Abstract
Purpose of review:
Levels of small, dense LDL (sdLDL) particles determined by several analytic procedures have been associated with risk of atherosclerotic cardiovascular disease (ASCVD). This review focuses on the clinical significance of sdLDL measurement.
Recent findings:
Results of multiple prospective studies have supported earlier evidence that higher levels of sdLDL are significantly associated with greater ASCVD risk, in many cases independent of other lipid and ASCVD risk factors as well as levels of larger LDL particles. A number of properties of sdLDL vs. larger LDL, including reduced LDL receptor affinity and prolonged plasma residence time as well as greater oxidative susceptibility and affinity for arterial proteoglycans, are consistent with their heightened atherogenic potential. Nevertheless, determination of the extent to which sdLDL can preferentially impact ASCVD risk compared with other apoB-containing lipoproteins has been confounded by their metabolic interrelationships and statistical collinearity, as well as differences in analytic procedures and definitions of sdLDL.
Summary:
A growing body of data points to sdLDL concentration as a significant determinant of ASCVD risk. While future studies should be aimed at determining the clinical benefit of reducing sdLDL levels, there is sufficient evidence to warrant consideration of sdLDL measurement in assessing and managing risk of cardiovascular disease.
Keywords: Small dense LDL, cholesterol, cardiovascular disease
Introduction
Plasma low density lipoproteins (LDL) are defined as particles of density 1.019–1.063 g/ml. Within this spectrum, there are multiple subspecies varying in size, density, charge, and lipid and protein composition (1). Among these, as discussed below, small, dense, lipid depleted LDL particles (sdLDL) have been found to be particularly strongly associated with risk of atherosclerotic cardiovascular disease (ASCVD) (2). This review focuses on issues that have arisen as to the clinical significance of sdLDL measurement by addressing these questions: 1) how are sdLDL defined and measured?; 2) are sdLDL levels predictive of ASCVD risk independent of LDL-cholesterol (LDL-C) and beyond standard risk algorithms?; 3) is there evidence that sdLDL and large buoyant LDL (lbLDL) differentially affect ASCVD risk?; and 4) is there a basis for selectively targeting sdLDL as a means of reducing ASCVD risk?
sdLDL Definition and Measurement
Multiple methodologies have been implemented for analysis of subclasses of LDL and other lipoprotein particle subspecies as a function of their density and/or diameter, including analytical and density gradient ultracentrifugation, non-denaturing gradient gel electrophoresis (GGE), nuclear magnetic resonance spectroscopy (NMR), and ion mobility (IM) (2–5). Table 1 describes properties of the LDL subspecies as a function of their analytic ultracentrifuge flotation rate (1), buoyant density (1), and particle size as assessed by IM (6). In this scheme, sdLDL can be considered to comprise the particle species spanning the range of small and very small LDL (vsLDL) from density 1.034 to 1.063 g/ml and size 214.1 to 180 Å. However, while there has been a report of correlations of LDL size subclass concentrations among several methodologies (4), their strengths varied considerably. Moreover, the subclass definitions have not been harmonized across the various analytic procedures, nor have their results been compared using common defined reference lipoprotein subfraction preparations. As has been pointed out (3, 5) these concerns have compromised the adoption of sdLDL concentration as a standard clinical laboratory measurement.
Table.
Classification of LDL subspecies based on analytic ultracentrifugal flotation rate (1), density range (1), and particle size interval (4).
| LDL Subclass Analytic ultracentrifugation flotation rate (Sf) |
LDL Size Subspecies | Density Range Density gradient ultracentrifugation (g/ml) |
Size Interval Ion mobility (Å) |
|---|---|---|---|
| Large LDL-I (Sf 7–12) | 1 | 1.019 – 1.023 | 224.6 – 233.3 |
| Medium LDL-II (Sf 5–7) | 2a | 1.023 – 1.028 | 220.0 – 224.6 |
| 2b | 1.028 – 1.034 | 214.1 – 220.0 | |
| Small LDL-III (Sf 3–5) | 3a | 1.034 – 1.041 | 208.2 – 214.1 |
| 3b | 1.041 – 1.044 | 204.9 –208.2 | |
| Very small LDL-IV (Sf 0–3) | 4a | 1.044 – 1.051 | 199.0 – 204.9 |
| 4b | 1.051 – 1.063 | 190.0 – 199.0 | |
| 4c | Not determined | 180.0 – 190.0 |
In addition to the methods for analyzing LDL particle concentrations noted above, a homogenous direct method for determination of plasma sdLDL-cholesterol (sdLDL-C) has been developed that has been shown to correlate well with levels of sdLDL defined as particles of d =1.044–1.063 g/mL (7, 8). However, based on the LDL density ranges shown in Table 1, it is likely that this fraction corresponds with very small LDL-IV (vsLDL) rather than the small LDL-III subclass, as demonstrated by correlations of vsLDL-cholesterol determined by GGE with vsLDL mass concentrations measured by both analytical ultracentrifugation and IM (2). Thus, the potential ASCVD risk related to levels of LDL-III may not be identified by this procedure. Another consideration is that the 1.044–1.063 g/mL density range includes a small proportion of atherogenic lipoprotein(a) particles, and therefore it is possible that the sdLDL-C assay is also detecting this component. However the particle diameter of Lp(a) significantly exceeds that of sdLDL (9), and thus Lp(a) is not included in size-based measurements of sdLDL (GGE, NMR, and IM).
sdLDL in the Prediction of CVD Risk
The assessment of the relationship of sdLDL to ASCVD risk is confounded by the fact that this LDL category is a component of a lipoprotein phenotype that includes elevated levels of triglycerides and very low density lipoproteins (VLDLs) and reduced levels of high density lipoprotein (HDL)-cholesterol (10), and it is correlated with plasma apolipoprotein B and other ASCVD risk factors comprising metabolic syndrome (1). The multiple co-linearity among these measures creates challenges for assessing the specific impact of sdLDL using standard statistical approaches, which are subject to potential over-adjustment. Nevertheless, the question can be raised as to the extent to which sdLDL predicts ASCVD beyond standard risk measures.
Multiple prospective studies have assessed the relation of baseline sdLDL level to incidence of clinical cardiovascular events. A recent systematic review and meta-analysis assembled data from prospective studies (cohort, case-control, nested case-control, and randomized controlled trial) published through January, 2020 that utilized measurements derived from GGE, NMR, and the homogeneous sdLDL-cholesterol assay (11). The composite relative risk/hazard ratios in 14 studies with measurements of sdLDL other than sdLDL-C were 1.46, 1.72, and 1.54 (all but the last statistically significant) in analyses adjusted for co-variates (age and sex; demographics plus lifestyle risk factors; demographics plus lifestyle risk factors and lipid fractions, respectively). In a second group of 7 studies in which sdLDL-C was determined using GGE or the direct homogeneous assay (11), the values were 1.21, 1.92, and 1.75, respectively (all significant).
The question as to whether the relation of sdLDL-C to ASCVD risk is independent of total LDL and other risk factors was addressed in two of the prospective cohort studies included in the meta-analysis, as well as in several more recent reports. In the Atherosclerosis Risk in Communities study of 10,225 individuals followed for an average of 11 years, the hazard ratio for coronary heart disease (CHD) was 1.51 (95% confidence interval [CI] 1.21–1.88) for the highest versus the lowest quartile, respectively, in a model that included established risk factors, with similar values for individuals with total LDL-C greater or less than 100 mg/dL (12). Moreover, among 3,334 normoglycemic participants in the Multiethnic Study of Atherosclerosis (MESA), those in the top sdLDL-C quartile showed higher risk of incident CHD (hazard ratio, 2.41; P=0.0037) compared with those in the bottom quartile in multivariable adjusted models, with very similar values in those with total LDL-C above and below 100 mg/dL (13). In another analysis of MESA data, the estimated plasma level of sdLDL-C derived from an algorithm based on standard lipid measurements was the strongest lipid predictor of ASCVD after multivariate adjustment for other known ASCVD risk factors (14). Notably, in both ARIC and MESA (12, 13) there were no significant ASCVD associations with quartiles of lbLDL-cholesterol, determined as the difference between total LDL-C and sdLDL-C.
Several recent studies not included in the meta-analysis also documented a relationship of sdLDL-C to ASCVD risk. Among 38,322 individuals participating in the Copenhagen General Population Study with a median 3.1 yr of follow-up, the hazard ratio for ASCVD per 1 mmol/l (39 mg/dl) higher sdLDL-C in a multivariable-adjusted Cox model was 1.62 (95% CI: 1.33–1.96) (15). In a nested prospective case-control study from the Women’s Health Study, sdLDL-C was significantly associated with myocardial infarction (HR for Quartile 4 vs 1: 3.71 [95% CI: 1.59 to 8.63]; p for trend < 0.001) (16). Additionally, in 3094 individuals initially free of ASCVD in the Framingham Offspring Study, the ASCVD HR for sdLDL-C was 1.28 (CI 1.04–1.58, p=0.021) in a multivariable model including total cholesterol and HDL-C and other standard ASCVD risk factors (17). Moreover, elevated sdLDL-C was the best lipoprotein-related measure of incident ASCVD and added significant risk information to the ACC/AHA pooled cohort equation (17). In another recent study of patients with angiographically documented coronary artery disease (CAD), elevated level of sdLDL-C, but not total LDL-C or non-HDL-C, was predictive of ASCVD events, a finding driven by the presence of diabetes mellitus (18). Finally, in a trial of patients with stable CAD randomized to low dose (1 mg/d) vs. high dose (4 mg/d) pitavastatin, there was a significant association of sdLDL-C quartiles with ASCVD events independent of LDL-C and other risk factors on the low dose, whereas there was no association on the high dose, suggested to reflect a significantly greater reduction of sdLDL-C in those with higher baseline levels (19).
A caveat to interpretation of the findings for sdLDL-C, as noted above, is that this may not represent the full spectrum of sdLDL particles (LDL-III + LDL-IV), as do, for example, the NMR and IM methods. Moreover, two studies employing detailed measurements of LDL size subfractions by IM have shown that levels of medium size LDL-II as well as small LDL-III (but not large LDL-I), are significantly predictive of ASCVD (20, 21).
Do sdLDL and lbLDL Differentially Impact ASCVD?
While the evidence reviewed above points to a significant association of sdLDL with ASCVD risk, in many cases independent of other risk factors, there has been controversy as to whether this represents differential atherogenicity of small and large LDL particles, and hence whether concentration of total LDL particles (LDL-Ps) or, as discussed below, apoprotein B (apoB), is sufficient as an LDL-related measure of ASCVD risk. A number of properties of sdLDL particles can be invoked to suggest heightened atherogenicity compared with larger LDL (22). These include prolonged plasma residence time (23) reflecting reduced LDL receptor binding (24) due to conformational changes in apoB (25), increased susceptibility to oxidative modification (26) and glycation (27), preferential enrichment in lipoprotein-associated phospholipase A2 (28) and the pro-inflammatory protein apoC-III (29), and content of specific components of the lipidome that can act to enhance the atherogenicity of sdLDL (30). On the other hand, it has been suggested that the higher cholesterol content of larger LDL particles should be considered as a feature that may promote atherogenesis to a similar degree as sdLDL (31). Nevertheless, despite these considerations, it cannot be assumed that the differing pathophysiologic properties of LDL subclasses translate into their impact on ASCVD outcomes.
Several additional arguments have been made in support of the premise that smaller and larger LDL particles are equally atherogenic, and hence that measurement of sdLDL level does not add to the risk information provided by total LDL-P concentrations. As described below, however, there are concerns with each of these arguments:
When both peak LDL diameter and LDL-P concentration have been included in models assessing their relation to carotid atherosclerosis (32) or ASCVD (33, 34), only LDL-P has been found to be significantly predictive of risk. However, while peak LDL diameter can be used to identify larger and smaller LDL subclass phenotypes, as discussed below, it does not represent the more pathologically relevant absolute concentrations of the subclasses.
Patients with familial hypercholesterolemia (FH) have a relative abundance of large LDL particles (35), and this has supported the case that large LDL-Ps as well as small LDL-Ps are atherogenic, and that thus the total number rather than the size of LDL-Ps is the key LDL determinant of CVD risk. This does not however consider that the total number of LDL-Ps is much greater in FH than is typically seen in patients with atherogenic dyslipidemia, and also that due to LDL receptor deficiency, levels of small as well as large LDL are increased. Importantly however, if, as is likely, prolonged plasma residence time of LDL particles is a significant determinant of ASCVD risk, this mechanism may apply both to sdLDL by virtue of their lower LDL receptor affinity, as noted above, and to the more general impairment of plasma LDL-P clearance due to reduced LDL receptor number or function in FH.
A related issue to consider is evidence that apoB, an established ASCVD index (36) which represents the total concentration of LDL, intermediate density lipoprotein, and VLDL particles, is strongly correlated with levels of small LDL-III [(12) and Figure 1], but not with large LDL-I (Figure 1). Hence it can be argued that apoB level may be sufficient to assess the impact of sdLDL, as well as other atherogenic lipoproteins, on ASCVD risk. The studies cited above indicating the relationship of sdLDL to ASCVD did not attempt to adjust for its collinearity with apoB. However, the variance in sdLDL explained by apoB [r2=~40%, (12) and Figure 1] is not sufficient to consider apoB as a surrogate for sdLDL, nor do the relationships with apoB address the relative atherogenicity of small vs. large LDL particles.
Since as noted above there is in general an inverse correlation between plasma levels of small and large LDL (4), it has been suggested that both subfractions should be included in regression models for assessing their independent relation to ASCVD risk. The application of such a model has indicated that while large LDL did not have a significant univariate relationship with risk, the association became significant when both fractions were included in the model (32, 33). On this basis, as well as observations in some studies that the association of sdLDL with ASCVD is reduced in significance with inclusion of total LDL-P in regression models, it has been concluded that large and small subfractions have similar atherogenic effects on a per-particle basis, and hence that total LDL-P is superior to sdLDL for risk stratification and lipid management (32, 33). This statistical method however obscures the confounding differential impact of LDL size phenotypes. It has been shown that peak LDL particle size and density generally follow a bimodal distribution in the population, with the modes representing individuals with predominance of either lbLDL (phenotype A) or sdLDL (phenotype B) (6, 37–39), and that these phenotypes have, in part, a genetic basis (40). Based on this bimodality, it would be considered appropriate to include both LDL size phenotype and LDL subfractions in multivariable regression models, as well as an interaction term for the phenotype. The results of including such an interaction in assessing the relationship between large and small LDL particle concentrations are illustrated in Figure 2, which is based on IM data from 158 overweight but otherwise healthy individuals [((41), (Krauss, R.M., personal communication)]. Figure 2A displays the modes in the peak LDL particle diameter distribution, and Figure 2B shows an inverse correlation between large and small LDL concentrations using a linear model. However, as shown in Figure 2C, a model employing a significant interaction term for LDL size phenotype (p<0.001) reveals that for phenotype B there is a significant positive association between large and small LDL concentrations (p<0.001), whereas there is not a significant association for phenotype A. Thus, it would not be appropriate for ASCVD risk prediction to employ a model including both large and small LDL with the aim of adjusting for the relationship between them without including an LDL phenotype interaction term which could unmask the effect modification by these phenotypes. Differing relationships of large and small LDL concentrations between the two LDL subclass phenotypes are consistent with the significant differences in the physical and compositional properties of LDL subfractions between the phenotypes (42, 43).
Figure 1. Correlations between plasma levels of immunochemically measured apoB and small LDL-III and large LDL-I.

LDL measurements were made by ion mobility in 804 healthy men and women using data derived from a previously published study (52). Similar results were obtained for small LDL defined as LDL-III plus LDL-IV. r: Pearson correlation coefficient. n.s.: not significant at p <0.05.
Figure 2. Statistical considerations in assessing interrelationships of large and small LDL.
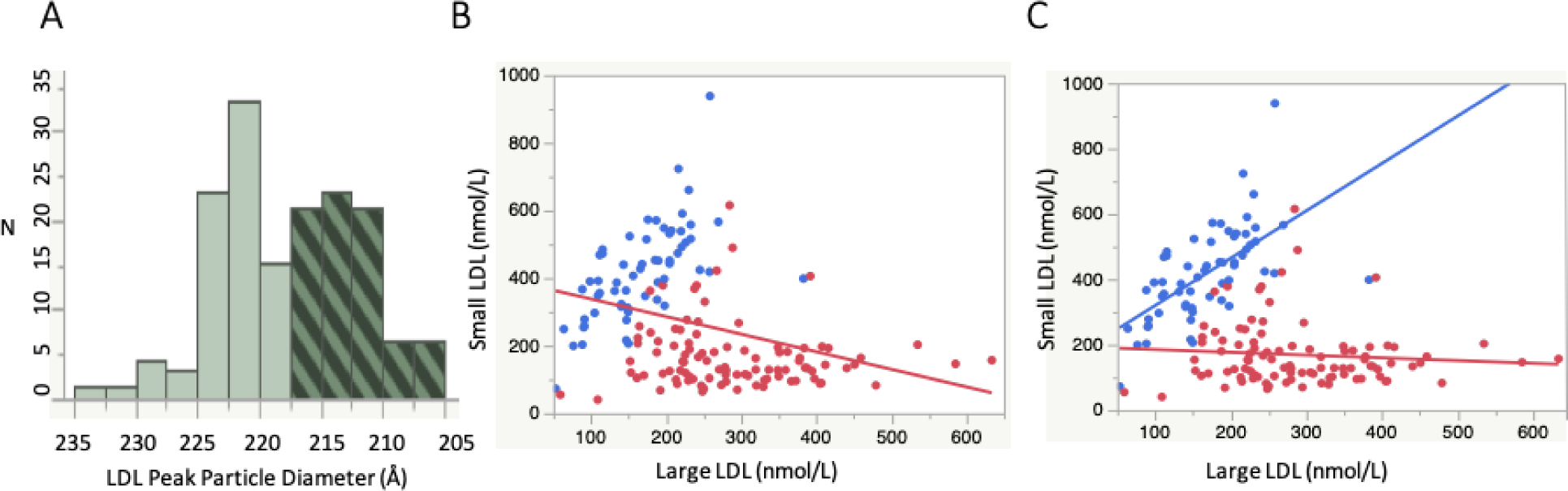
2A: Bimodality of the distribution LDL peak particle diameter determined by ion mobility in 158 overweight but otherwise healthy participants in a previously published study (41). The clear bars represent phenotype A (peak diameter >217.5 Å) and the cross-hatched bars represent phenotype B. 2B: Concentrations of lbLDL (LDL-I) vs. sdLDL (LDL-III). Red circles are individuals with phenotype B and blue circles are those with phenotype B. The line represents a significant inverse relationship (p<0.001) using a linear regression model. 1C: The same data as in Figure 1B with regression lines determined in a model incorporating an interaction for LDL size phenotype (p<0.001). The positive slope for phenotype B is significant (p<0.001), whereas the relationship for phenotype A is not.
In summary, these considerations, coupled with the results from the prospective studies reviewed above, do not provide strong support for the premise that all LDL particles are equally atherogenic. Multiple properties of sdLDL that differentiate them from larger LDL particles, as noted above, may contribute to a preferential impact on ASCVD risk.
Is there a basis for targeting sdLDL for reducing ASCVD risk?
Statins, the most commonly used lipid-lowering drugs, are effective in lowering levels of sdLDL, but the magnitude of reduction can differ between statin types (44) and doses (45), and may depend on a patient’s dyslipidemic phenotype (45–47). In this regard, it has been shown that the reduction in sdLDL is strongly correlated with baseline triglyceride level (46), likely reflecting reduced LDL formation as a result of statin-induced catabolism of TG-rich precursors (47). Moreover, the measurement used to assess sdLDL may be a factor influencing the results, in that statins lower cholesteryl ester content of all apoB-containing lipoproteins (46), and hence assays that measure sdLDL-C may manifest a disproportionally greater reduction relative to sdLDL particle concentration. Finally, there is variation in the capability of differing analytic techniques to clearly distinguish sdLDL and vsLDL subspecies from other LDL subclasses (3,48), and in this regard there are several reports based on high resolution ion mobility measurements that statin-induced reductions in levels of vsLDL particles are much smaller than those for larger LDL (20, 49, 50) likely reflecting, at least in part, their reduced LDL receptor affinity. For this reason, and also to assess the therapeutic benefit of reducing levels of sdLDL, it would be desirable to develop therapeutic approaches for specifically targeting these particles. However, the strong metabolic interrelationships of sdLDL with other lipids and lipoproteins creates a challenge for devising such an intervention. Alternatively, the impact of lowering sdLDL on ASCVD risk could be predicted using a Mendelian randomization analysis. In this regard, it is of interest that a common ASCVD risk-raising allele at a locus that regulates expression of the SORT1/CELSR2/PSRC1 gene cluster is associated with higher levels of sdLDL and vsLDL, but not with larger LDL particles (51). However, since this allele is also significantly associated with elevated levels of total LDL-C, it has not been feasible to conduct a Mendelian randomization analysis based on this genetic locus that specifically addresses the causality of the small and very small LDL subspecies.
Conclusion
There is a growing body of evidence for a strong relationship of sdLDLs to ASCVD risk, and this presents a case for their measurement in assessing and managing this risk. However there remain challenges for determining the utility of incorporating sdLDL concentration in standard clinical guidelines. These point to the need for standardization across sdLDL assays, as well as research aimed at testing the impact on ASCVD risk of selectively reducing plasma sdLDL concentrations.
Supplementary Material
Key points.
* Recent prospective studies have supported a significant association of plasma sdLDL concentration with risk of ASCVD.
There is evidence that this relationship is independent of plasma LDL cholesterol level and can add to the risk assessed by current algorithms.
The strong metabolic and statistical interrelationships of sdLDL with other lipoprotein and ASCVD risk markers confound the ability to determine the specific contribution of sdLDL to disease risk.
Several analytic methodologies are available for clinical measurement of sdLDL concentrations, but there remains the need to harmonize their results.
Taken together with the pathophysiologic properties of sdLDL, the available data do not support the premise that all LDL particles are equally atherogenic and suggest that measurement of sdLDL has a role in the clinical assessment and monitoring of ASCVD risk.
Acknowledgments
Thanks to Dr. Alan Remaley for helpful comments.
Footnotes
Conflict of Interest
Ronald Krauss has research funding from Quest Diagnostics, Inc., and has licensed patents for lipoprotein particle analysis by ion mobility.
References and recommended reading:
- 1.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43(9):1363–79. [DOI] [PubMed] [Google Scholar]
- 2.Krauss RM. All low-density lipoprotein particles are not created equal. Arterioscler Thromb Vasc Biol. 2014;34(5):959–61. [DOI] [PubMed] [Google Scholar]
- 3. Kanonidou C Small dense low-density lipoprotein: Analytical review. Clin Chim Acta. 2021;520:172–8. • This paper presents a critical analysis of various methodologies for sdLDL measurement.
- 4.Williams PT, Zhao XQ, Marcovina SM, Otvos JD, Brown BG, Krauss RM. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis. 2014;233(2):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson PWF, Jacobson TA, Martin SS, Jackson EA, Le NA, Davidson MH, et al. Lipid measurements in the management of cardiovascular diseases: Practical recommendations a scientific statement from the national lipid association writing group. J Clin Lipidol. 2021. [DOI] [PubMed] [Google Scholar]
- 6.Athinarayanan SJ, Hallberg SJ, McKenzie AL, Lechner K, King S, McCarter JP, et al. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albers JJ, Kennedy H, Marcovina SM. Evaluation of a new homogenous method for detection of small dense LDL cholesterol: comparison with the LDL cholesterol profile obtained by density gradient ultracentrifugation. Clin Chim Acta. 2011;412(7–8):556–61. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Fujimura M, Ohta M, Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem. 2011;57(1):57–65. [DOI] [PubMed] [Google Scholar]
- 9.O’Neal D, Grieve G, Rae D, Dragicevic G, Best JD. Factors influencing Lp[a]- particle size as determined by gradient gel electrophoresis. J Lipid Res. 1996;37(8):1655–63. [PubMed] [Google Scholar]
- 10.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82(2):495–506. [DOI] [PubMed] [Google Scholar]
- 11. Liou L, Kaptoge S. Association of small, dense LDL-cholesterol concentration and lipoprotein particle characteristics with coronary heart disease: A systematic review and meta-analysis. PLoS One. 2020;15(11):e0241993. • The authors have assembled a number of studies using various methodologies for addressing the relation of sdLDL levels to risk of coronary artery disease, and performed a meta-analysis for assessing relative risks and hazard ratios using data derived from these studies. This review does not however include studies using ion mobility methodology, as well as several published since January, 2020.
- 12.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(1):196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson M, Wolska A, Warnick R, Lucero D, Remaley AT. A New Equation Based on the Standard Lipid Panel for Calculating Small Dense Low-Density Lipoprotein-Cholesterol and Its Use as a Risk-Enhancer Test. Clin Chem. 2021;67(7):987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balling M, Nordestgaard BG, Langsted A, Varbo A, Kamstrup PR, Afzal S. Small Dense Low-Density Lipoprotein Cholesterol Predicts Atherosclerotic Cardiovascular Disease in the Copenhagen General Population Study. J Am Coll Cardiol. 2020;75(22):2873–5. [DOI] [PubMed] [Google Scholar]
- 16. Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. 2020;75(17):2122–35. •• Using data from the Women’s Health Study, this study makes the observation that both sdLDL cholesterol and triglyceride-rich lipoprotein cholesterol are strongly associated with risk of myocardial infarction independent of LDL cholesterol and high sensitivity C-reactive protein, whereas only triglyceride-rich lipoprotein cholesterol is associated with risk of peripheral artery disease. This speaks to the differential atherogenic effects of these particles in the two vascular beds.
- 17. Ikezaki H, Lim E, Cupples LA, Liu CT, Asztalos BF, Schaefer EJ. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the Prospective Framingham Offspring Study. J Am Heart Assoc. 2021;10(5):e019140. • Inclusion of sdLDL-cholesterol in various models for assessing ASCVD risk was shown to provide stronger hazard ratios than other lipoprotein measures, and to increase the C-statistic on top of the ACC/AHA pooled risk equation.
- 18.Jin JL, Zhang HW, Cao YX, Liu HH, Hua Q, Li YF, et al. Association of small dense low-density lipoprotein with cardiovascular outcome in patients with coronary artery disease and diabetes: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii J, Kashiwabara K, Ozaki Y, Takahashi H, Kitagawa F, Nishimura H, et al. Small Dense Low-Density Lipoprotein Cholesterol and Cardiovascular Risk in Statin-Treated Patients with Coronary Artery Disease. J Atheroscler Thromb. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mora S, Caulfield MP, Wohlgemuth J, Chen Z, Superko HR, Rowland CM, et al. Atherogenic Lipoprotein Subfractions Determined by Ion Mobility and First Cardiovascular Events After Random Allocation to High-Intensity Statin or Placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial. Circulation. 2015;132(23):2220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29(11):1975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–30. •• Using data from the Women’s Health Study, this study makes the observation that both sdLDL cholesterol and triglyceride-rich lipoprotein cholesterol are strongly associated with risk of myocardial infarction independent of LDL cholesterol and high sensitivity C-reactive protein, whereas only triglyceride-rich lipoprotein cholesterol is associated with risk of peripheral artery disease. This speaks to the differential atherogenic effects of these particles in the two vascular beds.
- 23.Thongtang N, Diffenderfer MR, Ooi EMM, Barrett PHR, Turner SM, Le NA, et al. Metabolism and proteomics of large and small dense LDL in combined hyperlipidemia: effects of rosuvastatin. J Lipid Res. 2017;58(7):1315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos H, Arnold KS, Balestra ME, Innerarity TL, Krauss RM. Differences in receptor binding of LDL subfractions. Arterioscler Thromb Vasc Biol. 1996;16(6):794–801. [DOI] [PubMed] [Google Scholar]
- 25.Lund-Katz S, Laplaud PM, Phillips MC, Chapman MJ. Apolipoprotein B-100 conformation and particle surface charge in human LDL subspecies: implication for LDL receptor interaction. Biochemistry. 1998;37(37):12867–74. [DOI] [PubMed] [Google Scholar]
- 26.Tribble DL, Holl LG, Wood PD, Krauss RM. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis. 1992;93(3):189–99. [DOI] [PubMed] [Google Scholar]
- 27.Soran H, Durrington PN. Susceptibility of LDL and its subfractions to glycation. Curr Opin Lipidol. 2011;22(4):254–61. [DOI] [PubMed] [Google Scholar]
- 28.McCall MR, La Belle M, Forte TM, Krauss RM, Takanami Y, Tribble DL. Dissociable and nondissociable forms of platelet-activating factor acetylhydrolase in human plasma LDL: implications for LDL oxidative susceptibility. Biochim Biophys Acta. 1999;1437(1):23–36. [DOI] [PubMed] [Google Scholar]
- 29.Shin MJ, Krauss RM. Apolipoprotein CIII bound to apoB-containing lipoproteins is associated with small, dense LDL independent of plasma triglyceride levels in healthy men. Atherosclerosis. 2010;211(1):337–41. [DOI] [PubMed] [Google Scholar]
- 30.Chapman MJ, Orsoni A, Tan R, Mellett NA, Nguyen A, Robillard P, et al. LDL subclass lipidomics in atherogenic dyslipidemia: effect of statin therapy on bioactive lipids and dense LDL. J Lipid Res. 2020;61(6):911–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, et al. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019;4(12):1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC Jr., et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2007;192(1):211–7. [DOI] [PubMed] [Google Scholar]
- 33.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113(12):1556–63. [DOI] [PubMed] [Google Scholar]
- 34.Jungner I, Sniderman AD, Furberg C, Aastveit AH, Holme I, Walldius G. Does low-density lipoprotein size add to atherogenic particle number in predicting the risk of fatal myocardial infarction? Am J Cardiol. 2006;97(7):943–6. [DOI] [PubMed] [Google Scholar]
- 35.Teng B, Thompson GR, Sniderman AD, Forte TM, Krauss RM, Kwiterovich PO, Jr. Composition and distribution of low density lipoprotein fractions in hyperapobetalipoproteinemia, normolipidemia, and familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1983;80(21):6662–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259(3):247–58. [DOI] [PubMed] [Google Scholar]
- 37.Dreon DM, Fernstrom HA, Williams PT, Krauss RM. A very low-fat diet is not associated with improved lipoprotein profiles in men with a predominance of large, low-density lipoproteins. Am J Clin Nutr. 1999;69(3):411–8. [DOI] [PubMed] [Google Scholar]
- 38.Georgieva AM, van Greevenbroek MM, Krauss RM, Brouwers MC, Vermeulen VM, Robertus-Teunissen MG, et al. Subclasses of low-density lipoprotein and very low-density lipoprotein in familial combined hyperlipidemia: relationship to multiple lipoprotein phenotype. Arterioscler Thromb Vasc Biol. 2004;24(4):744–9. [DOI] [PubMed] [Google Scholar]
- 39.Miller BD, Alderman EL, Haskell WL, Fair JM, Krauss RM. Predominance of dense low-density lipoprotein particles predicts angiographic benefit of therapy in the Stanford Coronary Risk Intervention Project. Circulation. 1996;94(9):2146–53. [DOI] [PubMed] [Google Scholar]
- 40.Austin MA, King MC, Vranizan KM, Newman B, Krauss RM. Inheritance of low-density lipoprotein subclass patterns: results of complex segregation analysis. Am J Hum Genet. 1988;43(6):838–46. [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu S, Williams PT, Krauss RM. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS One. 2017;12(2):e0170664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Belle M, Blanche PJ, Krauss RM. Charge properties of low density lipoprotein subclasses. J Lipid Res. 1997;38(4):690–700. [PubMed] [Google Scholar]
- 43.La Belle M, Krauss RM. Differences in carbohydrate content of low density lipoproteins associated with low density lipoprotein subclass patterns. J Lipid Res. 1990;31(9):1577–88. [PubMed] [Google Scholar]
- 44.Takagi H, Niwa M, Mizuno Y, Yamamoto H, Goto SN, Umemoto T. Effects of rosuvastatin versus atorvastatin on small dense low-density lipoprotein: a meta-analysis of randomized trials. Heart Vessels. 2014;29(3):287–99. [DOI] [PubMed] [Google Scholar]
- 45.Guerin M, Egger P, Soudant C, Le Goff W, van Tol A, Dupuis R, et al. Dose-dependent action of atorvastatin in type IIB hyperlipidemia: preferential and progressive reduction of atherogenic apoB-containing lipoprotein subclasses (VLDL-2, IDL, small dense LDL) and stimulation of cellular cholesterol efflux. Atherosclerosis. 2002;163(2):287–96. [DOI] [PubMed] [Google Scholar]
- 46.Caslake MJ, Stewart G, Day SP, Daly E, McTaggart F, Chapman MJ, et al. Phenotype-dependent and -independent actions of rosuvastatin on atherogenic lipoprotein subfractions in hyperlipidaemia. Atherosclerosis. 2003;171(2):245–53. [DOI] [PubMed] [Google Scholar]
- 47.Gaw A, Packard CJ, Murray EF, Lindsay GM, Griffin BA, Caslake MJ, et al. Effects of simvastatin on apoB metabolism and LDL subfraction distribution. Arterioscler Thromb. 1993;13(2):170–89. [DOI] [PubMed] [Google Scholar]
- 48.Remaley AT, Otvos JD. Methodological issues regarding: “A third of nonfasting plasma cholesterol is in remnant lipoproteins: Lipoprotein subclass profiling in 9293 individuals”. Atherosclerosis. 2020;302:55–6. [DOI] [PubMed] [Google Scholar]
- 49.Krauss RM, Pinto CA, Liu Y, Johnson-Levonas AO, Dansky HM. Changes in LDL particle concentrations after treatment with the cholesteryl ester transfer protein inhibitor anacetrapib alone or in combination with atorvastatin. J Clin Lipidol. 2015;9(1):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Postmus I, Trompet S, Deshmukh HA, Barnes MR, Li X, Warren HR, et al. Pharmacogenetic meta-analysis of genome-wide association studies of LDL cholesterol response to statins. Nat Commun. 2014;5:5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466(7307):714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, Shiboski S, et al. Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the Cholesterol and Pharmacogenetics (CAP) Study. Am J Cardiol. 2006;97(6):843–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


