Abstract
In this study we investigated the long-term survival of and morphological changes in Salmonella strains at low water activity (aw). Salmonella enterica serovar Enteritidis PT4 and Salmonella enterica serovar Typhimurium DT104 survived at low aw for long periods, but minimum humectant concentrations of 8% NaCl (aw, 0.95), 96% sucrose (aw, 0.94), and 32% glycerol (aw, 0.92) were bactericidal under most conditions. Salmonella rpoS mutants were usually more sensitive to bactericidal levels of NaCl, sucrose, and glycerol. At a lethal aw, incubation at 37°C resulted in more rapid loss of viability than incubation at 21°C. At aw values of 0.93 to 0.98, strains of S. enterica serovar Enteritidis and S. enterica serovar Typhimurium formed filaments, some of which were at least 200 μm long. Filamentation was independent of rpoS expression. When the preparations were returned to high-aw conditions, the filaments formed septa, and division was complete within approximately 2 to 3 h. The variable survival of Salmonella strains at low aw highlights the importance of strain choice when researchers produce modelling data to simulate worst-case scenarios or conduct risk assessments based on laboratory data. The continued increase in Salmonella biomass at low aw (without a concomitant increase in microbial count) would not have been detected by traditional microbiological enumeration tests if the tests had been performed immediately after low-aw storage. If Salmonella strains form filaments in food products that have low aw values (0.92 to 0.98), there are significant implications for public health and for designing methods for microbiological monitoring.
Members of the genus Salmonella are major international food-borne pathogens and reportedly cause approximately 30,000 cases of food-borne illness each year in England and Wales (4). In the United States, approximately 40,000 Salmonella infections are confirmed by culturing each year, and the true figure is estimated to be between 800,000 and 4 million infections, with approximately 500 fatalities (2, 13). Salmonella enterica serovar Enteritidis phage type 4 (PT4) and S. enterica serovar Typhimurium definitive type 104 (DT104) are the two most prevalent serotypes isolated from infected humans and together accounted for approximately 80% of all isolates obtained from 1990 to 1994 in England and Wales (PHLS Communicable Disease Surveillance Centre). It has been known for 10 years that S. enterica serovar Enteritidis PT4 is a key cause of infections associated with foods of animal origin, especially shelled eggs and poultry (2). More recently, S. enterica serovar Typhimurium DT104 has emerged as an important Salmonella strain in Europe and the United States, and the percentage of antibiotic-resistant S. enterica serovar Typhimurium isolates increased from 1% in 1979 and 1980 to 34% in 1996 in the United States (13). S. enterica serovar Typhimurium DT104 is of particular concern to the food industry due to the severity of the human disease which it causes, its multiple drug resistance, and the extensive animal reservoirs of the organism (40).
Reducing the available water in food is a long-established method for controlling bacterial growth. Desiccation or increasing the humectant content of a food results in a reduced water activity (aw) (5). Optimal growth of Salmonella strains occurs at an aw of 0.99, but these bacteria tolerate many stressful conditions and can survive in low-aw foods for long periods (36). Various solutes are incorporated into food in order to reduce the aw and maintain a reasonable safety margin before growth of microorganisms can occur. However, consumer pressure to reduce the levels of salt and sugar in food products has resulted in increases in the aw in intermediate-moisture-level foods (aw, 0.9 to 0.6 [24]). This affects primarily the spoilage stability of the products at the lower end of this aw range, but pathogens, including Salmonella spp., have caused illness following survival in foods at the higher end. For example, S. enterica serovar Napoli and S. enterica serovar Agona have caused international food poisoning outbreaks associated with low-aw chocolate (12, 15) and a low aw-crisp type of snack, respectively (3, 21, 35). In addition, it has been reported that the infectious dose of Salmonella cells is reduced (e.g., 10 to 100 cells) when the organism is present in low-aw foods (15, 32), possibly due to acid tolerance that occurs after sublethal osmotic stress, which has increased concern over the survival of the bacteria under these conditions. For these reasons it is important to continue to assess safety when either food composition or processing changes and to assess the ability of emerging pathogens, such as S. enterica serovar Typhimurium DT104, to survive at low aw.
Recent research has revealed that the virulence of S. enterica serotype Enteriditis PT4 in animal models infected orally is linked to greater tolerance of a range of stressful conditions (18, 19). In Escherichia coli, the major stationary-phase sigma factor (RpoS) encoded by the rpoS gene is important for survival during an osmotic upshift (16). Understanding the survival of Salmonella cells in low-aw foods should facilitate the design of safe preservation procedures. Since RpoS expression is important for survival under certain stressful conditions, it may influence the ability of Salmonella cells to survive at low aw.
In this paper we describe the long-term survival of isolates of S. enterica serovar Enteriditis PT4 and S. enterica serovar Typhimurium DT104 at reduced aw values (achieved by using various humectants), the associated morphological changes (as determined by microscopy and flow cytometry), and the involvement of the rpoS gene. To the best of our knowledge, this is the first report of filamentation in S. enterica serovar Enteriditis PT4 and S. enterica serovar Typhimurium DT104 in response to low-aw conditions achieved by using NaCl, glycerol, and sucrose.
MATERIALS AND METHODS
Salmonella strains.
S. enterica serovar Typhimurium DT104 strain 30 (41) was isolated from cattle feces. S. enterica serovar Typhimurium DT104 strain 10 (41) and S. enterica serovar Enteriditis PT4 strain E (18, 19) are human isolates. S. enterica serovar Enteriditis PT4 strain I (18, 19) was obtained from a chicken carcass, and strain LA5 was obtained from a natural chicken infection (1). Strain EAV54 is an otherwise isogenic rpoS mutant of LA5 (1) which was kindly provided by M. J. Woodward, Veterinary Laboratory Agency, Weybridge, United Kingdom. Strains 10 and I are naturally occurring rpoS mutants (F. Jørgensen, S. J. Wilde, G. S. A. B. Stewart, and T. J. Humphrey, submitted for publication). Strain I exhibits reduced virulence in animals infected orally and lower tolerance to certain environmental stresses (18, 19).
Preparation of log- and stationary-phase cultures.
Salmonella strains were recovered from storage at −20°C on Protect beads (Mast Diagnostics, Merseyside, United Kingdom). A single bead was streaked onto 5% horse blood agar (Columbia agar base [Oxoid, Basingstoke, United Kingdom] containing horse blood [E & O Laboratories, Bonnybridge, Scotland]) prior to incubation at 37°C for 24 h. Log-phase cultures were prepared by inoculating 9 ml of tryptone soya broth (TSB) (Oxoid) which contained 0.25% glucose or nutrient broth (NB) (Oxoid) which contained no glucose with a single typical colony. The broth was incubated at 37°C for 3 h before it was used. One microliter of a log-phase culture was used to inoculate 9 ml of TSB before incubation at 37°C for 15 h to produce a stationary-phase culture. Before cultures were used, their turbidities at 600 nm were measured with a spectrophotometer (Cecil model CE1010) and standardized to values of 0.2 for NB and 0.4 for TSB, which gave a final cell density of 108 CFU ml−1 in each case.
Preparation of low-aw broth media.
AnalaR grade NaCl, glycerol, and sucrose (BDH, Poole, Dorset, United Kingdom) were used as humectants to produce a range of low-aw TSB or NB preparations (2, 4, 8, and 12% [wt/vol] NaCl, corresponding to aw values of 0.99, 0.98, 0.95, and 0.92, respectively; 16, 32, 64, and 96% [wt/vol] sucrose, corresponding to aw values of 0.99, 0.98, 0.96, and 0.94, respectively; 16, 32, 64, and 96% [vol/vol] glycerol, corresponding to aw values of 0.96, 0.92, 0.86, and 0.79, respectively).
Broth preparations were steamed for 30 min to avoid caramelization due to the high humectant content. An aliquot of each batch of broth was incubated at 37°C to ensure that no viable microorganisms remained. The pH of each broth was adjusted to pH 7.0 ± 0.2 by using HCl and NaOH.
The aw values of the broth preparations were confirmed by using an Aqualab model CX-3 aw meter (Labcell, Basingstoke, Hampshire, United Kingdom) with an accuracy of ±0.003. The aw meter works on the dew point principle, which involves detection of condensation on a mirror during cooling-heating cycles.
Survival of Salmonella cells at low aw values (achieved by using sucrose, glycerol, or NaCl) over a 144-h period.
Ten-microliter portions of log- or stationary-phase cultures of strains E, I, 30, and 10 were added to 190-μl portions of NB or TSB with the aw reduced to between 0.79 and 0.99 by NaCl, glycerol, or sucrose in a microtiter plate. Cells inoculated into reduced-aw NB were previously grown in NB, and cells inoculated into reduced-aw TSB were previously grown in TSB. The microtiter plate was incubated statically at 21 or 37°C, and the number of CFU per milliliter was determined after 24, 72, and 144 h of incubation by using the method of Miles and Misra (28) and blood agar plates, which were incubated at 37°C for 24 h. The limit of detection was 40 CFU ml−1.
The role of rpoS was examined in more detail by repeating some of the survival studies in triplicate with LA5 and its isogenic rpoS mutant, EAV54. Ten-microliter portions of log- or stationary-phase cultures of strains E, I, 30, 10, LA5, and EAV54 were added to 190-μl portions of reduced-aw TSB containing NaCl (aw, 0.92 to 0.99) in triplicate in a microtiter plate. The plate was incubated statically at 37°C, and counts were determined as described above.
Long-term survival of Salmonella cells at low aw values (achieved by using NaCl) over a 5-month period.
Ten-milliliter aliquots of TSB preparations with the aw reduced to between 0.92 and 0.99 by NaCl were prepared in duplicate, and 100-μl portions of stationary-phase cultures of strains E, I, 30, and 10 were inoculated. The preparations were incubated statically at 21 and 37°C for up to 5 months. Counts were determined every 14 days by using the method described above.
Filamentation of Salmonella cells at low aw values and recovery from filamentation.
Ten-microliter portions of log- or stationary-phase cultures of strains E, I, 30, and 10 were added to 190-μl portions of NB or TSB with the aw reduced to between 0.79 and 0.99 by NaCl, glycerol, or sucrose in a microtiter plate, and the preparations were incubated statically at 21 or 37°C. Changes in cell morphology were assessed by examining wet preparations and by Gram staining; the preparations were viewed by using an oil immersion lens (magnification, ×100) and model CH-2 light microscope (Olympus Instruments, London, United Kingdom). Images of Gram-stained cells were passed via the microscope through a high-performance charge-coupled device video camera to a Mac 7200/90 computer. Image capture and editing were accomplished by using Scion Image LG5 software (available from zippy.nimh.nih.gov).
A Live-Dead (BACLIGHT; Molecular Probes, Inc.) bacterial viability assay kit (25) was used to assess the viability of the filamentous cells and typical small rods of Salmonella strains at low aw values. This assay kit uses a mixture of nucleic acid stains that rapidly distinguish live bacteria with intact plasma membranes, which fluoresce green, from dead bacteria with compromised membranes, which fluoresce red. Ten-milliliter portions of cultures of strains E, I, 30, and 10 that had been incubated for 144 h in TSB supplemented with 8% NaCl at 21°C were stained as recommended by the manufacturer, concentrated onto a membrane, and examined by using an oil immersion lens (magnification, ×100) as described above.
To determine the cell size distribution by flow cytometry, stationary-phase cultures of strains E, I, 30, and 10 were incubated in TSB with the aw reduced by NaCl for 144 h in triplicate. Cells were then fixed by adding a 0.1 volume of 40% filtered formalin and stored at 4°C for up to 1 week until it was convenient to process them. Each culture was diluted in phosphate-buffered saline to a concentration of approximately 106 particles ml−1 and was analyzed in triplicate by using a Becton Dickinson FACStar Plus flow cytometer set to trigger on forward scatter. The instrument was set up and aligned by using 0.5-μm latex beads (Molecular Probes, Inc.); filtered (pore size, 0.1 μm) distilled water was used as the sheath fluid, and a 100-μm-diameter nozzle was used. Side scatter data was collected at a photomultiplier tube voltage of 450 V by using logarithmic gains. Histograms of side scatter data were characterized in terms of means, medians, and coefficients of variation of (sub)populations by using the software provided with the instrument.
To assess the recovery of filamentous Salmonella cells, 10-μl portions of stationary-phase cultures of strains E, I, 30, and 10 in TSB were added to 190-μl portions of different preparations of TSB supplemented with NaCl in a microtiter plate and incubated statically at 21°C for 144 h. The organisms were then pelleted by centrifugation at 3,400 rpm (2,279 × g) for 15 min with a Jouan model CR312 centrifuge, and the supernatant was discarded. The organisms were resuspended in fresh TSB containing no humectant and incubated at 37°C. This procedure had no immediate visible effect on the average cell length. The preparations were examined with the microscope as described above at predetermined time intervals up to 8 h following rehydration.
Data analysis.
Data analysis was performed with Microsoft Excel 97.
RESULTS
Survival of Salmonella cells at low aw values over a 144-h period.
All of the Salmonella strains tested could survive for long periods at reduced aw values, although survival was greatest at an aw of 0.99 (in TSB or NB containing no added humectant). Concentrations at or above 8% NaCl (aw, 0.95), 96% sucrose (aw, 0.94), and 32% glycerol (aw, 0.92), were usually bactericidal for Salmonella cells (Tables 1 and 2), although this depended on the broth base and the incubation temperature. For example, after 72 h of incubation, 8% NaCl was always bactericidal when NB was the broth base but was not always bactericidal when TSB was the broth base, and 32% glycerol was always bactericidal at 37°C but was not always bactericidal at 21°C. The minimum aw for growth of Salmonella cells was between 0.95 (when NaCl was bactericidal) and 0.92 (when glycerol was bactericidal). Incubation at 37°C resulted in more rapid loss of viability than incubation at 21°C at lethal aw values. The variable minimum aw values for growth when different humectants were used supported the hypothesis that there was a specific solute effect (NaCl > sucrose > glycerol).
TABLE 1.
Log10 reductions in numbers of viable S. enterica serovar Enteriditis PT4 cells after 72 h of incubation in TSB or NB containing solutes (NaCl, sucrose, and glycerol) at 21 or 37°C
| Temp (°C) | Solute | Concn (%, wt/vol) | aw | Log10 reduction in no. of cellsa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain E
|
Strain Ib
|
||||||||||
| Log phase
|
Stationary phase
|
Log phase
|
Stationary phase
|
||||||||
| NB | TSB | NB | TSB | NB | TSB | NB | TSB | ||||
| 37 | NaCl | 8 | 0.95 | −0.5 | −1.1 | −2.0 | 0.0 | −1.3 | 1.0 | −1.8 | 0.3 |
| 12 | 0.92 | −2.4 | −3.3 | −2.5 | −2.7 | −2.4 | −2.6 | −4.1 | −2.5 | ||
| Sucrose | 64 | 0.96 | −0.3 | 1.3 | 0.4 | 0.0 | 1.4 | 1.2 | 0.4 | 0.3 | |
| 96 | 0.94 | −2.0 | −2.5 | −1.6 | −1.2 | −1.2 | −0.5 | −1.3 | −1.0 | ||
| Glycerol | 32 | 0.93 | −5.3 | −2.0 | −1.1 | −1.0 | −0.4 | 0.8 | −0.3 | −1.2 | |
| 64 | 0.86 | −5.3 | −6.3 | −2.0 | −2.1 | −5.3 | −3.4 | −2.1 | −1.5 | ||
| 21 | NaCl | 8 | 0.95 | −0.2 | 0.4 | −0.9 | 0.0 | −0.3 | 0.5 | −0.6 | 0.3 |
| 12 | 0.92 | −1.7 | −0.4 | −1.2 | −0.6 | −1.7 | −0.8 | −0.8 | −0.5 | ||
| Sucrose | 64 | 0.96 | 0.5 | 0.6 | −1.4 | −1.7 | 0.6 | 1.0 | −0.8 | −1.0 | |
| 96 | 0.94 | −0.2 | −0.3 | −0.6 | −1.0 | −0.4 | −0.2 | −0.5 | −0.6 | ||
| Glycerol | 32 | 0.93 | 0.8 | 1.2 | −0.2 | −0.2 | 1.1 | 1.5 | 0.3 | 0.3 | |
| 64 | 0.86 | −2.2 | −2.3 | −0.6 | −1.1 | −1.2 | −0.4 | −0.7 | −0.5 | ||
Boldface type indicates that growth occurred.
rpoS mutant.
TABLE 2.
Log10 reductions in numbers of viable S. typhimurium DT104 cells after 72 h of incubation in TSB or NB containing solutes (NaCl, sucrose, and glycerol) at 21 or 37°C
| Temp (°C) | Solute | Concn (%, wt/vol) | aw | Log10 reduction in no. of cellsa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain 30
|
Strain 10b
|
||||||||||
| Log phase
|
Stationary phase
|
Log phase
|
Stationary phase
|
||||||||
| NB | TSB | NB | TSB | NB | TSB | NB | TSB | ||||
| 37 | NaCl | 8 | 0.95 | −1.7 | 1.7 | −1.0 | −0.1 | −6.2 | 1.2 | −0.9 | −0.3 |
| 12 | 0.92 | −4.3 | −3.1 | −2.4 | −2.8 | −6.2 | −5.3 | −6.5 | −6.8 | ||
| Sucrose | 64 | 0.96 | 0.8 | 1.7 | 0.2 | −0.1 | 0.8 | 1.7 | 0.5 | 0.2 | |
| 96 | 0.94 | −2.1 | −1.1 | −1.9 | −1.2 | −2.9 | −3.1 | −2.6 | −1.8 | ||
| Glycerol | 32 | 0.93 | −2.5 | 0.0 | −3.7 | −0.7 | −3.3 | −0.9 | −3.0 | −1.7 | |
| 64 | 0.86 | −3.9 | −1.5 | −2.7 | −2.9 | −6.2 | −2.1 | −6.5 | −3.8 | ||
| 21 | NaCl | 8 | 0.95 | −1.8 | −1.0 | −0.8 | −0.1 | −3.2 | −1.7 | −0.7 | −0.4 |
| 12 | 0.92 | −3.0 | −1.2 | −1.9 | −0.7 | −6.2 | −2.7 | −1.8 | −0.5 | ||
| Sucrose | 64 | 0.96 | −0.4 | 0.2 | −0.9 | −0.7 | −0.9 | −0.2 | −0.1 | −0.5 | |
| 96 | 0.94 | −1.8 | −1.2 | −1.0 | −1.0 | −2.2 | −2.3 | −0.8 | −1.1 | ||
| Glycerol | 32 | 0.93 | −0.3 | 1.4 | −0.4 | −0.1 | −0.6 | 1.5 | −1.0 | 0.2 | |
| 64 | 0.86 | −2.6 | −1.1 | −1.1 | −1.1 | −3.4 | −1.1 | −2.3 | −1.1 | ||
Boldface type indicates that growth occurred.
rpoS mutant.
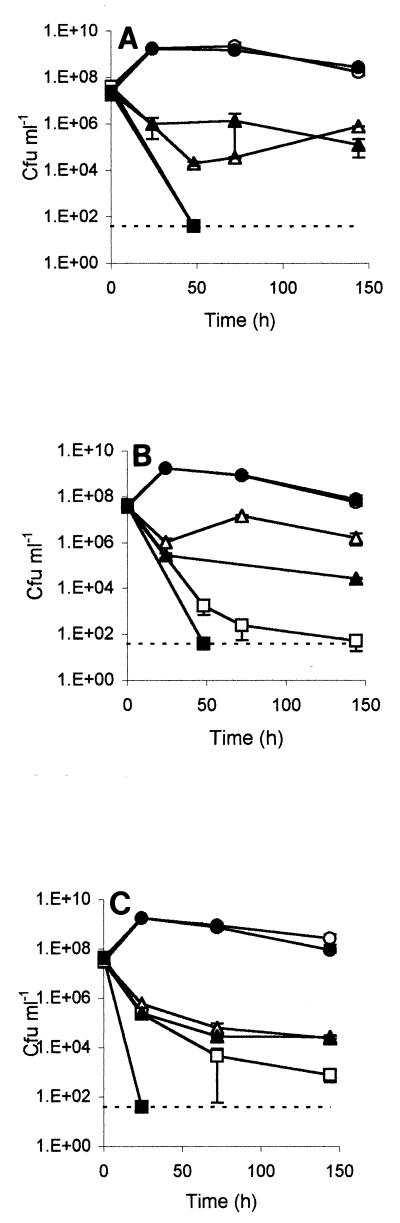
LA5 survived significantly better than its isogenic rpoS mutant (EAV54) at reduced aw values (achieved by using NaCl), particularly in the presence of 12% NaCl, and strain 30 survived better than the naturally occurring DT104 rpoS mutant strain 10. The survival of one naturally occurring PT4 rpoS mutant (strain I), however, did not differ from the survival of strain E, which exhibited normal RpoS expression (Fig. 1). The survival trends for Salmonella cells at low aw values were similar for the two broth bases, but in nearly all cases when there was a difference in the log10 reduction at 72 h between NB and TSB, the rate of death was greater in NB (Tables 1 and 2). Log-phase cells were often more sensitive to lethal low aw levels than stationary-phase cells were but exhibited shorter lags at growth-permissive humectant concentrations.
FIG. 1.
Survival of log-phase S. enterica serovar Enteriditis PT4 strain E (open symbols) and strain I (solid symbols) (A) S. enterica serovar Typhimurium DT104 strain 30 (open symbols) and strain 10 (solid symbols) (B), and S. enterica serovar Enteriditis PT4 strain LA5 (open symbols) and strain EAV54 (solid symbols) (C) at 37°C in TSB with the aw reduced by no NaCl (circles), 8% NaCl (triangles), or 12% NaCl (squares). The detection limits (40 CFU ml−1) are indicated by dashed lines. The error bars indicate the standard errors of the means.
Survival of Salmonella cells at low aw values over a 5-month period.
During incubation in TSB supplemented with 8% NaCl, the levels of all rpoS mutants became undetectable sooner than the levels of the strains that expressed RpoS became undetectable (Table 3). For example, strain E survived for 43 days in the presence of 8% NaCl before it became undetectable, whereas strain I survived for only 15 days. As with short-term survival, incubation at 37°C resulted in more rapid loss of viability than incubation at 21°C.
TABLE 3.
Time taken for the concentration of Salmonella cells to decrease from an initial value of 106 CFU ml−1 to an undetectable level (<40 CFU ml−1) when preparations were incubated at 21 or 37°C in TSB supplemented with NaCl
| NaCl concn (%, wt/vol) | aw | No of daysa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 37°C
|
21°C
|
||||||||
| Strain E | Strain I | Strain 30 | Strain 10 | Strain E | Strain I | Strain 30 | Strain 10 | ||
| 0 | 0.99 | 113 | 113 | 99 | 99 | 143 | 143 | 127 | 99 |
| 2 | 0.99 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 99 |
| 4 | 0.98 | 71 | 71 | 71 | 57 | 71 | 85 | 85 | 85 |
| 8 | 0.95 | 43 | 15 | 43 | 29 | 29 | 15 | 57 | 29 |
| 12 | 0.92 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Concentrations were determined on day 1 and then every 14 days thereafter.
Filamentation of Salmonella cells at low aw values over a 144-h period.
In response to an aw that was suboptimal for growth but not bactericidal (approximately 0.93 to 0.98, depending on the solute), all of the Salmonella strains tested formed filaments at 21 or 37°C, and some of these filaments were at least 200 μm long and had regularly spaced nucleoids visible by Gram staining (Fig. 2). Filaments were observed after approximately 24 h of incubation at 37°C in TSB supplemented with 8% NaCl. Filaments were observed in both NB and TSB but were longer and more numerous in the latter medium. Filaments formed whether sucrose, NaCl, or glycerol was used as the humectant, but with NaCl at aw values of 0.95 to 0.98 the filaments appeared to be longer and more numerous. TSB supplemented with NaCl at an aw of 0.95 to 0.98 was optimal for filament formation. The filaments had straight sides and no indentations visible by light microscopy.
FIG. 2.
Image analysis of S. enterica serovar Typhimurium DT104 strain 30, showing a filamentous cell formed during incubation at 21°C in TSB supplemented with 8% NaCl for 144 h (A) and subsequent recovery following 3 h (B) and 8 h (C) of rehydration in fresh TSB containing no added humectant.
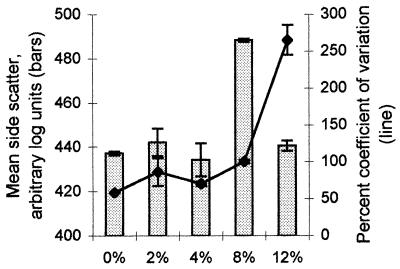
Filamentous cells were observed in TSB supplemented with NaCl at a concentration of 4% or higher. Filamentation was optimal in TSB supplemented with 8% NaCl, as determined by markedly increased light scatter during flow cytometry (Fig. 3). Data obtained by flow cytometry for both viable and nonviable cells indicated that after 144 h the cell size was most variable in TSB supplemented with 12% NaCl. Microscopy confirmed that a small proportion of cells formed very long filaments even under the extreme stress resulting from 12% NaCl, although by 144 h in the presence of 12% NaCl the vast majority of the cells were not viable (Table 3).
FIG. 3.
Light scattering of S. enterica serovar Enteriditis strain I, showing the mean particle sizes (bars) and the coefficients of variation (line) after incubation at 21°C in TSB supplemented with various concentrations of NaCl for 144 h.
Microscopic examination of concentrated cell preparations (incubated for 144 h in TSB supplemented with 8% NaCl at 21°C) stained with the Live-Dead kit revealed no green nonfilamentous cells. Approximately 20% of the filamentous cells were green, however, indicating that some filament-forming (but no non-filament-forming) cells survived under these conditions.
Following rehydration in TSB without added NaCl (aw, ∼0.99), the filaments developed septa along the cell length between nucleoids (Fig. 2). Rapid division into large numbers of viable daughter cells followed after incubation for 2 to 3 h at 37°C. After 8 h, no filaments remained, and the typical Salmonella short-cell morphology was observed.
DISCUSSION
In this study, S. enterica serovar Enteriditis PT4 and S. enterica serovar Typhimurium DT104 survived at reduced aw values for long periods. Under low-aw conditions, strains of S. enterica serovar Enteriditis and S. enterica serovar Typhimurium formed filaments, some of which were at least 200 μm long.
Previous work by Scott on the survival of microorganisms at low aw values revealed that the response to aw was solute independent (33). More recently, specific solute effects were described (6), and NaCl was found to be more inhibitory than glycerol for Salmonella cells at the same aw (27). Our estimates of the minimum aw for growth confirmed the findings of other researchers (10). The levels of survival at low aw values were greater at 21°C than at 37°C, which is consistent with research describing improved survival in the presence of high salt concentrations at a lower temperature (22). Our study revealed that optimal survival at a low aw requires RpoS expression. The survival data presented here highlight the importance of choosing the right strain to obtain mathematical modelling data to simulate worst-case scenarios. The results obtained for inactivation of PT4 and DT104 in this study were compared to the results predicted for Salmonella cells by the USDA Pathogen Modelling Program. Our results generally revealed less reduction in the number of Salmonella cells than would be predicted by the model. The discrepancies may have been due to differences in the conditions and strains used.
The filamentous cells observed in this study presumably formed as a result of a continued increase in biomass in the absence of cell septation during the low-aw stress. The filaments formed in this study had regularly spaced nucleoids and no indentations visible by light microscopy, which is consistent with an early block in the cell division genes involved in septation, the fts genes (26). Filamentous phenotypes have been found for cell division gene mutants (9) and, more recently, for wild-type organisms in response to a number of stresses. The latter organisms include Escherichia coli at a low temperature (34), S. enterica serovar Enteriditis at a low temperature (29), and Listeria monocytogenes under high osmotic stress conditions (20). Filamentation of S. enterica serovar Oranienburg in response to a low aw has been observed (34), and Yoshida et al. observed filamentation in S. enterica serovar Paratyphi in response to salts in solid media (42).
It is thought that low-aw stress results in inhibition of the production of (or action of) cell division proteins in Salmonella cells, which blocks septation but allows the proteins involved in biomass formation to function to some extent. The specific mechanism involved in filamentation remains unclear, but we believe that there are four main possibilities. First, a low aw affects the extent of DNA supercoiling, which in turn affects the regulation of osmotically controlled genes (14, 17) and may interfere with the regulation of cell division genes, thus causing filamentation. Second, the SOS response is a repair system induced by DNA damage (30) which upregulates SulA, an inhibitor of the initial protein involved in septation (FtsZ). If osmotic stress causes DNA damage, a filament with an early block in septation would be formed. Third, the heat shock response involves production of cell division inhibitors (8, 38). Cross-protection of osmotic shock and heat shock has been reported in non-Salmonella species (20, 37), and it has been demonstrated that common stress proteins occur in response to heat shock and osmotic shock in Bacillus subtilis (39). Therefore, it seems reasonable to suppose that the osmotic shock response could produce inhibitors of cell division. Finally, cell division is driven in part by turgor (11). If turgor is disturbed by osmotic stress, then there may be a “lack of signal” for cell division to proceed.
The longer, more numerous filaments and improved survival in reduced-aw TSB compared with NB could have been the result of acid habituation due to the presence of fermentable glucose in TSB. During overnight culture of Salmonella cells in TSB (containing 0.25% glucose), the pH of the culture decreased from around neutral to 4.5 (7). In any case, the presence of glucose can also lead to acid habituation at neutral pH by inducing a novel tolerance response (31). Acid habituation has been found to confer resistance to osmotic stress (23), so growth in TSB could cross-protect against osmotic stress.
The presence of live filaments after 144 h of incubation in TSB supplemented with 8% NaCl indicated that filamentation may improve survival under low-aw conditions which would kill cells that do not elongate. Further work is required to establish whether filamentation actually aids survival or if the normal (small-phenotype) cells that do survive go on to form filaments.
The filaments formed in response to a low aw are not dependent on rpoS expression since strains 10, I, and EAV54 (rpoS mutants) elongate; this is unlike the response to chilling, which is rpoS dependent (29). Thus, chilling appears to be more inhibitory to rpoS mutants than low aw is.
If filamentation is found to occur in foods, there are clear implications for low-aw food products. The DNA of a single contaminating Salmonella cell could continue to replicate, the biomass could increase, and long filaments could form in the food. Following an increase in the aw of the food (e.g., after rehydration with water), septation could resume and rapidly result in a large number of viable Salmonella cells, which could cause infection after consumption. The possible presence of filamentous Salmonella cells should also be considered when workers design methods for microbiological monitoring. The increase in Salmonella biomass (without a concomitant increase in the microbial count) would not be detected by traditional microbiological enumeration tests if a food product was tested by direct plating immediately after it was removed from low-aw storage conditions. The bacterial count would appear to be low, but under favorable conditions a rapid increase in the number of cells could occur in as little as 2 h. Note that testing for the presence of Salmonella cells by an enrichment method would probably not be negatively affected by the presence of filaments.
The survival and filamentation of Salmonella strains at low aw values clearly require further research to determine if the filamentation of Salmonella cells in food products (aw, 0.92 to 0.98) constitutes a risk to public health and to determine whether methods for microbiological monitoring are affected by the presence of filamentous Salmonella cells.
ACKNOWLEDGMENTS
We gratefully acknowledge funding provided by Nabisco Inc. and the Public Health Laboratory Service.
REFERENCES
- 1.Allen-Vercoe E, Dibb-Fuller M, Thorns C J, Woodward M J. SEF17 fimbriae are essential for the convoluted colonial morphology of Salmonella enteritidis. FEMS Microbiol Lett. 1997;153:33–42. doi: 10.1111/j.1574-6968.1997.tb10460.x. [DOI] [PubMed] [Google Scholar]
- 2.Angulo F J, Swerdlow D L. Salmonella Enteritidis infections in the United States. J Am Vet Med Assoc. 1998;213:1729–1731. [PubMed] [Google Scholar]
- 3.Anonymous. An outbreak of Salmonella agona due to contaminated snacks. Commun Dis Rep Weekly. 1995;5:29. , 32. [PubMed] [Google Scholar]
- 4.Anonymous. The rise and fall of salmonella? Commun Dis Rep Weekly. 1999;9:29. , 32. [PubMed] [Google Scholar]
- 5.Brown A D. Microbial water stress. Bacteriol Rev. 1976;40:803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan R L, Bagi L K. Effect of water activity and humectant identity on the growth kinetics of Escherichia coli O157:H7. Food Microbiol. 1997;14:413–423. [Google Scholar]
- 7.Buchanan R L, Edelson S G. Culturing enterohemorrhagic Escherichia coli in the presence and absence of glucose as a simple means of evaluating the acid tolerance of stationary-phase cells. Appl Environ Microbiol. 1996;62:4009–4013. doi: 10.1128/aem.62.11.4009-4013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukau B, Walker G C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989;171:2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano D A, Mouslim C, Ayala J A, Garcia-del Portillo F, Casadesus J. Cell division inhibition in Salmonella typhimurium histidine-constitutive strains: an ftsI-like defect in the presence of wild-type penicillin-binding protein 3 levels. J Bacteriol. 1998;180:5231–5234. doi: 10.1128/jb.180.19.5231-5234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian J H B, Scott W J. Water relations of salmonella at 30°C. Aust J Biol Sci. 1953;6:565–573. [PubMed] [Google Scholar]
- 11.Csonka L N, Hanson A D. Prokaryote osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 12.Gill O N, Sockett P N, Bartlett C L R, Vaile M S B. Outbreak of Salmonella napoli infection caused by contaminated chocolate bars. Lancet. 1983;i:574–577. doi: 10.1016/s0140-6736(83)92822-2. [DOI] [PubMed] [Google Scholar]
- 13.Glynn M K, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo F J. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 14.Graeme-Cook K A, May G, Bremer E, Higgins C F. Osmotic regulation of porin expression: a role for DNA supercoiling. Mol Microbiol. 1989;3:1287–1294. doi: 10.1111/j.1365-2958.1989.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood M H, Hooper W L. Chocolate bars contaminated with Salmonella napoli: an infectivity study. Br Med J. 1983;286:1394. doi: 10.1136/bmj.286.6375.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 17.Higgins C F, Dorman C J, Stirling D A, Waddell L, Booth I R, May G, Bremer E. A physiological role for DNA supercoiling in the osmoregulation of gene expression in S. typhimurium and E. coli. Cell. 1988;52:569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey T J, Williams A, McAlpine K, Lever M S, Guard-Petter J, Cox J M. Isolates of Salmonella enterica Enteritidis PT4 with enhanced heat and acid tolerance are more virulent in mice and more invasive in chickens. Epidemiol Infect. 1996;117:79–88. doi: 10.1017/s0950268800001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey T J, Williams A, McAlpine K, Jørgensen F, O'Byrne C. Pathogenicity in isolates of Salmonella enterica serotype Enteritidis PT4 which differ in RpoS expression: effects of growth phase and low temperature. Epidemiol Infect. 1998;121:295–301. doi: 10.1017/s0950268898001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jørgensen F, Stephens P J, Knochel S. The effect of osmotic shock and subsequent adaptation on the thermotolerance and cell morphology of Listeria monocytogenes. J Appl Bacteriol. 1995;79:274–281. [Google Scholar]
- 21.Killalea D, Ward L R, Roberts D, de Louvois J, Sufi F, Stuart J M, Wall P G, Susman M, Schweiger M, Sanderson P J, Fisher I S T, Mead P S, Gill O N, Bartlett C L R, Rowe B. International epidemiological and microbiological study of outbreak of Salmonella agona infection from a ready to eat savoury snack. I. England and Wales and the United States. Br Med J. 1996;313:1105–1107. doi: 10.1136/bmj.313.7065.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koelensmid W, Blanche A A, van Rhee R. Salmonella in meat products. Ann Inst Pasteur Lille. 1964;15:85–97. [PubMed] [Google Scholar]
- 23.Leyer G J, Johnson E A. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl Environ Microbiol. 1993;59:1842–1847. doi: 10.1128/aem.59.6.1842-1847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li K Y, Torres J A. Water activity relationships for selected mesophiles and psychrotrophs at refrigeration temperature. J Food Prot. 1993;56:612–615. doi: 10.4315/0362-028X-56.7.612. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd D, Hayes A J. Vigour, vitality and viability of microorganisms. FEMS Microbiol Lett. 1995;133:1–7. [Google Scholar]
- 26.Lutkenhaus J, Mukherjee A. Cell division. In: Neidhardt F C, editor. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1615–1626. [Google Scholar]
- 27.Marshall B J, Ohze D F, Christian J H B. Tolerance of bacteria to high concentrations of sodium chloride and glycerol in the growth medium. Appl Microbiol. 1971;21:363–364. doi: 10.1128/am.21.2.363-364.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles A A, Misra S S. The estimation of bacteriacidal power of blood. J Hyg. 1938;38:732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips L E, Humphrey T J, Lappin-Scott H M. Chilling invokes different morphologies in two Salmonella enteritidis PT4 strains. J Appl Microbiol. 1998;84:820–826. doi: 10.1046/j.1365-2672.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 30.Radman M. Phenomenonology of an inducible mutagenic DNA repair pathway in Escherichia coli: SOS repair hypothesis. In: Prakash L, Sherman F, Miller M, Lawrence C, Tabor H W, editors. Molecular and environmental aspects of mutagenesis. Springfield, Ill: Charles C Thomas; 1974. pp. 128–142. [Google Scholar]
- 31.Rowbury R J, Goodson M. Glucose-induced acid tolerance appearing at neutral pH in log-phase Escherichia coli and its reversal by cyclic AMP. J Appl Microbiol. 1998;85:615–620. doi: 10.1046/j.1365-2672.1998.853569.x. [DOI] [PubMed] [Google Scholar]
- 32.Rowe B, Begg N T, Hutchinson D N, Dawkins H C, Gilbert R J, Jacob M, Hales B H, Rae F A, Jepson M. Salmonella ealing infections associated with consumption of infant dried milk. Lancet. 1987;ii:900–903. doi: 10.1016/s0140-6736(87)91384-5. [DOI] [PubMed] [Google Scholar]
- 33.Scott W J. The water relations of Staphylococcus aureus at 30°C. Aust J Beef Sci. 1953;6:549. [PubMed] [Google Scholar]
- 34.Shaw M K. Formation of filaments and synthesis of macromolecules at temperatures below the minimum for growth of Escherichia coli. J Bacteriol. 1968;95:221–230. doi: 10.1128/jb.95.1.221-230.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shohat T, Green M S, Merom D, Gill O N, Reisfeld A, Matas A, Blau D, Gal N, Slater P E. International epidemiological and microbiological study of an outbreak of Salmonella agona infection from a ready to eat savoury snack. II. Israel. Br Med J. 1996;313:1107–1109. doi: 10.1136/bmj.313.7065.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamminga S K, Beumer R R, Kampelmacher E H. Survival of Salmonella eastborne and Salmonella typhimurium in chocolate. J Hyg. 1976;76:41–47. doi: 10.1017/s0022172400054929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trollmo C, André L, Blomberg A, Adler L. Physiological overlap between osmotolerance and thermotolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1988;56:321–326. [Google Scholar]
- 38.Tsuchido T, Van Bogelen R A, Neidhardt F C. Heat shock response in Escherichia coli influences cell division. Proc Natl Acad Sci USA. 1986;83:6959–6963. doi: 10.1073/pnas.83.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Völker U, Mach H, Schmid R, Hecker M. Stress proteins and cross-protection by heat shock and salt stress in Bacillus subtilis. J Gen Microbiol. 1992;138:2125–2135. doi: 10.1099/00221287-138-10-2125. [DOI] [PubMed] [Google Scholar]
- 40.Wall P G, Morgan D, Lamden K, Ryan M, Griffen M, Threlfall E J, Ward L R, Rowe B. A case control study of infection with an epidemic strain of multiresistant Salmonella typhimurium DT104 in England and Wales. Commun Dis Rep Rev. 1994;4:R130–R135. [PubMed] [Google Scholar]
- 41.Williams A, Davies A C, Wilson J, Marsh P D, Leach S, Humphrey T J. Contamination of the contents of intact eggs by Salmonella typhimurium DT104. Vet Rec. 1998;143:562–563. [PubMed] [Google Scholar]
- 42.Yoshida S, Udou T, Mizuguchi Y, Tanabe T. Salt-induced filamentous growth of a Salmonella strain isolated from blood. J Clin Microbiol. 1986;23:192–194. doi: 10.1128/jcm.23.1.192-194.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]