Abstract
Background:
Indoleamine 2, 3-dioxygenase 1 (IDO1) is the rate-limiting enzyme for tryptophan metabolism. IDO1 malfunction is involved in the pathogenesis of atherosclerosis. Vascular smooth muscle cells (VSMCs) with an osteogenic phenotype promote calcification and features of plaque instability. However, it remains unclear whether aberrant IDO1-regulated tryptophan metabolism causes VSMCs osteogenic reprogramming and calcification.
Methods:
We generated global apolipoprotein E (Apoe) and IDO1 double knockout mice, and Apoe knockout mice with specific deletion of IDO1 in VSMCs or macrophages. Arterial intimal calcification was evaluated by Western diet-induced atherosclerotic calcification model.
Results:
Global deficiency of IDO1 boosted calcific lesion formation without gender bias in vivo. Conditional IDO1 loss of function in VSMCs rather than macrophages promoted calcific lesion development and the abundance of runt-related transcription factor 2 (RUNX2). In contrast, administration of kynurenine via intraperitoneal injection markedly delayed the progression of intimal calcification in parallel with decreased RUNX2 expression in both Apoe−/− and Apoe−/− Ido1−/− mice. We found that IDO1 deletion restrained RUNX2 from proteasomal degradation, which resulted in enhanced osteogenic reprogramming of VSMCs. Kynurenine administration downregulated RUNX2 in an aryl hydrocarbon receptor (AhR)-dependent manner. Kynurenine acted as the endogenous ligand of AhR, controlled resultant interactions between cullin 4B and AhR to form an E3 ubiquitin ligase that bound with RUNX2, and subsequently promoted ubiquitin-mediated instability of RUNX2 in VSMCs. Serum samples from patients with coronary artery calcification had impaired IDO1 activity and decreased kynurenine catabolites, comparing with those without calcification.
Conclusions:
Kynurenine, an IDO1-mediated tryptophan metabolism main product, promotes RUNX2 ubiquitination and subsequently leads to its proteasomal degradation via an AhR-dependent non-genomic pathway. Insufficient kynurenine exerts the deleterious role of IDO1 ablation in promoting RUNX2-mediated VSMCs osteogenic reprogramming and calcification in vivo.
Keywords: arterial calcification, IDO1, kynurenine, osteogenic reprogramming, RUNX2
This study is the first to find that indoleamine 2, 3-dioxygenase 1 (IDO1) deletion in vascular smooth muscle cells but not macrophages exacerbated the progression of arterial calcification. The phenotype was rescued by supplementation with the major IDO1-produced catabolite, kynurenine. The suppression of calcification by kynurenine was runt-related transcription factor 2 (RUNX2) dependent. Mechanistically, kynurenine exerted the protective effects by activating the RUNX2 proteasome degradation. In the clinical setting, kynurenine derivatives and serum IDO1 activity were negatively correlated with coronary artery calcification progression in chronic kidney disease patients. These findings may provide new insights into therapeutic strategies for the relief of abnormal calcification in the cardiovascular system.
Twitter handle: @GSU_Research
Introduction
In the clinical setting, the leading cause of acute myocardial infarction and stroke events has been traced to atherosclerotic plaque rupture.1 Calcific vasculopathy, featured by ectopic deposition of hydroxyapatite crystal in vessels, is prevalent in advanced lesions and contributes to the increased vulnerability and probability of rupture.2 Previously considered passive and degenerative, arterial calcification is now recognized as an active osteogenic process resembling osteoblast differentiation during skeletal mineralization, and vascular smooth muscle cell (VSMC) is the major contributor.3 Due to the lack of a comprehensive understanding of the pathogenesis of arterial calcification, no effective interventions have been identified.
Indoleamine 2, 3-dioxygenase 1 (IDO1) is the determinant enzyme that catalyzes the degradation of the essential amino acid tryptophan to multiple catabolites, via which IDO1 exerts its biological effects.4 Kynurenine is the main, initial downstream product. The kynurenine-to-tryptophan (Kyn/Trp) ratio reflects IDO1 activity and is linked to disorders including cancer immuno-resistance, neurodegeneration, and intestinal inflammation.5 IDO1-related pathways exert dual biological effects (i.e., protective or deleterious) on cardiovascular diseases, including endothelial malfunction,6 myocardial infarction,7 cardiometabolic syndrome,8 hypertension,9 abdominal aortic aneurysm,10 and atherosclerosis.11 However, it is unknown whether IDO1-mediated tryptophan metabolism contributes to arterial calcification.
IDO1 activity and kynurenine derivatives are indispensable for osteoblastic differentiation of bone cells during skeletal metabolism. Kynurenine leads to decreased bone marrow mesenchymal stem cell (MSC) differentiation and bone mineralization through increased oxidative stress,12 altered energy production,13 and impaired autophagy.14 Quinolinic acid, the end product of the kynurenine pathway, exerts an osteogenic effect on human MSCs.15 Anthranilic acid and 3-hydroxyanthranilic acid imbalance is strongly associated with a higher risk of osteoporosis in humans.16 Given the overlap of mechanisms underlying bone and vasculature mineralization, we hypothesized that IDO1 and tryptophan-derived metabolites have potential effects on the development of arterial calcification.
Runt-related transcription factor 2 (RUNX2) is a hallmark for osteoblast differentiation and chondrocyte maturation. Mutations in the RUNX2 gene cause cleidocranial dysplasia in humans.17 Healthy vasculature expresses trace levels of RUNX2, but markedly increased RUNX2 levels have been identified in animal models and human aortic tissue specimens of calcified atheromatous plaques.18 RUNX2 expressed in vascular cells dominates the intrinsic signaling cascades of osteogenic reprogramming and arterial calcification.19 One study found an inhibitory effect of kynurenine on RUNX2 expression in MSCs but did not elucidate the precise mechanism.20 In this study, we found that the formation of calcific plaque was boosted by IDO1 deficiency but conversely prevented by kynurenine supplementation via a regulatory network of RUNX2 ubiquitin-dependent proteasomal degradation. Our findings reveal the previous unsuspected role of IDO1 and its metabolites in the repression of arterial calcification and suggest new approaches for potential treatments.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. The expanded methods are provided in the supplemental material.
Study population
This case-control study was conducted from March 2018 to January 2020 at Tungwah Hospital of Sun Yat-sen University, China. Of 198 participants with a diagnosis of chronic kidney disease (CKD), 10 participants who met the exclusion criteria, 10 participants who missed the blood collection, 22 patients without data from multidetector computed tomography (MDCT) scanning of the coronary artery, and 4 patients due to serum sample quality issue were excluded. The clinical study protocol followed ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the Tungwah Hospital of Sun Yat-sen University (2017DHLL017). All participants gave written informed consent before entering the study.
Animal experiments
The animal experiments were performed following protocols approved by the Georgia State University Institute Animal Care and Use Committee. Specific-pathogen-free mice were raised at the animal facility of Georgia State University in controlled, identical temperature, humidity, and 12-hour light-dark cycle conditions. They had free access to sterilized water and the assigned diet.
Statistical analyses
All results were expressed as mean ± standard error of the mean (SEM), median (25th-75th quartiles) for continuous variables, or n (%) for categorical variables, respectively. The Shapiro-Wilk test or D’Agostino-Pearson test was performed to assess data normality. The equality of group variances was assessed using F test or Brown-Forsythe test. For comparisons between two groups, significance was assessed using unpaired two-tailed Student’s t-tests, unpaired t-test with Welch’s correction, or nonparametric Mann-Whitney U test. For comparisons among multiple groups, analysis of variance (ANOVA) or Welch’s ANOVA test accompanied by Tukey’s post hoc test (equal variances assumed), Dunnett’s post hoc test, or Tamhane’s T2 post hoc test (equal variances not assumed) was performed. Correlation was assessed using Spearman’s rank correlation coefficient. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 28.0 (IL, USA) or GraphPad Prism 8.0 (CA, USA).
Results
IDO1 deficiency aggravates atherosclerotic calcification in vivo without gender bias
Ido1−/− mice were crossed with hyperlipidemic apolipoprotein E-deficient (Apoe−/−) mice to generate Apoe−/− Ido1−/− mice. Male Apoe−/− Ido1−/− and Apoe−/− mice were fed with Western diet for 24 weeks. Atherosclerotic calcification was confirmed using von Kossa staining. Apoe−/− Ido1−/− mice had a marked tendency for systemic calcification formation within lesion areas in the aortic root, aortic arch, femoral artery, and brachiocephalic artery, compared with Apoe−/− mice (Figure 1A–1E). Apoe−/− Ido1−/− mice exhibited markedly increased calcium content and ALP activity in aortas, compared with Apoe−/− mice (Figure 1F and 1G).
Figure 1. IDO1 deficiency promotes atherosclerotic calcification in vivo.
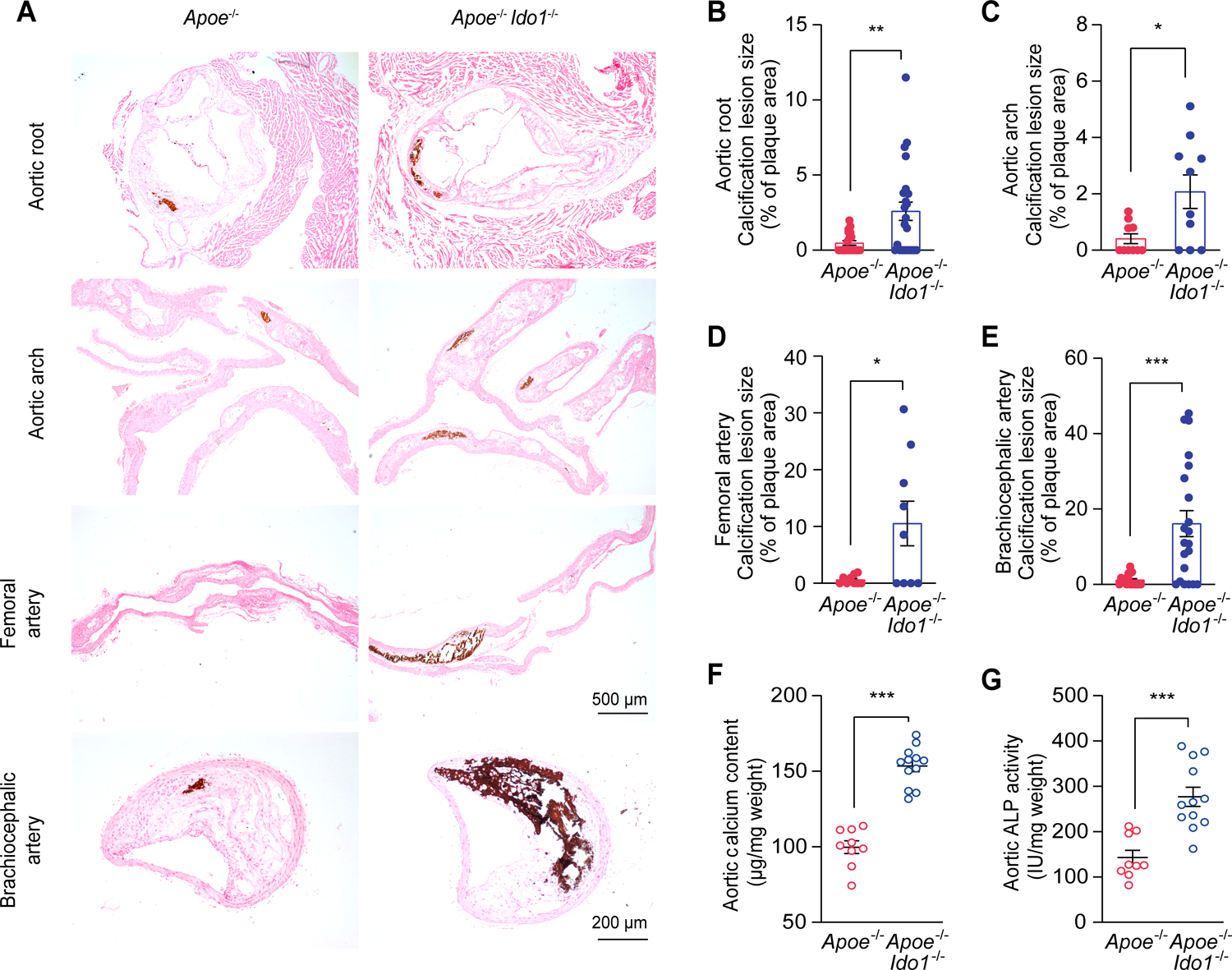
A-E, Representative von Kossa staining (A) and quantification performed in serial tissue sections of aortic root (B), aortic arch (C), femoral artery (D), and brachiocephalic artery (E) of Apoe−/− (n = 9–20) and Apoe−/− Ido1−/− (n = 9–24) mice that were fed with Western diet for 24 weeks. Scale bars: 200 μm for brachiocephalic artery; 500 μm for aortic root, aortic arch, and femoral artery.
F and G, Biochemical measurement of aortic calcium content (F) and ALP activity (G) of Apoe−/− and Apoe−/− Ido1−/− mice fed with Western diet for 24 weeks (n = 9–12).
Results are presented as mean ± SEM, and analyzed using Mann Whitney test for B and C, unpaired t-test with Welch’s correction for D and E, two-tailed unpaired Student’s t-test for F and G. *P < 0.05, **P < 0.01, ***P < 0.001.
The reasons for the sex-based differences in the effects of atherosclerotic calcification remain controversial. Thus, we fed female Apoe−/− and Apoe−/− Ido1−/− mice with the Western diet for 24 weeks. We found marked increases in aortic calcium content and ALP activity in the female IDO1-knockout mice, along with accumulated aortic calcification revealed by the von Kossa staining, which matched the results of male Apoe−/− Ido1−/− (Figure S1). Taken together, our findings suggest that IDO1 deficiency aggravates atherosclerotic calcification formation in vivo, without gender bias.
Calcification development in IDO1 knockout mice is atherosclerosis-independent
To exclude the effect of atherogenesis on calcification development in vivo, we next assessed absolute lesion areas and lesion area fractions in situ. We found no significant differences in these arteries between the Apoe−/− and Apoe−/− Ido1−/− mice, including gender differences (Figure S2). Male and female Apoe−/− and Apoe−/− Ido1−/− mice had similar metabolic parameters, including serum triglyceride, cholesterol, calcium, blood glucose, and body weight (Figure S3). Thus, we conclude that the enhanced calcification formation in IDO1 knockout mice is independent of atherosclerosis.
Serum kynurenine levels are inversely associated with calcification development in vivo
When we stratified all the male and female Apoe−/− and Apoe−/− Ido1−/− mice fed with Western diet into three groups according to severity of atherosclerotic calcification and compared serum kynurenine levels among groups, we found a negative correlation between serum kynurenine levels and extents of atherosclerotic calcification (Figure S4A–S4G). We also found a similar association between serum Kyn/Trp ratios and progression of atherosclerotic calcification (Figure S4H and S4I). Taken together, these results suggest that kynurenine is involved in IDO1-regulated arterial calcification.
IDO1 depletion in VSMCs but not in macrophages promotes RUNX2 expression and atherosclerotic calcification in vivo
VSMCs and macrophages are central regulators of arterial calcification.21 We postulated that VSMCs and macrophages, which both express IDO1,4 participated in the IDO1-relevant modulation of calcification. Therefore, we generated Apoe−/− background mice with a specific deletion of IDO1 in VSMCs (Apoe−/− Ido1fl/fl Myh11Cre [Apoe−/− Ido1△SMC]) or macrophages (Apoe−/− Ido1fl/fl lysMCre [Apoe−/− Ido1△MΦ]), and fed them and their littermates (Apoe−/− Ido1fl/fl) with Western diet for 24 weeks. Apoe−/− Ido1△SMC mice but not Apoe−/− Ido1△MΦ mice had higher levels of calcium content and ALP activity in aorta tissues than Apoe−/− Ido1fl/fl mice (Figure 2A and 2B). Resembling skeletal mineralization, VSMCs ossification relies on multistep intracellular pathways modulated by various transcription factors, such as RUNX2, msh homeobox 2 (MSX2), and SOX9.3 Accordingly, we next examined whether they were modulated in our system. The results indicated that only RUNX2 expression was subject to IDO1 depletion in vivo (Figure S5). Western blot analysis verified the elevations in RUNX2 expression in aortas of Apoe−/− Ido1△SMC mice but not in Apoe−/− Ido1△MΦ mice, compared with their controls (Figure 2C). Consistently, von Kossa staining and immunofluorescence staining revealed markedly increased calcium nodule deposition in both aortic root and brachiocephalic artery and coincident upregulated aortic expression of RUNX2 in Apoe−/− Ido1△SMC mice, compared with their littermates (Figure 2D–2G). Absolute lesion area and lesion area fraction in situ were unchanged between three groups (Figure S6A and S6B). Besides, serum triglyceride, cholesterol, calcium, blood glucose, and body weight also remained similar (Figure S6C–S6G). Moreover, we found that IDO1 deficiency in macrophages had a mild effect on aortic kynurenine levels in situ (Figure S6H). Taken together, these results indicate that VSMCs-expressed IDO1 is essential for regulation of arterial calcification progression.
Figure 2. VSMCs-specific IDO1 deficiency promotes calcification and RUNX2 expression.
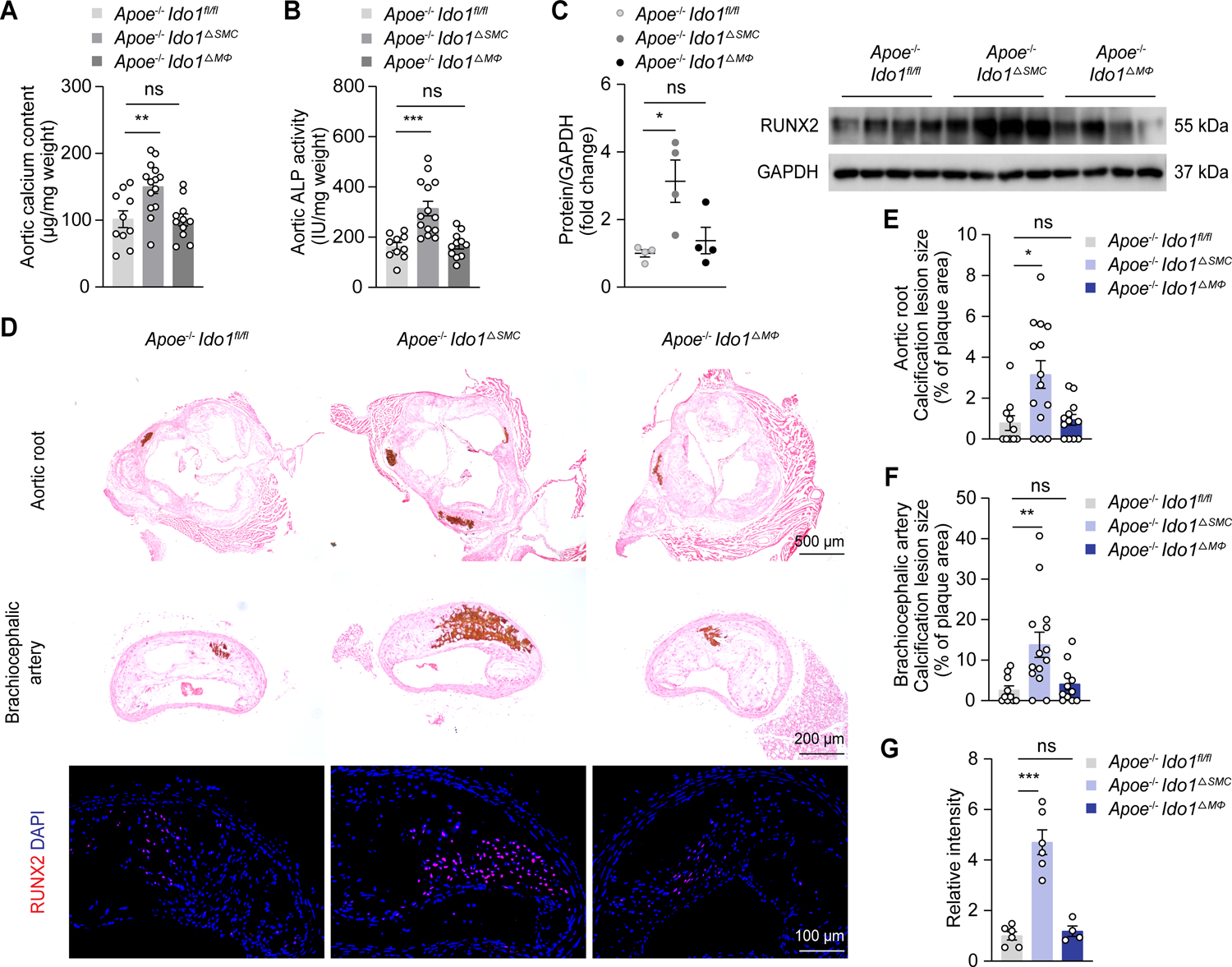
Apoe−/− Ido1fl/fl (n = 10), Apoe−/− Ido1fl/fl Myh11Cre (Apoe−/− Ido1△SMC, n = 14), and Apoe−/− Ido1fl/fl lysMCre (Apoe−/− Ido1△MΦ, n = 11–12) mice were fed with Western diet for 24 weeks.
A and B, Biochemical measurement of aortic calcium content (A) and ALP activity (B) for indicated mice.
C, Western blot analysis of RUNX2 expression in aorta lysates from indicated mice (n = 4).
D, Representative von Kossa staining and immunofluorescence staining of RUNX2 in aortic root and brachiocephalic artery tissue sections from indicated mice. Scale bars: 500 μm for von Kossa staining of aortic root; 200 μm for von Kossa staining of brachiocephalic artery; 100 μm for immunofluorescence staining.
E-G, Quantification of percentage of calcification lesions within plaque areas in aortic root (E) and brachiocephalic artery tissue sections (F) from Apoe−/− Ido1fl/fl, Apoe−/− Ido1△SMC, and Apoe−/− Ido1△MΦ mice, and RUNX2 immunofluorescence staining (G) in brachiocephalic artery tissue sections from indicated mice (n = 4–6 for G).
Results are presented as mean ± SEM. P values are assessed using one-way ANOVA with Dunnett’s post hoc test for A-C and G, Welch’s ANOVA with Dunnett’s post hoc test for E and F. *P < 0.05, **P < 0.01, ***P < 0.001, ns indicates P > 0.05.
Kynurenine alleviates oxLDL-induced RUNX2 expression in vitro
The aforementioned results indicated a strong link between kynurenine levels and arterial calcification. To identify IDO1-catablized metabolites that associated with IDO1-regulated calcification, we incubated primary VSMCs isolated from Ido1fl/fl Myh11Cre mice in osteogenic media for 14 days, supplemented with major metabolites of IDO1-catabolized tryptophan degradation. The post-screening western blot and alizarin red staining results indicated that only kynurenine down-regulated RUNX2 expression and suppressed calcified nodule deposition, compared with the DMSO-treated control group (Figure S7A–S7C). Moreover, quantitative RT-PCR results revealed that none of the metabolites, including kynurenine, altered Runx2 mRNA levels, compared to the DMSO group (Figure S7D). Elevated oxLDL exerted its deleterious effect on aggravation of atherosclerotic calcification by inducing RUNX2 expression in VSMCs.21 Thus, we treated primary VSMCs isolated from Ido1fl/fl and Ido1fl/fl Myh11Cre mice in the presence of oxLDL, with or without kynurenine. As expected, oxLDL successfully triggered upregulation of RUNX2, and kynurenine supplementation reversed this effect (Figure S7E). Altogether, these results suggest kynurenine had a crucial role in inhibition of calcification via RUNX2 reduction.
Kynurenine administration suppresses atherosclerotic calcification in vivo
To verify the beneficial effects of kynurenine on calcification development in vivo, Apoe−/− and Apoe−/− Ido1−/− mice were fed with Western diet for 16 weeks, then were supplemented with vehicle or kynurenine (100 mg/kg) via intraperitoneal injection along with the Western diet for another 8 weeks. Corresponding increased kynurenine were detected in serum from kynurenine-treated Apoe−/− and Apoe−/− Ido1−/− mice (Figure S8). Consistently, kynurenine supplementation ameliorated aortic calcium content and ALP activity in both Western diet-fed Apoe−/− and Apoe−/− Ido1−/− mice. It also resulted in reduced mineralization in atherosclerotic plaque areas of the aortic roots and brachiocephalic arteries (Figure 3A–3F). Immunofluorescence staining and western blot results indicated RUNX2 expression was also reduced in the kynurenine-treated groups, compared with the vehicle controls (Figure 3G and 3H). However, mRNA levels of Runx2 expression were not affected by IDO1 deletion or kynurenine (Figure 3I). Changes in atherogenesis in situ were not significant (Figure S9A and S9B), nor were there any changes in metabolic parameters, including serum lipid and calcium levels, blood glucose, and body weight after kynurenine administration (Figure S9C–S9G). Overall, these results indicate that kynurenine has a preventive effect on calcification development.
Figure 3. Kynurenine alleviates RUNX2 expression and calcification.
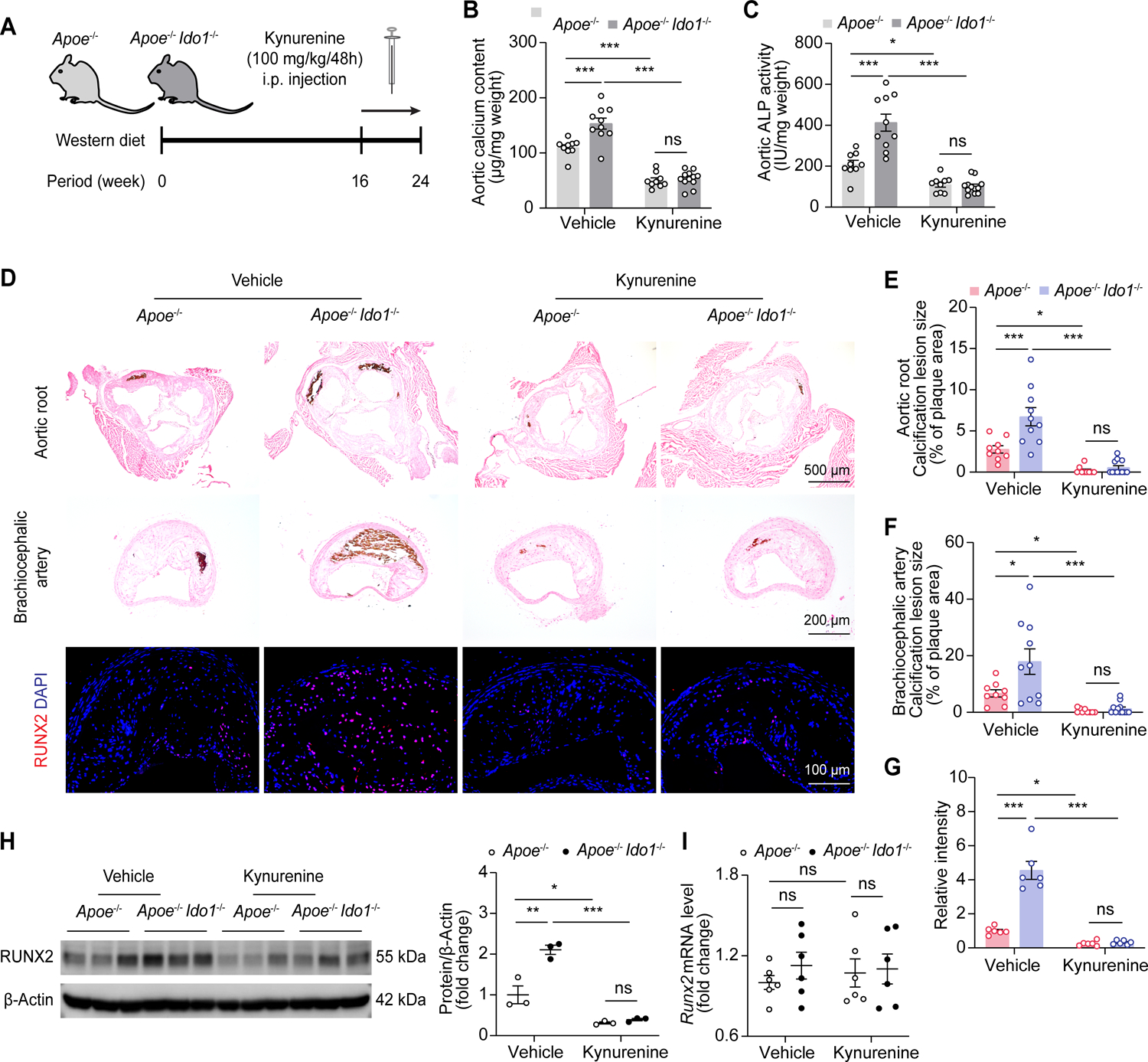
A, Experimental scheme, Apoe−/− and Apoe−/− Ido1−/− mice were fed with Western diet for 16 weeks, then supplemented with vehicle or kynurenine (100 mg/kg) via intraperitoneal (i.p.) injection every 48 hours together with the Western diet for another 8 weeks.
B and C, Biochemical measurement of aortic calcium content (B) and ALP activity (C) of indicated group (n = 9–12).
D, Representative von Kossa staining and immunofluorescence staining of RUNX2 in aortic root and brachiocephalic artery tissue sections from indicated mice. Scale bars: 500 μm for von Kossa staining of aortic root; 200 μm for von Kossa staining of brachiocephalic artery; 100 μm for immunofluorescence staining.
E-G, Quantification of percentages of calcification lesions within plaque areas in aortic root (E) and brachiocephalic artery tissue sections (F), and RUNX2 immunofluorescence staining (G) in brachiocephalic artery tissue sections from indicated mice (n = 6 for G).
H and I, Western blot (H, n = 3) and quantitative RT-PCR (I, n = 6) analysis of RUNX2 expression in aorta lysates from mice with or without kynurenine injection.
Results are presented as mean ± SEM, and analyzed using two-way ANOVA with Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ns indicates P > 0.05.
IDO1-related kynurenine modulates osteogenic reprogramming of VSMCs
RUNX2 is required for calcification development by mastering the osteogenic reprogramming of VSMCs without affecting atherogenesis.19 Next, we examined the VSMCs osteogenic phenotype switch in an IDO1 knockout setting. Western blot analysis revealed that IDO1 knockdown induced increased expression of osteogenic trans-differentiation markers (OCN and OPN), accompanied by suppression of contractile markers (SM22α and calponin1) in aorta tissues (Figure 4A). Immunofluorescence staining analyses indicated that the elevated RUNX2 and osteochondrogenic reprogramming marker of COL1A1 were mostly co-localized in aortic intimal calcification areas in vivo (Figure 4B–4D). We cultured aortic rings isolated from Ido1fl/fl and Ido1fl/fl Myh11Cre mice in osteogenic media ex vivo, with or without kynurenine supplementation. We found a marked shift towards less RUNX2 and more α-SMA expression in kynurenine-treated subjects, compared with vehicle-treated controls (Figure 4E and 4F). In summary, these results indicate that kynurenine has a protective role in calcification by rescuing IDO1 deficiency-promoted VSMCs osteogenic reprogramming.
Figure 4. IDO1-related kynurenine modulates osteogenic reprogramming of VSMCs.
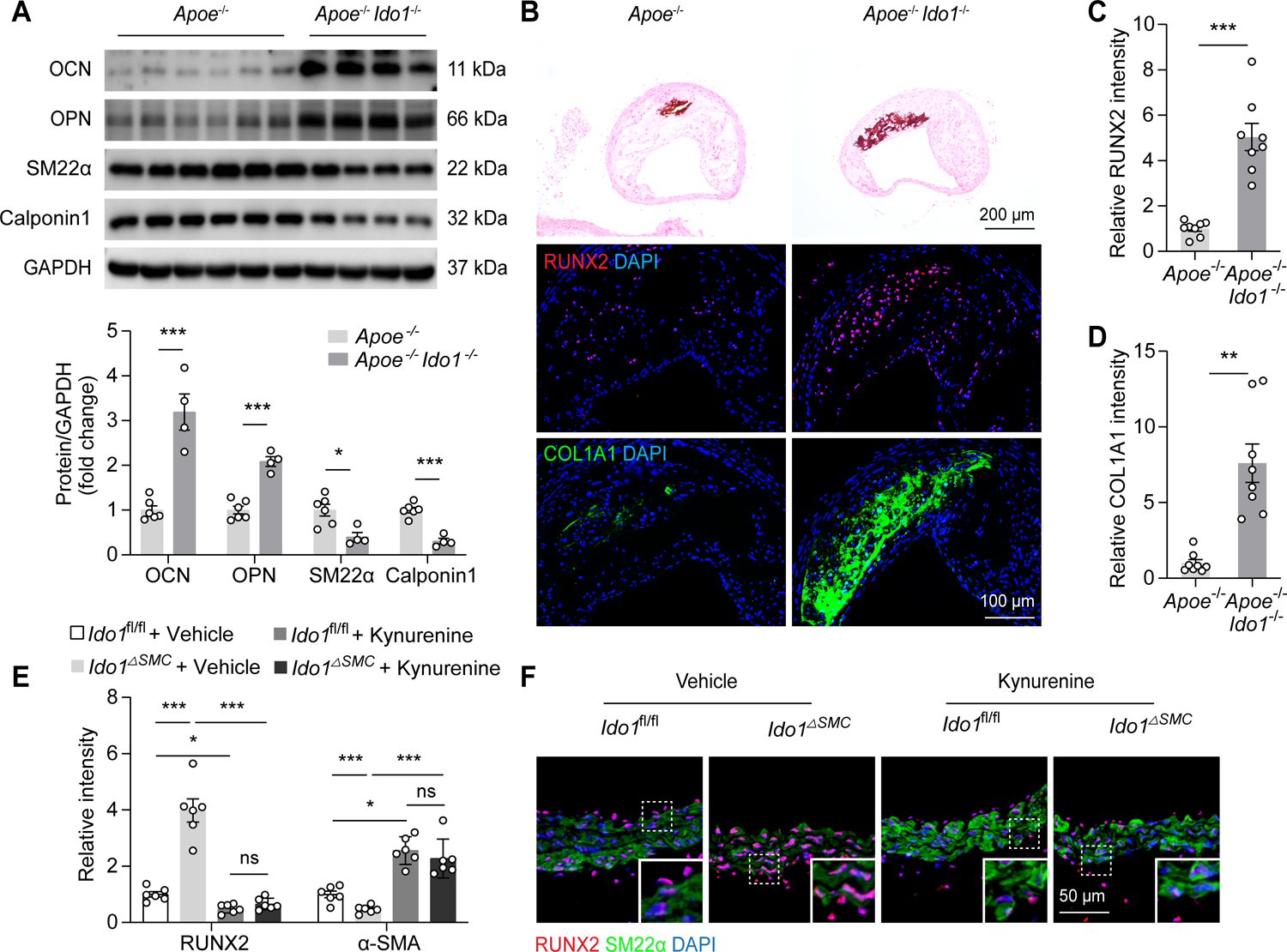
Apoe−/− and Apoe−/− Ido1−/− mice were fed with Western diet for 24 weeks.
A, Western blot analysis of OCN, OPN, SM22α, and calponin1 expression in whole aorta lysates from Apoe−/− (n = 6) and Apoe−/− Ido1−/− (n = 4) mice.
B, Representative von Kossa staining and immunofluorescence staining of RUNX2 in brachiocephalic artery tissue sections from male Apoe−/− and Apoe−/− Ido1−/− mice. Scale bars: 200 μm for von Kossa staining; 100 μm for immunofluorescence staining.
C and D, Quantification of RUNX2 (C) and COL1A1 (D) expression in brachiocephalic artery tissue sections from Apoe−/− and Apoe−/− Ido1−/− mice (n = 8).
E and F, Aortic rings isolated from Ido1fl/fl and Ido1△SMC mice were treated with DMSO or kynurenine (50 μmol/L) for 3 days in osteogenic media ex vivo. RUNX2 and α-SMA expression were detected using immunofluorescence staining (n = 6).
Results are presented as mean ± SEM, and analyzed using two-tailed unpaired Student’s t-test for A, unpaired t-test with Welch’s correction for C and D; Two-way ANOVA with Tukey’s post hoc test is used for E. *P < 0.05, **P < 0.01, ***P < 0.001, ns indicates P > 0.05.
IDO1 modulates VSMCs osteogenic reprogramming in a RUNX2-dependent manner
Our results indicated that RUNX2 expression is regulated by IDO1. To examine whether RUNX2 mediated the VSMCs calcification promoted by IDO1 deficiency, primary VSMCs or aortic rings were isolated from Ido1fl/fl and Ido1fl/fl Myh11Cre mice and then transfected with lentivirus carrying scramble shRNA or RUNX2 shRNA. Although IDO1 depletion markedly fostered calcium nodule formation, RUNX2 deficiency effectively suppressed VSMCs calcification and related expression of osteogenic reprogramming markers (OCN and OPN), accompanied by reciprocal changes in contractile markers (SM22α and calponin1) (Figure 5A and 5B). Cultured aortic rings also had reduced calcium deposition and ALP activity in the RUNX2 deficient groups (Figure 5C–5F). Lentivirus carrying RUNX2 shRNA infection abrogated calcium deposition in human coronary VSMCs stably encoding IDO shRNA (Figure S10). Taken together, these results indicate that IDO1-modulated VSMCs osteogenic reprogramming is RUNX2-dependent.
Figure 5. IDO1 modulates osteogenic reprogramming of VSMCs in a RUNX2-dependent manner.
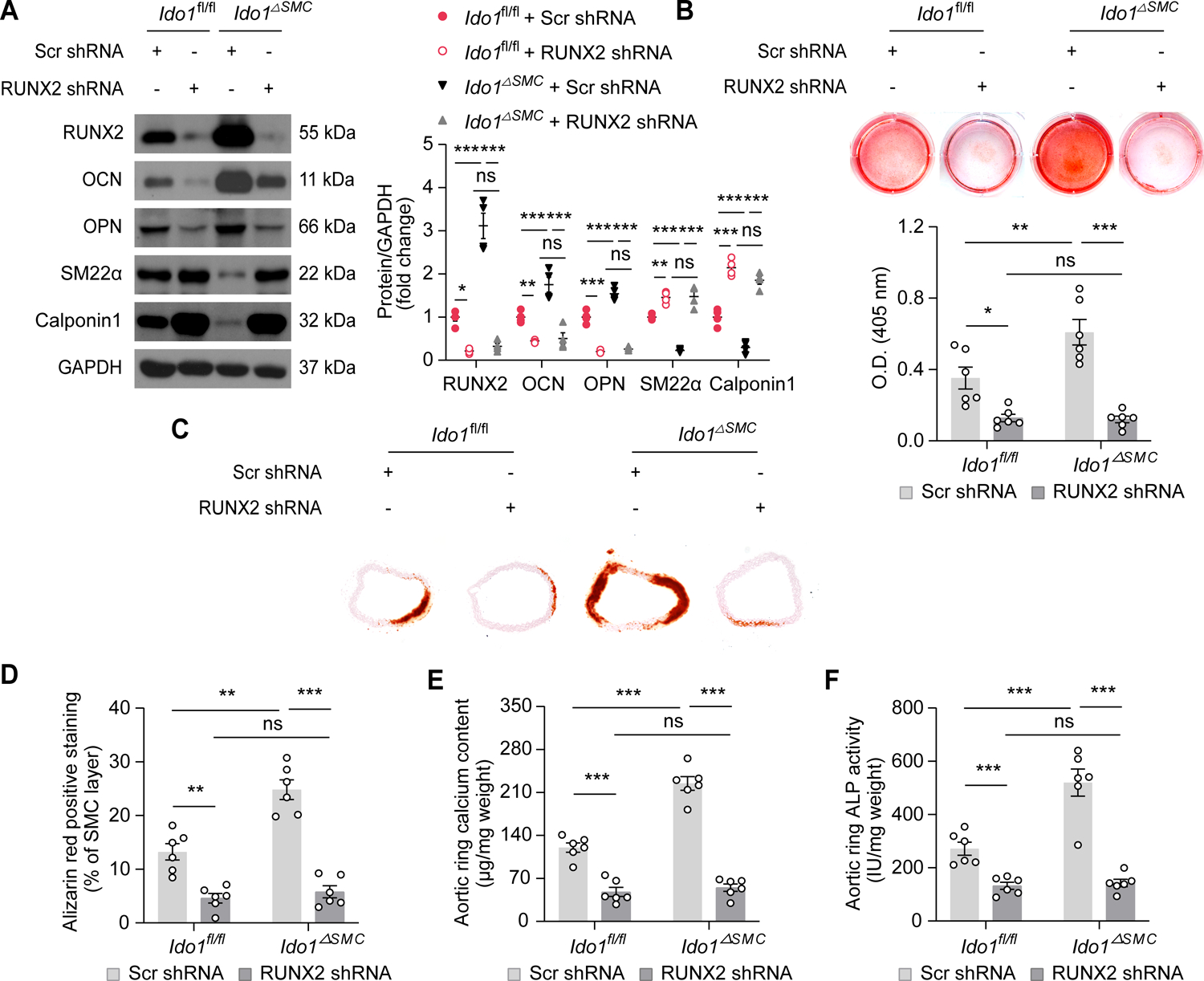
A and B, Western blot analysis of RUNX2, OCN, OPN, SM22α, and calponin1 expression (A), and quantitative analysis of alizarin red staining (B) in primary VSMCs isolated from Ido1fl/fl and Ido1fl/fl Myh11Cre mice infected with lentivirus encoding scramble shRNA or RUNX2 shRNA, and then challenged with osteogenic media for 14 days (n = 6).
C-F, Quantitative analysis of alizarin red staining (C and D), calcium content (E), and ALP activity (F) in primary aortic rings isolated from Ido1fl/fl and Ido1fl/fl Myh11Cre mice infected with lentivirus carrying Scr shRNA or RUNX2 shRNA, and then challenged with osteogenic media for 3 days (n = 6).
Results are presented as mean ± SEM, and analyzed using two-way ANOVA with Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ns indicates P > 0.05.
IDO1 promotes ubiquitination and proteasomal degradation of RUNX2 in VSMCs through kynurenine production
As rodents might differ from humans regarding the regulation of IDO1 activity, we then compared the results for rodents with results of human coronary VSMCs. Consistent with the murine results, silencing IDO1 resulted in significant upregulation of RUNX2, and kynurenine treatment reduced remnant RUNX2 in VSMCs in a time-dependent way (Figure S11). The aforementioned in vivo results indicated that Runx2 mRNA expression was not affected by IDO1 deletion or kynurenine. To examine potential mechanisms underlying IDO1-kynurenine-mediated RUNX2 reduction, we treated human coronary VSMCs with IDO1 silence or exogenous kynurenine administration. We found these treatments had no effects on RUNX2 mRNA expression levels (Figure 6A). These results suggest that IDO1-kynurenine regulates RUNX2 expression at the post-transcription level. RUNX2 is altered by mRNA decay or is degraded by lysosome or proteasome.22 Thus, we first applied transcription inhibitor actinomycin D to human coronary VSMCs pre-infected with scramble siRNA or IDO1 siRNA, but RUNX2 mRNA levels were not affected in either group (Figure 6B). Next, lysosome inhibitor chloroquine or proteasome inhibitor MG132 were incubated with human coronary VSMCs pretreated with scramble siRNA or IDO1 siRNA. Chloroquine had mild effects on RUNX2 expression (Figure 6C). MG132 time-dependently increased RUNX2 accumulation in the control group as early as 4 hours but only mildly altered RUNX2 expression in the IDO1-silenced group (Figure 6D). These results indicate IDO1 depletion prevents RUNX2 from proteasomal degradation, but not lysosome degradation.
Figure 6. Kynurenine promotes ubiquitin-mediated proteasomal degradation of RUNX2 in VSMCs.
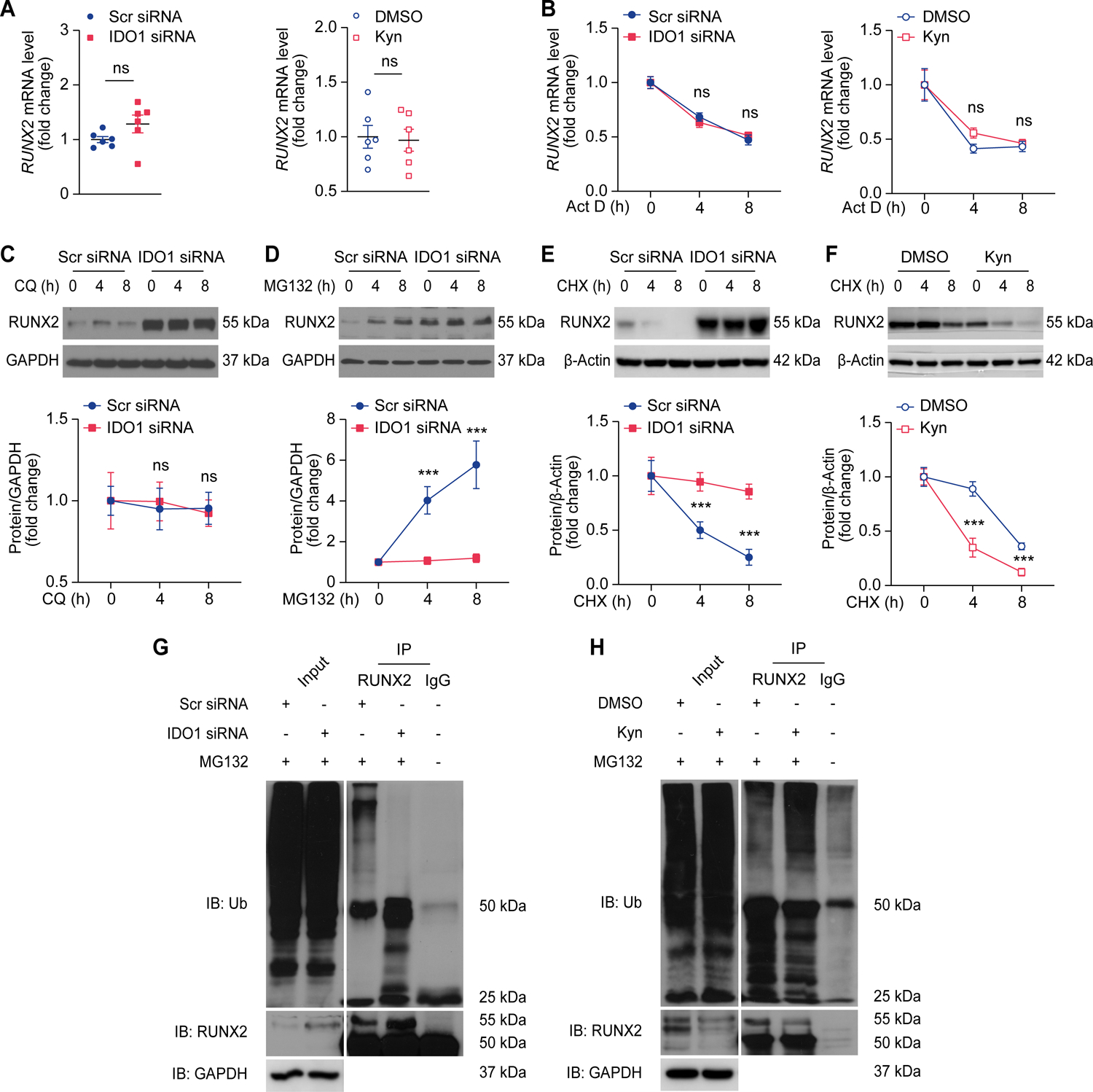
A, Quantitative RT-PCR analysis of RUNX2 expression in human coronary VSMCs transfected with scramble (Scr) siRNA or IDO1 siRNA (left panel), and human coronary VSMCs treated with DMSO or kynurenine (Kyn, 50 μmol/L) for 48 hours (right panel) (n = 6).
B, Quantitative RT-PCR analysis of RUNX2 expression in human coronary VSMCs transfected with scramble (Scr) siRNA or IDO1 siRNA (left panel), and human coronary VSMCs treated with DMSO or Kyn (50 μmol/L) for 48 hours, followed by treatment with actinomycin D (Act D, 1 μmol/L) for different periods (right panel) (n = 4–6).
C-E, Western blot analysis of RUNX2 expression in human coronary VSMCs transfected with Scr siRNA or IDO1 siRNA, and then treated with chloroquine (CQ, 20 μmol/L) (C), MG132 (1 μmol/L) (D), or cycloheximide (CHX, 50 μg/mL) (E) for the indicated periods (n = 4).
F, Western blot analysis of RUNX2 expression in RUNX2-overexpressed human coronary VSMCs treated with DMSO or Kyn (50 μmol/L), followed by treatment with CHX (50 μg/mL) for different periods (n = 4).
G and H, Human coronary VSMCs with Scr siRNA or IDO1 siRNA transfection (G), DMSO or Kyn (50 μmol/L) supplementation (H) were pretreated with MG132 (1 μmol/L). The expression of RUNX2, ubiquitin (Ub), and GAPDH was determined by Western blot analysis (Input). Immunoprecipitation was performed with RUNX2 or IgG antibody, RUNX2 and RUNX2-bound Ub was determined by Western blot analysis (IP; n = 3).
Results are presented as mean ± SEM. P values are assessed using two-tailed unpaired Student’s t-test used for A; Two-way ANOVA with Tukey’s post hoc test is used for B-F. ***P < 0.001, ns indicates P > 0.05.
Human coronary VSMCs were transfected with scramble siRNA or IDO1 siRNA and then treated with cycloheximide (an inhibitor of protein synthesis). IDO1 knockdown did not result in a decrease in the half-life of RUNX2 protein compared with the control group. In contrast, the half-life was markedly decreased in kynurenine-supplemented VSMCs, compared with the DMSO control (Figure 6E and 6F). Ubiquitination is crucial for RUNX2 degradation.22 Therefore, we examined the effects of endogenous IDO1 activity on RUNX2 ubiquitination in human coronary VSMCs treated with IDO1 siRNA or kynurenine. We found that compared with the control group, RUNX2 ubiquitination was markedly reduced in the IDO1-silenced group but was markedly induced in the presence of kynurenine (Figure 6G and 6H). Taken together, these results indicate that IDO1 facilitates RUNX2 ubiquitination and proteasomal degradation through kynurenine production in VSMCs.
Kynurenine activates assembly of RUNX2 and CUL4BAhR E3 ligase complex
Kynurenine acts as an endogenous ligand of the AhR. Their binding induces conformational changes in AhR that promote its nuclear translocation and subsequent modulation of the target genes.7 However, we found that despite the addition of kynurenine, AhR remained in the cytosol (Figure S12A) and that AhR depletion had mild effects on RUNX2 mRNA expression levels in vitro (Figure S12B). This result suggested kynurenine-activated AhR had a non-genomic function beyond transcriptional regulation.
The AhR is also characterized as an atypical component of the ubiquitin ligase complex involving ubiquitin ligase core component CUL4B, which regulates proteasomal degradation of target proteins.23 Therefore, we next verified the combination of RUNX2 and CUL4BAhR complex. As depicted in Figure 7A–7C, increased endogenous binding of RUNX2 to CUL4BAhR was evident in cell lysates of kynurenine-treated human coronary VSMCs, pulled down by either RUNX2, AhR, or CUL4B immunoprecipitation. As shown in Figure 7D and 7E, silencing of AhR or CUL4B stimulated RUNX2 expression in human coronary VSMCs. Moreover, with MG132 supplementation, the scramble siRNA-transfected group had markedly increased RUNX2 expression, but not the AhR- or CUL4B-silenced groups. We further examined the effect of the CUL4BAhR complex on RUNX2 stability. In the presence of kynurenine, overexpression of AhR and CUL4B together with RUNX2 in human coronary VSMCs led to instability of RUNX2, compared with the control group (Figure 7F). In contrast, CUL4B deficiency abolished the effect of kynurenine on decreasing RUNX2 expression in human coronary VSMCs (Figure 7G). In the context of kynurenine deficiency due to IDO1 silencing in human coronary VSMCs, CUL4B overexpression did not downregulate RUNX2 expression, compared to the control group (Figure 7H). Taken together, these results indicate that RUNX2 is a substrate for kynurenine-activated CUL4BAhR E3 ligase complex and that CUL4BAhR complex is vital for RUNX2 proteasomal degradation.
Figure 7. Kynurenine activates assembly of RUNX2 and CUL4BAhR E3 ligase complexes.
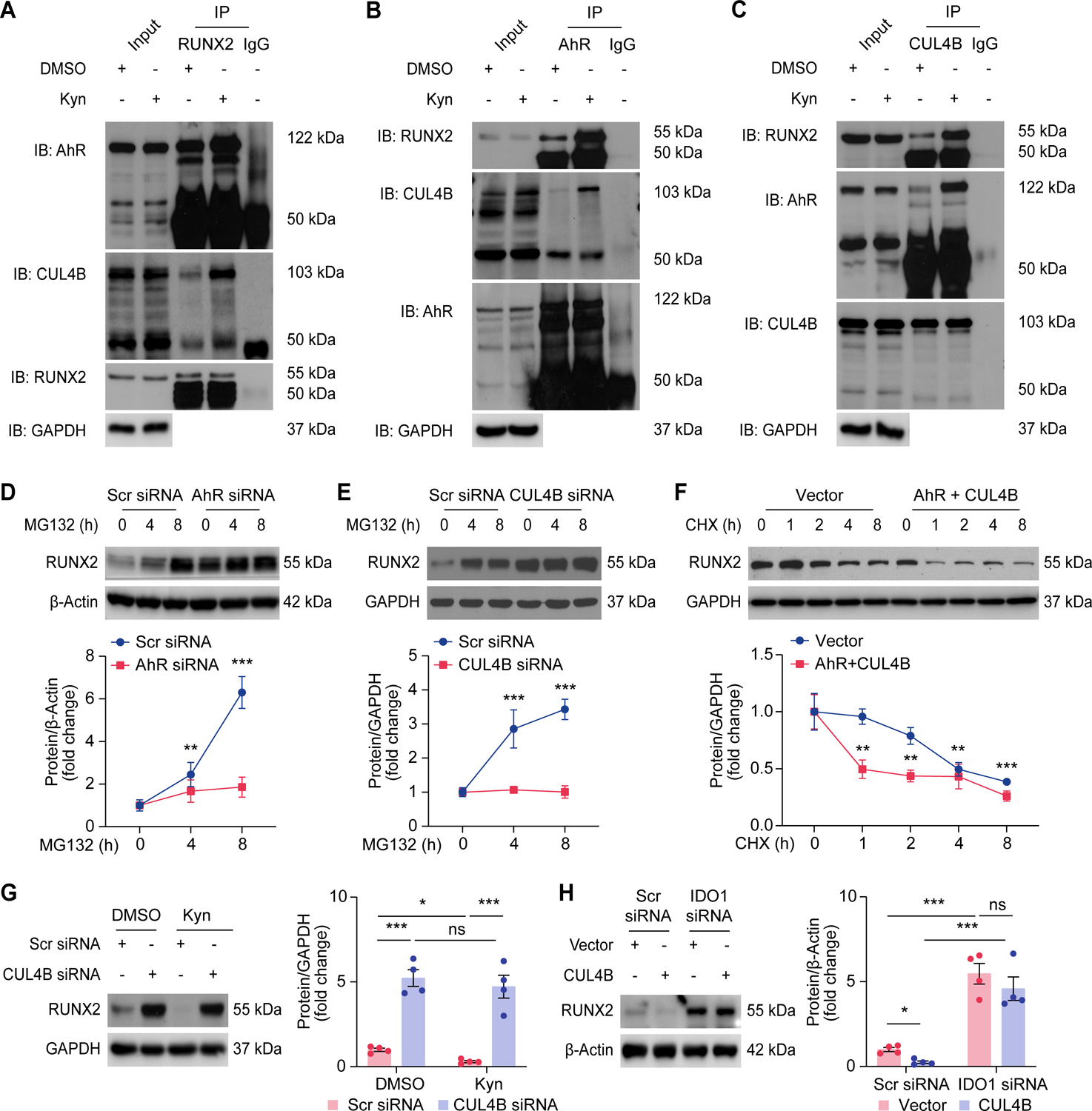
A-C, Western blot analysis of human coronary VSMCs supplemented with DMSO or Kyn (50 μmol/L) followed by pretreatment with MG132 (1 μmol/L) before immunoprecipitation with anti-RUNX2 (A) or anti-AhR (B) or anti-CUL4B (C) antibodies and detection with indicated antibodies (n = 3).
D, Western blot analysis of RUNX2 expression in human coronary VSMCs transfected with Scr siRNA or AhR siRNA, followed by treatment with MG132 (1 μmol/L) for indicated periods (n = 4).
E, Western blot analysis of RUNX2 expression in human coronary VSMCs transfected with Scr siRNA or CUL4B siRNA, followed by treatment with MG132 (1 μmol/L) for indicated periods (n = 4).
F, Western blot analysis of RUNX2 expression in human coronary VSMCs transfected with RUNX2 plasmid combined with or without AhR and CUL4B plasmids, followed by CHX (50 μg/mL) treatment for the indicated periods (n = 4).
G, Western blot analysis of RUNX2 expression in human coronary VSMCs transfected with Scr siRNA or CUL4B siRNA, followed by treatment with DMSO or Kyn (50 μmol/L) (n = 4).
H, Western blot analysis of RUNX2 expression in human coronary VSMCs transfected with Vector or CUL4B plasmid combined with Scr siRNA or IDO1 siRNA (n = 4).
Results are presented as mean ± SEM, and analyzed using two-way ANOVA with Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ns indicates P > 0.05.
Patients with coronary artery calcification exhibit aberrant tryptophan metabolism
To determine the clinical relevance of tryptophan catabolism and arterial calcification, we further performed LC-MS/MS detection in serum samples from CKD patients with or without coronary artery calcification. We found that the serum levels of Kyn/Trp ratio and kynurenine catabolites, including kynurenine, kynurenic acid, 3-hydroxyanthranilic acid, xanthurenic acid, and quinolinic acid, were negatively correlated with calcification Agatston scores of patients (Figure 8). Results of the multiple linear regression analyses of baseline clinical factors and CAC Agatston scores, with adjustments for age and sex, showed that serum kynurenine level was significantly associated with CAC scores in CKD patients (Table S1 and S2). These results suggest that patients with coronary artery calcification exhibit an aberrant IDO1-mediated tryptophan metabolism profile, and IDO1 is involved in the progression of calcification in the clinical setting.
Figure 8. Tryptophan metabolism in patients with or without coronary artery calcification.
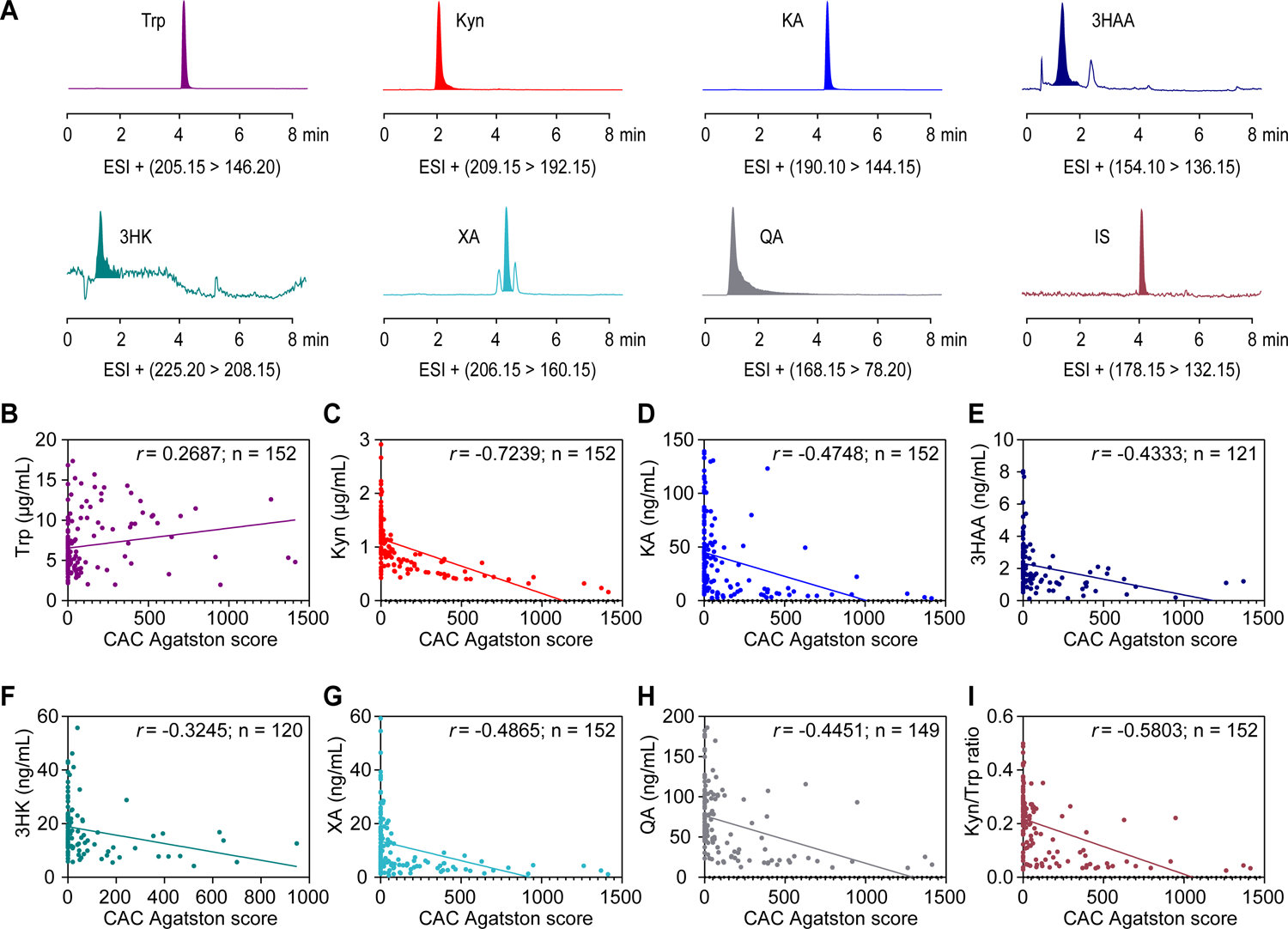
A, Representative multiple reaction monitoring chromatograms of serum catabolites from tryptophan metabolism and an internal standard (IS, 5-hydroxyindole-2-carboxylic acid) in a standard mixture solution at the limit of quantitation.
B-H, Correlation between serum levels of catabolites from tryptophan metabolism and coronary artery calcification (CAC) Agatston scores of CKD patients (n = 120–152). Catabolites presented as: tryptophan (B), kynurenine (C), kynurenic acid (KA) (D), 3-hydroxyanthranilic acid (3HAA) (E), 3-hydroxykynurenine (3HK) (F), xanthurenic acid (XA) (G), quinolinic acid (QA) (H).
I, Correlation between serum levels of Kyn/Trp ratios and CAC Agatston scores of CKD patients (n = 152).
Results are analyzed using Spearman’s rank correlation for B-I.
Discussion
This study was the first to find that IDO1 deletion in VSMCs but not macrophages exacerbated the progression of VSMCs osteogenic reprogramming and the consequent calcification. The phenotype was rescued by supplementation with the major IDO1-produced catabolite, kynurenine, both in vitro and in vivo. The suppression of calcification by kynurenine was RUNX2 dependent. Mechanistically, kynurenine exerted the protective effects by activating the AhR, which resulted in the formation of the CUL4BAhR E3 ubiquitin ligase complex and promotion of ubiquitin-mediated proteasomal degradation of RUNX2 and subsequent RUNX2 downregulation. In the clinical setting, kynurenine derivatives and serum IDO1 activity were negatively correlated with coronary artery calcification progression in CKD patients. Taken together, these results reveal the role of IDO1 as an endogenous regulator of arterial calcification, which is a ubiquitous vasculopathy with limited therapeutic treatment.
Using a long-term Western diet-induced calcification mouse model, we found that global IDO1 inhibition had a detrimental effect on calcification formation in vivo. Likewise, IDO1 has been identified in different vascular cells that can independently respond to stimuli.24 IDO1 from those cells can be activated and produce kynurenine. To determine the direct role of IDO1 in this context, we used a genetic loss-of-function approach and found that IDO1 deficiency in VSMCs promoted intimal ossification; IDO1 ablation in macrophages had mild effects. Despite the fact that IDO1 expression and activity were suppressed in macrophages, IDO1 deletion in these cells did not affect aortic kynurenine levels in situ. Assessed by the Kyn/Trp ratio, IDO1 activity in aortas of Apoe−/− Ido1△MΦ mice was much higher than that in Apoe−/− Ido1△SMC mice. These results suggest that IDO1 in VSMCs, but not in macrophages, is a major source of kynurenine in the vascular system. These findings provide the previously unknown effect of VSMCs IDO1 on calcific vasculopathy.
Upon lipid or other pro-calcification cues during atherosclerosis, VSMCs possess high plasticity to adopt alternative phenotypes. These phenotypes resemble foam cells, osteogenic cells, macrophages, and even rare subsets such as myofibroblast-like cells and MSC-like cells.25,26 A lineage-tracing study found that within lesions, 98% of osteogenic cells were VSMCs-derived.27 Our results showed that IDO1 deficiency aggravated catastrophic elevation of bone matrix proteins, with concurrent lower expression of contractile proteins, both in vivo and in vitro. Taken together, these characteristics support the hypothesis that IDO1 participates in VSMCs osteogenic phenotype switching. Compelling evidence indicates that VSMCs osteogenic differentiation is predominately regulated by the transcription factor RUNX2.22 We found that IDO1 deletion sustained the abundance of intrinsic RUNX2 in arteries, but not of other osteogenic factors such as SOX9 and MSX2. The elevated RUNX2 co-localized with COL1A1 and calcium granules within atherosclerotic lesions. The compromised effects upon RUNX2 deletion revealed that RUNX2 mediated IDO1 ablation-enhanced calcification. Taken together, these findings strongly suggest that IDO1 has an indispensable role as a “gatekeeper” of the fate of VSMCs during lineage reprogramming towards osteochondrogenesis.
IDO1-catabolized tryptophan metabolism generates a variety of end-products.4 We incubated VSMCs with major derivatives to identify catabolites responsible for the guidance of VSMCs trans-differentiation. The results indicated that only kynurenine could decrease the expression of RUNX2 and concomitant calcium deposits in vitro and in vivo. Conversely, silencing IDO1-induced endogenous kynurenine insufficiency resulted in the accumulation of RUNX2 in VSMCs. These findings suggest kynurenine is a key intermediary during anti-calcification of IDO1 via RUNX2 downregulation, and there were no increases in lipid levels, glucose fluctuation, weight change, or plaque formation after kynurenine treatment. Interestingly, recent reports indicate that kynurenine is connected with osteoblast proliferation and differentiation, and it can be related to the pathophysiology of chronic kidney disease.28 Besides, serotonergic drugs are demonstrated to have pharmacological inhibition on aortic calcific valvulopathy progression.29,30 Therefore, from the therapeutic perspective, kynurenine may be promisingly applicable to ameliorate sclerotic vascular complications in the clinical setting.
Kynurenine acts as an endogenous AhR ligand.31 AhR has been linked to RUNX2 regulation. Tong et al. found that AhR activation suppresses the expression of RUNX2 in MSCs.32 Smooth muscle cell lineage-specific AhR knockout mice contain cells modulated towards a chondrogenic phenotype with RUNX2 upregulation.33 However, the underlying mechanism remains obscure. Post-translational modifications are critical for maintaining intracellular RUNX2 homeostasis in osteoblasts.22 Our studies found that either IDO1 silencing or kynurenine administration modulated RUNX2 protein expression without a corresponding alteration in RUNX2 mRNA levels or stabilities. Furthermore, in vitro studies unambiguously revealed that kynurenine enhanced ubiquitin-mediated proteasomal degradation of RUNX2. Ligand-activated AhR is integrated as a component of the CUL4B ubiquitin ligase complex that targets proteins for degradation, including sex steroid receptors,23 cytoplasmic 1,34 and peroxisome proliferator-activated receptor γ.35 Consistently, immunoprecipitation experiments found that kynurenine activated endogenous assembly of RUNX2 and CUL4BAhR complexes. Overexpression of the AhR and CUL4B promoted further instability and degradation of RUNX2. However, CUL4B failed to repress RUNX2 elevation without AhR activation, for which kynurenine was insufficient due to IDO1 deletion. These results also explained why RUNX2 mRNA levels were not affected by AhR deficiency, and cytosolic localization of AhR in VSMCs under kynurenine application. Thus, these findings increased our understanding of atypical AhR functions. Kynurenine-activated AhR mediated RUNX2 degradation via cooperation with E3 ubiquitin ligase, but not the canonical genomic pathway. This study shed new light on the interaction between RUNX2 and AhR in the vascular disease setting. Clinically, AhR activation may constitute a novel approach for the reduction of calcification.
Although mineralization occurs in parallel in bone and vasculature, the paradox that repressed skeletal ossification coexisting with enhanced aortic ossification is often recognized in humans.22 IDO1-mediated kynurenine production has Janus-faced roles (i.e., both beneficial and toxic effects) with regard to MSC and VSMCs osteogenic differentiation.15,20,36 The reason for this inconsistency remains inconclusive, considering that the effects exerted by specific IDO1 metabolite or downstream enzymes can vary according to cell type and stimulus. The multifaceted regulatory network of AhR may also account for this discrepancy. Kynurenine signaling through the AhR-complex-associated Src kinase activity is implicated in the control of systemic inflammation.31 In the presence of receptor activator of nuclear factor-kappa B ligand, kynurenine induces macrophages to undergo osteoclastogenesis by binding to AhR that subsequently translocated to the nucleus to form a complex with AhR nuclear translocator.36 Kynurenic acid and xanthurenic acid have also been described as potential endogenous ligands for AhR. The binding of kynurenic acid to the AhR leads to recruitment of AhR nuclear translocator and induction of interleukin-6 mRNA expression.37 Within breast cancer cells, the AhR is activated by xanthurenic acid in an amplification feedback loop, maximizing tumor cell migration.38 Taken together, these findings suggest that the distinct role of the AhR pathway in osteoclastogenesis depends on the specific binding ligand, the co-factors interacted with, and the downstream signaling cascade triggered, ultimately, the specific genes targeted. Thus, whether kynurenine and related derivatives present synergistic or competing functions on AhR signaling for modulation of arterial calcification awaits further investigation.
Kynurenine is the precursor for downstream metabolites that exert multiple effects on vascular pathophysiology. The 3-hydroxyanthranilic acid acts as a potent factor for nuclear factor-kappa B activation in VSMCs,10 and kynurenic acid is responsible for the interleukin-10 reduction in macrophages39; both can directly upregulate RUNX2 expression.40 The xanthurenic acid treatment induces mitochondria malfunction and cellular apoptosis in VSMCs.41 The resultant apoptotic bodies can act as nucleating structures for crystallizing calcium.42 The quinolinic acid is involved in the neurotoxicity associated with inflammation, the well-known initiator for calcification.43 The findings from other studies provide the background needed to understand why kynurenine attenuated calcification whilst other catabolites had adverse outcomes.
Overall, we found a causal relationship between IDO1 activity and the development of arterial calcification. Current study highlights a potent effect of IDO1-catablized kynurenine production on protecting against VSMCs osteogenic reprogramming and subsequent calcification. These findings may provide new insights into therapeutic strategies for the relief of abnormal calcification in the cardiovascular system.
Supplementary Material
Clinical Perspective.
What is new?
This study reveals the previously unrecognized protective role of indoleamine 2, 3-dioxygenase 1 (IDO1) in arterial calcification, vascular smooth muscle cells defective of IDO1 result in enhanced runt-related transcription factor 2 (RUNX2) and ectopic calcium deposition in plaques.
Through kynurenine production, IDO1 exerts potent inhibitory effects on osteogenic activity in vasculature by promoting E3 ligase complex formation between an aryl hydrocarbon receptor and cullin 4B, thus driving ubiquitin-mediated degradation of RUNX2.
Patients with coronary artery calcification have abnormal tryptophan metabolism, and serum IDO1 activity is inversely associated with calcification development in the clinical setting.
What are the clinical implications?
This work reveals a protective role for IDO1 in mitigating arterial intimal calcification through kynurenine production.
Developing interventions towards the IDO1-kynurenine-RUNX2 axis may prevent the pathogenesis of arteriosclerotic complications.
Sources of Funding
This work was supported by funding from the National Heart, Lung, and Blood Institute HL089920 (M.H.Z.), HL142287 (M.H.Z.), HL153333 (Y.D) and HL140954 (P.S.), and the American Heart Association 19CDA34730035 (Y.D).
Non-standard abbreviations and acronyms
- AhR
Aryl hydrocarbon receptor
- ALP
Alkaline phosphatase
- Apoe
Apolipoprotein E
- COL1A1
Collagen I alpha1
- CUL4B
Cullin 4B
- IDO1
Indoleamine 2, 3-dioxygenase 1
- Kyn
Kynurenine
- ND
Normal diet
- OCN
Osteocalcin
- OPN
Osteopontin
- RUNX2
Runt-related transcription factor 2
- α-SMA
Alpha smooth muscle actin
- SM22α
Smooth muscle 22 alpha
- Trp
Tryptophan
- Ub
Ubiquitin
- VSMC
Vascular smooth muscle cell
- WD
Western diet
Footnotes
Disclosures
None.
References
- 1.Tomaniak M, Katagiri Y, Modolo R, de Silva R, Khamis RY, Bourantas CV, Torii R, Wentzel JJ, Gijsen FJH, van Soest G, et al. Vulnerable plaques and patients: state-of-the-art. Eur Heart J. 2020;41:2997–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentzon JF, Otsuka F, Virmani R and Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Zhao X and Wu H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler Thromb Vasc Biol. 2020;40:1078–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song P, Ramprasath T, Wang H and Zou MH. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol Life Sci. 2017;74:2899–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platten M, Nollen EAA, Rohrig UF, Fallarino F and Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379–401. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Zhang M, Ding Y, Wang Q, Zhang W, Song P and Zou MH. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ Res. 2014;114:480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melhem NJ, Chajadine M, Gomez I, Howangyin KY, Bouvet M, Knosp C, Sun Y, Rouanet M, Laurans L, Cazorla O, et al. Endothelial Cell Indoleamine 2, 3-Dioxygenase 1 Alters Cardiac Function After Myocardial Infarction Through Kynurenine. Circulation. 2021;143:566–580. [DOI] [PubMed] [Google Scholar]
- 8.Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S, Sovran B, Denis RGP, Dairou J, Cardellini M, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018;24:1113–1120. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Y, Christou H, Liu L, Visner G, Mitsialis SA, Kourembanas S and Liu H. Endothelial indoleamine 2,3-dioxygenase protects against development of pulmonary hypertension. Am J Respir Crit Care Med. 2013;188:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Ding Y, Song P, Zhu H, Okon I, Ding YN, Chen HZ, Liu DP and Zou MH. Tryptophan-Derived 3-Hydroxyanthranilic Acid Contributes to Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Mice In Vivo. Circulation. 2017;136:2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole JE, Astola N, Cribbs AP, Goddard ME, Park I, Green P, Davies AH, Williams RO, Feldmann M and Monaco C. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci U S A. 2015;112:13033–13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton S, Smith K, Singh K, Kaiser H, Kolhe R, Mondal AK, Khayrullin A, Isales CM, Hamrick MW, Hill WD, et al. Accumulation of kynurenine elevates oxidative stress and alters microRNA profile in human bone marrow stromal cells. Exp Gerontol. 2020;130:110800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce JL, Roberts RL, Yu K, Kendall RK, Kaiser H, Davis C, Johnson MH, Hill WD, Isales CM, Bollag WB, et al. Kynurenine suppresses osteoblastic cell energetics in vitro and osteoblast numbers in vivo. Exp Gerontol. 2020;130:110818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondrikov D, Elmansi A, Bragg RT, Mobley T, Barrett T, Eisa N, Kondrikova G, Schoeinlein P, Aguilar-Perez A, Shi XM, et al. Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway. Exp Gerontol. 2020;130:110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidal C, Li W, Santner-Nanan B, Lim CK, Guillemin GJ, Ball HJ, Hunt NH, Nanan R and Duque G. The kynurenine pathway of tryptophan degradation is activated during osteoblastogenesis. Stem Cells. 2015;33:111–121. [DOI] [PubMed] [Google Scholar]
- 16.Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stoy N and Stone TW. On the Biological Importance of the 3-hydroxyanthranilic Acid: Anthranilic Acid Ratio. Int J Tryptophan Res. 2010;3:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. [DOI] [PubMed] [Google Scholar]
- 18.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL and Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG and Chen Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Refaey ME, McGee-Lawrence ME, Fulzele S, Kennedy EJ, Bollag WB, Elsalanty M, Zhong Q, Ding KH, Bendzunas NG, Shi XM, et al. Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. J Bone Miner Res. 2017;32:2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Z, Ding Y, Zhang M, Lu Q, Wu S, Zhu H, Song P and Zou MH. Ablation of Adenosine Monophosphate-Activated Protein Kinase alpha1 in Vascular Smooth Muscle Cells Promotes Diet-Induced Atherosclerotic Calcification In Vivo. Circ Res. 2016;119:422–433. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhao X and Wu H. Transcriptional Programming in Arteriosclerotic Disease: A Multifaceted Function of the Runx2 (Runt-Related Transcription Factor 2). Arterioscler Thromb Vasc Biol. 2021;41:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–566. [DOI] [PubMed] [Google Scholar]
- 24.Yun TJ, Lee JS, Machmach K, Shim D, Choi J, Wi YJ, Jang HS, Jung IH, Kim K, Yoon WK, et al. Indoleamine 2,3-Dioxygenase-Expressing Aortic Plasmacytoid Dendritic Cells Protect against Atherosclerosis by Induction of Regulatory T Cells. Cell Metab. 2016;23:852–866. [DOI] [PubMed] [Google Scholar]
- 25.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K and Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. [DOI] [PubMed] [Google Scholar]
- 26.Collett GD and Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. [DOI] [PubMed] [Google Scholar]
- 27.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM and Speer MY. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res. 2012;94:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker JA, Richards S, Whelan SA, Yoo SB, Russell TL, Arinze N, Lotfollahzadeh S, Napoleon MA, Belghasem M, Lee N, et al. Indoleamine 2,3-dioxygenase-1, a Novel Therapeutic Target for Post-Vascular Injury Thrombosis in CKD. J Am Soc Nephrol. 2021;32:2834–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong F, Xian J, Demer LL and Tintut Y. Serotonin receptor type 2B activation augments TNF-alpha-induced matrix mineralization in murine valvular interstitial cells. J Cell Biochem. 2021;122:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joll JE 2nd, Clark CR, Peters CS, Raddatz MA, Bersi MR and Merryman WD. Genetic ablation of serotonin receptor 2B improves aortic valve hemodynamics of Notch1 heterozygous mice in a high-cholesterol diet model. PLoS One. 2020;15:e0238407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong Y, Niu M, Du Y, Mei W, Cao W, Dou Y, Yu H, Du X, Yuan H and Zhao W. Aryl hydrocarbon receptor suppresses the osteogenesis of mesenchymal stem cells in collagen-induced arthritic mice through the inhibition of beta-catenin. Exp Cell Res. 2017;350:349–357. [DOI] [PubMed] [Google Scholar]
- 33.Kim JB, Zhao Q, Nguyen T, Pjanic M, Cheng P, Wirka R, Travisano S, Nagao M, Kundu R and Quertermous T. Environment-Sensing Aryl Hydrocarbon Receptor Inhibits the Chondrogenic Fate of Modulated Smooth Muscle Cells in Atherosclerotic Lesions. Circulation. 2020;142:575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu WC, Shyu JF, Lim PS, Fang TC, Lu CL, Zheng CM, Hou YC, Wu CC, Lin YF and Lu KC. Concentration and Duration of Indoxyl Sulfate Exposure Affects Osteoclastogenesis by Regulating NFATc1 via Aryl Hydrocarbon Receptor. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dou H, Duan Y, Zhang X, Yu Q, Di Q, Song Y, Li P and Gong Y. Aryl hydrocarbon receptor (AhR) regulates adipocyte differentiation by assembling CRL4B ubiquitin ligase to target PPARgamma for proteasomal degradation. J Biol Chem. 2019;294:18504–18515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisa NH, Reddy SV, Elmansi AM, Kondrikova G, Kondrikov D, Shi XM, Novince CM, Hamrick MW, McGee-Lawrence ME, Isales CM, et al. Kynurenine Promotes RANKL-Induced Osteoclastogenesis In Vitro by Activating the Aryl Hydrocarbon Receptor Pathway. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ and Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novikov O, Wang Z, Stanford EA, Parks AJ, Ramirez-Cardenas A, Landesman E, Laklouk I, Sarita-Reyes C, Gusenleitner D, Li A, et al. An Aryl Hydrocarbon Receptor-Mediated Amplification Loop That Enforces Cell Migration in ER-/PR-/Her2- Human Breast Cancer Cells. Mol Pharmacol. 2016;90:674–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metghalchi S, Ponnuswamy P, Simon T, Haddad Y, Laurans L, Clement M, Dalloz M, Romain M, Esposito B, Koropoulis V, et al. Indoleamine 2,3-Dioxygenase Fine-Tunes Immune Homeostasis in Atherosclerosis and Colitis through Repression of Interleukin-10 Production. Cell Metab. 2015;22:460–471. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Li Y, Yang P, Liu X, Lu L, Chen Y, Zhong X, Li Z, Liu H, Ou C, et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-kappaB (Nuclear Factor kappaB) Signals. Arterioscler Thromb Vasc Biol. 2020;40:751–765. [DOI] [PubMed] [Google Scholar]
- 41.Malina HZ, Richter C, Mehl M and Hess OM. Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: activation of cell caspases but not cytoskeleton breakdown. BMC Physiol. 2001;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM and Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. [DOI] [PubMed] [Google Scholar]
- 43.Feng W, Wang Y, Liu ZQ, Zhang X, Han R, Miao YZ and Qin ZH. Microglia activation contributes to quinolinic acid-induced neuronal excitotoxicity through TNF-alpha. Apoptosis. 2017;22:696–709. [DOI] [PubMed] [Google Scholar]
- 44.Xu R, Zhang L, Zhang P, Wang F, Zuo L, Zhou Y, Shi Y, Li G, Jiao S, Liu Z, et al. Gender-specific reference value of urine albumin-creatinine ratio in healthy Chinese adults: results of the Beijing CKD survey. Clin Chim Acta. 2008;398:125–129. [DOI] [PubMed] [Google Scholar]
- 45.Chen F, Yang W, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, et al. Albuminuria: Prevalence, associated risk factors and relationship with cardiovascular disease. J Diabetes Investig. 2014;5:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skinner JS, Smeeth L, Kendall JM, Adams PC, Timmis A and Chest Pain Guideline Development G. NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart. 2010;96:974–978. [DOI] [PubMed] [Google Scholar]
- 47.Kramer CK, Zinman B, Gross JL, Canani LH, Rodrigues TC, Azevedo MJ and Retnakaran R. Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. BMJ. 2013;346:f1654. [DOI] [PubMed] [Google Scholar]
- 48.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P and Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. [DOI] [PubMed] [Google Scholar]
- 49.Ding Y, Zhang M, Zhang W, Lu Q, Cai Z, Song P, Okon IS, Xiao L and Zou MH. AMP-Activated Protein Kinase Alpha 2 Deletion Induces VSMC Phenotypic Switching and Reduces Features of Atherosclerotic Plaque Stability. Circ Res. 2016;119:718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han YM, Bedarida T, Ding Y, Somba BK, Lu Q, Wang Q, Song P and Zou MH. beta-Hydroxybutyrate Prevents Vascular Senescence through hnRNP A1-Mediated Upregulation of Oct4. Mol Cell. 2018;71:1064–1078 e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


