Abstract
Purpose:
Kaposi sarcoma (KS), an endothelial cell tumor associated with KS-herpesvirus (KSHV), remains among the most common malignancies occurring with HIV infection (HIV-KS). As an oral anti-inflammatory, anti-angiogenic, and immunomodulatory agent, lenalidomide is potentially an attractive alternative to standard chemotherapy for KS.
Experimental Design:
The primary objectives of this phase I/II trial were to determine the maximum tolerated dose (MTD) and response rates for lenalidomide in HIV-KS. Secondary objectives included correlating response with natural killer (NK) and T cell subsets, plasma cytokines, viral copy number, and KSHV gene expression in biopsies. Four dose levels of oral lenalidomide taken 21 consecutive days of 28-day cycles were evaluated in adults with HIV-KS on antiretroviral therapy with controlled viremia.
Results:
Fifteen and 23 participants enrolled in phase I and II, respectively, 76% of whom had received prior KS therapy. The MTD was not reached, declaring 25 mg as the recommended phase II dose (RP2D). The most frequent adverse events were neutropenia, fatigue, leukopenia and diarrhea. Of 25 evaluable participants receiving RP2D, 60% responded. Correlative studies performed in a subset of participants demonstrated a significant increase in proportions of blood T-cells with T-regulatory phenotype, and plasma cytokines trended toward a less inflammatory pattern. Clinical response was associated with loss of KSHV transcription.
Conclusions:
Lenalidomide is active in HIV-KS. The most common adverse events were manageable. With 60% of participants receiving RP2D obtaining a partial response and <10% discontinuing due to adverse events, the response and tolerability to lenalidomide support its use in HIV-KS.
Introduction
Kaposi sarcoma (KS) is an endothelial cell tumor characterized by formation of aberrant blood vessels that collectively present as purpuric lesions most commonly in the skin, gastrointestinal and respiratory systems. KS is caused by Kaposi sarcoma herpesvirus (KSHV), also known as human herpes virus-8 (HHV-8).(1) KS is common in settings of impaired cellular immunity, including iatrogenic (i.e. post-transplant immunosuppression), HIV infection, and aging.(2) When HIV-induced immunosuppression is ameliorated with antiretroviral therapy (ART), KS lesions often regress, but may not respond at all,(3) and can even suddenly proliferate, a condition termed KS-immune reconstitution syndrome (KS-IRIS).(4) These observations highlight the role of immunosurveillance in KS pathogenesis.
Although widespread use of ART is associated with significant reductions in the incidence of KS in persons with HIV (PWH), KS remains among the most common malignancies occurring in this population in the United States (US)(5) and Sub-Saharan Africa.(6) In the US, a third of KS develops in PWH with no detectable HIV viral load and normal or near-normal CD4+ cell counts, suggesting that the immune restoration upon ART is incomplete, and that the immune system in PWH remains compromised to a degree that allows KSHV and KS to emerge.(7) Immunosenescence as a consequence of aging, or perhaps chronic HIV infection, may serve as a predisposing factor in development of KS in the setting of normal absolute CD4 counts.(8)
There is evidence that endothelial cells are reprogrammed by KSHV into “spindle”-shaped KS cells, supported through various paracrine factors such as interleukin (IL)-1, IL-6, gamma interferon, granulocyte-macrophage colony stimulating factor, tumor necrosis factor alpha, basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), chemokines, and transforming growth factor β (TGF- β)(9) as well as KSHV microRNAs.(10)
Lenalidomide, thalidomide, and pomalidomide are oral immunomodulatory (IMiD) compounds with potent anti-inflammatory, anti-angiogenic, and immunomodulatory properties,(11) making IMiDs a promising class of therapy to address both the impaired cellular immunity and the KSHV- associated inflammation and angiogenesis that drive this disease.
Thalidomide was explored for management of HIV-related immunosuppression early in the HIV epidemic. Thalidomide caused inhibition of pro-inflammatory cytokines, IL-1β and IL-6 while stimulating anti-inflammatory IL-10 production.(12) Administration of thalidomide in AIDS participants resulted in increased production of IL-2 and IL-12.(13) As a defective IL-12 response is felt to play a role in progressive immune deficiency,(14) it was postulated that thalidomide therapy could improve immune function via restoration of IL-12 production. Without concurrent ART, thalidomide caused increasing HIV viremia, but was later found to have activity in the control of HIV-KS with partial response (PR) rates ranging between 35–47%.(15),(16) High doses of thalidomide were used to achieve these responses with associated adverse events - including fatigue, depression, neuropathy and rash - resulting in an early discontinuation rate of 20%. Pomalidomide was reported as active and tolerable in a phase I/II study including 22 participants with KS,(17) resulting in accelerated US Food and Drug Administration (FDA) approval for adults with KS. The response rate in the 15 participants with HIV infection participating in the study was 60%. Reported grade 3/4 adverse events attributable to pomalidomide included neutropenia, infection and edema.
The three different IMiDs differ subtly in their effects on immune health and angiogenesis modulation as well as their adverse event profiles, supporting a prospective Phase I/II trial of lenalidomide in HIV-KS.
Objectives
The primary objectives of the Phase I and II portions of the study were to determine the maximum tolerated dose (MTD), and overall response rates of lenalidomide with concurrent ART in HIV-KS, respectively. Secondary objectives included assessing the impact of lenalidomide on: peripheral blood T-lymphocyte and natural killer (NK) cell numbers, growth factors relevant to tumor proliferation and angiogenesis (i.e. IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-15, GM-CSF, TNFα, and IFNγ), HIV serum viral load, plasma KSHV copy number, as well as immune cell composition and viral gene expression in KS biopsies. Further, we planned to correlate any changes in these parameters with response to therapy.
Participants and Methods
This study was conducted in accordance with the Declaration of Helsinki. Institutional review boards at each of the study sites approved the study protocol in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services. All participants provided written informed consent prior to study procedures and treatment.
Protocol design and therapy:
This was an open label, dose escalation Phase I/II trial with Phase II expansion at the MTD determined in Phase I. Phase I evaluated 4 pre-specified dose levels of lenalidomide taken orally daily on days 1–21 of a 28-day cycle: 10 mg, 15 mg, 20 mg and 25 mg. The Phase I portion followed a 3+3 design with inter-cohort dose escalation allowed if no dose limiting toxicities (DLTs) occurred during the first 2 cycles of treatment (8 weeks). Simon’s 2-stage design was used in the Phase II portion to test the null hypothesis that the overall response is ≤20% against the alternative that it is 50% at the one-sided 0.05 significance level with power of 0.90. The study was designed to terminate early if ≤2 out of the first 10 participants responded, otherwise a total of 22 evaluable participants were planned for enrollment for the Phase II portion. Participants with disease meeting criteria for partial response (PR) after cycle 6 were allowed to continue therapy up to a total of 12 cycles.
Key Eligibility Criteria:
Participants were adults (≥18 years) with serologically documented HIV infection, and stable or progressive biopsy-proven KS. Participants were required to be on a stable ART regimen (excluding zidovudine given potential for overlapping myelosuppression) for at least 12 weeks prior to study entry, have a CD4+ count >50 cells/mm3 and an HIV viral load <2,000 copies/mL. Other key eligibility requirements included: life expectancy > 3 months, ECOG performance status <3, ANC>1,000, hemoglobin > 8gm/dL, platelets > 100K/mm3, AST/ALT < 3 x ULN, and creatinine clearance > 60 mL/min in the Phase I, and > 30 mL/min in the Phase II.
Individuals were ineligible if front-line cytotoxic KS therapy was indicated (i.e. symptomatic visceral or pulmonary KS or KS impairing functional status) or any of the following were present: uncontrolled comorbidity, including active opportunistic infection; history of second malignancy within the last 2 years; concurrent glucocorticoid therapy; thrombophilia unless receiving therapeutic anticoagulation; and pregnancy or nursing.
Lenalidomide Risk Evaluation and Mitigation:
In line with the Risk Evaluation and Mitigation Strategy (REMS) program for lenalidomide, contraceptive precautions and monitoring were required of females of childbearing potential (FCBP) and men who had sexual contact with FCBP. A study form assessing compliance with contraception or abstinence, drug handling precautions, as well as certification of counseling of the participant regarding risks, including teratogenicity, was completed to certify participant understanding and compliance prior to dispensing drug each cycle.
Participants with a history of thrombosis or any predisposing thrombotic risks were required to be on full-dose therapeutic anticoagulation. For all others, use of antiplatelet agents or anticoagulation was instituted at the discretion of the investigator according to individual institutional policy.
Response and Toxicity Evaluation:
Response assessment occurred every cycle through cycle 6, then every 2 cycles using modified AIDS Clinical Trials Groups (ACTG) criteria(18) outlined in the protocol (see Supplemental Methods).
Toxicity monitoring occurred through 1 month post completion of trial therapy, or resolution of toxicity to baseline. CD4+ T cell counts and HIV-1 plasma RNA copy number were measured at baseline, then every 3 cycles and at treatment discontinuation. Additional lymphocyte subset and cytokine testing was performed at baseline, cycle 1 days 8 and 15, and day 1 of subsequent cycles. Two 3-mm punch biopsies were collected from non-indicator lesion(s) at baseline and cycle 1, day 15 for correlative studies.
Correlative Studies:
CD4+ T cell counts and HIV viral load were measured using standard commercial assays.
Peripheral blood lymphocyte subset analysis:
Peripheral blood buffy coat was isolated, removed of red cells using RBC lysis buffer, and stained with antibodies specific for the following antigens: CD3, CD4, CD8, CD16, NKG2D, HLA-DR, CD56, CD25, CD69, CD127, αβ TCR, δγ TCR, CD20, B7H1, CD206, and Va24Ja18 (Biolegend). Intracellular FOXP3 staining was performed using the FOXP3 kit (eBioscience) according to the manufacturer’s instruction using ficoll-separated peripheral blood mononuclear cells with the exception of the antibody incubation duration which was adjusted to 24 hours at 4°C in the dark. Stained cells were collected using flow cytometry on a FACSCanto instrument and analyzed using the FACSDiva and FlowJO platforms.
Cytokines:
Cytokine measurements of levels of plasma IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-12p70, IL-15, GM-CSF, TNFα, and IFNγ were performed on blood samples collected at the start of each cycle, as well as day 8 and 15 of cycle 1, using multiplexed immunometric assays (Luminex platform, R&D Systems), as previously described(19),(20) (see Supplemental Methods).
KSHV plasma PCR:
KSHV PCR on DNA extracted from plasma collected at baseline, cycle 3 day 1, and treatment discontinuation was carried out as previously described(21).
Tumor KSHV gene expression:
Latent and lytic KSHV gene expression profiling was performed on cutaneous KS lesion 3 mm punch biopsies, results of which were evaluated for change between baseline and cycle 1 day 15, and correlated with response to therapy. The biopsies were obtained from separate representative cutaneous KS lesions at the different time points to exclude biopsy-induced changes and stored in RNAlater. RNA was isolated from each biopsy using RNAzol (Tel-Test, Inc.), followed by polyA selection (Qiagen, Inc.) according to the suppliers’ protocols, and reverse-transcribed using 120 pmol random hexanucleotide primers per reaction. The known KSHV and selected human mRNAs were quantitated by real-time qPCR using 190 individual primer pairs, as previously described(22). This included 8 “non-template control” reactions, 3 “reverse transcriptase untreated” reactions, and 4 “positive control” reactions. For analysis, cycle threshold (CT) values for each primer pair were evaluated. A CT >37 was considered as a non-detectable target mRNA. Primer pairs with a standard deviation of CT=0 across all samples were considered unchanged and removed.
T-regulatory (T-reg) cell immunohistochemistry:
Formalin-fixed sections of cutaneous KS lesion 3 mm punch biopsies of 4 participants were stained with anti-FOXP3 antibody (eBioscience)(23). The study was performed on the first 4 consecutive participants enrolled at the University of California, San Diego site, funded by a short-term pilot grant active during the period of their treatment. FOXP3+ cells were quantified at two different time points: pretreatment (baseline) and post-treatment (day 15) using ImageJ. The biopsies were obtained from separate representative cutaneous KS lesions at the different time points to exclude biopsy-induced changes.
Statistics
Analysis of variance was used to evaluate the changes from baseline to 4 weeks in CD4+ and CD8+ cell counts and percentages and levels of plasma HIV viral load. For each dose group and for all groups combined, the Wilcoxon signed rank test was used to evaluate the changes in HIV viral load and CD4+ cell count from baseline to day 29 (day 1 of cycle 2). Analysis of variance was used to assess the relationship between quantification of baseline levels of KSHV and HIV viral copy numbers with response.
Data Availability
The data generated in this study are available upon request from the corresponding author.
Results
A total of 38 evaluable participants were enrolled from 14 AMC sites: 15 in Phase I and 23 in Phase II. An additional Phase I participant was not included in the MTD determination or other analyses due to HIV viremia being above the threshold required by eligibility criteria.
Phase I
All of the participants in the Phase I portion of the study were male. The majority were White (80.0%) with 4 identifying as Hispanic. The median age of the participants was 46 years (30 – 74). Median baseline CD4+ count was 408 cells/m3 (170 – 799). The median HIV load at baseline was 48.0 (<40 – 707) (Table 1).
Table 1.
Demographics and Baseline Characteristics
| Demographic and Baseline Characteristics | Phase I | Phase II |
|---|---|---|
| N | 15 | 23 |
| N (%) | N (%) | |
| Gender | ||
| Male | 15 (100) | 23 (100) |
| Race | ||
| White | 12 (80) | 14 (61) |
| Black | 2 (14) | 5 (22) |
| Pacific Islander | 1 (7) | 1 (4) |
| Asian | 1 (7) | 1 (4) |
| Multiracial | 2 (9) | |
| Unknown | 1 (4) | |
| Ethnicity | ||
| Hispanic | 4 (26) | 4 (17) |
| Non-Hispanic | 11 (73) | 18 (78) |
| Tumor Stage | ||
| 0 – Tumor is confined to skin and/or to lymph nodes and/or minimal oral disease 1 – Tumor with edema, ulceration, or extensive oral KS or gastro KS or KS in other nonnodal viscera |
9 (60) 6 (40) |
13 (57) 10 (43) |
| Prior treatment for KS | 13 (87) | 16 (70) |
| Local | ||
| Surgery | 1 (7) | 2 (9) |
| Cryotherapy | 0 | 1 (4) |
| Radiation | 3 (20) | 3 (13) |
| Topical/Intralesional | 3(20) | 4 (14) |
| Systemic: | ||
| Adriamycin | 0 | 1 (4) |
| Interferon | 0 | 1 (4) |
| Bleomycin | 0 | 1 (4) |
| Bortezomib | 0 | 1 (4) |
| Etoposide | 1 (7) | 1 (4) |
| Liposomal doxorubicin | 13 | 15 (65) |
| Paclitaxel | 4 (28) | 8 (35) |
| Vincristine | 0 | 1 (4) |
| Vinblastine | 0 | 1 (4) |
| Other single agent therapy | 2 (14) | 4 (17) |
| Age in years | ||
| Median | 46 | 47 |
| Min - Max | 30 to 74 | 30 to 63 |
| CDC Risk Group | ||
| Homosexual/bisexual contact | 12 (80) | 22 (96) |
| Heterosexual contact | 3 (20) | 2 (9) |
| Baseline Absolute CD4 Count | ||
| Mean | 434 | 384 |
| SD | 196 | 157 |
| Median | 408 | 394 |
| Min – Max | 170 to 799 | 122 to 697 |
| Absolute CD8 Count | ||
| Mean | 919 | 949 |
| SD | 330 | 583 |
| Median | 852 | 841 |
| Min – Max | 445 to 1717 | 209 to 3032 |
| HIV Load | ||
| Median | 48 | 40 |
| Min - Max | 40 to 707 | 20 to 50 |
Of the 15 participants in the Phase I portion of the study, 6 (40%) completed the maximum of 12 cycles, 2 (13.3%) discontinued early due to PD, 3 (20%) stopped treatment due to an adverse event, 3 (20%) withdrew from the protocol voluntarily, and 1 (6.7%) chose an alternative therapy. No DLTs occurred at any of the 4 dose levels tested in phase I. There were no serious adverse events (SAEs) reported in the 10 mg/day, 15 mg/day or 20 mg/day dose groups. Two participants in the 25 mg/day dose cohort experienced an SAE: grade 1 facial nerve disorder and grade 4 lung infection.
Among the 15 participants in Phase I, 5 had a partial response (1 in the 20 mg/day cohort and 4 of 5 evaluable in the 25 mg/day cohort), 6 had stable disease, 2 had disease progression, and 2 were unevaluable for response due to withdrawal prior to receipt of 1 cycle of protocol therapy. A maximum tolerated dose was not identified, and the 25 mg dose level was chosen for Phase II.
Phase II
All 23 Phase II participants were male with a median age of 47 years (range 30–63), 70% of whom had received prior therapy for KS. Most were White (n= 14, 61%), with 5 Black, 2 multi-racial, 1 Pacific Islander, and 1 unreported race; 4 participants identified as Hispanic (17%). The median absolute CD4+ count at baseline was 394.0 cells/mm3 (122–697), and the median HIV load was 40.0 (<20–50). 10 participants had stage II disease, defined as KS tumors with edema, ulceration, or extensive oral KS, gastrointestinal KS or KS in other non-nodal visceral involvement (Table 1).
The median number of cycles completed by participants in Phase II was 7, with 13 of 23 Phase II participants completing the maximum of 12 cycles. Reasons for early discontinuation were disease progression (n=3), voluntary withdrawal (n=3), adverse event (n=2, both asymptomatic neutropenia), lost to follow-up (n=1) and non-compliance (n=1).
AEs occurred in 35 of 38 total participants, with 12 and 2 participants experiencing grade 3 or grade 4 toxicity, respectively. There were 185 AEs and 2 serious AEs (1 hypotension and 1 lung infection) considered at least possibly related to lenalidomide. The most frequently reported AE associated with lenalidomide were neutropenia, fatigue, leukopenia and diarrhea (Table 2). Dose reduction due to AE occurred in 6 participants (1 receiving 20 mg and 5 receiving 25mg lenalidomide). No deaths occurred. No participants developed KSHV-associated conditions (i.e. primary effusion lymphoma, multicentric Castleman disease, or KSHV inflammatory cytokine syndrome) while on study
Table 2.
| Organ System / Adverse Event | Severity Grade Number of Occurrences | TOTAL | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Blood and Lymphatic System Disorders | Anemia | 16 | 1 | 17 | |||
| Neutropenia | 6 | 1 | 7 | ||||
| Other (increased platelets) | 1 | 1 | |||||
| Cardiac Disorders | Chest Pain - Cardiac | 1 | 1 | ||||
| Ear and Labyrinth Disorders | Tinnitus | 2 | 2 | ||||
| Eye Disorders | Conjunctivitis | 1 | 1 | ||||
| Dry Eye | 1 | 1 | |||||
| Other (vision changes) | 2 | 2 | |||||
| Gastrointestinal Disorders | Abdominal Distension | 1 | 1 | ||||
| Abdominal Pain | 2 | 2 | |||||
| Bloating | 1 | 1 | |||||
| Constipation | 5 | 1 | 6 | ||||
| Diarrhea | 11 | 7 | 1 | 19 | |||
| Dry Mouth | 2 | 2 | |||||
| Dyspepsia | 3 | 3 | |||||
| Gastroesophageal Reflux Disease | 1 | 1 | |||||
| Nausea | 11 | 11 | |||||
| Salivary Duct Inflammation | 1 | 1 | |||||
| Vomiting | 3 | 3 | |||||
| General Disorders and Administration Site Conditions | Chills | 1 | 1 | ||||
| Edema Limbs | 2 | 2 | |||||
| Fatigue | 22 | 7 | 29 | ||||
| Fever | 3 | 3 | |||||
| Pain | 3 | 1 | 4 | ||||
| Immune System Disorders | Allergic Reaction | 2 | 2 | ||||
| Infections and Infestations | Lung Infection | 1 | 1 | 2 | |||
| Sinusitis | 1 | 1 | |||||
| Skin Infection | 1 | 2 | 3 | ||||
| Upper Respiratory Infection | 4 | 4 | |||||
| Investigations | Alanine Aminotransferase Increased | 7 | 7 | ||||
| Aspartate Aminotransferase Increased | 3 | 3 | |||||
| Lymphocyte Count Decreased | 1 | 2 | 3 | ||||
| Neutrophil Count Decreased | 2 | 23 | 15 | 1 | 41 | ||
| Platelet Count Decreased | 12 | 12 | |||||
| Weight Loss | 2 | 2 | |||||
| White Blood Cell Decreased | 13 | 6 | 19 | ||||
| Metabolism and Nutrition Disorders | Anorexia | 4 | 4 | ||||
| Bilirubin increased | 1 | 1 | |||||
| Hyperglycemia | 1 | 1 | |||||
| Hypocalcemia | 1 | 1 | |||||
| Hypoglycemia | 1 | 1 | |||||
| Hypophosphatemia | 3 | 1 | 4 | ||||
| Musculoskeletal and Connective Tissue Disorders | Arthralgia | 3 | 1 | 4 | |||
| Back Pain | 1 | 1 | |||||
| Myalgia | 2 | 1 | 3 | ||||
| Pain In Extremity | 1 | 1 | 2 | ||||
| Skin nodules | 1 | 1 | |||||
| Nervous System Disorders | Dizziness | 4 | 3 | 7 | |||
| Dysgeusia | 1 | 1 | |||||
| Facial Nerve Disorder | 1 | 1 | |||||
| Headache | 8 | 2 | 1 | 11 | |||
| Neuralgia | 1 | 1 | |||||
| Paresthesia | 1 | 1 | 2 | ||||
| Peripheral Motor Neuropathy | 2 | 2 | |||||
| Peripheral Sensory Neuropathy | 1 | 1 | |||||
| Psychiatric Disorders | Depression | 1 | 1 | ||||
| Insomnia | 1 | 1 | 2 | ||||
| Other (dysphoria) | 1 | 1 | |||||
| Respiratory, Thoracic And Mediastinal Disorders | Cough | 3 | 3 | ||||
| Dyspnea | 1 | 1 | |||||
| Nasal Congestion | 6 | 6 | |||||
| Pharyngolaryngeal Pain | 1 | 1 | |||||
| Sore Throat | 1 | 1 | |||||
| Voice Alteration | 1 | 1 | |||||
| Renal | Acute kidney injury | 1 | 1 | ||||
| Skin and Subcutaneous Tissue Disorders | Dry Skin | 3 | 1 | 4 | |||
| Hyperhidrosis | 1 | 1 | |||||
| Pruritus | 8 | 2 | 10 | ||||
| Rash, Maculo-Papular | 10 | 4 | 14 | ||||
| Other (Bleeding KS lesion, abnormal skin sensation) | 2 | 2 | |||||
| Vascular Disorders | Hypotension | 1 | 1 | ||||
| Lymphedema | 3 | 1 | 4 | ||||
| TOTAL (Occurrences) | 215 | 84 | 23 | 2 | 324 | ||
PR occurred in 11 of 20 (55%, 95% CI: 0.335, 0.797) evaluable Phase II participants with a median time to response of 12.0 weeks [95% CI: 8.0 to 23.29]. Seven (36%) additional participants had stable disease (SD). PD occurred in 2 participants (10%). The remaining 3 participants were not evaluable for response due to receiving less than 1 cycle of protocol therapy. In both Phase I and Phase II, the majority of participants experienced some degree of improvement in number (Figure 1a), quality (flattening of raised lesions, Figure 1b) or lesion size (Figure 1c), with all but 1 evaluable participant experiencing decrease in aggregate size of marker lesions at best response (Figure 1c). For all measures of response, the largest reductions in lesion count, number of raised lesions and aggregate lesion size occurred at the highest, 25mg dose level (Figure 1). Many lesions had modest changes in size but had resolution of purpuric changes, with residual hyperpigmentation consistent with a tatoo effect (Figure 2). No participants experiencing response had progression of their disease during a 13-month maximum duration of study therapy and follow-up.
Figure 1. Waterfall plots of response.
For all measures of response, the largest reductions in lesion count and size occurred at the highest dose level. A. Maximum change in the total number of cutaneous KS lesions represented as percent change from baseline by dose level. B. Maximum change in the number of raised cutaneous KS lesions represented as percent change from baseline by dose level. C. Percent change in aggregate area of cutaneous KS marker lesions from baseline to time of best response by dose level. All but 1 evaluable participant had decrease in aggregate marker lesion area at best response during study treatment.
Figure 2. Representative tumor response in a patient completing 12 cycles of lenalidomide at the 25 mg dose level.
Although the lesion here had only modest decrease in the bidimensional measurements, lenalidomide treatment was associated with resolution of induration and the color changed from erythematous to faintly tan. Such changes are consistent with a tattoo effect that is common with KS regression, and attributable to hemosiderin-laden macrophages.35 Although lesions with such an appearance are likely to represent resolution of KS, this is not completely captured by the ACTG KS response criteria18 unless biopsies are performed of residual pigmented areas confirming lack of KS tumor cells.
Immunology studies:
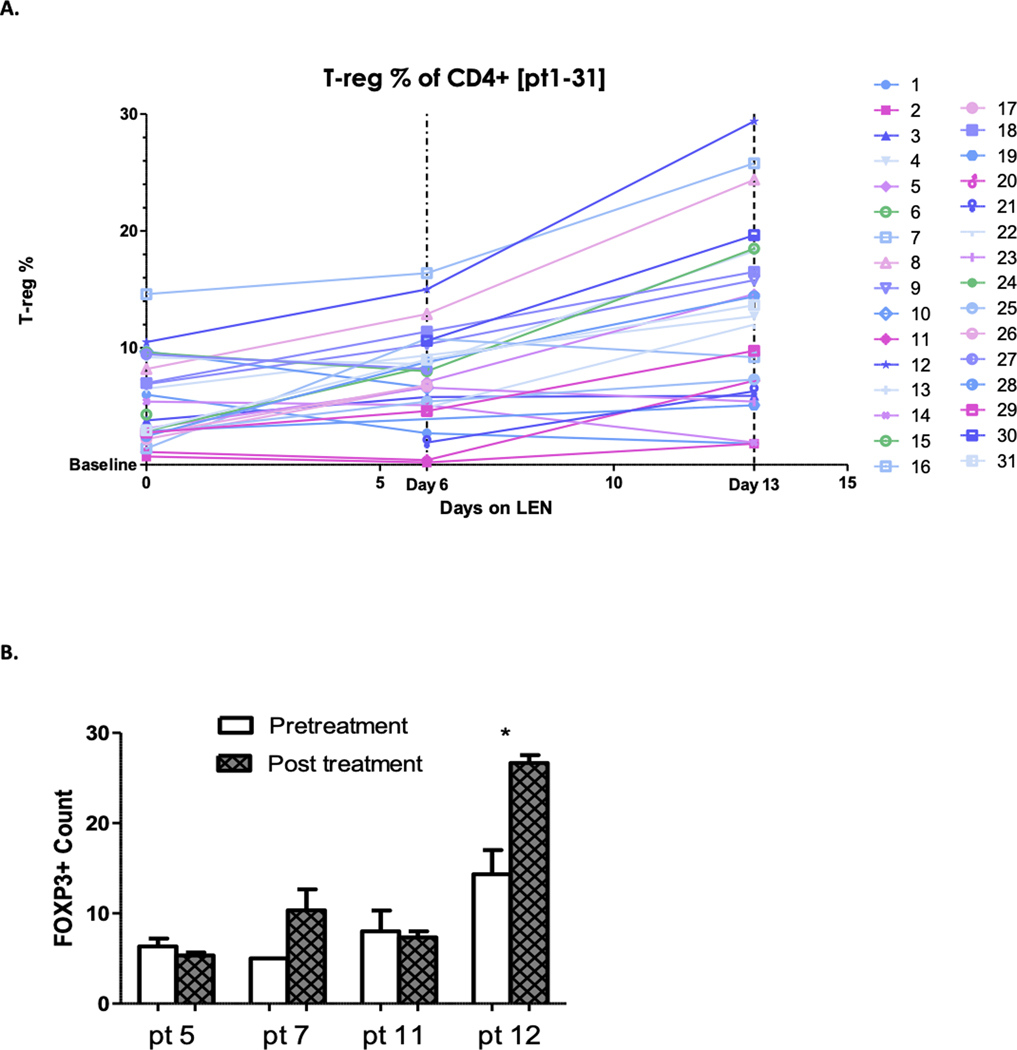
There was a statistically significant increase in the proportion of blood T cells with T regulatory (T-reg) phenotype during the first two weeks of lenalidomide (baseline mean, 5.5%; day 6 mean, 8.8%, p-value <0.01; day 13 mean, 13.7%, p-value <0.001) (Figure 3a). No consistent change in CD4+, CD8+ or NK cell fractions occurred in peripheral blood. In the 4 participants with tumor biopsies assessed, day 15 biopsy FOXP3 staining increased from baseline in two participants obtaining PR compared to no change in two participants with SD (Figure 3b).
Figure 3. Changes in peripheral blood and tumor T-reg cells from baseline.
A. Increase in proportion of peripheral blood CD4+ T cells with T-reg phenotype from baseline. Increase in the mean proportion of blood T cells with T regulatory phenotype during the first two weeks of lenalidomide treatment from baseline (mean 5.5): day 6 (mean 8.8, p-value <0.01), day 13 (mean 13.7, p-value <0.001). B. Increase in tumor FOXP3 staining at day 15 in participants with PR compared to SD. FOXP3 staining, a marker of T-reg cells, increased from baseline in day 15 biopsies of two participants obtaining PR (pt 7 and 12). In contrast, there was no significant change in FOXP3 staining in two participants with SD (pt 5 and 11).
HIV plasma viral load:
In the highest dose cohort, the median change during the study in HIV viral load from baseline was 0 (−1.2 to 1.6 log copies/ml). There was not a significant association between change in HIV viral load and response.
KSHV plasma DNA copy number:
The majority of participants had detectable plasma KSHV DNA, but below linear range of the assay (undetectable to >50,000 copies/ml). There was no correlation between baseline KSHV copy number and response, nor significant change in viral copy number with lenalidomide therapy.
KSHV tumor gene expression:
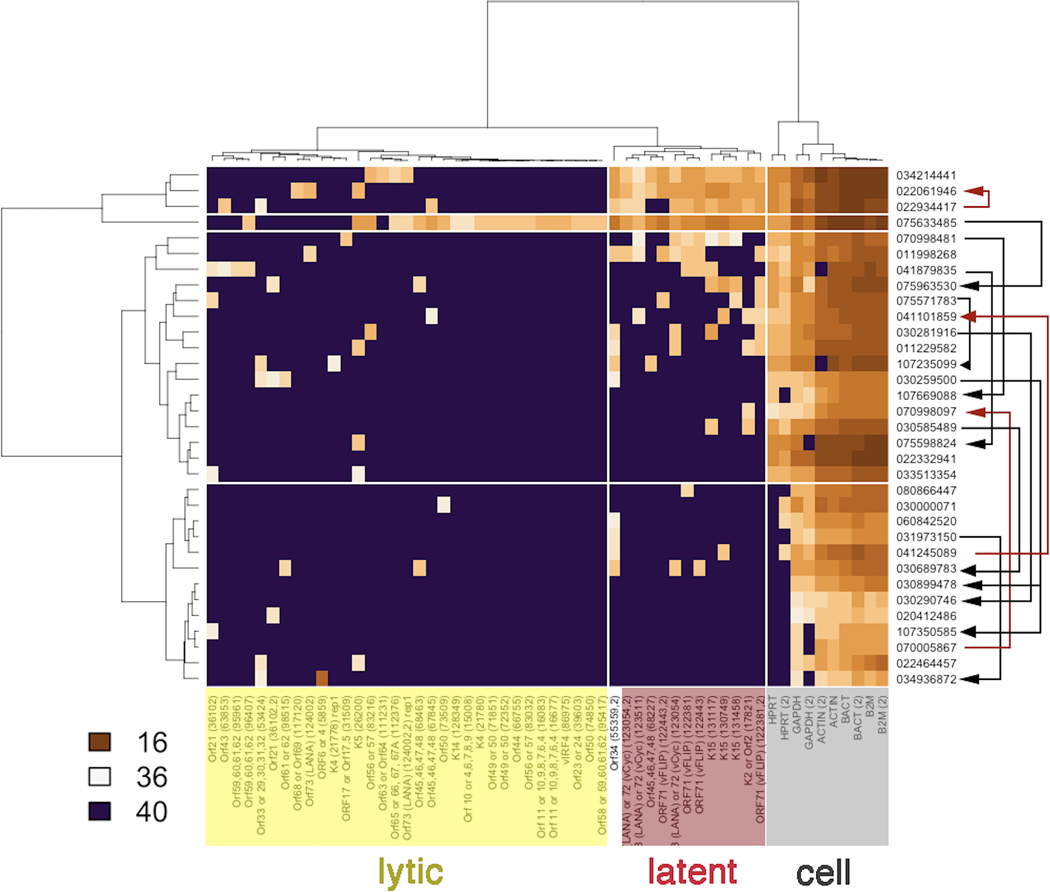
In 11 evaluable participants, the baseline KSHV viral transcription profile in tumor biopsies showed predominately latent KSHV gene expression (Figure 4), as has previously been reported in KS.(24) This heatmap shows the spectrum of viral gene expression in each lesion and the clustering of genes (horizontal) as well as biopsies (vertical). Unsupervised clustering divided the genes into three classes: “lytic” viral genes, “latent” viral genes and “cell” (i.e. non-viral genes). The “cell” genes are used to verify RNA quality, and also to correlate with the relative number of tumor cells in a KS biopsy. Genes labeled “Latent” are indicative of tumor biopsies with many viable, virus-infected tumor cells in the lesion, while genes labeled “Lytic” serve as biomarkers for viral replication.
Figure 4. KSHV gene expression:
Paired biopsy samples were evaluable for viral gene expression profiling in 11 participants collected at baseline and cycle 1 day 15. The heatmap is clustered by columns into KSHV lytic genes, KSHV latent genes and KS cell genes and organized by row based on degree of gene expression. Baseline KSHV viral expression profile in tumor biopsies demonstrated predominately latent viral protein expression. Eight demonstrated overall decrease in viral gene expression (black arrows) compared with baseline at day 15, 6 of whom eventually experienced PR; the remaining 2 had stable disease. Three participants had increased viral gene expression at day 15 (red arrows). Of these, 1 participant experienced PR and the other 2 had progression of KS. Brown coloration indicates the presence and violet the absence of a particular transcript. The data represent raw CT values in the range of 16.8 (abundant) to 40 (absent).
Unsupervised analysis identified four clusters of gene expression among the biopsies. Only a single biopsy (#075633485) showed evidence of robust lytic gene expression. Three participant biopsies showed evidence of consistent latent viral gene expression. The remaining two clusters contained biopsy samples for which only very few KSHV transcripts were detectable, yet cellular mRNAs, such as actin, were present. This suggests that in these biopsy specimens, very few true KS tumor cells were present. The arrows in Figure 3 designate samples from successive timepoints for the same participant. A decrease of KSHV transcription refers to changes in transcription between paired baseline and day 15 biopsy samples in the same participant. In these cases, the baseline biopsy of a participant demonstrated transcription across multiple gene loci on the viral genome, a pattern traditionally associated with lytic viral replication. By contrast, the same participant’s subsequent biopsy had expression restricted to the KSHV latency locus only, or no evidence of KSHV transcription at all. Comparing tumor biopsies collected from baseline and day 15, tumor biopsies from 8 participants demonstrated decrease in viral gene transcription (black arrows), of which 6 participants eventually experienced PR, while the remaining 2 participants had SD. Tumor biopsies in 3 of 11 participants had increased viral gene transcription at day 15. Of these, 1 participant experienced a PR and the other 2 had progression of KS.
Cytokines:
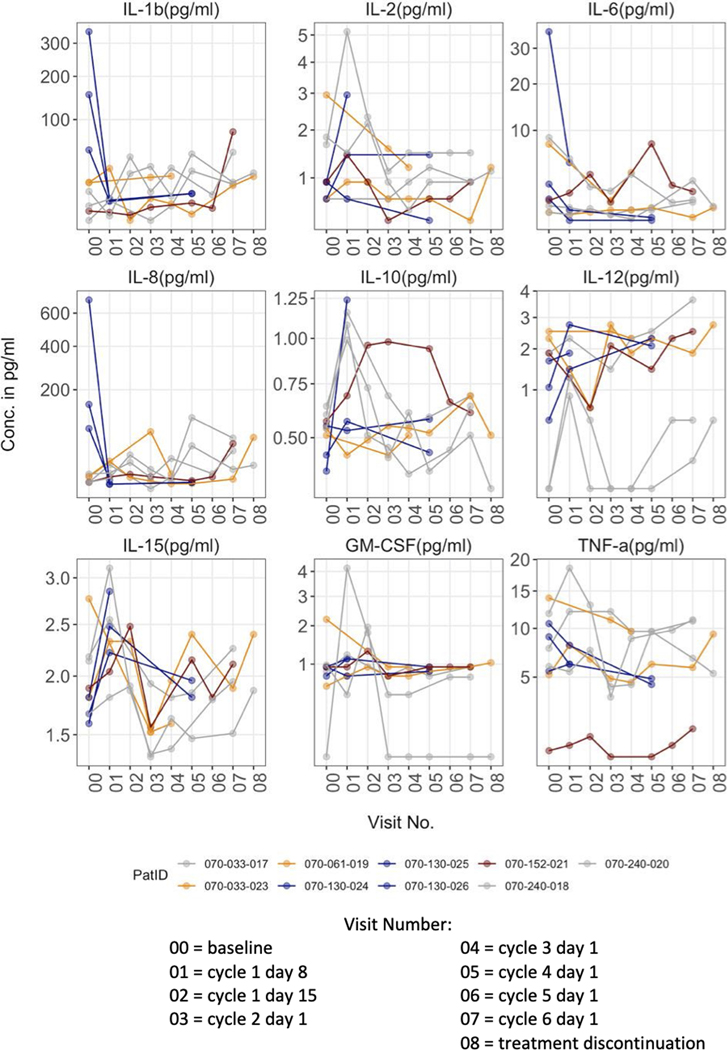
Changes in plasma cytokine levels with treatment varied between participants, as summarized in Figure 5. Of note, when participants had baseline high levels of the pro-inflammatory cytokines IL-1β, IL-6 or IL-8, these cytokine levels tended to decrease after initiation of lenalidomide. By contrast, increases in IL-10, an anti-inflammatory cytokine, and IL-12, a TH1-promoting immune stimulatory cytokine, occurred with lenalidomide treatment.
Figure 5. Changes in Cytokines:
Nine participants had serial plasma samples available for cytokine measurement. In participants with high baseline levels of the pro-inflammatory cytokines IL1b, IL6 or IL8, these cytokines trended down rapidly after initiation of lenalidomide. By contrast, IL10, an anti-inflammatory cytokine, and IL12, a TH1-promoting immune stimulatory cytokine, trended up.
Discussion
As the largest prospective trial of IMiDs in HIV-KS, this trial demonstrates lenalidomide is effective and tolerable for this condition. A smaller French study previously reported 4 partial responses to 25 mg lenalidomide dosing in 10 evaluable participants with HIV-KS(25), while the current study had an PR rate of 60% among the 25 evaluable participants (phase I+II) at the 25mg lenalidomide dose level. The most common AEs were consistent with known risks of lenalidomide and were manageable. Only 2 of 23 phase II participants withdrew from study due to AEs. These two participants withdrew due to asymptomatic neutropenia, which could have been alternatively managed with lenalidomide dose reduction and/or granulocyte growth factor support, which were allowable per the protocol.
Although small numbers of participants treated at the lower dose levels limit evaluation, 4 of the 5 PRs demonstrated in Phase I occurred at the highest dose level of 25 mg/day suggesting a possible dose-dependent or threshold response. The waterfall plots (Figure 1) of response metrics demonstrate that the highest degree of lesion improvement occurred at the highest dose level. Of note, 12 of 15 participants obtaining a PR initially met the response criteria through 50% or greater reduction in the number of raised lesions.
With a 60% PR rate and <10% discontinuation rate due to AEs at the 25 mg dose level (phase I+II), the response and tolerability rates found in this study are comparable or superior to other oral agents evaluated in HIV-associated KS(15),(16),(17),(26). A single-center trial of pomalidomide in KS demonstrated a similar overall response rate of 60% 15 participants with symptomatic HIV-KS, 3 of whom had complete remissions with a similar median time to response of 8 weeks(17). Since our trial only evaluated HIV-KS, the activity of lenalidomide in non-HIV KS remains to be demonstrated. However, given the reported activity of thalidomide(27) and pomalidomide(17) in non-HIV KS, activity of lenalidomide in this population is likely. As the three available IMiDs have not been compared prospectively in KS, it is difficult to conclude superiority of one agent, but lenalidomide and pomalidomide are generally more tolerable than thalidomide, particularly when considering peripheral neuropathy, which is more common with advanced HIV and in the elderly, and a common side effect of thalidomide. Finally, although IMiDs have been associated with a modest increased risk of secondary malignancies(28), including 3 reported in the trial of pomalidomide for KS,(17) none were reported in any of the current study participants.
Lenalidomide increased the numbers of T-regs in the peripheral blood. In addition, limited assessment of tumor biopsies suggests response may be associated with an increase in T-regs. FOXP3 staining, a marker of T-reg cells, was increased from baseline in biopsies of 2 participants obtaining PR compared to biopsies from 2 participants with SD. Although T-regs can be associated with cancer progression(29), the fact that KS represents an angioproliferative malignancy, whose growth could increase with inflammation, (30),(31) suggests that T-regs might actually hinder KS progression. In fact, several other cancers demonstrate a correlation between increased T-regs and better prognosis.(32),(33),(34)
In participants who had high baseline levels of inflammatory cytokines (i.e. IL-1β, IL-6, IL-8), there was a noticeable decrease in their level during lenalidomide treatment (Figure 4). In contrast, the level of IL-10, an anti-inflammatory cytokine, increased in response to lenalidomide, which could be associated with increased numbers of T-regs, as observed here. There were also increases in IL-12, a TH1-promoting immune stimulatory cytokine. The heterogeneity of the various cytokine levels in the current participant cohort precludes the use of individual cytokines as biomarkers of response.
KSHV gene expression patterns in tumor biopsies demonstrated decreased KSHV transcription at day 15 in 6 of 7 evaluable responders. Tissue from the other responder had increased KSHV transcription, as did biopsies from 2 participants with PD. There were no signs of lytic activation in response to treatment. Decrease in KSHV mRNA in tumor biopsies was associated with eventual tumor response to lenalidomide. A limitation to this analysis is that entire KS biopsies were analyzed rather than single cells. Hence, it was not possible to ascertain whether the decrease in KSHV transcription represented a loss of KSHV-positive tumor cells in the lesion, or suppression of KSHV transcription overall.
In summary, lenalidomide is feasible and active in HIV-KS with response rates comparable to those recently reported with pomalidomide. Given these therapies are available as oral formulations, they have the potential to improve access to KS therapy, particularly in resource limited settings, if the cost of these drugs is mitigated and their potential for teratogenicity managed. In follow-up to this study, multicenter domestic and international AMC trials (ClinicalTrials.gov Identifiers: NCT03601806, NCT04577755) are underway to confirm the tolerability and activity of IMiDs in KS. Other studies exploring the activity of IMiDs in conjunction with chemotherapy (NCT02659930) or immunotherapy (NCT04902443) are also underway. Further work is needed to understand the clinical and immunological differences among the different IMiDs and the implications of the lenalidomide-mediated alterations in cytokines and T-reg cells, to determine whether these may contribute to HIV, KSHV and KS control.
Supplementary Material
Translational Relevance:
As the largest study of immunomodulatory (IMiD) therapy in HIV-related Kaposi sarcoma (KS), this study demonstrates lenalidomide is active for this condition with response rates comparable to those reported with pomalidomide. Reported adverse events were consistent with the established safety profile of lenalidomide. The study also explored potential mechanisms of anti-KS activity in a subset of participants, providing data indicating blood and KS tumor T-regulatory cells increase in the setting of response to lenalidomide and showing changes in inflammatory cytokine patterns after lenalidomide as well as data suggesting response may be associated with loss of KS herpesvirus transcription. Given IMiDs are available as oral formulations, they have the potential to improve access to KS therapy, particularly in resource-limited settings. Following on this study, multicenter domestic and international AIDS Malignancy Consortium trials are underway to confirm the tolerability and activity of IMiDs in KS.
Acknowledgements:
We gratefully acknowledge the participants, research staff, additional statistical support by Eric Siegal, PhD, and additional AMC sub-investigators contributing to the conduct of this clinical trial: Mark Dickson, MD; Ayad Hamdan, MD; David Henry, MD; Juan Carlos Ramos, MD; Bruce Shiramizu, MD; Corey Casper, MD.
This study was supported by NCI U01 AMC grant (UM1CA121947), NIH Cancer Center Support Grant (NCI CCC 5P50 CA23100–25): UCSD Cancer Clinical Investigator Team Leadership Award (ER), UCLA Center for AIDS Research (P30 AI028697) (RM), UCLA Clinical Translational Sciences Institute (UL1 TR0001881) (RM), NIDCR RO1 DE018304 (DPD), and PHS grant CA019014 (DPD). This work was also supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply the endorsement by the U.S. government.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Dittmer DP, Damania B. Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Invest. 2016;126(9):3165–75. Epub 2016/09/01. doi: 10.1172/JCI84418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342(14):1027–38. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- 3.Pellet C, Chevret S, Blum L, Gauvillé C, Hurault M, Blanchard G, et al. Virologic and immunologic parameters that predict clinical response of AIDS-associated Kaposi’s sarcoma to highly active antiretroviral therapy. J Invest Dermatol. 2001;117(4):858–63. doi: 10.1046/j.0022-202x.2001.01465.x. [DOI] [PubMed] [Google Scholar]
- 4.Bower M, Nelson M, Young AM, Thirlwell C, Newsom-Davis T, Mandalia S, et al. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J Clin Oncol. 2005;23(22):5224–8. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- 5.Hessol NA, Whittemore H, Vittinghoff E, Hsu LC, Ma D, Scheer S, et al. Incidence of first and second primary cancers diagnosed among people with HIV, 1985–2013: a population-based, registry linkage study. Lancet HIV. 2018;5(11):e647–e55. Epub 2018/09/21. doi: 10.1016/S2352-3018(18)30179-6. [DOI] [PubMed] [Google Scholar]
- 6.Chinula L, Moses A, Gopal S. HIV-associated malignancies in sub-Saharan Africa: progress, challenges, and opportunities. Curr Opin HIV AIDS. 2017;12(1):89–95. doi: 10.1097/COH.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007;357(13):1352–3. Epub 2007/09/28. doi: 357/13/1352 [pii] 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 8.Unemori P, Leslie KS, Hunt PW, Sinclair E, Epling L, Mitsuyasu R, et al. Immunosenescence is associated with presence of Kaposi’s sarcoma in antiretroviral treated HIV infection. AIDS. 2013;27(11):1735–42. doi: 10.1097/QAD.0b013e3283601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavallin LE, Goldschmidt-Clermont P, Mesri EA. Molecular and cellular mechanisms of KSHV oncogenesis of Kaposi’s sarcoma associated with HIV/AIDS. PLoS Pathog. 2014;10(7):e1004154. Epub 2014/07/10. doi: 10.1371/journal.ppat.1004154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamara RP, Chugh PE, Bailey A, Costantini LM, Ma Z, Bigi R, et al. Extracellular vesicles from Kaposi Sarcoma-associated herpesvirus lymphoma induce long-term endothelial cell reprogramming. PLoS Pathog. 2019;15(2):e1007536. Epub 2019/02/04. doi: 10.1371/journal.ppat.1007536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shortt J, Hsu AK, Johnstone RW. Thalidomide-analogue biology: immunological, molecular and epigenetic targets in cancer therapy. Oncogene. 2013;32(36):4191–202. Epub 2013/01/14. doi: 10.1038/onc.2012.599. [DOI] [PubMed] [Google Scholar]
- 12.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–6. [PubMed] [Google Scholar]
- 13.Haslett PA, Klausner JD, Makonkawkeyoon S, Moreira A, Metatratip P, Boyle B, et al. Thalidomide stimulates T cell responses and interleukin 12 production in HIV-infected patients. AIDS Res Hum Retroviruses. 1999;15(13):1169–79. doi: 10.1089/088922299310269. [DOI] [PubMed] [Google Scholar]
- 14.Clerici M, Lucey DR, Berzofsky JA, Pinto LA, Wynn TA, Blatt SP, et al. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262(5140):1721–4. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 15.Fife K, Howard MR, Gracie F, Phillips RH, Bower M. Activity of thalidomide in AIDS-related Kaposi’s sarcoma and correlation with HHV8 titre. Int J STD AIDS. 1998;9(12):751–5. [DOI] [PubMed] [Google Scholar]
- 16.Little RF, Wyvill KM, Pluda JM, Welles L, Marshall V, Figg WD, et al. Activity of thalidomide in AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2000;18(13):2593–602. [DOI] [PubMed] [Google Scholar]
- 17.Polizzotto MN, Uldrick TS, Wyvill KM, Aleman K, Peer CJ, Bevans M, et al. Pomalidomide for Symptomatic Kaposi’s Sarcoma in People With and Without HIV Infection: A Phase I/II Study. J Clin Oncol. 2016;34(34):4125–31. Epub 2016/10/31. doi: 10.1200/JCO.2016.69.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1989;7(9):1201–7. doi: 10.1200/JCO.1989.7.9.1201. [DOI] [PubMed] [Google Scholar]
- 19.Epstein MM, Rosner B, Breen EC, Batista JL, Giovannucci EL, Magpantay L, et al. Pre-diagnosis plasma immune markers and risk of non-Hodgkin lymphoma in two prospective cohort studies. Haematologica. 2018;103(10):1679–87. Epub 2018/06/21. doi: 10.3324/haematol.2017.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, et al. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiol Biomarkers Prev. 2013;22(11):2009–15. Epub 2013/08/27. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Lee JY, Kaplan LD, Dezube BJ, Noy A, Krown SE, et al. Effects of chemotherapy in AIDS-associated non-Hodgkin’s lymphoma on Kaposi’s sarcoma herpesvirus DNA in blood. J Clin Oncol. 2009;27(15):2496–502. Epub 2009/04/06. doi: 10.1200/JCO.2008.20.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseinipour MC, Sweet KM, Xiong J, Namarika D, Mwafongo A, Nyirenda M, et al. Viral profiling identifies multiple subtypes of Kaposi’s sarcoma. MBio. 2014;5(5):e01633–14. Epub 2014/09/23. doi: 10.1128/mBio.01633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, et al. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. Epub 2008/04/22. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dittmer DP. Restricted Kaposi’s sarcoma (KS) herpesvirus transcription in KS lesions from patients on successful antiretroviral therapy. MBio. 2011;2(6):e00138–11. Epub 2011/11/01. doi: 10.1128/mBio.00138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pourcher V, Desnoyer A, Assoumou L, Lebbe C, Curjol A, Marcelin AG, et al. Phase II Trial of Lenalidomide in HIV-Infected Patients with Previously Treated Kaposi’s Sarcoma: Results of the ANRS 154 Lenakap Trial. AIDS Res Hum Retroviruses. 2017;33(1):1–10. Epub 2016/09/07. doi: 10.1089/AID.2016.0069. [DOI] [PubMed] [Google Scholar]
- 26.Koon HB, Krown SE, Lee JY, Honda K, Rapisuwon S, Wang Z, et al. Phase II trial of imatinib in AIDS-associated Kaposi’s sarcoma: AIDS Malignancy Consortium Protocol 042. J Clin Oncol. 2014;32(5):402–8. Epub 2013/12/30. doi: 10.1200/JCO.2012.48.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubegni P, Sbano P, De Aloe G, Flori ML, Fimiani M. Thalidomide in the treatment of Kaposi’s sarcoma. Dermatology. 2007;215(3):240–4. doi: 10.1159/000106583. [DOI] [PubMed] [Google Scholar]
- 28.Pan B, Lentzsch S. The application and biology of immunomodulatory drugs (IMiDs) in cancer. Pharmacol Ther. 2012;136(1):56–68. Epub 2012/07/14. doi: 10.1016/j.pharmthera.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18. Epub 2016/12/20. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douglas JL, Gustin JK, Dezube B, Pantanowitz JL, Moses AV. Kaposi’s sarcoma: a model of both malignancy and chronic inflammation. Panminerva Med. 2007;49(3):119–38. [PubMed] [Google Scholar]
- 31.Riva G, Barozzi P, Torelli G, Luppi M. Immunological and inflammatory features of Kaposi’s sarcoma and other Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8-associated neoplasias. AIDS Rev. 2010;12(1):40–51. [PubMed] [Google Scholar]
- 32.Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010;127(4):748–58. doi: 10.1002/ijc.25464. [DOI] [PubMed] [Google Scholar]
- 33.Alvaro T, Lejeune M, Salvadó MT, Bosch R, García JF, Jaén J, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–73. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 34.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–92. Epub 2008/12/08. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 35.Pantanowitz L, Dezube BJ, Pinkus GS, Tahan SR. Histological characterization of regression in acquired immunodeficiency syndrome-related Kaposi’s sarcoma. J Cutan Pathol. 2004;31(1):26–34. doi: 10.1046/j.0303-6987.2004.0132.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.