20.8) | .25 |
| International | 239 (72.9) | 173 (52.7) | 54 (16.5) | |||
| Phase† | ||||||
| 1 | 40 (71.4) | <.001 | 34 (60.7) | <.001 | 11 (19.6) | .02 |
| 1/2 | 31 (81.6) | 27 (71.1) | 12 (31.6) | |||
| 2 | 126 (75.9) | 104 (62.7) | 36 (21.7) | |||
| 2/3 | 39 (72.2) | 27 (50.0) | 9 (16.7) | |||
| 3 | 72 (69.9) | 49 (47.6) | 18 (17.5) | |||
| 4 | 22 (73.3) | 11 (36.7) | 4 (13.3) | |||
| N/A‡ | 20 (41.7) | 17 (35.4) | 1 (2.1) | |||
Trials that included both U.S. and international locations were excluded for this analysis (n = 23).
The p value represents the overall χ2 test.
Trials without phases.
Discussion
Despite evidence suggesting that pregnant individuals are at an increased risk of adverse outcomes from COVID-19, they were excluded from nearly all vaccine trials and a majority of treatment trials at the time of our data extraction (Ellington et al., 2020). These results emphasize the systematic exclusion of pregnant women and other individuals capable of pregnancy from COVID-19 clinical research. Smith et al. demonstrated that only 1.7% of COVID-19 treatment and vaccine clinical research included pregnant women in April 2020, a pattern demonstrated in previous studies (Shields & Lyerly, 2013; Smith et al., 2020; Taylor et al., 2021).
This study advanced our understanding of pregnancy exclusion patterns in COVID-19 clinical trials by studying its association with vaccine and treatment type, phase of trial, location, and sponsor. Despite the known safety profiles for each vaccine, there was no association between vaccine type and rates of pregnancy exclusion. Live-attenuated vaccines are usually not advised during pregnancy owing to the theoretical risk of vertical transmission, although studies on live-attenuated vaccines for rubella, measles, mumps, polio, and yellow fever administered during pregnancy have not found elevated risks of adverse outcomes (Global Advisory Committee on Vaccine Safety, 2014). However, inactivated and toxoid-based vaccines are not associated with an increased risk of fetal or maternal harm (Global Advisory Committee on Vaccine Safety, 2014). In fact, after studies were conducted on vaccines during pregnancy, influenza and Tdap vaccines are recommended in pregnant individuals (Munoz et al., 2015; Global Advisory on Vaccine Safety, 2014; Vaccinating Pregnant Women Protects Moms and Babies, 2019). The higher rates of exclusion in nucleic acid vaccine trials compared with live-attenuated vaccine trials demonstrate that study inclusion and exclusion criteria are not always based entirely on evidence-based reasoning.
Although the exclusion of lactating people was associated with vaccine type, this exclusion pattern is not supported by scientific evidence. According to the Centers for Disease Control and Prevention, neither live-attenuated nor inactivated vaccines pose any risk to a lactating mother or neonate (Vaccination Safety for Breastfeeding Mothers, 2021). Although live-attenuated viruses may replicate inside the maternal host, the virus has not been found in breast milk (Vaccination Safety for Breastfeeding Mothers, 2021). Furthermore, the exclusion of pregnant and lactating individuals from vaccine clinical trials was not associated with trial phase (Table 1). According to the U.S. Food and Drug Administration, it is recommended to include pregnant and lactating individuals during phase III clinical trials to improve our understanding of the impact on this patient population (Development and Licensure of Vaccines to Prevent COVID-19, 2020; Stewart et al., 2016). Additionally, U.S. Food and Drug Administration guidance specific to COVID-19 has explicitly encouraged the inclusion of pregnant and lactating individuals in these studies owing to the increased risk of severe symptoms and preterm birth (COVID 19: Developing Drugs and Biological Products for Treatment or Prevention, 2021). However, the Pfizer and Moderna COVID-19 mRNA vaccine trials excluded pregnant and lactating individuals with no biological evidence, suggesting their vaccines were harmful to fetuses or that they were transmitted in breastmilk (Van Spall, 2021). This highlights a disconnect between U.S. Department of Health and Human Services guidelines for safe research participation of pregnant and lactating individuals and the reality of clinical research during the COVID-19 pandemic. Prospective cohort studies have since compared the COVID-19 vaccine-induced antibodies in both pregnant and nonpregnant patients and concluded that vaccination generates robust immunity in pregnant and lactating individuals, with evidence of immune transfer to neonates via placenta (Gray et al., 2021). The Centers for Disease Control and Prevention now recommend COVID-19 vaccination for people who are pregnant, breastfeeding, trying to get pregnant now, or who might become pregnant in the near future (COVID-19 Vaccines while Pregnant or Breastfeeding, 2022). Correspondingly, the American College of Obstetricians and Gynecologists (ACOG) and Society for Maternal-Fetal Medicine enthusiastically encourage vaccination of pregnant individuals (ACOG and SMFM Recommend COVID-19 Vaccination for Pregnant Individuals, 2021). The inappropriate exclusion of this population in clinical trials may have resulted in preventable negative health implications for those infected by COVID-19 after vaccine availability.
Many clinical trials’ inclusion criteria required “adequate” or “effective” contraception in those capable of pregnancy, but often did not define those terms. Importantly, pharmaceutical-initiated trials for both vaccines and treatments were associated with an increased likelihood of contraception requirements. There are no current guidelines on specific birth control requirements during clinical trials, and there are documented disadvantages of contraception requirements, including reduced reproductive control and side effects (Sullivan et al., 2019). This inclusion criteria may serve as a potential barrier to study participation for women, highlighting the need for an individualized approach to inclusion criteria specific to the experimental agent. Additionally, research indicates that Black, Indigenous, and people of color (BIPOC) populations and those facing economic hardship have faced greater barriers to contraceptive care during the pandemic, in addition to experiencing higher COVID-19 infection and death rates (Diamond-Smith et al., 2021). Requiring the use of effective contraception at a time when BIPOC people are disproportionately affected by barriers to contraceptive care risks exacerbating existing racial and ethnic disparities in clinical trial participation and COVID-19 outcomes (Global Participation in Clinical Trials Report, 2017). The exclusion of these crucial research populations limits our knowledge of potential side effects on a subsequent pregnancy.
Although treatment with protease inhibitors, monoclonal antibodies, and stem cell therapies had inconclusive pregnancy safety recommendations, evidence suggests there are no adverse outcomes with breastfeeding (Antenatal Corticosteroid Therapy for Fetal Maturation, 2017; Development and Licensure of Vaccines to Prevent COVID-19, 2020; Smith et al., 2020; Stewart et al., 2016). Many of the COVID-19 treatments being studied are recommended for use in pregnant and breastfeeding individuals. The ACOG endorses the use of both glucocorticoids and hydroxychloroquine in pregnancy and breastfeeding clinical guidelines for other diseases (ACOG Committee Opinion No. 776: Immune Modulating Therapies in Pregnancy and Lactation, 2019). Azithromycin is a common antibiotic used during pregnancy (Antenatal Corticosteroid Therapy for Fetal Maturation, 2017). Remdesivir and interferons have widespread use during pregnancy (Taylor et al., 2021). Last, pregnancy is not a contraindication to blood component transfusion (Grisolia et al., 2020). Despite this evidence, pregnant and lactating individuals faced stringent exclusion from hydroxychloroquine, glucocorticoids, azithromycin, remdesivir, interferon, and convalescent plasma clinical trials. These findings demonstrate systematic exclusion patterns among pregnant, lactating, and reproductive-aged individuals regardless of location, sponsor, or phase of COVID-19 trials.
Limitations include the use of one clinical trial database (Clinicaltrials.gov) from May 1 through October 31, 2020. Although other studies demonstrate a similar trend of exclusion across multiple databases, our data is limited to the first 6 months of the rapidly changing pandemic (Blehar et al., 2013; Ellington et al., 2020; WHO Coronavirus Disease (COVID-19) Dashboard, 2020; Yang et al., 2020). Additionally, the data were limited to the criteria listed explicitly on the database. Our analysis was thorough for National Institutes of Health–determined relevant COVID-19 treatments during data extraction, but not comprehensive to all clinical trials. Our results adequately represent the universal exclusion of reproductive-aged women during the coronavirus pandemic.
Implications for Practice and/or Policy
If the medical treatment of women is based on studies from which women are excluded as research participants, then a concern for generalizability must be raised, and women are at risk of not receiving the same level of evidence-based care available to men. The inclusion of diverse groups in clinical trials strengthens our confidence in efficacy and safety for the entire public; this inadequate representation of reproductive-aged individuals with childbearing potential has perpetuated an absence of critical knowledge (Enhancing the Diversity of Clinical Trial Populations, 2020). Because drugs and vaccines recommended for pregnant women are not routinely tested in this population, contraception requirements inhibiting women's participation restrict the generalizability of clinical trials. Stricter monitoring of inclusion and exclusion criteria to better include women willing to participate in trials would benefit a larger population and improve the generalizability of the efficacy findings for drugs and vaccines. Although pregnant and lactating individuals are a protected population, given the benefits of discovering novel therapies with known safety profiles, inclusion of this population in trials is appropriate where the known benefits outweigh risks (Subpart B—Additional Protections for Pregnant Women, Human Fetuses and Neonates Involved in Research, 2001). The inappropriate exclusion of reproductive-aged women from COVID-19 vaccination and clinical trials leads to significant morbidity and mortality that could have been prevented with better design of clinical trial protocols.
Conclusions
Clinical trials for COVID-19 vaccines and treatments often exclude pregnant and lactating individuals or require use of effective contraception for inclusion. Significant sex-based differences in physiology and disease mean researchers must achieve appropriate representation of women in clinical research, especially during a global pandemic. When evidence of potential harm to pregnant people and fetuses is absent, allowing those capable of pregnancy to participate in potentially beneficial clinical trials without contraception requirements can expand clinical trial participation and advance equity. Women should be given the opportunity to consider the risks and benefits of a COVID-19 vaccine or treatment, and the autonomy to choose whether they should participate.
Biographies
Kelly M. Kons, BS, is an MD candidate in the Penn State College of Medicine class of 2023. She is a Global Health Scholar with research interests in adolescent/women's reproductive health and advocacy, primary care, global and public health, and physician wellness.
Megan L. Wood, BS, has affiliations with Pennsylvania State College of Medicine, Penn State Health, and Iona College. Research interests are varied, including women's health advocacy, preventive care, quality improvement, health care equity, and global health.
Lindsey C. Peck, BA, is an MD candidate for the class of 2023 at Penn State College of Medicine. Her research interests include reproductive health in homeless women, contraceptive requirements in clinical trials, adolescent eating disorders, and patient to provider discrimination.
Sarah M. Hershberger, BS, is a medical student at Penn State Hershey. Her research interests include women's health issues, access to care, provider/patient communication, and health equity.
Allen R. Kunselman, MA, is a Senior Instructor in the Division of Biostatistics and Bioinformatics, Department of Public Health Sciences at the Penn State College of Medicine. A research area of interest is statistical methodology for longitudinal data using mixed models.
Christina Stetter, BS, has a BS degree in mathematics and more than 20 years of experience as a statistical analyst in the PHS department, with a heavy emphasis on OB-GYN research.
Richard S. Legro, MD, FACOG, is Chair of the Department of Obstetrics and Gynecology and University Professor at Penn State Health in Hershey, PA. His research and clinical practice are primarily focused on polycystic ovary syndrome (PCOS).
Timothy A. Deimling, MD, FACOG, is an Associate Professor of Obstetrics and Gynecology at the University of Pittsburg Magee Women's Hospital. His research and clinical practice are primarily focused on endometriosis and fibroids.
Footnotes
Funding Statement: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Financial Disclosures: Dr. Legro has consulted with Bayer, Kindex, Fractyl, Ferring, AbbVie, Insud Pharma. Dr. Legro has accepted grants from Guerbet and Hass Avocado Board. Dr. Deimling has consulted with AbbVie and Myovant. Allen Kunselman has stock ownership in Merck.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.whi.2022.06.004.
Supplementary Data
Coronavirus Disease 2019 vaccine clinical trials.
Coronavirus Disease 2019 treatment clinical trials.
References
- ACOG Clinical; Washington, D.C.: 2021. ACOG and SMFM Recommend COVID-19 Vaccination for Pregnant Individuals. [Google Scholar]
- ACOG Committee Opinion No. 776: Immune Modulating Therapies in Pregnancy and Lactation. Obstetrics and Gynecology. 2019;133:e287–e295. doi: 10.1097/AOG.0000000000003176. [DOI] [PubMed] [Google Scholar]
- A. C. O. N. 713, editor. Antenatal Corticosteroid Therapy for Fetal Maturation. American College of Obstetricians and Gynecologists; Washington, D.C.: 2017. [Google Scholar]
- Blehar M.C., Spong C., Grady C., Goldkind S.F., Sahin L., Clayton J.A. Enrolling pregnant women: Issues in clinical research. Womens Health Issues. 2013;23:e39–e45. doi: 10.1016/j.whi.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health; Washington, DC: 2020. [PubMed] [Google Scholar]
- Draft guidance for industry. Food and Drug Administration, U.S. Department of Health and Human Services, Center for Biologics Evaluation and Research; Washington, DC: 2021. COVID-19: Developing drugs and biological products for treatment or prevention. [Google Scholar]
- Centers for Disease Control and Prevention; Atlanta: 2022. COVID-19 Vaccines while Pregnant or Breastfeeding. [Google Scholar]
- Draft guidance for industry. Food and Drug Administration, U.S. Department of Health and Human, Center for Biologics Evaluation and Research; Washington, DC: 2020. Development and licensure of vaccines to prevent COVID-19. [Google Scholar]
- Diamond-Smith N., Logan R., Marshall C., Corbetta-Rastelli C., Gutierrez S., Adler A., Kerns J. COVID-19's impact on contraception experiences: Exacerbation of structural inequities in women's health. Contraception. 2021;104:600–605. doi: 10.1016/j.contraception.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington S., Strid P., Tong V.T., Woodworth K., Galang R.R., Zambrano L.D.…Gilboa S.M. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United States, January 22–June 7, 2020. MMWR Morbidity and Mortalilty Weekly Reports. 2020;69:769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enhancing the Diversity of Clinical Trial Populations . Guidance for industry. Food and Drug Administration U.S. Department of Health and Human Services, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research; Washington, DC: 2020. Eligibility criteria, enrollment practices, and trial designs. [Google Scholar]
- Global Advisory Committee on Vaccine Safety . World Health Organization; Geneva: 2014. Safety of immunization during pregnancy: Review of the evidence. [Google Scholar]
- U.S. Food and Drug Administration; Washington, DC: 2017. Global Participation in Clinical Trials Report. [Google Scholar]
- Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N.…Edlow A.G. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. American Journal of Obstetrics & Gynecology. 2021;225:303.e301–303.e317. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisolia G., Franchini M., Glingani C., Inglese F., Garuti M., Beccaria M.…De Donno G. Convalescent plasma for coronavirus disease 2019 in pregnancy: A case report and review. American Journal of Obstetrics and Gynecology Materna and Fetal Medicine. 2020;2:100174. doi: 10.1016/j.ajogmf.2020.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz F.M., Eckert L.O., Katz M.A., Lambach P., Ortiz J.R., Bauwens J., Bonhoeffer J. Key terms for the assessment of the safety of vaccines in pregnancy: Results of a global consultative process to initiate harmonization of adverse event definitions. Vaccine. 2015;33:6441–6452. doi: 10.1016/j.vaccine.2015.07.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention; Atlanta: 2022. People with Certain Medical Conditions. [Google Scholar]
- Shields K.E., Lyerly A.D. Exclusion of pregnant women from industry-sponsored clinical trials. Obstetrics and Gynecology. 2013;122:1077–1081. doi: 10.1097/AOG.0b013e3182a9ca67. [DOI] [PubMed] [Google Scholar]
- Smith D.D., Pippen J.L., Adesomo A.A., Rood K.M., Landon M.B., Costantine M.M. Exclusion of pregnant women from clinical trials during the coronavirus disease 2019 pandemic: A review of international registries. Amercina Journal of Perinatology. 2020;37:792–799. doi: 10.1055/s-0040-1712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J., Breslin W.J., Beyer B.K., Chadwick K., De Schaepdrijver L., Desai M.…Chen C.L. Birth control in clinical trials: Industry survey of current use practices, governance, and monitoring. Therapeutic Innovation Regulatory Science. 2016;50:155–168. doi: 10.1177/2168479015608415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S Department of Health & Human Services Office for Human Research Protections; Washington, DC: 2001. Subpart B — Additional Protections for Pregnant Women, Human Fetuses and Neonates Involved in Research. [Google Scholar]
- Sullivan K.A., Little M.O., Rosenberg N.E., Zimba C., Jaffe E., Gilbert S.…Lyerly A.D. Women's views about contraception requirements for biomedical research participation. PLoS One. 2019;14:e0216332. doi: 10.1371/journal.pone.0216332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.M., Kobeissi L., Kim C., Amin A., Thorson A.E., Bellare N.B.…Broutet N. Inclusion of pregnant women in COVID-19 treatment trials: A review and global call to action. Lancet Global Health. 2021;9:e366–e371. doi: 10.1016/S2214-109X(20)30484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention; Atlanta: 2019. Vaccinating Pregnant Women Protects Moms and Babies. [Google Scholar]
- Centers for Disease Control and Prevention; Atlanta: 2021. Vaccination Safety for Breastfeeding Mothers. [Google Scholar]
- Van Spall H.G.C. Exclusion of pregnant and lactating women from COVID-19 vaccine trials: A missed opportunity. European Heart Journal. 2021;42:2724–2726. doi: 10.1093/eurheartj/ehab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization; Geneva: 2020. WHO Coronavirus Disease (COVID-19) Dashboard. [Google Scholar]
- Yang R., Mei H., Zheng T., Fu Q., Zhang Y., Buka S.…Zhou A. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: A population-based cohort study in Wuhan, China. BMC Medicine. 2020;18:330. doi: 10.1186/s12916-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronavirus Disease 2019 vaccine clinical trials.
Coronavirus Disease 2019 treatment clinical trials.