Abstract
Totipotent stem cells are transiently occurring in vivo cells that can form all cell types of the embryo including placenta, with their in vitro counterparts being actively pursued. Subsequently, totipotent-like stem cells are established with variable robustness and biological relevance. Here, we summarize current progress on capturing these cells in culture.
Keywords: Totipotent stem cells, 2 cell-like cells, expanded/extended pluripotent stem cells, Totipotent blastomere-like cells, totipotent-like stem cells (TLSCs), Blastoids
Totipotency is the ability of a single cell to form viable embryos including the embryo proper as well as extraembryonic placenta and yolk sac. However, mixed usage of different definitions have caused confusion in the field. Some groups have defined it as the capacity of a group of cells to contribute to both embryonic and extraembryonic tissues. In contrast, a relatively more stringent definition by others refers to the ability of a single cell to contribute to both embryonic and extraembryonic lineages of a developing embryo. By the strictest definition, only zygote and two-cell (2C) stages of developing embryos are genuinely totipotent for their ability to give rise to viable organisms, referred to as in vivo canonical totipotency. In contrast, those in vitro grown cells with less stringent definitions are referred to as in vitro experimental totipotency [1].
Totipotency exists transiently in zygote and 2-cell embryo stages during early development, which subsequently commit to two distinct lineages, i.e., the embryonic cell lineage (inner cell mass, ICM) that forms embryo proper and the extraembryonic cell lineage (trophectoderm, TE) that forms placental tissue (Figure 1A). Embryonic stem cells (ESCs) are derived from ICM and have the capacity to develop all three primary germ layers, a unique feature of pluripotency. However, they fall short of the extraembryonic lineage contribution associated with totipotency (Figure 1B, Table 1). The gold-standard approach for generating authentic totipotent cells is somatic cell nuclear transfer (SCNT) technique, pioneered by Sir J. G. Gurdon in 1962 in frogs and culminated in the cloning of Dolly the Sheep by Ian Wilmut in 1996 (Figure 1A). However, SCNT involves transferring a somatic nucleus into an enucleated oocyte and suffers from technical challenges due to its poor efficiency rate and ethical challenge for using oocytes in human studies. Therefore, alternative approaches to capture the in vitro experimental totipotency are actively pursued in the field.
Figure 1:
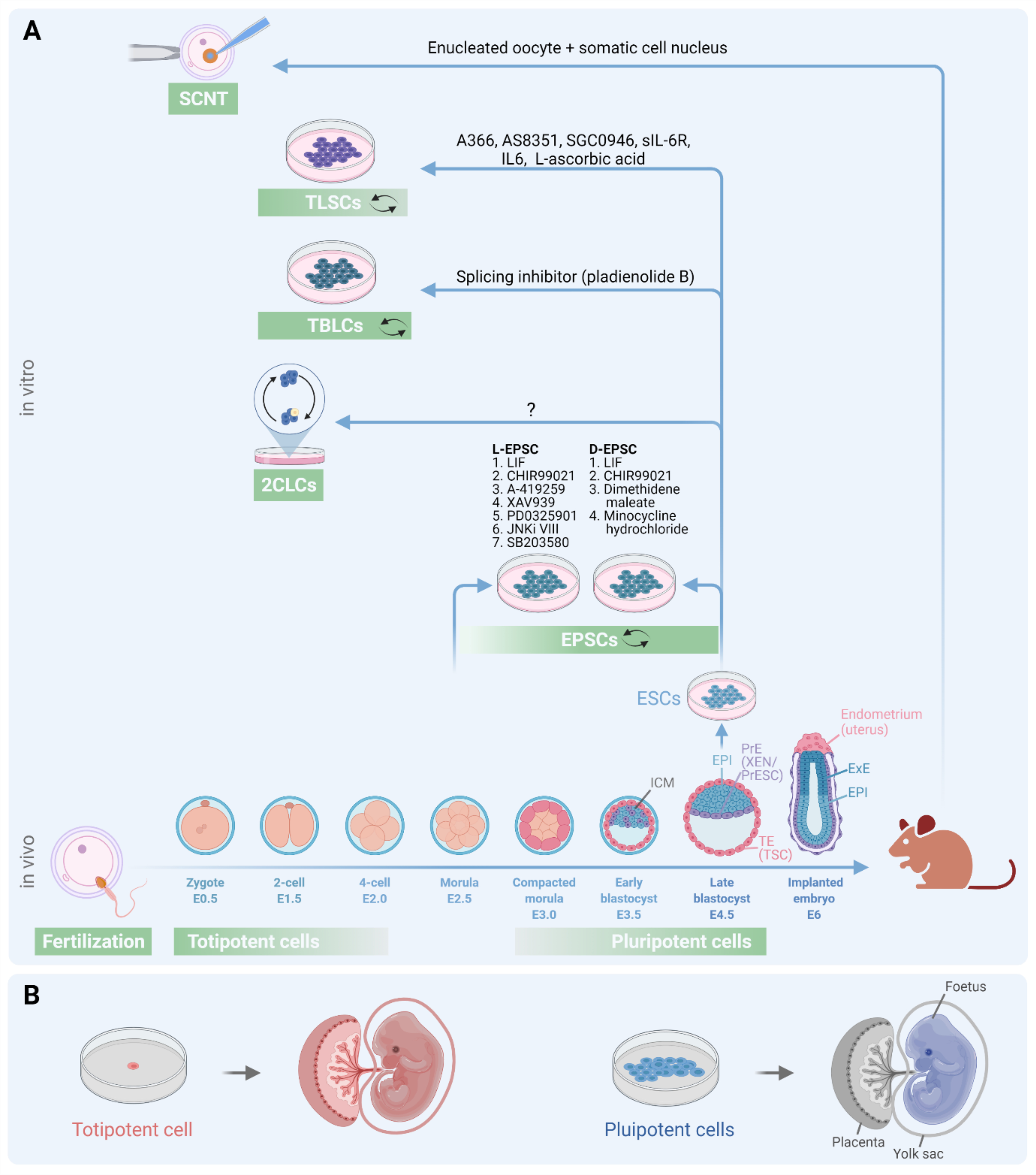
Overview of mouse early development and current status of totipotent-like cells cultured in vitro. (A) Mouse development begins with fertilization followed by the formation of a zygote (1-cell) and blastomere of a 2-cell embryo, both of which are totipotent. The zygotic genome is activated at the 2C stage in the mouse. The inner cell mass (ICM) cells in the blastocyst are pluripotent. The blastocyst has three distinct cell lineages: EPI-epiblast; PrE-primitive endoderm; TE-trophectoderm. EPI gives rise to all cells of the organism; TE forms placenta; PrE forms yolk sac. Embryonic stem cells (ESCs), extraembryonic endoderm (XEN) or primitive endoderm stem cells (PrESCsup ref 1) cells, trophoblast stem cells (TSCs) have been established from EPI, PrE and TE respectively, by isolating cells from mouse blastocysts. Differentiated cells can be reprogrammed to totipotent cells through somatic cell nuclear transfer (SCNT). ESCs have been reprogrammed to totipotent blastomere-like cells (TBLCs) via spliceosome suppression [12], to expanded/extended potential stem cells (EPSCs [8, 9]) and totipotent-like stem cells (TLSCs [13]) via small molecule inductions. In contrast, 2-cell like cells (2CLCs) are transiently appearing cells within ESC culture where ESCs dynamically enter and exit a 2CLC-like state with 1–5% cells at equilibrium at a given time [2, 6]. Key chemical inhibitors used for in vitro derivation of indicated totipotent-like stem cells are mentioned. Green rectangles roughly represent the molecular features matching with early mouse development stages. Two small, curved arrows represent self-renewal in vitro. E0.5 to E6 represent embryonic day numbers. ExE: extraembryonic endoderm. (B) Schematic depiction of totipotent (red) and pluripotent (blue) cells and their contributions to both embryonic and extraembryonic tissues or only to embryonic tissues, respectively. Drawn with BioRender.com.
Table 1:
Molecular features of pluripotent and in vitro experimental totipotent-like cells
| Features | mESCs | hESCs (naïve) | 2CLCs [2] | EPSCs [8, 9] | TBLCs [12] | TLSCs [13] |
|---|---|---|---|---|---|---|
| Embryonic tissue contribution | Yes | Yes | Yes | Yes | Yes | Yes |
| Extra embryonic contribution to trophectoderm (TE) | ||||||
| (i) Chimera assays (in vivo) | No | Ethically challenging | Yes# | Yes### | Yes | Yes |
| (ii) Blastoid-formation assay (in vitro) | No | Yes | Yes## | Yes### | Yes | Yes |
| (iii) Trophoblast stem cell (TSC) reprogramming | No | Yes | Not tested | Yes### | Not tested | Not tested |
| Transcriptomic profiles | ||||||
| Global comparison with mouse early embryonic states (Single-cell RNA-seq) | Similar to pre- implantation epiblast stage | Similar to pre- implantation epiblast stage | Not compared with in vivo totipotent cells | Similar to 4- and 8C stages### | Similar to 2- and 4-cell stages | Similar to 2- and 4-cell stages |
| Totipotency or 2C markers | No | No | High | No | High | High |
| Pluripotency markers | High | High | Low | High | Low | Low |
| MERVL | Low | Show HERV expression | High | No | High | High |
| Genomic landscape | ||||||
| Chromatin mobility | Low | comparable | High | Not tested | Not tested | Not tested |
| Chromatin accessibility | Low | comparable | High | Not tested | High; 8 cell and ICM-specific open ATAC-seq peaks | High, 2- & 4-cell specific open chromatin |
| DNA methylome | High | comparable | Low | Intermediate / Dynamic*; higher level of 5hmC | Low | Not tested |
| In vitro culture | ||||||
| Self-renewal | Yes | Yes | Transient | Yes | Yes | Yes |
but not tested with stringency [7]
Done using EpiSC derived iBLCs sup ref 2 which are molecularly similar to 2CLCs; also not done with single 2CLC
Challenged by [11]
Varying levels of 5mC were found between mESCs cultured in different media and EPSCs generated from mESCs of different genetic backgrounds [8]
Here high/low and yes/no are based on a comparison with mESCs
In vitro experimental totipotent-like cells and their biological properties
The journey towards capturing totipotent-like cells started with cultured pluripotent cells. In 2012, Pfaff lab identified a rare transient population of 2-cell like cells (2CLCs) within mouse ESCs [2] that shared transcriptomic, genomic, and metabolic features with that of totipotent 2-cell (2C) stage embryo (Figure 1A). Transcriptomic profiling during ESC to 2CLC transition showed the emergence of Zscan4+ cells, downregulation of pluripotency followed by activation of 2C-specific gene circuitry as intermediate states [3, 4]. Functional studies identified Myc, Dnmt1, Prc1.6, Ep400-Tip600 as barriers [3, 4] and Dux, Gata2, Eif3h, Dppa2/4, Nelfa, Atr as facilitators for 2CLC emergence (summarized in [5]). Mechanistically, Dux activates 2C related genes by binding directly or indirectly by binding and activating the miR-344 cluster, which post-transcriptionally silences Zmym2 and Lsd1 resulting in de-repression of MERVL and other 2C-specific genes [6]. Owing to their contribution to embryonic and extraembryonic lineages, 2CLCs are proposed to have an expanded cell fate potential [2, 7]. However, stable 2CLCs in vitro has not been captured in culture, and their totipotency status has not been rigorously tested according to current standards (Table 1).
Using small molecules, Liu and Deng labs generated expanded/extended potential stem cells (EPSCs) from mouse 8-cell embryos, mouse ESCs (mESCs), human ESCs (hESCs), and human iPSCs (hiPSCs) [8, 9] (Figure 1A). EPSCs show totipotency-associated marker gene expression and possess the ability to contribute to both embryonic and extraembryonic tissues, including trophoblast lineages in chimera generation (Table 1). While ESCs need trophoblast stem cells (TSCs) and/or extraembryonic endoderm (XEN) cells to form into a blastoid-like structure, even a single EPSC could do so [10]. Such potential fits the in vitro experimental totipotency definition. However, contrary to previous claims, single-cell transcriptomic profile comparison showed Liu-EPSCs correlated more with E4.5 or parental ESCs and Deng-EPSCs with E5.5 epiblast or EpiSCs rather than earlier embryonic states [11]. EPSCs also failed to facilitate TSC reprogramming. Although EPSCs contributed to both epiblast (EPI) and trophectoderm (TE) in chimera aggregation experiments, a careful examination of cells contributing to TE showed expression of pluripotency factors rather than TE or trophoblast lineage markers [11], suggesting the in vitro experimental totipotency of EPSCs could be limited (Table 1). Although EPSCs also underperformed in blastoid-forming assays, a small population could nonetheless contribute to TE or extra embryonic endoderm (ExE) like cells. This brings up a contrasting yet intriguing point that only the forced positional localization of donor EPSCs to surface in blastoid-forming assays, but not freely localized donor EPSCs to the host embryo in chimera aggregation experiments, may induce TE differentiation. These observations suggest that not all but only a tiny EPSC population might have expanded potential under specific experimental conditions. Thus, future totipotency capture and assessment should focus on the differentiation of proper cell types and their positions in the embryo to satisfy strict standards.
In the race to obtain in vitro totipotent cells, Du lab has made fascinating claims of generating totipotent blastomere-like cells (TBLCs) from mESCs (Figure 1A) using a splicing inhibitor [12]. TBLCs show epigenomic and transcriptomic profiles similar to 2- and 4-cell blastomeres but distinct from either EPSCs or 2CLCs and have robust bidirectional embryonic and extraembryonic differentiation potential (Table 1). However, in the current culture conditions, these cells suffer from a low proliferation rate, and the molecular basis for self-renewal of TBLC warrant further experiments. Nonetheless, this study uncovers another way to capture and maintain totipotent-like cells in vitro.
More recently, totipotent-like stem cells (TLSCs) were derived from mESCs using chemical reprogramming (Figure 1) that showed remarkably similar features to 2-cell embryos than any other totipotent-like cells derived so far [13]. TLSCs share 2C-stage transcriptomic, chromatin and broad-H3K4me3 profiles and contribute to both embryonic and extraembryonic tissues in in vivo chimera and in vitro blastoid-formation assays following the stringent criteria for totipotency assessment (Table 1). Although TLSCs present a suitable model for understanding establishment, maintenance, and exit of totipotency, they are heterogeneous cells in culture and, like all other totipotent-like cells established thus far, the blastoids derived from TLSCs are incapable of developing into an entire organism.
The last decade has seen a surge of in vitro cultured stem cell-based embryo models using human EPSCs, iPSCs and especially human naïve ESCs [14]. Compared to conventional human primed ESCs, naïve hESCs remarkably display a broader developmental potential to form blastocyst-like structure and contribute to both EPI and TE [15]. Naïve ESC-based blastoids, generated with triple inhibition of Hippo, TGFβ and ERK pathways, showed better similarity to their in vivo counterparts including implantation capability using hormone stimulated 2D open-faced endometrial layer (OFEL) system in vitro [15]. However, these human-blastoids do not represent early preimplantation (zygote to morula) stages thus making in vitro derived totipotent-like cells a more desirable system to capture early and late stages of embryogenesis.
The above-mentioned in vitro derived totipotent-like cells were all shown to have varying degrees of expanded lineage differentiation potential compared to ESCs. Yet they are molecularly and functionally distinct (Figure 1A; Table 1). The striking differences in the acquiring approaches and molecular features of these cells could suggest that there exist alternate routes to reach an authentic totipotent state or alternative totipotent states.
The ongoing capture of in vitro experimental totipotency
Lessons from in vivo embryogenesis and ground-breaking discovery for induction and maintenance of iPSCs should provide a guiding torchlight for establishing authentic totipotent cells in vitro. The forced ectopic expression of transcription factors (TFs) could similarly be used to induce totipotency in pluripotent and/or somatic cells following a rational selection of factors, involved in both induction and maintenance of totipotency. For example, although not essential for embryonic development(sup ref 3), the Dux family protein Dux (human homolog DUX4) could be a potential candidate as its overexpression in ESCs can partially activate ZGA and cause chromatin changes similar to 2-cell stage embryos (sup ref 4−5).
Extracellular signalling is also critical in regulating cell fates by circumventing limitations of low efficiency, slow kinetics, viral vector dependence, and multiple TF requirement. Additionally, small molecules are rapid, safe, dose-dependent, more controllable, and have the potential for high throughput screening and therapeutic interventions. High BMP4 and low Activin/Nodal signalling are speculated to be the reason for expanded potential of EPSCs [10, 11]. Similarly, using chemical inhibitors, triple inhibition of Hippo, TGF-β, and ERK pathways enhanced human blastoid generation [15]. Thus, improving chemically defined culture conditions will accelerate totipotency reprogramming and facilitate establishing bona fide in vitro experimental totipotency.
The success of all above-mentioned strategies largely relies on a robust reporter system. The most commonly used reporter system so far is MERVL-Zscan4 dual reporter for mouse 2CLCs [2, 4], yet a robust reporter for human system is currently lacking and thus a TBLC or TLSC like derivation approach has not been tested for hESCs. An urgent and unmet need is to identify and develop an ideal platform and authentic totipotent reporter for investigating the individual contribution of medium supplements and reprogramming factors that can directly convert somatic and pluripotent cells to acquire in vitro experimental totipotency.
Here, we have summarized the current status of existing totipotent-like cells and the technical challenges in generating authentic totipotent cells in vitro. The advent of multidisciplinary high-throughput approaches (e.g., genome-wide CRISPR and small-molecule screening) combined with computational (e.g., totipotency reprogramming time-course single-cells genomics data analysis) and experimental (e.g., in vivo contribution to extraembryonic tissues in chimera and in vitro single-cell based blastoid-formation) methods should enable us to expand our understanding of totipotency and thus empower us to capture and exploit these totipotent-like cells in developmental biology, fertility, designing new contraceptives, generating organs, and regenerative medicine.
Supplementary Material
Box1: Tests for assessing the authenticity of in vitro captured totipotent-like cells.
Since there is some confusion in defining the true nature of in vitro derived totipotent-like cells (Figure 1A), gold standard criteria for their assessment are crucial. Currently, totipotency is assessed using the following standards: (i) Transcriptomic profiling, especially using single-cell RNA-seq, determines the appropriate cellular state as well as heterogeneity relative to in vivo totipotent cells. (ii) Monitoring cellular epigenome during long-term cultures (not done for EPSCs, TBLCs and TLSCs, Figure 1A and Table 1). (iii) TSC differentiation tests the differentiation capacity of totipotent-like cells to trophoblast lineage by switching to TSC culture conditions (only done using EPSCs, Figure 1A, and Table 1). (iv) Assessment of differentiation potential for three embryonic germ layers, TE, PrE, and their derivatives in vitro; and chimera formation between donor cells and host embryos followed by lineage contribution analysis at various developmental stages (pre-implantation E4.5 and post-implantation E6.5/E12.5) for their in vivo contribution to all lineages including trophoblast lineage. Chimera generation is a suitable test to assess pluripotency but with a caveat for totipotency since the host cell stage has already passed the totipotency stage. Therefore, the paracrine signaling donor cells receive in the host environment isn’t same as they would have otherwise received in totipotent host stage. (v) The most stringent or gold standard test to date is blastoid-forming assay when a single cell type (even more stringent if only a single cell is used) can form the entire blastocyst and eventually form a live conceptus or entire fertile organism. This assay helps determine appropriate cell types generated and their spatial position in 3D blastoids, suggesting that localization is equally important as differentiation potential when assessing totipotency. However, such an assay could not be implemented for human studies because of ethical reasons, thus for pre-implantation morula-aggregation and for post-implantation inter-species chimera generation techniques are often conducted.
Acknowledgments
We thank Ralf Jauch and members in the Wang laboratory for their feedback on the manuscript. The totipotency research in the Wang laboratory is supported by NIH (HD097268; HD095938) and NYSTEM (C35583GG). We apologize to our colleagues whose work we could not cite due to space restrictions.
Glossary
- Totipotency
The ability of a single cell to give rise to all cell types in a body plus extraembryonic or placenta cells
- Pluripotency
The ability of a single cell to give rise to all cell types of a body but not the extraembryonic or placenta cells
- Blastocyst
The blastocyst of the mammalian embryo follows morula (solid ball of cells) stage by forming a hollow ball of cells with a fluid cavity (blastocyst), consisting of inner cell mass (ICM) and an outer layer trophoblast
- Inner cell mass (ICM)
A blastocyst cell population that contains the precursors of epiblast (EPI) and primitive endoderm (PrE) lineages
- Trophectoderm (TE)
Outer layer of the blastocyst, which will form foetal portion of placenta and outer layer of parietal yolk sac
- Epiblast (EPI)
Blastocyst cells that form almost all foetal tissues and the extraembryonic mesoderm
- Trophoblast stem cells (TSC)
TSCs are the precursor cells of placenta that contribute specifically to the trophoblastic component of placenta and parietal yolk sac
- Extraembryonic Endoderm (XEN) stem cells
XEN cells are derived from the PrE and act as a useful model of PrE and serve for differentiation into visceral and parietal endoderm
- 2-cell like cells (2CLCs)
2CLCs are transiently appearing cells within ESC culture where ESCs dynamically enter and exit a 2C-like state with 1–5% cells at equilibrium at a given time
- Expanded/extended potential stem cells (EPSCs)
EPSCs are in vitro totipotent-like cells that are derived from 8-cell or ESCs using small molecule inhibitors
- Totipotent blastomere-like cells (TBLCs)
TBLCs are in vitro totipotent-like cells derived from mouse ESCs using a splicing inhibitor and resemble more closely to 2- and 4-cell stages of early embryonic development
- Totipotent-like stem cells (TLSCs)
TLSCs are derived from mESCs using chemical reprogramming that show molecular and functional features similar to 2-cell embryos
Footnotes
Declaration of interests
No interests are declared.
References
- 1.Riveiro AR and Brickman JM (2020) From pluripotency to totipotency: an experimentalist’s guide to cellular potency. Development 147 (16). [DOI] [PubMed] [Google Scholar]
- 2.Macfarlan TS et al. (2012) Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487 (7405), 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu X et al. (2019) Myc and Dnmt1 impede the pluripotent to totipotent state transition in embryonic stem cells. Nat Cell Biol 21 (7), 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Terrones D et al. (2018) A molecular roadmap for the emergence of early-embryonic-like cells in culture. Nat Genet 50 (1), 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iturbide A and Torres-Padilla ME (2020) A cell in hand is worth two in the embryo: recent advances in 2-cell like cell reprogramming. Curr Opin Genet Dev 64, 26–30. [DOI] [PubMed] [Google Scholar]
- 6.Yang F et al. (2020) DUX-miR-344-ZMYM2-Mediated Activation of MERVL LTRs Induces a Totipotent 2C-like State. Cell Stem Cell 26 (2), 234–250 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genet M and Torres-Padilla ME (2020) The molecular and cellular features of 2-cell-like cells: a reference guide. Development 147 (16). [DOI] [PubMed] [Google Scholar]
- 8.Yang J et al. (2017) Establishment of mouse expanded potential stem cells. Nature 550 (7676), 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y et al. (2017) Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 169 (2), 243–257 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R et al. (2019) Generation of Blastocyst-like Structures from Mouse Embryonic and Adult Cell Cultures. Cell 179 (3), 687–702 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posfai E et al. (2021) Evaluating totipotency using criteria of increasing stringency. Nat Cell Biol 23 (1), 49–60. [DOI] [PubMed] [Google Scholar]
- 12.Shen H et al. (2021) Mouse totipotent stem cells captured and maintained through spliceosomal repression. Cell 184 (11), 2843–2859 e20. [DOI] [PubMed] [Google Scholar]
- 13.Yang M et al. (2022) Chemical-induced chromatin remodeling reprograms mouse ESCs to totipotent-like stem cells. Cell Stem Cell. [DOI] [PubMed] [Google Scholar]
- 14.Popovic M et al. (2021) Engineered models of the human embryo. Nat Biotechnol 39 (8), 918–920. [DOI] [PubMed] [Google Scholar]
- 15.Kagawa H et al. (2022) Human blastoids model blastocyst development and implantation. Nature 601 (7894), 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


