Abstract
Background:
Chimeric antigen receptor (CAR) T cells targeting B-cell maturation antigen (BCMA-CARTx) are an emerging treatment for relapsed or refractory multiple myeloma (R/R MM).
Objectives:
Characterize the epidemiology of infections, risk factors for infection, and pathogen-specific humoral immunity in patients receiving BCMA-CARTx for R/R MM.
Study Design:
We performed a retrospective cohort study in 32 adults with R/R MM enrolled in two single-institution phase 1 clinical trials of BCMA-CARTx administered after lymphodepleting chemotherapy alone (n=22) or with (n=10) a gamma secretase inhibitor (GSI). We tested serum prior to and up to approximately 180 days after BCMA-CARTx for measles-specific IgG and for any viral-specific IgG using a systematic viral epitope scanning assay to describe the kinetics of total and pathogen-specific IgG levels pre- and post-BCMA-CARTx. We identified microbiologically documented infections to determine infection incidence and used Poisson regression to explore risk factors for infections within 180 days after BCMA-CARTx.
Results:
Most individuals developed severe neutropenia, lymphopenia and hypogammaglobulinemia after BCMA-CARTx. Grade ≥3 cytokine release syndrome (CRS; Lee criteria) occurred in 16% of participants; corticosteroids and/or tocilizumab were administered to 50% of participants. Before BCMA-CARTx, 28/32 (88%) participants had an IgG <400 mg/dL and only 5/27 (19%) had seropositive measles antibody titers. After BCMA-CARTx, all participants had IgG <400 mg/dL and declining measles antibody titers; of the 5 individuals with baseline seropositive levels, 2 remained above the seroprotective threshold post-treatment. Participants with IgG MM (n=13) had significantly fewer antibodies to a panel of viral antigens compared to participants with non-IgG MM (n=6), both prior to and after BCMA-CARTx. In the first 180 days after BCMA-CARTx, 17 (53%) participants developed 23 infections, in whom 13/23 (57%) infections were mild-to-moderate viral infections. Serious infections were more frequent in the first 28 days. Infections appeared to be more common in individuals with higher-grade CRS.
Conclusion:
Individuals with R/R MM have substantial deficits in humoral immunity. These data demonstrate the importance of plasma cells in maintaining long-lived pathogen-specific antibodies and suggest that BCMA-CARTx recipients should have ongoing surveillance for late-onset infections. Most infections were mild-moderate severity viral infections. The early infection incidence appears to be lower than reported after CD19-CARTx for B-cell neoplasms, which may be due to differences in patient/disease characteristics and regimen related toxicities.
Keywords: CAR-T cell, BCMA, chimeric antigen receptor, infection
INTRODUCTION:
Immune function in patients with active malignancy receiving immunotherapy is an ongoing area of study, as it pertains to both treatment of malignancy and risk of infections. In multiple myeloma (MM), increased rates of infection are a consequence not only of the disease, but also of treatments that impair normal plasma cell function. Several studies demonstrate an increased risk for both bacterial and viral infections in patients with MM [1–8].
Chimeric antigen receptor T (CAR-T) cells are a promising treatment for MM. CAR-T-cells are genetically engineered host T-cells that redirect T-cell specificity for tumor antigens, and this therapeutic modality has been successful in inducing durable remissions in participants with relapsed and/or refractory CD19-positive B-cell malignancies [9]. For MM, CAR-T-cells have been engineered to target B-cell maturation antigen (BCMA), a cell surface antigen almost entirely restricted to expression on plasma cells and involved in myeloma cell survival and proliferation[10–12]. BCMA-directed CAR-T cell therapy (BCMA-CARTx) was recently approved for treatment of R/R MM based on a clinical trial demonstrating response rates as high as 70% and median progression free survival of 8 months [13]. Additionally, there is ongoing investigation of adjunctive therapy with a gamma secretase inhibitor (GSI) to evaluate the safety of the combination and to assess the impact of increased surface BCMA density on clinical response [14].
The impact of BCMA-CARTx on infectious risk is not well characterized. BCMA is expressed on healthy plasma cells, which are responsible for producing long-lived antibody responses to previously encountered pathogens or vaccine antigens[15–18]. Thus, depletion of plasma cells may result in substantial humoral immune deficits. In contrast, CD19 CARTx recipients demonstrate prolonged CD19-positive B cell aplasia but only a slight decrease in total serum immunoglobulin G (IgG) levels and preservation of pre-existing pathogen-specific antibody levels, hypothesized to be due to the persistence of CD19-negative plasma cells[19–21].
There are limited data regarding infectious complications, risk factors for infection, and pathogen-specific humoral immunity in individuals receiving BCMA-CARTx for MM. Characterizing risk is important for guiding short and long-term management of these individuals. We hypothesized that early infectious complications would be similar to CD19-CARTx recipients, but depletion of BCMA-expressing plasma cells would result in substantial decrements in total and pathogen-specific IgG, increasing risk for later-onset infections. In this study, we examine the epidemiology of infections, risk factors for infection, and the kinetics of pathogen-specific humoral immunity in the first 180 days after BCMA-CARTx for relapsed or refractory (R/R) MM.
MATERIALS AND METHODS:
Participants and treatment characteristics
Participants included in this analysis were over 21 years of age with R/R MM and persistent disease after >4 prior treatment regimens with a proteasome inhibitor and immunomodulatory drug and prior failed autologous hematopoietic cell transplant (HCT) or were ineligible for HCT. They were enrolled in one of two phase 1 dose-escalation clinical trials (NCT03338972, NCT03502577). One trial included the gamma secretase inhibitor (GSI) JSMD194 25 mg three times per week for three weeks starting on the day of BCMA-CARTx infusion to increase BCMA surface antigen density. Pre-CAR-T cell infusion lymphodepleting chemotherapy consisted of daily fludarabine 25 mg/m2 and cyclophosphamide 300 mg/m2 starting day −5 followed by infusion of CAR-T cells at one of three dose levels (50 ×106, 150 ×106 and 300 ×106 cells). Relapse was defined by response criteria from the International Myeloma Working Group.[22] This study was approved by the Fred Hutchinson Cancer Research Center institutional review board; informed consent was obtained in accordance with the Declaration of Helsinki.
Supportive care and monitoring
Antimicrobial prophylaxis included acyclovir for 6–12 months after lymphodepletion, dapsone or trimethoprim-sulfamethoxazole for 3–6 months starting after neutrophil recovery, and levofloxacin plus fluconazole during periods of severe neutropenia (ANC <500 cells/ul). Total serum IgG was tested pre-BCMA-CARTx and monthly thereafter. Intravenous immunoglobulin (IVIG) 400 mg/kg was recommended monthly for participants with serum IgG concentrations less than 400 mg/dL and who had recurrent infections but was limited for some patients by intermittent U.S. national shortages during the study period or insurance denial. Monitoring for disease activity with bone marrow sampling and serum protein electrophoresis were performed on days 14, 28, 60, 90, 180, 365 after cell infusion.
Infection categorization
We identified definite (microbiologically diagnosed) infections for up to 180 days after BCMA-CARTx. We categorized infections by pathogen, clinical site, and severity. Severity was graded as nonserious (grades 1–2) or serious (grade 3) as described in the Blood and Marrow Transplant Clinical Trials Network Manual of Procedures Version 3.0, Appendix 4-A[23].
Samples and testing
Serum was collected on days 14, 28, 60, 90, 180, 365 and stored at −80° Celsius. Samples obtained within 4 months after IVIG were excluded from immunoglobulin quantification and testing of pathogen-specific immunity. We obtained total serum IgG levels prior to enrollment from clinical records. In participants with IgG MM, total non-myeloma IgG was estimated by subtracting the monoclonal component from the gamma region of serum protein electrophoresis. We measured serum measles IgG levels prior to lymphodepletion and at approximately day 28, 90, and 180 after CAR-T-cell infusion. Measles antibodies were used for proof-of-concept to study the effect of BCMA-CARTx on pathogen-specific antibody production as most individuals have seroprotective measles antibody titers that are stable over the course of a lifetime[24]. Samples were batch-tested using a Food and Drug Administration approved indirect fluorescent antibody assay (Zeus Scientific Inc., Branchburg, NJ). Negative, indeterminate, and positive results for participant samples were based on values of ≤0.90, 0.91–1.09, and ≥1.10 mIU/mL, respectively.
Absolute lymphocyte and neutrophil counts were obtained prior to lymphodepletion, and at days 14, 28, 60, and 90. CD19+ B cell enumeration by peripheral blood flow cytometry was obtained at days 14, 28, 60 and 90, subject to sample availability. Detectable peripheral blood CD19+ B cell values were defined as ≥1 cell/uL.
Additionally, we performed a systematic viral epitope scanning assay (VirScan) to identify IgG antibodies to human pathogenic viruses described in the UniProt database (206 viruses with >1000 strains) as detailed in the Supplemental Methods[25,26]. The measured output is the number of IgG antibodies to unique viral epitopes, referred to as epitopes hits. We tested 2 samples per participant using the pre-lymphodepletion and latest available post-CAR-T-cell therapy samples.
Data collection and analysis
Data regarding infections were collected through 180 days after CAR-Tx. Data regarding objective markers of immunity were collected through approximately 180 days but subject to variability in sample collection time. Data collection was interrupted by loss to follow-up, new anti-tumor therapy, or death, whichever occurred first. We collected baseline participant characteristics and followed clinical events including infections, cytokine release syndrome (CRS; Lee Criteria according to protocol)[27], immune effector cell-associated neurotoxicity syndrome (ICANS; ASTCT criteria)[28], and administration of steroids or tocilizumab.
We reported frequencies and percentages for categorical variables, and medians and ranges for continuous variables. We plotted IgG levels over time by MM type. The total number of viral epitopes per participant before and after CARTx were represented using violin plot distributions. Two-tailed paired T tests were used to compare changes in number of viral epitopes between the pre- and post-CARTx samples overall and within categories defined by myeloma type. Independent samples T tests were used to compare number of viral epitopes between myeloma types at a given timepoint.
We calculated the number of infections per 100 participant days-at-risk (infection incidence rate) for time periods of 0–28, 29–90 and 91–180 days after CAR T cell infusion. Cumulative incidence was calculated for any, viral, bacterial, and fungal infections over the 180 days of follow-up, using the first of each of these infections per participant, with new anti-tumor therapy, new cancer requiring treatment, or death before infection treated as competing risk events. Univariable Poisson regression was used to explore associations between baseline characteristics and infection rate, including all infections per participant. For these models, the logarithm of follow-up days was included as an offset and the scale parameter was estimated using Pearson’s chi square statistic to account for overdispersion.
RESULTS:
Participant and treatment characteristics
We studied 32 individuals who received BCMA-CARTx for R/R MM from January 2018 to February 2020. Twenty-two participants were treated with BCMA-CARTx alone, and 10 participants additionally received BCMA-CARTx with GSI. The median duration of follow-up was 180 days (range, 35–180) during which time 1 participant died, 2 participants relapsed and required new therapy, 1 participant developed a new gastrointestinal malignancy requiring chemotherapy, and 2 participants were lost to follow-up before day 180. Baseline characteristics are detailed in Table 1. Sixty-three percent (20/32) of participants had IgG MM and 26 (81%) had a HCT a median of 3.6 years prior (range, 0.6 – 12.5 years). Participants were treated with a median of 8 anti-myeloma regimens (range, 4–18 regimens) prior to receiving BCMA-CARTx. Bridging chemotherapy was used in 22 (69%) participants. Notably bridging chemotherapy did not include any BCMA-targeted agents. Cytokine release syndrome and/or ICANS Grade ≥3 occurred in 13% and 16% of participants, respectively. Corticosteroids (n=15) and/or tocilizumab (n=14) were administered to 50% of participants.
Table 1.
Participant and treatment characteristics
| Baseline characteristics (N=32) | Overall (N=32) | IgG Myeloma (N=20) | Non-IgG Myeloma (N=12) |
|---|---|---|---|
|
| |||
| Age, median (range) | 64 (44– 77) | 66 (44–77) | 58 (44–72) |
| Female | 13 (41%) | 10 (50%) | 3 (25%) |
| IgG myeloma | 20 (63%) | 20 (100%) | 0 |
| IgM myeloma | 1 (3%) | 0 | 1 (8%) |
| IgA myeloma | 5 (16%) | 0 | 5 (42%) |
| Light chain myeloma | 6 (19%) | 0 | 6 (50%) |
| Prior CAR T cell therapy | 3 (9%) | 1 (5%) | 2 (17%) |
| Prior hematopoietic stem cell transplant (HCT) | 26 (81%) | 16 (80%) | 10 (83%) |
| Prior autologous HCT | 26 (81%) | 16 (80%) | 10 (83%) |
| Prior allogeneic HCT | 6 (19%) | 4 (20%) | 2 (17%) |
| Years since last transplant, median (range) | 3.6 (0.6 – 12.5) | 3.7 (0.6–12.5) | 2.7 (1.6–8) |
| IVIG at least once in 4 months prior to CARTx | 4 (13%) | 2 (10%) | 2 (17%) |
| IgG < 400 mg/dL pre-lymphodepletion | 28 (88%) | 19 (95%) | 9 (75%) |
| ALC < 200 cells/uL pre-lymphodepletion | 1 (3%) | 1 (5%) | 0 |
| ANC < 500 cells/uL pre-lymphodepletion | 0 | 0 | 0 |
| Receipt of gamma secretase inhibitor (GSI) | 10 (31%) | 8 (40%) | 2 (17%) |
|
| |||
| Treatment associated with CARTx | |||
|
| |||
| Bridging chemotherapy3 | 22 (69%) | 14 (70%) | 8 (67%) |
| CAR T dose - 50x10^6 EGFRt cells | 12 (38%) | 8 (40%) | 4 (33%) |
| CAR T dose - 150x10^6 EGFRt cells | 11 (34%) | 6 (30%) | 5 (42%) |
| CAR T dose - 300x10^6 EGFRt cells | 9 (28%) | 6 (30%) | 3 (25%) |
|
| |||
| Post-CARTx findings | |||
|
| |||
| Days to ANC > 500 cell/uL, median (range)1 | 10 (4 – 24) | 11 (5–24) | 6 (4–16) |
| Neurotoxicity (ICANS) grade 1–2 | 5 (16%) | 5 (25%) | 0 |
| Neurotoxicity (ICANS) grade 3–5 | 5 (16%) | 4 (20%) | 1 (8%) |
| CRS grade 1–2 | 23 (72%) | 16 (80%) | 7 (58%) |
| CRS grade 3–5 | 5 (16%) | 3 (15%) | 2 (17%) |
| Corticosteroids and tocilizumab2 | 13 (41%) | 10 (50%) | 3 (25%) |
| Corticosteroids alone2 | 2 (6%) | 2 (10%) | 0 |
| Tocilizumab alone2 | 1 (3%) | 0 | 1 (8%) |
| IVIG at least once in 180 days post CARTx | 20 (63%) | 12 (60%) | 8 (67%) |
All values listed as number (%) unless otherwise indicated.
Among 28 patients that achieved ANC>500 cell/uL. One participant did not have recovery of ANC during study follow-up.
Administered in the first 28 days after BCMA-CARTx
Regimens included: dexamethasone (n=1); carfilzomib, dexamethasone (n=1); carfilzomib, lenalidomide, dexamethasone (n=3); ixazomib, lenalidomide, dexamethasone (n=1); cyclophosphamide (n=1); carfilzomib, cyclophosphamide, dexamethasone (n=1); ixazomib, cyclophosphamide, dexamethasone (n=1); carfilzomib, cyclophosphamide, thalidomide, dexamethasone (n=1); bortezomib, cyclophosphamide (n=1); bortezomib, bendamustine (n=1); bortezomib, cyclophosphamide, cisplatin, doxorubicin, etoposide (n=1); bendamustine, lenalidomide, dexamethasone (n=1); bendamustine, thalidomide, dexamethasone (n=1); cisplatin, cyclophosphamide, doxorubicin, etoposide (n=1); venetoclax (n=1); venetoclax, dexamethasone (n=1); pomalidomide (n=1); pomalidomide, daratumumab (n=1); daratumumab, melphalan, prednisone, lenalidomide (n=1); elotuzumab, carfilzomib, bortezomib, cyclophosphamide, doxorubicin, etoposide (n=1); lenalidomide, dexamethasone (n=1)
Most participants developed severe neutropenia, lymphopenia, and hypogammaglobulinemia
Prior to lymphodepleting chemotherapy, no participants had severe neutropenia (absolute neutrophil count [ANC] <500 cells/uL) and one had severe lymphopenia (absolute lymphocyte count [ALC] < 200 cells/uL). After lymphodepleting chemotherapy and BCMA-CARTx, 28 participants (88%) had severe neutropenia for a median of 10 days (range, 4–24 days), and 31 participants (97%) had severe lymphopenia for a median of 12 days (range, 9–28 days). Three participants did not develop severe neutropenia. One participant had persistent neutropenia and lymphopenia until death on day 35. CD19+ B-cell enumeration was performed for at least one timepoint after BCMA-CARTx in 30 participants. All participants developed CD19+ B-cell aplasia (Figure S1). By approximately day 90, 17/24 (71%) participants with samples at that timepoint had detectable CD19+ B cells in their peripheral blood.
After excluding laboratory results obtained within 4 months after IVIG administration, total IgG levels before and after BCMA-CARTx were available for 29 (91%) participants. Prior to BCMA-CARTx, 25 (86%) participants had IgG <400 mg/dL. When stratified by MM type, 18/19 (95%) participants with IgG MM and 4/10 (40%) participants with non-IgG MM had serum IgG levels <400 mg/dL before BCMA-CARTx. After BCMA-CARTx, all participants had IgG levels <400mg/dL by day 28 (Figure 1A–1B). IgG data stratified by GSI receipt and among the subgroup who received bridging chemotherapy and did not receive prior CARTx are shown in Figure S2A–B and Figure S3A, respectively. Aside from one individual with IgA MM, no participants had an IgG level >400 mg/dL observed during follow up. One or more doses of IVIG were administered to 20/32 (63%) participants after BCMA-CARTx during the study period.
Figure 1: Kinetics of total IgG and measles-specific IgG after BCMA-CARTx by myeloma type.
The top two line-graphs (A, B) show total IgG per-participant in IgG myeloma (A, n=19) and non-IgG myeloma (B, n=10) over time. The horizontal solid line represents the lower limit of normal for total IgG (610mg/dL). The horizontal dashed line represents the threshold at which guidelines recommend IVIG replacement (400mg/dL). The bottom two line-graphs (C, D) show measles specific IgG in IgG myeloma (C, n=18) and non-IgG myeloma (D, n=9) over time. The horizontal solid line depicts the lower threshold for seroprotective measles IgG. The horizontal dashed line represents upper threshold for a seronegative measles IgG. The area between the two lines is an indeterminate value. Samples were excluded if obtained within 16 weeks after IVIG administration. On all four line-graphs, the vertical line represents day 0, the day of CAR T cell infusion.
Most individuals lacked protective serum antibody levels for measles at baseline, and levels declined further after BCMA-CARTx
To understand the impact of BCMA-CARTx on long-lived pathogen-specific antibodies, we quantified measles IgG levels before and after CARTx in 27 (84%) participants with samples beyond 4 months after IVIG administration. Before BCMA-CARTx, 22/27 (81%) participants lacked protective serum antibody levels for measles. When stratified by MM type, 17/18 (94%) participants with IgG MM and 5/9 (56%) participants with non-IgG MM lacked protective serum antibody levels for measles.
After BCMA-CARTx, the kinetics of measles IgG levels differed between IgG and non-IgG MM as shown in Figures 1C and 1D. In most participants with IgG MM, there was a paradoxical increase in measles IgG levels at the first (~day 28) time point with subsequent rapid decline back to or below baseline titers. The only participant with IgG MM who was seropositive at baseline became seronegative after CARTx. Of the 4 participants with non-IgG MM who had baseline seroprotective measles IgG, all had subsequent decline in measles IgG titers and only 2 remained seropositive. Results were similar when stratified by GSI receipt (Figure S2C–D) and when restricted to the subgroup who received bridging chemotherapy and no prior CARTx (Figure S3B).
Antibody reactivity to pathogenic human viruses was lower in individuals with IgG MM compared to non-IgG MM
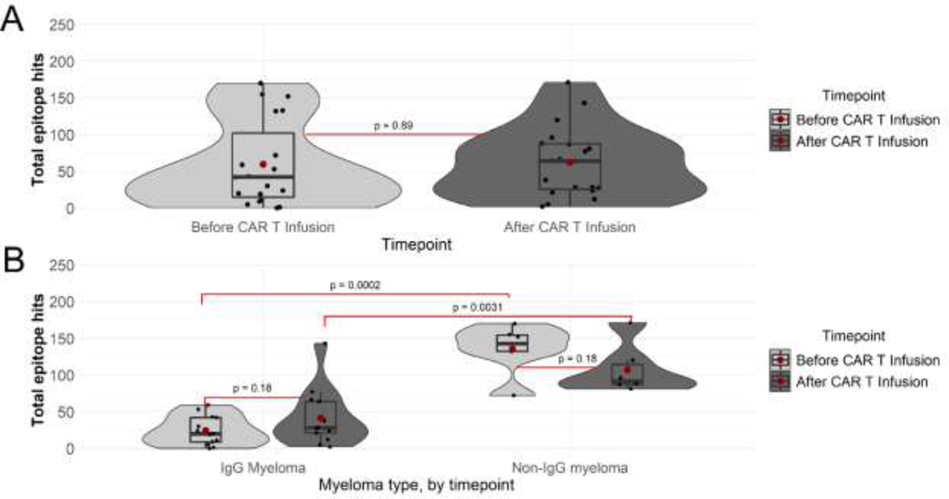
To further investigate the effect of BCMA-CARTx on pathogen-specific humoral immunity, we tested a pre-CARTx sample and the latest post-CARTx sample using the VirScan assay. There were 19 (59%) participants with available pre/post samples after excluding samples obtained within 4 months after IVIG. The median day of sample testing after CARTx was 98 days (range, 76–204) in 13 participants with IgG MM and 92 days (range, 87–177) in 6 participants with non-IgG MM. There was no significant difference in the total number of pathogen-specific antibodies to viral antigens (epitope hits) between pre- and post-CARTx samples overall (Figure 2A, Figure S4). When stratified by MM type, participants with IgG MM had significantly fewer viral epitope hits compared to non-IgG MM both prior to and after BCMA-CARTx (Figure 2B). After BCMA-CARTx, participants with IgG MM had more epitope hits compared to pre-CARTx, whereas participants with non-IgG MM had fewer epitope hits compared to pre-CARTx, although the differences were not statistically significant. Similar patterns were observed when stratified by GSI receipt and among the subgroup who received bridging chemotherapy and no prior CARTx (Figure S5, Figure S6).
Figure 2. Violin plots of ‘epitope hits’ based on viral pathogen-specific IgG measured by the VirScan assay before and after BCMA-CARTx.
Violin plots showing distribution of antibodies to unique viral antigens, ‘epitope hits’, per participant (n=19) before and after CAR T cell infusion (A), and by myeloma type, IgG myeloma (n=13), non-IgG myeloma (n=6) (B). The box within the violin represents the median and interquartile range, with red dots representing means. Individual data points are overlayed on violin plots, jittered on the horizontal axis to aid in visualization. The vertical line spans the upper and lower values. P-values are shown for relevant comparisons. Paired T tests were used to compare pre- and post-CARTx samples overall and within categories defined by myeloma type. Independent samples T tests were used to compare myeloma types at a given timepoint.
Serious infections were most frequent in the first 28 days after BCMA-CARTx
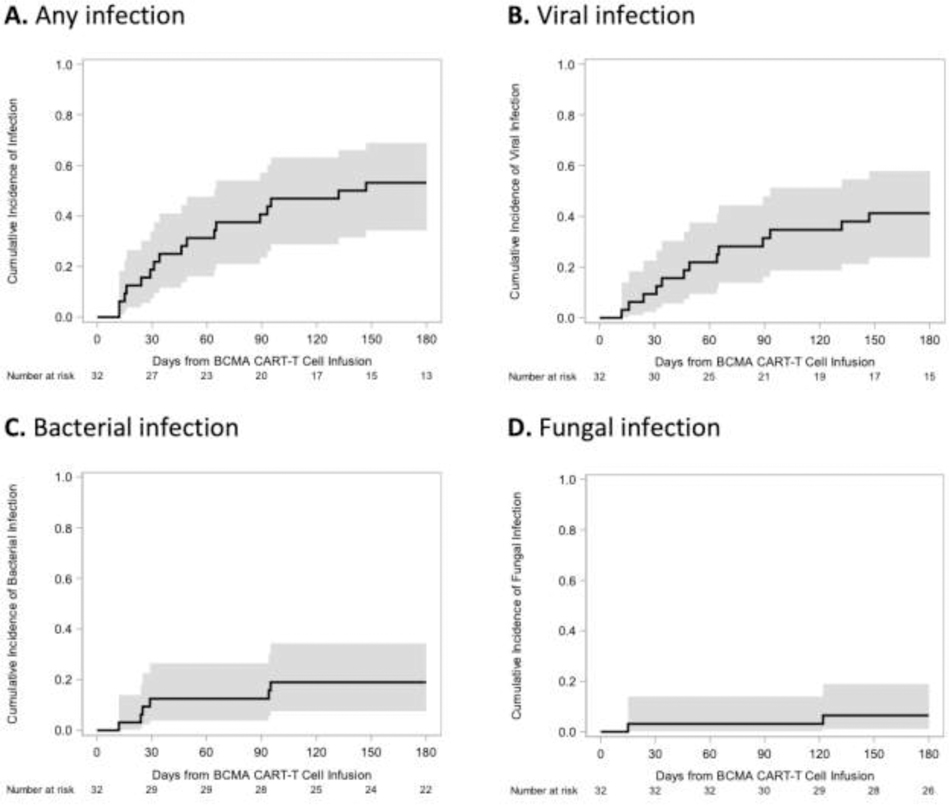
As we observed severe cytopenias with progressive and persistent hypogammaglobulinemia and loss of pathogen-specific antibody responses after lymphodepletion and CARTx, we were interested in characterizing concurrent infections. Cumulative incidence curves of any, bacterial, viral, and fungal infections are depicted in Figure 3. First infections occurred a median of 46 days (range, 12–147 days) after CAR–T-cell infusion. Overall, 23 microbiologically documented infections occurred in 17 participants (53%) by day 180; 6 participants had two infections and 11 participants had only one infection during the study follow-up period. Of these infections, 6 (26%) were serious and occurred in 4 individuals, including 3 (12%) bacterial bloodstream infections. Of all 23 infections, 7 (30%) infections occurred in the first 28 days, accounting for 75% of all bacterial bloodstream infections and 67% of all serious infections. Infection incidence rates over time, as well as infection type and severity, are depicted in Figure 3 and Table 2, respectively. Infection incidence rates decreased over time, and most infections beyond day 28 were mild to moderate severity viral infections. One participant died during the study period from a disseminated fungal infection starting on day 15 (with brain, gastrointestinal, and pulmonary abscesses) in the setting of grade 4 CRS.
Figure 3. Infection incidence rate after BCMA-CARTx in participants with relapsed/refractory multiple myeloma.
Infection incidence rate, defined as the number of infections per 100 person-days of risk, is shown for all participants (n=32) and stratified by type of infection and time period post CARTx.
Table 2.
Infections after BCMA-CARTx in participants with relapsed/refractory multiple myeloma
| Total participants | Total events | Mild events | Moderate events | Severe events | |
|---|---|---|---|---|---|
| Fungal | 2 (6) | 2 (9) | 0 | 0 | 2 (9) |
| Bacterial | 6 (19) | 7 (30) | 3 (13) | 1 (4) | 3 (13) |
| Viral | 13 (41) | 14 (61) | 3 (13) | 10 (44) | 1 (4) |
| Any infection | 17 (53) | 23 (100) | 6 (26) | 11 (48) | 6 (26) |
Data are shown as number (%). Percentages for Total participants were computed among n=32 total participants. All other percentages were computed among 23 total events.
Infections occurred more frequently among individuals with higher-grade CRS and associated treatment
As most severe infections occurred in the first 28 days, we were interested in exploring associations of baseline characteristics and post-treatment complications with infection. As shown in Table 3, there were no significant associations between baseline characteristics and infection rate. However, participants with IgG myeloma had a higher observed infection rate (0.50 per 100 person-days) compared to those with non-IgG myeloma (0.31 per 100 person-days).
Table 3.
Univariable Poisson Model Estimates for Associations with Infection Rate
| Baseline Variable | Univariable IRR (95% CI) | p-value |
|---|---|---|
| Age, per 10-year increase | 1.4 (0.7, 2.5) | 0.32 |
| CAR-T dose level | ||
| 50x10^6 EGFRt cells | 1.0 (Reference) | |
| 150x10^6 EGFRt cells | 0.7 (0.2, 2.3) | 0.57 |
| 300x10^6 EGFRt cells | 0.8 (0.2, 2.6) | 0.65 |
| History of HCT vs none | 0.7 (0.2, 2.2) | 0.53 |
| Bridging chemotherapy vs none | 0.8 (0.3, 2.2) | 0.65 |
| IgG vs non-IgG myeloma | 1.6 (0.5, 5.7) | 0.47 |
| Pre-CAR-T total IgG < 400 mg/dL vs ≥400 mg/dL | 1.4 (0.2, 8.7) | 0.71 |
| GSI administration vs none | 1.6 (0.6, 4.2) | 0.38 |
Abbreviations, IRR = incidence rate ratio, CI = confidence interval, HCT = hematopoietic stem cell transplant, GSI = gamma secretase inhibitor.
We next described infections in relation to concurrent treatment related events after BCMA-CARTx. Among five participants with CRS grade ≥3, all (100%) developed at least one infection after CRS onset, including three (60%) who developed a serious infection. In comparison, among the 27 other participants with CRS grade ≤2, 12 (44%) developed at least one infection after CRS onset including one (4%) who developed a serious infection. Similarly, among 16 participants who received corticosteroids and/or tocilizumab, 10 (63%) developed any infection including three (19%) who developed a serious infection compared to the 16 remaining individuals in whom 7 (44%) developed any infection including one (6%) who developed a serious infection.
DISCUSSION:
In this study of individuals receiving lymphodepleting chemotherapy and BCMA-CARTx for R/R MM, we observed early treatment-related severe neutropenia, lymphopenia and hypogammaglobulinemia in most participants. The minority of individuals had seropositive measles antibody titers before BCMA-CARTx, likely reflecting prior plasma-cell targeted therapies and prior HCT. Measles-specific humoral immunity further declined after CARTx, and together, these findings underscore the role of plasma cells in maintaining pathogen-specific antibody-mediated immunity and contrast with the findings in CD19-CARTx recipients.[19,21] Findings differed by MM type, wherein participants with non-IgG MM had fewer baseline deficits, although patterns were similar after BCMA-CARTx. The overall infection incidence rate was relatively low, and most serious infections occurred in the first 28 days after CAR-T-cell infusion. Serious infections appeared to be most frequent among individuals who developed and were treated for grade ≥3 CRS, similar to findings in CD19-CARTx recipients.[29]
We first characterized infectious risk via objective measures of the humoral immune system. This cohort was heavily pre-treated with a median of 8 treatment regimens, and 81% of participants had prior HCT. Most participants developed severe neutropenia, lymphopenia, and CD19+ B cell aplasia early after lymphodepleting chemotherapy and BCMA-CARTx, although the majority of tested individuals had detectable CD19+ B cells by day 90. At baseline, we also observed severe hypogammaglobulinemia in 88% of participants, with lower levels observed in participants with IgG MM. After BCMA-CARTx, severe hypogammaglobulinemia occurred and persisted in all participants. Most participants with normal baseline IgG levels reached their nadir by day 28, suggesting rapid destruction of IgG-producing plasma cells.
Measles IgG provided a pathogen-specific measure of immunity in our participant cohort. Strikingly, the majority (81%) of participants lacked protective antibody levels at baseline, which was more common in individuals with IgG versus non-IgG MM. In comparison, a cross-sectional study of cancer patients at a large academic cancer center found that 75% of patients were seropositive for measles, with 63% and 46% seropositive in hematologic malignancy and HCT patients, respectively.[30] In our participants with non-IgG MM, we observed further decrements in measles IgG levels after CARTx, consistent with destruction of antibody-producing normal plasma cells. In participants with IgG MM, we observed a paradoxical increase in measles IgG levels at the first (~day 28) post-CAR-T-cell infusion time point, with subsequent decrements back to or below baseline levels. The cause of this phenomenon is unclear. Overall, these findings align with one other study reporting antibodies to measles, mumps, and rubella in 3 BCMA-CARTx recipients.[31]
Prior studies demonstrate immunodeficiency in MM and other plasma cell neoplasms using pathogen-specific antibodies against various bacterial and viral pathogens[4]. We used the VirScan assay to provide a more global assessment of pathogen-specific humoral immunity in this study. Our cohort had overall fewer viral epitopes hits at baseline and after treatment compared to a cohort of CD19-CARTx recipients [19]. Surprisingly, we did not observe a significant change in the number of anti-viral antibodies (epitope hits) before and after BCMA-CARTx, but individuals with IgG MM had fewer epitope hits compared to those with non-IgG MM. These data support a higher degree of pathogen-specific humoral immunodeficiency in BCMA-CARTx recipients, which is likely multifactorial due to differences in the underlying disease and its treatment. Individuals with IgG MM may be at particularly increased risk for infection compared to other MM types, which is a novel observation.
We observed a 53% cumulative incidence of any infection in the 180 days after BCMA CARTx. Most infections were moderate severity viral infections. Most serious infections occurred in the first 28 days, immediately following the period of lymphopenia and neutropenia. These infections were more frequent in individuals receiving treatment for grade ≥3 CRS with glucocorticoids and/or tociluzumab. We did not identify significant associations between baseline characteristics and infection density, although our sample size was limited. Most experience pertaining to infections after CARTx are from studies of CD19-CARTx recipients. In that population, grade ≥3 CRS tends to occur more frequently and is described in up to 46% of recipients compared to 16% in our cohort of BCMA-CARTx recipients.[29,32–35] The infection incidence rate in the first 28 days after CD19-CARTx is typically higher (1.2–2.9 infections per 100 days-at-risk) than our observation of 0.75 infections per 100 days-at-risk after BCMA-CARTx.[29,34] In the KarMMa trial leading to FDA approval of a BCMA-CARTx for R/R MM, 67–75% of participants developed any infection, most of which were viral infections.[13,31] Our overall findings of infection incidence, type, and severity align with the three other published studies on epidemiology of infections in BCMA-CARTx recipients.[31,36,37]. Notably, our study represents the largest to date to comment on pathogen-specific immunity in this population. Additionally, we observe associations between CRS and associated steroid/tocilizumab administration with higher rates of infection, an association also noted after CD19-CARTx [29,35].
The duration of humoral immunodeficiency and its effect on long-term infection risk after BCMA-CARTx remains incompletely understood. As we learn more about CARTx persistence and the impact on humoral immunity, it will be important to compare to other forms of immunotherapy such as BCMA-directed bispecific antibodies [38]. In this era of emerging viral pathogens such as SARS-CoV-2, the impact of CARTx on humoral immunity is of increasing importance. Based on our observations of deficits in pathogen-specific antibodies in this population, one could consider monthly IVIG administration in BCMA-CARTx recipients with hypogammaglobulinemia, particularly those with recurrent infections [21,31]. However, there are no studies to date that definitively demonstrate a benefit of IVIG in this context. Recent data suggest that BCMA-CARTx recipients may respond to vaccinations before and/or after treatment [39]. Current guidance recommends considering routine vaccinations as early as 6 months after CARTx, although there are limited data to support this [40]. While awaiting further research in this area, it is appropriate to utilize these strategies in an individualized manner.
This is one of the first studies of epidemiology of infections in BCMA-CARTx recipients, which is a rapidly growing population given the recent approval of the first commercially available product. We also detail changes in pathogen-specific humoral immunity with novel observations of differences by MM subtype. There are several limitations related to the retrospective design, relatively small sample size, and patient heterogeneity. As such, the results of this study should be considered hypothesis generating. Although we did not observe any associations between baseline characteristics and infections, smaller effect sizes would be missed due to small sample size, and we were unable to perform a multivariate analysis. Nonetheless, this study documents early observations in this unique and rapidly growing patient population to better guide clinical care and future studies. Exclusion of participants who were receiving IVIG from analyses of pathogen-specific immunity may have biased our results to a relatively less immune compromised cohort. We were unable to correlate findings with objective metrics of plasma cell destruction due to data access restrictions from ongoing clinical trial conduct, although disease remission served as an indirect indicator of plasma cell destruction. Recovery of normal plasma cells may begin around 3 months after BCMA-CARTx, and with longer follow up, it is possible that antibody levels may recover.[31] Our study utilizes pathogen-specific antibodies as surrogates for pathogen-specific immunity. This correlation has been previously demonstrated in other populations[41]. However, antibody-mediated immunity may differ in this population and does not occur in isolation; future studies should also consider other mechanisms of host defense including cellular immunity.
In conclusion, we demonstrate that patients with R/R MM have substantial deficits in humoral immunity that is further exacerbated by lymphodepleting chemotherapy and BCMA-CARTx. Serious infections typically occur within the same time frame as treatment-related toxicities of CRS and cytopenias. These data support the need for further research on strategies to prevent infections in the first few months after BCMA-CARTx, promote immune reconstitution, and better understand the long-term risk in this patient population.
Supplementary Material
Figure 4. Cumulative incidence of infections in the first 180 days after BCMA-CARTx in participants with relapsed/refractory multiple myeloma.
Cumulative incidence of infections among all participants (n=32) for any (A), viral (B), bacterial (C), and fungal (D) infections. The shaded area indicates the 95% confidence interval. New anti-tumor therapy, new cancer, and death before infection were treated as competing risks.
Highlights.
32 individuals with relapsed/refractory multiple myeloma received BCMA-CAR-T cells
Most individuals in our study lack seroprotection to measles before CAR-T cells
BCMA-CAR-T cells further deplete pathogen-specific antibodies
Serious infections were most frequent in the first 28 days after treatment
Infections occurred more frequently in individuals with high-grade CRS
Acknowledgments
Funding considerations:
This work was supported by NIH/NCI (U01CA247548 to JAH and P01CA018029 to DJG), the NIH/NCI Cancer Center Support Grants (P30CA0087-48 and P30CA015704-44), and Juno Therapeutics/BMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions.
DISCLOSURE OF CONFLICTS OF INTEREST
Srirama Josyula, Jacob Keane-Candib, Xiaoling Song, Sushma Thomas, Gregory Pepper, Elizabeth Krantz, Sayan Dasgupta: none
Margot Pont: has equity interest in Lyell Immunopharma, is currently employed by and has equity interest in CellPoint B.V.
Stanley Riddell: is a founder of Juno Therapeutics, a Bristol-Myers Squibb company, and Lyell Immunopharma, and has served as an advisor to Juno Therapeutics, a Bristol-Myers Squibb company, Nohla, Adaptive Biotechnologies and Cell Medica
Andrew Cowan: has received research funding from Juno Therapeutics, a Bristol-Myers Squibb company, Janssen, AbbVie, and Sanofi; and has served as a consultant for Celgene.
Damian Green: has received research funding, has served as an advisor and has received royalties from Juno Therapeutics, a Bristol-Myers Squibb company; has served as an advisor and received research funding from Seattle Genetics; has served as an advisor to GlaxoSmithKline, Celgene, Janssen Biotech and Legend Biotech; and has received research funding from SpringWorks Therapeutics, Sanofi and Cellectar Biosciences.
Joshua Hill: has served as a consultant for Allogene and Gilead, with research support from Gilead.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].Blimark C, Holmberg E, Mellqvist U-H, Landgren O, Björkholm M, Hultkrantz ML, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica 2014;100:107–13. 10.3324/haematol.2014.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen M, Zhao Y, Xu C, Wang X, Zhang X, Mao B. Immunomodulatory drugs and the risk of serious infection in multiple myeloma: systematic review and meta-analysis of randomized and observational studies. Annals of Hematology 2018;97:925–44. 10.1007/s00277-018-3284-y. [DOI] [PubMed] [Google Scholar]
- [3].Dumontet C, Hulin C, Dimopoulos MA, Belch A, Dispenzieri A, Ludwig H, et al. A predictive model for risk of early grade ≥ 3 infection in patients with multiple myeloma not eligible for transplant: analysis of the FIRST trial. Leukemia 2018;32:1404–13. 10.1038/s41375-018-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Karlsson J, Andréasson B, Kondori N, Erman E, Riesbeck K, Hogevik H, et al. Comparative Study of Immune Status to Infectious Agents in Elderly Patients with Multiple Myeloma, Waldenstrom’s Macroglobulinemia, and Monoclonal Gammopathy of Undetermined Significance. Clinical and Vaccine Immunology 2011;18:969–77. 10.1128/cvi.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nahi H, Chrobok M, Gran C, Lund J, Gruber A, Gahrton G, et al. Infectious complications and NK cell depletion following daratumumab treatment of Multiple Myeloma. PLOS ONE 2019;14:e0211927. 10.1371/journal.pone.0211927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nucci M, Anaissie E. Infections in Patients With Multiple Myeloma. Seminars in Hematology 2009;46:277–88. 10.1053/j.seminhematol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- [7].Rahman S, Rybicki L, Hamilton BK, Pohlman B, Jagadeesh D, Cober E, et al. Early infectious complications after autologous hematopoietic cell transplantation for multiple myeloma. Transplant Infectious Disease 2019;21:105. 10.1111/tid.13114. [DOI] [PubMed] [Google Scholar]
- [8].Ying L, YinHui T, Yunliang Z, Sun H. Lenalidomide and the risk of serious infection in patients with multiple myeloma: a systematic review and meta-analysis. Oncotarget 2017;5:46593–600. 10.18632/oncotarget.16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].June CH, Sadelain M. Chimeric Antigen Receptor Therapy. New England Journal of Medicine 2018;379:64–73. 10.1056/nejmra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest 2019;129:2210–21. 10.1172/jci126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. New Engl J Med 2019;380:1726–37. 10.1056/nejmoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tai Y-T, Acharya C, An G, Moschetta M, Zhong MY, Feng X, et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016;127:3225–36. 10.1182/blood-2016-01-691162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Munshi NC Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. New Engl J Med 2021;384:705–16. 10.1056/nejmoa2024850. [DOI] [PubMed] [Google Scholar]
- [14].Pont MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 2019;134:1585–97. 10.1182/blood.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meinl E, Thaler FS, Lichtenthaler SF. Shedding of BAFF/APRIL Receptors Controls B Cells. Trends in Immunology 2018;39:673–6. 10.1016/j.it.2018.07.002. [DOI] [PubMed] [Google Scholar]
- [16].Sakai J, Akkoyunlu M. The Role of BAFF System Molecules in Host Response to Pathogens. Clinical Microbiology Reviews 2017;30:991–1014. 10.1128/cmr.00046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lightman SM, Utley A, Lee KP. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Frontiers in Immunology 2019;10:1903. 10.3389/fimmu.2019.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA Is Essential for the Survival of Long-lived Bone Marrow Plasma Cells. The Journal of Experimental Medicine 2004;199:91–8. 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hill JA, Krantz EM, Hay KA, Dasgupta S, Stevens-Ayers T, Ignacio RAB, et al. Durable preservation of antiviral antibodies after CD19-directed chimeric antigen receptor T-cell immunotherapy. Blood Advances 2019;3:3590–601. 10.1182/bloodadvances.2019000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 2016;128:360–70. 10.1182/blood-2016-01-694356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Walti CS, Krantz EM, Maalouf J, Boonyaratanakornkit J, Keane-Candib J, Joncas-Schronce L, et al. Antibodies to vaccine-preventable infections after CAR-T-cell therapy for B-cell malignancies. Jci Insight 2021;6. 10.1172/jci.insight.146743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17:e328–46. 10.1016/s1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- [23].Technical Manual of Procedures 3AD.
- [24].Amanna IJ, Carlson NE, Slifka MK. Duration of Humoral Immunity to Common Viral and Vaccine Antigens. New Engl J Medicine 2007;357:1903–15. 10.1056/nejmoa066092. [DOI] [PubMed] [Google Scholar]
- [25].Ignacio RAB, Dasgupta S, Stevens-Ayers T, Kula T, Hill JA, Lee SJ, et al. Comprehensive viromewide antibody responses by systematic epitope scanning after hematopoietic cell transplantation. Blood 2019;134:503–14. 10.1182/blood.2019897405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu GJ, Kula T, Xu Q, Li MZ, Vernon SD, Ndung’u T, et al. Comprehensive serological profiling of human populations using a synthetic human virome. Science 2015;348:aaa0698. 10.1126/science.aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–95. 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Tr 2019;25:625–38. 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- [29].Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor–modified T-cell immunotherapy. Blood 2018;131:121–30. 10.1182/blood-2017-07-793760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Marquis SR, Logue JK, Chu HY, Loeffelholz T, Quinn ZZ, Liu C, et al. Seroprevalence of Measles and Mumps Antibodies Among Individuals With Cancer. Jama Netw Open 2021;4:e2118508. 10.1001/jamanetworkopen.2021.18508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang Y, Li C, Xia J, Li P, Cao J, Pan B, et al. Humoral immune reconstitution after anti-BCMA CAR-T cell therapy in relapse/refractory multiple myeloma. Blood Adv 2021. 10.1182/bloodadvances.2021004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New Engl J Medicine 2017;377:2531–44. 10.1056/nejmoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New Engl J Medicine 2018;378:439–48. 10.1056/nejmoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vora SB, Waghmare A, Englund JA, Qu P, Gardner RA, Hill JA. Infectious complications following CD19 chimeric antigen receptor T-cell therapy for children, adolescents and young adults. Open Forum Infectious Diseases 2020. 10.1093/ofid/ofaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clinical Infectious Diseases 2018;67:533–40. 10.1093/cid/ciy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kambhampati S, Sheng Y, Huang C-Y, Bylsma SA, Lo M, Kennedy VE, et al. Infectious complications in relapsed refractory multiple myeloma patients after BCMA Car t-cell therapy. Blood Adv 2021. 10.1182/bloodadvances.2020004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang D, Mao X, Que Y, Xu M, Cheng Y, Huang L, et al. Viral infection/reactivation during long-term follow-up in multiple myeloma patients with anti-BCMA CAR therapy. Blood Cancer J 2021;11:168. 10.1038/s41408-021-00563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhou X, Einsele H, Danhof S. Bispecific Antibodies: A New Era of Treatment for Multiple Myeloma. J Clin Medicine 2020;9:2166. 10.3390/jcm9072166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Walti CS, Loes AN, Shuey K, Krantz EM, Boonyaratanakornkit J, Keane-Candib J, et al. Humoral immunogenicity of the seasonal influenza vaccine before and after CAR-T-cell therapy: a prospective observational study. J Immunother Cancer 2021;9:e003428. 10.1136/jitc-2021-003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood 2020;136:925–35. 10.1182/blood.2019004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Plotkin SA. Updates on immunologic correlates of vaccine-induced protection. Vaccine 2020;38:2250–7. 10.1016/j.vaccine.2019.10.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.